Abstract
Many studies of the response of colonic tumors to therapeutics use tumor multiplicity as the endpoint to determine the effectiveness of the agent. These studies can be greatly enhanced by accurate measurements of tumor volume. Here we present a quantitative method to easily and accurately determine colonic tumor volume. This approach uses a biocompatible alginate to create a negative mold of a tumor-bearing colon; this mold is then used to make positive casts of dental stone that replicate the shape of each original tumor. The weight of the dental stone cast correlates highly with the weight of the dissected tumors. After refinement of the technique, overall error in tumor volume was 16.9% ± 7.9% and includes error from both the alginate and dental stone procedures. Because this technique is limited to molding of tumors in the colon, we utilized the ApcPirc/+ rat, which has a propensity for developing colonic tumors that reflect the location of the majority of human intestinal tumors. We have successfully used the described method to determine tumor volumes ranging from 4 to 196 mm3. Alginate molding combined with dental stone casting is a facile method for determining tumor volume in vivo without costly equipment or knowledge of analytic software. This broadly accessible method creates the opportunity to objectively study colonic tumors over time in living animals in conjunction with other experiments and without transferring animals from the facility where they are maintained.
Colon cancer is the third leading cause of cancer in men and women, with more than 100,000 new cases diagnosed each year in the United States alone. This disease is not limited to humans—cancers of the colon and rectum also affect companion species, such as dogs, albeit less frequently than in humans.20 Colorectal cancers generally develop from precancerous polyps, which can be detected and removed during colonoscopy screening before they become invasive cancers. However, not all precancers will become cancerous,23 and a better understanding of early tumor growth dynamics in models of the disease can simultaneously increase the rate of detection of polyps destined to become cancerous and decrease the rate of unnecessary removal of benign polyps.
Sizing of tumors creates an additional dimension beyond studies examining tumor multiplicity alone. Terminal sizing of tumors uses an eyepiece reticule under a dissection microscope to measure the maximal diameter of each tumor. However, this method likely misrepresents tumor volume for several reasons. First, tumors often are not symmetrical in shape, thereby limiting the interpretation of even multiple linear measurements. When volume calculations rely on the use of a formula, the irregular shape of solid tumors may require the testing of many different formulas to find the optimal one for that particular measurement and model.8 Second, if tumor sizing occurs after fixation, the original shape of the tumor can be affected. However, when tumor sizing occurs before fixation, the added time to size the tumors may result in degradation of the intestinal tissue, limiting further analysis. An alternate method of tumor sizing involves using the surrogate of tumor weight, the current ‘gold standard,’ for terminal studies. Tumor weight correlates closely with tumor size, although tumor density may vary depending on the tumor type. In addition, this technique is limited to use at the terminal time point. Methods that determine true tumor volume are powerful; those that can be applied in vivo to study the tumor longitudinally are even more compelling.
It recently has been recognized that not all early colonic tumors grow; some remain static for years whereas a few spontaneously regress.23 Importantly, the early growth profile of a tumor may correlate with its eventual fate.23 This aspect of tumor biology is a newly emerging area that deserves deeper study. The current gold standard for determining longitudinal tumor volume is CT, given that tumor weight is available only through terminal experiments. In mice, microCT colonography can be used to detect a 16% change in tumor volume with 95% confidence in living animals.5 However, the cost of CT equipment limits this technology to shared facilities, and the pathogen status of these facilities may preclude returning animals to the place where they were original housed, limiting the opportunities for longitudinal study. Importantly, many institutions do not have access to microCT technology, and even if available, 3D renderings must be recreated to determine tumor volume, a process requiring specialized software and detailed computing knowledge. Furthermore, CT exposes animal subjects to radiation, which may interfere with the tumor biology. Although MRI can be used to determine tumor volume accurately in the absence of ionizing radiation, specialized scanners and software are required, and enemas or intravenous treatments are needed to visualize tumors clearly.26
Another imaging modality uses the surface area of signal due to proteins expressing a fluorescent marker, such as red fluorescent protein, as a surrogate for tumor volume.17 However, tumor volume measured by fluorescent surface area12 may not accurately represent tumor volume in irregularly shaped tumors. In addition, this method necessitates a surgical procedure to orthotopically transplant fluorophore-expressing cells, raising questions of immune interactions between the recipient animal and the donor cells or to the surgery itself. If nude or immunocompromised animals are used in the procedure, the ability to study the immune aspect of tumor biology is reduced or eliminated.
