Abstract
The substituted β-keto amphetamine mephedrone (4-methylmethcathinone) was banned in the UK in April 2010 but continues to be used recreationally in the UK and elsewhere. Users have compared its psychoactive effects to those of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’). This review critically examines the preclinical data on mephedrone that have appeared over the last 2–3 years and, where relevant, compares the pharmacological effects of mephedrone in experimental animals with those obtained following MDMA administration. Both mephedrone and MDMA enhance locomotor activity and change rectal temperature in rodents. However, both of these responses are of short duration following mephedrone compared with MDMA probably because mephedrone has a short plasma half-life and rapid metabolism. Mephedrone appears to have no pharmacologically active metabolites, unlike MDMA. There is also little evidence that mephedrone induces a neurotoxic decrease in monoamine concentration in rat or mouse brain, again in contrast to MDMA. Mephedrone and MDMA both induce release of dopamine and 5-HT in the brain as shown by in vivo and in vitro studies. The effect on 5-HT release in vivo is more marked with mephedrone even though both drugs have similar affinity for the dopamine and 5-HT transporters in vitro. The profile of action of mephedrone on monoamine receptors and transporters suggests it could have a high abuse liability and several studies have found that mephedrone supports self-administration at a higher rate than MDMA. Overall, current data suggest that mephedrone not only differs from MDMA in its pharmacological profile, behavioural and neurotoxic effects, but also differs from other cathinones.
Keywords: mephedrone, cathinones, MDMA, locomotion, body temperature, drug metabolism, dopamine, 5-hydroxytryptamine
Introduction
Mephedrone (4-methylmethcathinone; Figure 1) was first synthesized in 1929 as a homologue of ephedrine, a year after the publication of the synthesis of another homologue, methcathinone (Figure 1). These two compounds have stimulant properties and methcathinone was actually marketed in the USSR as an antidepressant in the 1930s but became illegal in the USA and several other countries in the 1990s following evidence of widespread abuse (see Kelly, 2011). Bupropion, another chemically related compound, has been available in many countries since the mid 1980s, being initially marketed as an antidepressant, a treatment for attention deficit hyperactivity disorder and subsequently as an aid to smoking cessation. Cathinone (Figure 1) itself is known to be the major active constituent of khat, the leaf from the Catha edulis plant that has been chewed recreationally in East Africa and parts of the Middle East for centuries. Khat is outlawed in the USA, Canada and many European countries, but remained legal in the UK. However, the government announced on 3 July 2013 that it intended to make this herbal stimulant a controlled class C drug like anabolic steroids and ketamine. For a more detailed historical overview on the history of the synthesis and clinical use of cathinone derivatives, see Kelly (2011).
Figure 1.
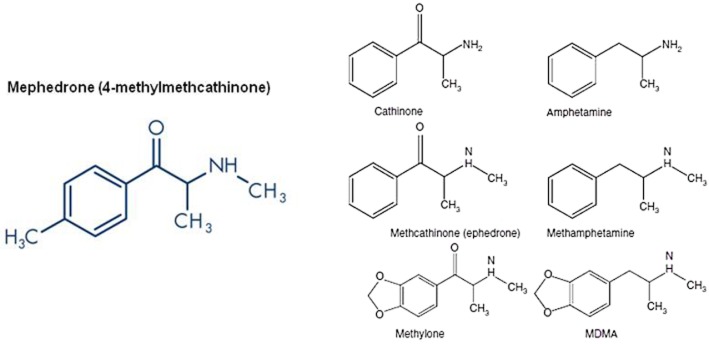
Structure of mephedrone and other β-keto amphetamines (cathinones) and their related amphetamine congeners.
Scientific interest in mephedrone was resurrected around the turn of the 21st century when the psychoactive effects of mephedrone were discovered and it became widely available as a party drug in Israel, selling under the nickname of ‘plant food’. During this period, a number of other synthetic cathinones appeared as ‘legal highs’. These so-called ‘designer drugs’ are compounds that are chemically related to known psychoactive substances but because of their novel structure have not been listed as controlled substances. 3,4-Methylenedioxymethcathinone (methylone; Figure 1), a cathinone with a close structural similarity to 3,4-methylenedioxymethamphetamine (MDMA), became available around 2004 in Japan and the Netherlands and its availability was enhanced by it being sold in head shops and on the Internet. Mephedrone with street names that include m-cat, drone, bubbles, bath salts and meow-meow (although the last has been suggested to have originated in the popular press) subsequently became available via these same sales outlets often being marketed as ‘plant food’ or ‘bath salts’.
Because of the extensive recreational use of mephedrone it received substantial media attention in the UK, particularly as it was implicated in a number of adverse events and unexplained deaths and was banned in April 2010 following the advice of the Advisory Committee on the Misuse of Drugs (ACMD, 2010). The chair of the committee was reported to have stated that mephedrone ‘is an amphetamine by another name’ (Dyer, 2010). This was perhaps a surprising conclusion given that we have been unable to find in PubMed a single preclinical neuropharmacological publication on mephedrone before 2011 and very few on methcathinone. Mephedrone was also classified as a controlled substance in many other European countries in December 2010 (EMCDDA, 2011) despite the ACMD (2010) report opening with: ‘There are no formal pharmacokinetic and pharmacodynamic studies on mephedrone. There are no published formal studies assessing the psychological or behavioural effects of mephedrone in humans. In addition, there are no animal studies on which to base an extrapolation of potential effects.’ In the USA, President Barack Obama signed a Federal law banning mephedrone in July 2012 (Haggin, 2012). Interestingly, this law is similar to those enacted in Europe in that it covers many related cathinone substances.
Nevertheless, mephedrone continued (and continues) to be available for illicit recreational consumption (Brandt et al., 2010). Before being banned, mephedrone was the cathinone derivative with the highest recreational use. An online survey of 2289 experienced polydrug users found that 42% had tried mephedrone at least once, with approximately 30% using it every 2 weeks or more frequently (Winstock et al., 2011b). The increased use of mephedrone coincided with a decrease in both the availability and also in the purity of ‘ecstasy’ tablets. Fewer than 50% of ecstasy tablets confiscated in the Netherlands in 2009 contained MDMA, compared with 90% in previous years (Brunt et al., 2011). In many of these tablets MDMA was substituted by other compounds, and in 2009 mephedrone was found to be the most prevalent new designer drug to be misleadingly sold as MDMA/ecstasy. Both the decline in purity and availability of ecstasy tablets and the fact that mephedrone had initially been legal are thought to be the main reasons for its increased popularity (EMCDDA, 2010).
What was striking about the legislation that made mephedrone illegal was the way it was constructed. The original UK Misuse of Drugs Act (1971) only allowed a specific compound to be controlled. In contrast, not only mephedrone but also many other chemically related cathinone compounds were also banned with it, presumably in an attempt to outlaw the development and use of structurally related designer drugs. It was believed that this was the first time that a generic ban based purely on chemical structure had been enforced on a group of compounds (Morris, 2010). The advantages and problems of this type of generic approach to legislation have recently been reviewed (Van Amsterdam et al., 2013).
What many may consider should be a matter of some concern is that the ban on mephedrone and related compounds appeared to have been driven more by information given in the media rather than peer-reviewed scientific knowledge gained from relevant clinical and preclinical pharmacological studies. Deaths and severe adverse reactions that were widely reported in the press as being the result of mephedrone ingestion were subsequently found to be due to other drugs or even natural causes (Measham et al., 2010; Sare, 2011). Many of the newspaper reports on the effects of the drug in recreational users were hyperbolic, speculative or just incorrect. For example, one story in a major newspaper on a severe adverse event was actually an Internet hoax (Davey et al., 2010). Needless to say, retraction of the false information seldom occurred. Even scientific papers on the adverse effects of the drug sometimes disclosed that evidence on the physiological and psychological consequences of ingesting the drug was based solely on the fact that the subjects under investigation ‘believed’ that they had taken mephedrone (Dargan et al., 2010; James et al., 2011; Regan et al., 2011; Wood et al., 2011). No forensic blood samples were taken to confirm that exposure to mephedrone had occurred. Interestingly, a recent study still found that a significant proportion of ‘mephedrone fatalities’ was likely to be due to the other drugs that had been subsequently identified post-mortem (Schifano et al., 2012).
The last 2–3 years has seen the publication of a reasonable body of preclinical work on the pharmacology of mephedrone and this review is a critical appraisal of these studies. Clinical reviews on the drug are available elsewhere (Dargan et al., 2010; 2011,; Schifano et al., 2011; Prosser and Nelson, 2012; Wood et al., 2012; Wood and Dargan, 2012; Zawilska and Wojcieszak, 2013). Where relevant, we have compared the data on mephedrone with that obtained in studies on MDMA (‘ecstasy’; Figure 1), because this is a substituted amphetamine and also because recreational users have subjectively reported that the stimulant, euphoric and empathogenic effects of mephedrone are similar to MDMA (Carhart-Harris et al., 2011). Some users even consider mephedrone to be superior to MDMA in terms of the desired experience (Vardakou et al., 2011; Winstock et al., 2011b).
What is now becoming clear is that mephedrone has its own very specific pharmacology that is distinct from MDMA and also other amphetamines. As Dal Cason et al. (1997) concluded several years ago: ‘caution [should] be used in attempting to draw conclusions or make predictions about the activity and potency of novel cathinone analogues by analogy to the structure–activity relationships derived from amphetamine-related agents; it would appear that each new cathinone analogue will require individual investigation.’
For simplicity, the information is grouped in subsections that examine its main pharmacokinetic and pharmacodynamic effects in experimental animals.
Metabolism and pharmacokinetics of mephedrone in rats and humans
Until recently, a major problem in assessing the pharmacological effects of MDMA was that few detailed pharmacokinetic studies had been performed in either animals or humans. Consequently, it was difficult to translate most of the preclinical pharmacodynamic studies of this drug in terms of their likely functional and toxicological importance. Recent pharmacokinetic studies have shown that MDMA has a much faster rate of metabolism in rats compared with humans (Baumann et al., 2009). Consequently, many studies on both the pharmacological and toxicological effects of this drug in experimental animals have limited translational value and may even be misleading (Green et al., 2012a). In contrast, there are already several good pharmacokinetic studies on mephedrone in rats (Hadlock et al., 2011; Aarde et al., 2013; Martínez-Clemente et al., 2013; Miller et al., 2013) and some useful results on the plasma concentrations of the drug in recreational users which allow a degree of confidence in the translational value of preclinical studies in rodents.
The normal routes of mephedrone administration in recreational users are reported to be oral and insufflation. Extrapolation from dosing to plasma levels is difficult as there are no detailed dose–concentration curves available and pharmacokinetic studies on the drug in humans have yet to be performed. However, it is suggested that a ‘normal’ recreational oral dose is 100–200 mg, while somewhat lower doses are used when the drug is insufflated (EROWID, 2013). This oral dose is similar to the usual oral MDMA dose typically resulting from ingestion of two tablets (140–180 mg), but an important difference with mephedrone is that the reported short duration of the psychoactive response often leads to rapid repeat dosing (Schifano et al., 2012). Interestingly, plasma mephedrone concentrations in subjects suffering a fatal overdose have been reported to be in the region of 2000 ng·mL−1 (Maskell et al., 2011; Schifano et al., 2012) which is very similar in range to the concentration of MDMA seen post-mortem in persons suffering from fatal acute toxicity (Dowling et al., 1987; Henry et al., 1992). Therefore, extrapolation from MDMA recreational use would suggest that administration of mephedrone in the 5–10 mg·kg−1 range to rats is comparable to doses used recreationally and therefore appropriate when simulating a human recreational dose. However, this dose may not be sufficient to reflect the drug exposure that must occur when humans engage in binge dosing. In mimicking that situation, repeat dosing of animals must also be performed.
