Abstract
Three breeding colonies of Myd88−/− mice had a history of significant morbidity and mortality. Although strain-specific poor reproductive performance might explain neonatal death and dystocia, mice were found dead or required euthanasia because of moribundity, distended abdomen, head tilt, and seizures. Histopathology results included bacteremia, placentitis, metritis, peritonitis with abscess formation, and suppurative meningoencephalitis. Intralesional gram-negative coccobacilli were present, often in extremely high number. Cultures of samples of the cardiac blood of a mouse and from water-bottle sipper tubes provided to some affected mice grew Pseudomonas aeruginosa. In addition, affected tissues from 2 mice and feces from a third tested PCR-positive for P. aeruginosa. Although the mice had received autoclaved reverse-osmosis–purified drinking water, we suspect that the mice were inoculated with P. aeruginosa through contaminated sipper tubes. Because of the deficiency in most of the Toll-like receptor signaling pathways, these Myd88−/− mice were unlikely to have developed competitive innate and adaptive immune responses, resulting in bacterial infections. These clinical cases underscore the importance of understanding how genotype, phenotype and environment affect animal health. Sound husbandry and experimental practices are needed to prevent the exposure of immunodeficient mice to pathogens.
Abbreviations: TLR, Toll-like receptor
Rodent colonies typically are managed on a ‘herd-health’ basis, with prevention, treatment, and control measures at the colony level rather than the individual level. Although sporadic cases of sick and dead mice are not unusual in large breeding colonies, unexpectedly high morbidity and mortality rates typically warrant clinical investigation and analysis of the disease triangle: the conceptual model of the interactions among the host, environment, and pathogen. Of these 3 factors, the host usually is well-characterized pertaining to its genotype, phenotype, and experimental manipulations. Similarly, the environmental conditions in a laboratory animal setting typically are controlled. However, infections with adventitious pathogens can occur and cause disease outbreak in susceptible animals. For example, poorly sanitized cages can act as fomites for pathogens that may cause clinical infections in susceptible mice.
Immunodeficient mice are prone to exhibit clinical signs when infected with a pathogen. One such strain is Myd88−/−, which lack the myeloid differentiation factor 88 (MyD88) protein, a cytoplasmic adaptor molecule essential for the signaling of IL1 and Toll-like receptor (TLR) family.14,27 MyD88 plays a central role in innate and adaptive immune response, because it is essential for cellular responses to IL1, IL18, and many bacterial cell wall components including lipopolysaccharide, peptidoglycan, and lipopeptide.1,10,27 Because of their phenotype, Myd88−/− mice are commonly used for infectious disease and immunology studies.
Here, we present a case series of Myd88−/− mice that had a history of morbidity and mortality attributed to bacterial infections caused by gram-negative coccobacilli; the organism was identified to be Pseudomonas aeruginosa in one case. We underscore the importance of understanding host responses to pathogens and the provision of sound husbandry procedures to prevent infections in immunodeficient mice, such as the Myd88−/− strain.
Case Reports
Three breeding colonies of experimentally naïve B6.129P2(SJL)-Myd88tm1.1Defr mice were housed under IACUC-approved protocols at an AAALAC-accredited research facility. The mice originally were obtained from Dr Shizuo Akira (Department of Host Defense, Osaka University, Japan). Quarterly soiled-bedding sentinel-program results indicated that colonies were negative for pinworms and furmites and seronegative for mouse hepatitis virus, minute virus of mice, mouse parvovirus, mouse rotavirus, murine norovirus, pneumonia virus of mice, Sendai virus, lymphocytic choriomeningitis virus, ectromelia virus, Hantaan virus, mouse adenovirus, mouse cytomegalovirus, respiratory enteric orphan virus 3, K virus, lactic dehydrogenase elevating virus, polyoma virus, mouse thymic virus, Prospect Hill virus, Mycoplasma pulmonis, Encephalitozoon cuniculi, and cilia-associated respiratory bacillus. Mice were provided with irradiated rodent chow (Harlan Teklad 7912, Indianapolis, IN), and autoclaved reverse-osmosis–purified water, corncob bedding material (Harlan Teklad), and an environmental enrichment device in positive-pressure autoclaved IVC (Mouse IVC Green Line, Tecniplast, Exton, PA). Cages were changed and experimental and husbandry procedures were performed in cage-changing stations (NuAire, Plymouth, MN) by personnel wearing personal protective equipment, including gloves that were disinfected routinely before handling animals and cages. Environmental conditions in housing rooms were 12:12-h light:dark cycle, 30% to 50% relative humidity, and 20 to 23 °C.
