SUMMARY
Cholangiocarcinoma (CCA) is an orphan cancer with limited understanding of its genetic and genomic pathogenesis. Typically, it is highly treatment-refractory and patient outcome is dismal. Currently, there are no approved therapeutics for CCA and surgical resection remains the only option with curative intent. Clinical trials are currently being performed in a mixed cohort of biliary tract cancers that includes intrahepatic CCA, extrahepatic/perihilar CCA, distal extrahepatic CCA, gallbladder carcinoma and, in rare cases, even pancreatic cancers. Today, clinical trials fail primarily because they are underpowered mixed cohorts and designed without intent to enrich for markers to optimize success for targeted therapy. This review aims to emphasize current clinical attempts for targeted therapy of CCA, as well as highlight promising new candidate pathways revealed by translational genomics.
Practice Points.
Patient classification and characterization may be needed regardless of successful implementation of individualized treatment. This may guide secondary or tertiary treatment options in the case of drug resistance or disease recurrence.
Detailed genetic, genomic and/or epigenomic data from large cohorts in combination with genomic analyses of individual patients will facilitate both faster clinical decision-making and the appropriate choice of therapies among the available drug options and pathways to target.
Implementation of either conventional monotherapy or a single pathway-specific targeted therapy is unlikely to be successful, particularly in drug-resistant heterogeneous cancers such as cholangiocarcinoma (CCA).
Coadministration of either unique targeted therapy based on molecular characteristics of the patient's tumor, with cytotoxic standard chemotherapy or combination of several targeted therapies inhibiting multiple dysregulated pathways, is likely to achieve a synergistic effect and be successful. However, the concern with simultaneously targeting multiple pathways is the increased risk of drug toxicity and adverse effects for the patient.
CCA is characterized by diverse anatomical locations – that is, intrahepatic and extrahepatic bile ducts – as well as clinical and molecular heterogeneity that complicates assessment of treatment efficacy. This is further confounded when patients with biliary tract cancer are considered altogether as a group.
Recent interest in understanding the molecular complexity of CCA has been evident; however, access to well-preserved and annotated cohorts for detailed genetic and genomic assessments is a limiting factor. Mainly due to the anatomical complexity of this malignancy, heterogeneity of the tumor samples/cohorts studied may be considered as a confounding factor that should be addressed in each study according to the 2013 International Liver Cancer Association guidelines.
Cholangiocarcinoma (CCA) is a rare and devastating malignancy with a dismal outcome. Whereas the incidence of most malignancies has declined in the past two decades, the incidence of CCA has increased worldwide [1], and the mortality rate of CCA has risen by more than 9% in the USA [2]. Intrahepatic CCA (iCCA) is classified as a peripheral-type tumor of the interlobular bile ducts, whereas tumors designated perihilar (pCCA)/hilar are generally considered extrahepatic, and originate in the main hepatic ducts or at the bifurcation of the common hepatic duct. iCCA is often diagnosed at a late stage in the disease progression; partly due to the anatomical location, diverse growth pattern and underlying pathobiological heterogeneity. In addition, at diagnosis, chemotherapy and radiation therapy are generally ineffective. Thus, the clinical management of this disease offers limited therapeutic options – that is, in the absence of unresectable advanced and metastatic disease, surgical resection remains the only curative treatment for patients with CCA [3]. Standard therapy administered to these patients is therefore typically palliative. In addition, intrahepatic recurrence is frequent following attempted curative resection and serves as a confounding variable [4].
The etiology of CCA remains partly undetermined [5]. A background of chronic liver inflammation, for example, primary biliary cirrhosis or primary sclerosing cholangitis are linked to increased risk of developing CCA. Other risk factors include bile duct injury (cholestasis), hepatitis B and C viral infection, alcohol consumption, diabetes or more regional specific hazards such as parasitic liver infestation.
Multitarget tyrosine kinase inhibitor (TKI) sorafenib, which is used as first-line therapy for advanced hepatocellular carcinoma (HCC), has had limited success in trials of CCA patients [6]. The lack of therapeutic efficacy in the clinical management of CCA is, in part, the result of inadequate molecular and pathobiological understanding of this disease. Stratification of class-specific risk groups or focus on individual patients may be essential for clinical success in treating CCA patients in the future.
Conventional chemotherapy: current standard of care
Standard of care is usually administered to CCA patients with palliative intend. Only few Phase III randomized controlled trials have been conducted in CCA, and typically these studies are in mixed biliary tract cancer (BTC) cohorts. Surgical resection remains the only clinical option with curative intent in which an estimated 30% 5-year survival rate may be achieved. Patients with unresectable iCCA have a 0–5% 5-year survival rate [7].
Systemic chemotherapy, as well as targeted therapies, have typically had limited success in CCA. A randomized Phase III trial in 90 patients with advanced BTC and pancreatic cancers compared 5-flurouricil and leucovorin to best supportive care [8]. This trial demonstrated improved quality of life and prolonged survival as a result of the treatment. Interestingly, more than 100 Phase II trials that included >2800 patients were conducted between 1985 and 2006; however, they only had an average cohort size of 25 subjects [9]. This meta-analysis demonstrated an overall response rate – that is, partial plus complete response – following systemic chemotherapy of 22.6%, time to progression of 4.1 months and median overall survival (OS) was 8.2 months. In a small study including 23 treatment-naive unresectable BTC patients, gemcitabine was given as a single agent [10]. In this nonrandomized Phase II trial, gemcitabine alone was generally well tolerated, showed limited adverse events and had clinical efficacy with 26.1% rate partial. In addition, gemcitabine in combination with either irinotecan [11] or capecitabine [12] showed limited adverse events, but overall modest improved efficacy. The recent ABC-02 Phase III randomized controlled trial included 410 patients and established a new guideline for the standard of care in advanced and metastatic BTC [13,14]. In this trial, the authors reported a significant benefit in time to progression and increase in the median OS by 11.7 months after combined chemotherapy of gemcitabine plus cisplatin (CisGem) versus gemcitabine alone [14]. This trial was validated in a smaller Japanese Phase II multicenter study (BT22) of CisGem in patients with advanced BTC [15], reporting similar efficacy as observed in the ABC-02 trial. Although most clinical trials include patients with BTC and are typically underpowered, nonrandomized, single-center studies, these recent studies are encouraging as a baseline to design combination trials with molecular-targeted therapies in unique well-characterized patient groups.
The potential of neoadjuvant chemotherapy to achieve operable margins in patient downstaging would also represent a promising step to improve management of the disease. As for adjuvant therapy, currently there is limited evidence of any clinical efficacy. However, in a Phase III randomized clinical trial with mitomycin C plus 5-flurouricil, clinical benefit was reported, but only in the patient subgroup diagnosed with gallbladder cancer [16]. However, we are currently awaiting the ongoing UK NCRI BILCAP trial that will report its findings in 2014 on postoperative capecitabine versus observation alone.
Despite some encouraging progress, there are clearly still many limitations and unmet needs. Discovery of new systemic agents with clinical efficacy and actionable targets are urgently required. To achieve this goal in a rare neoplasms, such as CCA, with diverse clinicopathology, multicenter studies are necessary to acquire the required cohorts. Future trials may include CisGem or derivatives in the control arm of the study as current standard of care. Detailed molecular evaluation is recommended and, as such, it is suggested that future clinical trials plan for procurement of tissue samples for molecular characterization to: evaluate the biomarker and/or drugable target network; establish a drug response or resistance signature; and identify genetic events pre- and post-treatment, for example, difference in the mutational profile caused following the treatment either directly drug-related or due to accrual of new or underlying somatic mutations, which would indicate resistance to the treatment.
