Introduction
Parkinson's disease
Parkinson's disease (PD) is the most common age-related motor deteriorating neurodegenerative disease characterized by four cardinal signs: rigidity, bradykinesia, postural instability, and tremor (Samii, Nutt et al. 2004). It affects 1-2% of the population above 65 years of age. It is characterized by the progressive loss of dopaminergic neurons of the substantia nigra pars compacta (SN) with motor symptoms appearing when approximately 50-60% of these neurons degenerate leading to a 70-80% depletion of dopamine (DA) levels in the dorsal striatum where these neurons project (Fearnley and Lees 1991). Other than decreases in striatal DA, another major pathological identifier of PD is α-synuclein positive neuronal inclusions called Lewy bodies. There is no cure and the best treatment option, L-DOPA, only provides symptomatic relief with no abatement for the progression of PD. A large majority of diagnosed PD cases are idiopathic and sporadic; however, autosomal dominant and recessive familial forms of the disease have been identified.
Mitochondrial dysfunction is often cited as a primary or secondary contributor to neurondegenerative events, especially in PD. Mitochondria are unique in that they contain multiple copies of their own ∼16.5 kbp genome. Mitochondrial DNA (mtDNA) is transcribed and translated within the mitochondria and contributes subunits to all complexes of the oxidative phosphorylation (OXPHOS) pathway, except complex II (Anderson, Bankier et al. 1981). OXPHOS is a metabolic pathway used by mitochondria to produce adenosine triphosphate (ATP), whose production is vital for cellular function, signaling pathways, and overall cell viability. This is true for all cells; however, the reliance on proper mitochondrial function is particularly high for neurons due to their post-mitotic status, unique electrophysiological properties, and high ATP demand.
How can an organelle that is essential for all neurons play a role in a selective neuron loss when it becomes dysfunctional? Understanding how and why certain neuronal populations, such as those in the SN, are more sensitive to mitochondrial dysfunctions will help develop treatments to prevent and delay neurodegenerative events.
In this review, we will focus on transgenic mouse models of PD that are associated with mitochondrial defects. We will examine, in particular, how mitochondria become dysfunctional in these models and look for commonalities and possible contributors that would lead to a better understanding of the OXPHOS function in the pathophysiology of PD.
Transgenic Mouse Models of PD
Complex I Based Models
Early descriptions suggested that mitochondrial dysfunction played an important role in PD. PD post-mortem brains had decreased mitochondrial complex I activity in the affected SN (Schapira, Cooper et al. 1990; Schapira, Mann et al. 1990). Also, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and its metabolized toxic byproduct 1-methyl-4-phenylpyridinium (MPP+) were originally shown to cause atypical Parkinsonism by inhibiting complex I selectively in DA neurons (Burns, LeWitt et al. 1985; Nicklas, Vyas et al. 1985). Dopaminergic neurodegeneration and Parkinsonism phenotypes also appeared in rodents after exposure to complex I inhibiting pesticides (Betarbet, Sherer et al. 2000; Thiruchelvam, Richfield et al. 2000). These observations suggested that complex I inhibition was a major player in the risk, development, and progression of PD.
Although these first original findings occurred in the early 90's, not until recently genetic complex I knockout mouse models were available to test complex I's involvement in PD. The first complex I deficient mouse model was the Ndufs4 (a complex I subunit) knockout mouse (Kruse, Watt et al. 2008). The systemic Ndufs4−/− mouse has a very severe phenotype dying prematurely at 7 weeks of age, and although these mice display motor coordination phenotypes with decreased Complex I assembly in the brain, the central nervous system (CNS) does not show any major gross neuroanatomical defects (Kruse, Watt et al. 2008). A follow up study from the same laboratory utilized a Nestin driven-cre to specifically knockout Ndufs4 in glia and neurons (Quintana, Kruse et al. 2010). These mice showed a progressive degeneration of the vestibular nuclei, olfactory bulb, and cerebellum due to neuroinflammation, abnormal mitochondrial morphology, and high levels of oxidative damage in these same neuroanatomical regions (Quintana, Kruse et al. 2010). However, in both of these models, there was no detectable regional vulnerability or degeneration of the midbrain region. This mouse was crossed with the dopamine transporter (DAT) promoter driven-Cre recombinase line to inactivate complex I specifically in DA neurons; although there was no SN degeneration or Parkinsonism phenotype in these mice, they showed signs of DA dysregulation and increased sensitivity to MPTP treatment (Sterky, Hoffman et al. 2012). These findings led to a reconsideration of previous thoughts that complex I deficiency can be a sole causing factor in PD pathogenesis.
General Mitochondrial Dysfunction Mouse Models
Although complex I defects contribute to PD, it appears that, to mimic PD, it is also important to model a general OXPHOS complex deficiency in dopaminergic neurons. In PD post-mortem and healthy aged SN neurons, mtDNA mutation loads were found to reach ∼60% (ratio of mtDNA deleted molecules to wild type molecules) and positively correlated with cytochrome c oxidase (complex IV) deficiency (Bender, Krishnan et al. 2006; Kraytsberg, Kudryavtseva et al. 2006; Reeve, Krishnan et al. 2008). Other findings also point to a more global mitochondrial-related energy disruption in PD. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) is a transcriptional co-activator that regulates nuclear and mtDNA related gene expression, increasing mitochondrial biogenesis (Wu, Puigserver et al. 1999). PGC-1α was found to be down-regulated in PD post-mortem brains (Zheng, Liao et al. 2010). These evidences suggest that general mitochondrial dysfunctions contribute in conferring a risk for PD or in exacerbating its pathophysiology.