In Sprague-Dawley rats, the uptake and elimination of a single dose of mephedrone (5.6 mg·kg−1 s.c.) is rapid. The peak plasma concentration (Cmax) observed was 1206 ng·mL−1 with a tmax of 0.25 h (Miller et al., 2013). In binge dosing studies, plasma levels of 384.2 ± 62.2, and 1294.3 ± 145.5 ng·mL−1 were recorded 1 h after, respectively, 4 × 10 or 4 × 25 mg·kg−1 s.c., given at 2 h intervals. Whole brain tissue levels of 2.1 ± 0.2 ng·mg−1 and 7.8 ± 0.9 ng·mg−1 were found 1 h after these dose schedules (Hadlock et al., 2011). A further study in Sprague-Dawley rats given i.v. mephedrone (10 mg·kg−1) reported plasma concentrations fitted a two-compartment model (α = 10.23 h−1, β = 1.86 h−1). The same study showed that after oral administration (30 and 60 mg·kg−1), the peak mephedrone concentration occurred between 0.5 and 1 h later and the drug was undetectable at 9 h. The bioavailability of mephedrone was about 10% and the plasma protein binding value was 21% (Martínez-Clemente et al., 2013).
The drug is rapidly taken up into the brain (peak levels were observed 2 min after i.v. injection) and almost totally cleared within an hour (Aarde et al., 2013). Peak brain tissue concentrations were 4 ng·mg−1 2 min after a 1 mg·kg−1 i.v. dose. The concentration had fallen to less than 1 ng·mg−1 within 30 min and less than 0.4 ng·mg−1 60 min later (Aarde et al., 2013). Consistent with this profile, Simmler et al. (2013) noted that in rats mephedrone had a twofold greater blood–brain barrier permeability than MDMA.
The Aarde et al. (2013) study found that mephedrone is cleared rapidly from both Sprague-Dawley and Wistar rats and the in vitro assay confirmed that the drug undergoes extensive hepatic metabolism. Martínez-Clemente et al. (2013) also concluded that the drug is subject to first pass metabolism. Pedersen et al. (2012) reported that cytochrome P450 2D6 is the main metabolic enzyme responsible for degradation of mephedrone in humans, the same enzyme that metabolizes MDMA (Tucker et al., 1994).
A few investigations have examined the metabolic pathways of mephedrone in both rodents and man. Meyer et al. (2010) examined the metabolite pattern after oral administration of the drug to Wistar rats and the primary metabolites identified were nor-mephedrone, nor-dihydro-mephedrone, hydroxytolyl-mephedrone and nor-hydroxytolyl-mephedrone (Figure 2; Dybdal-Hargreaves et al., 2013). Identification was primarily in plasma, but also in urine. Based on this information, the partly overlapping metabolic pathways presented in Figure 2 have been postulated: N-demethylation to the primary amine, reduction of the keto moiety to the respective alcohol, and oxidation of the tolyl moiety to the corresponding alcohols. Because nor-hydroxytolyl-mephedrone and hydroxytolyl-mephedrone were more abundant after glucuronidase and sulphatase hydrolysis, it was concluded that they were partly excreted as glucuronides and/or sulphates. The same metabolites were identified in human plasma and urine but additionally 4-carboxy-dihydro-mephedrone was also identified in urine. These major metabolites were also detected by Martínez-Clemente et al. (2013) in their study on Sprague-Dawley rats. It is unclear at present whether any of the metabolites possess pharmacological activity. Further evidence for the formation of these metabolites both in vitro and in vitro has been presented by Pedersen et al. (2012), who used cDNA-expressed CYP enzymes and human liver microsomal preparations and found cytochrome CYP2D6 to be the main enzyme responsible for the in vitro metabolism of mephedrone, with some minor contribution from other NADPH-dependent enzymes. They also found both hydroxytolyl-mephedrone and nor-mephedrone were formed. In four forensic traffic accident cases where mephedrone was detected in blood, hydroxytolyl-mephedrone and nor-mephedrone, 4-carboxy-dihydro-mephedrone, dihydro-mephedrone, and 4-carboxy-mephedrone were all also detected.
Figure 2.
The major metabolites of mephedrone and proposed pathways of their formation. [Reproduced from Dybdal-Hargreaves et al. (2013) with permission from Elsevier Press].
MDMA is metabolized to catechol metabolites which can undergo oxidation to o-quinones that are highly redox-active molecules and produce free reactive oxygen species or nitrogen species radicals (Capela et al., 2006). It is widely believed that it is these oxidation products which may be responsible for the toxicity exerted by MDMA (Capela et al., 2009; Song et al., 2010; Green et al., 2012a), a view supported by the observation that administration of the free radical trapping agent α-phenyl-N-tert-butyl nitrone attenuated the long-term loss of 5-HT in the rat brain induced by MDMA (Colado and Green, 1995). In contrast to MDMA, catechol and quinone metabolites do not appear to be formed as the result of mephedrone metabolism (Figure 2). This distinction may explain why most studies have failed to observe any similar mephedrone-induced neurotoxicity in rat brain (see later).
The common practice of rapid binge dosing of mephedrone by recreational users (see Schifano et al., 2011; Winstock et al., 2011a) to sustain its psychoactive action is likely to reflect its rapid metabolism in humans. Furthermore, this is consistent with the proposal that the general pharmacokinetic profile of mephedrone is similar in rats and humans. This makes mephedrone markedly different from MDMA which has a rapid rate of metabolic clearance in rats and several other species but a much slower rate of metabolism in humans (Green et al., 2012a). MDMA also has a major metabolite 3,4-methylenedioxyamphetamine (MDA) which has the same general pharmacological activity as MDMA (Green et al., 2003; 2012a,), while no active metabolites of mephedrone have yet been identified.
Locomotor activity
Increased locomotion following mephedrone administration has been observed in several strains of rats and mice. Kehr et al. (2011) noted that the locomotor effect of mephedrone in Sprague-Dawley rats was similar in intensity and duration to MDMA at the same dose (3 mg·kg−1 s.c.), but modest and short lasting compared with a lower dose of amphetamine (1 mg·kg−1 s.c.). The brief period of increased activity is consistent with the previously discussed short plasma half-life (t½) of mephedrone, an interpretation supported by a recent study demonstrating a clear relationship between plasma mephedrone concentration and locomotor activity (Martínez-Clemente et al., 2013).
Lisek et al. (2012) also reported that mephedrone (3–30 mg·kg−1 i.p.) increased ambulatory activity in Sprague-Dawley rats and showed that locomotor hyperactivity was inhibited by pretreatment with the dopamine D1 receptor antagonist SCH23390, but enhanced by pretreatment with sulpiride, a dopamine D2 receptor antagonist (receptor nomenclature conforms to BJP's Guide to Pharmacology, Alexander et al., 2013). Shortall et al. (2013c) also observed a dose-dependent (1–10 mg·kg−1 i.p.) increase in locomotion in Lister Hooded rats lasting around 60 min after the highest dose. A subsequent study (S.E. Shortall et al., unpubl. obs.) using a greater dose range (4–30 mg·kg−1 i.p.) found that the highest dose enhanced further both the activity peak and AUC, but had only a small effect on the duration of the locomotor response (Figure 3). Oral administration also induces a dose-dependent increase in locomotion but with a more sustained duration of action of around 2 h (Martínez-Clemente et al., 2013).
Figure 3.
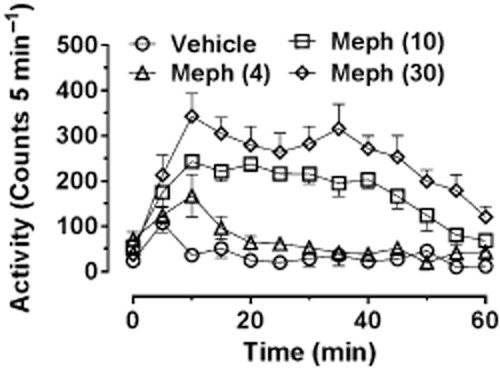
Locomotor response of individually housed male Lister-hooded rats following various doses of (±)-mephedrone HCl (4, 10 and 30 mg·kg−1 i.p.) administered at time 0. Rats were habituated to the test arena for 60 min prior to injection. Data are shown as mean ± SEM infrared beam breaks in each 5 min bin. Mephedrone-treated groups different from control group (P < 0.01 or better) as follows: mephedrone (4 mg·kg−1) at 10 min; mephedrone (10 mg·kg−1) from 10 to 45 min; and mephedrone (30 mg·kg−1) from 10 to 55 min.
Wright et al. (2012a) examined the locomotor response in both Wistar and Sprague-Dawley strains in two different ambient temperature conditions (23 and 27°C). Although mephedrone increased locomotor activity for a similar duration in both strains, significantly more activity was observed in Sprague-Dawley rats. The locomotor activity was similar when the rats were examined in either ambient temperature condition, in contrast to the effect of mephedrone on body temperature (see later). However, in Sprague-Dawley rats, a higher ambient temperature of 30°C has been reported to enhance mephedrone-induced locomotion compared with that seen at 20°C (Miller et al., 2013).
Motbey et al. (2012a) administered the high dose of 30 mg·kg−1 i.p and noted a marked hyperlocomotion response over the next 60 min in Wistar rats which was not sensitized (enhanced in amplitude) even after this dose had been given once daily for 10 days. However, they also reported that no sensitization occurred in a parallel cohort given methamphetamine using the same dosing schedule. Because methamphetamine, amphetamine and MDMA are all known to produce sensitization when given at a low dose and with an abstinence period (Vanderschuren and Kalivas, 2000; Aberg et al., 2007; Bradbury et al., 2012), this suggests that the protocol employed may not have been appropriate for investigating sensitization, particularly as sensitization to the locomotor effects of mephedrone has been reported by several other groups as detailed below. Lisek et al. (2012) gave mephedrone (0.5 mg·kg−1 i.p.) once daily for 5 days followed by a 10 day abstinence period while Shortall et al. (2013c) gave 10 mg·kg−1 i.p. on 2 consecutive days each week for 3 weeks in order to mimic likely weekend recreational dosing in humans. Both groups observed robust enhancement of the locomotor response on the final test day compared with that seen following the first injection. In a further study, Shortall et al. (unpublished) gave a total of 5 doses of either mephedrone (10 mg·kg−1 i.p.) or MDMA (5 mg·kg−1 i.p.) to reflect weekend human use (2 consecutive days in weeks 1 and 2, and a final dose after a further week) and saw a robust enhancement (sensitization) of the locomotor response to both compounds following the final versus the first dose (Figure 4). Gregg et al. (2013) have also recently reported that two different dose schedules (both fixed and variable dose schedules) to Sprague-Dawley rats produced clear evidence of locomotor sensitization.
Figure 4.
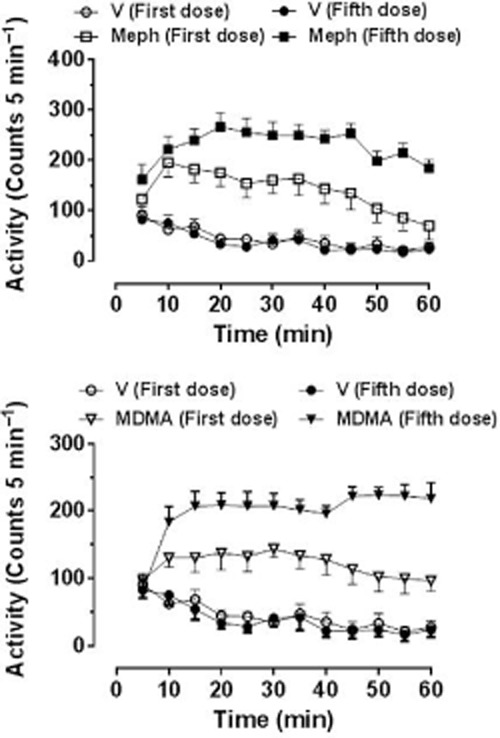
Locomotor response of individually housed rats following the first and fifth doses of (±)-mephedrone HCl (10 mg·kg−1) or (±)-MDMA HCl (5 mg·kg−1), doses being given on 2 consecutive days on weeks 1 and 2, and the final dose 1 further week later. Rats were habituated to the test arena for 60 min prior to injection. Data are shown as mean ± SEM infrared beam breaks in each 5 min bin. Total beam breaks are first dose: saline 534 ± 74, mephedrone 1695 ± 300*, MDMA 1447 ± 181*; fifth dose: saline 467 ± 74, mephedrone 2742 ± 213*†, MDMA 2629 ± 319*†. *Compared with the respective dose of the saline injection; †P < 0.001 compared with the first dose of the same drug challenge injection.