Rationale for targeting candidate oncogenic signaling pathways
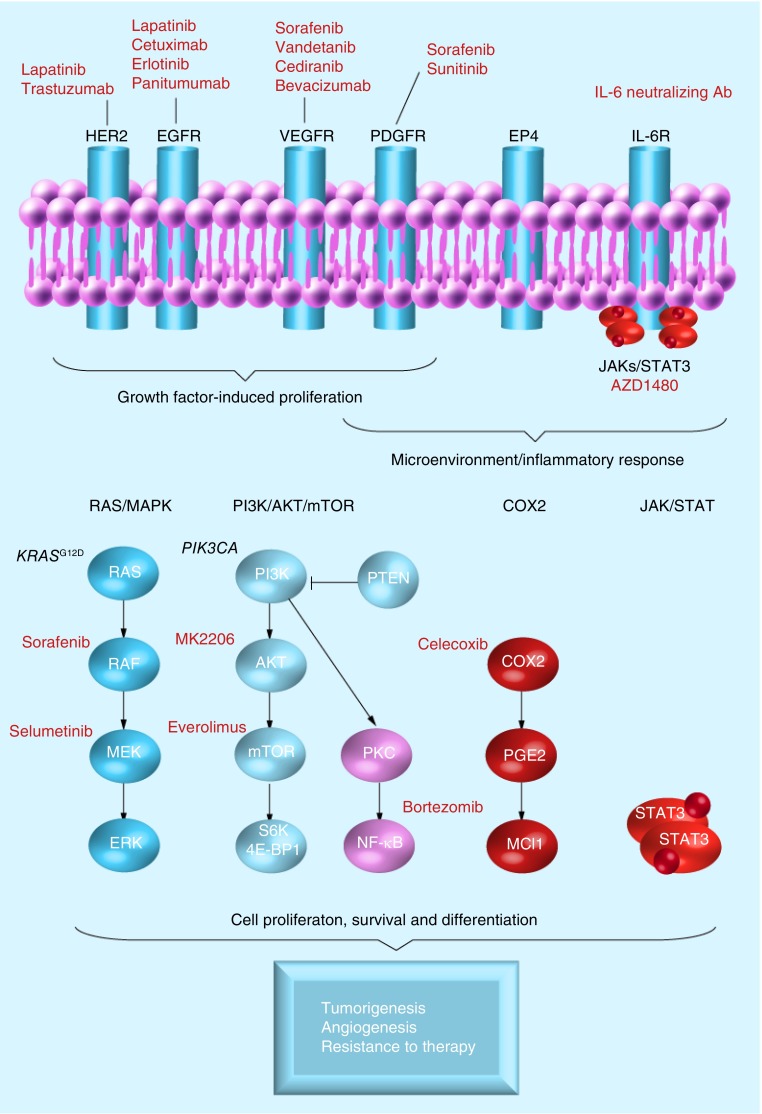
To date, some of the most interesting approaches include strategies targeting the EGF receptor (EGFR) family (EGFR and HER2), the RAS/RAF/MEK/ERK signaling network and angiogenesis (VEGF) (Figure 1). Besides these pathways, well-described molecular alterations include the MET proto-oncogene and inflammatory stimuli from the tumor microenvironment – that is, the tumor stromal compartment, such as IL-6/STAT3, as well as COX2. However, integration of the new genomic data [17–20], for example, genetic, epigenomic and/or transcriptomic [21] patterns that may improve patient stratification may emerge and help guide clinical decisions and provide new opportunities to treat CCA, as is the case for other malignancies.
Figure 1. Canonical pathways and classical targeted therapy.
EGFR: EGF receptor; IL-6R: IL-6 receptor; PDGFR: PDGF receptor; VEGFR: VEGF receptor.
▪ EGFR family
The EGFR family of receptor tyrosine kinases (RTKs) is comprised of ERBB1–4. However, ERBB1 (EGFR) and ERBB2 (HER2/Neu) are the two RTKs most frequently dysregulated in the molecular pathogenesis of CCA [22,23]. In addition, this is an attractive pathway for therapeutic targeting since TKIs are effective antitumor agents in other cancers.
Aberrant EGFR and HER2 expression is found in many cancers, for example, breast, gastric and ovarian. In CCA, overexpression of EGFR and HER2 was reported in 10–32% and 20–30% of tumors, respectively [22,23]. BTC with increased EGFR and HER2 expression displayed copy number (CN) gains in 77 and 79% of cases, respectively [24]. However, two recent large genomic studies of CCA, showed overexpression of EGFR and HER2 signaling in patient subgroups associated with poor outcome and KRAS mutations, despite a lack of CN amplification [17,18,25]. Although HER2 and EGFR are rarely mutated in CCA, genetic variants in EGFR (0–13.6%) have been reported [17,18,26]. In the case of ERBB3 and ERBB4, information is limited, although these receptors were shown by immunohistochemistry to be moderate-to-strongly positive in 40 and 11% of cases, respectively [27]. Following laser-capture microdissection in a subset of CCAs [17], the authors found a significant HER2–ERBB3 gene network associated with the epithelial tumor compartment, suggesting cooperation between the two receptors. Recently, ERBB3 was shown in association with HER2 to correlate with survival in a subset of pCCA patients [28].
Preclinical evidence supports the oncogenic role of the ERBB receptor family, particularly HER2, in the development and treatment of CCA. Kiguchi et al. have developed a transgenic mouse model where expression of the rat ErbB2, under the control of the bovine keratin-5 promoter (BK5.erbB2), is targeted to the basal layer of multiple epithelial tissues, which include the bile ducts, giving rise to CCA in 30% of cases as a consequence of the elevated HER2 expression [29]. Other evidence for the involvement of HER2 originates from chemical-induced animal models using furan [30], thioacetamide [31], p53-deficient/CCL4 [32], as well as the orthotopic transplantation BDEneu model (in which adult rat BDE1 cholangiocytes are transfected with a mutated constitutively active HER2 gene, resulting in the cells becoming tumorigenic) [33]. Elevated expression levels of EGFR and COX2, as well as activation of ERK and AKT, generally characterize these models, which are similar to human CCA with poor prognosis.
To assess RTKs as potential drug targets [23], cell culture and orthotopic animal models using anti-EGFR therapies, such as erlotinib and cetuximab, have demonstrated the ability of these drugs to inhibit proliferation of CCA cell lines [34]. Furthermore, simultaneous targeting of EGFR and HER2 by lapatinib has demonstrated antiproliferative effects, as well as the ability to reduce tumor growth [17,35]. A multi-TKI (vandetanib), which targets EGFR, VEGF receptor (VEGFR) 2 and RET was demonstrated in vivo to cause significant tumor growth inhibition and prolong the time to metastasis [36]. Currently, several clinical Phase I/II trials are ongoing, evaluating vandetanib alone or in combination with standard chemotherapy.
The clinical experience with molecular-targeted therapies against EGFR in CCA has been inadequate compared with other therapeutic areas, suggesting that future trials may require improved patient stratification and likely combination strategies, targeting several oncogenic pathways simultaneously. Interestingly, COX2 activation may be involved in the regulation of RTKs and the MAPK signaling network. As such, it was demonstrated that bile acid from patients with congenital choledochal cysts has a growth promoting effect on CCA cell lines and that this proliferation can be inhibited by the COX2 inhibitor, celecoxib [37]. Other preclinical models include the EGFR inhibitor iressa as a radio sensitizer [38]. Interestingly, introduction of radiation therapy in CCA was shown to activate AKT, which in turn was suppressed by administration of iressa.
▪ MAPK signaling cascade
The RAS/RAF/MEK/ERK tyrosine kinases pathway is frequently dysregulated in cancers, including CCA, supporting unrestricted growth and survival of cancer cells [39,40]. This dysregulation is driven by numerous growth promoting signals, including RTKs, PDGF and inflammatory stimuli.
Activating mutations in KRAS are one of the most frequently reported disease-causing genetic events in CCA, with an incidence ranging from 8 to 100% [17–20,41–43]. The authors previously reported that 24.6% of CCA patients have mutations in the KRAS gene [17]. However, when classifying patients according to tumor site, 53.3% of hilar-type (pCCA) versus 16.7% of peripheral-type CCAs (iCCA) were positive for KRAS mutations, suggesting the importance of the anatomical location for the tumor in selecting for KRAS mutations, and the subsequent choice of therapy.
Preclinical animal models, such as the genetically engineered tissue-specific activation of the hotspot mutation KRAS G12D, display low penetrance and long latency for the development of invasive iCCA [44]. In combination with p53 inactivation the penetrance is greatly increased, suggesting these genes are important ‘drivers’ in the development of iCCA [44]. To date, there are no proven KRAS-targeted therapies. Furthermore, the therapeutic efficacy of EGFR-targeted therapies, for example, erlotinib, cetuximab and panitumumab, is restricted to patients with wild-type KRAS gene status. Similarly, patients with rare NRAS mutations showed limited respond to EGFR inhibition [45]. However, we have shown the potential of sensitizing CCA to TKI-based therapies using a murine xenograft model designed to mimic TKI-resistant human CCA with a mutated KRAS gene (KRASG12D). In this model, mice treated with the HER2 inhibitor (trastuzumab) in combination with celecoxib demonstrate a significant decrease in tumor growth and benefit on survival, whereas trastuzumab administered alone is ineffective, suggesting that celecoxib sensitize these tumors to HER2 inhibition [Andersen JB et al., Unpublished Data]. Interestingly, preclinical mouse models of orthotopic transplanted gallbladder carcinoma with KRAS mutations also showed a significant survival benefit following treatment with downstream MEK inhibitors (U0126 and PD98059) [46]. Targeting MEK is an attractive strategy, particularly since concerns of drug resistance similar to what is observed with EGFR inhibitors are limited in patients with KRASG12D CCAs. Several clinical trials evaluating drugs targeting MEK (selumetinib [AZD6244] [47], ARRAY-438162 and GSK1120212) are either ongoing or concluded.