Over 1 y, cases of morbidity and mortality were reported for 9 breeding cages, mostly affecting adult female mice but occasionally affecting neonatal, weanling, and male breeder mice. Most mice were found lethargic or moribund (n = 3) or dead (n = 21). Other clinical presentations included dystocia (n = 3), head tilt (n = 1), severe abdominal distention (n = 1), and seizures (n = 3). All mice were observed to be healthy the day before clinical presentation. Differential diagnoses for cases affecting neonatal and pregnant mice included strain-associated poor reproductive performance and dystocia.
In one colony, several dams were reported for dystocia. For diagnostic workup, a gravid moribund female mouse was euthanized. Necropsy revealed areas filled with dark blood and 2 dead feti in the right uterine horn. On histopathology, the dam had multifocal myocardial abscesses and suppurative and necrotizing nephritis and placentitis; intralesional colonies of gram-negative coccobacilli were present. PCR assay of the uterine tissue block was positive for Pseudomonas aeruginosa. Fecal sample and oral and skin swabs from another moribund dam also were analyzed by PCR assays (PCR Rodent Infectious Agent, Charles River Laboratories, Wilmington, MA); results indicated that the mouse was fecal PCR-positive for Helicobacter spp., P. aeruginosa, and Staphylococcus xylosus.
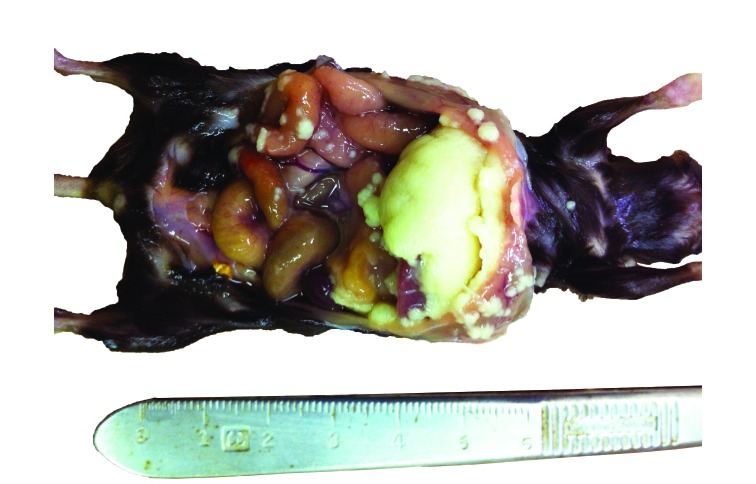
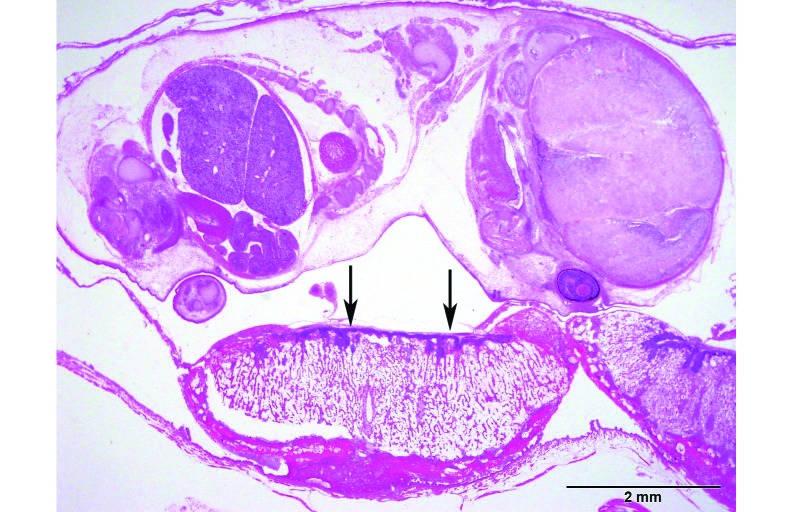
In another colony in a separate vivarium, a female mouse that recently had a litter presented with severe abdominal distention resulting from peritoneal abscesses (Figure 1). Histopathology revealed infection of the liver, spleen, kidneys, urinary bladder, uterus, stomach, and intestines with gram-negative bacterial coccobacilli. A second moribund pregnant dam was euthanized, and CBC revealed moderate leukocytosis and neutrophilia. On histopathology, the placental disc of one fetus had extremely high numbers of plump bacterial rods admixed with moderate numbers of degenerate neutrophils (Figure 2). The bacteria and inflammation