Alternatively, tumor volume can be estimated from endoscopic images. The study of tumors by colonoscopy has become routine for both mouse6,10 and rat1,15 models of the disease. In contrast to terminal assessments, colonoscopy allows tumors to be visualized in vivo over time, capturing the dynamics of tumor growth. Documentation of this aspect of tumor biology can greatly enrich studies evaluating chemopreventive or therapeutic agents.6,15 Quantitative methods for determining tumor volume take this benefit a step further, allowing the investigation of the effects of background strain, therapeutic agents, environmental factors, or other modifiers of tumor growth pattern. One method to estimate tumor size uses the fraction of luminal cross-section occluded by tumor.2 However, the colonic lumen expands as the animal grows, and its size often increases to accommodate the growing tumor, to prevent intestinal blockage. Optical methods to extrapolate tumor sizes from 2D images obtained in vivo during colonoscopy are achieved by inserting a flexible metal rod of known dimensions into the working channel of the endoscope.10 However, because colonic tumors can differ in shape (some are flat whereas others are pedunculated), area measurements may not translate accurately to tumor volume.
To overcome these limitations and to add another tool to the growing cancer-research toolbox, we have developed a method using a biologically inert alginate to create negative molds of colonic tumors. These molds are filled with dental stone to achieve a positive cast of each tumor. A conversion factor then is used to calculate the volume of the original tumor from the dry weight of the dental stone cast. This procedure, which requires no specialized or expensive equipment and no complicated analytical methods, can be performed within the facility where the rats are housed and takes less than 15 min, including the 8 to 12 min during which the alginate sets. Therefore, our new method offers possibilities to study the dynamics of tumor growth in virtually any animal facility, regardless of the health status of subject animals or equipment availability.
Materials and Methods
Animal breeding and maintenance.
The ApcPirc/+ rat model for familial intestinal cancer (Taconic, Hudson, NY) was used in this study.1 As reported previously, tumor multiplicity and intestinal distribution differs between Pirc strains and between male and female Pirc rats.1,24 Therefore, both male and female F344 and (F344 × ACI) F1 rats were used in the study. Rats of diverse ages were used to mold and cast a broad spectrum of tumor sizes. The effect of age as a potential confounder was controlled whenever possible, given that both the multiplicity and size of tumors increase with age. Rats were SPF (negative for Helicobacter spp., pinworms, mites, rat pneumonia virus, H1 virus, Kilham rat virus, rat minute virus, rat coronavirus–sialodacryoadenitis virus, and rat theilovirus) and purpose-bred inhouse for research only. The pathogen status of the facility is monitored by using a sentinel monitoring program. Rats were housed in single-sex groups of 2 or 3 rats per standard shoebox caging containing corncob bedding. Rats were allowed free access to a commercial diet (5020 chow, Harlan, Madison, WI) and acidified water (hydrochloric acid to pH 2.5) delivered via an automatic watering system. Rats were maintained in an AAALAC-accredited facility, in which rat room temperature was maintained at an average of 71.5 °F (29.5 °C), humidity was maintained between 30% and 70%, and an automated timer system was used to maintain a 12:12-h light:dark cycle. All protocols were approved by the Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health and were consistent with the Guide for the Care and Use of Laboratory Animals.14
Endoscopy.
Rats were anesthetized with 3% isoflurane and placed ventral side down on a sterile surgical field above a heating pad to maintain a consistent body temperature during the procedure. The colon was flushed with 37 °C PBS (catalog no. 14190, Invitrogen, Grand Island, NY) to remove any fecal material and to lubricate the colon. A video from the proximal to distal colon was recorded, and a still image of each tumor was acquired by using a Hopkins 0° 1.9-mm endoscope (catalog no. 1232AA, Karl Storz, Tuttlingen, Germany) contained within a sheath (catalog no. 61029D, Karl Storz).
Alginate negative molds.