▪ BRAFV600E
BRAF is an upstream component of the MAPK pathway. The genetic variant BRAFV600E was identified in 0–22% of CCA, dependent on the population studied [17,18,48,49]. Several BRAF-targeted therapies, for example, GSK2118436 and vemurafenib (PLX-4032), are in clinical development; however, vemurafenib is thus far the only US FDA-approved BRAF inhibitor for metastatic melanoma. The V600E mutation in the BRAF gene was recently highlighted as a promising target in appropriate iCCA patients – limited to Europe, with a potential of an estimated 3000 iCCA patients annually [18].
▪ Angiogenesis: VEGF
VEGF, a key factor in promoting tumor growth and metastasis via its important role in neo-angiogenesis, is overexpressed in >50% of CCA [50,51]. Moreover, high expression of VEGF is associated with poor prognosis. Experimental studies have shown that sorafenib, a multi-TKI primarily targeting VEGF, but also PDGF receptor (PDGFR) and RAF kinases, has potent activity in vitro and in vivo against iCCA [52]; however, clinical trials in BTC have failed [6,53]. Evaluation of other VEGF inhibitors includes, sunitinib (against VEGFR, PDGFR and KIT), vandetanib (against VEGFR, EGFR and RET), bevacizumab (against VEGF-a) and cediranib (against VEGFR) has either failed or is ongoing.
Targeted therapies in CCA have mainly in the past focused on inhibition of RTKs (EGFR and HER2), VEGFR and MAPK pathways. However, several promising ‘classical’ targets include the HGF receptor (MET), the PI3K/AKT pathway and mTOR.
▪ MET proto-oncogene signaling
Binding of the HGF to the MET receptor triggers an activation cascade of multiple downstream signal transduction pathways, for example, MAPK, PI3K/AKT and STAT [54]. Aberrant signaling and constitutive MET activation caused by either mutation or CN amplification was demonstrated in numerous malignancies [48]. Increased expression of MET is observed in 12–58% of CCA patients [24,55,56] and is associated with poor prognosis [55,57]. Both human data [57] and preclinical experimental models [55,58] suggest an oncogenic signaling loop between overexpression of MET and members of the ERBB-family, suggesting a likelihood of drug resistance to EGFR inhibitors in patients with aberrant MET expression.
Several MET-targeted inhibitors are in clinical development [59]. Data from a recent Phase II randomized clinical trial evaluating efficacy of tivantinib (ARQ-196) in advanced HCC as second line after sorafenib failure are very encouraging [60]. The trial demonstrated an approximate doubling of progression-free survival and OS compared with placebo in patients with MET overexpression, which includes approximately 25% of patients. A dose escalation Phase I trial for MET inhibitor LY2801653 is currently enrolling patients with advanced cancers in three different tumor types, including CCA (NCT01285037) [201]. This safety and later efficacy trial is, unfortunately, not expected to report before late 2015. MET inhibitors (tivantinib, cabozantinib, golvatinib, foretinib or MetMAb) have not been evaluated in CCA; however, future clinical trials evaluating MET-targeted therapies alone or in combination with standard of care, combined with EGFR/HER2 inhibitors or sorafenib in patients stratified for MET expression, would be warranted.
▪ PI3K/PTEN/AKT/mTOR network
The PI3K/AKT/mTOR pathway is activated downstream of RTKs. Altered regulation of this pathway results in proliferation, changes in cell adhesion and motility, as well as angiogenesis and, ultimately, tumorigenesis. Subgroups of CCA patients with poor outcome are characterized by activation and elevated expression of RTKs, in particular, EGFR, IGF receptor, HER2 and MET, which result in phosphorylation of AKT observed in 22–65% of tumors [61,62]. Data obtained from transcriptomic profiling show that the AKT/mTOR pathway is activated in a subset of CCAs [17,63]. This is supported by immunohistochemical analyses of the downstream targets of mTOR, RPS6, eIF4-E and the 4E-BP1 [17,63,64].
The gene PIK3CA is a subunit (P110α) of PI3K, which is an activator of the downstream AKT/mTOR pathway. Mutations in PIK3CA were identified in many cancers and shown to result in constitutive activation of AKT signaling. Recent studies in CCA suggest that mutations in PIK3CA (0–32%) are relatively rare, with the exception of in the Chinese population [20,49,64,65]. Reiner et al. identified hotspot PIK3CA mutations in one out of 11 iCCAs [64]. Similarly, Deshpande et al. found no mutations (none out of 44) in iCCA and pCCA [65], whereas 13% of the examined gallbladder carcinomas were positive for PIK3CA mutations. By contrast, a study in the Chinese population reported PIK3CA mutations in 32% (11 out of 32) of CCA patients [49].
A genetically engineered mouse model of liver-specific PTEN and SMAD4 disruption was found to develop aggressive iCCA [66]. Given the strong activation of downstream mTOR and MAPK pathways in this animal model, as well as in CCA patients with poor outcome, targeting either of the nodes in the PI3K/AKT/mTOR pathway may present an alternative therapeutic strategy in the clinical management of CCA. Preclinical evaluation of inhibition of AKT (MK2206) and mTOR (RAD001/everolimus) alone or in combination demonstrated a synergistic effect of both targeted therapies in CCA, significantly reducing the tumor growth [67]. In addition, we have shown that MK2206 alone is sufficient to inhibit tumor growth in a TKI-resistant xenograft model of KRAS G12D CCA [Andersen JB et al., Unpublished Data]. The efficacy of MK2206 in unresectable, advanced and metastatic refractory biliary cancer is currently ongoing and results from this Phase II trial are anticipated in early 2014 (NCT01425879) [202].
Although a recent review suggested that more than 26 PI3K inhibitors are in development and used in >150 clinical trials, we found no current clinical trials using PIK3CA-targeted therapy in CCA [68]. The only clinical trial, which planned to enroll patients with CCA, was a Phase II study scheduled to evaluate BKM120 in PI3KCA-mutated cancers. However, this trial was terminated owing to limited accrual (NCT01501604) [203]. Although multiple trials evaluating mTOR-targeted therapies in HCC are ongoing [69], implementation of these inhibitors in the treatment of CCA is currently limited to two ongoing single-arm Phase II studies (RADiChol and CRAD001T) using everolimusin first-line treatment against advanced CCA (NCT00973713 [204] and NCT01525719 [205]).
Molecular-targeted therapies
The standard of care for patients with advanced inoperable CCA is palliative chemotherapy. Since the efficacy of systemic drugs is limited to marginal prolonged survival compared with best supportive care, recent years have led to intensified investigations into molecular-targeted therapies. The implementation of these therapies in the treatment of CCA is difficult, largely because the majority of clinical trials are designed for BTC and, as such, the CCA patient group is typically too underpowered to allow for subgroup analysis and there is an absence of a comprehensive genomic and genetic understanding of the pathogenesis of CCA.
▪ Tyrosine kinase inhibitors
In past years, clinical trials have mainly been focused on TKIs as single agents in first-line treatment or in combination with standard chemotherapy. Results from multiple Phase II clinical trials that have assessed targeted agents – for example, erlotinib (against EGFR) [70], lapatinib (against EGFR and HER2) [71], sorafenib (multi-TKI against VEGFR2/3, PDGFR and RAF kinases) [6], sunitinib (against VEGFR, PDGFR and KIT) [72] and selumetinib (against MEK) [47], have unfortunately not warranted further investigation. It is important to mention that these were all relatively small monotherapy-driven trials performed in mixed BTC cohorts. Furthermore, clinical trials designed to evaluate efficacy of targeted therapy should be encouraged to include molecular analysis of the particular pathway inhibited by the drug (i.e., marker-driven study). In this regard, the study design of the selumetinib trial [47] required tissue sampling with analysis of BRAF and KRAS mutational status, as well as quantification of the activation of ERK and AKT. The level of ERK phosphorylation was determined to significantly correlate with drug response.
