Abstract
We recently reported that levulinate (4-ketopentanoate) is converted in the liver to 4-hydroxypentanoate, a drug of abuse, and that the formation of 4-hydroxypentanoate is stimulated by ethanol oxidation. We also identified 3 parallel β-oxidation pathways by which levulinate and 4-hydroxypentanoate are catabolized to propionyl-CoA and acetyl-CoA. We now report that levulinate forms three seven-carbon cyclical CoA esters by processes starting with the elongation of levulinyl-CoA by acetyl-CoA to 3,6-diketoheptanoyl-CoA. The latter gamma-diketo CoA ester undergoes two parallel cyclization processes. One process yields a mixture of tautomers, i.e., cyclopentenyl- and cyclopentadienyl-acyl-CoAs. The second cyclization process yields a methyl-pyrrolyl-acetyl-CoA containing a nitrogen atom derived from the epsilon nitrogen of lysine, but without carbons from lysine. The cyclic CoA esters were identified in rat livers perfused with levulinate, and in livers and brains from rats gavaged with calcium levulinate ± ethanol. Lastly, 3,6-diketoheptanoyl-CoA, like 2,5-diketohexane, pyrrolates free lysine and, presumably, lysine residues from proteins. This may represent a new pathway for protein pyrrolation. The cyclic CoA esters and related pyrrolation processes may play a role in the toxic effects of 4-hydroxypentanoate.
Keywords: gamma diketones, CoA esters, lysine, pyrrolation, 4-hydroxypentanoate
Introduction
We recently reported on new pathways of levulinate (4-ketopentanoate) metabolism.1 This compound, as a calcium salt, is used as an oral or intravenous source of calcium. We found that levulinate is converted in the liver to 4-hydroxypentanoate, a drug of abuse, an analogue of 4-hydroxybutyrate.2 However, 4-hydroxypentanoate has a weaker drug effect and is more toxic than 4-hydroxybutyrate.3 The formation of 4-hydroxypentanoate is stimulated by ethanol oxidation.1 We also identified 3 parallel β-oxidation pathways by which levulinate and 4-hydroxypentanoate are catabolized to propionyl-CoA and acetyl-CoA. One of the three β-oxidation pathways involves the formation of 4-phosphopentanoyl-CoA, an intermediate in the isomerization of 4-hydroxypentanoyl-CoA to 3-hydroxypentanoyl-CoA. Using a combination of metabolomics and mass isotopomer analysis,4 we identified ten C5 acyl-CoA esters involved in the catabolism of levulinate and 4-hydroxypentanoate. Of these ten acyl-CoAs derived from levulinate, three accumulate in large concentrations: levulinyl-CoA, 4-hydroxypentanoyl-CoA and 4-phosphopentanoyl-CoA, resulting in substantial CoA trapping. The latter occurs from the activation of some drugs, and as a result of inborn disorders of metabolism, such as fatty acid oxidation disorders.5 Sequestration of CoA can inhibit CoA-dependent processes such as the oxidation of fatty acids,5,6 ketogenesis,7 and the metabolism of some drugs.8
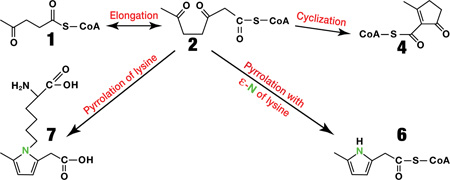
In this subsequent study, we identified four C7 CoA esters derived from levulinate. The first ester derives from the elongation of levulinyl-CoA to 3,6-diketoheptanoyl-CoA. The latter undergoes (i) reduction to 3-hydroxy-6-ketoheptanoyl-CoA, (ii) pyrrolation with lysine, and (iii) two parallel cyclization processes. One cyclization process yields a mixture of tautomers, i.e., cyclopentenyl- and cyclopentadienyl-acyl-CoAs. The second cyclization process yields a methyl-pyrrolyl-acetyl-CoA containing a nitrogen atom derived from the epsilon nitrogen of lysine. These cyclic CoA esters were identified in rat livers perfused with levulinate, and in livers and brains from rats gavaged with calcium levulinate ± ethanol.
EXPERIMENTAL PROCEDURES
Nomenclature of Mass Isotopomers
Mass isotopomers are molecules containing zero to n heavy atoms over the natural enrichment.9,10 They are designated as M, M1, M2, … Mn, where n is the number of heavy atoms in the molecule. The isotopic enrichment of each mass isotopomer is expressed as mol percent.
Materials
Sigma-Aldrich-Isotec supplied most chemicals and the following isotopically labeled compounds: [13C6]glucose, 15NH4Cl, [ε-15N]lysine, [13C6,15N2]lysine, [2H7]butyric acid, [2H9]pentanoic acid, sodium [13C2]acetate. 4-Hydroxy-[3,3,4,5,5,5-2H6]pentanoate and [2H9]pentanoyl-CoA internal standards were prepared as previously described.1,11[13C5]Levulinate was prepared as in Ref12. 3,6-Diketoheptanoate ethyl ester (Scheme 1, 2') was synthesized as in Ref13. Its purity was assessed by 1H NMR (400 MHz, CDCl3): δ 1.25 (t, 3H, J = 7.2 Hz), 2.19 (s, 3H), 2.69–2.83 (m, 4H), 3.49 (s, 2 H), 4.15 (q, 2 H, J = 7.2 Hz);13C NMR (100 MHz, CDCl3): δ 14.3, 30.0, 36.6, 37.1, 49.6, 61.6, 167.3, 201.8, and 206 (Figure S1). This compound is stable at −80°C, but slowly cyclizes to 4' + 5' at room temperature. 2' was used as such in perfused rat livers and as a synthon for the preparations of (i) 2-methyl-5-oxocyclopent-1-enecarboxylic acid ethyl ester 4', (ii) 2-(5-methyl-1H-pyrrol-2-yl)acetic acid (free acid of 6), and (iii) of 2-amino-6-(2-(carboxymethyl)-5-methyl-1H-pyrrol-1-yl)hexanoic acid (pyrrolated lysine 7) via Paal-Knorr reactions with ammonium acetate or lysine.14,15
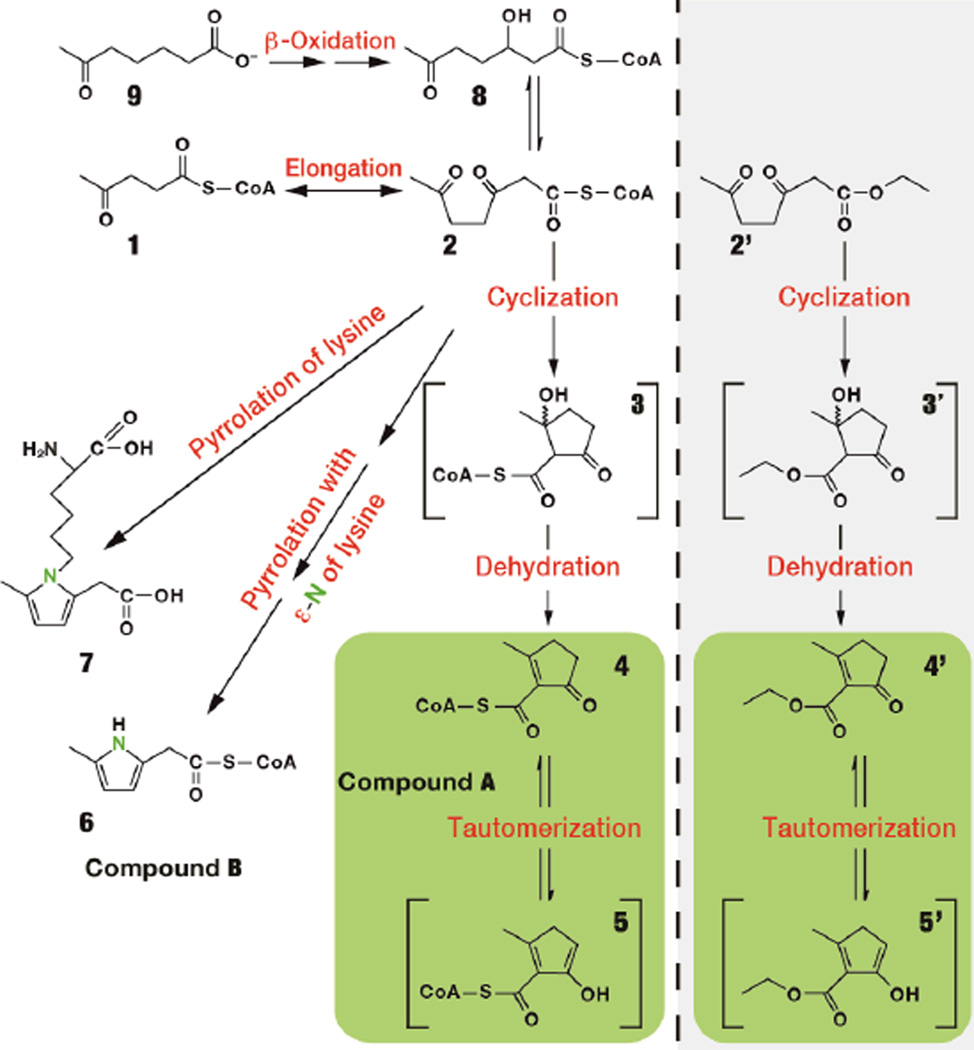
Scheme 1.
