Abstract
The objective of this study was to investigate the effects of liraglutide, an analog of human glucagon-like peptide 1 (GLP1), on WBN/Kob-Leprfa (fa/fa) rats, which spontaneously develop type 2 diabetes mellitus with pancreatic disorder and obesity. Male fa/fa rats (age, 7 wk) were allocated into 4 groups and received liraglutide (37.5, 75, 150 μg/kg SC) or saline (control group) once daily for 4 wk. All rats in the control group became overweight and developed hyperglycemia as they aged. Although the rats given liraglutide showed a dose-dependent reduction in food intake, no significant effects on body weight or fat content occurred. In the liraglutide groups, the development of hyperglycemia was suppressed, even as plasma insulin concentrations increased in a dose-dependent manner. Intravenous glucose tolerance testing of the liraglutide-treated rats confirmed improvement of glucose tolerance and enhanced insulin secretion. Histologic examination revealed increased numbers of pancreatic β-cell type islet cells and increased proliferation of epithelial cells of the small ducts in the liraglutide-treated groups. Although our study did not reveal a significant decrease in obesity after liraglutide administration, the results suggest a marked antidiabetic effect characterized by increased insulin secretion in fa/fa rats with pancreatic disorders.
Abbreviations: GLP1, glucagon-like peptide-1; IVGTT, intravenous glucose tolerance testing; T2DM, type 2 diabetes mellitus
The number of patients diagnosed with diabetes has more than doubled over the last 30 y, and diabetes has become an important public health concern worldwide.6 Approximately 90% of patients with diabetes are diagnosed with type 2 diabetes mellitus (T2DM).31 The onset of T2DM is determined by multiple factors that lead to reduced insulin secretion or insulin resistance, including genetic predisposition and lifestyle-associated habits such as lack of exercise, overeating, and obesity. Many drugs are already used clinically to treat T2DM;9,11 nevertheless, the search and development of more efficient and safe drugs is currently underway. In this regard, incretin has recently gained attention as a member of a class of drugs used to treat T2DM.9,11
Enteroendocrine cells secret incretin hormones, which augment glucose-induced insulin secretion in response to food ingestion, in a glucose-dependent manner. Currently, the 2 confirmed incretins are glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 (GLP1). Research has shown that GLP1 derivatives have functions in addition to the promotion of insulin secretion, including facilitation of β-cell proliferation,28 suppression of β-cell apoptosis,12 and promotion of β-cell differentiation or de novo formation of β cells.29,30 GLP1 derivatives have been reported to have multiple nonpancreatic functions, including suppression of appetite and body weight,7,13 suppression of gastric secretions,19 reduction of lipid accumulation in the liver,17 and promotion of sensitivity to insulin in adipose cells and skeletal muscle.8,22
WBN/Kob-type male rats are a relevant animal model for diabetes without obesity. These rats typically show disease conditions including chronic pancreatitis and pancreatic endocrine disorders.18,26 A new model rat for diabetes was established recently by crossing rats carrying the Leprfa obesity gene with wild-type WBN/Kob rats, yielding a fa/fa congenic strain.1 The obesity gene (Leprfa) is a spontaneous mutation derived from Zucker-fatty rats that leads to dysfunction of the leptin receptor. Rats homozygous for this gene are obese, resistant to insulin, and hyperinsulinemic.4,16,32 Male WBN/Kob-Leprfa rats (hereafter referred to as fa/fa rats) represent a new animal model in which the animals spontaneously develop diabetes in addition to endogenous insulin resistance. Compared with the parental strains, fa/fa rats are characterized by an earlier onset of diabetes and more severe pancreatic complications.1,2 Our previous investigations have revealed that fa/fa rats present with hyperinsulinemia at a prediabetic stage as a compensatory response to insulin resistance, but these rats show high blood glucose levels because of a difficulty in maintaining blood insulin concentrations as a consequence of declining pancreatic β-cell function associated with advancing age.14
In the current study, to further validate fa/fa rats as a T2DM model, we investigated the effects of a GLP1 analog in fa/fa rats with hyperglycemia (age, 7 to 11 wk). We used liraglutide, a human GLP1 analog, which has been shown to be clinically effective in T2DM patients.9,11
Materials and Methods
Test animals and growth conditions.
We obtained male fa/fa rats from Japan SLC (Shizuoka, Japan). The rats were housed individually in plastic cages under the following conditions: temperature, 21 ± 2 °C; humidity, 55% ± 5%; and lights on for 12 h daily from 0800 to 2000. They were allowed free access to food (CRF1, Charles River Laboratories Japan, Kanagawa, Japan) and tap water from a plastic water bottle. All animal studies were approved by the Azabu University Animal Research Ethics Committee.