A combination therapy comprising both erlotinib and bavacizumab showed marginal benefit over previous trials with 12% rate partial, time to progression was 4.4 months and median OS was 9.9 months [73]. As a secondary end point, the trial was designed to integrate molecular evaluation of EGFR and KRAS mutational status, as well as VEGF serum levels. Several clinical trials that are designed to evaluate VEGFR inhibitor (vandetinib) alone or in combination with conventional chemotherapy are ongoing (NCT00753675 and NCT00551096) [206,207]. Similarly, two trials designed to study the efficacy of cediranib in advanced BTC are currently in progress (NCT01229111 and NCT00939848) [208,209]. The UK-based trial ABC-003 is a randomized Phase II study with 136 enrolled patients, which will compare cediranib in combination with CisGem to CisGem alone. This study is active but not recruiting, and expected to report in 2014.
In a continuation of monotherapy-based trials using sorafenib or erlotinib, a combination of both drugs in first-line treatment also failed to meet the primary end point [74]. Interestingly, the study concluded that improved patient selection may be required. Analysis of molecular markers prior to enrollment as part of inclusion/exclusion criteria may be beneficial for the outcome of targeted therapies in future clinical trials.
In recent years, combination trials including both chemotherapy – for example, gemcitabine and platinum-based drugs with targeted therapies, for example, bevacizumab [75], cetuximab (EGFR monoclonal antibody) [76] and erlotinib [77], have given rise to more encouraging results. The erlotinib trial is currently the only randomized Phase III trial, with 268 patients being administered GEMOX plus erlotinib versus standard chemotherapy alone [77]. This trial demonstrated a significant improved objective response (40 vs 25 patients in either arm, p < 0.005); marginal benefit in progression-free survival was 4.2 versus 5.4 months and a comparable median OS of 9.5 months was achieved. The two single-arm Phase II trials both demonstrated better objective response rates (40 vs 63%) and median OS (12.7 vs 15.2 months) [75,76]. Particularly, GEMOX plus cetuximab showed encouraging results as three patients experienced complete response and a total of nine patients downstaged after therapy and subsequently underwent surgical resection with curative intent.
To improve clinical efficacy of targeted therapies, strengthened patient selection may be required either based on the tumor biology – that is, mixed BTC cohorts and/or evaluation of molecular targets as part of the inclusion criteria. A small Phase II trial recently reported significant efficacy of GEMOX, capecitabine plus panitumumab (EGFR monoclonal antibody) in first-line treatment of BTC with a KRAS wild-type gene status as an inclusion criteria [78]. However, the evaluation of the study is complicated by the addition of capecitabine in the single-arm trial compared with previous trials. Since the study met its efficacy criteria with a primary end point of 6 months progression-free survival at 74%, it may warrant a larger randomized trial. A randomized Phase II trial (PiCCA) evaluating the efficacy of panitumumab and CisGem compared with CisGem alone is currently only recruiting patients with KRAS wild-type tumor status and no prior systemic treatment; however, results from this trial are not scheduled until mid-2014. Beside this study, only few trials are currently ongoing [22].
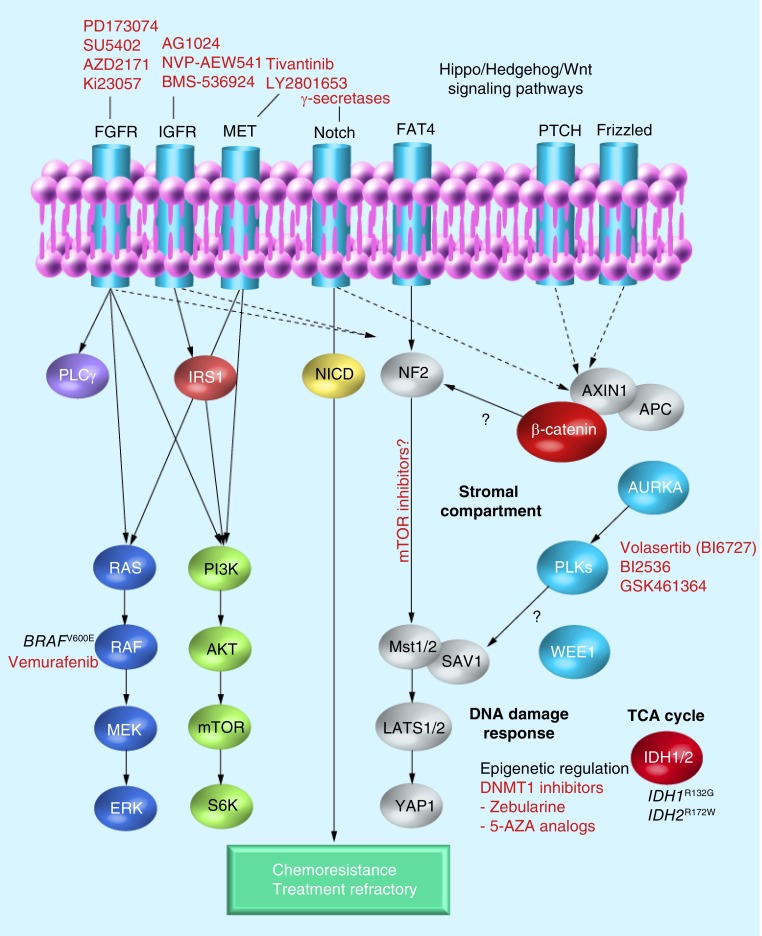
Emerging molecular targets
In recent years, promising new candidate targets have been uncovered by detailed genomic characterization and patient stratification, which may result in ‘innovative therapeutics’ and strategies to improve clinical outcome in CCA (Figure 2). We recently showed that a stromal-specific gene signature following microdissection of CCAs is associated with poor prognosis and that predominant gene networks correlate with hepatic stellate cells activation, chemokine and cytokine expression [17]. In another stromal gene signature of iCCA, osteopontin was identified as an independent predictor of survival [79]. CCA is a very desmoplastic cancer type [80], and the microenvironment is a key component of the tumor development and progression in many cancers [81]. The CCA microenvironment has received attention in recent years and various studies suggest that this area could be of therapeutic interest in the future [10,11,29,79,82–84].
Figure 2. Emerging-targeted therapy.
APC: Antigen-presenting cell; FGFR: FGF receptor; IGFR: IGR receptor; TCA: Tricarboxylic acid cycle.
▪ Polo-like kinase
This is a family of serine/threonine kinases involved in key regulatory processes, such as cell cycle progression (G2/M transition) and cytokinesis. Interestingly, Polo-like kinase (PLK)1 is overexpressed in several cancers and correlates with poor outcome [85]. In a large genomic subgroup analysis, the authors found PLK1 differentially expressed in CCA from patients with overall poor prognosis [17]. Preclinical evaluation found that the selective PLK1 inhibitor (BI2536) reduced proliferation of 14 BTC cell lines by inducing G2/M arrest and apoptosis, whereas the phosphorylation status of AKT and ERK was unchanged by the drug [86]. Recently, a role of PLK2 linked PLK and Hedgehog (Hh) signaling in TRAIL-induced cell death, by demonstrating the ability of Hh to regulate PLK2 expression, stabilize Mcl-1 and thus, convene resistance to TRAIL [83]. Fingas et al. concluded that targeting either Hh or PLK signaling may restore CCA cell susceptibility to TRAIL-induced apoptosis [83].
▪ Hh & Notch signaling pathways
The Hh signaling pathway is important in the regulation of proliferation, survival, self-renewal and development of embryo and adult tissues. The pathway is implicated in many cancer types, including biliary tract cancers [87].