Formation of cyclic CoA esters and pyrrolated lysine from levulinate. The left and right panels refer to compounds present in tissues and in an in vitro chemical experiment, respectively.
Synthesis of 2-methyl-5-oxocyclopent-1-enecarboxylic acid ethyl ester 4'. 2' was incubated at room temperature in 50 mM sodium phosphate buffer pH 7.4. The reaction was followed spectrophotometrically (see Results). The identity of the product was confirmed by NMR [1H NMR (CDCl3, 400 MHz) δ 4.28 (q, 2H), 2.62 (t, 2H), 2.44 (t, 2H), 2.35 (s, 3H), 1.31 (t, 3H). 13C NMR (CDCl3, 400 MHz) δ 203.77 (CO), 184.56 (COO), 163.48 (C), 132.95 (C), 60.99 (CH2), 35.19 (CH2), 32.86 (CH2), 19.53 (CH3), 14.45(CH3)] and LC/MS [a single peak at 27.2 min with positive precursor ion at m/z 169 and fragments at m/z 141, 123, 95, 67 and 65] (Figure S2).
Synthesis of 2-(5-methyl-1H-pyrrol-2-yl)acetic acid (free acid of 6). Ammonium acetate (332.7 mg, 4.32 mmol) in 5 ml of methanol was slowly added to the solution of 3,6-diketoheptanoate ethyl ester (268 mg, 1.44 mmol) in 10 ml methanol solution. The resulting mixture was stirred for 1 hour at room temperature. The solvent was removed. Flash chromatography of the residue (100% methylene dichloride, TLC: Rf= 0.3) gave the pure ethyl ester of 2-(5-methyl-1H-pyrrol-2-yl)acetic acid (150 mg, 62.5%) as a colorless oil.1H NMR (CDCl3, 400 MHz) δ 8.36 (s, 1H), 5.88 (d, 1H), 5.78 (d, 1H), 4.20 (q, 2H), 3.61 (s, 2H), 2.26 (s, 3H), 1.29 (t, 3H).
Sodium hydroxide (66.4 mg, 1.66 mmol) in 0.5 ml of ultrapure H2O was slowly added to the purified ethyl ester (50 mg, 0.29 mmol) in 6 ml of methanol solution. The reaction mixture was stirred for 4 hr at room temperature. Then 1N HCl aqueous solution was added dropwise into the solution to adjust the pH value near 7. The solvent was then removed. Flash chromatography of the residue (Rf = 0.3 in 10% methanol in chloroform) gave the pure 2-(5-methyl-1H-pyrrol-2-yl)acetic acid (30 mg, 72%) as yellow powder.1H NMR (CDCl3, 400 MHz) δ 10.99 (s, 1H), 8.28 (s, 1H), 5.91 (d, 1H), 5.79 (d, 1H), 3.65 (s, 2H), 2.25 (s, 3H).13C NMR (CDCl3, 400 MHz) δ 177.15 (COO), 128.26 (C), 121.34 (C), 108.13 (CH), 106.17 (CH), 33.73 (CH2), 13.26 (CH3).
Synthesis of 2-amino-6-(2-(carboxymethyl)-5-methyl-1H-pyrrol-1-yl)hexanoic acid 7. A solution of lysine monochloride (300 mg, 1.64 mmol) and 3,6-diketoheptanoate ethyl ester (100 mg, 0.54 mmol) in 5 ml H2O was stirred in the dark under argon at 60°. After 3 hours, the solvent was removed and the residue was purified by flash chromatography (10% methanol, 90% EtOAc) afforded the pure 2-amino-6-(2-(2-ethoxy-2-oxoethyl)-5-methyl-1H-pyrrol-1-yl)hexanoic acid (110 mg, 69.1%) as a yellow oil.1H NMR (CD3OD, 400 MHz) δ 5.81 (d, 1H), 5.71 (d, 1H), 4.14 (q, 2H), 3.83 (t, 2H), 3.62 (s, 2H), 3.57 (t, 1H), 2.21 (s, 3H), 1.90-1.85 (2H), 1.66-1.62 (2H), 1.48-1.46 (2H), 1.25 (t, 3H). Lithium hydroxide (10 mg, 0.42 mmol) in 1 ml of H2O was added to the purified ethyl ester (22 mg, 0.074 mmol) in 4 ml H2O. The reaction mixture was stirred for 30 minutes at room temperature. Then 1N HCl aqueous solution was added dropwise into the solution to adjust the pH value near 7. The mixture was concentrated and purified by preparative HPLC. This afforded the pure 2-amino-6-(2-(carboxymethyl)-5-methyl-1H-pyrrol-1-yl)hexanoic acid (8 mg, 88%) as red oil. 1H NMR (CD3OD, 400 MHz) δ 5.73 (d, 1H), 5.64 (d, 1H), 3.80 (t, 2H), 3.53 (t, 1H), 3.39 (s, 2H), 2.17 (s, 3H), 1.89-1.79 (2H), 1.65-1.61 (2H), 1.48-1.42 (2H).
In vivo experiments
Overnight fasted male Sprague-Dawley rats weighing 200–250 g were divided into 4 groups (9 rats per group) and given an oral gavage of one of the following: (i) 2 mmol/kg calcium levulinate; (ii) 2 mmol/kg calcium chloride (control for (i)); (iii) 2 mmol/kg calcium levulinate + 13.4 mmol/kg ethanol, and (iv) 2 mmol/kg calcium chloride + 13.4 mmol/kg ethanol (control for (iii)). The dose of calcium levulinate per kg is equivalent to twice the recommended daily allowance of calcium for adult humans. The dose of ethanol was calculated to induce 20 mM in total body water, assuming instantaneous diffusion. Rats were given the gavage at 0 min, and were killed at 7 min, followed by 15 min intervals up to 120 min (n = 1 rat per time point in each group). At the time of euthanasia, the following samples were taken: (i) portal and peripheral plasma, (ii) a lobe of the liver (quick-frozen), (iii) the whole brain (quick-frozen), and (iv) urine by bladder puncture.