Research protocol.
We allocated 7-wk-old fa/fa rats (n = 32) into 4 groups; a control group (n = 8) and 3 liraglutide-treatment groups (doses: 37.5, 75, and 150 μg/kg group; 8 rats per group). Saline or liraglutide (Victoza, Novo Nordisk Pharma, Tokyo, Japan) was administered subcutaneously once daily for 4 wk. These study protocols were selected in light of the results from our previous14 and preliminary studies, in which bolus administration of liraglutide (37.5 to 150 μg/kg SC) caused a dose-dependent increase in plasma insulin levels. At the end of the study, when the rats were 11 wk old, we performed intravenous glucose tolerance testing (IVGTT), after which the rats were euthanized by exsanguination under anesthesia with pentobarbital sodium (50 to 60 mg/kg IP; Kyoritsu Seiyaku, Tokyo, Japan).
Blood sampling and plasma separation.
Blood was drawn from the tail vein of the rats every week prior to administration of either saline or liraglutide. At the end of the experiment, we obtained blood from the abdominal aorta under pentobarbital anesthesia. Heparin sodium (Mitsubishi Tanabe Pharma, Tokyo, Japan) was used as an anticoagulant, and plasma was separated from the collected blood by centrifugation at 3000 × g for 10 min.
IVGTT.
IVGTT was performed at the end of the study, when the rats were 11 wk old. After being fasted for 18 h, the rats were anesthetized by using pentobarbital sodium (50 to 60 mg/kg IP), and 0.2 mL of blood was obtained from the jugular vein. The rats then underwent glucose loading (0.5 g/kg) via injection of a glucose solution (20% w/v; Otsuka Pharmaceutical, Tokyo, Japan) into the femoral vein. Equivalent volumes of blood were sampled from the jugular vein at 2, 5, 10, and 20 min after glucose loading. Heparin sodium was used as an anticoagulant, and plasma was separated by centrifugation (2000 × g for 15 min). We used the AUC of the plasma insulin values, determined by IVGTT, as an indicator of insulin secretory ability.
Measurement of blood biomarkers.
Plasma glucose levels were measured by using a blood glucose self-monitoring device (Accu-Chek Aviva, Roche Diagnostics, Indianapolis, IN) and Glucose CII-Testwako (Wako Pure Chemical Industries, Osaka, Japan). Plasma insulin concentrations were quantitated by ELISA (Super-Sensitive Rat Insulin Measurement Kit, Morinaga Institute of Biologic Science, Kanagawa, Japan).
Measurements of body weight, food consumption, and water intake.
Body weight was measured once weekly before the start of liraglutide or saline administration. After the initiation of drug administration, the body weight of the rats and their food and water intakes were measured between 1000 and 1400 twice each week.
Measurements of fat content.
After IVGTT, anesthetized rats were euthanized by exsanguination, and the liver, pancreas, epididymal fat, and mesenteric fat were harvested and weighed.
Pancreatic histopathologic examination.
Pancreatic tissue isolated during necropsy was fixed in a 10% neutral buffered formalin solution and processed according to standard histologic techniques. After paraffin-embedding, 4-μm sections were cut and stained with hematoxylin and eosin for histopathologic evaluation. In addition, tissue sections were stained with antibodies against insulin and the cell-proliferation marker Ki67.
Pancreatic tissue sections were deparaffinized and rehydrated according to previously published methods,20 and immunohistochemical staining was conducted for insulin or Ki67. Antigen retrieval for Ki67 was performed in citrate-buffered solution (pH 6.0) for 10 min at 121 °C in an autoclave. The sections slated for insulin immunohistochemical staining were rinsed with 50 mM Tris-buffered saline (pH 7.6), whereas the sections used for Ki67 immunohistochemistry were rinsed with 10 mM PBS (pH 7.2). All sections subsequently were exposed to 1% hydrogen peroxide in methanol and rinsed again with the buffer used previously. The sections then were incubated in 8% nonfat milk for 30 min (to block nonspecific antibody binding) followed by a polyclonal guinea pig antiinsulin antibody (dilution, 1:100; catalog no. A0564, Dako Japan, Kyoto, Japan) for 1 h at 4 °C or a rabbit antiKi67 polyclonal antibody (dilution, 1:100; catalog no. ab66155, Abcam, Cambridge, MA) overnight at 4 °C. After slides were washed, antiinsulin and antiKi67 immunoreactivities were determined by using the avidin–biotin–peroxidase complex method (LSAB2 Kit, catalog no. K0609, Dako Japan) and the peroxidase-labeled polymer method (Histofine Simple Stain Rat MAX-PRO [MULTI], Nichirei Bioscience, Tokyo, Japan). All sections were counterstained with hematoxylin.
