Abstract
Background and Purpose
Eye exposure to the organophosphorus (OP) irreversible cholinesterase inhibitor sarin results in long-term miosis and impaired visual function. We have previously shown that tropicamide is better at ameliorating this insult than topical atropine or cyclopentolate. However, to minimize side effects associated with repeated tropicamide applications and high treatment doses, we evaluated the effects of oximes (ChE re-activators) alone and combined with tropicamide at ameliorating OP-induced ocular impairments.
Experimental approach
Rats were topically exposed to sarin, followed by topical treatment with various oximes alone or in combination with tropicamide. Pupil width and light reflex were measured by an infrared-based digital photograph system, while visual performance was assessed by employing the cueing version of the Morris water maze (MWM).
Key Results
Oxime treatment following sarin ocular exposure induced a slow persistent pupil widening with efficacy in the order of HLö-7 > HI-6 > obidoxime = TMB-4 = MMB-4. In the light reflex test, the ability of the iris to contract following oxime treatment was mostly impaired at 1 h and was back to normal at 4 h following sarin exposure. All oxime treatments ameliorated the sarin-induced visual impairment as tested in the visual task (MWM). The combined topical treatment of tropicamide with an oxime induced a rapid improvement in pupil widening, light reflex and visual performance, and enabled a reduction in tropicamide dose.
Conclusions and Implications
The use of tropicamide combined with an oxime should be considered as the topical treatment of choice against the toxic effects of ocular OP exposure.
Keywords: anticholinergic treatments, cued MWM, HI-6, HLö-7, Long-Evans rats, miosis, MMB-4, organophosphates, oxim, pupillary light reflex, sarin, TMB-4, obidoxime, tropicamide, visual impairment
Introduction
Ocular symptoms following organophosphorus (OP) exposure are essentially due to direct contact of the nerve agent vapour with the ocular surface rather than systemic exposure via skin or inhalation (Sidell and Groff, 1974; Soli et al., 1980; Yanagisawa et al., 2006). This OP penetration leads to a local measurable inhibition of cholinesterase (ChE) in the ocular tissues (Lund-Karlsen and Fonnum, 1976; Dabisch et al., 2005) with subsequent accumulation of the neurotransmitter ACh (Mattio et al., 1984). This local rise of ACh leads to the contraction of the papillary sphincter muscle of the iris and of the ciliary muscle (Rengstorff, 1994). Consequently, pupil diameter is reduced, causing dim vision and reduction in visual field (Rengstorff, 1994). The light reflex response may also be reduced due to desensitization of muscarinic receptors in the iris (Lund-Karlsen and Fonnum, 1976; Shiraishi and Takayanagi, 1993; Dabisch et al., 2008; Genovese et al., 2008). In addition to miosis, ciliary muscle spasm may cause ocular pain that may lead to headaches, nausea, blurred vision and myopia (Smith and Smith, 1980; Rengstorff, 1985; Nohara and Segawa, 1996; Cannard, 2006; Yanagisawa et al., 2006). Miosis is considered one of the most sensitive clinical signs following OP exposure, evident even following minimal exposure to sublethal OP vapour (Genovese et al., 2008).
Systemic anticholinergic treatment shows no benefit in ameliorating OP-induced ocular impairment (Ohbu et al., 1997; Yanagisawa et al., 2006) unless given at very high doses (Cannard, 2006) and may even induce systemic side effects in mild casualties (Hurst et al., 2007). Therefore, only topical anticholinergic treatment should be used to facilitate ocular symptom relief (Ohbu et al., 1997; Okudera, 2002). Most OP whole body exposure casualties also suffer from ocular symptoms (Yanagisawa et al., 2006), which will probably result in visual impairment and anxiety that accompanies stressful ocular pathologies, and in these cases pharmacological intervention is critical.
In the past, topical treatment with 2-pyridine aldoxime methyl chloride or obidoxime has been shown to re-activate ocular ChE, which was shown to correlate with pupil diameter (Lund-Karlsen and Fonnum, 1976). Topical treatment with HI-6 was also shown to have a beneficial effect on pupil widening following OP ocular exposure (Macic et al., 1990). In contrast, 10% (w v−1) phenylephrine, an α1 adrenoceptor agonist (Alexander et al., 2013), has shown no beneficial role in pupil widening following topical sarin exposure (unpubl. obs. from our lab).
We have shown recently that the short-acting ophthalmic anticholinergic drug, tropicamide, is beneficial at ameliorating the sarin-induced miosis and visual impairment, with no side effects of mydriasis and visual impairment as noticed when using topical atropine or cyclopentolate eye drops (Gore et al., 2012). However, because ocular sarin exposure may result in long-lasting miosis (days to weeks) (Munro et al., 1994; Rengstorff, 1994; Yanagisawa et al., 2006), a repeated application of tropicamide eye drops may be required until ocular toxic effects fade. To avoid this, we hypothesized that adding an oxime to tropicamide will be beneficial, as tropicamide will rapidly widen the iris and reduce ciliary spasm, while the oxime will reactivate ocular ChE, preventing a recurrence of the ocular toxic symptoms. In addition, this combined treatment may enable a lower dose of tropicamide dose to be used, so reducing the intensity and duration of mydriasis and partial cycloplegia side effects in cases of misuse (sign-free exposed casualties).
Because the potential effect of the combined treatment has not yet been determined, in the present study, we aimed to examine the concept of the combined topical treatment with the different pharmacological properties (anticholinergic and ChE re-activators).
For this purpose, we evaluated the efficacy of various oximes (HI-6, TMB-4, obidoxime, MMB-4 and HLö-7) alone or in combination with the short-acting anticholinergic drug tropicamide at ameliorating the sarin-induced ocular impairment in the rat model by assessing pupil width, iris contraction ability and visual performance.
