Abstract
Background and Purpose
Type 2 diabetes impairs the healing process because of an exaggerated and persistent inflammatory response, and an altered expression pattern of angiogenic molecules. We investigated the effects of inflammasome blockade in diabetes-related wound-healings defects, in genetically diabetic mice.
Experimental Approach
An incisional skin wound model was produced on the back of female diabetic C57BL/KsJ-m +/+ Leptdb mice (db+/db+) and their normal littermates (db+/m+). Animals were treated daily with two inflammasome blocking agents, BAY 11-7082 (20 mg·kg−1 i.p.), or Brilliant Blue G (BBG, 45.5 mg·kg−1 i.p.), or vehicle. Mice were killed on 3, 6 and 12 days after skin injury to measure expression of the NOD-like receptor NLRP3, caspase-1, VEGF, the inflammasome adapter protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) and the chemokine CXCL12. Wound levels of IL-1β and IL-18 were also measured, along with histological assessments of wound tissue and the time to complete wound closure.
Key Results
During healing, the diabetic mice exhibited increased activation of NLRP3, caspase-1, ASC, IL-1β and IL-18. They also showed a reduced expression of VEGF and CXCL12.Treatment with BAY 11-7082 or BBG, to block activation of the inflammasome, decreased the levels of pro-inflammatory molecules. Histological evaluation indicated that inflammasome blockade improved the impaired healing pattern, at day 12 in diabetic mice, along with a decreased time to complete skin healing.
Conclusions and Implications
These data strongly suggest that activation of the NLRP3 inflammasome is one of the key contributors to the delayed healing of wounds in diabetic mice.
Keywords: inflammasome, diabetes, wound healing
Introduction
A disturbed wound-healing process characterizes diabetes and may enhance the overall morbidity and mortality of type 2 diabetic patients (Falanga, 2005; Singh et al., 2005). Generally, skin repair includes a complex programmed sequence of cellular and molecular processes including inflammation, cell migration, angiogenesis, provisional matrix synthesis, collagen deposition and re-epithelization (Falanga, 2005; Reiber and Raugi, 2005). Angiogenesis plays a central role in wound healing and is associated with expression of several cytokines and angiogenic factors, such as VEGF and the chemokine CXCL12 (SDF-1α) (Ferrara et al., 2003; Tammela et al., 2005). Healing impairment in diabetics is characterized by an increased and persistent inflammatory response, which in turn delays cellular infiltration and granulation tissue formation, with a consequently decreased collagen organization and reduced angiogenesis (Goodson and Hunt, 1977; Bohlen and Niggl, 1979).
The inflammasomes are multiprotein oligomers that respond to inflammatory stimuli by initiating an intracellular inflammatory cascade, particularly by increasing the cleavage of the inactive precursor of IL-1β to its active form (Schroder and Tschopp, 2010). One of the largest cytosolic inflammasomes is the NLRP3 inflammasome which comprises the NOD-like receptor NLRP3, the adapter protein termed ‘apoptosis-associated speck-like protein containing a caspase recruitment domain’ (ASC) and procaspase-1. During type 2 diabetes, an increased accumulation of endogenous damage-associated molecular pattern molecules (DAMPs), such as free cholesterol and membrane complex lipids (i.e. ceramides), serve as activators of the NLRP3 inflammasome. These activators cause inflammasome assembly by interactions in the protein domains of NLRP3, ASC and procaspase-1, with subsequent autolytic cleavage of the inactive pro-caspase into its active form. Active caspase-1 then can cleave the inactive stored forms of IL-1 and IL-18 into the active cytokines, which further cause insulin resistance and organ dysfunction. IL-1β is predominantly produced by monocytes, macrophages and neutrophils, and is implicated in the pathogenesis of many inflammatory diseases including diabetes (Dinarello, 2011). In addition, activation of the membrane-bound purinergic P2X7 receptor participates in the processing and release of IL-1β, and this receptor has been proposed as the upstream activator of NLRP3 inflammasome assembly (Ferrari et al., 2006).