Currently, there are two mouse models of Parkinsonism that are based on mtDNA disruption to create an OXPHOS deficiency in dopaminergic neurons. The first was the “Mito-Park” mouse which employs the usage of a DAT driven-Cre to inactivate the TFAM gene (mitochondrial transcription factor A) in dopaminergic neurons (Ekstrand, Terzioglu et al. 2007). TFAM maintains mtDNA function by initiating transcription of its encoded genes and replicating the mtDNA genome; its loss leads to an mtDNA depletion (Dairaghi, Shadel et al. 1995; Kanki, Ohgaki et al. 2004). This mouse model recapitulates key features of human PD with loss of SN neurons, depletion of DA in the striatum, loss of voluntary movement, tremors, responsiveness to L-DOPA treatment, and abnormal mitochondrial aggregates (Ekstrand, Terzioglu et al. 2007). Another model, which was developed in our laboratory, causes double strand breaks in the mtDNA via the expression of a mitochondrial targeted endonuclease (mito-PstI) in dopaminergic neurons (Pickrell, Pinto et al. 2011). Mito-PstI expression leads to both the formation of rearranged and depleted mtDNA causing a subsequent OXPHOS deficiency and disruption in the mitochondrial membrane potential (Δψm) (Srivastava and Moraes 2005; Fukui and Moraes 2009; Pickrell, Fukui et al. 2011). This “PD-mito-PstI” mouse has similar behavioral and biochemical phenotypes to the Mito-Park mouse except for a slower progression of dopaminergic neuron loss with ∼60% SN neurons lost after 9 months as opposed to a more severe degenerative phenotype in the Mito-Park mouse (Ekstrand, Terzioglu et al. 2007; Pickrell, Pinto et al. 2011). The PD-mito-PstI model also showed that motor behavior disruptions appear prior to the SN cell body loss at time points where DA axonal loss and DA depletion in the dorsal striatum occur (Pickrell, Pinto et al. 2011). The Mito-Park and PD-mito-PstI mouse clearly demonstrated the importance of mitochondrial integrity in PD.
Mouse Models Manipulating Genes Found to be Mutated in Familial PD
A majority of PD cases (over ∼95%) are sporadic with unknown etiology. However, there are reported early-onset familial PD patients (comprising the other 5%) caused by mutations in genes that encode products that localize to or affect mitochondria integrity and function. Mouse models that either knockout the endogenous genes or overexpress the mutant forms of these genes have been created to study their effects in vivo. Most of these models poorly recapitulated the motor phenotypes and the typical neurodegeneration present in human PD patients. The most common proteins affecting mitochondrial function that are mutated in familial cases of PD are LRRK2, Pink1, Parkin, DJ-1 and α-synuclein.
The most common autosomal dominant mutations for PD are in the gene PARK8, encoding the protein leucine-rich repeat kinase 2 (LRRK2) (Paisan-Ruiz, Jain et al. 2004; Zimprich, Biskup et al. 2004). The familial mutations in LRRK2 appear to increase its kinase activity, which may confer its toxicity (Smith, Pei et al. 2006). LRRK2 has been shown to localize in the cytoplasm but also to associate with the mitochondrial outer membrane (West, Moore et al. 2005). In cell culture, LRRK2 mutations led to OXPHOS dysfunction and abnormalities in mitochondrial dynamics (Mortiboys, Johansen et al. 2010; Wang, Yan et al. 2012). However, the specific role that LRRK2 exerts on mitochondrial function and whether these observations are true in vivo is still unclear.
To answer this question, LRRK2 transgenic mouse models were created that express point mutations in the GTPase or mixed-lineage kinase (MLK) domains, modeling common disease-causing PARK8 mutations. The first reported LRRK2 PD transgenic mouse model overexpressed human mutant LRRK2 (R1441G) from a bacterial artificial chromosome (BAC) (Li, Liu et al. 2009). This mouse model showed progressive motor coordination abnormalities reversed with L-DOPA or apomorphine (a dopamine agonist); it showed defects in striatal DA release and dystrophic tyrosine hydroxylase (the rate limiting enzyme in DA synthesis) (TH+) neurites, but lacking DA cell body degeneration (Li, Liu et al. 2009). A knock-in LRRK2 mouse with the R1441C point mutation was developed around the same time as the R1441G BAC mouse; however, this model showed no behavioral phenotypes, no DA neurodegeneration, and only mild alterations in DA release and compromised D2 auto-receptor function (Tong, Pisani et al. 2009). Another BAC transgenic LRRK2 mouse with the G2019S mutation displayed no clear motor phenotypes or DA neurodegeneration, with only a slight ∼25% reduction in striatal DA levels at 12 months-of-age (Li, Patel et al. 2010). None of these transgenic strains clearly represent the human PD diseased state.
LRRK2 point mutations are autosomal dominant suggesting that they were cases where the protein had a gain of function. In fact, the LRRK2 knockout mouse created by the removal of exons 39-40 showed no overt phenotype, nigrostriatal defects, or DA neuronal loss (Andres-Mateos, Mejias et al. 2009). LRRK2 null mice displayed a lack of hypersensitivity to MPTP treatment questioning the significance of LRRK2's association with the outer mitochondrial membrane (Andres-Mateos, Mejias et al. 2009). It has yet to be determined if any mitochondrial deficits occur as a result of LRRK2 mutations in vivo. With the observations made from these transgenic LRRK2 mice and the LRRK2−/− mouse, LRRK2's functional role associating the pathogenetic mechanism with the mitochondria is unclear.