Methods
Chemicals
Isopropyl methylphosphono-fluoridate (sarin) was synthesized by the Department of Organic Chemistry (Israel Institute for Biological Research, IIBR) receiving purity of >95% determined by quantitative NMR. Sarin stock solution of 30 mg mL−1 in propylene glycol (PG) was prepared and stored at −20°C. Sarin solutions in saline were freshly prepared before each experiment. Tropicamide 0.5% (w v−1) was purchased as commercial eye drops: Mydramide 0.5% (w v−1) [benzeneacetamide, N-ethyl-α-(hydroxymethyl)-N-(4-pyridinylmethyl)-] from Fischer Medical Israel. Saline was purchased as a commercial product from Teva Medical, Ashdod, Israel. HI-6 [1-(2-hydroxyiminomethylpyridinium)-3-(4-karbamoylpyridinium)-2-oxapropan dimethanesulfonate], MMB-4 [bis-1, 1-(4-hydroxyiminomethylpyridinium)methane dichloride] and obidoxime [bis-1,3-(4-hydroxyiminomethylpyridinium)-2-oxapropane dichloride] were purchased from Chemprotect, Prague, Czech Republic. Trimedoxime [TMB-4; 1,3-bis(4-hydroxyiminomethylpyridinium)-propanedichloride] and benzalkonium chloride (alkylbenzyldimethylammonium chloride) were purchased from Sigma, Rehovot, Israel. PBS was purchased from Biological Industries Beit-Haemek, Kibbuts Beit-Haemek, Israel. HLö-7 (pyridinium, 1-[[[4-(aminoarbonyl)pyridinio] methoxy]methyl]-2,4-bis-[(hydroxyimino)methyl]diiodide) was received as a gift from Dr G Amitai (IIBR). Oxime solutions were prepared at a concentration of 2.5% (w v−1) in a solution consisting of potassium chloride 0.534 mM, potassium phosphate monobasic 0.94 mM, sodium chloride 27.6 mM and sodium phosphate dibasic 1.62 mM (diluted in PBS; pH 7.2) in order to reduce the solution's osmolarity. Osmolarity of the solutions was 278–338 mOsM, within the physiological range. All treatments were given at a volume of 5 μL per eye. Concentrations of the oxime treatment were: HLö-7 2.5% (w v−1; 47.1 mM; 0.2355 μmol per 5 μL), HI-6 2.5% (w v−1; 69.6 mM; 0.348 μmol per 5 μL), obidoxime 2.5% (w v−1; 69.6 mM; 0.348 μmol per 5 μL), MMB-4 2.5% (w v−1; 59.8 mM; 0.299 μmol per 5 μL), TMB-4 2.5% (w v−1; 56 mM; 0.28 μmol per 5 μL). In addition, the low volume of oxime solution was administered and diluted on the rat ocular tear film surface (∼100 μL volume of rat ocular surface), which was rapidly washed by the lacrimal system, decreasing the oxime solution osmolarity. In order to improve the oxime penetration via the ocular surface (Lund-Karlsen and Fonnum, 1976), oxime solutions were prepared with the ophthalmic preservative benzalkonium chloride 0.02% (w v−1) (Sweetman, 2002; Bartlett et al., 2008b). The drugs/chemicals used in this manuscript conform to the British Journal of Pharmacology's Concise Guide to PHARMACOLOGY (Alexander et al., 2013).
Animals
Pigmented male Long-Evans rats (250–300 g) were purchased from Charles River, Boston, MA, USA. Animals were housed in ambient conditions of three animals per cage, temperature-controlled environment at 21 ± 2°C and 40–70% humidity, 12 h light per dark cycle. Rats were provided with altromin 1324 pellets (Altromin, Lage, Germany) and tap water ad libitum during the study and were maintained in accordance with the principles enunciated in the Guide for the Care and Use of Laboratory Animals, Eighth Edition, National Academy Press, Washington DC, 2010. Experimental procedure began at least 1 week following animal arrival. The protocols of all experiments were approved by the IIBR committee for animal care and use, and were designed to prevent or minimize any unnecessary animal discomfort. Animals were killed 24 h following experimental procedure by carbon dioxide asphyxiation. This work is reported in accordance with ARRIVE guidelines for reporting experiments involving animals (Kilkeny et al., 2010; McGrath et al., 2010). Long-Evans pigmented male rats were used in this study due to their superior physiological retina function, which includes a higher number of rod photoreceptors compared with non-pigmented rats, thus enabling higher visual performance (Imai et al., 1983; Prusky et al., 2000; 2002). Long-Evans rats are frequently used in Morris water maze (MWM) tasks (Whishaw and Petrie, 1988).
IR photography
Pupil width was measured by an IR-based system, adapted from the ‘ViewPoint Eye Tracker’ taken from humans to small animals as previously described (Gore et al., 2012). The pupil was identified in the visual field designated according to the contrast distinction compared with its surroundings. The pupil width is indicated by a yellow spherical mark and determined throughout the measurement in pixel units. Measurements are presented as ratio of pupil width to the permanent parameter of window length of the eye display. The ratios of measurements were used in order to correct distance variability from the camera to the eye.
Topical eye sarin exposure protocol and ocular measurements
The left eye of each rat (n = 12 for each exposure group) was digitally photographed to determine the degree of miosis produced by topical eye exposure to sarin. Volume of all eye drops used in this study was 5 μL. Images were taken under controlled low-light conditions (2 lux) and only after the animals had been allowed to adjust to darkness for 15 min. Hand-restrained animals were topically exposed to 0.2 or 1 μg sarin per eye in a fume hood, where they were held for 10 min after exposure to prevent environmental contamination. Exposure doses of 0.2 and 1 μg sarin were selected in this work based on previous results (Gore et al., 2012) showing pinpoint pupils and a reduction in visual performance, with no systemic intoxication. The width of the pupil was measured from 15 min following exposure and continued for up to 8 h.
Eye treatment protocol and ocular measurements
Efficacy evaluations of oxime treatment alone or in combination with tropicamide on pupil width were made after a single exposure to 0.2 or 1 μg sarin in the left eye. Fifteen minutes following sarin or control [0.66% (w v−1) PG] exposure, pupil width measurement was performed (n = 12 for each treatment group) under controlled low-light conditions (2 lux). Twenty minutes after sarin exposure, animals received topical oxime alone or in combination with tropicamide (volume of 5 μL for each substance in all experiments). The treatments assessed and compared in this study were: HLö-7 2.5%, HI-6 2.5%, obidoxime 2.5%, MMB-4 2.5%, TMB-4 2.5% and tropicamide 0.5%.
The results from a preliminary study with HI-6 demonstrated the increased efficacy of two drops compared with one drop (X1). Thus, two drops of oximes were used in all the studies that followed. An oxime concentration of 2.5% was used in this work, as performed previously (Macic et al., 1990). Preparation in % (w v−1) was done as accepted for ophthalmic ocular solutions.
In most experiments, a double drop of oxime was used with an interval of 30 s (2 × 5 μL), while in some experiments only one drop of oxime was used (indicated by ×1). Tropicamide treatment (one drop) was given 30 s following oxime treatment in the combined treatments. Pupil diameter was measured 10 min following treatment (∼30 min after exposure) and at 1, 2, 4 and 8 h following exposure. Light reflex measurements were assessed at 1 and 4 h following treatment as described previously (Gore et al., 2012). Briefly, the left eye was illuminated with 350 lux for 2–3 s and pupil diameter was immediately determined. Light reflex is presented as % pupil width constriction change of pre-reflex level. The physiological range of light reflex change was determined in this study as 35–57%. These values were determined following examination of 36 naïve eyes presenting a mean of 46% change with a SD of 11. All treatments were compared with the saline-treated animals.