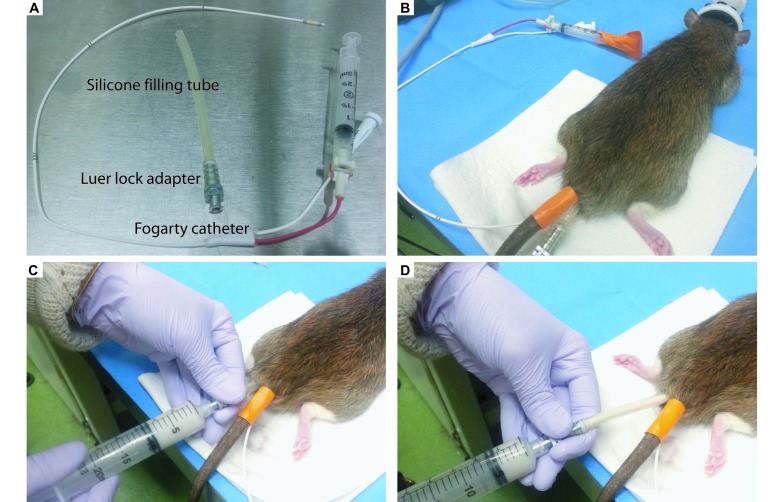
After anesthesia with isoflurane, a rat was prepared by inserting a 5.5 French (1.83 mm) Fogarty catheter (catalog no. 12TLW405F35, Edwards Lifesciences, Irvine, CA) about 10 to 12 cm into the colon and inflating (Figure 1 A and B). A 13.5-cm silicone filling tube (catalog no. 14-176-332B, Fisher Scientific, Waltham, MA) with a stainless steel luer-lock adapter (catalog no. VN530, Vita Needle, Needham, MA) was lubricated with mineral oil (ensuring that the mineral oil did not contact any of the colonoscopy equipment) and gently inserted into the rectum to the inflated Fogarty balloon (Figure 1 B). Then GenesisV alginate (1.5 g; Accu-cast, Bend, OR) was mixed by vigorous shaking with 6 mL room-temperature deionized water in a 20-mL luer-lock syringe (catalog no. 309661, Becton Dickenson, Franklin Lakes, NJ). The syringe then was attached to the adapter on the filling tube, and alginate was pushed through the tube while it was slowly withdrawn from the colon (Figure 1 C and D). A paper towel was held over the anus to ensure that no alginate leaked out before it solidified (about 8 to 12 min, depending on the ambient temperature and humidity). To remove the mold, the Fogarty balloon was deflated and removed from the colon. A hand on the underside of the rat was used to guide the alginate mold from the colon, which was accomplished easily owing to the mineral oil lubrication and flexible nature of solidified alginate. The endoscope was used to ensure that no alginate material had been left in the colon. Each mold was rinsed of fecal material and stored in a conical tube until dental stone casts could be made (within 1 wk). To determine the reproducibility of the alginate process, 2 or 3 molds were made on the same day for 41 tumors from 14 rats.
Figure 1.
Alginate molding procedure. (A) Equipment needed for the procedure includes a Fogarty catheter and flexible silicone filling tube attached to a luer-lock adapter. (B) After the rat is anesthetized, a Fogarty catheter is placed inside the colon, secured to the tail with tape, and the balloon inflated and secured by taping down the syringe plunger. (C) The syringe is attached to the luer-lock adapter on the filling tube inside the rat. (D) As the alginate is pushed through the filling tube into the colon, the tube is slowly removed from the colon. A paper towel is held at the anus for several minutes to ensure the alginate does not leak out. The alginate is left to set for 8 to 12 min before removal.
Dental stone positive casts.
The alginate molds were removed from storage and the tumor impressions gently dried by using laboratory tissues (KimWipes, Kimberly-Clark, Irving, TX). Yellow dental stone (2.5 g; The Plaster Guys, Glenside, PA) then was added to a small beaker containing 1.0 mL deionized water and mixed completely. A small scoopula was used to fill the impressions in the alginate mold, with care to avoid bubbles and completely fill the impression. The dental stone was left to set for 15 to 20 min at room temperature before it was removed gently from the mold. Casts were then put into labeled multiwell cell culture plates and placed uncovered in a 90 °C hybridization oven for 48 h before being weighed. To determine the reproducibility of the dental stone casting process, 3 individual stone casts were made from each alginate tumor mold for a subset of samples. Dental stone casts can be stored indefinitely in sealed tubes at room temperature.
Conversion factor and tumor volume calculations.
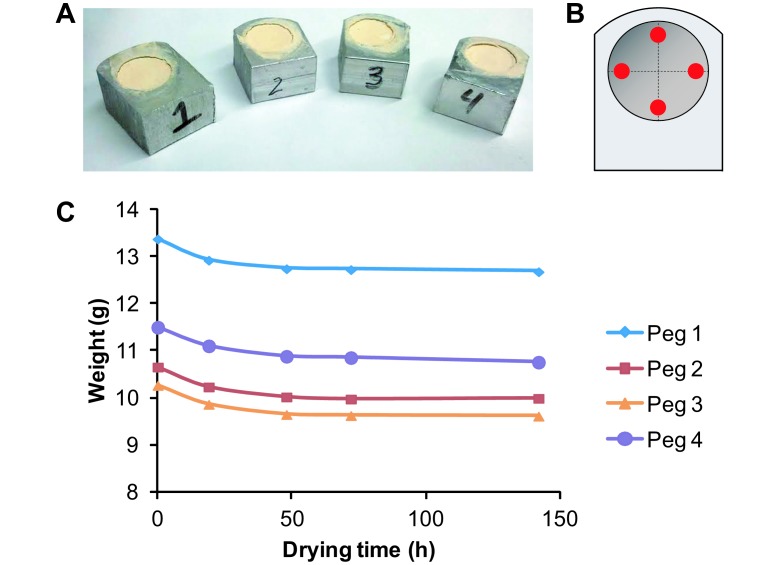
Four aluminum pegs each containing a cylindrical hole were machined and their initial empty weights determined (Figure 2 A). The volume of each hole was determined by using the formula for the volume of a cylinder (π × radius2 × height), where the radius was determined by averaging 2 diameter measurements perpendicular to one another and dividing by 2, and the height was determined by averaging 4 depth measurements for each hole (Figure 2 B). The holes then were filled completely with dental stone and dried in a 90 °C hybridization oven. Filled pegs were then reweighed at 0, 19, 48, 72, and 142 h of drying, and the empty peg weight subtracted to yield the cast weight (Figure 2 C). A conversion factor then was determined from the initial volume of a peg hole and weight of its dry cast. The weight of each dental stone cast was divided by the average conversion factor to determine the original tumor volume.