Our group has already demonstrated the efficacy of several therapeutic approaches in improving the altered healing and increasing angiogenesis, in the well known db/db mouse model (Altavilla et al., 2001; 2011; Galeano et al., 2001; 2003; 2008; 2011,). In this study, we evaluated the effects of inflammasome NLRP3 blockade, as a new strategy to ameliorate wound healing in this model.
Methods
Animals
All animal care and experimental procedures were in accordance with the Principles of Laboratory Animal Care (NIH publication no.85-23, revised 1985) and authorized by our Local Institution. They are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 111 animals were used in the experiments described here.
Genetically diabetic female (30–35 g) C57BL/KsJ-m +/+ Leptdb mice (db+/db+) and their normal littermates (22–25 g; db+/m+), were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Animals were 10 weeks old at the start of the experiments. During the experiments, the animals were kept one per cage, under controlled environmental conditions (12 h light/dark cycle, 23°C), and provided with standard food and water ad libitum. After general anaesthesia with sodium pentobarbital (80 mg·kg−1 i.p.), the hair on the back was shaved and two parallel 4 cm incisions were produced with a scalpel (Figure 1A), on the back of all mice as previously described (Altavilla et al., 2001; 2011; Galeano et al., 2001; 2003; 2008; 2011,).
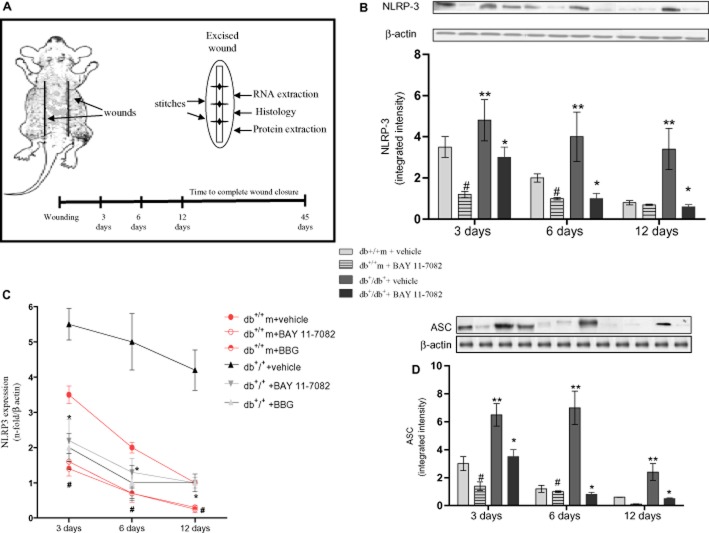
Figure 1.
(A) The diagram represents the back of a mouse with the site of the two wounds. The right hand side shows an excised wound (ellipse shape), subdivided to perform the analyses. At the bottom the experimental timeline is shown. (B) NLRP3 protein expression in skin wound samples, collected at 3, 6 and 12 days, from either normoglycaemic (db+/m+) and diabetic (db+/db+) mice, given either vehicle or BAY 11-7082 (20 mg·kg−1 i.p.). Each bar represents the mean ± SD of seven animals. #P < 0.01 versus db+/m+ + vehicle; **P < 0.01 versus db+/m+ + vehicle; *P < 0.05 versus db+/db+ + vehicle. (C) NLRP3 mRNA expression in skin wound samples, collected at 3, 6 and 12 days, from either normoglycaemic (db+/m+) and diabetic (db+/db+) mice, given either vehicle or BAY 11-7082 (20 mg·kg−1 i.p.) or BBG (45.5 mg·kg−1 i.p.). Each bar represents the mean ± SD of seven animals. #P < 0.01 versus db+/m+ + vehicle; *P < 0.05 versus db+/db+ + vehicle. (D) ASC protein expression in skin wound samples, collected at 3, 6 and 12 days, from either normoglycaemic (db+/m+) and diabetic (db+/db+) mice, given either vehicle or BAY 11-7082 (20 mg·kg−1 i.p.). Each bar represents the mean ± SD of seven animals. #P < 0.01 versus db+/m+ + vehicle; **P < 0.005 versus db+/m+ + vehicle; * P < 0.05 versus db+/db+ + vehicle.