Perfused liver experiments
Additional data were obtained from 2 groups of 6 rat livers perfused for 2 hr with recirculating bicarbonate buffer containing 4% dialyzed, fatty acid-free, bovine serum albumin, 4 mM glucose, 2 mM [13C5]levulinate ± 20 mM ethanol.1 In addition, rat livers were perfused16 with 2 mM unlabeled levulinate and either (i) 4 mM [13C6]glucose, (ii) 4 mM unlabeled glucose + 10 mM [13C2]acetate, (iii) 4 mM unlabeled glucose + 5 mM 15NH4Cl, (iv) 4 mM unlabeled glucose + 0.4 mM [ε-15N]lysine (1 h), (v) 4 mM glucose + 1 mM [13C6,15N2]lysine, or (vi) 4 mM glucose + 1 mM unlabeled lysine. Additional perfusions were conducted with (i) 4 mM unlabeled glucose + 1 mM 3,6-diketoheptanoate ethyl ester (2') + 0.4 mM [ε-15N]lysine (1 h) or 1 mM [13C6,15N2]lysine, or (ii) unlabeled glucose + 1 mM 2-(5-methyl-1H-pyrrol-2-yl)acetate (as a way to generate 6 by CoA activation). Perfusate was sampled every 20 min. Livers were quick-frozen at the end of the experiment. The Case IACUC approved all animal experiments.
Analytical procedures
The concentrations and mass isotopomer distributions of the various acids, ketoacids and hydroxyacids were assayed by GC/MS of pentafluorobenzyl derivatives, using analog unlabeled or labeled compounds as internal standards. The concentrations and mass isotopomer distributions of acyl-CoA esters were assayed as in Ref17, except that the mass spectrometer was set to monitor masses up to 1050. The chromatographic conditions optimize the separation of short- and medium-chain acyl-CoAs. Because the peaks of levulinyl-CoA and 4-hydroxypentanoyl-CoA partially overlapped (retention times: 14.3 and 14.7 min, respectively), peak integrations were conducted on the front 50% of the levulinyl-CoA peak and the rear 50% of the 4-hydroxypentanoyl-CoA peak. For the assay of the concentrations of new acyl-CoA esters for which unlabeled and labeled standards are not available, we used a calibration curve of acetyl-CoA concentration with an internal standard of [2H9]pentanoyl-CoA prepared from the acid1. High-resolution LC/MS of CoA esters was run on a Thermo Scientific Q-Exactive mass spectrometer operated under positive electrospray ionization, with a resolution of 140,000. Theoretical m/z were calculated with the Thermo Xcalibur software. Samples were introduced into the mass spectrometer via a Thermo Accela 1250 UPLC pump, and a Thermo Gold column (2.1 × 100 mm, 1.9 µm) kept at 40°C. The mobile phase components were 10 mM ammonium bicarbonate (A) and methanol (B). The gradient programmed was 95% A from 0 to 10 min, 75% A from 10 to 22 min, 9% A from 22 to 23.1 min, 95%A from 23.1 to 30 min (flow rate: 0.3 ml/min).
To identify pyrrolated lysine 7, 400 µl of liver perfusate or 100 µl of urine was deproteinized with acetonitrile. The supernatant was dried with nitrogen gas and dissolved in 120 µl of water, and 40 µl was injected on a Phenomenex ODS 5 µm (150 × 2 mm), protected by a guard column (Phenomenex, 10 × 2.1 mm), in an Agilent 1100 liquid chromatograph. The chromatogram was developed at 0.2 ml/min (i) for 15 min with 5% buffer A (0.1% formic acid in methanol) and 95% buffer B (0.1% formic acid in water), (ii) from 15 to 16 min with buffer A increasing from 73% to 100% and maintained until 22 min, and (iv) for 10 min of stabilization with 5% buffer A and 95% buffer B before the next injection. The liquid chromatograph was coupled to a 4000 QTrap mass spectrometer (Applied Biosystems, Foster City, CA) operated under positive ionization mode with the following source settings: turbo-ion-spray source at 600°C under N2 nebulization at 65 p.s.i., N2 heater gas at 55 p.s.i., curtain gas at 30 p.s.i., collision-activated dissociation gas pressure held at high, turbo ion-spray voltage at 5,500 V, declustering potential at 70 V, entrance potential at 10 V, collision energy at 51 V, and collision cell exit potential at 10 V. The mass spectrometer was set to analyze the precursor/product ion pair 269/84.
Calculations
Correction of measured mass isotopomer distributions for natural enrichment was performed using the CORMAT software.18 The data points shown in the figures represent means of duplicate GC/MS or LC/MS/MS injections, which differed by <2%. The statistical differences between some profiles were tested using an unpaired t test (Graph Pad Prism Software, version 3).
RESULTS and DISCUSSION
Detection of cyclical CoA esters in perfused livers (Figure S3)
In reviewing the raw data of the assays of acyl-CoA profiles in livers perfused with M or M5 levulinate1, we noticed two peaks (A and B) for which the masses of the precursor/product pairs (i) differed by 507 Da (as in all acyl-CoAs and in free CoA19), (ii) were 24 and 23 Da heavier than levulinyl-CoA 1, respectively, (iii) increased by 5 or 7 Da when unlabeled levulinate was replaced by M5 levulinate, and (iv) were not present in control livers perfused without levulinate. Product ion scan analyses confirmed that the two compounds were CoA esters because of the presence of fragments that are typical of CoA and all CoA esters (m/z 428, 261 and 16019). The following evidence shows that compounds A and B are the 4/5 tautomers, and 6, respectively (Scheme 1, left panel).
The two unknown CoA esters contain more than the five carbons of levulinate, in addition to the CoA moiety. This is because, in livers perfused with M5 levulinate, the two compounds were M5 and M7 labeled (Table 1, rows c and d). This suggested that CoA esters A and B contained the five carbons of levulinate, and two carbons derived from the metabolism of M5 levulinate. We had previously shown that M5 levulinate is catabolized to M2 acetyl-CoA.1 In livers perfused with M5 levulinate, the M5 enrichment of levulinyl-CoA and the M2 enrichment of acetyl-CoA were 95%1 and 4.2%, respectively (Table 1, row c).
Table 1.
Distribution of mass isotopomers of C7 CoA esters and acetyl-CoA in rat livers perfused with levulinate and substrates labeled with 13C, 15N or both
| Row | Substrates | n | Percent Labeled Mass Isotopomers | ||
|---|---|---|---|---|---|
| Unknown A (4/5) |
Unknown B (6) |
Acetyl-CoA | |||
| a | Glucose | 6 | undetected | undetected | unlabeled |
| b | LEV + glucose | 2 | unlabeled | unlabeled | unlabeled |
| c | [13C5]LEV + glucose | 6 | M5 = 78 ± 0.78 | M5 = 79 ± 0.80 | M2 = 4.2 ± 0.53 |
| M7 = 10 ± 0.75 | M7 = 10 ± 0.63 | ||||
| d | [13C5]LEV + glucose + ETOH | 6 | M5 = 72 ± 1.4* | M5 = 75 ± 1.1* | M2 = 8 ± 0.86* |
| M7 = 15 ± 1.1* | M7 = 14 ± 1.2* | ||||
| e | LEV + [13C6]glucose | 1 | M2 = 12.5 | M2 = 12.9 | M2 = 11.5 |
| 1 | M2 = 3.3 | M2 = 3.1 | M2 = 3.5 | ||
| 1 | M2 = 3.2 | M2 = 3.0 | M2 = 3.5 | ||
| f | LEV + [1,2-13C2]acetate | 1 | M2 = 3.30 | M2 = 3.40 | M2 = 3.90 |
| 1 | M2 = 16.9 | M2 = 14.8 | M2 = 20.8 | ||
| 1 | M2 = 21.6 | M2 = 22.9 | M2 = 23.2 | ||
| g | LEV + glucose + 15NH4Cl | 3 | unlabeled | unlabeled | unlabeled |
| h | LEV + glucose + lysine | 1 | unlabeled | unlabeled | unlabeled |
| i | LEV + glucose + [ε-15N]lysine | 1 | unlabeled | M1 = 6.2 | unlabeled |
| j | LEV + glucose + [15N2 -13C6]lysine | 3 | unlabeled | M1 = 7.3 ± 0.54 | unlabeled |
| k | 3,6-Diketoheptanoate ethyl ester (2') + glucose + [ε-15N]lysine | 1 | unlabeled | M1 = 7.4 | unlabeled |
| l | 3,6-Diketoheptanoate ethyl ester (2') + glucose + [13C6 -15N2]lysine | 1 | unlabeled | M1 = 5.2 | unlabeled |
| m | 2-(5-methyl-1H-pyrrol-2-yl)acetate (free acid of 6) | 1 | undetected | M detected | unlabeled |
Enrichments of compounds are expressed as molar percent enrichment ± SE.