Visual performance assessment: the cued MWM
Visual performance in rats was evaluated by using the cueing version of the MWM (Morris, 1984; Brandeis et al., 1989). In this version, target identification is done by using a single clue, which signs the target location (Hamilton et al., 2004). As previously described (Gore et al., 2012), animal performance in the maze was monitored by an overhead tracking video camera connected to an image analyser (HVSImage, Hampton, UK) and analysed by the water maze software HVS WATER 2020. Briefly, rats were evaluated during a 1 day experiment of eight trials (four trials per block). In each block, rats departed from all start points (in each quadrant), while the sequence of locations was randomly selected. The rat was expected to find the platform by identifying the cue (green rod) indicating the platform location, which was also altered randomly between trials to minimize spatial learning. Escape latency (time to reach the platform), path length and swimming speed were recorded in each trial.
Visual performance evaluation by cued MWM following ocular sarin exposure and treatments
Rats' eyes were exposed (n = 12) topically to 1 μg sarin or 0.66% (w v−1) PG (control) at a volume of 5 μL per eye, in both eyes. Ten minutes following exposure, rats were taken out of the fume hood and 5 min later pupil width was determined, and the appropriate treatment was given to both eyes. Treatments included two drops of: HI-6 2.5% (w v−1), obidoxime 2.5% (w v−1), MMB-4 2.5% (w v−1) or TMB-4 2.5% (w v−1) alone or in combination with one drop of tropicamide 0.5% (w v−1). Control animals were exposed to sarin or PG, and received a treatment of saline or tropicamide 0.5% (w v−1). A second pupil width examination was done 35 min after sarin exposure (∼15 min following treatment administration), and a visual performance test was conducted immediately. All examinations and tests were done in a room with controlled light conditions (∼170 lux).
Data analysis
Pupil width at the various time points following exposure and treatments are presented as a % of baseline determined a day before sarin exposure and presented as mean ± SEM. Pupil diameter score and light reflex (% pupil width change relative to pre-reflex level) were analysed by a two-way or a three-way anova, with one repeated variable (time), and one or two non-repeated variables (group, or group and treatment). Specific comparisons were performed using the simple main effects contrasts analysis (Winer, 1971), which was specifically suited for determining significant interactions when some of the variables involved were of repeated measurement type. Visual performance scores (escape latency, path length and swimming speed) were analysed by a three-way multiple analysis of variance (manova), with one repeated variable (blocks of trials) and two non-repeated variables (group and treatment). Specific comparisons were performed as discussed earlier. All scores are reported as mean ± SEM.
Results
Effect of topical oxime treatments on sarin-induced miosis
Oximes alone had no effect on pupil width in control animals (PG application) (Figure 1A) indicating that this treatment has no anticholinergic properties. Sarin exposure induced an immediate reduction in pupil width, which finally widened to differing sizes depending on the dose of exposure (Figure 1B and C). Ocular oxime treatment following 0.2 (Figure 1B) or 1 μg (Figure 1C) sarin exposure gradually ameliorated the miotic effect depending on the oxime used and time following treatment. The oxime HLö-7 was graded as the most potent oxime compared with the other oximes evaluated, showing a higher pupil widening following 0.2 or 1 μg sarin exposure at all time points (P < 0.01). Treatment with two or one drop of the oxime HI-6 was graded second and third, respectively, in the potency of pupil widening compared with the other oximes evaluated (P < 0.01), which presented a similar efficacy in pupil widening following ocular sarin exposure. All oximes showed a significant higher efficacy in pupil widening when applied against the lower dose of sarin (P < 0.01) (efficacy comparison among the various oximes following 0.2 or 1 μg sarin is not presented on the same graph in Figure 1).
Figure 1.
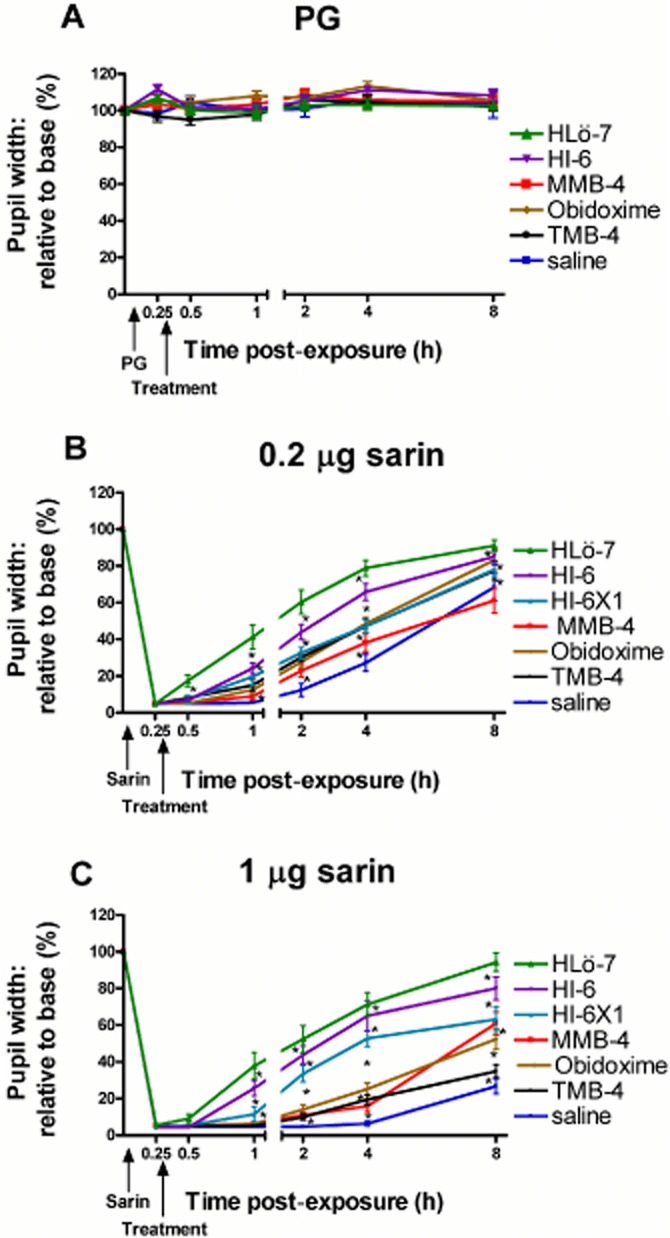
Pupil width alteration following topical sarin exposure and oxime treatment. Rats were exposed topically to PG (A), 0.2 (B) or 1 μg (C) sarin followed by two drops of topical oxime (2.5%) treatment (except for one treatment group, in which one drop of HI-6 was used, indicated as HI-6X1). Pupil width is presented as % relative to baseline of the same animal before exposure. Each point is the mean ± SEM of 12 rats. All differences, between groups, at different time points post-exposure are statistically significant (by anova), at a level of *P < 0.01.
Light reflex assessment following topical sarin exposure and oxime treatments
Control groups treated with the oximes HLö-7, HI-6 one (X1) or two drops, obidoxime, TMB-4, and MMB-4 presented a light reflex ability within the normal range at 1 and 4 h after treatment, slightly beneath the light reflex ability of 54% presented in control animals following saline treatment (Figure 2A).