Normoglycaemic and diabetic mice, received either BAY 11-7082 (20 mg·kg−1 day i.p.), or Brilliant Blue G (45.5 mg·kg−1 i.p.) every 2 days, or vehicle (a 1:3 solution of DMSO and 0.9% NaCl) for up to 12 days. Ten animals from each strain and treatment arm were killed after 3, 6 and 12 days after wounding and the wounds removed using a scalpel to cut the shape of an ellipse around the lesion (Figure 1A). Of the two wounds, one was used for Western blot, gene expression and elisa assays, and the other one for histology. Other groups of normoglycaemic and diabetic mice (n = 21 in each group) were subjected to wounding and used to measure the time needed to complete wound closure (up to 45 days).
Expression of NLRP3, caspase-1, ASC, and VEGF by Western blot analysis
Expression of NLRP3, caspase-1 and VEGF proteins was evaluated at day 3, 6 and 12 days by Western Blot, as previously described (Galeano et al., 2008; 2011,; Altavilla et al., 2011). Primary antibodies for NLRP3 and VEGF were purchased from Abcam (Cambridge, UK), pro-caspase-1 and caspase-1 were obtained from Cell Signaling (Danvers, MA), ASC was purchased from Millipore (Billerica, MA, USA). Secondary peroxidase conjugated antibodies were obtained by Thermo Scientific (Waltham, MA, USA). The protein signals were visualized by chemiluminescence (ECL plus, Thermo Scientific), quantified by scanning densitometry, using a bio-image analysis system (Bio-Profil Celbio, Milan, Italy), and were expressed as integrated intensity, relative to β-actin (Cell Signaling), measured on stripped blots.
Determination of IL-1β and IL-18 in wound lysates
The wound lysates were prepared, at 3, 6 and 12 days, by homogenizing wound tissue (30 mg) in 1 mL PBS, pH 7.4 using an Ultra Turrax (IKA, Staufen, Germany) homogenizer. The homogenate was centrifuged at 15,000 × g for 15 min at 4 °C. The supernatant was collected and used for elisa assays. IL-1β and IL-18 in these lysates were determined with commercially available mouse-specific elisa assay kits (from Abcam and eBioscience Inc., San Diego, CA, USA; respectively) and following the manufacturer's instructions. IL-1β (detection range 2.74–2000 pg·mL−1) and IL-18 (detection range 31.3–2000 pg·mL−1) levels were expressed as pg·mL−1 of wound lysate.
Levels of mRNA for NLRP3, pro-caspase-1, and CXCL12
Total RNA was extracted from wounds by cell lysis with Trizol (Invitrogen, Carlsbad, CA, USA), and quantified as described previously (Galeano et al., 2008). RNA was reverse transcribed to cDNA and used to quantify the amount of NLRP3, pro-caspase-1 and CXCL12 mRNA by real-time PCR using TaqMan probes (Applied Biosystems, Foster City, CA, USA); β-actin was used as endogenous control. The results were expressed as the n-fold difference relative to controls (relative expression levels).
Histological examination and time to complete wound closure
Wound samples were fixed in 10% neutral buffered formalin, and processed as previously described (Galeano et al., 2008). All slides were examined by a pathologist, unaware of the treatment, using an eye-piece grid under the microscope from ×20 to ×100 magnification. The following parameters were evaluated and graded for re-epithelialization, dermal matrix deposition and regeneration, granulation tissue formation and remodelling. The histological specimens were scored as earlier reported (Altavilla et al., 2011; Galeano et al., 2011). The time to complete wound closure, as shown by a closed linear healing ridge, was determined as described previously (Altavilla et al., 2011; Galeano et al., 2011).