Data from individual livers are shown in rows e, f, h, i, k, l, m. In row d, values different from the corresponding values in row c are indicated with an * (p < 0.02).
One would predict based on probability analysis that, if unknowns A and B derived from the elongation of levulinyl-CoA by acetyl-CoA, their M5 and M7 enrichments should be 91% and 4% in livers perfused with M5 levulinate. In fact, the M5 and M7 enrichments of unknowns A and B were 78–80% and 10%, respectively (Table 1, row c). A likely explanation for the discrepancy is that, at the site of thiolase, the acetyl-CoA derived from M5 levulinate (via thiolase, see Figure 1 of Ref1) has not fully been diluted by unlabeled acetyl-CoA derived from unlabeled substrates. Thus, the M2 enrichment of acetyl-CoA used to elongate levulinyl-CoA is greater than the 4% measured in the total liver extract.
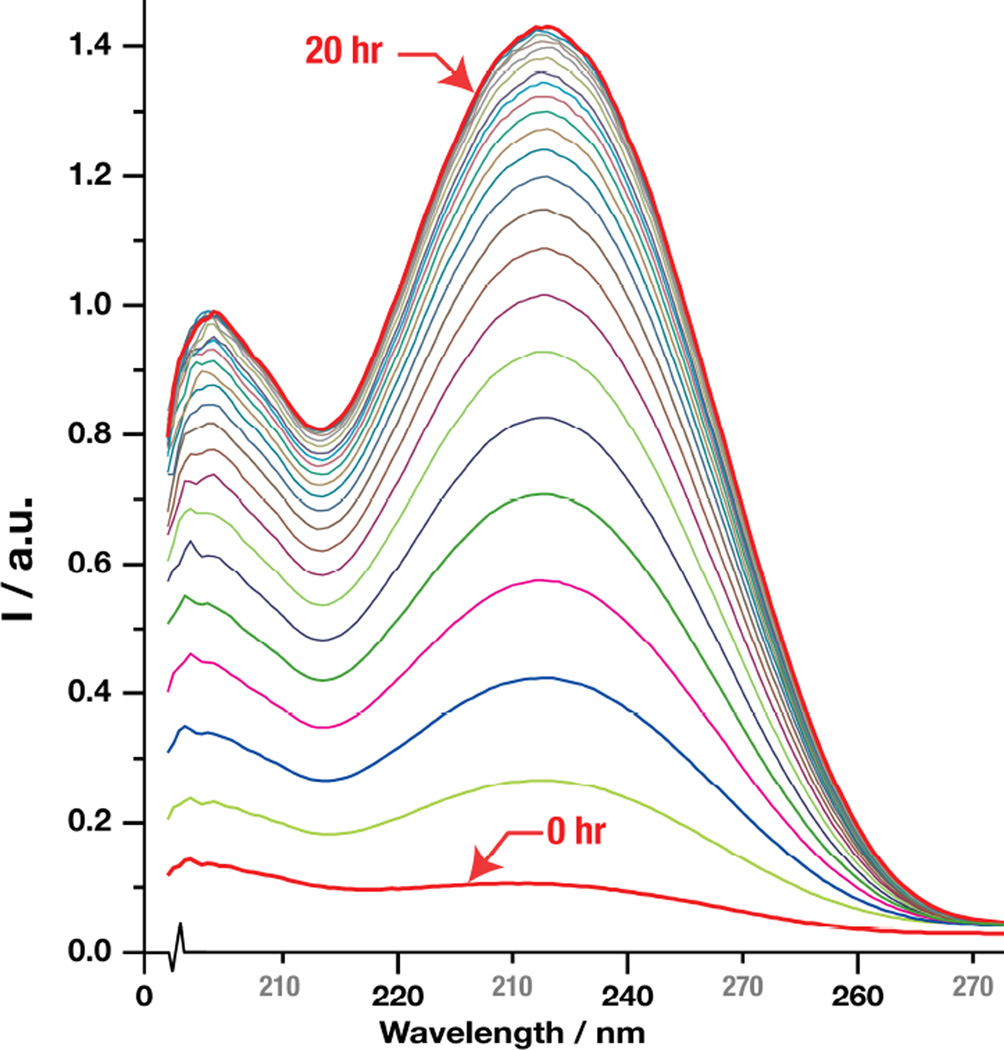
Figure 1.
Time profile of UV absorbance of 2' at pH 7.4
Similar calculations apply to the labeling of the unknown CoA esters in livers perfused with M5 levulinate + ethanol (Table 1, row d). There, the M5 enrichment of levulinyl-CoA and the M2 enrichment of acetyl-CoA were 95%1 and 8.0%, respectively. The predicted M5 and M7 enrichments of unknowns A and B would be 87.4% and 7.6% (vs. the measured 72–75% and 14–15% (Table 1, row d)). The m/z of the precursor-product pair of unknown A (890/383) is 24 Da greater than levulinyl-CoA (866/359). So we considered what process(es) could add 24 Da to levulinyl-CoA. Addition of one acetyl unit (+42) followed by aldol addition and subsequent condensation (−18) would yield the observed 24 mass unit difference. This suggested that unknown A is formed via the sequence 1 to 2 to 3 to 4/5. First, levulinyl-CoA 1 is elongated to a seven carbon intermediate, presumably 3,6-diketoheptanoyl-CoA 2. The elongation could be catalyzed by an elongase using malonyl-CoA or by the reverse reaction of 3-ketoacyl-CoA thiolase using acetyl-CoA. Second, 2, as a gamma-diketo compound, undergoes spontaneous cyclization through an aldol addition to a hydroxy-cyclopentanone intermediate 3. Third, 3 undergoes spontaneous dehydration to a mixture of keto/enol tautomers 4 and 5.
The m/z of the precursor-product pair of unknown B (889/382) is 23 Da more than levulinyl-CoA (866/359). There is no clear combination of addition and subtraction of Cx, Hy and Oz that would account for the addition of 23 Da to levulinyl-CoA by standard reactions starting with elongation (+ 42 Da). This suggested thatan other atom, possibly nitrogen (14 Da), is part of the 23 increase in Da of unknown B versus levulinyl-CoA. Because nitrogen is trivalent or pentavalent, it would allow for the addition of an odd-number of mass units, such as 23, in the conversion of levulinyl-CoA to unknown B. This suggested that unknown B (i) is formed via the gamma-diketo compound 2, prone to cyclization, and (ii) contains a nitrogen atom in its acyl moiety. This evoked the formation of pyrrolic compounds from (i) the reaction between 2,5-diketohexane (a product of hexane oxidation) and either free lysine or lysine residues of proteins via a Paal-Knorr type reaction,20–23 and (ii) from gamma-ketoaldehydes formed from cyclooxygenase reaction on arachidonate.24,25 We thus hypothesized that unknown B is a pyrrolated acyl-CoA, i.e.,6.