PARK2 and PARK6 genes, encoding Parkin and Pink1 (PTEN induced kinase 1) proteins respectively, are mutated in early-onset familial cases of PD (Kitada, Asakawa et al. 1998; Valente, Bentivoglio et al. 2001). Parkin and Pink1 interact within the same pathway, affecting mitochondrial function and influencing its morphology (Deng, Dodson et al. 2008; Poole, Thomas et al. 2008). Parkin and Pink1 also promote mitophagy, the removal of dysfunctional mitochondria, by targeting those with low mitochondrial membrane potential (Δψm) or pathogenic mtDNA mutations (Narendra, Tanaka et al. 2008; Geisler, Holmstrom et al. 2010; Narendra, Jin et al. 2010; Suen, Narendra et al. 2010; Vives-Bauza, Zhou et al. 2010). New studies also suggest an interaction between mitochondrial dynamics and Parkin-mediated mitophagy as Parkin and Pink1 have been found to affect Miro levels, a mitochondrial motor adaptor protein, to enhance mitophagic clearance (Wang, Winter et al. 2011; Liu, Sawada et al. 2012). Mutations in Pink1 and Parkin confer a loss of function causing defects in the surveillance of mitochondrial quality (Geisler, Holmstrom et al. 2010). Although most of the molecular mechanisms of Parkin-mediated mitophagy have been revealed, mouse models have not been able to answer how Parkin and Pink1 function in vivo.
Multiple labs have independently created Parkin germline knockout mice. One of the first mice described lacked Parkin exon 3. It exhibited only mild phenotypes on beam traverse or sticker removal examinations monitoring fine motor skills, and slight DA abnormalities or loss; it did not show any neurodegeneration and only its medium spiny neurons seemed to be affected, being less sensitive to evoked action potentials (Goldberg, Fleming et al. 2003). Similarly, the deletion of exon 7 of Parkin did not cause motor behavioral phenotypes nor DA neurodegeneration, but a heightened startle response with a loss of TH+ neurons in the locus coeruleus with concordant reductions in norephiphrine in the CNS (Von Coelln, Thomas et al. 2004). Perez and Palmiter performed an exhaustive behavioral study on their Parkin null mouse, lacking exon 2, to arrive to the conclusion that the knockout mouse had no real progressive motor phenotype, no DA neurochemical defects in the striatum, and no DA neurodegeneration of the SN or ventral tegmental area (VTA) regions (Perez and Palmiter 2005). Parkin knockout mice disappointingly modeled PD questioning how loss of function mutations cause disease in humans.
Pink1−/− mice have also been created to mimic the loss of function mutations seen in familial PD causes. A deletion in exons 4-7 of Pink1 resulted in animals showing no signs of DA neurodegeneration or defects in striatal DA synthesis or degradation; however, the authors reported deficits in long term potentiation and depression (LTP and LTD) attributed to diminished evoked DA release (Kitada, Pisani et al. 2007). A Pink1 null mouse with an exon 4-5 deletion displayed a progressive loss of DA in the striatum; however, there was no nigrostriatal degeneration leaving the loss of DA unexplained (Akundi, Huang et al. 2011).
Calcium regulation/homeostasis and mitochondrial impairments have been reported as features in some of the Pink1 knockout mouse models. Pink1−/− derived primary neurons and MEFs displayed reduced Δψm, impairments in respiration, Ca++ overload in mitochondria, and heightened reactive oxygen species production (Gandhi, Wood-Kaczmar et al. 2009; Heeman, Van den Haute et al. 2011). Pink1−/− mice also have reduced ATP levels in the striatum during in vivo imaging (Heeman, Van den Haute et al. 2011). In isolated mitochondria from this region of older Pink1 null mice, there were defects in complex I activity during state 3 and 4 respiration (Gautier, Kitada et al. 2008). Mitochondria derived from Pink1 null mice also have reduced calcium buffering capacity (Akundi, Huang et al. 2011). Although these mitochondrial dysfunctions have been described, it is unclear how Pink1−/− DA neurons are still viable and able to overcome these bioenergetic crisises.
Mutations in PARK7, that encodes the protein DJ-1, are another cause of the autosomal recessive forms of early-onset PD (Bonifati, Rizzu et al. 2003). DJ-1, particularly its oxidized form, has been found to localize to mitochondria in the inner membrane space and matrix, as well as in the cytoplasm (Canet-Aviles, Wilson et al. 2004; Zhang, Shimoji et al. 2005). DJ-1 is thought to be an oxidative stress sensor modulating different cellular defense pathways such as Akt and Cu-Zn superoxide dismutase 1 (SOD1) to mediate protection (Aleyasin, Rousseaux et al. 2010; Wang, Liu et al. 2011). DJ-1 binds mitochondrial and glutathione peroxidase transcripts, altering their expression levels under oxidative stress (van der Brug, Blackinton et al. 2008). It also acts as an atypical peroxidase to scavenge hydrogen peroxide (Andres-Mateos, Perier et al. 2007). Finally, DJ-1 can also interact with Pink1 and Parkin in an E3 ligase complex to mediate the ubiquitination of Parkin in the cytoplasm and mitochondria (Xiong, Wang et al. 2009). This multi-dimensional protein confers many effects, directly or indirectly upon mitochondria, relevant to dysfunctions reported in PD.
As with the Parkin and Pink1 knockout mice, multiple groups have created variations of the DJ-1 knockout mice with similar findings between the independent strains. The DJ-1 knockout mouse with a deleted exon 2 showed no nigrostriatal degeneration, and no disruption in DA synthesis or metabolism; however, these mice did display some deficits in spontaneous movement with reductions in DA uptake in the striatum due to impaired D2 receptors (Goldberg, Pisani et al. 2005). Independently, another DJ-1 knockout mouse with a deletion of exons 3-5 was found to have no dopaminergic neurodegeneration or overall DA depletion in the striatum (Kim, Smith et al. 2005). A DJ-1 knockout mouse with exons 2-3 deleted showed similar characteristics with a lack of a progressive DA neurodegeneration, but was also described to have a compensatory increase in oxidative defenses (such as glutathione peroxidase, catalase, and manganses superoxide dismutase) that further solidified DJ-1's role as a oxidative stress sensor (Andres-Mateos, Perier et al. 2007).