Figure 2.
Determining the conversion factor to calculate tumor volume from dry dental stone weight. (A) Four pegs each containing a cylindrical hole were filled with dental stone and dried for various periods of time. (B) Peg volume was calculated by averaging 2 measurements for diameter (dashed lines) and 4 measurements for depth (red dots). (C) After 48 h of drying at 90 °C the weight of the dental stone changed very little. The volume of the pegs and dry weight of the dental stone was used to determine the conversion factor (1.3236 gm/cm3).
Terminal tumor wet and dry weight.
Rats were euthanized by CO2 asphyxiation by using a restricted flow rate. Cervical dislocation was used as a secondary method, and death was confirmed by a lack of respiration, movement or reflexes and by the presence of fixed pupils. The colon was removed, flushed with PBS, opened longitudinally, and laid flat. Tumors that underwent the alginate molding procedure were cut at the base of the stalk and weighed before fixation to determine the wet weight. Tumors were transferred to 10% neutral buffered formalin for 24 h, removed, air-dried for 10 min, and reweighed to determine the dry weight.
Statistical methods.
All statistical tests were performed using the free software MSTAT (version 5.5.7, available from http://www.mcardle.wisc.edu/mstat/). To test for a correlation between error rate and the experimenter's increase in skill level performing the procedure over time, the Jonckheere–Terpstra test was used. To test for a difference between 2 groups, a 2-sided Wilcoxon rank-sum test was used. To test for a correlation between 2 variables, such as error rate and tumor size, a 2-sided Kendall rank-correlation test was used. For each of these tests, a P value of 0.05 or less was considered significant. Regression analysis was used to determine how closely the weight of the dental stone casts correlated with the weight of the dissected tumors. Means, standard deviations, and P values for each comparison are provided.
Results
To determine the time required to dry casts to a constant weight, 4 pegs each containing a cylindrical hole with volumes ranging from 1224.6 to 1259.6 mm3 were filled with wet dental stone and heated to 90 °C to speed drying (Figure 2 A and B). On average, the weight of the dental stone decreased by 3.7% ± 0.3% between 0 and 19 h, by 1.9% ± 0.3% between 19 and 48 h but only by 0.3% ± 0.4% or less after 48 h (Figure 2 C). Thus, we determined that drying for 48 h was sufficient to obtain a stable dry dental stone weight. Then, based on the known volume of each peg hole and the weight of the corresponding dry dental stone in a series of 3 experiments, we determined the conversion factor to be 0.001324 ± 0.000310 g/mm3. To determine the initial tumor volume (in mm3), the weight (in grams) of the dry dental stone cast was divided by 0.001324.
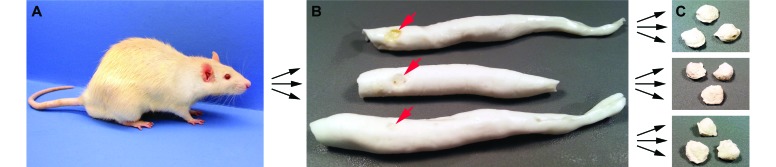
Multiple alginate molds and dental stone casts were made from 21 rats, each containing 1 to 5 colonic tumors (Figure 3). A total of 66 tumors were used to create 133 molds and more than 300 casts. The overall average errors were 17.9% ± 11.6% for the molding procedure and 20.9% ± 18.9% for the casting procedure, which resulted in an overall tumor-volume error of 22.7% ± 16.7%. The errors from both procedures decreased significantly (Jonckheere–Terpstra test P < 0.0001) over time as the skill level and experience of the experimenter increased. The mean error of the alginate molding procedure during the first compared with the second half of the study was not statistically significant (P = 0.42) by a 2-sided Wilcoxon rank-sum test. However, the standard deviation from the mean was greater in the first half of the study (17.2% ± 14.5%) compared with the second half (18.4% ± 8.8%). Similarly, the average dental stone cast error was significantly (P < 0.0001, 2-sided Wilcoxon rank sum test) greater in the experiments run during the first half of the study (30.1% ± 21.6%) compared with the second half (11.5% ± 7.8%). Therefore, the overall calculated tumor-volume error after refinement of the technique was 16.5% ± 7.9%.
Figure 3.