Figure 2.
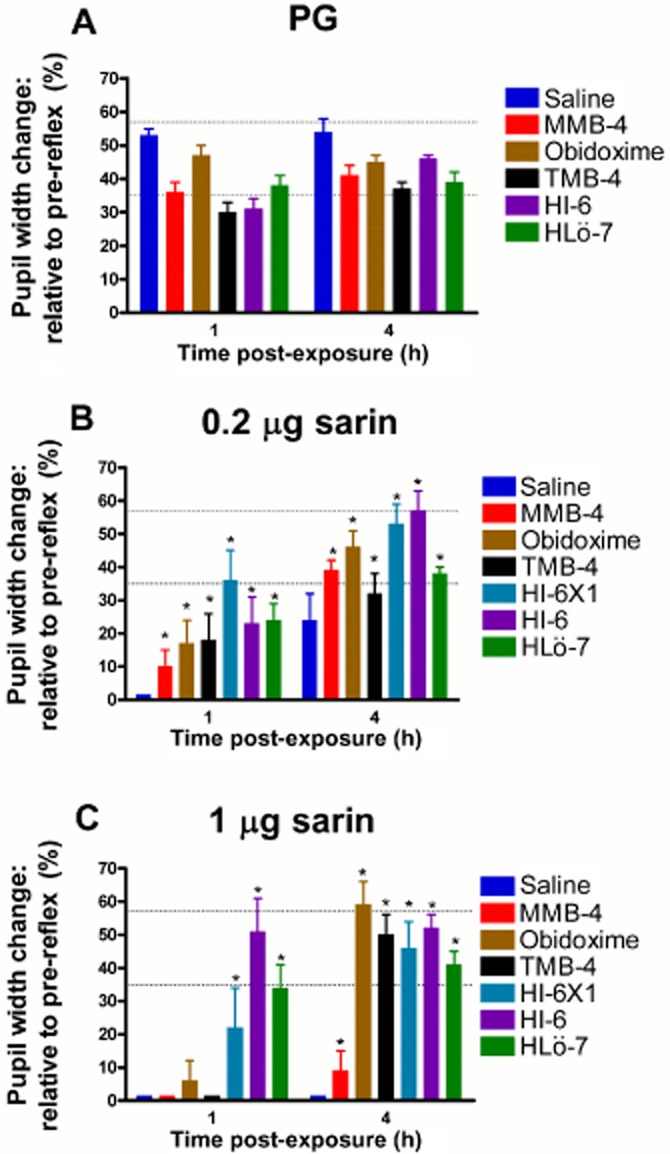
Effect of oxime treatments on the pupillary light reflex following sarin exposure. Treated eyes, as described in Figure 1 (two drops of topical 2.5% oxime), were illuminated with 350 lux for 2–3 s, 1 and 4 h following exposure to PG (A), 0.2 μg (B) or 1 μg (C) sarin. Each point is presented as % change relative to the pre-reflex pupil width and represents the mean ± SEM of 12 animals. Normal light reflex range is indicated by the dotted horizontal lines. Differences between treatments following exposure of 0.2 or 1 μg sarin at both time points post-exposure are statistically significant (by anova), at a level of *P < 0.01 (vs. saline at the same time point).
Sarin-induced pinpoint pupils showed no light reflex after saline treatment at 1 h following both 0.2 or 1 μg sarin exposure, and at 4 h only following 1 μg sarin exposure (Figure 2B and C). Miotic pupils treated with the oxime HI-6 or HLö-7 showed more potent iris contraction ability compared with the oximes TMB-4, MMB-4 and obidoxime (Figure 2B and C). The oximes TMB-4, MMB-4 and obidoxime showed a weak light response at 1 h following exposure, which was more pronounced following exposure of 1 μg sarin (Figure 2B and C).
Visual performance assessment following ocular sarin exposure and oxime treatments
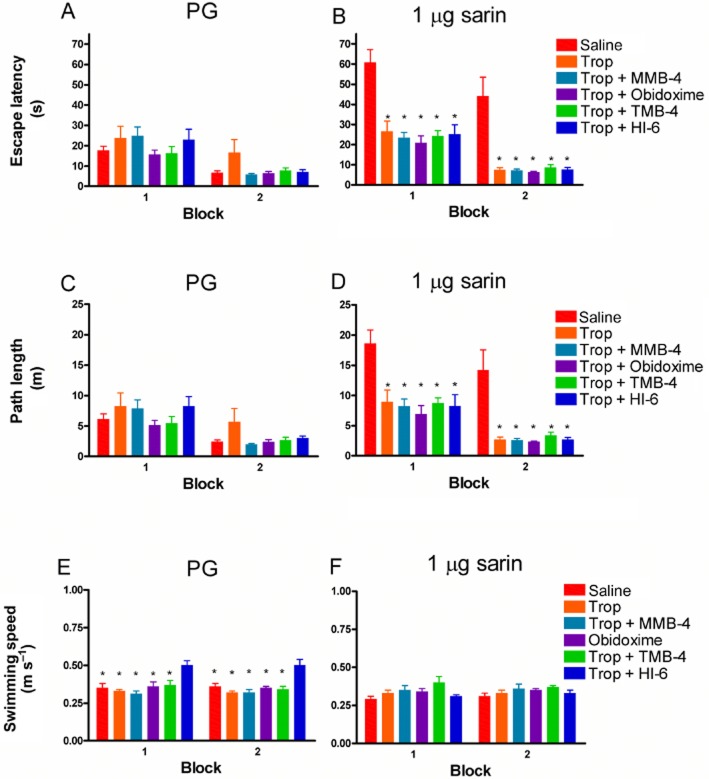
In order to determine the beneficial role of oxime treatment in altering visual dysfunction, a visual performance test was conducted following ocular sarin exposure and oxime treatment. For these experiments, two representative oximes MMB-4 and obidoxime were selected based on their low efficacy in pupil widening (Figure 1). Control animals treated only with MMB-4 or obidoxime showed no change in the escape latency (Figure 3A) or path length (Figure 3B) parameters similar to control animals treated with saline. Following sarin exposure (1 μg), a significant increase in escape latency and path length parameters were noticed in the saline-treated group, indicating visual performance deficits in this group (P < 0.001; Figure 3A and B). In contrast, sarin-induced visual impairment was ameliorated following treatment with the oximes MMB-4 or obidoxime, presenting a visual performance similar to the control group (Figure 3A and B). No significant differences were observed in the swimming speed parameter between treated, non-treated, sarin-exposed or -non-exposed groups (Figure 3C).
Figure 3.
