Abstract
Retinoic acid is a widely used factor in both mouse and human embryonic stem cells. It suppresses differentiation to mesoderm and enhances differentiation to ectoderm. Fibroblast growth factor 2 (FGF2) is widely used to induce differentiation to neurons in mice, yet in primates, including humans, it maintains embryonic stem cells in the undifferentiated state. In this study, we established an FGF2 low-dose-dependent embryonic stem cell line from cynomolgus monkeys and then analyzed neural differentiation in cultures supplemented with retinoic acid and FGF2. When only retinoic acid was added to culture, neurons differentiated from FGF2 low-dose-dependent embryonic stem cells. When both retinoic acid and FGF2 were added, neurons and astrocytes differentiated from the same embryonic stem cell line. Thus, retinoic acid promotes the differentiation from embryonic stem cells to neuroectoderm. Although FGF2 seems to promote self-renewal in stem cells, its effects on the differentiation of stem cells are influenced by the presence or absence of supplemental retinoic acid.
Abbreviations: EB, embryoid body; ES, embryonic stem; ESM, embryonic stem cell medium; FGF, fibroblast growth factor; GFAP, glial fibrillary acidic protein; LIF, leukemia inhibitory factor; MBP, myelin basic protein; RA, retinoic acid; SSEA, stage-specific embryonic antigen; TRA, tumor-related antigen
Pluripotent stem cells are potential sources of material for cell replacement therapy and are useful experimental tools for in vitro models of human disease and drug screening. Embryonic stem (ES) cells are capable of extensive proliferation and multilineage differentiation, and thus ES-derived cells are suitable for use in cell-replacement therapies.18,23 Reported ES cell characteristics including tumorigenic potential, DNA methylation status, expression of imprinted genes, and chromatin structure were elucidated by using induced pluripotent stem cells.2,11,17 Because the social expectations of regeneration medicine are growing, we must perform basic research with ES cells, which differ from induced pluripotent stem cells in terms of origin, differentiation ability, and epigenetic status.2,8
Several advances in research have been made by using mouse ES cells. Furthermore, primate ES cell lines have been established from rhesus monkeys (Macaca mulatta),24 common marmosets (Callithrix jacchus),25 cynomolgus monkeys (M. fascicularis),20 and African green monkeys (Chlorocebus aethiops).19 Mouse and other mammalian ES cells differ markedly in their responses to the signaling pathways that support self-renewal.8,28 Mouse ES cells require leukemia inhibitory factor (LIF)–STAT3 signaling.14 In contrast, primate ES cells do not respond to LIF. Fibroblast growth factor 2 (FGF2) appears to be the most upstream self-renewal factor in primate ES cells. FGF2 also exerts its effects through indirect mechanisms, such as the TGFβ–Activin–Nodal signaling pathway, in primate ES cells.21 In addition to the biologic similarities between monkeys and humans, ES cells derived from cynomolgus monkeys or human blastocysts have extensive similarities that are not apparent in mouse ES cells.8,14,21,28 Numerous monkey ES cell lines are now available, and cynomolgus monkeys are an efficient model for developing strategies to investigate the efficacy of ES-cell–based medical treatments in humans.
Several growth factors and chemical compounds, including retinoic acid (RA),4,9,13,22,26 FGF2,9,10,16,22 epidermal growth factor,9,22 SB431542,1,4,10 dorsomorphin,10,27 sonic hedgehog,12,13,16,27,29 and noggin,1,4,9,27 are essential for the differentiation and proliferation or maintenance of neural stem cells derived from primate ES cells. Of these factors, active RA signaling suppresses a mesodermal fate by inhibiting Wnt and Nodal signaling pathways during in vitro culture and leads to neuroectoderm differentiation in ES cells.4,13,26 RA is an indispensable factor for the specialization to neural cells. FGF2 is important during nervous system development,12 and FGF2 and RA both are believed to influence the differentiation to neural cells. The current study was done to clarify the mechanism of RA and FGF2 in the induction of differentiation along the neural lineage.
We recently established a monkey ES cell line that does not need FGF2 supplementation for maintenance of the undifferentiated state. This ES cell line allowed us to study the role of differentiation to neural cells with RA and enabled us to compare ES cell differentiation in the context of supplementation with RA or FGF2 in culture. To this end, we established a novel cynomolgus monkey cell line derived from ES cells and maintained it in an undifferentiated state in the absence of FGF2 supplementation.
Materials and Methods
Animals.
Mature cynomolgus monkeys (Macaca fascicularis) are maintained in our facility according to guidelines set by the National Institute of Biomedical Innovation for the care, use, and biohazard countermeasures of laboratory animals. This study was approved by the institutional Animal Welfare and Animal Care Committee.
Derivation and culture of an FGF2 low-dose-dependent (Fld-) ES cell line.