Determining the error rate of the alginate molding and dental stone casting procedures. (A) A total of 21 rats underwent the alginate molding procedure, with several rats examined at multiple time points. (B) Then 1 to 3 alginate molds were made per time point for each rat; multiple alginate molds made on the same day of the same rat were used to test the reproducibility of the molding procedure. The negative impression left by the tumor in each alginate mold is indicated with an arrow. (C) From each alginate mold, 1 to 3 dental stone casts were made; multiple dental stone casts of each tumor were used to test the reproducibility of the casting procedure.
Tumors as small as 4 mm3 (about 2 mm × 2 mm × 1 mm) and as large as 196 mm3 (about 7 mm × 7 mm × 4 mm) were molded successfully by using this technique (Figure 4). Alginate molding error did not correlate (P = 0.87, 2-sided Kendall rank correlation test) with tumor size, indicating that this procedure worked as well for small tumors as for large tumors. However, the dental stone cast error increased (P < 0.00001, 2-sided Kendall rank correlation test) with tumor size.
Figure 4.
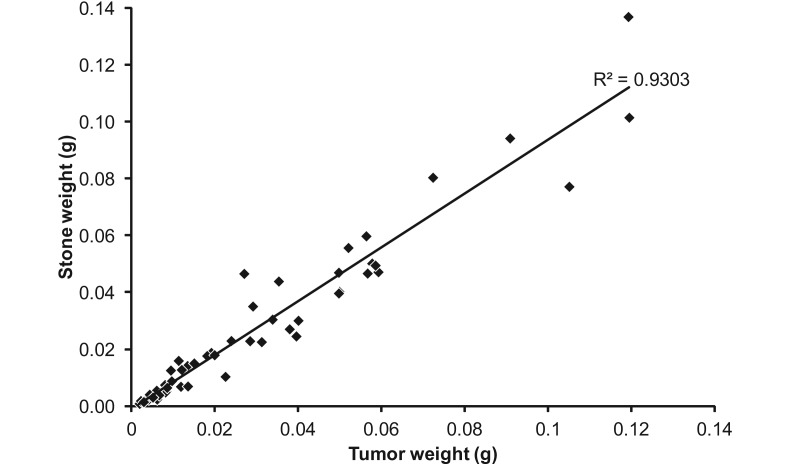
From 16 rats, a total of 50 tumors were molded, cast, and directly weighed. Tumor weights in this experiment ranged from 0.008 g to 0.137 g (corresponding to tumor volumes of about 6 to 104 mm3) and were highly correlated with dry dental stone weight (R2 = 0.9303), the surrogate for tumor size.
To determine whether tumor volume derived from the alginate method correlated with actual tumor weight, a cohort of rats underwent alginate molding as a terminal procedure and subsequently were dissected to harvest individual tumors. From a subset of 16 rats, a total of 50 tumors were both cast and directly weighed, 40 of which had both wet and dry weights determined. The correlation between tumor wet weight and dry weight was very high (R2 = 0.999). In this experiment, tumor weights ranged from 0.008 to 0.137 g (corresponding to tumor volumes of approximately 6 to 104 mm3), and the correlation between the weights of the dental stone cast and tumor was high (R2 = 0.93, Figure 4). These data were based on a single dental stone cast from a single alginate mold. This correlation between tumor volume derived from dental stone weight and terminal tumor weight is similar to that reported between microCT and terminal tumor weight (R2 = 0.87).5
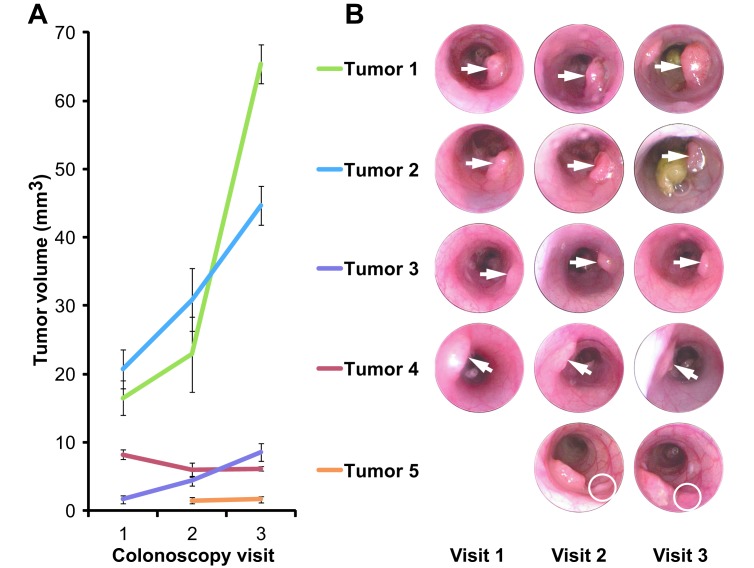
Our technique is even more powerful when applied longitudinally. To test this method longitudinally, alginate molds were made every 1 to 2 wk over a period of 4 to 5 wk in 6 rats. Throughout the study, all rats appeared healthy, active, and recovered after the procedure similarly to those who underwent colonoscopy alone. Of the 10 tumors in these 6 rats, 8 grew, 1 remained static, and 1 regressed during the study (Figure 5).