The function of cancer-associated fibroblasts or myofibroblasts (MFs) has recently demanded more attention since a cross-talk between MFs and the tumor epithelium itself may be tumor promoting, and even correlate with CCA survival [88,89]. In the liver, MFs are derived from – for example, activated hepatic stellate cells, which transition is stimulated by Hh signaling [90]. The MF-to-CCA cross-talk involves several signaling pathways, PLK [83], PDGF [82,91], Hh [92] and Notch [84,92]. SHH expression was shown to regulate both PDGF-BB signaling [93] and Jagged-1 [92]. Recently, in a model of bile duct ligation, Xie et al. demonstrated that impaired Hh signaling inhibited the Notch pathway, and that Hh and Notch cooperate to control cell fate in adult liver repair [84]. Overexpression of both Hh and Notch has been implicated in chemoresistance and, as such, blocking Hh signaling may enhance therapeutic efficacy [88]. Preclinical models have shown that Notch signaling is critical in cholangiocarcinogenesis [94,95] and that γ-secretase inhibitors blocking Notch signaling may reduce tumor growth [94] and also attenuate liver fibrosis by affecting the gene profile in MFs [96]. In addition, macrophages are involved in the promotion of liver inflammation and profibrotic signals. Interestingly, activated hepatic stellate cells were recently demonstrated to cause the differentiation of macrophages [97]. The interplay between inflammation, profibrotic signals and tumorigenesis mediated in part through MFs and tumor-associated macrophages may present promising treatment options in the future.
▪ IL-6/STAT3 signaling cascade
Binding of IL-6 to its receptor (gp130) triggers the heterodimerization with the Janus kinases (JAK1, JAK2 or TYK2), thus facilitating a signaling cascade and activation of STAT3 – that is, the JAK/STAT pathway – and/or the MAPK pathway.
In experimental models, IL-6 was found to function in an autocrine manner, leading to increased expression of the antiapoptotic protein Mcl-1, TRAIL resistance through AKT and growth [98–100]. Continued activation of IL-6/STAT3 signaling was linked to epigenetic silencing of the key inhibitor of STAT3, SOCS3 [101], whereas restoring the SOCS3 expression using a demethylating drug sensitized CCA to TRAIL-induced apoptosis [101]. Inhibition of IL-6, by incubating cells with a neutralizing antibody, abrogated proliferation in an ERK and p38 MAPK-dependent pathway [100,101]. As part of the inflammatory response, COX2 expression is often increased in CCA with poor prognosis, and correlates with both EGFR and HER2. COX2-derived prostaglandin (PGE2) induces IL-6 levels, suggesting a link between the COX2 and IL-6/STAT3 signaling pathways [102]. Targeting COX2 downstream of the RTKs, MAPK, IL-6/STAT3 and PI3K/AKT/mTOR pathways is very compelling and celecoxib has successfully been used to inhibit CCA cell proliferation and reduce tumor growth in preclinical models [103–105].
The expression of the proinflammatory cytokine IL-6 is often increased in CCA as well as in serum and bile from CCA patients [99]. Serum level of IL-6 correlates with tumor size, and following photodynamic therapy the level of IL-6 is decreased, suggesting that IL-6 may be used both as a metric of tumor size and response. Recently, the JAK/STAT pathway was estimated to affect more than 70% of the inflammation subclass in iCCA [18]. This subclass corresponds to the patient subgroup with good prognosis and is characterized by enrichment in cytokine pathway signatures, overexpression of IL-6 and activation of STAT3 [17,18,25,106]. Sia et al. proposed targeting the JAK/STAT pathway, taking advantage of novel STAT3 and JAK1–JAK2 inhibitors [18]. JAK inhibitors such as AZD1480 have been shown to potently and concomitantly block JAK/STAT signaling [107], as well as the activation of JAK2, STAT3, MAPK and Fgfr3 by phosphorylation [108].
▪ The Hippo pathway
The Hippo signaling pathway is a novel tumor-suppressor pathway involved in development and stem cell biology [109]. Dysregulation of this pathway is implicated in tumorigenesis [110], and deletion of Mst1/2 was recently shown to cause both HCC and CCA [111]. Inactivation of Mst1/2 results in activation of the oncogene YAP1 and resistance to FAS-induced apoptosis. Interestingly, acute inactivation of Mst1/2 results in activation of mTORC1 and increased mTORC1-dependent phosphorylation of p70 S6 Thr389/412 phosphorylation. It was also suggested that overexpression of YAP1 may mediate activation of Notch and Wnt signaling through β-catenin transcriptional activity and induce the expression of downstream Notch targets. This suggests a cross-talk between these pathways, which needs to be investigated further.
Recently, Sia et al. examined the landscape of chromosomal aberration in iCCA [18]. The majority of regional CN alterations showed no apparent accumulation when analyzed compared with the prognostic gene classification. However, one interesting focal deletion containing the Hippo pathway gene, SAV1, which is enriched in the proliferation subclass, was also significantly associated with reduced SAV1 gene expression. Lately this pathway has attracted attention in numerous genetic studies, detailing its involvement in primary liver cancers [112]. However, validation of this may be required before it warrants a large translational study into the Hippo network in iCCA. To date, no Hippo–YAP-targeted therapy/inhibitor is available; however, recent evidence suggests a potential role for mTOR inhibitors in Hippo-dependent cancers [113].
Conclusion
CCA is an orphan malignancy with no current approved therapy. Currently, standard chemotherapy provides improved survival and the promise of potential downstaging, which would make surgery with curative intention possible. In the past few years, our understanding of the underlying biology of this heterogeneous cancer type has improved and provided target-enriched patient classification. While the molecular understanding of this malignancy is improving, clinical trials are still designed in mixed BTC cohorts. Besides the need to consider the anatomical locations of the tumor, the ethnic background of the patient may further complicate the clinical decision-making by differences in key genetic variants – for example, KRAS, PIK3CA, TP53, IDH1 and IDH2 – suggesting the need to involve translational genomics-driven evaluation as a part of the clinical management.
We have suggested several emerging molecular pathways with compelling treatment options, which are encouraging, but require further investigation. These include targeting ‘oncogenic drivers’, for example, mutations in the IDH1 and IDH2 genes [114,115]. Alternatively to targeted therapy, since IDH genes influence epigenetic control, this may suggest a potential benefit of DNA methyl transferase inhibitors. In a preclinical translational genomics/epigenomics study, we recently demonstrated the effect of zebularine on HCC and CCA [116]. Other promising pathways, in addition to the classical growth factor signaling such as FGF receptor and IGF receptor, include Wnt/β-catenin, Notch, Hippo, Hh and PLK. These pathways may involve targeting the microenvironment – that is, the stromal compartment of the tumor, including inhibition of signals from MFs and tumor-associated macrophages. These interlinked networks are complex and require further investigation. However, these observations emphasize the benefit and need to treat the underlying inflammation, targeting, for example, NF-κB (curcumin or bortezomib) or COX2 (celecoxib). Dysregulated expression of Wnt/β-catenin and genetic alterations – for example, CTNNB1, AXIN1 and APC – are rare events in CCA, compared with HCC [117,118]. Currently, the only exome-sequencing project in CCA is in a small group of patients whose disease is related to liver flukes [19]. We are confident that additional sequencing projects will significantly enrich our understanding of ‘drivers’ and concurrent genetic variants in this malignancy, and that this may advance patient classification, enlighten future clinical trial designs and improve outcome of this disease.