Huang et al. (2012) compared the locomotor stimulant effects of mephedrone (1–10 mg·kg−1 s.c.), d-methamphetamine (0.5–5.6 mg·kg−1 s.c.) and MDMA (1–7.5 mg·kg−1 s.c.) on voluntary wheel running activity in Wistar rats. Methamphetamine induced a biphasic pattern of counts with relatively higher activity following lower doses, and lower counts following the highest dose, probably due to the induction of stereotyped behaviour at the high dose (Huang et al., 2012). In contrast, both mephedrone and MDMA, neither of which has been reported to induce stereotypic behaviour, produced a monophasic, dose-dependent reduction in counts compared with saline-treated controls. Although such a decrease seems paradoxical when compared with the consistent increase in locomotion recorded in activity boxes, this is because spontaneous wheel running represents a different form of activity involving divergent behavioural processes from spontaneous activity.
Following mephedrone, Baumann et al. (2011) observed reciprocal forepaw treading, which is one component of the 5-HT syndrome in rats (Green and Grahame-Smith, 1976). Components of this syndrome also occur following MDMA, and although the response is more robust, with the expression of other components of the syndrome, it is only apparent after a high dose (Colado et al., 1993). The fact that MAO is also inhibited by MDMA (Leonardi and Azmitia, 1994) may assist in the production of the syndrome. In the absence of a MAO inhibitor or a 5-HT re-uptake inhibitor, it is difficult to induce the syndrome in rats (Green and Grahame-Smith, 1976). Interestingly, there is one clinical case report of the 5-HT syndrome in a mephedrone user, but the patient was also taking fluoxetine so it is likely that it is the combination that was responsible (Garrett and Sweeney, 2010). It has also been reported that ‘bath salts’ can induce the syndrome in recreational users (Joksovic et al., 2012; Rasimas, 2013).
Mephedrone administration also increases locomotor activity in mice. Both López-Arnau et al. (2012) and Marusich et al. (2012) observed a dose-dependent increase in locomotion without any accompanying increase in rearing behaviour (López-Arnau et al., 2012). Pretreatment with the 5-HT2 receptor antagonist ketanserin or the non-selective dopamine receptor antagonist haloperidol, given at doses that did not affect basal locomotor activity, partly inhibited mephedrone-induced hyperactivity (by about 53 and 65% respectively). Pretreatment with p-chlorophenylalanine (PCPA), an inhibitor of 5-HT synthesis, also reduced the hyperlocomotor effect of mephedrone. In contrast, PCPA does not alter MDMA-induced hyperactivity in the mouse (Fantegrossi et al., 2005) and the role of dopamine is also unclear. Benturquia et al. (2008) reported that the selective D1 receptor antagonist SCH23390 antagonized the MDMA-induced locomotor response, but Risbrough et al. (2006) demonstrated that D1 receptor activation inhibited straight-line activity. They also noted that D2 receptor activation appeared to contribute to the repetitive circling behaviour produced by MDMA.
Body temperature and cardiovascular function
Hyperthermia is a major acute adverse event that can follow ingestion of MDMA by recreational users (Green et al., 2003; Docherty and Green, 2010; Halpern et al., 2011; Parrott, 2012a), and is sometimes marked in young persons who have ingested the drug at dance clubs or parties where the ambient temperature is high. These individuals sometimes also present with problems associated with hyperthermia, including rhabdomyolysis, myoglobinuria, renal failure, liver damage and disseminated intravascular coagulopathy, which can be fatal. Although administration of high or repeated doses of MDMA to rats usually causes hyperthermia, it can produce hypothermia particularly following a low dose or when the animals are housed singly or in a cool ambient temperature (Docherty and Green, 2010). Nevertheless, both MDMA-induced hyper-and hypothermia result from monoamine release in the brain (Docherty and Green, 2010).
Cathinone has been reported to induce hyperthermia and thermogenesis in urethane-anaesthetized rats (Tariq et al., 1989), and hyperthermia in freely moving animals (Shortall et al., 2013a). It also induces hyperthermia in the Siberian hamster (Jones et al., 2014). Methcathinone also produces hyperthermia in both individually restrained (Rockhold et al., 1997) and freely moving rats (Shortall et al., 2013a). These reports confirm that cathinones have effects on temperature regulation at similar doses to those that affect locomotor behaviour. Furthermore, several reports indicate that recreational users of mephedrone can suffer from apparent changes in body temperature with cold or blue fingers commonly featuring among the recorded adverse events (ACMD, 2010; Schifano et al., 2011; Winstock et al., 2011a). Although incidences of hot flushes and sweating are sometimes reported (Schifano et al., 2011) with mephedrone, severe hyperthermia has not been recorded (Wood et al., 2010; 2011,; Dargan et al., 2011). Consequently, these indications that mephedrone might be altering thermoregulation in humans, coupled with earlier reports that both cathinone and methcathinone administration produces hyperthermia in rodents, spurred several groups to examine in detail the effect of mephedrone on body temperature and thermoregulation in rats.
At normal ambient room temperature (20°C), mephedrone, like MDMA, produces a hypothermic response in individually housed rats, although the mephedrone effect was transient (Figure 5; Shortall et al., 2013a). Miller et al. (2013) also observed a body temperature decrease following mephedrone when the rats were housed at 20°C, but this decrease was abolished by housing at 30°C. Yet hyperthermia did not occur as would be expected with MDMA. Group housing also abolished the hypothermic response to mephedrone in rats and failed to induce hyperthermia (Shortall et al., 2013a) as would have occurred following MDMA (Green et al., 2003; Docherty and Green, 2010).
Figure 5.
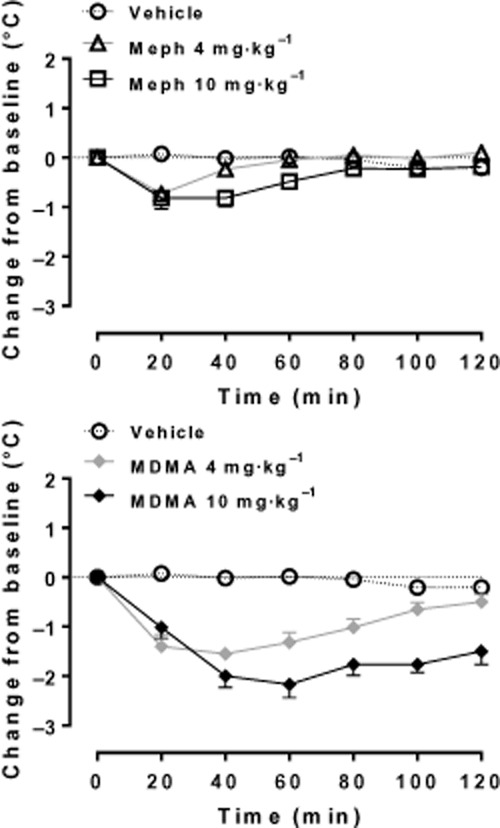
Effect of MDMA and mephedrone on rectal temperature in individually housed male Lister-hooded rats (n = 5–6 per group). Compounds (4 or 10 mg·kg−1 HCl salt) or saline vehicle (1 mL·kg−1) were injected i.p. at 0 min and temperature assessed at 20 min intervals for the next 2 h. Data are shown as change in temperature (°C, mean ± SEM) from the baseline reading taken at the time of injection. [Reproduced from data presented in Shortall et al. (2013a)].
Two recent studies found that repeated dosing of methedrone on the same day (binge dosing) induced hyperthermia. One study examined individually housed rats at normal ambient temperatures (three doses of 3–10 mg·kg−1 s.c.; Baumann et al., 2011) while the other investigated group-housed rats in a warm (≥27°C) environment (four doses of 1–25 mg·kg−1 s.c.; Hadlock et al., 2011). In contrast, in our own recent study on repeated dosing in individually housed rats, we again observed hypothermia (S.E. Shortall et al., unpubl. obs.). The reason for the discrepancy between these findings is unclear but may relate either to dosing [the studies of Baumann et al. (2011) and Hadlock et al. (2011) both employed high cumulative dosing] or to strain differences. Wright et al. (2012a) found mephedrone-induced hypothermia in Wistar rats housed at 23 or 27°C but that even the highest dose given (10 mg·kg−1) produced little body temperature change in Sprague-Dawley rats. Because both strains responded with a similar hypothermic response to the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), this suggests that there is a strain-specific difference in the body temperature response to mephedrone rather than some generalized difference in their ability to respond to temperature-changing drugs.
Shortall et al. (2013a) reported several differences between the pharmacology of MDMA-and mephedrone-induced changes in body temperature. The mephedrone-induced hypothermia was prolonged by the α1-adrenoceptor antagonist prazosin, but unaffected by the α2A-adrenoceptor antagonist BRL 44408 that potentiates and prolongs MDMA-induced hypothermia (Bexis and Docherty, 2006). Mephedrone-induced hypothermia was unaffected by dopamine D2 receptor blockade, which prevents MDMA-induced hypothermia (Green et al., 2005). However, it was prolonged and potentiated by the dopamine D1 receptor antagonist SCH23390, which fails to affect MDMA-induced hypothermia in a cool environment (Green et al., 2005). These pharmacological differences are surprising given that mephedrone and MDMA share similar affinities for the human α2A-adrenoceptor and possibly also the human D1 and D2 receptors (Simmler et al., 2013).
In an attempt to further understand the mechanisms involved in the induction of hypothermia following mephedrone, several groups have investigated its effect on cardiovascular function. Shortall et al. (2013a) compared the effect of MDMA and mephedrone on tail temperature because this is a major heat loss organ in the rat (Redfern et al., 1995) and also provides an indication of peripheral vascular tone. MDMA administered to singly housed rats at normal ambient temperature decreased tail temperature, indicative of peripheral vasoconstriction. This would aid heat conservation and may be mediated via a direct effect on α2A-adrenoceptors (Bexis and Docherty, 2006; Simmler et al., 2013) that regulate tail blood flow and heat loss in the rat. However, the fact that the MDMA-induced decrease in tail temperature was both short in duration and modest in size compared with the concomitant long-lasting and major decrease in rectal temperature demonstrates that centrally regulated heat conservation mechanisms are disrupted after a single dose of MDMA (Green et al., 2005), as discussed in detail elsewhere (Docherty and Green, 2010). Under these same conditions, mephedrone also produced hypothermia, but the small and short-lasting decrease in rectal temperature was associated with a prolonged decrease in tail temperature, and therefore differed from the temporal profile of MDMA-induced thermoregulatory response. The decrease in tail temperature following mephedrone is consistent with its affinity for both α1-and α2A-adrenoceptors (Simmler et al., 2013), the recently reported hypertension produced in rats (Meng et al., 2012) and the side effects of cold or blue fingers experienced by recreational users (ACMD, 2010; Schifano et al., 2011; Winstock et al., 2011a).
Mephedrone, like MDMA (Hysek et al., 2012a,2012b,2012c,), increases plasma noradrenaline levels (Shortall et al., 2013a), and in the case of mephedrone this effect is sensitive to α1-adrenoceptor, α2A-adrenoceptor and dopamine D1 receptor blockade.