Additional isotopic data confirming the structure of cyclical CoA esters
Togather more information on the structures of unknowns A and B, we conducted additional experiments with unlabeled levulinate and substrates labeled with 13C, 15N or both (Table 1). In livers perfused with unlabeled levulinate + either [13C6]glucose (row e) or [13C2]acetate (row f), unknowns A and B were only M2 labeled to a degree similar to the corresponding M2 enrichments of acetyl-CoA. Because [13C6]glucose and [13C2]acetate are both precursors of M2 acetyl-CoA, the data of rows e and f of Table 1 confirm that unknowns A and B contain two carbons derived from acetyl-CoA.
To check whether unknown B contains a nitrogen atom, we first perfused livers with unlabeled glucose + unlabeled levulinate + 5 mM 15NH4Cl (Table 1, row g). No M1 label was detected in compound B, as would be expected if 15N from 15NH4Cl were the precursor to a N atom in B. However, as expected in livers perfused with 15NH4Cl26, perfusate glutamate was 35% M1 labeled at 60 min. Therefore, if unknown B contains a nitrogen atom, this atom does not derive from non-essential amino acid(s), which become rapidly labeled from 15NH4Cl. To test whether unknown B contains a N atom derived from the essential aminoacid lysine, we perfused livers with unlabeled glucose + unlabeled levulinate + either [ε-15N]lysine or [13C6,15N2]lysine (Table 1, rows i and j). In the presence of [ε-15N]lysine, unknown B was approximately 6% M1 labeled, as would be expected if the N atom in B were derived from the ε-N of lysine. In the presence of [13C6,15N2]lysine, unknown B was approximately 7% M1 labeled, and no heavier mass isotopomers of B were detected. Thus, unknown B contains a N atom derived from the ε-position of lysine, but no carbon from lysine. The identity of compound B as 6 was verified by perfusing one liver with 4 mM glucose + 1 mM 2-(5-methyl-1H-pyrrol-2-yl)acetate, the synthetic free acid corresponding to 6. In the frozen tissue, we identified compound B by its retention time and its multiple reaction monitoring precursor/product ion pair which was identical to that observed in perfusions with unlabeled glucose and unlabeled levulinate (Table 1, rows m and b).
To confirm the identity of 3,6-diketoheptanoyl-CoA 2, we synthesized 3,6-diketoheptanoate ethyl ester 2' and perfused two livers with unlabeled glucose + unlabeled 2' + either [ε-15N]lysine or [13C6,15N2]lysine (no levulinate). We detected unlabeled 2, unlabeled 1 and M1 labeled 6 (Table 1, rows k and l). Unknown A was detected but was unlabeled. In livers perfused with 2'(and no levulinate), the simultaneous presence of 1 and 2 strongly supports the hypothesis that levulinyl-CoA is elongated via a thiolase.γ
Lastly, to generate 3,6-diketoheptanoyl-CoA 2 from a third source, i.e., β-oxidation, we perfused one liver with unlabeled 6-ketoheptanoate 9. In the liver extract, we identified 1, 2, 4/5, and 6.
To confirm the identity of all CoA esters shown in Scheme 1 (left panel), we used high-resolution Q-Exactive LC/MS on extracts from three livers perfused with unlabeled levulinate, M5 levulinate, and unlabeled levulinate + [ε-15N]lysine. On this instrument, we identified 1, 2, 4/5, 6, and 8, i.e., 3-hydroxy-6-ketoheptanoyl-CoA which was not detectable on regular LC/MS. 8 is formed presumably by reversible reduction of compound 2 via 3-hydroxy-acyl-CoA dehydrogenase. It is also an intermediate of the β-oxidation of 6-ketoheptanoate 9. 3 was not detected. Table S1 shows the differences between the theoretical and the measured m/z ranged from 0.1 to 1.3 ppm. The exact mass assays strongly support the identity of the compounds shown in Scheme 1 (left panel), except for the putative intermediate 3, which was not detected.
In vitro modeling of the cyclization of 2 to 4/5
To confirm that 2 undergoes spontaneous cyclization/dehydration to 4/5, we used the ethyl ester analog of 2, i.e., 2' and incubated it in 50 mM sodium phosphate buffer pH 7.4 at room temperature (initial concentration of 2' was 0.1 mM). Figure 1 shows the development of the UV absorbance of the solution over 20 hr. A time dependent increase in absorbance is observed in the UV portion of the spectrum. The interpretation of this result is that a UV-specific chromophore is being formed during the course of the reaction, in this case the enone of the aldol condensation product. The insert shows the time course of absorbance of the main peak at 233 nm (k = 0.41 hr−1). The same rate constant was measured for the peak at 204 nm. Our interpretation of the UV data was confirmed as the structure of the product as 2-methyl-5-oxocyclopent-1-enecarboxylic acid ethyl ester 4' was confirmed by NMR (see Methods). Compounds such as 4' are known to undergo keto/enol tautomerism. We did not identify the tautomer 5' by NMR or LC/MS (one peak only). Presumably, the proportion of 5' in the 4'/5' mixture is very low. By analogy, we can assume that 4, identified by LC/MS in perfused livers, undergoes tautomerization with 5, with the primary tautomer being 4.
Pyrrole-lysine adduct7
γ-Diketones react with the ε-N of lysine to form pyrrolic compounds.22,23,27 We tested whether 3,6-diketoheptanoyl-CoA 2' (or the corresponding free acid) reacts with lysine to form 2-amino-6-(2-(carboxymethyl)-5-methyl-1H-pyrrol-1-yl)hexanoate 7. In perfused rat livers, lysine is formed by proteolysis of tissue proteins. We synthesized 7 from 2' by a Paal-Knorr reaction,14,15 and identified it by LC/MS in the perfusate of a number of livers perfused with levulinate, 3,6-diketoheptanoate ethyl ester, or 6-ketoheptanoate (three precursors of 2). We also found 7 in the urine of rats gavaged with calcium levulinate (see below).