occasionally extended into superficial endometrium and myometrium. No bacteria were present in or on the feti. Fetal distress was evident by the presence of aspirated keratinized squamous epithelial cells in the bronchi of one pup. A third dam had bacterial infection and necrosis of the placenta and endometrium with minimal neutrophilic infiltrate; one fetus had bacterial rods in the lungs. A fourth dam was euthanized due to moribundity, and histopathology revealed neutrophilic meningoencephalitis with bacterial coccobacilli in the pia–arachnoid and superficial Virchow–Robbins spaces of the cerebrum, cerebellum, trigeminal nerve, and thalamus. Gram staining of affected tissues in all 4 dams revealed intralesional plump gram-negative rods.
Figure 1.
Severe peritonitis with abscess formation. There were marked fibrinosuppurative exudates on the liver capsule and peritoneum.
Figure 2.
Placentitis and metritis. The gravid uterus had large numbers of bacteria within the chorionic disc, with moderate necrosis and mild neutrophilic infiltrate (arrows). Hematoxylin and eosin stain; bar, 2 mm.
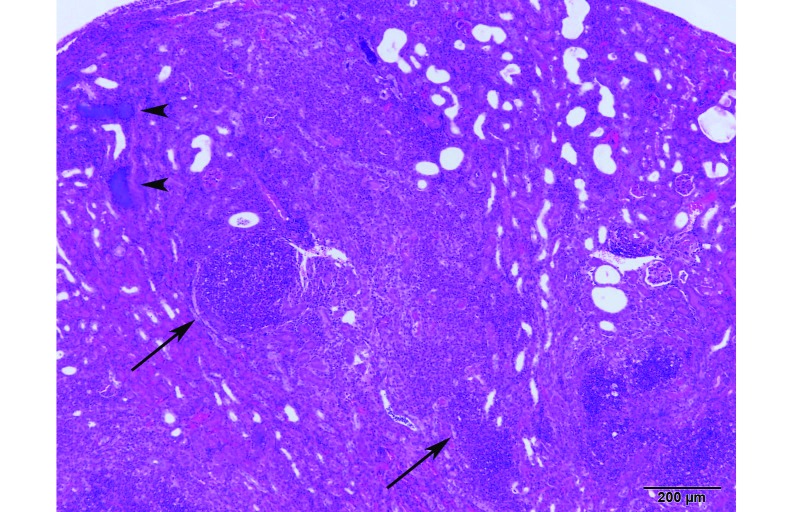
In the same colony, a dam was found dead 6 d after parturition. Thereafter, the 2 dams and their breeding mates and litters used for cross-fostering of the orphaned litter were found moribund or dead. Multiple mice of various ages were submitted for histopathology; most mice had organs that were autolyzed or normal. However, 2 mice had significant findings. A dead weanling's spleen had moderate multifocal recent hemorrhage, with bacteria, fibrin, and karyorrhectic nuclear dust in the red and white pulp. PCR assay results of the spleen tissue block from this mouse were equivocal for P. aeruginosa, indicating that the bacterial DNA copy number was estimated to be very low. In addition, a male breeder mouse had pinpoint white nodules on the right kidney. On histopathology, the renal cortex and medulla had multiple coalescing abscesses consisting of degenerate neutrophils admixed with abundant necrotic cellular debris (Figure 3).
Figure 3.