Although these DJ-1 deletion mice did not display cardinal signs of PD or have neurodegeneration, mitochondrial abnormalities have been found to be associated with these mice. They have been reported to have higher levels of mitochondrial ROS thought to result from the down-regulation of uncoupled proteins in the SN neurons (Guzman, Sanchez-Padilla et al. 2010). Fragmented mitochondria and abnormal mitochondrial dynamics are present in primary cortical neurons cultures and mouse embryonic fibroblasts (MEFs) derived from DJ-1−/−mice (Irrcher, Aleyasin et al. 2010). Moreover, DJ-1 null mouse models have shown hypersensitivity to MPTP and paraquat treatment indicating a role for DJ-1's involvement in oxidative stress defense in vivo (Kim, Smith et al. 2005; Yang, Chen et al. 2007).
Alpha-synuclein (α-syn) is one of the major components of intracytoplasmatic inclusions, a characteristic pathology of PD post-mortem brains called Lewy bodies (Spillantini, Schmidt et al. 1997). Lewy bodies are also not solely recognized as part of the pathology for PD, but are also present in other synucleinopathies such as Multiple Systems Atrophy and Lewy Body disease, where other neurons are targeted for degeneration (Papka, Rubio et al. 1998; Goedert 2001). Familial cases of PD derived from mutations, the most common being A53T and A30P, in the α-syn gene (SNCA) are autosomal dominant, thus gain of function has been associated to α-syn mutations (Polymeropoulos, Lavedan et al. 1997; Kruger, Kuhn et al. 1998). This protein, of unknown function, is mainly cytoplasmic but it localizes partially to the mitochondrial membranes, in particular at the synapses and is known to impair complex I activity in a dose dependent manner (Li, Yang et al. 2007; Devi, Raghavendran et al. 2008; Nakamura, Nemani et al. 2008; Liu, Zhang et al. 2009).
There have been several mouse models that have been created to overexpress wild-type or mutant forms of α-syn under the control of various promoters in attempts to model Parksinson's. The creation of independent strains utilizing the prion protein promoter (PrP)-driven human α-syn with the A53T point mutation transgenic mouse model present debilitating motor phenotypes causing premature death with signs of α-syn accumulation and astrogloisis (Giasson, Duda et al. 2002; Lee, Stirling et al. 2002). Another model drove mutant α-synuclein under the Thy-1 neuronal specific promoter reporting progressive motor coordination decline, degeneration of the neuromuscular junction, and α-synuclein aggregates throughout the CNS and spinal cord (van der Putten, Wiederhold et al. 2000). These A53T transgenic mice develop mitochondria with abnormal morphology, Wallerian degeneration, and display a complex IV deficiency in spinal cord homogenates, which is one of the most affected tissues in this model (Giasson, Duda et al. 2002; Martin, Pan et al. 2006). One transgenic model driving the human A53T α-syn gene under the platelet derived growth factor β promoter displayed moderate reductions in DA neurons and TH levels in the striatum with a mild motor coordination phenotype at 12 months of age, nevertheless there was no neuroanatomical restrictions on where the α-synuclein inclusions were located (Masliah, Rockenstein et al. 2000). Most of these past models displayed no dopaminergic death or caused no direct nigrostriatal abnormalities, even a transgenic model driving the human A53T α-syn by the tyrosine hydroxylase promoter (van der Putten, Wiederhold et al. 2000; Matsuoka, Vila et al. 2001; Giasson, Duda et al. 2002; Lee, Stirling et al. 2002). A recent publication, Lin et al. expressed mutant A53T α–synuclein selectively in dopaminergic neurons after birth. The mice developed Parkinsonism motor phenotypes and had an age-related loss of SN and VTA neurons; however, mitochondrial function was not extensively studied (Lin, Parisiadou et al. 2012). A previous study from the same group using their conditional A53T α–synuclein transgenic mouse under a different neuronal promoter found evidence of mitochondrial damage in those neurons. Therefore, it is plausible that mitochondrial function is disturbed in these dopaminergic neuron-targeted synuclein model (Lin, Parisiadou et al. 2009).
Although it is known that α-synuclein exerts a gain of function effect, knockout α-syn mice were created in attempts to elucidate its endogenous function. A model removing exons 4-5 in the α-syn gene showed no neurodegeneration or gross abnormalities of the CNS, but reductions in the number undocked synaptic vesicles were observed, which led to disruptions during repetitive stimulation and replenishment of the reserve pool was slow (Cabin, Shimazu et al. 2002). Another model targeting α-syn first two exons described similar gross CNS findings, but found reduced DA content in the striatum and defects in post-synaptic depression due to altered DA neurotransmission (Abeliovich, Schmitz et al. 2000). Studies found that the exposure to MPTP and pesticides, complex I inhibitors, attenuated the drugs' toxic effects in α-syn null mice leading to modest decreases in striatal dopamine loss and SN degeneration (Dauer, Kholodilov et al. 2002; Klivenyi, Siwek et al. 2006). These results suggest that α-synuclein can influence mitochondrial function.
Limitations of Using Mouse Models of PD
One of the main concerns in using rodents as models to mimic and study PD pathogenesis is that none of the genetic models based on familial PD mutations created until now, recapitulates all the PD features. One of the potential problems connected with this issue is the inactivation of PD-linked genes in the germline. The catecholamine neurotransmitter system has the propensity to compensate during development for gene inactivation that would otherwise cause degeneration and dysfunction in an adult animal as observed with the conditional knockout of glial derived nerve factor (Pascual, Hidalgo-Figueroa et al. 2008). In PD-mito-PstI mouse for example, we observed alterations of other neurotransmitter systems that could compensate for the disruption in the DA circuitry. This could perhaps explain why this mouse model does not show a worsening of the motor phenotype even when more SN neurons degenerate (Pickrell, Pinto et al. 2011). A possible explanation comes from the novel conditional Parkin knockout mouse, where DA degeneration in the SN was reported. This contrasts with Parkin models with germline inactivation, which have little or no phenotype (Perez and Palmiter 2005). Therefore, it seems important to understand how germline inactivation and possible compensatory mechanisms affect the CNS.