In vivo experiments
Because levulinate is ingested by humans as a calcium salt, we investigated the impact of gavaging rats with Ca-levulinate ± ethanol on the CoA ester profile of liver and brain. The plasma concentration of levulinate followed similar profiles in the absence or presence of ethanol (Figure S4A). The plasma concentration of 4-hydroxypentanoate, derived from reduction of levulinate (Figure S4B), increased faster in the presence of ethanol, based on linear regression of the data over 80 min: Ca-levulinate experiments: slope = 0.0026; R2 = 0.91; Ca-levulinate + ethanol experiment: slope = 0.0044, R2 = 0.97. In previous in vivo and perfused rat liver experiments, the reduction of levulinate to 4-hydroxypentanoate was also stimulated by ethanol.1
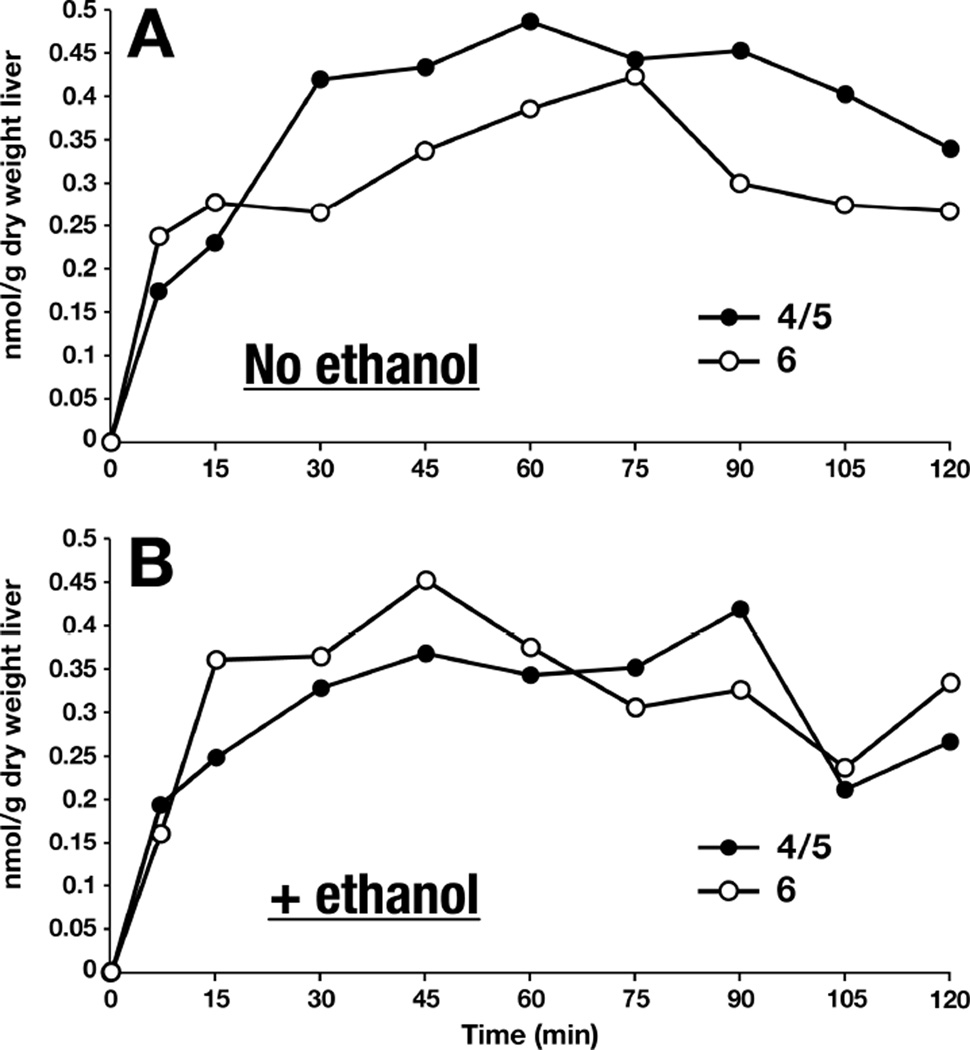
In the livers of rats gavaged with Ca-levulinate, the concentrations of the three most abundant CoA esters derived from levulinate (levulinyl-CoA, 4-hydroxypentanoyl-CoA and 4-phosphopentanoyl-CoA) peaked at 30–45 min (Figure S5A). A similar profile was observed in livers of rats gavaged with Ca-levulinate + ethanol (Figure S5B). The sum of the three concentrations peaked at 45 min at levels of 645 and 755 nmol/(g dry wt) in the absence and presence of ethanol, respectively (Figure S5C). This represents major trapping of CoA. Figures 2A and 2B show the concentrations of 4/5 and 6 in livers of rats gavaged with Ca-levulinate ± ethanol. These C7 compounds are present at much lower concentrations than the three main C5 acyl-CoAs derived from levulinate.
Figure 2.
Liver concentrations of 4/5 and 6 after gavage with calcium levulinate ± ethanol.
In the brains of rats gavaged with Ca-levulinate ± ethanol, the concentrations of the three most abundant CoA esters derived from levulinate peaked at 45 – 75 min (Figures S6A and S6B). The sum of the three concentrations peaked at 60 – 75 min at levels of 3.1 and 4.1 nmol/(g wet wt) in the absence and presence of ethanol, respectively (Figure S6C). Although the total concentrations of the esters were 20 to 60% higher in the presence than in the absence of ethanol, statistical analysis could not be conducted because we had only one rat per time point. Traces of the C7 acyl-CoAs were detected in the brains of both groups of rats, but their concentrations could not be measured accurately (range 0.004 to 0.01 nmol/(g wet weight)).
CONCLUSIONS
We identified two types of cyclical CoA esters derived from levulinate via elongation of levulinyl-CoA 1 with acetyl-CoA to a gamma-diketo-CoA ester 2. The 4/5 pair of tautomers clearly derive from the spontaneous cyclization/dehydration of 2, as demonstrated with the analogous ethyl ester 2' which forms the 4'/5' pair of tautomers. Walsh’s group had described the formation of a similar cyclopentenone compound (termed C5N) from the cyclization of 5-aminolevulinyl-CoA (a gamma-dicarbonyl compound) in microorganisms.28
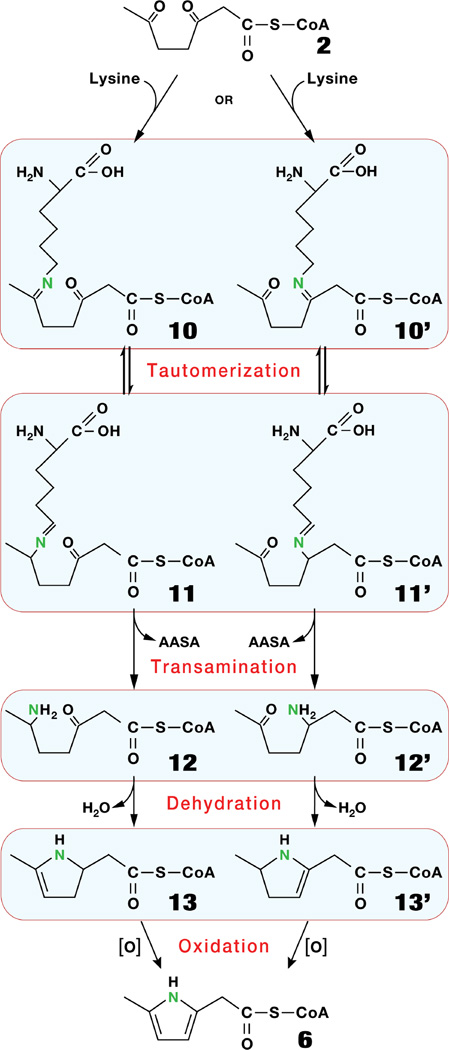
The pyrrolic CoA ester 6 is formed from the carbon skeleton of 2 and the ε-N of lysine, without the carbons of lysine. This appears to be a new mechanism of pyrrolation. Conceivably, 2 could be processed by a string of non-enzymatic reactions starting by a transamination that converts one of the keto groups of 2 to a primary amine with the ε-N of lysine (See hypothetical Scheme 2, 10 to 12, or 10' to 12'). This would be accompanied by the release of 2-aminoadipate semialdehyde. 12 or 12' would undergo a cyclization with loss of water, and spontaneous oxidation driven by the aromatization of 6. In support of the spontaneous character of the conversion of 2 to 6, is the Paal-Knorr reaction we used in vitro to convert 2' (the ethyl ester analog of 2) to the ethyl ester analog of 6 (see Experimental Procedures).
Scheme 2.
Hypothetical scheme for the formation of 6 from (2 + lysine) by a tautomerization / dehydration / oxidation mechanism.