Future perspective
Traditional clinical trials, and consequently treatment schemes, against CCA with conventional chemotherapy have, at best, resulted in modest successes. Characteristically, the disease is ‘clinically silent’, presenting at a late stage where surgical resection is not an option and with limited or no therapeutic options. The clinical challenges for CCA, besides its treatment-refractory nature, gross anatomical and tumor heterogeneity and late-stage diagnosis, resides in essentially unknown disease etiology; limited understanding of the genetic, genomic and epigenomic abnormalities in the pathogenesis of the disease; and lack of standardized and well-powered clinical trials. To achieve successful outcome, future clinical trial designs should require multicenter, homogeneous and well-powered cohorts; biopsies taken pre- and post-therapy to extract maximum molecular information; consider possible metastatic sites, as these tissues may provide a ‘window’ into potential tumor clones, which may determine/alter treatment decisions; and include translational genomics specialists as a part of the multidisciplinary clinical decision team. These approaches should resolve if future clinical success will result from treatment of class-specific patient subgroups with innovative multidrug approaches combining, for example, conventional cytotoxic chemotherapy with well-designed precision therapy targeting several molecular pathways simultaneously to achieve drug synergy or, if indeed, the future will bring well-designed treatment approaches tailored for the individual patient.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 3.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011;8(9):512–522. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miwa S, Miyagawa S, Kobayashi A, et al. Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J. Gastroenterol. 2006;41(9):893–900. doi: 10.1007/s00535-006-1877-z. [DOI] [PubMed] [Google Scholar]
- 5.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin. Gastroenterol. Hepatol. 2013;11(1):13–21.e1. doi: 10.1016/j.cgh.2012.09.009. quiz e3–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bengala C, Bertolini F, Malavasi N, et al. Sorafenib in patients with advanced biliary tract carcinoma: a Phase II trial. Br. J. Cancer. 2010;102(1):68–72. doi: 10.1038/sj.bjc.6605458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin. Liver Dis. 2004;24(2):115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 8.Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann. Oncol. 1996;7(6):593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 9.Sookprasert A, Chindaprasert J, Wirasorn K. Systemic therapy for locally advanced and metastatic cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2012;13(Suppl.):S3–S6. [PubMed] [Google Scholar]
- 10.Park JS, Oh SY, Kim SH, et al. Single-agent gemcitabine in the treatment of advanced biliary tract cancers: a Phase II study. Jpn J. Clin. Oncol. 2005;35(2):68–73. doi: 10.1093/jjco/hyi021. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava P, Jani CR, Savarese DM, O’Donnell JL, Stuart KE, Rocha Lima CM. Gemcitabine and irinotecan in locally advanced or metastatic biliary cancer: preliminary report. Oncology (Williston Park) 2003;17(9 Suppl. 8):S23–S26. [PubMed] [Google Scholar]
- 12.Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a Phase II trial. J. Clin. Oncol. 2005;23(10):2332–2338. doi: 10.1200/JCO.2005.51.008. [DOI] [PubMed] [Google Scholar]
- 13.Furuse J, Okusaka T, Bridgewater J, et al. Lessons from the comparison of two randomized clinical trials using gemcitabine and cisplatin for advanced biliary tract cancer. Crit. Rev. Oncol. Hematol. 2011;80(1):31–39. doi: 10.1016/j.critrevonc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 15.Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br. J. Cancer. 2010;103(4):469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A Phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95(8):1685–1695. doi: 10.1002/cncr.10831. [DOI] [PubMed] [Google Scholar]
- 17.Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142(4):1021–1031. doi: 10.1053/j.gastro.2011.12.005. e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Describes the first prognostic classification and molecular characterization of a large cohort of cholangiocarcinomas.
- 18.Sia D, Hoshida Y, Villanueva A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144(4):829–840. doi: 10.1053/j.gastro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Prognostic classification of intrahepatic cholangiocarcinoma.
- 19.Ong CK, Subimerb C, Pairojkul C, et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat. Genet. 2012;44(6):690–693. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]; ▪▪ First exome-sequencing study in cholangiocarcinoma.
- 20.Voss JS, Holtegaard LM, Kerr SE, et al. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum. Pathol. 2013;44(7):1216–1222. doi: 10.1016/j.humpath.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Andersen JB, Thorgeirsson SS. Genetic profiling of intrahepatic cholangiocarcinoma. Curr. Opin. Gastroenterol. 2012;28(3):266–272. doi: 10.1097/MOG.0b013e3283523c7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faris JE, Zhu AX. Targeted therapy for biliary tract cancers. J. Hepatobiliary Pancreat. Sci. 2012;19(4):326–336. doi: 10.1007/s00534-011-0496-0. [DOI] [PubMed] [Google Scholar]
- 23.Sirica AE. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2008;14(46):7033–7058. doi: 10.3748/wjg.14.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakazawa K, Dobashi Y, Suzuki S, Fujii H, Takeda Y, Ooi A. Amplification and overexpression of c-ErbB-2, epidermal growth factor receptor, and c-MET in biliary tract cancers. J. Pathol. 2005;206(3):356–365. doi: 10.1002/path.1779. [DOI] [PubMed] [Google Scholar]
- 25.Andersen JB, Thorgeirsson SS. Genomic decoding of intrahepatic cholangiocarcinoma reveals therapeutic opportunities. Gastroenterology. 2013;144(4):687–690. doi: 10.1053/j.gastro.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Gwak GY, Yoon JH, Shin CM, et al. Detection of response-predicting mutations in the kinase domain of the epidermal growth factor receptor gene in cholangiocarcinomas. J. Cancer Res. Clin. Oncol. 2005;131(10):649–652. doi: 10.1007/s00432-005-0016-1. [DOI] [PubMed] [Google Scholar]
- 27.Ito Y, Takeda T, Sasaki Y, et al. Expression and clinical significance of the ErbB family in intrahepatic cholangiocellular carcinoma. Pathol. Res. Pract. 2001;197(2):95–100. doi: 10.1078/0344-0338-00016. [DOI] [PubMed] [Google Scholar]
- 28.Lee HJ, Chung JY, Hewitt SM, Yu E, Hong SM. HER3 overexpression is a prognostic indicator of extrahepatic cholangiocarcinoma. Virchows Arch. 2012;461(5):521–530. doi: 10.1007/s00428-012-1321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiguchi K, Carbajal S, Chan K, et al. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61(19):6971–6976. [PubMed] [Google Scholar]
- 30.Sirica AE, Radaeva S, Caran N. Neu overexpression in the furan rat model of cholangiocarcinogenesis compared with biliary ductal cell hyperplasia. Am. J. Pathol. 1997;151(6):1685–1694. [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh CN, Maitra A, Lee KF, Jan YY, Chen MF. Thioacetamide-induced intestinal-type cholangiocarcinoma in rat: an animal model recapitulating the multi-stage progression of human cholangiocarcinoma. Carcinogenesis. 2004;25(4):631–636. doi: 10.1093/carcin/bgh037. [DOI] [PubMed] [Google Scholar]
- 32.Farazi PA, Zeisberg M, Glickman J, Zhang Y, Kalluri R, Depinho RA. Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Res. 2006;66(13):6622–6627. doi: 10.1158/0008-5472.CAN-05-4609. [DOI] [PubMed] [Google Scholar]
- 33.Sirica AE, Zhang Z, Lai GH, et al. A novel “patient-like” model of cholangiocarcinoma progression based on bile duct inoculation of tumorigenic rat cholangiocyte cell lines. Hepatology. 2008;47(4):1178–1190. doi: 10.1002/hep.22088. [DOI] [PubMed] [Google Scholar]
- 34.Jimeno A, Rubio-Viqueira B, Amador ML, et al. Epidermal growth factor receptor dynamics influences response to epidermal growth factor receptor targeted agents. Cancer Res. 2005;65(8):3003–3010. doi: 10.1158/0008-5472.CAN-04-3586. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Oyesanya RA, Campbell DJ, Almenara JA, Dewitt JL, Sirica AE. Preclinical assessment of simultaneous targeting of epidermal growth factor receptor (ErbB1) and ErbB2 as a strategy for cholangiocarcinoma therapy. Hepatology. 2010;52(3):975–986. doi: 10.1002/hep.23773. [DOI] [PubMed] [Google Scholar]
- 36.Yoshikawa D, Ojima H, Kokubu A, et al. Vandetanib (ZD6474), an inhibitor of VEGFR and EGFR signalling, as a novel molecular-targeted therapy against cholangiocarcinoma. Br. J. Cancer. 2009;100(8):1257–1266. doi: 10.1038/sj.bjc.6604988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu GS, Zou SQ, Luo XW, Wu JH, Liu ZR. Proliferative activity of bile from congenital choledochal cyst patients. World J. Gastroenterol. 2003;9(1):184–187. doi: 10.3748/wjg.v9.i1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyata H, Sasaki T, Kuwahara K, Serikawa M, Chayama K. The effects of ZD1839 (Iressa), a highly selective EGFR tyrosine kinase inhibitor, as a radiosensitiser in bile duct carcinoma cell lines. Int. J. Oncol. 2006;28(4):915–921. [PubMed] [Google Scholar]