Visual performance test following topical sarin exposure and oxime treatment. Both eyes were topically exposed to 1 μg sarin or to 0.66% PG (control). Fifteen minutes following exposure, pupil diameter was measured to confirm pupil constriction (not shown). Eyes were then treated with saline or 2.5% oximes as indicated. Twenty minutes following treatment, visual performance was evaluated under controlled room light conditions (∼170 lux) using the cued MWM paradigm. The parameters examined and compared between groups were escape latency (A), path length (B) and swimming speed (C). The first block shows the average of the initial four trials of 12 rats, and the second block represents the following four trials average for the same animals. Differences in escape latency and path length between non-treated sarin-exposed group and each of the other groups are statistically significant (by manova) at a level of *P < 0.001 (vs. sarin; saline in the same block).
Effect of combined oxime and tropicamide treatment on sarin-induced miosis
Control eyes treated with oximes and tropicamide showed a sharp pupil width widening (mydriasis) within a few minutes, followed by gradual constriction, returning to baseline at 8 h similar to eyes treated with tropicamide alone (Figure 4A). Eyes exposed to 1 μg sarin presented pinpoint pupils for up to 4 h, with a slight pupil widening of ∼26% at 8 h following exposure (Figure 4B). Sarin-induced pinpoint pupils were significantly widened at all time points following the combined treatment of oximes with the short-acting anticholinergic drug, tropicamide, compared with sarin-exposed eyes (P < 0.05). All combined treatments in Figure 4B showed a significant, higher efficacy in pupil widening compared with each compound alone presented in Figure 1B and C, or tropicamide alone (P < 0.05–0.001). The various oximes given with tropicamide presented a significant, prolonged (P < 0.05) action in counteracting the sarin-induced pinpoint pupils, with an efficacy order of HI-6 > obidoxime>TMB-4 > MMB-4 (Figure 4B).
Figure 4.
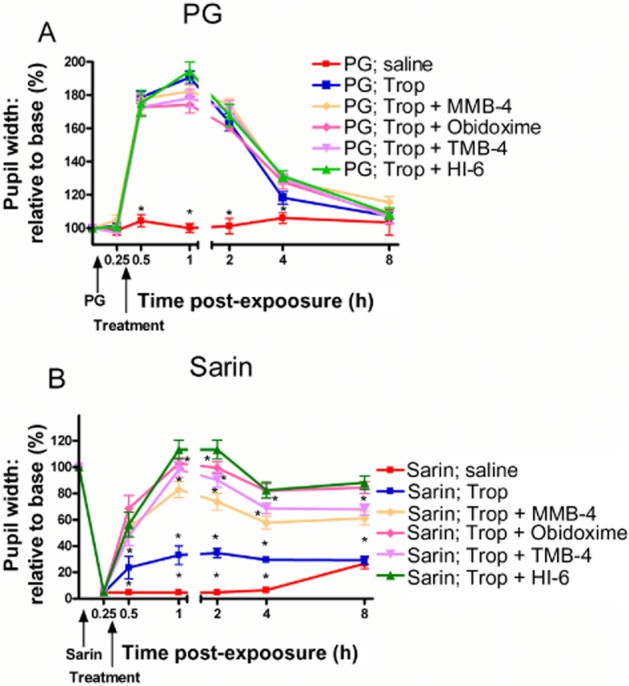
Evaluation of the combined tropicamide and oxime treatment on pupil width following topical sarin exposure. Rats were exposed topically to PG (A) or 1 μg sarin (B) and 20 min later were given a combined topical treatment of tropicamide (Trop) with two drops of 2.5% TMB-4, 2.5% obidoxime, 2.5% MMB-4 or 2.5% HI-6. Pupil width was determined at the indicated time points and presented as % relative to baseline of the same animal before exposure. Each point is the mean ± SEM of 12 rats. Differences between groups, at various time points post-exposure, are statistically significant (by anova) at a level of *P < 0.05.
Light reflex assessment following topical sarin exposure and combined treatments
In order to determine the efficacy of the combined treatment in ameliorating the deficit in physiological iris constriction ability, light reflex assay was utilized. Light reflex of the non-sarin-exposed animals treated with the combined treatment was within the physiological range (Figure 5A). Non-treated exposed eyes presenting pinpoint pupils showed no light response (Figure 5B). Following treatment, light reflex in sarin-exposed eyes returned to normal contraction ability within the physiological range at the two appointed times (Figure 5B).
Figure 5.
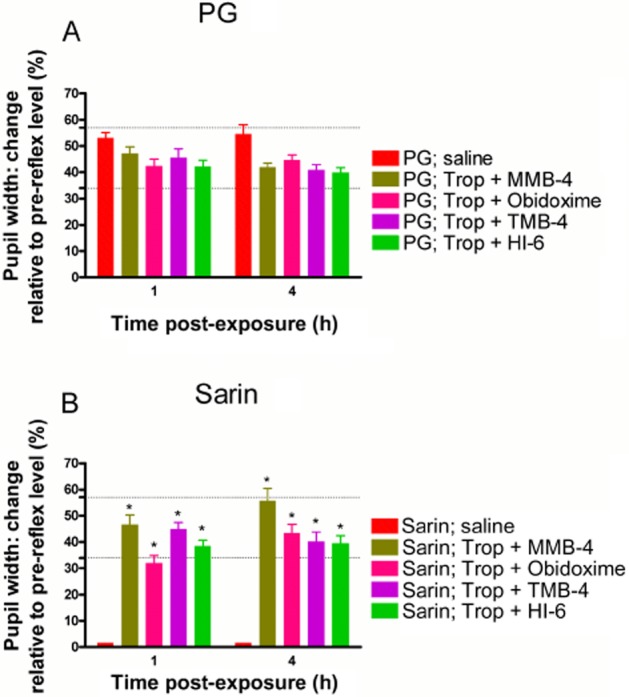
Effect of the combined tropicamide and oxime treatments on the pupillary light reflex following sarin exposure. Eyes were illuminated with 350 lux for 2–3 s, 1 and 4 h following the combined treatment (tropicamide with two drops of 2.5% oxime as indicated) and after exposure to PG (A) or 1 μg (B) sarin. Each point (mean ± SEM) is presented as % change relative to the pre-reflex pupil width and represents the average performance of 12 animals. Physiological light reflex range is indicated by the dotted horizontal lines. Differences in pupillary light reflex between groups are statistically significant (by anova) at a level of *P < 0.001 (vs. sarin; saline in the same block) as indicated.