In perfused livers, the gamma-diketo CoA ester 2 (formed from 1, 2' or 9) reacted with free lysine forming pyrrolated lysine 7 via 10 or 10' by reactions previously described.22,23 Also, 7 was detected in the urine of rats gavaged with calcium levulinate ± ethanol. The formation of 7 occurred by the same mechanism through which 2,5-diketohexane (derived from hexane oxidation) pyrrolates free lysine and lysine residues of proteins. Given the documented relevance of pyrrolation in understanding the toxicity of hexane in humans and animals, and our evolving understanding of the importance of pyrroles in immune-cell signaling, the relevance of the current studies is clear.29,30
The present study extends the number of potentially toxic processes resulting from the metabolism of levulinate ± ethanol, or 4-hydroxypentanoate. Our previous study, conducted in perfused rat livers, had revealed major CoA trapping in C5 CoA esters derived from levulinate: levulinyl-CoA, 4-hydroxypentanoyl-CoA and 4-phosphopentanoyl-CoA.1 The same CoA esters accumulated in the livers and brains of rats gavaged with calcium levulinate ± ethanol (Figures S5 and S6). Such trapping of CoA could impact on CoA-dependent reactions in liver.5 In brain, the total concentration of levulinyl-CoA + 4-hydroxypentanoyl-CoA + 4-phosphopentanoyl-CoA was much lower than in liver: 4 nmol/(g wet wt of brain) vs. 700 nmol/(g dry wt of liver), (Figures S6C and S5C). Note that the total CoA content of brain is three to ten times lower than in liver.31 It is not clear whether CoA trapping contributes to the brain toxicity of 4-hydroxypentanoate.
4-Phosphopentanoyl-CoA is an analog of 4-phosphobutyryl-CoA, which accumulates in large concentrations in the brain of mice deficient in succinic semialdehyde dehydrogenase.32 This suggested that 4-phosphobutyryl-CoA might contribute to the perturbation of brain metabolism in these mice that experience severe epileptic seizures.33 Also 4-phosphobutyryl-CoA may contribute to the mental retardation of patients with 4-hydroxybutyric aciduria.34 By analogy, mental dysfunction may be caused by 4-phosphopentanoyl-CoA in the brain of subjects ingesting either 4-hydroxypentanoate or calcium levulinate + ethanol. This concern is heightened by the fact that, after gavage with calcium levulinate ± ethanol, the peak concentration of 4-phosphopentanoyl-CoA in rat brain (0.8 to 1.4 nmol/g, Figures S6A and S6B) is up to 56 times higher than the brain concentration of 4-phosphobutyryl-CoA in mice deficient in succinic semialdehyde dehydrogenase (0.025 nmol/g).17
The C7 cyclic CoA esters derived from levulinate (4/5, 6), or their corresponding free acids, may also interfere with metabolic processes in liver and brain. Lastly, 3,6-diketoheptanoyl-CoA 2 (or its free acid equivalent) may, like 2,5-diketohexane, form pyrrole adducts with lysine residues of proteins.20–23 The formation of pyrrole-lysine adducts in neurofilaments is a major contributor to the toxicity of 2,5-diketohexane derived from hexane.35
4-Hydroxypentanoate has become a popular substitute for 4-hydroxybutyrate, which is now a controlled substance in the US.2 However, 4-hydroxypentanoate has a weaker drug effect and is more toxic than 4-hydroxybutyrate.3 Thus, addicts may use very toxic doses of 4-hydroxypentanoate to achieve the desired levels of drug effect. Alternatively, addicts may ingest large doses of calcium levulinate (which is freely available) with an alcoholic beverage to stimulate the conversion of levulinate to 4-hydroxypentanoate. Our new findings suggest additional potential deleterious metabolic effects of levulinate over those previously reported.1 Investigations of these effects will require the development of techniques to synthesize the CoA esters we have identified, and exploration of the hypothetical mechanism leading to the formation of 6. Lastly, proteomic studies should be conducted on brains of rats treated with levulinate ± ethanol to test for the presence of pyrrole adducts on lysine residues. The data of the present report emphasize the public health relevance of further mechanistic investigations on the metabolic perturbations induced by levulinate and 4-hydroxypentanoate.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Case Mouse Metabolic and Phenotyping Center for help with the in vivo experiments.
Funding
This work was supported by NIH Roadmap Grant R33DK070291 and Grant R01ES013925 (to H.B.), NIH Grant R01CA157735 and NSF Grant MCB-084480 (to G.P.T.), and NIH Grant R01GM021249 and EY016813(to R.G.S.).
Footnotes
ASSOCIATED CONTENT
[SI] Supporting Information
Additional table and figures as described in the text.
Table S1: Exact mass assays of CoA esters derived from levulinate in perfused rat livers.
Figure S1: 13C-NMR spectrum of synthesized 3,6-diketoheptanoate ethyl ester 2'. (See Experimental Procedures)
Figure S2: 1H-NMR and LC/MS spectra of synthesized 2-methyl-5-oxocyclopent-1-enecarboxylic acid ethyl ester 4'.
Figure S3: LC/MS data of 1, 2, 4/5, 6, 7 (Scheme 1, left panel).
Figure S4AB: Plasma concentrations of levulinate and 4-hydroxypentanoate in rats injected with levulinate ± ethanol.
Figure S5ABC: Liver concentrations of the main C5 acyl-CoAs derived from levulinate in rats gavaged with calcium levulinate ± ethanol.
Figure S6ABC: Brain concentrations of the main C5 CoA esters derived from levulinate in rats gavaged with calcium levulinate ± ethanol.
This material is available free of charge via the Internet at http://pubs.acs.org
Author Contributions
The manuscript was written through contributions of all authors. All authors approved the final version of the manuscript.
The authors declare no competing financial interest.
REFERENCES
- 1.Harris SR, Zhang GF, Sadhukhan S, Murphy AM, Tomcik KA, Vazquez EJ, Anderson VE, Tochtrop GP, Brunengraber H. Metabolism of levulinate in perfused rat livers and live rats: conversion to the drug of abuse 4-hydroxypentanoate. J. Biol. Chem. 2011;286:5895–5904. doi: 10.1074/jbc.M110.196808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson IB, Kim SY, Dyer JE, Burkhardt CB, Iknoian JC, Walsh MJ, Blanc PD. Trends in gamma-hydroxybutyrate (GHB) and related drug intoxication 1999 to 2003. Ann. Emerg. Med. 2006;47:177–183. doi: 10.1016/j.annemergmed.2005.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter LP, Chen W, Wu H, Mehta AK, Hernandez RJ, Ticku MK, Coop A, Koek W, France CP. Comparison of the behavioral effects of gamma-hydroxybutyric acid (GHB) and its 4-methyl-substituted analog, gamma-hydroxyvaleric acid (GHV) Drug Alcohol Depend. 2005;78:91–99. doi: 10.1016/j.drugalcdep.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Zhang G-F, Sadhukhan S, Tochtrop G, Brunengraber H. Metabolism of γ-hydroxybutyrate in perfused rat livers. J. Biol. Chem. 2011;286 doi: 10.1074/jbc.M110.196808. 32631-23635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell GA, Gauthier N, Lesimple A, Wang SP, Mamer O, Qureshi I. Hereditary and acquired diseases of acyl-coenzyme A metabolism. Mol. Genet. Metab. 2008;94:4–15. doi: 10.1016/j.ymgme.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Otto DA, Chatzidakis C, Kasziba E, Cook GA. Reciprocal effects of 5-(tetradecyloxy)-2-furoic acid on fatty acid oxidation. Arch. Biochem. Biophys. 1985;242(1):23–31. doi: 10.1016/0003-9861(85)90475-8. [DOI] [PubMed] [Google Scholar]