Procedures for the development of embryos and the establishment of ES cells from blastocysts that were developed in vitro were described previously.28,29 Briefly, prominent inner cell masses derived from blastocysts of cynomolgus monkeys were cultured with mitomycin C-treated mouse embryonic fibroblast cell monolayers at a concentration of 4 × 104 cells/cm2 on gelatin-coated dishes in embryonic stem cell medium (Dulbecco modified Eagle medium–Ham F12 nutrient mixture [1:1; Sigma, St Louis, MO] supplemented with 20% Knockout serum replacement [Invitrogen, Carlsbad, CA], 1 mM GlutaMax [Invitrogen], 1% nonessential amino acids [Invitrogen]) containing 4 ng/mL human recombinant basic FGF (FGF2; Wako Pure Chemical Industries, Osaka, Japan), 10 ng/mL human recombinant LIF (Merck Millipore, Billerica, MA), and 0.1 mM β-mercaptoethanol (Sigma). ES-like cell colonies were kept stable in culture with FGF2, human LIF, and β-mercaptoethanol for a total of 10 passages. Next, we transferred the ES-like cell colonies to modified ES cell medium without FGF2, human LIF, or β-mercaptoethanol, thus creating the first generation of the FGF2 low-dose-dependent cell line (that is, Fld-ES line). The medium was changed daily, and ES-like cell colonies that proliferated were split every 7 d, either manually or by disaggregation in collagenase and replating of collected cells onto dishes with fresh feeder layers.
ES cells that had been maintained in culture without FGF2, human LIF and β-mercaptoethanol for 15 to 20 passages were karyotyped as previously described at the International Council for Laboratory Animal Science Monitoring Center (Kanagawa, Japan) or the Chromosome Science Lab (Hokkaido, Japan). Chromosome spreads were Giemsa-banded, photographed, and sorted.
For pluripotency analysis, embryoid bodies (EB) and teratomas were prepared from ES cells. EB were developed in a suspension culture from collagenase-treated ES cells grown in suspension. After 2 wk, EB were transferred to 6-cm dishes coated with 0.1% gelatin in modified ES cell medium for attachment culture. For the evaluation of teratoma formation, small-cluster subcultured ES cells from a 6-cm dish were transferred into the right hindleg muscles of 2 NOD/SCID mice. The tumors that formed were fixed with 4% paraformaldehyde in PBS, embedded in paraffin, and sectioned for histologic and immunohistochemical analysis. Histologic analysis was done with hematoxylin and eosin staining. Immunohistochemical analysis was done by visualizing the localization of primary antibodies by using horseradish-peroxidase–labeled secondary antibody and diaminobenzidine. Primary antibodies were α-smooth muscle actin (dilution, 1:100; Dako, Glostrup, Denmark), synaptophysin (1:200; Dako) and α-fetoprotein (1:1000; Dako).
EB formation and attachment assay.
The percentage of attached EB that developed from Fld-ES cells was determined by microscopic observation. EB of 300 to 500 µm and 500 to 700 µm in diameter were counted and separated into 6-cm dishes coated with 0.1% gelatin. After 3 d of culture, attachment was confirmed, and the 2 size groups were compared.
Effect of RA dose on EB attachment and differentiation.
To induce the neural differentiation of Fld-ES cells, we assessed various doses of RA (all-trans-retinoic acid, catalog no. R2625, Sigma Aldrich) from 0.5 µM to 100 µM in the medium. After 3 d, we counted the attached EB, and after 10 d we counted the neural-like cell colonies from the outgrowth of the cultured EB and recorded the dose-dependent effect of RA.
Induction of neural differentiation from Fld-ES cells.