- 39.O’Neill E, Kolch W. Conferring specificity on the ubiquitous Raf/MEK signalling pathway. Br. J. Cancer. 2004;90(2):283–288. doi: 10.1038/sj.bjc.6601488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wellbrock C, Marais R. Elevated expression of MITF counteracts B-RAF-stimulated melanocyte and melanoma cell proliferation. J. Cell Biol. 2005;170(5):703–708. doi: 10.1083/jcb.200505059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen TC, Jan YY, Yeh TS. K-ras mutation is strongly associated with perineural invasion and represents an independent prognostic factor of intrahepatic cholangiocarcinoma after hepatectomy. Ann. Surg. Oncol. 2012;19(Suppl. 3):S675–S681. doi: 10.1245/s10434-012-2224-7. [DOI] [PubMed] [Google Scholar]
- 42.Furubo S, Harada K, Shimonishi T, Katayanagi K, Tsui W, Nakanuma Y. Protein expression and genetic alterations of p53 and ras in intrahepatic cholangiocarcinoma. Histopathology. 1999;35(3):230–240. doi: 10.1046/j.1365-2559.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- 43.Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47(5):721–727. doi: 10.1136/gut.47.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Dell MR, Huang JL, Whitney-Miller CL, et al. Kras(G12D) and p53 mutation cause primary intrahepatic cholangiocarcinoma. Cancer Res. 2012;72(6):1557–1567. doi: 10.1158/0008-5472.CAN-11-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11(8):753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 46.Horiuchi H, Kawamata H, Fujimori T, Kuroda Y. A MEK inhibitor (U0126) prolongs survival in nude mice bearing human gallbladder cancer cells with K-ras mutation: analysis in a novel orthotopic inoculation model. Int. J. Oncol. 2003;23(4):957–963. [PubMed] [Google Scholar]
- 47.Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional Phase II study of selumetinib in patients with metastatic biliary cancers. J. Clin. Oncol. 2011;29(17):2357–2363. doi: 10.1200/JCO.2010.33.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52(5):706–712. doi: 10.1136/gut.52.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu RF, Sun JP, Zhang SR, et al. KRAS and PIK3CA but not BRAF genes are frequently mutated in Chinese cholangiocarcinoma patients. Biomed. Pharmacother. 2011;65(1):22–26. doi: 10.1016/j.biopha.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer. 2008;98(2):418–425. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park BK, Paik YH, Park JY, et al. The clinicopathologic significance of the expression of vascular endothelial growth factor-C in intrahepatic cholangiocarcinoma. Am. J. Clin. Oncol. 2006;29(2):138–142. doi: 10.1097/01.coc.0000204402.29830.08. [DOI] [PubMed] [Google Scholar]
- 52.Sugiyama H, Onuki K, Ishige K, et al. Potent in vitro and in vivo antitumor activity of sorafenib against human intrahepatic cholangiocarcinoma cells. J. Gastroenterol. 2011;46(6):779–789. doi: 10.1007/s00535-011-0380-3. [DOI] [PubMed] [Google Scholar]
- 53.El-Khoueiry AB, Rankin CJ, Ben-Josef E, et al. SWOG 0514: a Phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest. New Drugs. 2012;30(4):1646–1651. doi: 10.1007/s10637-011-9719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010;11(12):834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 55.Miyamoto M, Ojima H, Iwasaki M, et al. Prognostic significance of overexpression of c-MET oncoprotein in cholangiocarcinoma. Br. J. Cancer. 2011;105(1):131–138. doi: 10.1038/bjc.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum. Pathol. 1998;29(2):175–180. doi: 10.1016/s0046-8177(98)90229-5. [DOI] [PubMed] [Google Scholar]
- 57.Marquardt JU, Andersen JB. Next-generation sequencing: application in liver cancer: past, present and future? Biology (Basel) 2012;1(2):383–394. doi: 10.3390/biology1020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radaeva S, Ferreira-Gonzalez A, Sirica AE. Overexpression of C-NEU and C-MET during rat liver cholangiocarcinogenesis: a link between biliary intestinal metaplasia and mucin-producing cholangiocarcinoma. Hepatology. 1999;29(5):1453–1462. doi: 10.1002/hep.510290524. [DOI] [PubMed] [Google Scholar]
- 59.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat. Rev. Cancer. 2012;12(2):89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 60.Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled Phase 2 study. Lancet Oncol. 2013;14(1):55–63. doi: 10.1016/S1470-2045(12)70490-4. [DOI] [PubMed] [Google Scholar]
- 61.Javle MM, Yu J, Khoury T, et al. Akt expression may predict favorable prognosis in cholangiocarcinoma. J. Gastroenterol. Hepatol. 2006;21(11):1744–1751. doi: 10.1111/j.1440-1746.2006.04373.x. [DOI] [PubMed] [Google Scholar]
- 62.Schmitz KJ, Lang H, Wohlschlaeger J, et al. AKT and ERK1/2 signaling in intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2007;13(48):6470–6477. doi: 10.3748/wjg.v13.i48.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansel DE, Rahman A, Hidalgo M, et al. Identification of novel cellular targets in biliary tract cancers using global gene expression technology. Am. J. Pathol. 2003;163(1):217–229. doi: 10.1016/S0002-9440(10)63645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riener MO, Bawohl M, Clavien PA, Jochum W. Rare PIK3CA hotspot mutations in carcinomas of the biliary tract. Genes Chromosomes Cancer. 2008;47(5):363–367. doi: 10.1002/gcc.20540. [DOI] [PubMed] [Google Scholar]
- 65.Deshpande V, Nduaguba A, Zimmerman SM, et al. Mutational profiling reveals PIK3CA mutations in gallbladder carcinoma. BMC Cancer. 2011;11:60. doi: 10.1186/1471-2407-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu X, Kobayashi S, Qiao W, et al. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and PTEN in mice. J. Clin. Invest. 2006;116(7):1843–1852. doi: 10.1172/JCI27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ewald F, Grabinski N, Grottke A, et al. Combined targeting of AKT and mTOR using MK-2206 and RAD001 is synergistic in the treatment of cholangiocarcinoma. Int. J. Cancer. 2013;133(9):2065–2076. doi: 10.1002/ijc.28214. [DOI] [PubMed] [Google Scholar]
- 68.Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA. Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit. Rev. Oncog. 2012;17(1):69–95. doi: 10.1615/critrevoncog.v17.i1.60. [DOI] [PubMed] [Google Scholar]
- 69.Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J. Hepatol. 2013 doi: 10.1016/j.jhep.2013.11.031. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J. Clin. Oncol. 2006;24(19):3069–3074. doi: 10.1200/JCO.2005.05.3579. [DOI] [PubMed] [Google Scholar]
- 71.Ramanathan RK, Belani CP, Singh DA, et al. A Phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother. Pharmacol. 2009;64(4):777–783. doi: 10.1007/s00280-009-0927-7. [DOI] [PubMed] [Google Scholar]
- 72.Yi JH, Thongprasert S, Lee J, et al. A Phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur. J. Cancer. 2012;48(2):196–201. doi: 10.1016/j.ejca.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 73.Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter Phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a Phase II Consortium study. J. Clin. Oncol. 2010;28(21):3491–3497. doi: 10.1200/JCO.2010.28.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Khoueiry AB, Rankin CJ, Iqbal S, et al. SWOG 0941: a Phase II study of sorafenib and erlotinib in patients (pts) with advanced gallbladder cancer or cholangiocarcinoma. J. Clin. Oncol. 2012;30(Suppl.) doi: 10.1038/bjc.2013.801. Abstract 4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu AX, Meyerhardt JA, Blaszkowsky LS, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a Phase 2 study. Lancet Oncol. 2010;11(1):48–54. doi: 10.1016/S1470-2045(09)70333-X. [DOI] [PubMed] [Google Scholar]
- 76.Gruenberger B, Schueller J, Heubrandtner U, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a Phase 2 study. Lancet Oncol. 2010;11(12):1142–1148. doi: 10.1016/S1470-2045(10)70247-3. [DOI] [PubMed] [Google Scholar]
- 77.Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, Phase 3 study. Lancet Oncol. 2012;13(2):181–188. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 78.Jensen LH, Lindebjerg J, Ploen J, Hansen TF, Jakobsen A. Phase II marker-driven trial of panitumumab and chemotherapy in KRAS wild-type biliary tract cancer. Ann. Oncol. 2012;23(9):2341–2346. doi: 10.1093/annonc/mds008. [DOI] [PubMed] [Google Scholar]
- 79.Sulpice L, Rayar M, Desille M, et al. Molecular profiling of stroma identifies Osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology. 2013 doi: 10.1002/hep.26577. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 80.Kajiyama K, Maeda T, Takenaka K, Sugimachi K, Tsuneyoshi M. The significance of stromal desmoplasia in intrahepatic cholangiocarcinoma: a special reference of ‘scirrhous-type’ and ‘nonscirrhous-type’ growth. Am. J. Surg. Pathol. 1999;23(8):892–902. doi: 10.1097/00000478-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Declerck YA. Desmoplasia: a response or a niche? Cancer Discov. 2012;2(9):772–774. doi: 10.1158/2159-8290.CD-12-0348. [DOI] [PubMed] [Google Scholar]
- 82.Fingas CD, Bronk SF, Werneburg NW, et al. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54(6):2076–2088. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Highlights the significance of the tumor microenvironment.