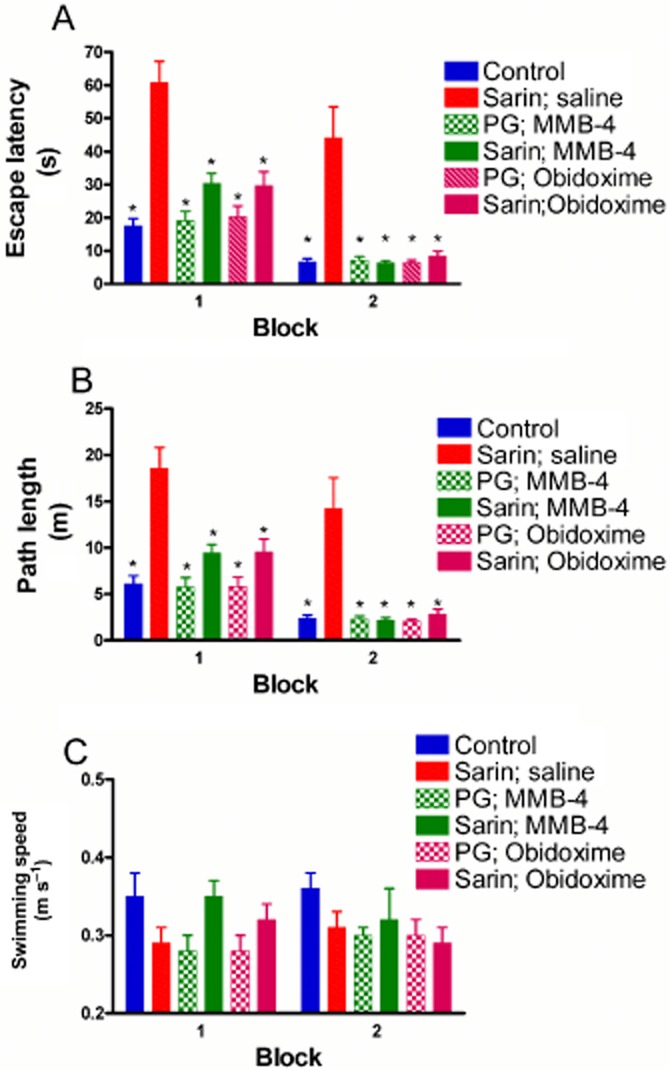
Visual performance following ocular sarin exposure and combined treatments
Control groups (PG-exposed) treated with tropicamide or with the combined treatment of oxime with tropicamide showed a similar escape latency (Figure 6A) and path length (Figure 6C) as the control group treated with saline. A significant reduction in the escape latency (Figure 6B) and in the path length (Figure 6D) was observed following ocular sarin exposure (P < 0.01, vs. sarin; saline in the same block). The combined treatments ameliorated the sarin-induced visual impairment, showing an escape latency (Figure 6B) and path length (Figure 6D) similar to that of the control animals. No significant differences in the swimming speed were observed between the sarin-exposed and -non-exposed groups (Figure 6 E and F). Changes in the swimming speed in the PG-exposed group treated with tropicamide with HI-6 (Figure 6E) showed no correlation to the escape latency and path length.
Figure 6.
Visual performance evaluation using the combined treatment following sarin exposure. Both rat eyes were treated topically with the indicated drugs, tropicamide (Trop) with two drops of various oximes (2.5%), 20 min following PG (A, C, E) or 1 μg sarin (B, D, F) exposure. Ocular measurements and the visual test were conducted 15 min later. Visual performance was evaluated under controlled light conditions (170 lux) using the ‘cued MWM’ paradigm. The parameters examined and compared between groups were escape latency (A), path length (B) and swimming speed (C); n = 12 for each group. The first block shows the average of the initial four trials of 12 rats, and the second block represents the following four trials average for the same animals. Significant (by manova) differences between groups are indicated by asterisks at a level of *P < 0.001. (B, D) *P < 0.001, versus sarin; saline in the same block; (E) *P < 0.001, versus Trop + HI-6 in the same block.
Discussion
The ophthalmic anticholinergic eye drops, atropine or homatropine are currently used in order to counteract the ocular symptoms following OP exposure (Hurst et al., 2007). These potent anticholinergic drugs widen the miotic pupils and reduce ocular pain by inducing ciliary muscle relaxation (Malthe-Sorenssen et al., 1979; Holstege and Baer, 2004). Side effects of these drugs may result in prolonged mydriasis and cycloplegia, leading to photophobia and blurred vision (Holstege and Baer, 2004). These side effects, which may persist for several days (Bartlett et al., 2008a), result in long-term visual impairment. In our previous work, we showed that the use of a short-acting anticholinergic drug, tropicamide, had a beneficial role in rapid pupil widening, enhancing physiological light reflex response and ameliorating visual impairment with no side effects of mydriasis in sarin-exposed animals (Gore et al., 2012). Because of the prolonged ocular effect of sarin (Munro et al., 1994), a repeated tropicamide treatment may be inevitable (Ohbu et al., 1997). In order to minimize repeated treatments and mistreatment side effects of mydriasis and cycloplegia (Bartlett et al., 2008a), a combined topical treatment of low-dose tropicamide with ChE re-activators (oximes) was evaluated in this study.
Oxime treatment efficacy was evaluated following topical exposure to 0.2 or 1 μg sarin and it was found to induce a slow rate of pupil widening. This effect was dependent on time, exposure dose, treatment dose and type of oxime. This dependency was also observed following the light reflex test. Efficacy order of the various topical oxime treatments in counteracting the ocular impairment was HLö-7 > HI-6 > obidoxime = TMB-4 = MMB-4. In the visual performance experiments, using the cued MWM, the oximes examined showed a similar beneficial effect at counteracting the visual impairment and restoring visual performance.
Despite the prolonged ChE inhibition (Lund-Karlsen and Fonnum, 1976) and the subsequent hypercholinergic process (Grob and Harvey, 1958; Mattio et al., 1984), an iris papillary muscle relaxation is observed in animals at the first hours following ocular OP exposure (Dabisch et al., 2007; Gore et al., 2012). This mechanism may be explained by the desensitization of the local muscarinic receptors (Dabisch et al., 2007). Previously, it had been reported in animals that topical oxime treatment following OP ocular exposure led to a significant re-activation of the ChE enzyme in a few hours, accelerating pupil widening (Lund-Karlsen and Fonnum, 1976). The pharmacological mechanism is apparently due to the provision of sufficient enzyme activity to hydrolyze the excessive synaptic ACh, stopping the hypercholinergic process, with no direct role in relaxation of the iris papillary spasm.
The same phenomenon of gradual iris relaxation was observed following topical O-ethyl-S-[2-(diisopropylamino)ethyl] methylphosphonothioate or sarin exposure in the rabbit model (Macic et al., 1990) as that observed in the Long-Evans rat model in this study following topical treatment with various ChE re-activators. In addition, in the present study we demonstrated that the oxime treatment had no anticholinergic activity in the control animals. This indicates that the physiological effect on iris papillary muscle relaxation accelerated by oximes is mediated by an indirect (re-activation of the ChE) rather than direct mechanism. Thus, variations in the re-activation efficacies of the various oximes may account for their different effects on pupil-widening following topical sarin exposure; the strong re-activators HLö-7 and HI-6 (Worek et al., 1998; 2007; Jokanovic and Stojilkovic, 2006) rapidly reactivate the sarin-inhibited ChE compared with TMB-4, obidoxime and MMB-4, which apparently possess a weaker ability to reduce the primary hypercholinergic outburst. Therefore, the rate of miotic recovery depends on the intensity and the duration of the initial hypercholinergic effect.