Data analysis
All data were expressed as means (± SD). Comparisons between different treatment groups were analysed by one-way anova followed by Tukey's multiple comparison test. In all cases, P < 0.05 was selected as the criterion for statistical significance. Graphs were drawn using GraphPad Prism (La Jolla, CA, USA; version 5.0 for Windows).
Materials
The following compounds were supplied as shown: BAY 11-7082 by Adipogene (San Diego, CA); BBG by Sigma Aldrich (Milan, Italy) and sodium pentobarbital by Intervet (Milan, Italy).
Results
Inflammasome blockade and activation of downstream pathways
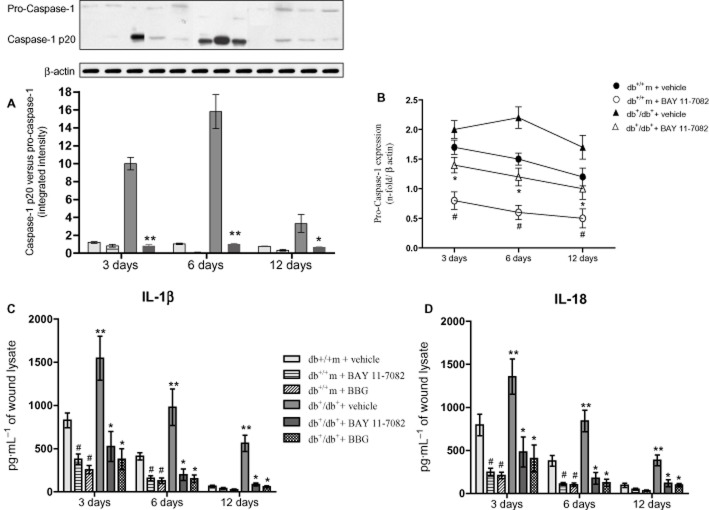
Diabetic animals exhibited an increased and persistent activation of the NLRP3 inflammasome during the 12 day observation period (Figure 1B; P < 0.01 vs. db+/m+). NLRP3 blockade either directly by BAY 11-7082 or via the upstream receptor, by BBG, was confirmed by the blunted protein and mRNA expression in either normoglycaemic and db+/db+ mice (Figure 1B,C; P < 0.05), at all time points. As a direct consequence of NLRP3 blockade, a reduction in ASC activation (Figure 1D) was observed, at all time points. Also p20 caspase-1, which is proteolytically activated from a pro-enzyme, was also significantly reduced in BAY 11-7082-treated diabetic and normoglycaemic animals (Figure 2A, P < 0.05). Pro-caspase-1 mRNA expression was also significantly reduced in BAY 11-7082-treated diabetic and normoglycaemic animals (Figure 2B). Raised levels of the active forms of IL-1β and IL-18 in wound lysates were sustained at 3, 6 and 12 days in db+/db+ animals, compared with controls (Figure 2C,D; P < 0.01). NLRP3 blockade either by BAY 11-7082 or by BBG markedly reduced both cytokines in wound lysates, at each time point (Figure 2C,D; P < 0.05).
Figure 2.