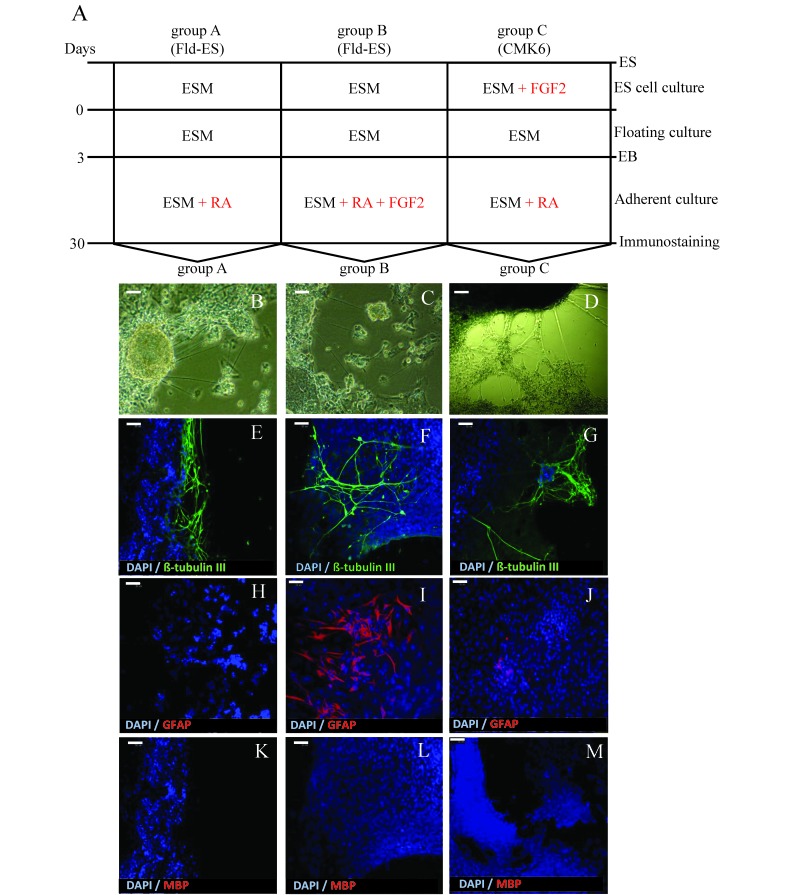
To assess neural differentiation, we used Fld-ES and CMK6, a stable cynomolgus monkey ES cell line.5,20 CMK6 cells were cultured on mitomycin-C-treated mouse embryonic fibroblast monolayers at a concentration of 4 × 104 cells/cm2 on gelatin-coated dishes in ES cell medium. Before the Fld-ES and CMK6 ES cells differentiated into neural cells, we divided them into 3 groups according to the differentiation-inducing factors used. Briefly, EB that formed from Fld-ES and CMK6 cells after 3 d of passage were transferred into 6-cm dishes coated with 0.1% gelatin, and attachment culture was initiated in the 3 groups. To group A (Fld-ES cells) and group C (CMK6 cells), we added only 1 µM RA. To group B (Fld-ES cells), we added 1 µM RA and 10 ng/mL FGF2. After 3 d, we confirmed the attachment of EB to the dishes in each group.
The attachment cultures of EB were maintained in fresh medium that was changed every 3 d. After 30 d of attachment culture of the EB derived from the Fld-ES and CMK6 cell lines, we confirmed their microtubular structure under the microscope. At the same time, we determined the neural cells in each colony through immunoassay of the 3 groups by using a confocal laser scanning microscope (Digital Eclipse C1, Nikon, Tokyo, Japan).
Immunofluorescence microscopy.
Undifferentiated and differentiation-induced cells were fixed with 4% paraformaldehyde in PBS for 30 min. After permeabilization with 0.1% Triton X100 in PBS for 10 min and blocking with 3% BSA in PBS for 30 min, cells were incubated with primary antibodies overnight at 4 °C and visualized by using IgG or IgM conjugated with Alexa 488 or 555 (1:1000; Invitrogen). Primary antibodies for staining undifferentiated cells were Oct-3/4 (1:50; Becton Dickinson, Franklin Lakes, NJ); stage-specific embryonic antigen (SSEA) -1, -3, and -4; and tumor-related antigens (TRA)-1-60 and -1-81 (1:80; Chemicon). Primary antibodies for staining differentiated cells were β-tubulin III (1:200, Dako); glial fibrillary acidic protein (GFAP, 1:1000; Dako); and myelin basic protein (MBP, 1:1000; Dako). Nuclei were stained with Hoechst 33342 (10 μg/mL; Calbiochem, La Jolla, CA) in PBS and DAPI (1 µg/mL, 1:1000; Dako). Fluorescent images were produced by using a confocal laser scanning microscope (Digital Eclipse C1, Nikon).
Results
Establishment and characterization of the Fld-ES cell line.
We established the Fld-ES cell line from cynomolgus monkeys by using blastocysts with appropriate morphology that contained distinct, prominent inner cell masses. The morphology of these derived cells was very similar to that of other primate ES cells.5,20 Finally, ES-like cells were generated in sufficient numbers to establish Fld-ES (Figure 1 A). This stable ES cell line did not require FGF2 supplementation to maintain an undifferentiated state during culture. However, we could not exclude the possibility that a small amount of FGF2 that might be derived from serum or feeder cells could have maintained the undifferentiated status of the ES cell line. Therefore, we putatively considered the cell line to be an FGF2 low-dose-dependent ES cell line.