Due to lack of techniques to monitor in vivo processes like mitophagy, it is also not known whether a rodent model is useful to study the removal of dysfunctional mitochondria by the Pink1/Parkin pathway. Dysfunctional mitochondria clearance seems to be an important process in post-mitotic neurons as in ATG7 and ATG5 null mice, where autophagy is blocked in the central nervous system (CNS). In these models, there was an accumulation of amorphous mitochondria with irregular morphology in neurons (Hara, Nakamura et al. 2006; Komatsu, Waguri et al. 2006). Curiously, when Sterky et al. crossed a Parkin −/− mouse with the Mito-Park mouse, they found no exacerbation of the phenotype, concluding that Parkin involvement in the clearance of dysfunctional mitochondria may not be important in mice (Sterky, Lee et al. 2011). An alternative explanation would be that the mito-Park mouse has no way for the mitochondria to compensate for the loss of both mtDNA replication and transcription with the mitochondrial defect being so severe that even a impairment in mitophagy would not worsen the already serious phenotype. Development of new techniques or new reporter mouse strains may be needed to fully conclude the role of Pink1 and Parkin in the mouse CNS.
Another major difference between mouse and humans SN is the absence of neuromelanin in the mice. Neuromelanin positive SN cells are often more susceptible in the pathology of human PD (Hirsch, Graybiel et al. 1988). Neuromelamin binds iron and accumulates as a byproduct of dopamine synthesis due to levels of increased cytosolic DA leading to the formation of toxic quinones and semiquinones (Sulzer, Bogulavsky et al. 2000; Zecca, Stroppolo et al. 2004). In rodent models, the expression of neuromelanin leads to DA neuronal loss due to oxidative stress and microglial activation (Zhang, Phillips et al. 2011). It has also been shown in aged human and PD patients that neuromelamin positive neurons harbor more mtDNA deletions than in SN that are non-pigmented in the same area (Elstner, Muller et al. 2011). Because the impact of neuromelanin is often not recapitulated in mouse models, its effect on mitochondrial function or exact contribution to PD patholophysiology is uncertain, although it raises the possibility that it could be part of the explanation for the differences in susceptibility between humans and mice.
Mitochondrial Dysfunction and Select Neuronal Vulnerability in PD
As mentioned above, in the brains of PD patients, there is only a substantial loss of SN neurons whereas neighboring mesolimbic-derived DA neurons of the ventral tegmental area (VTA) are spared. The Mito-Park mouse and PD-mito-PstI mouse showed preferential progressive degeneration of the SN over VTA regions (Ekstrand, Terzioglu et al. 2007; Pickrell, Pinto et al. 2011). It has been shown that SN neurons have higher metabolic transcript levels, suggesting that they are more reliant on mitochondrial function and that mitochondria from these areas could vary greatly in their response to OXPHOS dysfunction, especially after compromised mtDNA stability (Chung, Seo et al. 2005; Greene, Dingledine et al. 2010). Clearly, more research is needed to elucidate how mitochondrial function confers a selective risk for SN neurons to degenerate.
Future Directions and Perspectives: Increased Mitochondrial Biogenesis
It has been suggested that increasing mitochondrial biogenesis through the overexpression or pharmacological stimulation of PGC-1α could ameliorate mitochondrial dysfunction in PD and provide a potential therapy (Zheng, Liao et al. 2010). In cell culture models exposed to rotenone or overexpressing α-synuclein, PGC-1α overexpression has been shown to provide protective effects for TH+ neurons (Zheng, Liao et al. 2010). The protective role of this protein was further confirmed in MPTP treated rodents and in a conditional Parkin KO mouse model (Shin, Ko et al. 2011; Mudo, Makela et al. 2012). However, viral overexpression of PGC-1α caused unexpected DA dysregulation and neurodegeneration (Ciron, Lengacher et al. 2012). From these conflicting descriptions, it seems that differences in the expression levels of this protein are very important to mitigate or to enhance its protective effects. Often, PD patients are not diagnosed until over 60% of SN neurons are lost, so it is unclear if PGC-1α will provide benefits after diagnosis in humans. Unfortunately, the study of protective role of this protein in rodents have some technical problems, as it causes off target effects, such as transcriptionally co-regulating the gene expression of parvalbumin, a calcium binding protein present in a particular GABAergic neuronal population (Lucas, Markwardt et al. 2010; Ciron, Lengacher et al. 2012).
Also, increasing the translation of complex I subunits Ndufs2 and Ndufs3 by the transcription factor Engrailed was protective in MPTP PD mouse models (Alvarez-Fischer, Fuchs et al. 2011). It remains unknown if Engrailed is an alternative viable option, as it also controls other biological functions like axonal guidance (Brunet, Weinl et al. 2005). Researchers are looking towards alternative ways to boost mitochondrial function producing the protective effects of these proteins without all of the possible risks derived from multiple downstream effects.
Final Considerations
We reviewed transgenic mouse models of PD mainly focused on those with or associated with mitochondrial dysfunction (Table 1). Complex I knockout mice appear quite resistant to degeneration of the nigrostriatal system while other neuroanatomical regions appear to be more sensitive to the deficiency (Quintana, Kruse et al. 2010; Sterky, Hoffman et al. 2012). Complex I alone probably is not the sole causal factor in the pathophysiology of PD and could be a secondary side effect as this deficiency is even present in platelets and other tissues unaffected in PD patients (Mann, Cooper et al. 1992; Gu, Gash et al. 1997). When examining OXPHOS deficient mouse models, they appeared similar, with L-DOPA reversible motor disturbances, age related SN degeneration, and DA depletion in the striatum mimicking closely progression of human PD (Ekstrand, Terzioglu et al. 2007; Pickrell, Pinto et al. 2011). These transgenic mouse models are based on a global disruption of mitochondrial function increasing their validity; however, it is unclear how differences between Complex I inhibition alone and mtDNA depletion lead to a more disrupted dopaminergic neuron with less viability as seen in the latter case. Future studies are needed to elucidate how mitochondrial dysfunction affects particular neuronal subtypes in vivo and how other surrounding neuron types and glial cells react to this progressive loss of dopaminergic neurons.