(A) Caspase-1 active protein (p20) versus caspase-1 expression in skin wound samples collected at 3, 6 and 12 days from either normoglycaemic (db+/m+) and diabetic mice (db+/db+) given either vehicle or BAY 11-7082 (20 mg·kg−1 i.p.). Each bar represents the mean ± SD of seven animals. #P < 0.01 versus db+/m+ + vehicle; **P = 0.0178 versus db+/db+ + vehicle; * P < 0.05 versus db+/db+ + vehicle. (B) Pro-caspase-1 mRNA expression in skin wound samples collected at 3, 6 and 12 days from either normoglycaemic (db+/m+) and diabetic mice (db+/db+) given either vehicle or BAY 11-7082 (20 mg·kg−1 i.p.). Each bar represents the mean ± SD of seven animals. #P < 0.01 versus db+/m+ + vehicle; *P < 0.05 versus db+/db+ + vehicle. (C) IL-1β protein levels studied by elisa in skin wound samples collected at 3, 6 and 12 days from either normoglycaemic (db+/m+) and diabetic mice (db+/db+) given either vehicle, or BAY 11-7082 (20 mg·kg−1 i.p.), or BBG (45.5 mg kg−1 i.p.). Each bar represents the mean ± SD of seven animals. #P < 0.01 versus db+/m+ + vehicle; **P < 0.005 versus db+/m+ + vehicle; *P < 0.05 versus db+/db+ + vehicle. (D) IL-18 protein levels studied by elisa in skin wound samples collected at 3, 6 and 12 days from either normoglycaemic (db+/m+) and diabetic mice (db+/db+) given either vehicle, or BAY 11-7082 (20 mg·kg−1 i.p.), or BBG (45.5 mg·kg−1 i.p.). Each bar represents the mean ± SD of seven animals. #P < 0.01 versus db+/m+ + vehicle; **P < 0.005 versus db+/m+ + vehicle; * P < 0.05 versus db+/db+ + vehicle.
VEGF and CXCL12 expression in wounds
In the wounds of non-diabetic animals, high levels of VEGF protein and CXCL12 mRNA (Figure 3A, B respectively) were found, especially at days 3 and 6, with CXCL12 expression remaining high at day 12.
Figure 3.
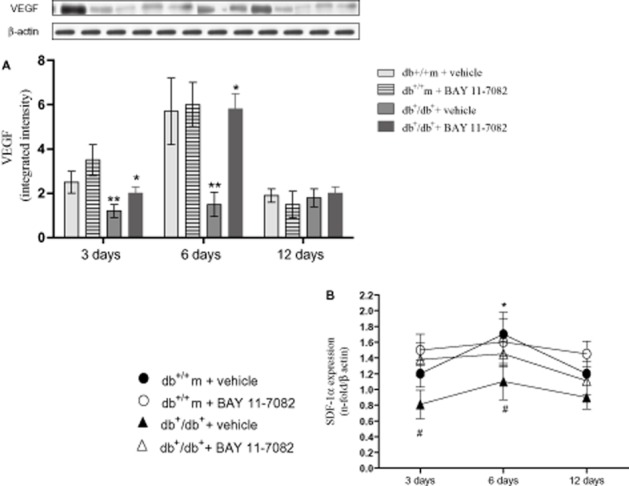
(A) VEGF expression in skin wound samples collected at 3, 6 and 12 days from either normoglycaemic (db+/m+) and diabetic mice (db+/db+) given either vehicle or BAY 11-7082 (20 mg·kg−1 i.p.). Each bar represents the mean ± SD of seven animals. **P < 0.01 versus db+/m+ + vehicle; * P < 0.05 versus db+/db+ + vehicle. (B) CXCL12 mRNA expression in skin wound samples collected at 3, 6 and 12 days from either normoglycaemic (db+/m+) and diabetic mice (db+/db+) given either vehicle, or BAY 11-7082 (20 mg·kg−1 i.p.). Each bar represents the mean ± SD of seven animals. #P < 0.01 versus db+/m+ + vehicle; *P < 0.05 versus db+/db+ + vehicle.
Wounds from diabetic mice showed a markedly reduced expression of CXCL12 mRNA, and protein levels of VEGF, compared with values from non-diabetic mice at days 3 and 6 following wounding. Treatment of diabetic mice with BAY 11-7082 enhanced expression of CXCL12 mRNA and of VEGF protein at days 3 and 6. (Figure 3A, B).
Histology
Figure 4 shows the histological scores (Figure 4A) of all groups, and representative histological pictures (Figure 4B–D) of diabetic animals treated with vehicle, or BAY 11-7082, or BBG at day 12. In normoglycaemic animals, the dermis remodelling and wound closure process were complete (Figure not shown). However, the administration of BAY 11-7082 or BBG further improved wound healing (Figure not shown).