Transverse renal section from a male breeder mouse showing multiple coalescing abscesses (arrows) consisting of degenerate neutrophils admixed with abundant necrotic cellular debris and large colonies of plump bacterial rods (arrowheads). Hematoxylin and eosin stain; bar, 200 µm.
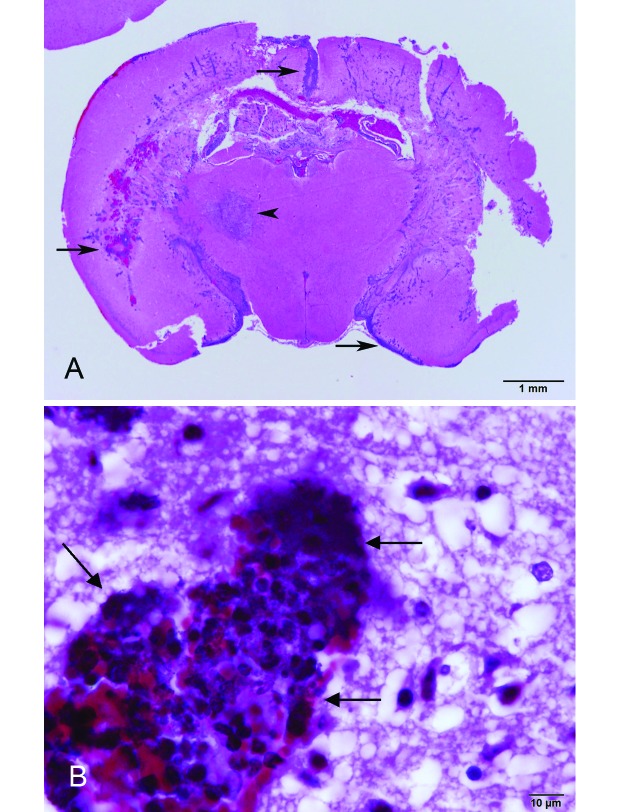
In another colony in the same mouse room as the second colony, 3 mice from 2 cages had violent continuous seizures and were euthanized. Three other mice in the cages were found dead without apparent clinical signs a few days afterward. Histopathology of one mouse with seizures revealed recent infarct in the thalamus and acute, severe, suppurative meningoencephalitis and ventriculitis (Figure 4 B) with short gram-negative bacterial rods (Figure 4 A). Swab culture of the cardiac blood of this mouse and the sipper tube of the water bottle given to the mouse were positive for P. aeruginosa.
Figure 4.
Meningoencephalitis in a mouse that had seizures. (A) Severe suppurative meningoencephalitis (arrows) with a unilateral infarct in the thalamus (arrowhead). Hematoxylin and eosin stain; bar, 1 mm. (B) Gram-negative rods in the cerebral cortex (arrows). Brown and Hopps stain; bar, 10 µm.
Discussion
The impaired immune phenotype of the Myd88−/− mice reported in these cases likely predisposed them to bacterial infections. Morbidity and mortality were more prevalent for the Myd88−/− colony than for others of the various mouse strains and stocks (for example, C56Bl/6J and Swiss Webster) housed in the same holding rooms and vivaria. The presence of aspirated squames in the lung of one fetus was consistent with fetal stress due to dystocia, and dystocia and neonatal death could be due to innate poor reproductive performance per strain characteristics. However, some of the euthanized mice and those found dead (including 3 pregnant dams initially diagnosed with dystocia) had bacterial infections according to gross and histopathologic findings.
Pathogens may be introduced into rodent colonies by various means, including incoming rodent shipments, unscreened biologics, and fomites such as people and contaminated food and bedding. Common murine adventitious pathogens were excluded from the list of our differential diagnoses in light of results from sentinel mice screening. The husbandry practices in our facility should have provided the mice with a sterile microenvironment. However, mice could have been contaminated during handling, even though procedures were supposed to be done aseptically. In addition, mice were used only for breeding and were not removed from the animal rooms.