Table 1. Summary of the Mouse Models of Parkinson 's Disease Associated with Mitochondrial Dysfunction.
The table describes the genetic manipulation used to create the PD mouse model and the phenotypes and features that are present in each model.
| Parkinson's Disease Mouse Models | Genetic manipulation | Motor Phenotypes | PD Pathology | DA Defects | SN DA Neurodegeneration | References |
|---|---|---|---|---|---|---|
| Parkin −/− | germline inactivation | Conflicting: either absent or subtle fine motor movement disturbed | NO | NO | NO | (Goldberg et. al. 2003; Perez and Palmiter 2005; Von Coelln et. al. 2004) |
| Conditional Parkin −/− | Lentivirus-GFP Cre injected in SN at 6-8 weeks of age; loxed p Parkin | ? | ? | ? | Yes, 10 months after injection | (Shin et. al. 2011) |
| DJ-1 −/− | germline inactivation | Absent | NO | Impaired DA uptake | NO | (Goldberg et. al. 2003; Kim et. al. 2005) |
| Pink1 −/− | germline inactivation | Absent | NO | Impaired evoked DA release | NO | (Akundi et. al. 2011; Kitada et. al. 2007) |
| LRRK2 −/− | germline inactivation | Absent | ? | NO | NO | (Andres-Mateos et. al. 2009) |
| Alpha synuclein −/− | germline inactivation | Absent | NO | Impared DA release Slightly lowered DA levels in the striatum |
NO | (Abeliovich et. al. 2000; Cabin et. al. 2002) |
| LRRK2 transgenics | BAC human LRRK2 R1441G, G2019S BACKnockin LRRK2 R1441C |
Varies from Absent to Severe (L-DOPA responsive) | NO | DA reduction in the striatum Impaired DA release |
DA Neurite Degeneration | (Li et. Al. 2010; Li et.al. 2009; Tong et. al. 2009) |
| alpha synuclein transgenics | hA53T α-synuclein, PDGFβ promoter hA53T α-synuclein, Thy1 promoter hA53T α-synuclein, PrP promoter |
Severe leading to paralysis and premature death | YES | NO | NO | (Giasson et al. 2002; Kruse et al. 2008; Martin et. al. 2006; van der Puttern et al. 2000) |
| Ndusf4 −/− | DAT driven cre; loxed p Ndsuf4 (Complex I subunit) | Absent | ? | Impared DA release | NO | (Sterky et. al. 2011) |
| “Mito-Park” | DAT driven cre; loxed p TFAM | begins at 3-4 months; declines in spontaneous activity and rearing tremors; poor conditions at 8mo (sacrificed) (L-DOPA reversible) |
Abnormal mitochondrial aggregates | DA reduction in the striatum | Yes, progressive | (Ekstrand et. al. 2007) |
| PD-mito-PstI | trangenic mitochondrial targetted restriction endonuclease to mtDNA | begins at 2-4 months; declines in spontaneous activity(L-DOPA reversible) |
Absent | DA reduction in the striatum | Yes, progressive | (Pickrell, Pinto et. al. 2011) |
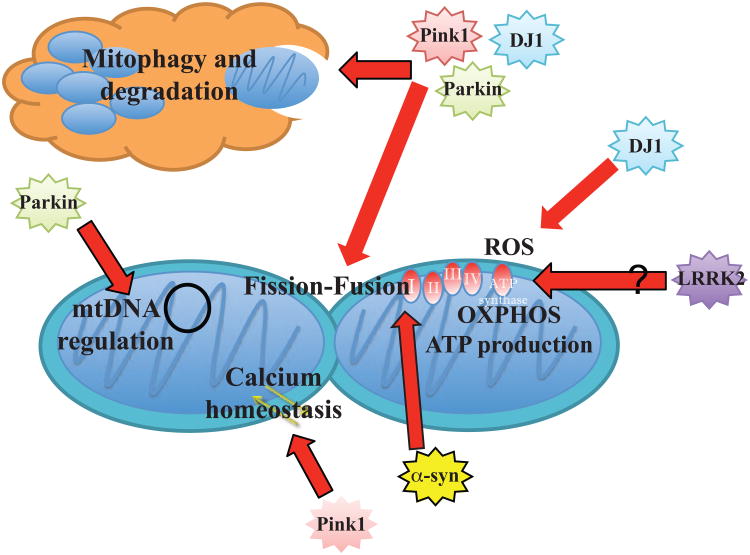
We also discussed transgenic mouse models that show gain or loss of function mutations present in familial PD cases (Figure 1). All of the DJ-1, Parkin, and Pink1 knockout mice lack significant degeneration of dopaminergic neurons, present little to no depletion of DA in the striatum, and have either absent or mild motor phenotypes. Another study that crossed all of the strains generating a triple knockout resulted in similar findings with no DA neurodegeneration (Kitada, Tong et al. 2009). Researchers need to invest in the creation of conditional knockout models for DJ-1 and Pink1 to understand if their loss of function alone is sufficient to cause DA neuron loss. Until then, it will remain unclear how the loss of DJ-1, Parkin, and Pink1 cause PD in familial cases. These models reported problems in the evoked DA response in the striatum, and evoked DA release was also attenuated in presymptomatic Mito-Park mice suggesting a strong involvement for mitochondria (Good, Hoffman et al. 2011). However, researchers have yet to fully understand how mitochondrial dysfunction impacts DA synaptic transmission and how that may progress to cause cell death in DA neurons. The different LRRK2 mice generated with different point mutations displayed more nigrostriatal defects and more severe motor deficits; however, whether its localization to the mitochondria and affects its function remains to be seen.