Figure 4.
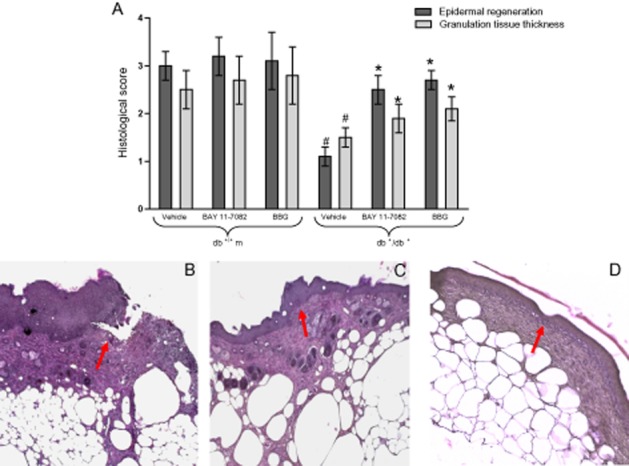
(A) Histological scores in wound samples, collected at 12 days, from either normoglycaemic (db+/m+) and diabetic mice (db+/db+) given either vehicle, or BAY 11-7082 (20 mg·kg−1 i.p.), or BBG (45.5 mg·kg−1 i.p.). Each bar represents the mean ± SD of seven animals. #P < 0.01 versus db+/m+; * P < 0.05 versus db+/db+ + vehicle. (B) Histological photomicrographs, haematoxylin-eosin stain, vehicle-treated diabetic (db+/db+) wounds. Incomplete re-epithelialization (see arrow), and poorly formed granulation tissue. (C) Histological photomicrographs, haematoxylin-eosin stain, BAY 11–7088 treated diabetic (db+/db+) wounds. Re-epithelialization is almost complete (see arrow), well-organized granulation tissue and no evidence of inflammation or oedema. (D) Histological photomicrographs, haematoxylin-eosin stain, BBG-treated diabetic (db+/db+) wounds. Re-epithelialization is complete (see arrow), the granulation tissue is well-organized and inflammation is absent.
Wounds of diabetic mice treated with vehicle showed poor to mild re-epithelialization at day 12, with only partly organized granulation tissue (Figure 4B). In contrast, moderate to complete re-epithelialization and well-formed granulation tissue were observed in the wounds of diabetic mice following NLRP3 blockade either with BAY 11-7082 or BBG (Figure 4C, D).
Wound closure
Complete wound closure was indicated by a closed linear ridge, with no open areas between stitches. Mice were observed for up to 45 days and complete wound closure was observed at day 22 ± 2 in vehicle-treated normoglycaemic animals, while wounds in diabetic mice closed completely only after 39 ± 3 days (Figure 5). Treatment of diabetic mice with BAY 11-7082 or BBG accelerated wound closure, reducing the time to complete closure to almost normoglycaemic values (Figure 5, P < 0.001 vs. vehicle).
Figure 5.
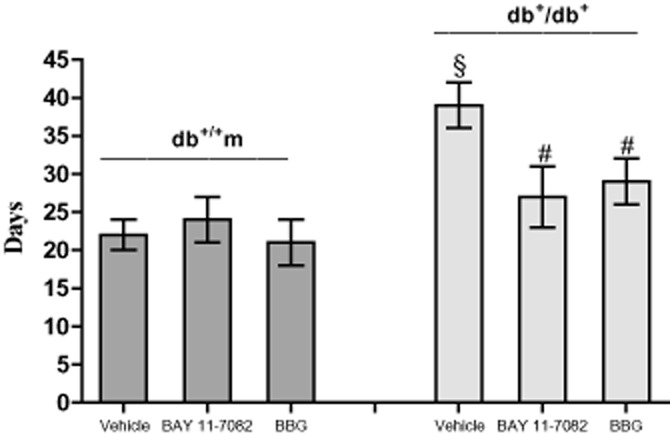
Time to complete the wound closure in normoglycaemic (db+/m+) and diabetic mice (db+/db+) given either vehicle, or BAY 11-7082 (20 mg·kg−1 i.p.), or BBG (45.5 mg·kg−1 i.p.). Each bar represents the mean ± SD of seven animals. §P < 0.001 versus db+/m+; #P < 0.001 versus db+/db+ + vehicle.