- 7.Thurston JH, Hauhart RE. Amelioration of adverse effects of valproic acid on ketogenesis and liver coenzyme A metabolism by cotreatment with pantothenate and carnitine in developing mice: possible clinical significance. Pediatr. Res. 1992;31(4 Pt 1):419–423. doi: 10.1203/00006450-199204000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Ponchaut S, Van HF, Veitch K. In vitro effects of valproate and valproate metabolites on mitochondrial oxidations. Relevance of CoA sequestration to the observed inhibitions. Biochem Pharmacol. 1992;43:2435–2442. doi: 10.1016/0006-2952(92)90324-c. [DOI] [PubMed] [Google Scholar]
- 9.Brunengraber H, Kelleher JK, Des Rosiers C. Applications of mass isotopomer analysis to nutrition research. Annu. Rev. Nutr. 1997;17:559–596. doi: 10.1146/annurev.nutr.17.1.559. [DOI] [PubMed] [Google Scholar]
- 10.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am. J. Physiol. 1999;276:E1146–E1170. doi: 10.1152/ajpendo.1999.276.6.E1146. [DOI] [PubMed] [Google Scholar]
- 11.Kasumov T, Martini WZ, Reszko AE, Bian F, Pierce BA, David F, Roe CR, Brunengraber H. Assay of the concentration and (13)C isotopic enrichment of propionyl-CoA, methylmalonyl-CoA, and succinyl-CoA by gas chromatography-mass spectrometry. Anal Biochem. 2002;305:90–96. doi: 10.1006/abio.2002.5639. [DOI] [PubMed] [Google Scholar]
- 12.Joubert C, Beney C, Marsura A, Luu-Duc C. Synthesis of labelled [13C6]testosterone and [13C5]19-nortestosterone. J. Labelled Compounds Radiopharm. 1995;36:745–754. [Google Scholar]
- 13.Padwa A, Kulharni YS, Zhang Z. Reaction of carbonyl compounds with ethyl lithiodiazoacetate. Studies dealing with the rhodium(II)-catalyzed behavior of the resulting adducts. J. Org. Chem. 1990;55:4144–4153. [Google Scholar]
- 14.Paal C. Ueber die Derivate des Acetophenonacetessigesters und des Acetonylacetessigesters. C. Ber. 1884;17:2756–2767. [Google Scholar]
- 15.Knorr L. Synthese von Furfuranderivaten aus dem Diacetbernsteinsaureester. C. Ber. 1984;17:2863–2870. [Google Scholar]
- 16.Brunengraber H, Boutry M, Lowenstein JM. Fatty acid and 3-beta-hydroxysterol synthesis in the perfused rat liver. J. Biol. Chem. 1973;248:2656–2669. [PubMed] [Google Scholar]
- 17.Zhang GF, Kombu RS, Kasumov T, Han Y, Sadhukhan S, Zhang J, Sayre LM, Ray D, Gibson KM, Anderson VA, Tochtrop GP, Brunengraber H. Catabolism of 4-hydroxyacids and 4-hydroxynonenal via-4-hydroxy-4-phosphoacyl-CoAs. J Biol. Chem. 2009;284:33521–33534. doi: 10.1074/jbc.M109.055665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez CA, Des Rosiers C, Previs SF, David F, Brunengraber H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J Mass Spectrom. 1996;31:255–262. doi: 10.1002/(SICI)1096-9888(199603)31:3<255::AID-JMS290>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Dalluge JJ, Gort S, Hobson R, Selifonova O, Amore F, Gokarn R. Separation and identification of organic acid-coenzyme A thioesters using liquid chromatography/electrospray ionization-mass spectrometry. Anal. Bioanal. Chem. 2002;374:835–840. doi: 10.1007/s00216-002-1554-x. [DOI] [PubMed] [Google Scholar]
- 20.Graham DG, St Clair MB, Amarnath V, Anthony DC. Molecular mechanisms of gamma-diketone neuropathy. Adv. Exp. Med. Biol. 1991;283:427–431. doi: 10.1007/978-1-4684-5877-0_58. [DOI] [PubMed] [Google Scholar]
- 21.Monaco S, Wongmongkolrit T, Shearson CM, Patton A, Schaetzle B, utilio-Gambetti L, Gambetti P, Sayre LM. Giant axonopathy characterized by intermediate location of axonal enlargements and acceleration of neurofilament transport. Brain Res. 1990;519:73–81. doi: 10.1016/0006-8993(90)90062-g. [DOI] [PubMed] [Google Scholar]
- 22.Sayre LM, Shearson CM, Wongmongkolrit T, Medori R, Gambetti P. Structural basis of gamma-diketone neurotoxicity: non-neurotoxicity of 3,3-dimethyl-2,5-hexanedione, a gamma-diketone incapable of pyrrole formation. Toxicol Appl Pharmacol. 1986;84:36–44. doi: 10.1016/0041-008x(86)90414-x. [DOI] [PubMed] [Google Scholar]
- 23.LoPachin RM, Jortner BS, Reid ML, Monir A. Gamma-Diketone central neuropathy: quantitative analyses of cytoskeletal components in myelinated axons of the rat rubrospinal tract. Neurotoxicology. 2005;26:1021–1030. doi: 10.1016/j.neuro.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Boutaud O, Li J, Chaurand P, Brame CJ, Marnett LJ, Roberts LJ, Oates JA. Oxygenation of arachidonic acid by cyclooxygenases generates reactive intermediates that form adducts with proteins. Adv. Exp. Med. Biol. 2001;500:133–137. doi: 10.1007/978-1-4615-0667-6_16. [DOI] [PubMed] [Google Scholar]
- 25.Brame CJ, Salomon RG, Morrow JD, Roberts LJ. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J. Biol. Chem. 1999;274:13139–13146. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- 26.Brosnan JT, Brosnan ME, Charron R, Nissim I. A mass isotopomer study of urea and glutamine synthesis from 15N-labeled ammonia in the perfused rat liver. J Biol. Chem. 1996;271:16199–16207. doi: 10.1074/jbc.271.27.16199. [DOI] [PubMed] [Google Scholar]
- 27.DeCaprio AP, Jackowski SJ, Regan KA. Mechanism of formation and quantitation of imines, pyrroles, and stable nonpyrrole adducts in 2,5-hexanedione-treated protein. Mol. Pharmacol. 1987;32:542–548. [PubMed] [Google Scholar]
- 28.Zhang W, Bolla ML, Kahne D, Walsh CT. A three enzyme pathway for 2-amino-3-hydroxycyclopent-2-enone formation and incorporation in natural product biosynthesis. J Am. Chem Soc. 2010;132:6402–6411. doi: 10.1021/ja1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Gavin T, DeCaprio AP, LoPachin RM. Gamma-diketone axonopathy: analyses of cytoskeletal motors and highways in CNS myelinated axons. Toxicol. Sci. 2010;117:180–189. doi: 10.1093/toxsci/kfq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, Podrez EA, Salomon RG, Byzova TV. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tholen H, Mordhorst R. Hepatic and cerebral coenzyme A contents after intravenous injection of coenzyme A in rats. Experientia. 1976;32:830–832. doi: 10.1007/BF02003711. [DOI] [PubMed] [Google Scholar]
- 32.Gibson KM, Jakobs C, Pearl PL, Snead OC. Murine succinate semialdehyde dehydrogenase (SSADH) deficiency, a heritable disorder of GABA metabolism with epileptic phenotype. IUBMB. Life. 2005;57:639–644. doi: 10.1080/15216540500264588. [DOI] [PubMed] [Google Scholar]
- 33.Nylen K, Velazquez JL, Likhodii SS, Cortez MA, Shen L, Leshchenko Y, Adeli K, Gibson KM, Burnham WM, Snead OC., III A ketogenic diet rescues the murine succinic semialdehyde dehydrogenase deficient phenotype. Exp. Neurol. 2008;210:449–457. doi: 10.1016/j.expneurol.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson KM, Hoffmann GF, Hodson AK, Bottiglieri T, Jakobs C. 4-Hydroxybutyric acid and the clinical phenotype of succinic semialdehyde dehydrogenase deficiency, an inborn error of GABA metabolism. Neuropediatrics. 1998;29:14–22. doi: 10.1055/s-2007-973527. [DOI] [PubMed] [Google Scholar]
- 35.DeCaprio AP. n-Hexane neurotoxicity: a mechanism involving pyrrole adduct formation in axonal cytoskeletal protein. Neurotoxicology. 1987;8:199–210. Review. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.