Figure 5.
Longitudinal tumor volume measurements. Five representative tumors from 3 rats that were followed longitudinally are shown. (A) Error bars (1 SD) represent cumulative error from both the alginate molding procedure and the dental stone casting procedure. (B) Over a 5-wk period, 3 tumors grew (tumors 1, 2, and 3), one was static (tumor 5), and one regressed (tumor 4).
This method has proven highly successful for determining in vivo tumor volume. However, it was essential to explore whether the procedure had any overt effect on tumor growth or multiplicity in longitudinal studies. Indeed, alginates are able to bind iron, and high iron levels have been associated with increased rates of colorectal cancer.22 To determine whether alginate had any effect on tumor multiplicity, 6 rats were followed longitudinally for 5 wk; 3 of the 6 rats underwent both colonoscopy and alginate molding at weeks 0, 3, and 5, whereas the other group of 3 rats underwent colonoscopy only. Although the number of animals examined was small, tumor multiplicity and size did not differ markedly, and the means for each were within 1 SD. At dissection, those undergoing colonoscopy only had 2.0 ± 1.7 tumors whereas those undergoing both colonoscopy and repeat alginate exposure had 2.7 ± 0.6 tumors (P = 0.5, 2-sided Wilcoxon rank sum test). To determine whether alginate molding had a significant effect on tumor size, 8 control rats underwent alginate molding as a terminal procedure only. The tumor sizes of these control rats were compared with those of 6 rats that had undergone 3 alginate moldings at least 1 wk apart over a period of 4 to 5 wk. No significant difference in tumor size was noted between control rats and those that underwent repeat alginate molding (22.8 ± 23.9 mm3 compared with 16.0 ± 18.4 mm3; P = 0.27, 2-sided Wilcoxon rank sum test).
Discussion
We have developed a method to monitor the growth of colonic tumors in vivo by using alginate molding coupled with dental stone casting. This method has successfully measured the volume of a wide range of tumor sizes (4 to 196 mm3). The weight of dental stone casts made from tumors correlated highly with the weight of the dissected tumor (R2 = 0.93). Beyond single time-point measurements, this method has been applied longitudinally to follow the growth trajectory of tumors in a quantitative manner. An added benefit of performing longitudinal studies is the ability to reduce significantly the number of animals needed for a study. When individual tumors can be followed over time, the ‘sample’ for statistical analysis becomes each tumor rather than each animal.
Biocompatible alginate, or alginic acid, is an anionic polysaccharide found in the cell walls of brown algae.4 When treated with Ca2+ ions and D-glucono-d-lactone, a homogenous, strong gel can be prepared, with gelation rates proportional to the concentration of Ca2+ ions.18 This material is not absorbed or fermented in the gastrointestinal tract, is approved for human consumption, and is often used to encapsulate pharmaceutical compounds.3 When solidified, alginate is strong yet flexible, making it easy to extrude from the colon without undue stress to the subject. It is these properties that make alginate a useful candidate material to mold tumors of the colon. The gel-setting time depends on the ratio of water to alginate powder and on the water temperature, ambient temperature, and humidity. For our procedure, we created a mixture that flowed freely into the colon yet had an in situ set time of about 10 min, thereby necessitating only brief anesthesia of the rats. This brevity and simplicity is in contrast to CT procedures, which can take 30 min or more to obtain images while the subject is anesthetized with a colon insufflated with air.
As we and others continue to refine the described method, additional detailed analyses are required to assess any effects on normal colonic tissue and tumors. Although we have not detected any adverse effects on the growth of colonic tumors, any method for measuring colonic tumor volume interferes with the biologic system. Only by additional use of this method can investigators at large detect any unanticipated biologic perturbation. The proportion of growing, static, and regressing tumors noted by using the alginate method was similar to those previously observed based on subjective scoring of images by blinded observers.15 Thus it appears that alginate does not have an overt effect on tumor growth profiles. Similarly, we observed no significant difference in tumor multiplicity or size between animals undergoing colonoscopy only and those also undergoing several alginate procedures. However, the potential for effects at the molecular or cellular level should be investigated further.