Further evidence for an action of mephedrone on peripheral adrenergic mechanisms has been obtained by two investigations on cardiovascular function in the rat. Varner et al. (2013) showed that mephedrone elicited dose-related increases in arterial pressure lasting around 1.5 h. This pressor response was accompanied by tachycardia that reached a plateau after 1 mg·kg−1 i.v. 2–5 min after drug administration. Similarly, Meng et al. (2012) reported that mephedrone (3 or 15 mg·kg−1 s.c.) significantly increased arterial pressure and heart rate in the conscious rat. The responses following s.c. injection had a slower onset and much longer duration (up to 5 h) than those following i.v. injection. The pressor response was significantly attenuated by phentolamine, indicating that activation of peripheral α-adrenoceptors plays an important role in mediating mean arterial pressure (MAP) responses. In contrast, the tachycardia produced by mephedrone was blocked by atenolol, suggesting that it was elicited by β-adrenoceptor activation. The authors proposed that mephedrone might release noradrenaline from peripheral sympathetic nerves innervating the vasculature; this is supported by the observation that mephedrone increases plasma noradrenaline (Shortall et al., 2013a). However, because Meng et al. (2012) showed that mephedrone elicits pressor responses and tachycardia in reserpine-treated rats, this suggests that the cardiovascular responses may not directly result from the release of noradrenaline from peripheral sympathetic nerves. Varner et al. (2013) therefore proposed that mephedrone has a substrate activity at noradrenaline transporters (NET) resulting in the release of cytoplasmic stores of noradrenaline, an idea supported by evidence of the affinity of mephedrone for these transporters (see later). All these observations in animals are consistent with the clinical reports that mephedrone can produce hypertension and tachycardia in humans (Wood et al., 2010; Regan et al., 2011).
Meng et al. (2012) extended their observations to examine the effects of mephedrone at a high concentration (30 μM) on cardiac electrophysiology and found little or no effect on the cardiac action potential waveform or L-type Ca2+ channels using ventricular myocytes isolated from the guinea pig, or on the transfected human ether-a-go-go-66 related gene cardiac K+ channel. They also examined the action of mephedrone on cardiac function in rats in real time using echocardiography. Mephedrone produced dose-dependent effects on the heart that were consistent with sympathomimetic stimulation. A dose of 10 mg·kg−1 s.c. produced a clear increase in heart rate, stroke volume and cardiac output. Ejection fraction and fractional shortening, both indicators of cardiac contractility, were also significantly increased. The effects of mephedrone on the heart after i.v. injection were rapid but also short lived, with many of the parameters returning to near pre-dose values 10 min after administration.
MDMA also increases heart rate, MAP and cardiac output in both rats and humans (Lester et al., 2000; O'Cain et al., 2000; Pedersen and Blessing, 2001; Badon et al., 2002; Cole and Sumnall, 2003). In the rat-isolated right ventricle, MDMA potentiated contractions mediated by noradrenaline, but not those mediated by isoprenaline, consistent with an action at the NET (Al-Sahli et al., 2001). These results suggest that any cardiac stimulant actions of MDMA and mephedrone may involve indirect sympathomimetic effects. It is also possible that an increase in locomotor activity may increase heart rate, but this is unlikely in human studies on MDMA where low doses were given in controlled clinical conditions.
Effect on brain monoamine concentrations
Only two studies have been published on the effect of mephedrone on brain tissue monoamine concentrations measured shortly after drug administration. Motbey et al. (2012a) examined concentrations of dopamine, 5-HT, and their major metabolites in striatum and hippocampus 60 min after either a single 30 mg·kg−1 dose or 60 min after the final of 10 once daily injections of this dose. Dopamine was significantly elevated, while 5-HT was significantly decreased, in the striatum and hippocampus following a single dose. A similar pattern was seen 60 min after 10 once daily injections. These data have recently been supported by Shortall and colleagues (unpublished) who gave mephedrone (10 mg·kg−1 i.p.) on 2 consecutive days a week for 3 weeks and also observed reduced hippocampal 5-HT levels 60 min after the final dose.
These results are similar to the acute effect of MDMA on cerebral monoamine content. Colado and Green (1994) showed that MDMA produced a rapid decrease in 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) content and increased striatal dopamine. While Motbey et al. (2012a) observed a modest increase in 5-HIAA following mephedrone, Shortall et al. (unpublished) observed a decrease in 5-HIAA in both the hippocampus and the striatum, and also found that mephedrone and MDMA produced a similar decrease in striatal 3,4-dihydroxyphenylacetic acid (Shortall et al., 2013c). Any discrepancies in the levels of monoamine or metabolites between studies are almost certainly due to rapid changes in their synthesis, release, metabolism and clearance shortly after drug-administration. Although metabolite concentrations cannot be used to indicate turnover rates as suggested by Motbey et al. (2012a), because neither 5-HT nor dopamine metabolism is at steady state (see Neff et al., 1969; Costa et al., 1972), we concur that their data suggest mephedrone releases dopamine and 5-HT from nerve terminals, results also strongly supported by several microdialysis studies.
By implanting microdialysis probes in the nucleus accumbens, Kehr et al. (2011) found that mephedrone (3 mg·kg−1 s.c.) rapidly increased extracellular dopamine levels in conscious rats by almost 500% above basal, while the same dose of MDMA induced a more modest increase (235%). The increase in extracellular 5-HT was 941% following mephedrone and 911% after MDMA. Baumann et al. (2011) obtained very similar results in the same brain region, showing that 0.3 and 1.0 mg·kg−1 of mephedrone i.v. produced a dose-related increase in extracellular dopamine and 5-HT, with the magnitude of the effect on 5-HT being greater. The microdialysis studies of Wright et al. (2012a) also observed that mephedrone induced a larger increase in 5-HT compared with dopamine in the nucleus accumbens. These data present an interesting contrast to the effect of MDMA on monoamine release in this region, because this drug produces twofold greater release of dopamine than of 5-HT (O'Shea et al., 2005). Recently, we have found that mephedrone also increases the extracellular concentration of dopamine in the striatum (S.E. Shortall et al., unpubl. obs.).
Neurotoxicity
It is well established that high-dose amphetamine or methamphetamine administration to rats or mice induces neurotoxic damage to both dopamine and 5-HT nerve endings in the brain (Hotchkiss and Gibb, 1980; Armstrong and Noguchi, 2014; Cadet et al., 2007). In contrast, repeated MDMA administration to the rat, guinea pig and monkey induces a selective neurotoxic loss of 5-HT in forebrain regions (see Green et al., 2003). The severity of this loss is dependent both on dose and frequency of administration (O'Shea et al., 1998) and the ambient temperature at the time of drug administration. By contrast, MDMA fails to produce damage to 5-HT neurons in mouse brain, instead causing damage to dopamine nerve terminals (see Green et al., 2003). However, the different pharmacokinetics of MDMA in humans compared with rats makes it unlikely that this compound is neurotoxic in human brain (Green et al., 2012a). Nevertheless, neurotoxicity may occur in humans when it has been taken in conjunction with other drugs, as generally occurs (Green et al., 2012b; Parrott, 2012b).
This association of amphetamines with neurotoxic damage to amine nerve terminals in the brain encouraged several groups to examine whether mephedrone also induced neurotoxicity in the rodent brain. The first study used an intense dosing regimen (four doses of 10 or 25 mg·kg−1 s.c. at 2 h intervals) and reported neurotoxic loss of 5-HT in the hippocampus of Sprague-Dawley rats (Hadlock et al., 2011). The neurotoxic damage reported by Hadlock et al. (2011) also produced a loss in the 5-HT transporter (SERT), analogous to the situation with MDMA (O'Shea et al., 2006).
However, several subsequent investigations have failed to confirm the Hadlock et al. (2011) finding that mephedrone produces neurotoxic loss of 5-HT in the rat brain. A similar binge-type dosing schedule of three injections of mephedrone (3 or 10 mg·kg−1 s.c.), one being given every 2 h, produced no loss of 5-HT, dopamine or noradrenaline in the cortex or striatum 2 weeks after dosing (Baumann et al., 2011). Similarly, administration of mephedrone (10 mg·kg−1) on 2 consecutive days a week for 3 weeks (to mimic weekend-type recreational use in humans) also failed to alter tissue concentrations of dopamine or 5-HT in the hippocampus, striatum or frontal cortex (Shortall et al., 2013c). A dose schedule of 7.5, 15 or 30 mg·kg−1 of mephedrone once daily for 10 days also failed to produce any long-term loss of 5-HT or dopamine in the striatum or hippocampus (Motbey et al., 2012a). Another binge dosing study in rats giving mephedrone (30 mg·kg−1 twice daily for 4 days) also failed to produce any loss of 5-HT, dopamine, noradrenaline or their metabolites, or noradrenaline in the frontal cortex, hippocampus or striatum 2 weeks later (den Hollander et al., 2013). This study also found that 5-HT and dopamine transporter levels were unchanged in these in vivo studies (den Hollander et al., 2013).
Baumann et al. (2013a) suggested that a possible reason for apparent neurotoxicity reported by Hadlock et al. (2011) might be group housing of the rats, but this seems unlikely as den Hollander et al. (2013) also grouped their animals. Furthermore, Motbey et al. (2012a) and den Hollander et al. (2013) gave high repeated doses that would have been more than sufficient to cause toxicity if it had been MDMA that had been administered at the same dose (O'Shea et al., 1998).
Studies in mice by Angoa-Pérez et al. (2012) and den Hollander et al. (2013) failed to detect any loss in striatal dopamine terminal integrity, as indicated by measuring dopamine levels, tyrosine hydroxylase activity and dopamine transporter (DAT) protein levels 7 days after administering a total of four doses of mephedrone (40 mg·kg−1) at 2 h intervals (Angoa-Pérez et al., 2012) or 30 mg·kg−1 twice daily for 4 days (den Hollander et al., 2013). Furthermore, mephedrone did not cause microglial activation in the striatum or increase glial fibrillary acidic protein (GFAP) levels, both reliable markers of neurodegeneration (Angoa-Pérez et al., 2012; den Hollander et al., 2013). This contrasts strongly with MDMA where a similar dose regime results in a substantial loss of striatal dopamine (Logan et al., 1988; O'Shea et al., 2001) and an increase in GFAP (Miller and O'Callaghan, 1995; Johnson et al., 2002a,b).
In a subsequent study, Angoa-Pérez et al. (2013) administered mephedrone (10, 20 or 40 mg·kg−1) to mice before each injection of methamphetamine (four injections of 2.5 or 5.0 mg·kg−1 at 2 h intervals). This methamphetamine dosing regime produced the expected dopamine neurotoxicity in the striatum, decreasing dopamine, DAT and tyrosine hydroxylase levels. Mephedrone failed to produce any neurotoxic damage, but enhanced the methamphetamine-induced dopamine neurotoxicity. Mephedrone also enhanced the neurotoxic effects of amphetamine and MDMA on dopamine nerve endings, suggesting that a potentially dangerous interaction might occur if mephedrone is taken either intentionally or unintentionally with other illicit amphetamines.
Effect on monoamine receptors and transporters
There is now good evidence that mephedrone interacts with plasma membrane transporters, including the DAT, NET and 5-HT (SERT) (Baumann et al., 2011; Hadlock et al., 2011; López-Arnau et al., 2012; Martínez-Clemente et al., 2012; Simmler et al., 2013). As Baumann et al. (2013a) emphasized, drugs acting on these transporters can be classified as either substrates (e.g. amphetamine) or blockers (e.g. cocaine). Substrates (but not blockers) are transported into the cell where they disrupt vesicular storage and stimulate non-exocytotic monoamine release by reversing the transporter flux (Rothman and Baumann, 2003; Sitte and Freissmuth, 2010), and may also interact with vesicular monoamine transporters. Blockers, in contrast, produce sustained deficits including monoamine depletion and loss of transporter function (Baumann et al., 2007; Fleckenstein et al., 2007). Several groups have reported that mephedrone inhibits the uptake of [3H]-dopamine, [3H]-noradrenaline and [3H]-5-HT into brain tissue, suggesting it functions as a transporter blocker (Baumann et al., 2011; 2013a,; Hadlock et al., 2011; López-Arnau et al., 2012; Martínez-Clemente et al., 2012; Simmler et al., 2013). However, as Baumann et al. (2013a) point out, assays measuring inhibition of uptake do not discriminate between drugs acting as transporter substrates or as blockers. Using in vitro release assays in rat brain synaptosomes (Rothman et al., 2001; Rothman and Baumann, 2003; Nagai et al., 2007), mephedrone was found to be a substrate for monoamine transporters, stimulating the release of [3H]-1-methyl-4-phenylpyridinium ([3H]-MPP+) via DAT and NET and release of [3H]-5-HT via SERT (Baumann et al., 2011). Their results showed that mephedrone and MDMA cause non-selective release of monoamines by being substrates for all the transporters, while amphetamine is a selective substrate at both DAT and NET. Furthermore, mephedrone and MDMA both had a similar potency to each other as a releaser at all three monoamine transporters. The work of Baumann et al. (2011; 2013a,) and Eshleman et al. (2013) on mephedrone and other cathinones makes it clear that their pharmacology can differ both from each other and from MDMA and amphetamine.