Discussion
The structure and function of the skin are maintained at the epidermal surface by an equilibrium between keratinocyte survival and death, which requires continual regeneration of this tissue. Among the early phases of a wound-healing response is infiltration of immune cells at the wound site (Martin and Leibovich, 2005). The accumulation of DAMPs during chronic inflammatory diseases is thought to contribute to systemic inflammation and disease pathogenesis. During the pathogenesis of type 2 diabetes, the NLRP3 inflammasome has been proposed to sense and mediate downstream inflammatory events of ‘glucotoxicity’ (Zhou et al., 2010), and thus be responsible for a constant pro-inflammatory status.
Our work clearly demonstrates the efficacy of NLRP3 inflammasome blockade on healing of wounds, via reduced activation of the inflammatory cascade. The I-κB kinase-β inhibitor, BAY 11-7082, selectively inhibited NLRP3 inflammasome activity in macrophages, independently of its inhibitory effect on NF-κB activity (Juliana et al., 2010). In our experiments, this inhibitor decreased the inflammatory pathways downstream of the NLRP3 inflammasome and, consequently, improved angiogenesis. However in a complex biological process such as wound healing, it is difficult to completely separate the role of NF-kB blockade from inflammasome blockade. In order to clarify this point, we used another way of blocking activation of NLRP3 inflammasomes, by blocking its upstream activator, the purinergic P2X7 receptor (Ferrari et al., 2006; Juliana et al., 2010; Zhao et al., 2013). This approach also resulted in inhibition of NLRP3 inflammasomes and in normalisation of the healing in diabetic mice.
In diabetes, the deficiency in neovascularization arises from inadequate VEGF production and release in wounds. Consequently, the normal angiogenic process is delayed and poorly functional. Disturbance of the mechanisms underlying angiogenesis and vasculogenesis, including altered VEGF and CXCL12 expression, has been previously described in db/db animals (Nguyen et al., 2010; Bitto et al., 2013). The present findings further strengthen these observations, suggesting that reduced inflammation at the wound site improves both angiogenesis and vasculogenesis, improving, in turn, the healing of wounds in the diabetic mice.
To our knowledge this is the first report that actually demonstrates a clear involvement of the NLRP3 inflammasome and its downstream cascade, in the impaired wound healing in obese-diabetic animals. Increased NLRP3 expression in adipose tissue has been correlated with obesity-associated insulin–resistance in type 2 diabetic patients and in obese mice, with strong caspase-1 auto activation and consequent IL-1β production in adipose tissue (Vandanmagsar et al., 2011). In agreement with these findings, we found that mice treated with BAY 11-7082 demonstrated a marked reduction in active caspase-1 expression and reduced IL-1β and IL-18 production, at the wound site. These cytokines were also markedly reduced when the upstream P2X7 receptor was pharmacologically blocked, emphasizing that activation of the NLRP3 inflammasome is one of the mechanisms involved in impaired healing during glucotoxicity.
All these effects normalised skin remodelling, as suggested by the histological data and reduced the time to complete closure, which represents the main outcome for any therapeutic intervention aimed at improving the healing of wounds in diabetics.
In conclusion, results from this study demonstrate for the first time, that direct inflammasome blockade can ameliorate the altered wound healing in diabetic mice, not only by blunting the inflammatory cascade, but also by increasing angiogenesis-related molecules, at least in obese-diabetic animals.
Glossary
- DAMPs
damage-associated molecular pattern molecules
- NLRP3
NOD-like receptor family, pyrin domain containing 3
Funding
This work has been supported by departmental funding assigned to Professor Francesco Squadrito.