The results of fecal PCR analysis of one moribund mouse indicated the presence of 2 gram-negative bacteria in the colony: Helicobacter spp. and P. aeruginosa. Helicobacter has been detected in the reproductive organs of mice, including the ovary, uterus, testis and epididymis,23,26 and markedly decreased pregnancy rates and pup survival in IL10−/− mice.26 However, Helicobacter has helical shape in contrast to P. aeruginosa’s rod shape, a morphology consistent with the intralesional bacteria in our cases. In addition, a swab culture of the cardiac blood of an affected mouse was positive for P. aeruginosa. Tissue-block PCR assays revealed that only 2 more mice from 7 other affected mice were positive or equivocal for P. aeruginosa. Other gram-negative bacilli that could be the causative agent include Escherichia coli, Proteus mirabilis, and Klebsiella spp.
P. aeruginosa is commonly found in warm, moist environments and has been demonstrated to survive in autoclaved drinking water.5 In addition, P. aeruginosa can form biofilms in sipper tubes, posing a sanitation challenge. Historical results of our microenvironmental monitoring program using RODAC plates indicated that some swabs of sanitized sipper tubes occasionally grew bacteria, which had not been identified. The mice reported here could have been orally inoculated with P. aeruginosa through inadequately sanitized sipper tubes. In light of our findings, we offer 3 possibilities for the pathogenesis involving P. aeruginosa. First, the ingested organisms could penetrate the nasal and oral mucous membranes, as previously reported,3 and invade surrounding tissues including the brain, causing meningoencephalitis, and the regional lymph nodes, causing systemic infections. Second, the organisms could have invaded the intestinal tract, particularly the cecum and colon, and caused systemic disease.18 Third, given that 2 dams had metritis and placentitis without bacteremia, fecal shedding of the organisms is possible, leading to an ascending infection through the open cervix (transcervical route) at parturition. For the unusual case of the mouse with peritoneal abscesses, rupture of hollow viscus (gastrointestinal tract or uterus) is possible, especially given that the animal had uterine implantation sites consistent with recent pregnancy. In one mouse, suppurative meningoencephalitis could have caused the seizures directly, leading to a thalamic infarct. Because Myd88−/− mice are immunodeficient and have severely impaired bacterial recognition,27 the mice may have been unable to mount the required immune response to the bacteria.
A literature search revealed that placentitis and meningoencephalitis have never been reported as nonexperimental conditions in mice. In one experiment, ingestion by mice of Listeria monocytogenes in drinking water caused purulent panmetritis and necrotizing placentitis,15 2 conditions seen in the mice we report here. In other animal species, P. aeruginosa has been identified to cause equine placentitis, a well-described disease entity that is considered the single most important factor in abortion and stillbirth in horses.9 Meanwhile, experimental meningoencephalitis in mice has been produced by intracerebral inoculation of P. aeruginosa.6,17
In mammals, bacterial antigen recognition is mediated by several membrane receptors.29 TLRs have been identified as pattern recognition receptors that recognize different pathogen-associated molecular patterns, and the interactions between TLRs and bacteria or their components, such as the LPS of gram-negative bacteria and lipoteichoic acid of gram-positive bacteria, have been studied.7,19 TLR induce distinct patterns of host responses through MyD88-dependent or -independent pathways, depending on the pathogen's nature. MyD88-dependent pathway has been reported to be essential for the host defense against infections by S. aureus,27 Toxoplasma gondii,21 and L. monocytogenes.24