- 83.Fingas CD, Mertens JC, Razumilava N, et al. Polo-like kinase 2 is a mediator of Hedgehog survival signaling in cholangiocarcinoma. Hepatology. 2013;58(4):1362–1374. doi: 10.1002/hep.26484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie G, Karaca G, Swiderska-Syn M, et al. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate. Hepatology. 2013 doi: 10.1002/hep.26511. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schoffski P. Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist. 2009;14(6):559–570. doi: 10.1634/theoncologist.2009-0010. [DOI] [PubMed] [Google Scholar]
- 86.Thrum S, Lorenz J, Mossner J, Wiedmann M. Polo-like kinase 1 inhibition as a new therapeutic modality in therapy of cholangiocarcinoma. Anticancer Res. 2011;31(10):3289–3299. [PubMed] [Google Scholar]
- 87.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425(6960):846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 88.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T, Thuwajit C. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol. Rep. 2009;21(4):957–969. doi: 10.3892/or_00000309. [DOI] [PubMed] [Google Scholar]
- 90.Choi SS, Omenetti A, Witek RP, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am. J. Physiol. Gastrointest. Liver. Physiol. 2009;297(6):G1093–G1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fingas CD, Mertens JC, Razumilava N, Bronk SF, Sirica AE, Gores GJ. Targeting PDGFR-beta in cholangiocarcinoma. Liver Int. 2012;32(3):400–409. doi: 10.1111/j.1478-3231.2011.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panman L, Galli A, Lagarde N, et al. Differential regulation of gene expression in the digit forming area of the mouse limb bud by SHH and gremlin 1/FGF-mediated epithelial-mesenchymal signalling. Development. 2006;133(17):3419–3428. doi: 10.1242/dev.02529. [DOI] [PubMed] [Google Scholar]
- 93.Omenetti A, Popov Y, Jung Y, et al. The Hedgehog pathway regulates remodelling responses to biliary obstruction in rats. Gut. 2008;57(9):1275–1282. doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 94.Zender S, Nickeleit I, Wuestefeld T, et al. A critical role for Notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2013;23(6):784–795. doi: 10.1016/j.ccr.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 95.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128(5):1354–1368. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 96.Chen Y, Zheng S, Qi D, et al. Inhibition of Notch signaling by a gamma-secretase inhibitor attenuates hepatic fibrosis in rats. PLoS ONE. 2012;7(10):e46512. doi: 10.1371/journal.pone.0046512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang J, Hisamatsu T, Shimamura K, et al. Activated hepatic stellate cells mediate the differentiation of macrophages. Hepatol. Res. 2013;43(6):658–669. doi: 10.1111/j.1872-034X.2012.01111.x. [DOI] [PubMed] [Google Scholar]
- 98.Meng F, Yamagiwa Y, Ueno Y, Patel T. Over-expression of interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J. Hepatol. 2006;44(6):1055–1065. doi: 10.1016/j.jhep.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheon YK, Cho YD, Moon JH, et al. Diagnostic utility of interleukin-6 (IL-6) for primary bile duct cancer and changes in serum IL-6 levels following photodynamic therapy. Am. J. Gastroenterol. 2007;102(10):2164–2170. doi: 10.1111/j.1572-0241.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128(7):2054–2065. doi: 10.1053/j.gastro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 101.Isomoto H, Mott JL, Kobayashi S, et al. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132(1):384–396. doi: 10.1053/j.gastro.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Han C, Demetris AJ, Stolz DB, Xu L, Lim K, Wu T. Modulation of Stat3 activation by the cytosolic phospholipase A2alpha and cyclooxygenase-2-controlled prostaglandin E2 signaling pathway. J. Biol. Chem. 2006;281(34):24831–24846. doi: 10.1074/jbc.M602201200. [DOI] [PubMed] [Google Scholar]
- 103.Sirica AE, Lai GH, Endo K, Zhang Z, Yoon BI. Cyclooxygenase-2 and ERBB-2 in cholangiocarcinoma: potential therapeutic targets. Semin. Liver Dis. 2002;22(3):303–313. doi: 10.1055/s-2002-34507. [DOI] [PubMed] [Google Scholar]
- 104.Wu GS, Zou SQ, Liu ZR, Tang ZH, Wang JH. Celecoxib inhibits proliferation and induces apoptosis via prostaglandin E2 pathway in human cholangiocarcinoma cell lines. World J. Gastroenterol. 2003;9(6):1302–1306. doi: 10.3748/wjg.v9.i6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu T, Leng J, Han C, Demetris AJ. The cyclooxygenase-2 inhibitor celecoxib blocks phosphorylation of Akt and induces apoptosis in human cholangiocarcinoma cells. Mol. Cancer Ther. 2004;3(3):299–307. [PubMed] [Google Scholar]
- 106.Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013;32(41):4861–4870. doi: 10.1038/onc.2012.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hedvat M, Huszar D, Herrmann A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16(6):487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scuto A, Krejci P, Popplewell L, et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia. 2011;25(3):538–550. doi: 10.1038/leu.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22(7):339–346. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee KP, Lee JH, Kim TS, et al. The Hippo–Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc. Natl Acad. Sci. USA. 2010;107(18):8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16(5):425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Avruch J, Zhou D, Fitamant J, Bardeesy N. Mst1/2 signalling to Yap: gatekeeper for liver size and tumour development. Br. J. Cancer. 2011;104(1):24–32. doi: 10.1038/sj.bjc.6606011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chiang J, Martinez-Agosto JA. Effects of mTOR inhibitors on components of the salvador-warts-hippo pathway. Cells. 2012;1:886–904. doi: 10.3390/cells1040886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17(1):72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang P, Dong Q, Zhang C, et al. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2013;32(25):3091–3100. doi: 10.1038/onc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andersen JB, Factor VM, Marquardt JU, et al. An integrated genomic and epigenomic approach predicts therapeutic response to zebularine in human liver cancer. Sci. Transl. Med. 2010;2(54):54ra77. doi: 10.1126/scitranslmed.3001338. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Novel pharmacogenomics/epigenomics approach that details the potential for demethylating agents in the treatment of primary liver cancers.
- 117.Sugimachi K, Taguchi K, Aishima S, et al. Altered expression of beta-catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod. Pathol. 2001;14(9):900–905. doi: 10.1038/modpathol.3880409. [DOI] [PubMed] [Google Scholar]
- 118.Tokumoto N, Ikeda S, Ishizaki Y, et al. Immunohistochemical and mutational analyses of Wnt signaling components and target genes in intrahepatic cholangiocarcinomas. Int. J. Oncol. 2005;27(4):973–980. [PubMed] [Google Scholar]
▪ Websites
- 201.www.clinicaltrials.gov/ct2/show/NCT01285037 A Study of LY2801653 in Advanced Cancer ( NCT01285037)
- 202.www.clinicaltrials.gov/ct2/show/NCT01425879 MK2206 in Treating Patients With Advanced Refractory Biliary Cancer That Cannot Be Removed by Surgery ( NCT01425879)
- 203.http://clinicaltrials.gov/ct2/show/NCT01501604 BKM120 in Cancers With PIK3CA Activating Mutations.
- 204.www.clinicaltrials.gov/ct2/show/NCT00973713 Study of RAD001 in Advanced Cholangiocarcinoma: RADiChol ( NCT00973713)
- 205.www.clinicaltrials.gov/ct2/show/NCT01525719 Single Arm Study of RAD001 as Monotherapy in Treatment in Advanced Cholangiocarcinoma (CRAD001T) ( NCT01525719)
- 206.http://clinicaltrials.gov/ct2/show/NCT00753675 Vandetanib Gemcitabine Or Placebo Plus Gemcitabine Or Vandetanib Monotherapy In Advanced Biliary Tract Cancer (VANGOGH)
- 207.http://clinicaltrials.gov/ct2/show/NCT00551096 Gemcitabine/Capecitabine/ZD6474 in Advanced Solid Tumors.
- 208.http://clinicaltrials.gov/ct2/show/NCT01229111 Cediranib Maleate and Combination Chemotherapy in Treating Patients With Advanced Biliary Cancers.
- 209.http://clinicaltrials.gov/ct2/show/NCT00939848 Cediranib Versus Placebo Plus Cisplatin/Gemcitabine Chemotherapy for Patients With Advanced Biliary Tract Cancers (ABC-03)