Figure 1. Endogenous Roles the Familial PD-implicated Proteins have on Mitochondria Function.
Stars represent the different proteins found with mutations that are linked with familial PD cases. Grouped stars indicate a shared interaction between different individual proteins. Red arrows point to outcomes that affect aspects of mitochondrial biology. LRRK2's localization and effects on mitochondria are unknown.
Mitochondrial dysfunction is a reoccurring feature in PD and with the appropriate mouse models we are coming closer to understanding how mitochondria play a role in the selective degeneration of DA neurons.
Acknowledgments
This work was supported in part by the National Institutes of Health Grant EY010804, AG036871, NS041777 and the Muscular Dystrophy Association (C.T.M). A.M.P. was supported by 5T32NS007492, 5T32NS007459, American Heart Association Grant 11Pre7610007, and Lois Pope LIFE Fellowship.
Footnotes
Conflicts of Interest Statement: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abeliovich A, Schmitz Y, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25(1):239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Akundi RS, Huang Z, et al. Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS One. 2011;6(1):e16038. doi: 10.1371/journal.pone.0016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleyasin H, Rousseaux MW, et al. DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc Natl Acad Sci U S A. 2010;107(7):3186–3191. doi: 10.1073/pnas.0914876107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Fischer D, Fuchs J, et al. Engrailed protects mouse midbrain dopaminergic neurons against mitochondrial complex I insults. Nat Neurosci. 2011;14(10):1260–1266. doi: 10.1038/nn.2916. [DOI] [PubMed] [Google Scholar]
- Anderson S, Bankier AT, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Andres-Mateos E, Mejias R, et al. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) J Neurosci. 2009;29(50):15846–15850. doi: 10.1523/JNEUROSCI.4357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres-Mateos E, Perier C, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci U S A. 2007;104(37):14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38(5):515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3(12):1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Brunet I, Weinl C, et al. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438(7064):94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns RS, LeWitt PA, et al. The clinical syndrome of striatal dopamine deficiency. Parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) N Engl J Med. 1985;312(22):1418–1421. doi: 10.1056/NEJM198505303122203. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22(20):8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, et al. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101(24):9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Seo H, et al. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14(13):1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciron C, Lengacher S, et al. Sustained expression of PGC-1alpha in the rat nigrostriatal system selectively impairs dopaminergic function. Hum Mol Genet. 2012;21(8):1861–1876. doi: 10.1093/hmg/ddr618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dairaghi DJ, Shadel GS, et al. Human mitochondrial transcription factor A and promoter spacing integrity are required for transcription initiation. Biochim Biophys Acta. 1995;1271(1):127–134. doi: 10.1016/0925-4439(95)00019-z. [DOI] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, et al. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99(22):14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Dodson MW, et al. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105(38):14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, et al. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283(14):9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104(4):1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner M, Muller SK, et al. Neuromelanin, neurotransmitter status and brainstem location determine the differential vulnerability of catecholaminergic neurons to mitochondrial DNA deletions. Mol Brain. 2011;4:43. doi: 10.1186/1756-6606-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fukui H, Moraes CT. Mechanisms of formation and accumulation of mitochondrial DNA deletions in aging neurons. Hum Mol Genet. 2009;18(6):1028–1036. doi: 10.1093/hmg/ddn437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, et al. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33(5):627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier CA, Kitada T, et al. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A. 2008;105(32):11364–11369. doi: 10.1073/pnas.0802076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12(2):119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34(4):521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2(7):492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278(44):43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45(4):489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Good CH, Hoffman AF, et al. Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson's disease. FASEB J. 2011;25(4):1333–1344. doi: 10.1096/fj.10-173625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JG, Dingledine R, et al. Neuron-selective changes in RNA transcripts related to energy metabolism in toxic models of parkinsonism in rodents. Neurobiol Dis. 2010;38(3):476–481. doi: 10.1016/j.nbd.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Gash MT, et al. Mitochondrial respiratory chain function in multiple system atrophy. Mov Disord. 1997;12(3):418–422. doi: 10.1002/mds.870120323. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468(7324):696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Heeman B, Van den Haute C, et al. Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J Cell Sci. 2011;124(Pt 7):1115–1125. doi: 10.1242/jcs.078303. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, et al. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334(6180):345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Irrcher I, Aleyasin H, et al. Loss of the Parkinson's disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet. 2010;19(19):3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- Kanki T, Ohgaki K, et al. Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol Cell Biol. 2004;24(22):9823–9834. doi: 10.1128/MCB.24.22.9823-9834.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Smith PD, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102(14):5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104(27):11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Tong Y, et al. Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J Neurochem. 2009;111(3):696–702. doi: 10.1111/j.1471-4159.2009.06350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klivenyi P, Siwek D, et al. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21(3):541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, et al. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38(5):518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kruse SE, Watt WC, et al. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metab. 2008;7(4):312–320. doi: 10.1016/j.cmet.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Stirling W, et al. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99(13):8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Yang R, et al. Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport. 2007;18(15):1543–1546. doi: 10.1097/WNR.0b013e3282f03db4. [DOI] [PubMed] [Google Scholar]
- Li X, Patel JC, et al. Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson's disease mutation G2019S. J Neurosci. 2010;30(5):1788–1797. doi: 10.1523/JNEUROSCI.5604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu W, et al. Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson's disease. Nat Neurosci. 2009;12(7):826–828. doi: 10.1038/nn.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant alpha-synuclein. Neuron. 2009;64(6):807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, et al. Conditional Expression of Parkinson's Disease-Related Mutant alpha-Synuclein in the Midbrain Dopaminergic Neurons Causes Progressive Neurodegeneration and Degradation of Transcription Factor Nuclear Receptor Related 1. J Neurosci. 2012;32(27):9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zhang C, et al. alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci Lett. 2009;454(3):187–192. doi: 10.1016/j.neulet.2009.02.056. [DOI] [PubMed] [Google Scholar]