Another substantial study on the effect of a range of cathinones and MDMA is that of Simmler et al. (2013) who used transfected cells expressing human DAT, NET and SERT as their assay system. Again, their results pointed to mephedrone functioning as a transportable substrate. The potency of inhibition (IC50) of mephedrone at DAT and SERT was similar, in agreement with the earlier studies of Hadlock et al. (2011) obtained using rat synaptosomes. MDMA was approximately 10 times more effective at the SERT than the DAT in Simmler et al.'s (2013) study and four times more in the synaptosomal study of Hadlock et al. (2011).
Mephedrone also binds to the 5-HT2A receptor with low micromolar affinity (López-Arnau et al., 2012; Martínez-Clemente et al., 2012; Simmler et al., 2013) while MDMA has a slightly lower affinity (Simmler et al., 2013). Mephedrone has little affinity for the 5-HT1A, 5-HT2C or any dopamine receptor subtypes, in common with MDMA (Simmler et al., 2013). However, mephedrone (Simmler et al., 2013) and MDMA (Bexis and Docherty, 2006) both bind to the α2A-adrenoceptor in the 1–10 μM range which may explain the peripheral vasoconstriction produced by both drugs as discussed earlier.
In a study to determine rat brain areas activated by mephedrone administration, Motbey et al. (2012b) examined the effects of mephedrone (15 and 30 mg·kg−1 i.p.) and methamphetamine (2 mg·kg−1 i.p.) on the expression of the c-fos transcription factor, an established marker of neuronal activation (Kovacs, 2008). The pattern of Fos expression induced by mephedrone resembled those expected for a drug combining the properties of methamphetamine and MDMA, with particularly strong Fos expression in the cortex, dorsal and ventral striatum, ventral tegmental area (typical of both MDMA and methamphetamine) and supraoptic nucleus (typical of MDMA), as demonstrated in earlier studies with methamphetamine (Carson et al., 2010) and MDMA (Hargreaves et al., 2007).
Effects in behavioural tests
Repeated use of MDMA by humans can lead to cognitive deficits (Parrott, 2013). However, meta-analysis does not suggest a clear dose-related association but implies that a combination of MDMA with other recreational drugs may be more problematic (Verbaten, 2003; Laws and Kokkalis, 2007). Preclinical studies indicated that MDMA can impair working memory (Piper and Meyer, 2004; Rodsiri et al., 2011) and sensorimotor gating in rats (Vollenweider et al., 1999) without any concomitant neurotoxic damage (Rodsiri et al., 2011). Therefore, Shortall et al. (2013c) compared the effect of mephedrone and MDMA on these measures. Mephedrone (1, 4 or 10 mg·kg−1) or MDMA (10 mg·kg−1) was injected on 2 consecutive days a week for 3 weeks (to mimic weekend-type recreational use in humans), and novel object discrimination (NOD; day 2), conditioned emotional response (CER; days 8 and 9) and prepulse inhibition of the acoustic startle (PPI; day 15) were evaluated. Rats that had received two previous treatments with mephedrone or MDMA (at 24 h and 30 min before testing) were unable to distinguish between the novel and familiar object during the choice trial, in contrast to controls. However, during the familiarization trial, mephedrone (4 and 10 mg·kg−1) and MDMA decreased total object directed exploration, making it difficult to attribute the absence of discrimination to a specific memory impairment. Although MDMA had no influence on associative memory in the CER test, the highest dose of mephedrone significantly reduced freezing on re-exposure to the context used for conditioning, but had no effect on freezing produced by representation of the light and tone cue, suggesting mephedrone attenuated contextual but not cued association, which are mediated by different neuroanatomical substrates. Neither drug altered PPI (sensorimotor gating) assessed 30 min after the fifth injection. Taking all these data together, Shortall et al. (2013c) suggested that while mephedrone may impair cognitive performance, it is unclear whether this is due to a deficit in learning and memory and/or attention.
In rhesus monkeys, a pronounced improvement in visual-spatial memory and learning occurred after a 0.32 mg·kg−1 dose of both methamphetamine and mephedrone, although spatial working memory was not improved by either drug. This suggests mephedrone can improve spatial memory and learning in monkeys analogous with classical psychomotor stimulants (Wright et al., 2012b).
Motbey et al. (2012b) also performed a battery of tests on rats given a higher dose of mephedrone (30 mg·kg−1) once daily for 10 days but with a significant time gap (11–35 days) following the last dose and the start of the behavioural tests. Although repeated mephedrone did not cause any lasting changes in anxiety (elevated plus maze) or social preference, it caused a clear deficit in NOD 36 days after drug treatment. den Hollander et al. (2013) used a similar protocol to examine the lasting consequences of repeated mephedrone (30 mg·kg−1 twice daily for 4 days) in mice, examining anxiety (elevated plus maze), spatial working memory (T-maze spontaneous alternation), long-term spatial memory (Morris water maze) and depressive behaviour (tail suspension test) 2 weeks after injections. Anxiety and depression-related behaviours were unaffected by mephedrone, but consistent with Motbey et al. (2012b) mephedrone did reduce working memory.
In acute dose studies, low to medium doses of MDMA (≤10 mg·kg−1) cause an anxiogenic response in rats in the x-maze, whereas higher doses (≥15 mg·kg−1) tend to reduce anxiety-related behaviour (Ho et al., 2004).
Abuse liability
It has been proposed that the predominant action of all cathinones on DAT is probably associated with a risk of addiction (Simmler et al., 2013). This is consistent with common reports from recreational users that they became addicted or dependent on the drug (Dargan et al., 2010). This risk contrasts with MDMA where users may suffer from some adverse events on acute withdrawal, but unequivocal reports of dependence or withdrawal are completely absent. Simmler et al. (2013) proposed that the twofold greater blood–brain barrier permeability of mephedrone over both methamphetamine and MDMA may produce a relatively greater reinforcing effect of the drug.
Aarde et al. (2013) showed that mephedrone supports i.v. self-administration in rats, resulting in consistent levels of drug intake from session to session and reward lever selectivity greater than 80% in most rats. Recently, Motbey et al. (2013) also showed that mephedrone robustly supported self-administration in rats. In contrast, MDMA is not readily self-administered by rats (De La Garza et al., 2007) and produces considerable inter-individual heterogeneity in acquisition (Schenk, 2009; Colussi-Mas et al., 2010; Bird and Schenk, 2013). The report of Aarde et al. (2013) supports the findings of Hadlock et al. (2011) that mephedrone supports self-administration in rats housed in high-ambient temperature conditions. Interestingly, high-ambient room temperature also increases MDMA self-administration (Cornish et al., 2003).
Lisek et al. (2012) recently reported that mephedrone produced changes in conditioned place preference in rats and mice. The preference shift detected following mephedrone conditioning suggests that the drug displays rewarding properties consistent with a risk of abuse liability. However, this shift was only seen at a very high dose.
Intracranial self-stimulation that measures the behavioural effects of psychoactive compounds on brain reward circuitry was used by Robinson et al. (2012) to investigate the ability of mephedrone and cocaine to alter responding for electrical stimulation of the medial forebrain bundle in mice. Adult male mice with unipolar stimulating electrodes implanted in the lateral hypothalamus responded for varying frequencies of brain stimulation reward (BSR). The frequency that supported half maximal responding (EF50), the BSR threshold and the maximum response rate were determined before and after saline, mephedrone (1, 3 or 10 mg·kg−1) or cocaine at the same doses. Mephedrone produced a dose-dependent decrease in EF50, threshold and the maximum response rate beginning 15 min after administration. Cocaine dose-dependently lowered the EF50 and threshold beginning immediately after administration, but did not affect maximum response rate. These results suggest that mephedrone, like cocaine, potentiates BSR, which the authors concluded may indicate its potential for abuse.
Drug interactions
Currently, very few studies have investigated possible interactions of mephedrone with other drugs. This is important as the combination of MDMA with other drugs, taken knowingly or unknowingly by recreational users, has been suggested to contribute to severe adverse events and possibly long-term neurotoxicity (Green et al., 2012b). Furthermore, most mephedrone users admit previous or concurrent illicit use of MDMA (Carhart-Harris et al., 2011; Moore et al., 2013), so possible interactions of mephedrone with other psychoactive drugs, including MDMA, are likely. Our own preliminary study found that MDMA pre-exposure altered the subsequent temperature response to a challenge dose of mephedrone, suggesting cross-sensitivity of some functional responses in rats (S.E. Shortall et al., unpubl. data). The study of Angoa-Pérez et al. (2013) in mice demonstrated that while mephedrone alone failed to produce any neurotoxic damage, it did enhance methamphetamine-induced neurotoxicity in dopamine nerve endings. It also enhanced the neurotoxic effects of amphetamine and MDMA on dopamine neurons, suggesting that a potentially dangerous interaction might occur when mephedrone is taken with other recreational drugs. In a subsequent study, the same group found that mephedrone did not cause 5-HT toxicity in the hippocampus even when co-administered with methamphetamine or MDMA (Angoa-Pérez et al., 2014).
The term ‘bath salts’, which seems prevalent in the U.S. recreational drug scene, often refers to some of the newer cathinone-related compounds that are now being distributed using names to hide the fact that they are intended for human ingestion (Baumann et al., 2013a,b). The content of ‘bath salt’ powders can include mephedrone, methylone (3,4-methylenedioxymethcathinone; Figure 1) and 3,4-methylenedioxypyrovalerone (MDPV). The different pharmacology of these compounds at monoamine receptor sites (Simmler et al., 2013) means that mixing them could have serious adverse effects in humans. Recently, for example, Cameron et al. (2013) showed that mephedrone and MDPV, due to their different actions at the dopamine nerve ending, might be expected initially to release dopamine and subsequently prevent its re-uptake via DAT. This combined action could have serious adverse effects on brain function.
Over the last few years, there has been increasing evidence that caffeine, an adulterant sometimes found in ‘ecstasy’ tablets and an ingredient in coffee, tea, and in many soft and ‘energy drinks’ such as Red Bull, enhances both the hyperthermia and the neurotoxicity induced in rats by MDMA raising concerns about its possible effects in humans (Vanattou-Saïfoudine et al., 2012). We have now found that the mephedrone-induced decrease in rectal temperature was reversed by combined caffeine administration, and a sustained hyperthermia occurred which had not returned to baseline levels even at 120 min post-injection (Shortall et al., 2013b). One possibility is that this elevation in temperature observed when both drugs are administered may explain the hot flushes sometimes reported by mephedrone users (Schifano et al., 2011). Importantly, this drug combination did not appear to induce 5-HT neurotoxicity in the brain (Shortall et al., 2013b) as occurs when caffeine is given with MDMA (Vanattou-Saïfoudine et al., 2012).
Conclusions
As detailed in the Introduction, although recreational users have stated that the psychoactive effects of mephedrone are similar to those of MDMA, the preclinical studies detailed in this review make it clear that these two drugs have a rather different, albeit related pharmacology. Table 1 provides a subjective overview of the behavioural pharmacokinetic and pharmacological effects of mephedrone and MDMA in rodents indicating some key supporting references for each comparator; however, full information is provided in the text of this review. Mephedrone has some properties that suggest its adverse effect profile might be less than MDMA, but its use by humans still raises significant safety concerns.