Statistical analysis.
Data are represented as means ± SE. Statistical analysis was conducted by using the Dunnett test, with significance defined as a P value of less than 0.05 (Prism 6, GraphPad Software, San Diego, CA).
Results
Changes to blood glucose and insulin levels.
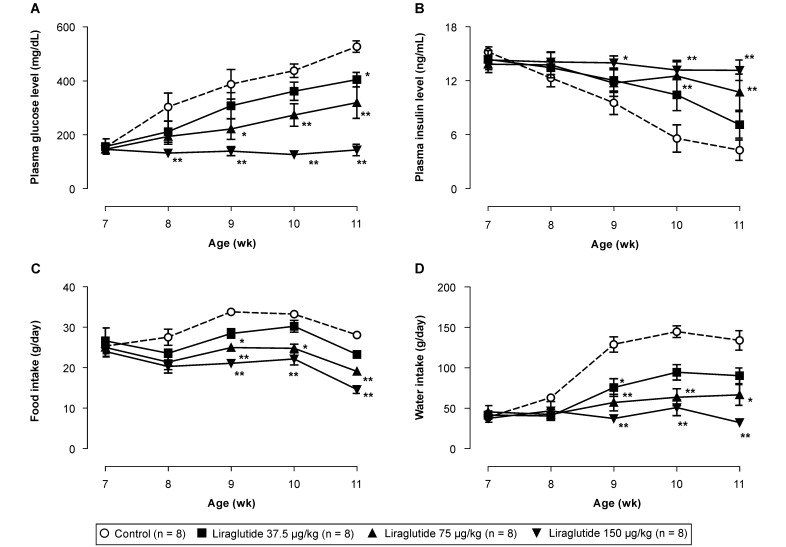
No significant intergroup differences were observed prior to the start of drug administration. The blood glucose levels in the control group (153 ± 11 mg/dL at 7 wk of age) increased with age, and at 11 wk of age, a high blood glucose level of 527 ± 21 mg/dL (P < 0.01 vs 7 wk of age) was observed (Figure 1). In contrast, the liraglutide groups showed dose-dependent reduction of blood glucose levels. Specifically, blood glucose levels at 7 wk of age did not differ from those at older ages in the high-dose liraglutide-treated groups. There were no differences among the 4 groups with regard to blood insulin concentrations in 7-wk-old rats prior to liraglutide administration. In the control group, blood insulin concentrations decreased gradually beginning at 8 wk of age. Alternatively, the reduction in insulin concentrations was attenuated dose-dependently in the liraglutide groups (Figure 1).
Figure 1.
Effects of liraglutide on (A) plasma glucose concentrations, (B) plasma insulin concentrations, (C) daily food intake, and (D) daily water intake in male fa/fa rats 7 to 11 wk of age. Data are expressed as means ± SE (n = 8). *, P < 0.05; **, P < 0.01 compared with control value.
Changes to food and water intake.
No intergroup differences in food and water intakes of the 7-wk-old rats were observed during the preadministration period. After the initiation of drug administration, food and water intakes were reduced dose-dependently in the liraglutide-treated rats, with significant (P < 0.05) differences observed after 8 wk of age in the high-dose (150 µg/kg) liraglutide group (Figure 1).
IVGTT.
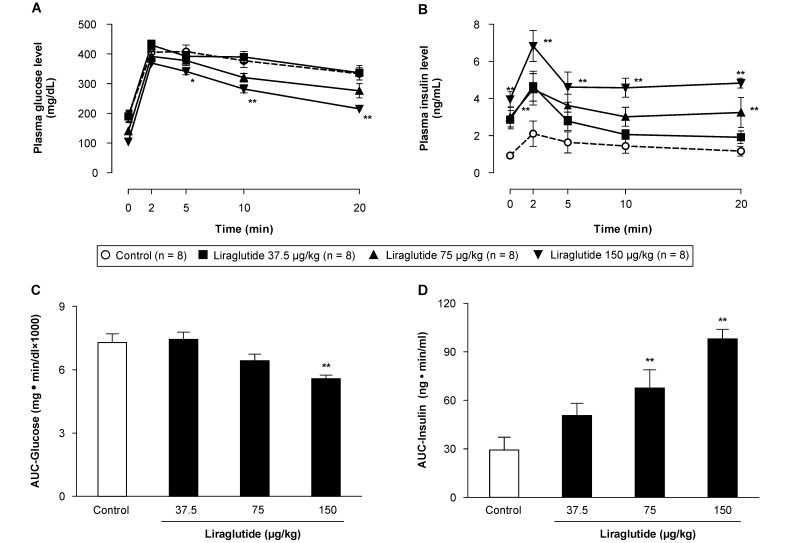
Blood glucose levels peaked 2 to 5 min after glucose loading in all groups and gradually decreased thereafter. The blood glucose levels at 5 to 20 min decreased in a dose-dependent manner in the liraglutide groups, and statistical significances (P < 0.05) were evident in the high-dose (150 µg/kg) group. Blood plasma insulin concentrations increased in response to blood glucose levels and peaked 2 min after glucose loading. Compared with the control group, the liraglutide-treated rats showed consistently and significantly (P < 0.05) higher blood plasma insulin concentrations before and after glucose loading (Figure 2).