Conflict of interest
None of the authors have any conflict of interest.
Significance of the work
The healing of wounds during diabetes is hardly impaired by an exaggerated inflammatory reaction as well as by a reduced expression of angiogenic molecules. The pharmacological blockade of the inflammasome could be a strategy to halt inflammation and increase angiogenesis, thus restoring the healing pattern.
References
- Altavilla D, Saitta A, Cucinotta D, Galeano M, Deodato B, Colonna M, et al. Inhibition of lipid peroxidation restores impaired vascular endothelial growth factor expression and stimulates wound healing and angiogenesis in the genetically diabetic mouse. Diabetes. 2001;50:667–674. doi: 10.2337/diabetes.50.3.667. [DOI] [PubMed] [Google Scholar]
- Altavilla D, Squadrito F, Polito F, Irrera N, Calò M, Lo Cascio P, et al. Activation of adenosine A2A receptors restores the altered cell-cycle machinery during impaired wound healing in genetically diabetic mice. Surgery. 2011;149:253–261. doi: 10.1016/j.surg.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Bitto A, Irrera N, Minutoli L, Calò M, Lo Cascio P, Caccia P, et al. Relaxin improves multiple markers of wound healing and ameliorates the disturbed healing pattern of the genetically diabetic mice. Clin Sci (Lond) 2013;125:575–585. doi: 10.1042/CS20130105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bohlen HG, Niggl BA. Adult microvascular disturbances as a result of juvenile-onset diabetes in db/db mice. Blood Vessels. 1979;16:269–276. doi: 10.1159/000158215. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Galeano M, Torre V, Deodato B, Campo GM, Colonna M, Sturiale A, et al. Raxofelast, a hydrophilic vitamin E-like antioxidant, stimulates wound healing in genetically diabetic mice. Surgery. 2001;129:467–477. doi: 10.1067/msy.2001.112072. [DOI] [PubMed] [Google Scholar]
- Galeano M, Deodato B, Altavilla D, Cucinotta D, Arsic N, Marini H, et al. Adeno-associated viral vector-mediated human vascular endothelial growth factor gene transfer stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetologia. 2003;46:546–555. doi: 10.1007/s00125-003-1064-1. [DOI] [PubMed] [Google Scholar]
- Galeano M, Bitto A, Altavilla D, Minutoli L, Polito F, Calò M, et al. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008;16:208–217. doi: 10.1111/j.1524-475X.2008.00361.x. [DOI] [PubMed] [Google Scholar]
- Galeano M, Polito F, Bitto A, Irrera N, Campo GM, Avenoso A, et al. Systemic administration of high-molecular weight hyaluronan stimulates wound healing in genetically diabetic mice. Biochim Biophys Acta. 2011;18127:752–759. doi: 10.1016/j.bbadis.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Goodson WH, Hunt TK. Studies of wound healing in experimental diabetes. J Surg Res. 1977;22:221–227. doi: 10.1016/0022-4804(77)90137-8. [DOI] [PubMed] [Google Scholar]
- Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285:9792–9802. doi: 10.1074/jbc.M109.082305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15:599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PD, Tutela JP, Thanik VD, Knobel D, Allen RJ, Jr, Chang CC, et al. Improved diabetic wound healing through topical silencing of p53 is associated with augmented vasculogenic mediators. Wound Repair Regen. 2010;18:553–559. doi: 10.1111/j.1524-475X.2010.00638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber GE, Raugi GJ. Preventing foot ulcers and amputations in diabetes. Lancet. 2005;366:1676–1677. doi: 10.1016/S0140-6736(05)67674-X. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, et al. P2X7 blockade attenuates lupus nephritis by inhibiting NLRP3/ASC/caspase-1 activation. Arthritis Rheum. 2013;65:3176–3185. doi: 10.1002/art.38174. doi: 10.1002/art.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]