Table 1.
Comparator overview of some of the major properties of mephedrone and MDMA
| Behavioural measurements | Mephedrone | MDMA |
|---|---|---|
| Locomotor activity(1–3)2010 | ||
| • Duration | ↑ | ↑↑ |
| • Magnitude | ↑ | ↑ |
| • 5-HT syndrome | ✓ | ✓ |
| • Sensitization to same drug | ✓ | ✓ |
| Body temperature(4–6)2010 | ||
| • Singly housed | ↓ | ↓↓ |
| • Group housed | ↔ | ↑↑ |
| • High-ambient temperature | ↔↑§2010 | ↑↑↑ |
| Drug self-administration(6)2010 | ↑↑↑ | ↑ |
| Chemical measurements | ||
| Pharmacokinetics(7,8) | ||
| • Plasma half-life (h) | ∼0.4 | ∼0.8 |
| • Half-life similar to humans | ✓ | Χ |
| • Active metabolites | ? | ✓ |
| • Brain penetration | ↑↑ | ↑ |
| Brain monoamine release in vivo(9–11)2010 | ||
| • 5-HT | ↑↑↑ | ↑↑↑ |
| • Dopamine | ↑↑ | ↑ |
| Brain monoamine content at 60 min(12–13) | ||
| • 5-HT | ↓ | ↓↓↓ |
| • Dopamine | ↑ | ↑ |
| Brain neurotoxicity(14–16)2010 | ||
| • 5-HT in rats | ?*2010 | ✓ |
| • Dopamine in mice | – | ✓ |
| Monoamine uptake inhibition (IC50)(18,19) | ||
| • SERT | 422 | 125 |
| • DAT | 762 | 1009 |
| • NET | 487 | 450 |
| Monoamine release (EC50)(20–22)2010 | ||
| • SERT | 122 | 39 |
| • DAT | 51 | 42 |
| • NET | 58 | 53 |
Data compiled to compare the behavioural effects and pharmacokinetic measurements were derived from studies using a single acute s.c. or i.p. injection of either drug (typically 1–10 mg·kg−1 but up to 30 mg·kg−1 for mephedrone) in rats and where available in mice, but in some microdialysis studies i.v. administration was used. Studies examining a neurotoxic effect have used multiple doses (either on the same day or over a few weeks) typically administering 10–30 mg·kg−1 s.c. or i.p. Arrows show relative size of behavioural response, either increase (↑) or decrease (↓), ↔ indicates no significant change from control. Check mark (✓) shows that a response or change occurs, dash (−) means change not seen, and X means the value is different from humans. IC50 and EC50 values are both reported in nM and taken from Baumann et al. (2013a).
Indicates that one of five studies reported neurotoxic damage. Combinations of other drugs with either MDMA or mephedrone and have been reported to, respectively, enhance or induce toxicity as detailed in the text.
Indicates conflicting results. Key references supporting the synopsis for each parameter are indicated as a numerical superscript and below, but further references are provided in the relevant section of the review: 1Kehr et al. (2011), 2Lisek et al. (2012), 3Green et al. (2003), 4Shortall et al. (2013a, b,), 5Miller et al. (2013), 6Docherty and Green (2010), 7Baumann et al. (2009), 8Martínez-Clemente et al. (2013), 9Kehr et al. (2011), 10Wright et al. (2012a), 11O'Shea et al. (2005), 12Motbey et al. (2012a), 13Colado and Green (1994), 14Shortall et al. (2013a, c,), 15Motbey et al. (2012a), 16Green et al. (2003), 17Hadlock et al. (2011), 18Martínez-Clemente et al. (2012), 19Baumann et al. (2013a), 20Nagai et al. (2007), 21Baumann et al. (2011), 22Simmler et al. (2013).
On the positive side, mephedrone, at least when given at a dose to rats that may have translational relevance, does not appear to induce monoamine neurotoxicity or produce hyperthermia in the majority of investigations. However, hyperthermia did occur when mephedrone was combined with caffeine. Of note are the indications that mephedrone has a short plasma half-life in rats and probably in humans, which is probably the reason why many recreational users take repeated doses over a short period. This binge use may induce more severe adverse consequences. What is also becoming clear from preclinical studies is that mephedrone has high-abuse liability resulting from several pharmacokinetic and pharmacodynamic differences from MDMA. Firstly, it has high brain penetration, rapid metabolism and brain clearance all of which are likely to lead to an acute withdrawal phenomenon. This does not occur with MDMA which has slower brain penetration, metabolism and clearance, both in rats and crucially in humans. Secondly, its interaction with monoamine neurotransmitters is very different from that of MDMA with greater potency at DAT and causing more dopamine release, leading Simmler et al. (2013) to suggest that mephedrone acts as a mixed ‘cocaine-MDMA’ type drug having some properties of both compounds. The 5-HT releasing property produces the ‘entactogenic’ subjective effects desired by many users, but because of its ability to potently activate the noradrenaline and dopamine systems, mephedrone is also likely to have a high psychostimulant and abuse liability. In contrast, MDMA has greater potency at SERT than mephedrone, and its dopamine/5-HT release potency will produce positive mood with little psychostimulant effect. Self-administration studies in rats show that mephedrone, unlike MDMA, robustly supports this behaviour.
Emerging data suggest that, like amphetamine and its derivatives, the cathinones all have their own specific pharmacological profile. Consequently, the pharmacology of mephedrone reviewed here cannot be taken as a template for the properties of other illicit cathinone derivatives that are appearing. The medical problem is that recreational users of the newest compounds are acting as ‘laboratory animals’, because none of the drugs have undergone any thorough preclinical evaluation similar to those now being published on mephedrone.
Glossary
- 5-HIAA
5-hydroxyindoleacetic acid
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- ACMD
Advisory Committee on the Misuse of Drugs
- BRL44408
2-((4,5-dihydro-1H-imidazol-2-yl)methyl)-2,3-dihydro-1-methyl-1H-isoindole
- BSR
brain stimulation reward
- Cmax
peak plasma concentration
- DAT
dopamine transporter
- EF50
half maximal response
- EMCDDA
European Monitoring Centre for Drugs and Drug Addiction
- MDMA
3,4-methylenedioxymethamphetamine
- PCPA
p-chlorophenylalanine
- SCH23390
R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzapine
- SERT
5-HT transporter
- tmax
time of drug peak
Conflict of interest
The authors declare no conflicts of interest.
References
- Aarde SM, Angrish D, Barlow DJ, Wright Jr MJ, Vandewater SA, Creehan KM, et al. Mephedrone (4-methylmethcathinone) supports intravenous self-administration in Sprague-Dawley and Wistar rats. Addict Biol. 2013;18:786–799. doi: 10.1111/adb.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg M, Wade D, Wall E, Izenwasser S. Effect of MDMA (ecstasy) on activity and cocaine conditioned place preference in adult and adolescent rats. Neurotoxicol Teratol. 2007;29:37–46. doi: 10.1016/j.ntt.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACMD. 2010. Consideration of the cathinones. Home office report. Available at: http://www.homeoffice.gov.uk/publications/agencies-public-bodies/acmd1/acmd-cathinodes-report-2010?view=Standard&pubID=878838(accessed 21/3/2014)
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G-Protein Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sahli W, Ahmad H, Kheradmand F, Connolly C, Docherty JR. Effects of methylenedioxymethamphetamine on noradrenaline-evoked contractions of rat right ventricle and small mesenteric artery. Eur J Pharmacol. 2001;422:169–174. doi: 10.1016/s0014-2999(01)01070-6. [DOI] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, et al. Mephedrone, an abused psychoactive component of ‘bath salts’ and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2012;120:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Sykes CE, Shah MM, et al. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J Neurochem. 2013;125:102–110. doi: 10.1111/jnc.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Herrera-Mundo N, Francescutti DM, Kuhn DM. Effects of combined treatment with mephedrone and methamphetamine or 3,4-methylenedioxymethamphetamine on serotonin nerve endings of the hippocampus. Life Sci. 97:31–36. doi: 10.1016/j.lfs.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong BD, Noguchi KK. The neurotoxic effects of 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine on serotonin, dopamine, and GABA-ergic terminals: an in-vitro autoradiographic study in rats. Neurotoxicology. 2004;25:905–914. doi: 10.1016/j.neuro.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Badon LA, Hicks A, Lord K, Ogden BA, Meleg-Smith S, Varner KJ. Changes in cardiovascular responsiveness and cardiotoxicity elicited during binge administration of ecstasy. J Pharmacol Exp Ther. 2002;302:898–907. doi: 10.1124/jpet.302.3.898. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. Effects of dose and route of administration on pharmacokinetics of (±)-3,4-methylenedioxymethamphetamine (MDMA) in the rat. Drug Metab Dispos. 2009;37:2163–2170. doi: 10.1124/dmd.109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2011;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive ‘bath salts’: not so soothing. Eur J Pharmacol. 2013a;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013b;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benturquia N, Courtin C, Noble F, Marie-Claire C. Involvement of D1 dopamine receptor in MDMA-induced locomotor activity and striatal gene expression in mice. Brain Res. 2008;1211:1–5. doi: 10.1016/j.brainres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Effects of MDMA, MDA and MDEA on blood pressure, heart rate, locomotor activity and body temperature in the rat involve α-adrenoceptors. Br J Pharmacol. 2006;147:926–934. doi: 10.1038/sj.bjp.0706688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird J, Schenk S. Contribution of impulsivity and novelty seeking to the acquisition and maintenance of MDMA self-administration. Addict Biol. 2013;18:654–664. doi: 10.1111/j.1369-1600.2012.00477.x. [DOI] [PubMed] [Google Scholar]
- Bradbury S, Gittings D, Schenk S. Repeated exposure to MDMA and amphetamine: sensitization, cross-sensitization, and response to dopamine D1-and D2-like agonists. Psychopharmacology (Berl) 2012;223:389–399. doi: 10.1007/s00213-012-2726-9. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Sumnall HR, Measham F, Cole J. Analyses of second-generation ‘legal highs’ in the UK: initial findings. Drug Test Anal. 2010;2:377–382. doi: 10.1002/dta.155. [DOI] [PubMed] [Google Scholar]
- Brunt TM, Poortman A, Niesink RJM, Brink van den W. Instability of the ecstasy market and a new kid on the block: mephedrone. J Psychopharmacol. 2011;25:1543–1547. doi: 10.1177/0269881110378370. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Verkariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of ‘bath salts,’ produce opposite effects at the human dopamine transporter. Psychopharmacology (Berl) 2013;227:493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela JP, Meisel A, Abreu AR, Branco PS, Ferreira LM, Lobo AM, et al. Neurotoxicity of ecstasy metabolites in rat cortical neurons, and influence of hyperthermia. J Pharmacol Exp Ther. 2006;316:53–61. doi: 10.1124/jpet.105.092577. [DOI] [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol. 2009;39:210–271. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, Nutt DJ. A web-based survey on mephedrone. Drug Alcohol Depend. 2011;118:19–22. doi: 10.1016/j.drugalcdep.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, et al. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addict Biol. 2010;15:448–463. doi: 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Colado MI, Green AR. A study of the mechanism of MDMA (‘ecstasy’)-induced neurotoxicity of 5-HT neurones using chlormethiazole, dizocilpine and other protective compounds. Br J Pharmacol. 1994;111:131–136. doi: 10.1111/j.1476-5381.1994.tb14034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colado MI, Green AR. The spin trap reagent α-phenyl-N-tert-butyl nitrone prevents ‘ecstasy'-induced neurodegeneration of 5-hydroxytryptamine neurons. Eur J Pharmacol. 1995;280:343–346. doi: 10.1016/0014-2999(95)00298-y. [DOI] [PubMed] [Google Scholar]