Figure 2.
Effects of liraglutide on plasma (A) glucose and (B) insulin concentrations and (C, D) their respective AUC in male fa/fa rats undergoing intravenous glucose tolerance testing (IVGTT). Data are expressed as means ± SE (n = 8). *, P < 0.05; **, P < 0.01 compared with control values.
As an index of glucose intolerance, we calculated the glucose AUC from the plasma glucose concentrations obtained after IVGTT. A similar calculation was performed to obtain the insulin AUC from plasma insulin concentrations, which facilitated the derivation of an index for assessing insulin secretion. The high-dose (150 µg/kg) liraglutide group showed a significant (P < 0.05) reduction in glucose AUC, confirming improved glucose tolerance. The insulin AUC in the liraglutide groups increased in a dose-dependent manner, with significant (P < 0.01) differences between the intermediate (75 µg/kg)- and high-dose groups as compared with the control group (Figure 2).
Changes in body weight.
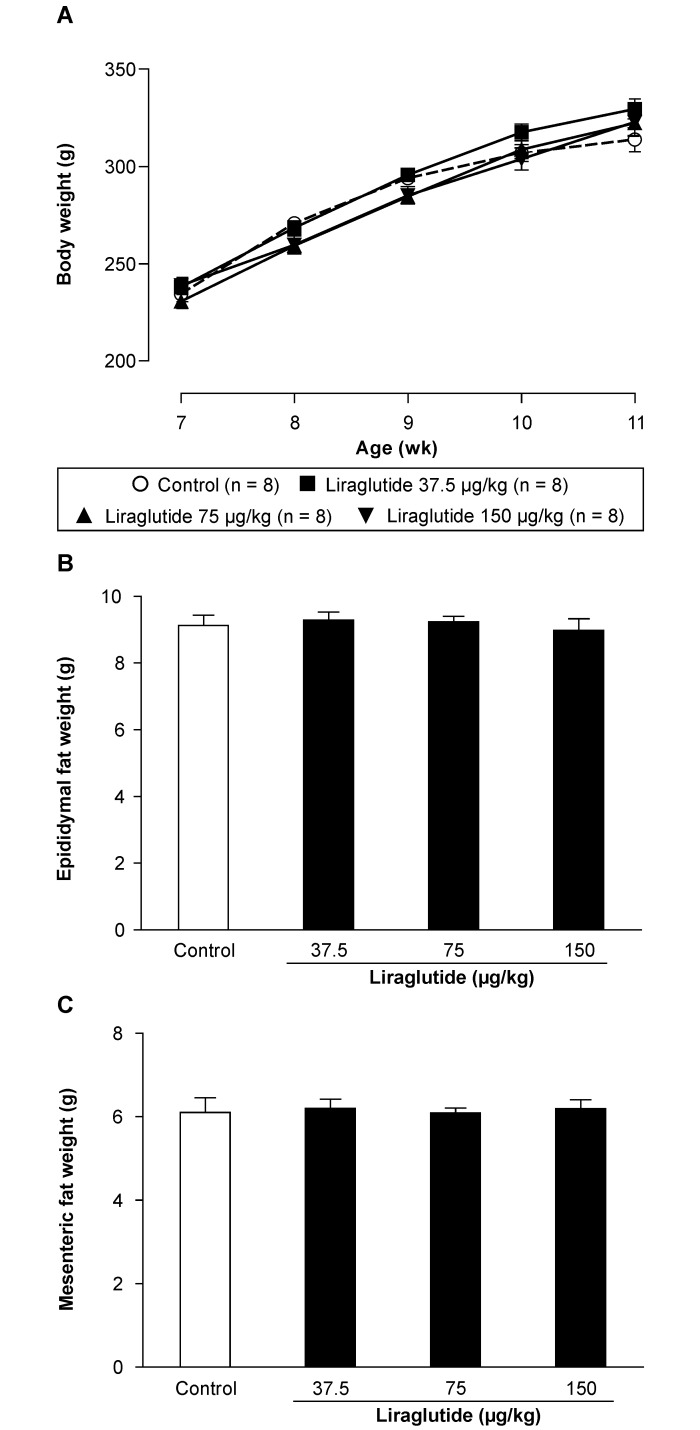
Intergroup differences in body weight at the start of the experimental period were not significant. The body weight of all groups continued to increase during the experiment with no significant differences between the control and liraglutide groups (Figure 3).