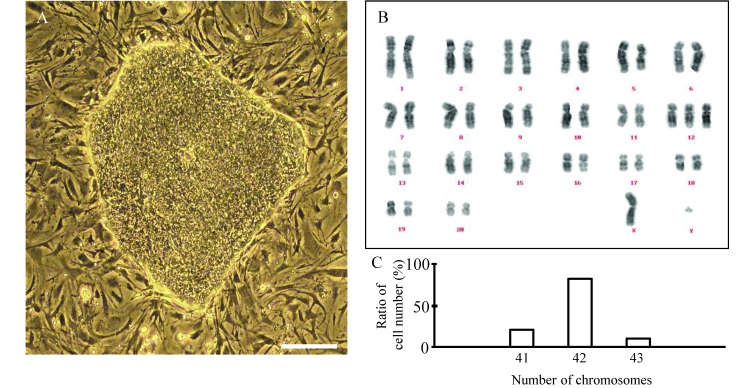
Figure 1.
Established FGF2 low-dose–dependent Fld-ES cells and their karyotype analysis. (A) The colony was flat and had the features of a primate ES cell colony. (B) The 18 generations of Fld-ES cells. The chromosome number was normal (42). The sex chromosome was XY. (C) About 80% of cells had a normal chromosome number. Scale bar, 100 µm.
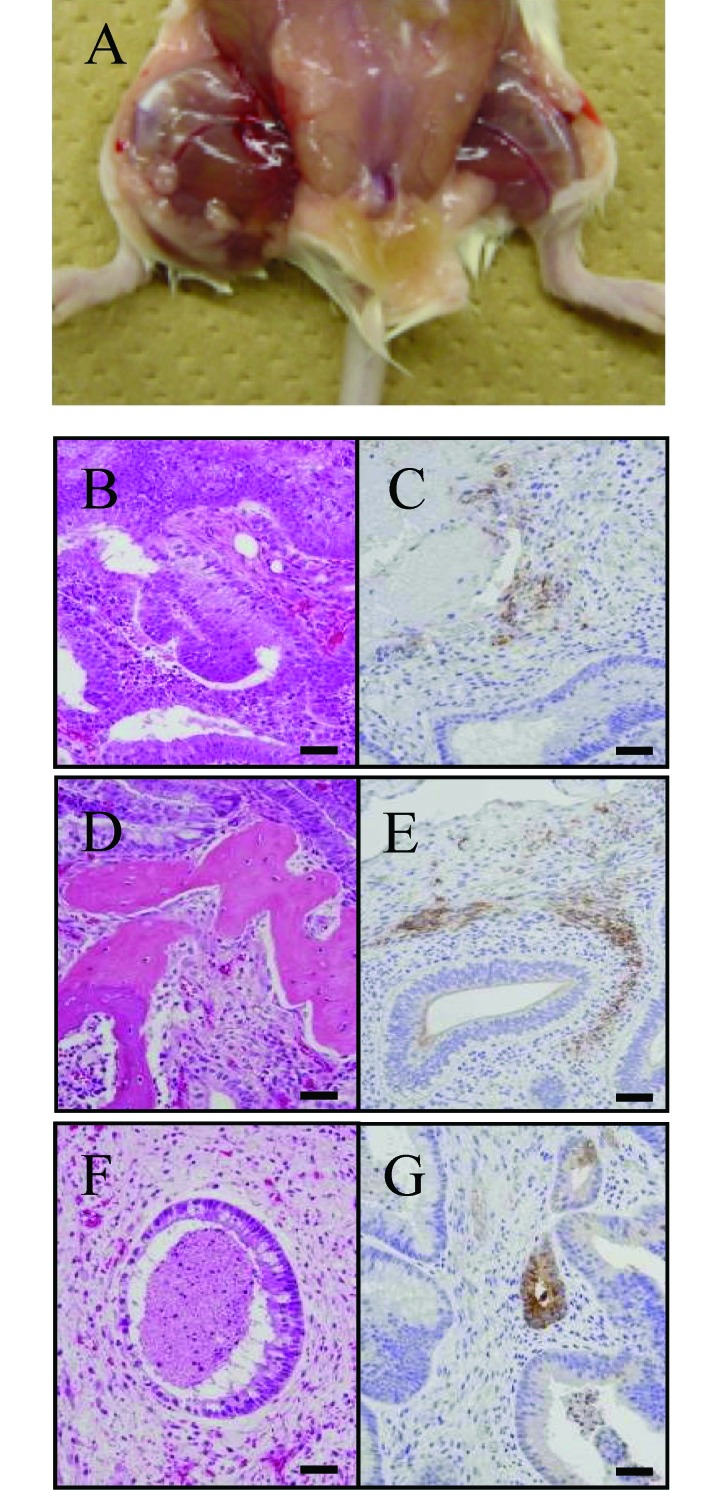
We examined the karyotype and expression of undifferentiated markers in Fld-ES. Karyotyping analysis revealed that approximately 80% of cells examined after 18 passages had the normal chromosome number of 42, and the sex chromosomes were XY (Figure 1 B and C). Immunofluorescence revealed that Fld-ES strongly expressed Oct-3, SSEA-4, and TRA-1-60 and -1-81. ALP activity was observed, and SSEA-3 was expressed very weakly in some cells; no signals were observed for SSEA-1 (Table 1). To examine the pluripotency of Fld-ES, we induced the development of EB and teratomas in vivo (Figure 2). Immunohistochemical analysis revealed that ES cells transferred into 2 immunodeficient mice formed teratomas consisting of tissues from 3 embryonic germ layers (ectoderm, mesoderm, and endoderm; Figure 2 B through G).