We note that tumor volume determination by alginate molding does have some limitations. Tumors that cluster closely together may not be resolved individually, and large tumors that occlude the lumen may be difficult to cast because the alginate may not flow freely around the entire tumor. In our experience, dental stone error increased as tumor size increased, presumably a result of incomplete filling of large deep holes in the alginate mold. Each stone must be examined when removed from the mold to ensure that no bubbles are present and that it accurately represents the impression within the alginate mold. Furthermore, completely flat lesions would not be detected with this technique; however, these tumors can be visualized by other methods, such as endoscopy. Although alginate can mold the entire colon, we have found that the rigid endoscope is limited to visualization of only the distal two-thirds of the rat colon, to the point where the colon turns to meet the cecum. Therefore, impressions made at the anterior end of the mold may not have corresponding endoscopic images. However, endoscopy is not essential to the alginate molding technique. Although endoscopic visualization of tumors gives added confidence, making several replicate molds from the same animal can give a similar level of confidence. The reproducibility of our described method has proven to be favorable, but room for improvement remains. The current level of error for an individual tumor may not meet regulatory criteria for an approved method for determining tumor volume. Additional work is needed to improve the precision of our technique and to compare this method directly with CT.
Attempts were made to adapt this procedure to the mouse, by using the ApcMin/+ model. We assessed several ratios of alginate to water to find a mixture that flowed through a narrow tube that accommodated the small diameter of the mouse colon yet solidified in a reasonable amount of time; however, set times exceeding 20 min were tested without success. Attempts to fill the mouse colon without a filling tube resulted in alginate molding of the distal portion of the colon only. Furthermore, the mouse colonic wall is much more fragile than that of the rat and is easily pierced. Nonetheless, with further procedural refinement, alginate molding may hold potential for determining accurate tumor volume measurements in the mouse colon.
Although the deep understanding of mouse genetics and the ability to manipulate the mouse genome have historically made this species a model of choice, the rat has recently made dramatic strides in this area and for biologic reasons is an increasingly preferred choice to model human disease.7,13,16 In some cases, rats may recapitulate the human disease more closely than do mice. The azoxymethane and dextran sodium sulfate models of rat colonic carcinogenesis have been used for several decades and are still used to research environmentally induced colon cancers.11,25 In the last several years, 2 rat models of familial colon cancer have been developed: the ApcPirc/+ rat, which we used here, and the KAD rat, a homozygously viable Apc-mutant model.25 Tumors form preferentially in the colons in both of these models, similar to features of the human disease and distinct from those of genetic mouse models, which develop tumors primarily in the small intestine. The recently acquired ability to readily manipulate the rat genome through the use of zinc-finger nucleases, transcription activator-like effector nucleases, and clustered, regularly interspaced, short palindromic repeat-associated proteins all have rapidly accelerated the study and use of genetically modified rat models.9,19,21 The growing prominence of rat models makes our method essential for the study of colonic neoplasia in experimental animals.
In summary, we have shown that alginate molding combined with dental stone casting is a facile and reliable method to determine tumor volume in vivo without the need for expensive equipment and software. MicroCT measurements can cost several hundred dollars per animal for each scan, and the data analysis can be time consuming and requires detailed command of the software. In contrast, the methods we describe here cost only a few cents per alginate mold and even less for the dental stone casts. Only an accurate balance and calculator are required to determine tumor volume from dental stone weight. Importantly, this method can be done directly in the animal facility or laboratory in conjunction with other experiments. Such a simple method opens up possibilities for researchers worldwide to study tumor dynamics. Furthermore, we have shown that the ApcPirc/+ rat, readily available to researchers throughout the world, is an appropriate model in which to perform longitudinal studies of the dynamics of colonic tumorigenesis.
Acknowledgments
We thank the Biomedical Engineering (BME) Design Curriculum and Amit Nimunkar for advising the BME undergraduate students who first developed this technique; Norman Drinkwater for statistical advice; and Alexandra Shedlovsky, Kevin Eliceiri, and Erik Toraason for critical reading of the manuscript.
This work was supported by grants from the National Cancer Institute (R01 CA63677 and R01 CA125591 to WD) and a National Institute of Environmental Health Sciences Pre-Doctoral Training Grant (T32ES007015-033 to A Irving). This paper is subject to the NIH Public Access Policy.