Figure 3.
(A) Changes of body weight in male fa/fa rats treated with saline or liraglutide from 7 to 11 wk of age. Comparison of (B) epididymal fat weight and (C) mesenteric fat weight in fa/fa rats at 11 wk of age. Data are expressed as means ± SE (n = 8).
Fat content.
Fat contents in the epididymis and mesentery did not differ between the control and liraglutide groups. No significant reduction in fat content was detected after liraglutide administration (Figure 3).
Histopathology of the pancreas.
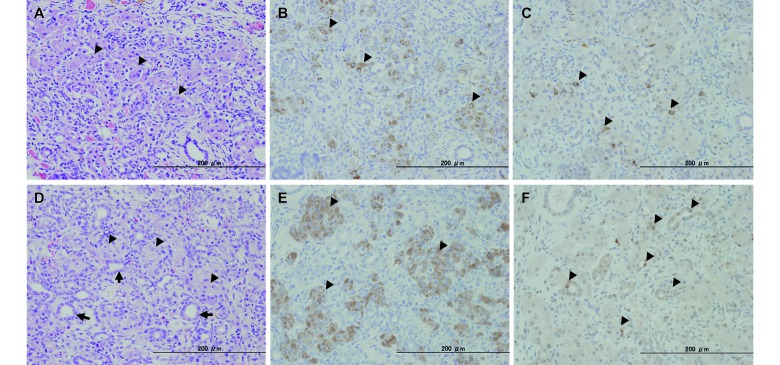
In the pancreata of the rats in the control and liraglutide groups, we noted evidence of mild-to-severe chronic interstitial pancreatitis, including interstitial fibrosis, proliferation of small ducts, acinar cell atrophy, hemosiderosis, and lymphocytic infiltration (Figure 4). Interstitial fibrosis spread to the islets, with clear evidence of damage to the islet structure. Furthermore, vacuolar degeneration was present in the remaining islet cells. We did not observe a significant effect of liraglutide on interstitial pancreatitis. In the regions where the small ducts had clear signs of proliferation or accumulation, large cells with weakly acidophilic cytoplasm (similar to pancreatic islet cells) were found scattered throughout the tissue. The number of these hypertrophic and pancreatic islet-like cells increased dose-dependently in the liraglutide groups. Immunohistochemical analysis revealed that the pancreatic islet-like cells stained positively for antiinsulin antibodies, indicating that these cells produced insulin. In contrast, these cells stained negatively for antiKi67 antibodies; however, the epithelial cells of the small and proliferative ducts, which were observed around the islet-like cells, were positive for antiKi67 antibodies (Figure 4).
Figure 4.
Histopathology and immunohistochemistry of the pancreas in male fa/fa rats treated with (A through C) saline or (D through F) 150 µg/kg liraglutide at 11 wk of age. (A and D) Hematoxylin and eosin staining. (B and E) Immunostaining for insulin. (C and F) Immunostaining for Ki67. Hematoxylin and eosin staining revealed disrupted islet cells (A, arrowheads), increased hypertrophic and islet-like cells (D, arrowheads), and proliferative and small ducts (D, arrows). Disrupted islet cells immunostained very weakly for insulin (B, arrowheads), but numerous hypertrophic and islet-like cells stained strongly for insulin (E, arrowheads). Immunostaining revealed few Ki67-positive interstitial cells (C, arrowheads). Many epithelial cells of proliferative and small ducts stained positive for Ki67 (F, arrowheads), but hypertrophic and islet-like cells were negative by Ki67 immunohistochemistry (F).