MyD88 has been reported to be essential for innate immunity to P. aeruginosa.25 In contrast to leukopenic or neutropenic mice that are highly susceptible to P. aeruginosa,11,22 Myd88−/− mice have normal numbers of polymorphonuclear neutrophils12 that have normal migratory ability.31 However, Myd88−/− mice are defective in mounting an early cytokine or inflammatory response or to control bacterial replication after experimental infection with P. aeruginosa.12,20,25 In these reports, the mice had reduced levels of inflammatory mediators (for example, macrophage inflammatory protein 2, TNF, and IL1β), leading to the defective influx of neutrophils into the airways. This is especially important, given that neutrophils comprise a major component of host resistance to P. aeruginosa infection, particularly in acute settings of bacteremia and sepsis.12,16 This defect is also consistent with our findings of Virchow–Robbins spaces with pure bacterial populations without attendant inflammatory cells in a mouse with meningoencephalitis and extremely high numbers of bacteria with only mild neutrophilic infiltrate in the placenta of a dam with dystocia that was euthanized. In addition, our cases could be characterized as acute, occurring even hours after the mice had been observed to be clinically healthy. This pattern suggests that MyD88-mediated immune events may play a vital role in host protection against acute infection caused by P. aeruginosa. Although Myd88−/− mice are resistant to LPS-induced endotoxin shock,10 TLR4 could signal through TRIF, an MyD88-independent pathway.30
An ounce of prevention is truly worth a pound of cure, especially concerning rodent pathogens. Strict barrier conditions and sound husbandry practices are important, especially with immunodeficient rodent strains like Myd88−/− mice. Other practices may include the provision of prophylactic antibiotics either in the drinking water or feed. For example, drinking water supplemented with trimethoprim (0.032 mg/mL) and sulfamethoxazole (0.04 mg/mL) has been used in Myd88−/− colonies.2,4 Alternatively, postsanitization autoclaving of bottles and the use of acidified (pH 2.5)28 or chlorinated (7 to 10 ppm)8,13 water have been shown to be effective in preventing growth of P. aeruginosa. Although we have not provided the mice with treated drinking water, we instituted procedures to prevent cross-contamination in the animal colonies, including changes in traffic flow, and to ensure that sipper tubes are disinfected and autoclaved properly. In addition, reverse-osmosis water systems are now flushed regularly, yielding passing results for water culture using eosin-methylene blue agar plates.
In summary, we report cases of morbidity and mortality due to bacterial infections in Myd88−/− mice. The immune phenotype and instigating environmental circumstances likely contributed to these cases. Sound husbandry and experimental practices are necessary to prevent the exposure of immunodeficient mice to pathogens that could affect colony health.
Acknowledgments
We thank Dr Karen Vargas, Ms Corina Rodriguez, and Dr Natalie Williams-Bouyer. The study for which the animals were used is funded by the National Institute for Allergy and Infectious Diseases (grant no. AI021242 to David Walker).
References
- 1.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. 1998. Targeted disruption of the MyD88 gene results in loss of IL1- and IL18-mediated function. Immunity 9:143–150 [DOI] [PubMed] [Google Scholar]
- 2.Bolz DD, Sundsbak RS, Ma Y, Akira S, Weis JH, Schwan TG, Weis JJ. 2006. Dual role of MyD88 in rapid clearance of relapsing fever Borrelia spp. Infect Immun 74:6750–6760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownstein DG. 1978. Pathogenesis of bacteremia due to Pseudomonas aeruginosa in cyclophosphamide-treated mice and potentiation of virulence of endogenous streptococci. J Infect Dis 137:795 –801 [DOI] [PubMed] [Google Scholar]
- 4.Buchholz BM, Billiar TR, Bauer AJ. 2010. Dominant role of the MyD88-dependent signaling pathway in mediating early endotoxin-induced murine ileus. Am J Physiol Gastrointest Liver Physiol 299:G531–G538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd JJ, Xu HS, Colwell RR. 1991. Viable but nonculturable bacteria in drinking water. Appl Environ Microbiol 57:875–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campanile F, Fioretti MC, Bonmassar E, Puccetti P. 1985. Evaluation of antibacterial activity in experimental meningoencephalitis in mice. J Antibiot (Tokyo) 38:1083–1087 [DOI] [PubMed] [Google Scholar]
- 7.Flo TH, Ryan L, Latz E, Takeuchi O, Monks BG, Lien E, Halaas Ø, Akira S, Skjåk-Braek G, Golenbock DT, Espevik T. 2002. Involvement of Toll-like receptor (TLR) 2 and TLR4 in cell activation by mannuronic acid polymers. J Biol Chem 277:35489–35495 [DOI] [PubMed] [Google Scholar]