- Colado MI, Murray TK, Green AR. 5-HT loss in rat brain following 3,4 methylenedioxymethamphetamine, p-chloroamphetamine and fenfluramine administration and effects of chlormethiazole and dizocilpine. Br J Pharmacol. 1993;108:583–589. doi: 10.1111/j.1476-5381.1993.tb12846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. Altered states: the clinical effects of ‘ecstasy’. Pharmacol Ther. 2003;98:35–58. doi: 10.1016/s0163-7258(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Colussi-Mas J, Wise RJ, Howard A, Schenk S. Drug seeking in response to a priming injection of MDMA in rats: relationship to initial sensitivity to self-administered MDMA and dorsal striatal dopamine. Int J Neuropsychopharmacol. 2010;13:1315–1327. doi: 10.1017/S1461145710000283. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Shahnawaz Z, Thompson MR, Wong S, Morley KC, Hunt GE, et al. Heat increases 3,4-methylenedioxymethamphetamine self-administration and social effects in rats. Eur J Pharmacol. 2003;482:339–341. doi: 10.1016/j.ejphar.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Costa E, Green AR, Koslow SH, LeFevre HF, Revuelta AV, Wang C. Dopamine and norepinephrine in noradrenergic axons: a study in vivo of their precursor product relationship by mass fragmentography and radiochemistry. Pharmacol Rev. 1972;24:167–190. [PubMed] [Google Scholar]
- Dal Cason TA, Young R, Glennon RA. Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol Biochem Behav. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- Dargan PI, Albert S, Wood DM. Mephedrone use and associated adverse effects in school and college/university students before the UK legislation change. QJM. 2010;103:875–879. doi: 10.1093/qjmed/hcq134. [DOI] [PubMed] [Google Scholar]
- Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone) Drug Test Anal. 2011;3:454–463. doi: 10.1002/dta.312. [DOI] [PubMed] [Google Scholar]
- Davey Z, Corazza O, Schifano F, Deluca P. Mass-information: mephedrone, myths, and the new generation of legal highs. Drugs Alcohol Today. 2010;10:24–28. [Google Scholar]
- De La Garza R, 2nd, Fabrizio KR, Gupta A. Relevance of rodent models of intravenous MDMA self-administration to human MDMA consumption patterns. Psychopharmacology (Berl) 2007;189:425–434. doi: 10.1007/s00213-005-0255-5. [DOI] [PubMed] [Google Scholar]
- Docherty JR, Green AR. The role of monoamines in the changes in body temperature induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and its derivatives. Br J Pharmacol. 2010;160:1029–1044. doi: 10.1111/j.1476-5381.2010.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GP, McDonough ET, Bost RO. ‘Eve’ and ‘Ecstasy’. A report of five deaths associated with the use of MDEA and MDMA. J Am Med Assoc. 1987;257:1615–1617. doi: 10.1001/jama.257.12.1615. [DOI] [PubMed] [Google Scholar]
- Dybdal-Hargreaves NF, Holder ND, Ottoson PE, Sweeney MD, Williams T. Mephedrone: public health risk, mechanisms of action, and behavioral effects. Eur J Pharmacol. 2013;714:32–40. doi: 10.1016/j.ejphar.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Dyer C. Mephedrone is an amphetamine ‘by another name,’ drug adviser tells MPs. BMJ. 2010;340:c1690. doi: 10.1136/bmj.c1690. [DOI] [PubMed] [Google Scholar]
- EMCDDA. 2010. Annual report 2010: the state of the drugs problem in Europe. Available at: http://www.emcdda.europa.eu/publications/annual-report/2010(accessed 21/3/2014)
- EMCDDA. 2011. Report on the risk assessment of mephedrone in the framework of the Council Decision on new psychoactive substances. Available at: http://www.emcdda.europa.eu/html.cfm/index116639EN.html(accessed 21/3/2014)
- EROWID. 2013. Doses. Available at: http://www.erowid.org/chemicals/4_methylmethcathinone/4_methylmethcathinone_dose.shtml(Accessed 21/3/2014)
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in receptor and transported interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, De la Garza R, 2nd, Woods JH. Serotonin synthesis inhibition reveals distinct mechanisms of action for MDMA and its enantiomers in the mouse. Psychopharmacology (Berl) 2005;181:529–536. doi: 10.1007/s00213-005-0005-8. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Garrett G, Sweeney M. The serotonin syndrome as a result of mephedrone toxicity. BMJ Case Rep. 2010 doi: 10.1136/bcr.04.2010.2925. doi: 10.1136/bcr.042010.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Grahame-Smith DG. Effects of drugs on the processes regulating the functional activity of brain 5-hydroxytryptamine. Nature. 1976;260:487–491. doi: 10.1038/260487a0. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Green AR, O'Shea E, Saadat KS, Elliott JM, Colado MI. Studies on the effect of MDMA (‘ecstasy’) on the body temperature of rats housed at different ambient room temperatures. Br J Pharmacol. 2005;146:306–312. doi: 10.1038/sj.bjp.0706318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, King MV, Shortall SE, Fone KC. Lost in translation: preclinical studies on MDMA provide information on mechanisms of action, but do not allow accurate prediction of adverse events in humans. Br J Pharmacol. 2012a;166:1523–1536. doi: 10.1111/j.1476-5381.2011.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, King MV, Shortall SE, Fone KC. Ecstasy cannot be assumed to be 3,4-methylenedioxyamphetamine (MDMA) Br J Pharmacol. 2012b;166:1521–1522. [Google Scholar]
- Gregg RA, Tallarida CS, Reitz A, McCurdy C, Rawls SM. Mephedrone (4-methylmethcathinone), a principal constituent of psychoactive bath salts, produces behavioral sensitization in rats. Drug Alcohol Depend. 2013;133:746–750. doi: 10.1016/j.drugalcdep.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, et al. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggin P. 2012. Obama signs federal ban on ‘bath salt’ drugs. TIME online. Available at: http://newsfeed.time.com/2012/07/10/obama-signs-federal-ban-on-bath-salt-drugs(accessed 17/9/2013)
- Halpern P, Moskovich J, Avrahami B, Bentur Y, Soffer D, Peleg K. Morbidity associated with MDMA (ecstasy) abuse: a survey of emergency department admissions. Hum Exp Toxicol. 2011;30:259–266. doi: 10.1177/0960327110370984. [DOI] [PubMed] [Google Scholar]
- Hargreaves GA, Hunt GE, Cornish JL, McGregor IS. High ambient temperature increases 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’)-induced Fos expression in a region-specific manner. Neuroscience. 2007;145:764–774. doi: 10.1016/j.neuroscience.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Henry JA, Jeffreys KJ, Dawling S. Toxicity and deaths from 3,4 methylenedioxymethamphetamine (‘ecstasy’) Lancet. 1992;340:384–387. doi: 10.1016/0140-6736(92)91469-o. [DOI] [PubMed] [Google Scholar]
- Ho YJ, Pawlak CR, Guo L, Schwarting RK. Acute and long-term consequences of single MDMA administration in relation to individual anxiety levels in the rat. Behav Brain Res. 2004;149:135–144. doi: 10.1016/s0166-4328(03)00220-1. [DOI] [PubMed] [Google Scholar]
- Hollander den B, Rozov S, Linden AM, Uusi-Oukari M, Ojanperä I, Korpi ER. Long-term cognitive and neurochemical effects of ‘bath salt’ designer drugs methylone and mephedrone. Pharmacol Biochem Behav. 2013;103:501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Brugger R, Simmler LD, Bruggisser M, Donzelli M, Grouzmann E, et al. Effects of the α2-adrenergic agonist clonidine on the pharmacodynamics and pharmacokinetics of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Pharmacol Exp Ther. 2012a;340:286–294. doi: 10.1124/jpet.111.188425. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Ineichen M, Grouzmann E, Hoener MC, Brenneisen R, et al. The norepinephrine transporter inhibitor reboxetine reduces stimulant effects of MDMA (‘ecstasy’) in humans. Clin Pharmacol Ther. 2012b;90:246–255. doi: 10.1038/clpt.2011.78. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Nicola VG, Vischer N, Donzelli M, Krähenbühl S, et al. Duloxetine inhibits effects of MDMA (‘ecstasy’) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS ONE. 2012c;7:e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, et al. Clinical characteristics of mephedrone toxicity reported to the U.K. National Poisons Information Service. Emerg Med J. 2011;28:686–689. doi: 10.1136/emj.2010.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EA, O'Callaghan JP, Miller DB. Chronic treatment with supraphysiological levels of corticosterone enhances D-MDMA-induced dopaminergic neurotoxicity in the C57BL/6J female mouse. Brain Res. 2002a;933:130–138. doi: 10.1016/s0006-8993(02)02310-7. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Shvedova AA, Kisin E, O'Callaghan JP, Kommineni C, Miller DB. d-MDMA during vitamin E deficiency: effects on dopaminergic neurotoxicity and hepatotoxicity. Brain Res. 2002b;933:150–163. doi: 10.1016/s0006-8993(02)02313-2. [DOI] [PubMed] [Google Scholar]
- Joksovic P, Mellos N, Wattum van PJ, Chiles C. ‘Bath salts’-induced psychosis and serotonin toxicity. J Clin Psychiatry. 2012;73:1125. doi: 10.4088/JCP.12cr07819. [DOI] [PubMed] [Google Scholar]
- Jones S, Fileccia EL, Murphy M, Fowler MJ, King MV, Shortall SE, et al. Cathinone increases body temperature, enhances locomotor activity, and induces striatal c-fos expression in the Siberian hamster. Neurosci Lett. 2014;559:34–38. doi: 10.1016/j.neulet.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, et al. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- Kovacs K. Measurement of immediate-early gene activation-c-fos and beyond. J Neuroendocrinol. 2008;20:665–672. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- Laws KR, Kokkalis J. Ecstasy (MDMA) and memory function: a meta-analytic update. Hum Psychopharmacol. 2007;22:381–388. doi: 10.1002/hup.857. [DOI] [PubMed] [Google Scholar]
- Leonardi ET, Azmitia EC. MDMA (ecstasy) inhibition of MAO type A and type B; comparisons with fenfluramine and fluoxetine (Prozac) Neuropsychopharmacology. 1994;10:231–238. doi: 10.1038/npp.1994.26. [DOI] [PubMed] [Google Scholar]
- Lester SJ, Baggott M, Welm S, Schiller NB, Jones RT, Foster E, et al. Cardiovascular effects of 3,4-methylenedioxymethamphetamine: a double blind, placebo-controlled trial. Ann Intern Med. 2000;133:969–973. doi: 10.7326/0003-4819-133-12-200012190-00012. [DOI] [PubMed] [Google Scholar]
- Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, et al. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. Br J Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan BJ, Laverty R, Sanderson WD, Yee YB. Differences between rats and mice in MDMA (methylenedioxymethylamphetamine) neurotoxicity. Eur J Pharmacol. 1988;152:227–234. doi: 10.1016/0014-2999(88)90717-0. [DOI] [PubMed] [Google Scholar]
- Martínez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur Neuropsychopharmacol. 2012;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Martínez-Clemente J, López-Arnau R, Carbó M, Pubill D, Camarasa J, Escubedo E. Mephedrone pharmacokinetics after intravenous and oral administration in rats: relation to pharmacodynamics. Psychopharmacology (Berl) 2013;229:295–306. doi: 10.1007/s00213-013-3108-7. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in ‘bath salts’ on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell PD, De Paoli G, Seneviratne C, Pounder DJ. Mephedrone (4-methylmethcathinone)-related deaths. J Anal Toxicol. 2011;35:188–191. doi: 10.1093/anatox/35.3.188. [DOI] [PubMed] [Google Scholar]
- Measham F, Moore K, Newcombe R, Smith Z. Tweaking, bombing, dabbing and stockpiling: the emergence of mephedrone and the perversity of prohibition. Drugs Alcohol Today. 2010;10:14–21. [Google Scholar]
- Meng H, Cao J, Kang J, Ying X, Ji J, Reynolds W, et al. Mephedrone, a new designer drug of abuse, produces acute hemodynamic effects in the rat. Toxicol Lett. 2012;208:62–68. doi: 10.1016/j.toxlet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Wilhelm J, Peters FT, Maurer HH. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal Bioanal Chem. 2010;397:1225–1233. doi: 10.1007/s00216-010-3636-5. [DOI] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol. 1995;11:177–192. doi: 10.1007/BF02740694. [DOI] [PubMed] [Google Scholar]