Discussion
In this study, to further validate fa/fa rats as a T2DM model, we investigated the effects of liraglutide, a human GLP1 analog that has been shown to be clinically effective in T2DM patients.9,11 Research to date has revealed that fa/fa rats possess genetic factors that cause insulin resistance. These rats can maintain normal blood glucose levels because of a compensatory increase in plasma insulin levels resulting from insulin resistance at a prediabetic stage. However, progression of pancreatitis eventually leads to islet cell destruction and a concomitant decrease in the ability to secrete insulin, culminating in the onset of diabetes.2,14 Our current findings supported these results, and we found that fa/fa rats were hyperinsulinemic at the age of 7 wk, and the onset of increase in blood glucose levels occurred concomitantly with reduction in plasma insulin concentrations in control rats 8 wk of age or older. Moreover, histologic examination of the pancreas revealed interstitial fibrosis, with little normal pancreatic islet tissue remaining. However, the previous studies14,21 also showed that plasma insulin levels in fa/fa rats at the onset of hyperglycemia were similar to those in normal rats. T1DM and T2DM are defined as a lack of insulin release and the action of insulin, respectively. Therefore, for fa/fa rats to represent a T1DM model is inconsistent with this definition. In contrast, insulin resistance is evident in fa/fa rats before and after the reduction in plasma insulin levels.14,21 In addition, an insulin sensitizer exerted prophylactic and therapeutic effects on the hyperglycemia in fa/fa rats, indicating that insulin resistance plays a key role in this model.21 These results suggest that fa/fa rats represent a model of T2DM that is associated with pancreatitis and obesity.
One reported action of GLP1 on β cells is the promotion of insulin secretion.10 The present study evaluated whether a GLP1 derivative promoted insulin secretion in fa/fa rats with advanced pancreatic dysfunction. The liraglutide-treated rats demonstrated dose-dependent increases in plasma insulin concentrations. This finding suggests that GLP1 derivatives can effectively promote insulin secretion in fa/fa rats, despite their advanced pancreatic dysfunction.
This study also demonstrated that liraglutide administration increases the number of insulin-positive islet-like cells, which resemble pancreatic β cells. Positive staining for antiKi67 antibodies and proliferative activity were not observed in the islet-like cells. However, these features were present in the epithelial cells of regenerated pancreatic ducts. We hypothesize that these epithelial cells are precursors to pancreatic β-cells.23 We conclude that the increase in the number of islet-like cells that we observed was not due to the proliferation of existing β cells but rather to the proliferation of precursor cells and their subsequent differentiation or de novo transformation into β cells.
Studies involving Sprague–Dawley rats with partial pancreatic resection or Goto–Kakizaki rats have demonstrated that β-cell proliferation and generation of β-cells can be induced by GLP1 derivatives.25,29 In contrast to this finding, we did not observe definitive proliferation of β cells, consistent with results in diabetic Zucker-fatty rats.24 One reason for this difference is that fa/fa rats show severe islet degeneration, which leads to a decreased number of normal β cells that are able to proliferate. In contrast, our study revealed proliferation of β-cell precursors. This finding suggests that GLP1 derivatives effectively induce β-cell generation despite advanced pancreatic dysfunction. Prolonged administration of sulphonylurea derivatives, which promote insulin release, can lead to secondary failure and a reduction in insulin secretion, but such failure is less common with GLP1 derivatives.5 The inductive effect of liraglutide on pancreatic β cells that we observed in our study could be related to the fact that secondary failures in efficacy are less frequent with GLP1 derivatives.
A factor that may contribute to the suppressed hyperglycemia in the liraglutide-treated rats is the liraglutide-induced reduction of food intake, which occurs in addition to the direct effect of liraglutide on increasing the number of insulin-containing pancreatic cells. The inhibitory effects of GLP1 derivatives, including liraglutide, on food intake are reportedly mediated by the activation of GLP1 receptors expressed on subdiaphragmatic vagal afferents, as well as in the brain.15 A previous report demonstrated that limiting food intake in male fa/fa rats at a young age reduced the severity of pancreatitis and led to the subsequent suppression of increased blood glucose levels.3 Therefore, liraglutide-induced suppression of hyperglycemia in the present study may reflect its intake-inhibitory effect in addition to its insulin-releasing effect. Water intake in the control group increased at 9 wk of age, when hyperglycemia developed; this effect was attenuated by liraglutide in a dose-dependent manner. The attenuated increase of water intake by liraglutide is likely secondary to T2DM prevention.