The molar concentration of each oxime varied, yet the effective dose range was narrow and did not affect efficacy. For instance, the oxime HLö-7 presented the highest efficacy at pupil widening probably due to its higher re-activation efficacy (Jokanovic and Stojilkovic, 2006; Worek et al., 2007), although its molar dose was less than that of HI-6. The oximes HI-6 and obidoxime were given at the same molar concentration, yet the more potent oxime HI-6 showed a higher ability to induce pupil widening. The oxime MMB-4, which was given at a higher molar dose than TMB-4, showed a weaker pupil widening ability and the oxime TMB-4, which was given at a lower dose than obidoxime showed the same effect, although both oximes are known to possess a similar re-activation efficacy. The different efficacies of the various oximes at inducing pupil widening may be mainly attributed to the re-activation ability of each oxime, although other factors such as ocular environment (osmolarity or pH) or changes in in vivo re-activation ability may explain the lower efficacy of MMB-4 compared with TMB-4 and obidoxime. Differences in oxime efficacy are probably not due to variations in permeability via the ocular surface (cornea, conjunctiva and sclera) because the use of benzalkonium chloride enables good oxime permeability.
By employing the visual performance test, we showed that all the treatments used were able to restore visual function causing no visual deterioration, as has been observed when using topical cyclopentolate treatment (Gore et al., 2012). Changes in visual performance could not be explained by motor alterations observed in some of the experimental groups in the cued MWM. Although few of these minor differences in swimming speed showed significant changes between various treatments, these differences showed no correlation with the visual differences (presented by escape latency and path length). Additionally, no change in peripheral blood ChE activity was found following 1 μg ocular topical sarin exposure (data not shown), indicating no systemic involvement. Altogether, these findings indicate that the observed escape latency and path length changes were apparently as a result of changes in visual and not in motor functions.
Unlike the indirect effect of oximes, the use of anticholinergic treatment alone in a hypercholinergic state creates competition on the muscarinic receptors between the anticholinergic compound and ACh. Thus, the change in pupil width will be determined by the potency and dose of the anticholinergic drug administered. Following this therapeutic approach, miosis may recur in time following a decrease in anticholinergic effect, while ChE is still inhibited. Therefore, a combination of both the oxime and the anticholinergic treatments will lead to a hypercholinergic reduction, inducing spontaneous muscle relaxation. The decrease in synaptic ACh will endorse the competitive advantage of the anticholinergic treatment over ACh on muscarinic receptors.
In this study, it was shown for the first time that the combined topical treatment of tropicamide with the various oximes induced a rapid improvement in terms of the pupil widening as well as functional return of light reflex. In addition, this treatment ameliorated the visual impairment following ocular sarin exposure. Because most sarin-exposed casualties in the past suffered from prolonged miosis (Munro et al., 1994; Rengstorff, 1994; Nozaki et al., 1997; Yanagisawa et al., 2006), the synergistic effect seen here after using the combined treatment could considerably ameliorate these symptoms in OP-exposed casualties.
As the combined treatment has two separate mechanisms, re-activating ChE and an antagonist effect on the muscarinic receptor, a combination of the two kinds of drugs provides a more potent effect than if given separately. This eliminates the need for repeated treatment, allowing a reduction in the dose of the anticholinergic component and limits the risk of side effects.
Although this work did not relate to the effect of the combined therapy on the ciliary muscle due to the anatomical proximity to the iris, it is highly reasonable that OP-exposed victims with ciliary spasm will benefit from this treatment, thus reducing ocular pain (Malthe-Sorenssen et al., 1979; Kato and Hamanaka, 1996; Cannard, 2006) and improving blurred distance vision. Therefore, the use of this combined treatment should be considered as the treatment of choice for sarin-induced ocular impairment.
Glossary
- IF
infrared
- MWM
Morris water maze
Conflict of interest
None.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G-Protein Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JD, Jaanus SD, Fiscella RG, Holdeman NR, Prokopich CL. Clinical Ocular Pharmacology. 5. St. Louis: Elsevier; 2008a. Chapter Nine. [Google Scholar]
- Bartlett JD, Jaanus SD, Fiscella RG, Holdeman NR, Prokopich CL. Clinical Ocular Pharmacology. 5. St. Louis: Elsevier; 2008b. Chapter Two. [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Cannard K. The acute treatment of nerve agent exposure. J Neurol Sci. 2006;249:86–94. doi: 10.1016/j.jns.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Dabisch PA, Burnett DC, Miller DB, Jakubowski EM, Muse WT, Forster JS, et al. Tolerance to the miotic effect of sarin vapor in rats after multiple low-level exposures. J Ocul Pharmacol Ther. 2005;21:182–195. doi: 10.1089/jop.2005.21.182. [DOI] [PubMed] [Google Scholar]
- Dabisch PA, Horsmon MS, Muse WT, Mioduszewski RJ, Thomson S. Muscarinic receptor dysfunction induced by exposure to low levels of soman vapor. Toxicol Sci. 2007;100:281–289. doi: 10.1093/toxsci/kfm213. [DOI] [PubMed] [Google Scholar]
- Dabisch PA, Horsmon MS, Taylor JT, Muse WT, Miller DB, Sommerville DR, et al. Gender difference in the miotic potency of soman vapor in rats. Cutan Ocul Toxicol. 2008;27:123–133. doi: 10.1080/15569520802064376. [DOI] [PubMed] [Google Scholar]
- Genovese R, Benton BJ, Oubre JL, Fleming PJ, Jakubowski EM, Mioduszewski RJ. Determination of miosis threshold from whole-body vapor exposure to sarin in African green monkeys. Toxicology. 2008;244:123–132. doi: 10.1016/j.tox.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Gore A, Brandeis R, Egoz I, Peri D, Turetz J, Bloch-Shilderman E. Efficacy assessment of various anticholinergic agents against topical sarin-induced miosis and visual impairment in rats. Toxicol Sci. 2012;126:515–524. doi: 10.1093/toxsci/kfs009. [DOI] [PubMed] [Google Scholar]
- Grob D, Harvey JC. Effects in man of the anticholinesterase compound sarin (isopropyl methyl phosphonofluoridate) J Clin Invest. 1958;37:350–368. doi: 10.1172/JCI103615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton D, Rosenfelt CS, Whishaw IQ. Sequential control of navigation by local and taxon cues in the Morris water task. Behav Brain Res. 2004;154:385–397. doi: 10.1016/j.bbr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Holstege CP, Baer AB. Insecticides. Curr Treat Options Neurol. 2004;6:17–23. doi: 10.1007/s11940-004-0035-2. [DOI] [PubMed] [Google Scholar]
- Hurst G, Tuorinsky S, Madsen J, Newmark J, Hill B, Boardman C, et al. 2007. Medical management of chemical casualties handbook. Chemical Casualty Care Division, US Army Medical Research Institute of Chemical Defense 3100 Ricketts Point Road Aberdeen Proving Ground, MD 21010-5400.