Table 1.
ALP activity and evaluation of undifferentiation markers in Fld-ES cells
| Marker | FGF2 added | No FGF2 added | |||
| Before passage | Passage 1 | Passage 10 | Passage 20 | ||
| ALP | + | + | + | + | |
| SSEA-1 | – | – | – | – | |
| SSEA-3 | – | – | – | – | |
| SSEA-4 | + | + | + | + | |
| TRA-1-60 | + | + | + | + | |
| TRA-1-81 | + | + | + | + | |
| Formation of EB | + | + | + | + | |
P, passage numbers.
Figure 2.
Teratoma formation from Fld-ES cells transplanted into immunodeficient mice (NOD/SCID). (A) The teratomas that formed from a small cluster of ES cells were transferred into the right hindleg muscle of NOD/SCID mice. (B–G) In vivo analysis of the multipotency of Fld-ES cells by histologic (left panel) and immunohistochemical (right panel) methods. Various tissues of the 3 germ-layer origins—neural tissue (B, ectoderm), bone (D, mesoderm), and gland (F, endoderm)— are identified. (C) The ectodermal marker is neuron-specific enolase. (E) The mesodermal marker is smooth-muscle actin. (G) The endodermal marker is α-fetoprotein. Scale bars, 100 µm.
EB of different sizes have different attachment rates.
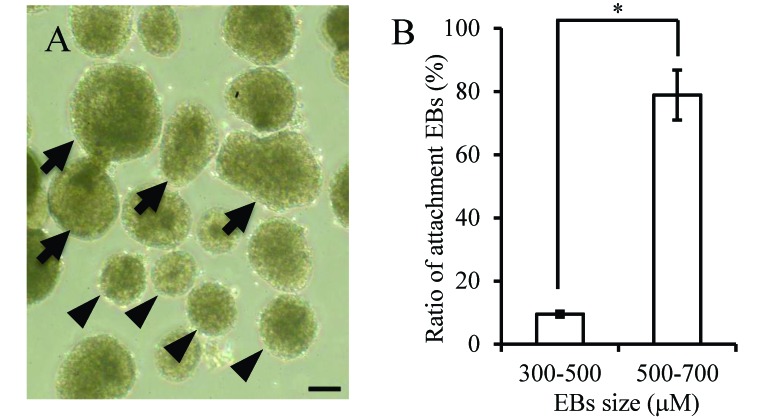
The rate of attachment on gelatin-coated dishes of EB of 500 to 700 µm in diameter (78.9%, 45 of 57) was higher than that of EB of 300 to 500 µm (9.5%, 6 of 63; Figure 3 A and B).
Figure 3.
EB derived from the Fld-ES cell line. (A) Arrows indicate EB of 500 to 700 µm; arrowheads indicate EB of 300 to 500 µm. (B) The rates of attachment were 78.9% and 9.5% for EB of 500 to 700 µm and 300 to 500 µm, respectively. Data are expressed as means ± 1 SD. An asterisk indicates a significant (P < 0.05) difference. Scale bar, 200 µm.
Dose-dependent effect of RA in EB differentiation.