A problem in the treatment of T2DM is the increase in body weight that occurs concurrently with the use of sulphonylurea or insulin. One advantage afforded by the use of GLP1 derivatives as a treatment for diabetes is the suppressive effect on food intake and body weight.7,13,27 However, in contrast to previous reports, our current experiments did not demonstrate any effect of liraglutide administration on body weight. Our findings indicate that obesity in young fa/fa rats that develop T2DM cannot be inhibited solely by liraglutide-induced reduction of food intake. Although the mechanism of the discrepancy between food intake and body weight has yet to be elucidated, there are a couple of possible explanations. First, abnormal energy metabolism that is resistant to liraglutide could play a critical role in the obesity of fa/fa rats. Second, the effect of the reduction in food intake would have been negated by the hyperinsulinemia-induced promotion of glucose uptake in the periphery in the liraglutide groups. Third, the antihyperglycemic and antiobesity effects of liraglutide may be dependent on the liraglutide treatment period or the pathophysiologic state of fa/fa rats. We currently are investigating the therapeutic effects of liraglutide on hyperglycemia and obesity in fa/fa rats older than 11 wk.
The current study used the fa/fa rat model of diabetes, in which rats develop advanced pancreatic dysfunction, to demonstrate that the GLP1 derivative liraglutide effectively retards diabetes development and promotes blood glucose control. Furthermore, we provide strong evidence that fa/fa rats are useful as an animal model of pancreatitis as well as T2DM.
Acknowledgment
This study was supported in part by a research project grant awarded by Azabu University.
References
- 1.Akimoto T, Nakama K, Katsuta Y, Zhang XJ, Ohsuga M, Ishizaki M, Sawai N, Ozawa H. 2008. Characterization of a novel congenic strain of diabetic fatty (WBN/Kob-Leprfa) rat. Biochem Biophys Res Commun 366:556–562 [DOI] [PubMed] [Google Scholar]
- 2.Akimoto T, Terada M, Shimizu A. 2012. Progression of pancreatitis prior to diabetes onset in WBN/Kob-Leprfa rats. J Vet Med Sci 74:65–70 [DOI] [PubMed] [Google Scholar]
- 3.Akimoto T, Terada M, Shimizu A, Sawai N, Ozawa H. 2010. The influence of dietary restriction on the development of diabetes and pancreatitis in female WBN/Kob-fatty rats. Exp Anim 59:623–630 [DOI] [PubMed] [Google Scholar]
- 4.Chua SC, White DW, Wu-Peng XS, Liu SM, Okada N, Kershaw EE, Chung WK, Power-Kehoe L, Chua M, Tartaglia LA, Leibel RL. 1996. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr). Diabetes 45:1141–1143 [DOI] [PubMed] [Google Scholar]
- 5.Cook MN, Girman CJ, Stein PP, Alexander CM. 2007. Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with Type 2 diabetes in UK primary care. Diabet Med 24:350–358 [DOI] [PubMed] [Google Scholar]
- 6.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang Y-H, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. 2011. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country–years and 2.7 million participants. Lancet 378:31–40 [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ. 2006. The biology of incretin hormones. Cell Metab 3:153–165 [DOI] [PubMed] [Google Scholar]
- 8.González N, Acitores A, Sancho V, Valverde I, Villanueva-Peñacarrillo ML. 2005. Effect of GLP1 on glucose transport and its cell signalling in human myocytes. Regul Pept 126:203–211. [DOI] [PubMed] [Google Scholar]
- 9.Evans M, McEwan P, O'Shea R, George L. 2013. A retrospective, case-note survey of type 2 diabetes patients prescribed incretin-based therapies in clinical practice. Diabetes Ther 4:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gromada J, Holst JJ, Rorsman P. 1998. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch 435:583–594 [DOI] [PubMed] [Google Scholar]
- 11.Henry RR, Buse JB, Sesti G, Davies MJ, Jensen KH, Brett J, Pratley RE. 2011. Efficacy of antihyperglycemic therapies and the influence of baseline hemoglobin A(1C): a meta-analysis of the liraglutide development program. Endocr Pract 17:906–913 [DOI] [PubMed] [Google Scholar]
- 12.Hui H, Nourparvar A, Zhao X, Perfetti R. 2003. Glucagon-like peptide 1 inhibits apoptosis of insulin-secreting cells via a cyclic 5′-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 144:1444–1455 [DOI] [PubMed] [Google Scholar]
- 13.Jendle J, Nauck MA, Matthews DR, Frid A, Hermansen K, Düring M, Zdravkovic M, Strauss BJ, Garber AJLEAD-2 and LEAD-3 Study Groups. 2009. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab 11:1163–1172 [DOI] [PubMed] [Google Scholar]