- Liu S, Sawada T, et al. Parkinson's disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8(3):e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EK, Markwardt SJ, et al. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1alpha. J Neurosci. 2010;30(21):7227–7235. doi: 10.1523/JNEUROSCI.0698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann VM, Cooper JM, et al. Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson's disease. Brain. 1992;115(Pt 2):333–342. doi: 10.1093/brain/115.2.333. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Pan Y, et al. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26(1):41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287(5456):1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Vila M, et al. Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis. 2001;8(3):535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- Mortiboys H, Johansen KK, et al. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology. 2010;75(22):2017–2020. doi: 10.1212/WNL.0b013e3181ff9685. [DOI] [PubMed] [Google Scholar]
- Mudo G, Makela J, et al. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson's disease. Cell Mol Life Sci. 2012;69(7):1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, et al. Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria. J Neurosci. 2008;28(47):12305–12317. doi: 10.1523/JNEUROSCI.3088-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183(5):795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8(1):e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, et al. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36(26)(26):2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44(4):595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Papka M, Rubio A, et al. A review of Lewy body disease, an emerging concept of cortical dementia. J Neuropsychiatry Clin Neurosci. 1998;10(3):267–279. doi: 10.1176/jnp.10.3.267. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, et al. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11(7):755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci U S A. 2005;102(6):2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Fukui H, et al. The striatum is highly susceptible to mitochondrial oxidative phosphorylation dysfunctions. J Neurosci. 2011;31(27):9895–9904. doi: 10.1523/JNEUROSCI.6223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Pinto M, et al. Striatal dysfunctions associated with mitochondrial DNA damage in dopaminergic neurons in a mouse model of Parkinson's disease. J Neurosci. 2011;31(48):17649–17658. doi: 10.1523/JNEUROSCI.4871-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105(5):1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana A, Kruse SE, et al. Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc Natl Acad Sci U S A. 2010;107(24):10996–11001. doi: 10.1073/pnas.1006214107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve AK, Krishnan KJ, et al. Nature of mitochondrial DNA deletions in substantia nigra neurons. Am J Hum Genet. 2008;82(1):228–235. doi: 10.1016/j.ajhg.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samii A, Nutt JG, et al. Parkinson's disease. Lancet. 2004;363(9423):1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, et al. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54(3):823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Mann VM, et al. Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson's disease. J Neurochem. 1990;55(6):2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- Shin JH, Ko HS, et al. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell. 2011;144(5):689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WW, Pei Z, et al. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9(10):1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Moraes CT. Double-strand breaks of mouse muscle mtDNA promote large deletions similar to multiple mtDNA deletions in humans. Hum Mol Genet. 2005;14(7):893–902. doi: 10.1093/hmg/ddi082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Hoffman AF, et al. Altered dopamine metabolism and increased vulnerability to MPTP in mice with partial deficiency of mitochondrial complex I in dopamine neurons. Hum Mol Genet. 2012;21(5):1078–1089. doi: 10.1093/hmg/ddr537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Lee S, et al. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci U S A. 2011;108(31):12937–12942. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen DF, Narendra DP, et al. Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells. Proc Natl Acad Sci U S A. 2010;107(26):11835–11840. doi: 10.1073/pnas.0914569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Bogulavsky J, et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A. 2000;97(22):11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, et al. The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease. J Neurosci. 2000;20(24):9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Pisani A, et al. R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc Natl Acad Sci U S A. 2009;106(34):14622–14627. doi: 10.1073/pnas.0906334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Bentivoglio AR, et al. Localization of a novel locus for autosomal recessive early-onset parkinsonism, PARK6, on human chromosome 1p35-p36. Am J Hum Genet. 2001;68(4):895–900. doi: 10.1086/319522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Brug MP, Blackinton J, et al. RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc Natl Acad Sci U S A. 2008;105(294):10244–10249. doi: 10.1073/pnas.0708518105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putten H, Wiederhold KH, et al. Neuropathology in mice expressing human alpha-synuclein. J Neurosci. 2000;20(16):6021–6029. doi: 10.1523/JNEUROSCI.20-16-06021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107(1):378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Coelln R, Thomas B, et al. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci U S A. 2004;101(29):10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, et al. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147(4):893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yan MH, et al. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet. 2012;21(9):1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu J, et al. DJ-1 modulates the expression of Cu/Zn-superoxide dismutase-1 through the Erk1/2-Elk1 pathway in neuroprotection. Ann Neurol. 2011;70(4):591–599. doi: 10.1002/ana.22514. [DOI] [PubMed] [Google Scholar]
- West AB, Moore DJ, et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102(46):16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Xiong H, Wang D, et al. Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation. J Clin Invest. 2009;119(3):650–660. doi: 10.1172/JCI37617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Chen L, et al. Paraquat induces dopaminergic dysfunction and proteasome impairment in DJ-1-deficient mice. Hum Mol Genet. 2007;16(23):2900–2910. doi: 10.1093/hmg/ddm249. [DOI] [PubMed] [Google Scholar]
- Zecca L, Stroppolo A, et al. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc Natl Acad Sci U S A. 2004;101(26):9843–9848. doi: 10.1073/pnas.0403495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shimoji M, et al. Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14(14):2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- Zhang W, Phillips K, et al. Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson's disease. Neurotox Res. 2011;19(1):63–72. doi: 10.1007/s12640-009-9140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Liao Z, et al. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson's disease. Sci Transl Med. 2010;2(52):52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]