- 8.Homberger FR, Pataki Z, Thomann PE. 1993. Control of Pseudomonas aeruginosa infection in mice by chlorine treatment of drinking water. Lab Anim Sci 43:635–637 [PubMed] [Google Scholar]
- 9.Hong CB, Donahue JM, Giles RC, Jr, Petrites-Murphy MB, Poonacha KB, Roberts AW, Smith BJ, Tramontin RR, Tuttle PA, Swerczek TW. 1993. Etiology and pathology of equine placentitis. J Vet Diagn Invest 5:56–63 [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115–122 [DOI] [PubMed] [Google Scholar]
- 11.Koh AY, Priebe GP, Pier GB. 2005. Virulence of Pseudomonas aeruginosa in a murine model of gastrointestinal colonization and dissemination in neutropenia. Infect Immun 73:2262–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh AY, Priebe GP, Ray C, Van Rooijen N, Pier GB. 2009. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun 77:5300–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahabir E, Bulian D, Bensch S, Schmidt J. 2009. Elimination of P. aeruginosa in mice by treatment with chlorine and the use of microbiological and PCR analyses. Scand J Lab Anim Sci 36:355–361 [Google Scholar]
- 14.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr 1998. MyD88 is an adaptor protein in the hToll/IL1 receptor family signaling pathways. Mol Cell 2:253–258 [DOI] [PubMed] [Google Scholar]
- 15.Miller JK, Burns J. 1970. Histopathology of Listeria monocytogenes after oral feeding to mice. Appl Microbiol 19:772–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizgerd JP. 2002. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol 14:123–132 [DOI] [PubMed] [Google Scholar]
- 17.Padeıskaia EN, Mnatsakanian VE. 1991. [Activity of fluoroquinolones studied on a model of suppurative meningoencephalitis caused by Pseudomonas aeruginosa in mice.] Antibiot Khimioter 36:25–28 [Article in Russian] [PubMed] [Google Scholar]
- 18.Percy DH, Barthold SW. 2007. Mouse, p 69. In: Pathology of laboratory rodents and rabbits, 3rd ed. Ames (IO): Blackwell Publishing. [Google Scholar]
- 19.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088 [DOI] [PubMed] [Google Scholar]
- 20.Power MR, Peng Y, Maydanski E, Marshall JS, Lin TJ. 2004. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J Biol Chem 279:49315–49322 [DOI] [PubMed] [Google Scholar]
- 21.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL12 production by dendritic cells. J Immunol 168:5997–6001 [DOI] [PubMed] [Google Scholar]
- 22.Scarff JM, Goldberg JB. 2008. Vaccination against Pseudomonas aeruginosa pneumonia in immunocompromised mice. Clin Vaccine Immunol 15:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scavizzi F, Raspa M. 2006. Helicobacter typhlonius was detected in the sex organs of 3 mouse strains but did not transmit vertically. Lab Anim 40:70–79 [DOI] [PubMed] [Google Scholar]
- 24.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early-phase clearance of Listeria monocytogenes in mice. J Immunol 169:3863–3868 [DOI] [PubMed] [Google Scholar]
- 25.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol 172:3377–3381 [DOI] [PubMed] [Google Scholar]
- 26.Sharp JM, Vanderford DA, Chichlowski M, Myles MH, Hale LP. 2008. Helicobacter infection decreases reproductive performance of IL10-deficient mice. Comp Med 58:447–453 [PMC free article] [PubMed] [Google Scholar]
- 27.Takeuchi O, Hoshino K, Akira S. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 165:5392–5396 [DOI] [PubMed] [Google Scholar]
- 28.Trentin JJ, Van Hoosier GL, Jr, Shields J, Stephens K, Stenback WA. 1966. Establishment of a caesarean-derived, gnotobiote foster nursed inbred mouse colony with observations on the control of Pseudomonas. Lab Anim Care 16:109–118 [PubMed] [Google Scholar]
- 29.Watanabe K, Shin EK, Hashino M, Tachibana M, Watarai M. 2010. Toll-like receptor 2 and class B scavenger receptor type I are required for bacterial uptake by trophoblast giant cells. Mol Immunol 47:1989–1996 [DOI] [PubMed] [Google Scholar]
- 30.Watts C. 2008. Location, location, location: identifying the neighborhoods of LPS signaling. Nat Immunol 9:343–345 [DOI] [PubMed] [Google Scholar]
- 31.Weighardt H, Kaiser-Moore S, Vabulas RM, Kirschning CJ, Wagner H, Holzmann B. 2002. Cutting edge: myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J Immunol 169:2823–2827 [DOI] [PubMed] [Google Scholar]