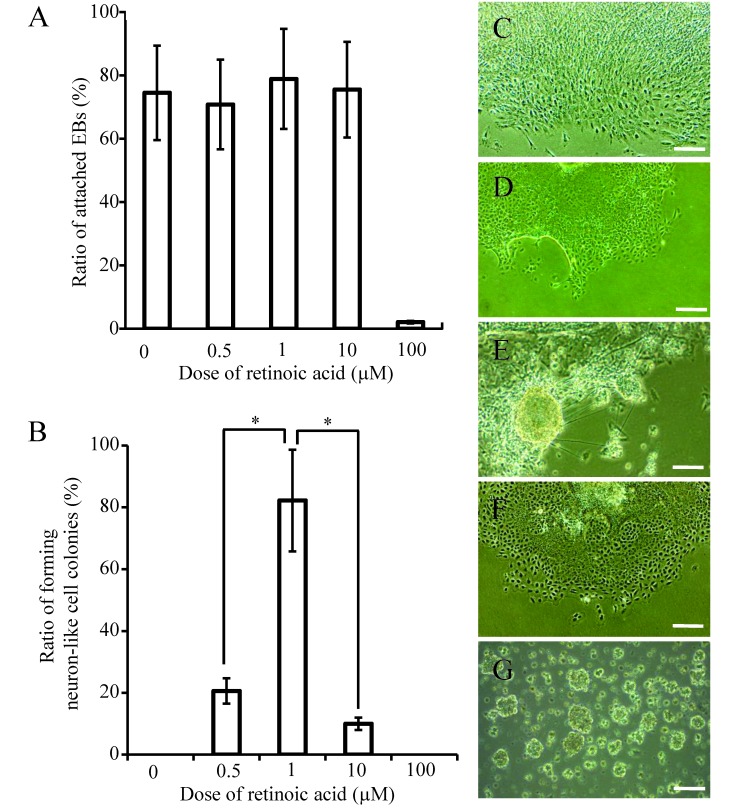
During attempts to induce neural differentiation, we observed that 1 µM RA remarkably accelerated the development of microtubules (the ratio of microtubule formation was 84%) to a level comparable to that in Fld-ES cells along with a high attachment ratio, whereas 100 µM RA did not accelerate differentiation but showed a high dose effect (Figure 4 A through G). Accordingly, for differentiation of ES cells, we used ES cell medium supplemented with 1 µM RA for all 3 groups studied.
Figure 4.
The attachment and neural differentiation ratio of EB derived from Fld-ES cells at each concentration of RA. (A) The ratio of attached EB on gelatin-treated dishes after the addition of retinoic acid at 4 concentrations. (B) The cells (except for the neural fibrillar structure) have extended like a sheet in the absence of retinoic acid. The colony with the structure of a microtubule was confirmed to have a high ratio of attached EB at 1 µM retinoic acid. The ratios of attached EB at 0.5 µM and 10 µM RA were low and that at 100 µM RA was negligible. The ES cells of all groups showed comparable results. (C through G) Colony formation at (C) 0, (D) 0.5, (E) 1, (F) 10, and (G) 100 µM retinoic acid. Scale bars, 200 µm. Data in panels A and B are expressed as means ± 1 SD. Asterisks in panel B indicate significant (P < 0.05) differences.
Induction of neural differentiation in Fld-ES cells by RA and FGF2.
Neural differentiation was induced in Fld-ES and CMK6 cells in culture from attached EB according to the schedule shown in Figure 5 A. During differentiation, microtubules with cells that were beyond the attached EB colonies were present (Figure 5 B through D). We induced neural differentiation with RA and FGF2 in the medium from Fld-ES and CMK6 cells. While inducing differentiation of ES cells to neural cells after 10 d of culture (Figure 5 A), we confirmed the structure of the neuron fibers (Figure 5 B through D) emanating from the attached EB outgrowths in each defined culture. In the immunostaining analysis, adding only RA to the medium of Fld-ES and CMK6 cells resulted in microtubules positive for β-tubulin III (Figure 5 E and G). In addition, RA-treated Fld-ES cells were negative for GFAP (that is, astrocytes) whereas CMK6 cells were weakly positive (Figure 5 H and J); MBP staining for oligodendrocytes was negative in all groups (Figure 5 K and M). Combined treatment of Fld-ES cells with RA and FGF2 led to cells positive for both β-tubulin III and GFAP but negative for MBP (Figure 5 F, I, and L). Therefore, treatment of Fld-ES with only RA led primarily to the generation of microtubules positive for β-tubulin III, whereas treating Fld-ES cells with both FGF2 and RA induced the growth of large numbers of GFAP-expressing astrocytes that appeared outside of attached EB.
Figure 5.
(A) Schematic illustration outlining the protocol for neural differentiation from Fld-ES and CMK6 cells. Groups A and B were Fld-ES cells cultured without added FGF2; group C was CMK6 cells cultured with FGF2. All groups formed EB after suspension cultures in ESM on untreated dishes for 3 d. EB were plated on gelatin-coated dishes at a density of 10 EB per dish. During this time, neural differentiation was induced by using RA only in groups A and C and both RA and FGF2 in group B. At 30 d after the initial formation of EB, neural cell markers were detected by immunostaining. (B–M) Morphologic changes in Fld-ES colonies after induction of neural differentiation and immunostaining analysis. (B–D) Nerve fibroid in clumps of cells from all groups was confirmed after 10 d in suspension culture. (E–G) Expression of the neuron marker β-tubulin III (green) was confirmed in all groups. (H–J) Expression of the astrocyte marker GFAP (red) was confirmed in groups B and C but not in group A. In group B, Fld-ES-derived EB with added FGF2 demonstrated marked neural differentiation into astrocytes, whereas in group C, CMK6 cells cultured with FGF2 showed only weak differentiation into developing astrocytes. (K–M) Expression of the oligodendrocyte cell marker MBP (red) was not detected in any group. ESM, embryonic stem cell culture medium; FGF2, fibroblast growth factor 2; GFAP, glial fibrillary acidic protein; MBP, myelin basic protein; RA, retinoic acid. Scale bars, 50 µm.