- 14.Kaji N, Okuno A, Ohno-Ichiki K, Oki H, Ishizawa H, Shirai M, Asai F. 2012. Plasma profiles of glucose, insulin and lipids in the male WBN/Kob-Leprfa rat, a new model of type 2 diabetes with obesity. J Vet Med Sci 74:1185–1189 [DOI] [PubMed] [Google Scholar]
- 15.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. 2011. Peripheral and central GLP1 receptor populations mediate the anorectic effects of peripherally administered GLP1 receptor agonists, liraglutide and exendin-4. Endocrinology 152:3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leonard BL, Watson RN, Loomes KM, Phillips ARJ, Cooper GJ. 2005. Insulin resistance in the Zucker diabetic fatty rat: a metabolic characterisation of obese and lean phenotypes. Acta Diabetol 42:162–170 [DOI] [PubMed] [Google Scholar]
- 17.Mells JE, Fu PP, Sharma S, Olson DE, Cheng L, Handy JA, Saxena NK, Sorescu D, Anania FA. 2011. GLP1 analogue liraglutide ameliorates hepatic steatosis and cardiac hypertrophy in C57BL/6J mice fed a western diet. Am J Physiol Gastrointest Liver Physiol 302:G225–G235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakama K, Shichinohe K, Kobayashi K, Naito K, Uchida O, Yasuhara K, Tobe M. 1985. Spontaneous diabetes-like syndrome in WBN/Kob rats. Acta Diabet Lat 22: 335–342 [DOI] [PubMed] [Google Scholar]
- 19.Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Ørskov C, Ritzel R, Schmiegel WH. 1997. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol 273:E981–E988 [DOI] [PubMed] [Google Scholar]
- 20.Ogawa T, Sugidachi A, Tanaka N, Fujimoto K, Fukushige J, Tani Y, Asai F. 2005. Effects of R102444 and its active metabolite R96544, selective 5-HT2A receptor antagonists, on experimental acute and chronic pancreatitis: additional evidence for possible involvement of 5-HT2A receptors in the development of experimental pancreatitis. Eur J Pharmacol 521:156–163. [DOI] [PubMed] [Google Scholar]
- 21.Okuno A, Kaji N, Takahashi A, Nagakubo D, Ohno-Ichiki K, Shirai M, Asai F. 2013. Role of insulin resistance in the pathogenesis and development of type 2 diabetes in WBN/Kob-Leprfa rats. J Vet Med Sci 75:1557–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sancho V, Nuche B, Arnés L, Cancelas J, González N, Díaz-Miguel M, Martín-Duce A, Valverde I, Villanueva-Peñacarrillo ML. 2007. The action of GLP1 and exendins upon glucose transport in normal human adipocytes and on kinase activity as compared to morbidly obese patients. Int J Mol Med 19:961–966. [PubMed] [Google Scholar]
- 23.Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. 1999. The homeodomain protein IDX1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 48:507–513 [DOI] [PubMed] [Google Scholar]
- 24.Sturis J, Gotfredsen CF, Rømer J, Rolin B, Ribel U, Brand CL, Wilken M, Wassermann K, Deacon CF, Carr RD, Knudsen LB. 2003. GLP1 derivative liraglutide in rats with beta-cell deficiencies: influence of metabolic state on β-cell mass dynamics. Br J Pharmacol 140:123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tourrel C, Bailbe D, Lacorne M, Meile M-J, Kergoat M, Portha B. 2002. Persistent improvement of type 2 diabetes in the Goto–Kakizaki rat model by expansion of the β-cell mass during the prediabetic period with glucagon-like peptide 1 or exendin 4. Diabetes 51:1443–1452 [DOI] [PubMed] [Google Scholar]
- 26.Tsuchitani M, Saegusa T, Narama I, Nishikawa T, Gonda T. 1985. A new diabetic strain of rat (WBN/Kob). Lab Anim 19:200–207 [DOI] [PubMed] [Google Scholar]
- 27.Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CMB, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JPH, Smith DM, Ghatei MA, Herbert J, Bloom SR. 1996. A role for glucagon-like peptide 1 in the central regulation of feeding. Nature 379:69–72 [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Brubaker P. 2002. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8-week-old db/db mice. Diabetologia 45:1263–1273 [DOI] [PubMed] [Google Scholar]
- 29.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. 1999. Exendin 4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48:2270–2276 [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Pineyro MA, Wang X, Doyle ME, Egan JM. 2002. Exendin 4 differentiation of a human pancreatic duct cell line into endocrine cells: involvement of PDX1 and HNF3β transcription factors. J Cell Physiol 192:304–314 [DOI] [PubMed] [Google Scholar]
- 31.Zimmet P, Alberti KGMM, Shaw J. 2001. Global and societal implications of the diabetes epidemic. Nature 414:782–787 [DOI] [PubMed] [Google Scholar]
- 32.Zucker LM. 1965. Hereditary obesity in the rat associated with hyperlipemia. Ann N Y Acad Sci 131:447–458 [DOI] [PubMed] [Google Scholar]