- Imai H, Miyata M, Uga S, Ishikawa S. Retinal degeneration in rats exposed to an organophosphate pesticide (fenthion) Environ Res. 1983;30:453–465. doi: 10.1016/0013-9351(83)90231-1. [DOI] [PubMed] [Google Scholar]
- Jokanovic M, Stojilkovic MP. Current understanding of the application of pyridinium oximes as cholinesterase reactivators in treatment of organophosphate poisoning. Eur J Pharmacol. 2006;553:10–17. doi: 10.1016/j.ejphar.2006.09.054. [DOI] [PubMed] [Google Scholar]
- Kato T, Hamanaka T. Ocular signs and symptoms caused by exposure to sarin gas. Am J Ophthalmol. 1996;121:209–210. doi: 10.1016/s0002-9394(14)70587-2. [DOI] [PubMed] [Google Scholar]
- Kilkeny C, Browne W, Cuthill I, Emerson M, Altman D. NC3Rs reporting guidelines working group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund-Karlsen R, Fonnum T. The effect of locally applied cholinesterase inhibitors and oximes on the acetylcholinesterae activity in different parts of the guinea-pig eye. Acta Pharmacol Toxicol (Copenh) 1976;38:299–307. doi: 10.1111/j.1600-0773.1976.tb03124.x. [DOI] [PubMed] [Google Scholar]
- Macic I, Vojvodic V, Knezevic D, Maksimovic M. The effect of the oxime HI-6 on miosis induced by topical administration of sarin and VX in the rabbit eye. Vojnosanit Pregl. 1990;47:399–401. [PubMed] [Google Scholar]
- Malthe-Sorenssen D, Soli NE, Fonnum F. 1979. The effect of locally applied organophosphates on miosis and acetylcholinesterase adaption to chronic treatment. Maintenance of air operations while under attack with chemical agents. N80–14731#, pp. 5.
- Mattio TG, Richardson JS, Giacobini E. Effects of DFP on iridic metabolism and release of acetylcholine and on pupillary function in the rat. Neuropharmacology. 1984;23:1207–1214. doi: 10.1016/0028-3908(84)90241-7. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, MeLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Development of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Munro NB, Ambrose KR, Warson AP. Toxicity of the organophosphate chemical warfare agents GA, GB, and VX: implications for public protection. Environ Health Perspect. 1994;102:13–37. doi: 10.1289/ehp.9410218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara M, Segawa K. Ocular symptoms due to organophosphorus gas (sarin) poisoning in Matsumoto. Br J Ophthalmol. 1996;80:1023–1027. doi: 10.1136/bjo.80.11.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki H, Hori S, Shinozawa Y, Fujishima S, Takuma K, Suzuki M, et al. Relationship between pupil size and acetylcholinesterase activity in patients exposed to sarin vapor. Intensive Care Med. 1997;23:1005–1007. doi: 10.1007/s001340050447. [DOI] [PubMed] [Google Scholar]
- Ohbu S, Yamashina A, Takasu N, Yamaguchi T, Murai T, Nakano K, et al. Sarin poisoning on Tokyo subway. South Med J. 1997;90:587–593. doi: 10.1097/00007611-199706000-00002. [DOI] [PubMed] [Google Scholar]
- Okudera H. Clinical features on nerve gas terrorism in Matsumoto. J Clin Neurosci. 2002;9:17–21. doi: 10.1054/jocn.2001.1020. [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res. 2000;40:2201–2209. doi: 10.1016/s0042-6989(00)00081-x. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Harker KT, Douglas RM, Whishaw IQ. Variation in visual acuity within pigmented and between pigmented and albino rat strains. Behav Brain Res. 2002;136:339–348. doi: 10.1016/s0166-4328(02)00126-2. [DOI] [PubMed] [Google Scholar]
- Rengstorff RH. Accidental exposure to sarin: vision effects. Arch Toxicol. 1985;56:201–203. doi: 10.1007/BF00333427. [DOI] [PubMed] [Google Scholar]
- Rengstorff RH. Vision and ocular changes following accidental exposure to organophosphates. J Appl Toxicol. 1994;14:115–118. doi: 10.1002/jat.2550140213. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Takayanagi I. Subtype of muscarinic receptors mediating relaxation and contraction in the rat iris dilator smooth muscle. Gen Pharmacol. 1993;24:139–142. doi: 10.1016/0306-3623(93)90024-r. [DOI] [PubMed] [Google Scholar]
- Sidell FR, Groff WA. The reactivatibility of cholinesterase inhibited by VX and sarin in man. Toxicol Appl Pharmacol. 1974;27:241–252. doi: 10.1016/0041-008x(74)90195-1. [DOI] [PubMed] [Google Scholar]
- Smith SA, Smith SE. Subsensitivity to cholinoceptor stimulation of the human iris sphincter in situ following acute and chronic administration of cholinomimetic miotic drugs. Br J Pharmacol. 1980;69:513–518. doi: 10.1111/j.1476-5381.1980.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soli NE, Karlsen RL, Opsahl M, Fonnum F. Correlations between acetylcholinesterase activity in guinea-pig iris and pupillary function: a biochemical and pupillographic study. J Neurochem. 1980;35:723–728. doi: 10.1111/j.1471-4159.1980.tb03712.x. [DOI] [PubMed] [Google Scholar]
- Sweetman SCE. Martindale. The Complete Drug Reference. 33. London: Pharmaceutical Press; 2002. [Google Scholar]
- Whishaw IQ, Petrie BF. Cholinergic blockade in the rat impairs strategy selection but not learning and retention of nonspatial visual discrimination problems in a swimming pool. Behav Neurosci. 1988;102:662–677. doi: 10.1037//0735-7044.102.5.662. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. pp. 559–571. [Google Scholar]
- Worek F, Widmann R, Knopff O, Szinicz L. Reactivating potency of obidoxime, pralidoxime, HI 6 and HLo 7 in human erthrocyte acetylcholinesterase inhibited by highly toxic organophosphorus compounds. Arch Toxicol. 1998;72:237–243. doi: 10.1007/s002040050495. [DOI] [PubMed] [Google Scholar]
- Worek F, Eyer P, Aurbek N, Szinicz L, Thiermann H. Recent advances in evaluation of oxime efficacy in nerve agent poisoning by in vitro analysis. Toxicol Appl Pharmacol. 2007;219:226–234. doi: 10.1016/j.taap.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci. 2006;249:76–85. doi: 10.1016/j.jns.2006.06.007. [DOI] [PubMed] [Google Scholar]