Discussion
We established a cynomolgus monkey ES cell line that requires the addition of FGF2 to the medium for only 10 passages; after that, it can be maintained in an undifferentiated state without FGF2. This implies FGF2 low-dose dependence, and so we named the cell line Fld-ES. The Fld-ES cell line expresses primate ES-cell-specific markers, forms teratomas, and has a normal chromosome number,20 suggesting that it is an important primate cell line for the study of the role of FGF2 during in vitro differentiation.
Before inducing in vitro differentiation, we observed that the attachment rate of EB formed from Fld-ES cells differed depending on their size: EB sizes of 500 to 700 µm had excellent adhesive capacity. Therefore, we used uniform EB of 500 to 700 µm, which showed strong adhesiveness, during neural differentiation. High-quality EB formation plays an important role in ES research for spontaneous differentiation into various cell types and even organ development.3 Although specific reasons for the influence of size on EB adherence are unknown, perhaps larger EB have high numbers of functional cells that are important for attachment and subsequent differentiation.
Our findings regarding the concentration of RA in the differentiation of neural cells from Fld-ES were different from those in the mouse ES neural induction study.3 The optimal concentration of RA to add to mouse ES cells to maximize the adhesiveness of EB was 0.5 µM,4 whereas 1 µM RA had the best effect on neural differentiation in our monkey Fld-ES cell line. In mice, RA directly interacts with the retinoid receptor RAR/RXR and downregulates Wnt signaling to inhibit the mesodermal fate and adopt an ectodermal fate for neural differentiation.15 We have demonstrated that the addition of RA to the culture medium of EB formed from Fld-ES cells made it possible for the cells to differentiate into neurons at 30 d after EB attachment began. In contrast, EB did not differentiate into astrocytes and oligodendrocytes. Moreover, the addition of both RA and FGF2 to the culture of EB from Fld-ES cells induced the differentiation of both neurons and astrocytes. We have not yet been able to induce oligodendrocytes from Fld-ES EB.
Another cynomolgus monkey ES cell line, CMK6, which was maintained in medium supplemented with FGF2, differentiated to neurons and a few astrocytes after the addition of RA only—that is, without an additional supply of FGF2 during differentiation. This difference may be due to the effect of FGF2, which was added to the medium of undifferentiating cultures before the start of the neural differentiation culture.
Because active RA signaling inhibits Wnt and Nodal signaling pathways to prevent the mesodermal fate of ES during differentiation into germ layer,4 it can also stimulate the differentiation of neuroectodermal progenitor cells. However, the mechanisms of differentiation may differ among neurons, astrocytes, and oligodendrocytes derived from neuroectodermal progenitor cells, and perhaps RA enables the smooth induction of neurons. We also showed that the combined actions of RA and FGF2 yielded cultures containing both progenitor cells and neural cells, that is, both astrocytes and neurons. However, RA and FGF2 did not prompt oligodendrocyte induction at a specific step during the neural differentiation of cynomolgus monkey ES cells. Several researchers have reported that, in both mouse and primate pluripotent stem cells, inducing factors such as sonic hedgehog, noggin, and SB431542 regulate oligodendrocyte differentiation at different times by inhibiting BMP4/TGFβ and Activin/Nodal.6,9,10 Therefore, oligodendrocyte differentiation seems to require specialized conditions and the presence of several factors at appropriate times.
In the present study, we established a novel monkey ES cell line that can maintain pluripotency without FGF2 supplementation of the culture medium. In addition, supplementation of our monkey Fld-ES line with both RA and FGF2 led to its differentiation to neural cells and astrocytes. The Fld-ES cell line may be useful for the study of neural differentiation-related signaling pathways.