References
- 1.Amos-Landgraf JM, Kwong LN, Kendziorski CM, Reichelderfer M, Torrealba J, Weichert J, Haag JD, Chen KS, Waller JL, Gould MN, Dove WF. 2007. A target-selected Apc-mutant rat kindred enhances the modeling of familial human colon cancer. Proc Natl Acad Sci USA 104:4036–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker C, Fantini MC, Neurath MF. 2006. High-resolution colonoscopy in live mice. Nat Protoc 1:2900–2904 [DOI] [PubMed] [Google Scholar]
- 3.Bodmeier R, Chen HG, Paeratakul O. 1989. A novel approach to the oral delivery of micro- or nanoparticles. Pharm Res 6:413–417 [DOI] [PubMed] [Google Scholar]
- 4.Draget KI, Smidsrod O, Skjak-Braek G. 2005. Alginates from algae. In: Biopolymers Online. Wiley Online Library. [Cited 08 February 2014]. Available at: http://onlinelibrary.wiley.com/doi/10.1002/3527600035.bpol6008/abstract [Google Scholar]
- 5.Durkee BY, Mudd SR, Roen CN, Clipson L, Newton MA, Weichert JP, Pickhardt PJ, Halberg RB. 2008. Reproducibility of tumor-volume measurement at microCT colonography in living mice. Acad Radiol 15:334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durkee BY, Shinki K, Newton MA, Iverson C, Weichert J, Dove WF, Halberg RB. 2009. Longitudinal assessment of colonic tumor fate in mice by computed tomography and optical colonoscopy. Acad Radiol 16:1475–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwinell MR, Lazar J, Geurts AM. 2011. The emerging role for rat models in gene discovery. Mamm Genome 22:466–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faustino-Rocha A, Oliveira PA, Pinho-Oliveira J, Teixeira-Guedes C, Soares-Maia R, da Costa RG, Colaco B, Pires MJ, Colaco J, Ferreira R, Ginja M. 2013. Estimation of rat mammary tumor volume using caliper and ultrasonography measurements. Lab Anim (NY) 42:217–224 [DOI] [PubMed] [Google Scholar]
- 9.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Menoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. 2009. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hensley HH, Merkel CE, Chang WC, Devarajan K, Cooper HS, Clapper ML. 2009. Endoscopic imaging and size estimation of colorectal adenomas in the multiple intestinal neoplasia mouse. Gastrointest Endosc 69:742–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirono I, Kuhara K, Hosaka S, Tomizawa S, Golberg L. 1981. Induction of intestinal tumors in rats by dextran sulfate sodium. J Natl Cancer Inst 66:579–583 [PubMed] [Google Scholar]
- 12.Hoffman RM. 2005. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer 5:796–806 [DOI] [PubMed] [Google Scholar]
- 13.Iannaccone PM, Jacob HJ. 2009. Rats! Dis Model Mech 2:206–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press [Google Scholar]
- 15.Irving AA, Halberg RB, Albrecht DM, Plum LA, Krentz KJ, Clipson L, Drinkwater N, Amos-Landgraf JM, Dove WF, Deluca HF. 2011. Supplementation by vitamin D compounds does not affect colonic tumor development in vitamin-D–sufficient murine models. Arch Biochem Biophys 515:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob HJ. 2010. The rat: a model used in biomedical research. Methods Mol Biol 597:1–11 [DOI] [PubMed] [Google Scholar]
- 17.Katz MH, Takimoto S, Spivack D, Moossa AR, Hoffman RM, Bouvet M. 2003. A novel red fluorescent protein orthotopic pancreatic cancer model for the preclinical evaluation of chemotherapeutics. J Surg Res 113:151–160 [DOI] [PubMed] [Google Scholar]
- 18.Kuo CK, Ma PX. 2001. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate, and mechanical properties. Biomaterials 22:511–521 [DOI] [PubMed] [Google Scholar]
- 19.Li D, Qiu Z, Shao Y, Chen Y, Guan Y, Liu M, Li Y, Gao N, Wang L, Lu X, Zhao Y, Liu M. 2013. Heritable gene targeting in the mouse and rat using a CRISPR-Cas system. Nat Biotechnol 31:681–683 [DOI] [PubMed] [Google Scholar]
- 20.Lingeman CH, Garner FM. 1972. Comparative study of intestinal adenocarcinomas of animals and man. J Natl Cancer Inst 48:325–346 [PubMed] [Google Scholar]
- 21.Mashimo T, Kaneko T, Sakuma T, Kobayashi J, Kunihiro Y, Voigt B, Yamamoto T, Serikawa T. 2013. Efficient gene targeting by TAL effector nucleases coinjected with exonucleases in zygotes. Sci Rep 3:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson RL. 2001. Iron and colorectal cancer risk: human studies. Nutr Rev 59:140–148 [DOI] [PubMed] [Google Scholar]
- 23.Pickhardt PJ, Kim DH, Pooler BD, Hinshaw JL, Barlow D, Jensen D, Reichelderfer M, Cash BD. 2013. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol 14:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Washington MK, Powell AE, Sullivan R, Sundberg J, Wright N, Coffey RJ, Dove WF. 2013. Pathology of rodent models of intestinal cancer: progress report and recommendations. Gastroenterology 144:705–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimi K, Tanaka T, Takizawa A, Kato M, Hirabayashi M, Mashimo T, Serikawa T, Kuramoto T. 2009. Enhanced colitis-associated colon carcinogenesis in a novel Apc mutant rat. Cancer Sci 100:2022–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young MR, Ileva LV, Bernardo M, Riffle LA, Jones YL, Kim YS, Colburn NH, Choyke PL. 2009. Monitoring of tumor promotion and progression in a mouse model of inflammation-induced colon cancer with magnetic resonance colonography. Neoplasia 11:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]