- Miller ML, Creehan KM, Angrish D, Barlow DJ, Houseknecht KL, Dickerson TJ, et al. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone) Drug Alcohol Depend. 2013;127:248–253. doi: 10.1016/j.drugalcdep.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K, Dargan PI, Wood DM, Measham F. Do novel psychoactive substances displace established club drugs, supplement them or act as drugs of initiation? The relationship between mephedrone, ecstasy and cocaine. Eur Addict Res. 2013;19:276–282. doi: 10.1159/000346678. [DOI] [PubMed] [Google Scholar]
- Morris K. UK places generic ban on mephedrone drug family. Lancet. 2010;375:1333–1334. doi: 10.1016/s0140-6736(10)60559-4. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Karanges E, Li KM, Wilkinson S, Winstock AR, Ramsey J, et al. Mephedrone in adolescent rats: residual memory impairment and acute but not lasting 5-HT depletion. PLoS ONE. 2012a;7:e45473. doi: 10.1371/journal.pone.0045473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, ‘meow’): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict Biol. 2012b;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Clemens K, Apetz N, Winstock AR, Ramsey J, Kong KM, et al. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: neural consequences and comparison with methamphetamine. J Psychopharmacol. 2013;27:823–836. doi: 10.1177/0269881113490325. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Kamimura K, Satoh H. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Neff NH, Lin RC, Ngai SH, Costa E. Turnover rate measurements of brain serotonin in unanesthetized rats. Adv Biochem Psychopharmacol. 1969;1:91–109. [PubMed] [Google Scholar]
- O'Cain PA, Hletko SB, Ogden BA, Varner KJ. Cardiovascular and sympathetic responses and reflex changes elicited by MDMA. Physiol Behav. 2000;70:141–148. doi: 10.1016/s0031-9384(00)00235-3. [DOI] [PubMed] [Google Scholar]
- O'Shea E, Granados R, Esteban B, Colado MI, Green AR. The relationship between the degree of neurodegeneration of rat brain 5-HT nerve terminals and the dose and frequency of administration of MDMA (‘ecstasy’) Neuropharmacology. 1998;37:919–926. doi: 10.1016/s0028-3908(98)00029-x. [DOI] [PubMed] [Google Scholar]
- O'Shea E, Esteban B, Camarero J, Green AR, Colado MI. Effect of GBR 12909 and fluoxetine on the acute and long term changes induced by MDMA (‘ecstasy’) on the 5-HT and dopamine concentrations in mouse brain. Neuropharmacology. 2001;40:65–74. doi: 10.1016/s0028-3908(00)00106-4. [DOI] [PubMed] [Google Scholar]
- O'Shea E, Escobedo I, Orio L, Sanchez V, Navarro M, Green AR, et al. Elevation of ambient room temperature has differential effects on MDMA-induced 5-HT and dopamine release in striatum and nucleus accumbens of rats. Neuropsychopharmacology. 2005;30:1312–1323. doi: 10.1038/sj.npp.1300673. [DOI] [PubMed] [Google Scholar]
- O'Shea E, Orio L, Escobedo I, Sanchez V, Camarero J, Green AR, et al. MDMA-induced neurotoxicity: long-term effects on 5-HT biosynthesis and the influence of ambient temperature. Br J Pharmacol. 2006;148:778–785. doi: 10.1038/sj.bjp.0706783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. MDMA and temperature: a review of the thermal effects of ‘Ecstasy’ in humans. Drug Alcohol Depend. 2012a;121:1–9. doi: 10.1016/j.drugalcdep.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Parrott AC. MDMA and 5-HT neurotoxicity: the empirical evidence for its adverse effects in humans – no need for translation. Br J Pharmacol. 2012b;166:1518–1520. doi: 10.1111/j.1476-5381.2012.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. MDMA, serotonergic neurotoxicity, and the diverse functional deficits of recreational ‘Ecstasy’ users. Neurosci Biobehav Rev. 2013;37:1466–1484. doi: 10.1016/j.neubiorev.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Pedersen AJ, Reitzel LA, Johansen SS, Linnet K. In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test Anal. 2012;5:430–438. doi: 10.1002/dta.1369. [DOI] [PubMed] [Google Scholar]
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–8865. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Meyer JS. Memory deficit and reduced anxiety in young adult rats given repeated intermittent MDMA treatment during the periadolescent period. Pharmacol Biochem Behav. 2004;79:723–731. doi: 10.1016/j.pbb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasimas JJ. ‘Bath salts’ and the return of the serotonin syndrome. J Clin Psychiatry. 2013;73:1126–1127. doi: 10.4088/JCP.12com07965. [DOI] [PubMed] [Google Scholar]
- Redfern WS, MacLean MR, Clague RU, McGrath JC. The role of alpha 2-adrenoceptors in the vasculature of the rat tail. Br J Pharmacol. 1995;114:1724–1730. doi: 10.1111/j.1476-5381.1995.tb14963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan L, Mitchelson M, Macdonald C. Mephedrone toxicity in a Scottish emergency department. Emerg Med J. 2011;28:1055–1058. doi: 10.1136/emj.2010.103093. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Masten VL, Caldwell S, Paulus MP, Low MJ, Geyer MA. Differential contributions of dopamine D1, D2, and D3 receptors to MDMA-induced effects on locomotor behavior patterns in mice. Neuropsychopharmacology. 2006;31:2349–2358. doi: 10.1038/sj.npp.1301161. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga CJ. Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behav Brain Res. 2012;234:76–81. doi: 10.1016/j.bbr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhold RW, Carlton FB, Jr, Corkern R, Derouen L, Bennett JG, Hume AS. Methcathinone intoxication in the rat: abrogation by dextrorphan. Ann Emerg Med. 1997;29:383–391. doi: 10.1016/s0196-0644(97)70351-2. [DOI] [PubMed] [Google Scholar]
- Rodsiri R, Spicer C, Green AR, Marsden CA, Fone KC. Acute concomitant effects of MDMA binge dosing on extracellular 5-HT, locomotion and body temperature and the long-term effect on novel object discrimination in rats. Psychopharmacology (Berl) 2011;213:365–376. doi: 10.1007/s00213-010-1921-9. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Sare J. How the media helped ban mephedrone. Br Med J. 2011;342:d1138. doi: 10.1136/bmj.d1138. [DOI] [PubMed] [Google Scholar]
- Schenk S. MDMA self-administration in laboratory animals: a summary of the literature and proposal for future research. Neuropsychobiology. 2009;60:130–136. doi: 10.1159/000253549. [DOI] [PubMed] [Google Scholar]
- Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, et al. Mephedrone (4-methylmethcathinone; ‘meow meow’): chemical, pharmacological and clinical issues. Psychopharmacology (Berl) 2011;214:593–602. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- Schifano F, Corkery J, Ghodse AH. Suspected and confirmed fatalities associated with mephedrone (4-methylmethcathinone, ‘meow meow’) in the United Kingdom. J Clin Psychopharmacol. 2012;32:710–714. doi: 10.1097/JCP.0b013e318266c70c. [DOI] [PubMed] [Google Scholar]
- Shortall SE, Green AR, Swift KM, Fone KC, King MV. Differential effects of cathinone compounds and MDMA on body temperature in the rat, and pharmacological characterization of mephedrone-induced hypothermia. Br J Pharmacol. 2013a;168:966–977. doi: 10.1111/j.1476-5381.2012.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortall SE, Byatt S, Kaufman N, Lipman H, Smith G, Green AR, et al. Caffeine alters the behavioural and thermoregulatory responses to mephedrone without causing long-term neurotoxicity. J Psychopharmacol. 2013b;27:A63. doi: 10.1177/0269881116650408. [DOI] [PubMed] [Google Scholar]
- Shortall SE, Macerola AE, Swaby RT, Jayson R, Korsah C, Pillidge KE, et al. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur Neuropsychopharmacol. 2013c;23:1085–1095. doi: 10.1016/j.euroneuro.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitte HH, Freissmuth M. The reverse operation of Na(+)/Cl(–)-coupled neurotransmitter transporters – why amphetamines take two to tango. J Neurochem. 2010;112:340–355. doi: 10.1111/j.1471-4159.2009.06474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BJ, Moon KH, Upreti VV, Eddington ND, Lee IJ. Mechanisms of MDMA (ecstasy)-induced oxidative stress, mitochondrial dysfunction, and organ damage. Curr Pharm Biotechnol. 2010;11:434–443. doi: 10.2174/138920110791591436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tariq M, Islam MW, al-Meshal IA, el-Feraly FS, Ageel AM. Comparative study of cathinone and amphetamine on brown adipose thermogenesis. Life Sci. 1989;44:951–955. doi: 10.1016/0024-3205(89)90494-3. [DOI] [PubMed] [Google Scholar]
- Tucker GT, Lennard MS, Ellis SW, Woods HF, Cho AK, Lin LY, et al. The demethylenation of methylenedioxymethamphetamine (‘ecstasy’) by debrisoquine hydroxylase (CYP2D6) Biochem Pharmacol. 1994;47:1151–1156. doi: 10.1016/0006-2952(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Van Amsterdam J, Nutt D, Brink van den W. Generic legislation of new psychoactive drugs. J Psychopharmacol. 2013;27:317–324. doi: 10.1177/0269881112474525. [DOI] [PubMed] [Google Scholar]
- Vanattou-Saïfoudine N, McNamara R, Harkin A. Caffeine provokes adverse interactions with 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) and related psychostimulants: mechanisms and mediators. Br J Pharmacol. 2012;167:946–959. doi: 10.1111/j.1476-5381.2012.02065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou CH. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2011;201:191–195. doi: 10.1016/j.toxlet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Varner KJ, Daigle K, Weed PF, Lewis PB, Mahne SE, Sankaranarayanan A, et al. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology (Berl) 2013;225:675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbaten MN. Specific memory deficits in ecstasy users? The results of a meta-analysis. Hum Psychopharmacol. 2003;18:281–290. doi: 10.1002/hup.482. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Remensberger S, Hell D, Geyer MA. Opposite effects of 3,4-methylenedioxymethamphetamine (MDMA) on sensorimotor gating in rats versus healthy humans. Psychopharmacology (Berl) 1999;143:365–372. doi: 10.1007/s002130050960. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011a;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011b;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wood DM, Dargan PI. Mephedrone (4-methylmethcathinone): what is new in our understanding of its use and toxicity. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:227–233. doi: 10.1016/j.pnpbp.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Wood DM, Davies S, Greene SL, Button J, Holt DW, Ramsey J, et al. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin Toxicol (Phila) 2010;48:924–927. doi: 10.3109/15563650.2010.531021. [DOI] [PubMed] [Google Scholar]
- Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J. 2011;28:280–282. doi: 10.1136/emj.2010.092288. [DOI] [PubMed] [Google Scholar]
- Wood DM, Hunter L, Measham F, Dargan PI. Limited use of novel psychoactive substances in South London nightclubs. QJM. 2012;105:959–964. doi: 10.1093/qjmed/hcs107. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, et al. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS ONE. 2012a;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Vandewater SA, Angrish D, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) and d-methamphetamine improve visuospatial associative memory, but not spatial working memory, in rhesus macaques. Br J Pharmacol. 2012b;167:1342–1352. doi: 10.1111/j.1476-5381.2012.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawilska JB, Wojcieszak J. Designer cathinones – an emerging class of novel recreational drugs. Forensic Sci Int. 2013;231:42–53. doi: 10.1016/j.forsciint.2013.04.015. [DOI] [PubMed] [Google Scholar]