References
- 1.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. 2009. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27:275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE. 2009. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5:111–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi YY, Chung BG, Lee DH, Khademhosseini A, Kim JH, Lee SH. 2010. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials 31:4296–4303 [DOI] [PubMed] [Google Scholar]
- 4.Engberg N, Kahn M, Petersen DR, Hansson M, Serup P. 2010. Retinoic acid synthesis promotes development of neural progenitors from mouse embryonic stem cells by suppressing endogenous, Wnt-dependent nodal signaling. Stem Cells 28:1498–1509 [DOI] [PubMed] [Google Scholar]
- 5.Furuya M, Yasuchika K, Mizutani K, Yoshimura Y, Nakatsuji N, Suemori H. 2003. Electroporation of cynomolgus monkey embryonic stem cells. Genesis 37:180–187 [DOI] [PubMed] [Google Scholar]
- 6.Gil JE, Woo DH, Shim JH, Kim SE, You HJ, Park SH, Paek SH, Kim SK, Kim JH. 2009. Vitronectin promotes oligodendrocyte differentiation during neurogenesis of human embryonic stem cells. FEBS Lett 583:561–567 [DOI] [PubMed] [Google Scholar]
- 7.Honda A, Hirose M, Hatori M, Matoba S, Miyoshi H, Inoue K, Ogura A. 2010. Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine. J Biol Chem 285:31362–31369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda A, Hirose M, Ogura A. 2009. Basic FGF and activin/modal but not LIF signaling sustain undifferentiated status of rabbit embryonic stem cells. Exp Cell Res 315:2033–2042 [DOI] [PubMed] [Google Scholar]
- 9.Izrael M, Zhang P, Kaufman R, Shinder V, Ella R, Amit M, Itskovitz-Eldor J, Chebath J, Revel M. 2007. Human oligodendrocytes derived from embryonic stem cells: effect of noggin on phenotypic differentiation in vitro and on myelination in vivo. Mol Cell Neurosci 34:310–323 [DOI] [PubMed] [Google Scholar]
- 10.Kim DS, Lee JS, Leem JW, Huh YJ, Kim JY, Kim HS, Park IH, Daley GQ, Hwang DY, Kim DW. 2010. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev 6:270–281 [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. 2010. Epigenetic memory in induced pluripotent stem cells. Nature 467:285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch P, Opitz T, Steinbeck JA, Ladewig J, Brüstle O. 2009. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci USA 106:3225–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, Zhang SC. 2008. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells 26:886–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niwa H, Ogawa K, Shimosato D, Adachi K. 2009. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 460:118–122 [DOI] [PubMed] [Google Scholar]
- 15.Okada Y, Shimazaki T, Sobue G, Okano H. 2004. Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev Biol 275:124–142 [DOI] [PubMed] [Google Scholar]
- 16.Patani R, Compston A, Puddifoot CA, Wyllie DJ, Hardingham GE, Allen ND, Chandran S. 2009. Activin/nodal inhibition alone accelerates highly efficient neural conversion from human embryonic stem cells and imposes a caudal positional identity. PLoS ONE 4:e7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li Y, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K. 2010. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol 28:848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. 2000. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 18:399–404 [DOI] [PubMed] [Google Scholar]
- 19.Shimozawa N, Nakamura S, Takahashi I, Hatori M, Sankai T. 2010. Characterization of a novel embryonic stem cell line from an intracytoplasmic sperm injection-derived blastocyst in the African green monkey. Reproduction 139:565–573 [DOI] [PubMed] [Google Scholar]
- 20.Suemori H, Tada T, Torii R, Hosoi Y, Kobayashi K, Imahie H, Kondo Y, Iritani A, Nakatsuji N. 2001. Establishment of embryonic stem cell lines from cynomolgus monkey blastocysts produced by IVF or ICSI. Dev Dyn 222:273–279 [DOI] [PubMed] [Google Scholar]
- 21.Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. 2008. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/β-catenin, activin/nodal, and BMP signaling. Development 135:2969–2979 [DOI] [PubMed] [Google Scholar]
- 22.Terashima M, Kobayashi M, Motomiya M, Inoue N, Yoshida T, Okano H, Iwasaki N, Minami A, Matsuoka I. 2010. Analysis of the expression and function of BRINP family genes during neuronal differentiation in mouse embryonic stem cell-derived neural stem cells. J Neurosci Res 88:1387–1393 [DOI] [PubMed] [Google Scholar]
- 23.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 24.Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. 1995. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA 92:7844–7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. 1996. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod 55:254–259 [DOI] [PubMed] [Google Scholar]
- 26.Tonge PD, Andrews PW. 2010. Retinoic acid directs neuronal differentiation of human pluripotent stem cell lines in a non-cell-autonomous manner. Differentiation 80:20–30 [DOI] [PubMed] [Google Scholar]
- 27.Wada T, Honda M, Minami I, Tooi N, Amagai Y, Nakatsuji N, Aiba K. 2009. Highly efficient differentiation and enrichment of spinal motor neurons derived from human and monkey embryonic stem cells. PLoS ONE 4:e6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Wang S, Yang S, Li T, Ji S, Chen H, Li B, Jin L, Xie Y, Hu Z, Chi J. 2006. Feeder layer and serum-free culture of rhesus monkey embryonic stem cells. Reprod Biomed Online 13:412–420 [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Su P, Li D, Tsang S, Duan E, Wang F. 2010. High-efficiency induction of neural conversion in human ESCs and human induced pluripotent stem cells with a single chemical inhibitor of transforming growth factor βsuperfamily receptors. Stem Cells 28:1741–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]