Abstract
Background:
Despite the evidence that women world-wide are using methamphetamine (MA) during pregnancy little is known about the neurodevelopment of their children.
Design:
The controlled, prospective longitudinal New Zealand (NZ) Infant Development, Environment and Lifestyle (IDEAL) study was carried out in Auckland, NZ. Participants were 103 children exposed to MA prenatally and 107 not exposed. The Mental Developmental Index (MDI) and the Psychomotor Developmental Index (PDI) of the Bayley Scales of Infant Development, Second Edition (BSID-II) measured cognitive and motor performance at ages 1, 2 and 3, and the Peabody Developmental Motor Scale, Second Edition (PDMS-II) measured gross and fine motor performance at 1 and 3. Measures of the child’s environment included the Home Observation of Measurement of the Environment and the Maternal Lifestyle Interview. The Substance Use Inventory measured maternal drug use.
Results:
After controlling for other drug use and contextual factors, prenatal MA exposure was associated with poorer motor performance at 1 and 2 years on the BSID-II. No differences were observed for cognitive development (MDI). Relative to non-MA exposed children, longitudinal scores on the PDI and the gross motor scale of the PDMS-2 were 4.3 and 3.2 points lower, respectively. Being male and of Maori descent predicted lower cognitive scores (MDI) and being male predicted lower fine motor scores (PDMS-2)
Conclusions:
Prenatal exposure to MA was associated with delayed gross motor development over the first 3 years, but not cognitive development. However, being male and of Maori descent were both associated with poorer cognitive outcomes. Males in general did more poorly on tasks related to fine motor development.
Keywords: Prenatal exposure, Methamphetamine, Neurodevelopment, Longitudinal
1. Introduction
Amphetamine-type stimulants (ATS) are the second most widely used illicit drug, after marijuana with an estimated prevalence of 0.3 – 1.2 percent in 2010, or between 14 and 52.5 million global users [58]. International trends of ATS show an increase in their initiation of use in the United States (US), East Asia and Oceania [56,57]. Oceania is the region with the highest prevalence of ATS use with New Zealand (NZ) having the second highest annual estimate (2.1%) after Australia (2.7%) [57]. Methamphetamine (MA) is a particularly potent form of ATS and reports suggest a substantial number of women are using it during pregnancy resulting in an escalation of women seeking treatment for prenatal MA dependence [1,55,60].
MA is a CNS stimulant that acts predominantly on the sympathetic nervous system. Acute administration of MA causes the release of monoamines dopamine, serotonin and norepinephrine. The psychoactive and potential neurotoxic effects from prolonged or high doses of MA may occur due to the following mechanisms that increase the synaptic or extracellular levels of these monoamines, primarily dopamine. 1) displacement of monoamines from synaptic vesicles to the cytosol; 2) reverse transport of neurotransmitters through plasma membrane transporters and through blocking the activity of dopamine transporters as well as decreasing the expression of these transporters at the cell surface; 3) increased activity and expression of tyrosine hydroxylase; and 4) inhibition of monoamine oxidase. [39,40,45,47].
Preclinical studies have shown MA to be neurotoxic to mature dopaminergic and serotonergic axons and terminal arbors and possibly glutaminergic axons through the production of reactive oxygen species and nitric oxide, p53 activation resulting in cell death and mitochondrial dysfunction.[42] The neurotoxicity of MA on the developing brain is not well understood, however, given the widespread influence of dopaminergic, serotonergic and glutaminergic systems on neuronal growth and connectivity, prenatal exposure may directly alter developing neural circuitry. Additionally, neurotoxic effects could occur indirectly through maternal anorexic effects of MA, through vasoconstrictive effects resulting in reduced uteroplacental blood flow and fetal hypoxia or through a cascade of events that repeatedly challenge the stress-response systems during gestation leading to an alteration in the hypothalamic-pituitary-adrenal axis [36,46,54].
Evidence from animal studies have revealed that prenatal administration of MA is toxic to dopaminergic and serotonergic neurons [27,41]; and results in motor and learning impairment.[32,50,59] Prenatal exposure in mice has been found to enhance conceptual DNA oxidation and lead to long-term and possibly permanent postnatal neurodevelopmental deficits in motor coordination. [33] Deficits originated from a single exposure to MA during either the embryonic period of organogenesis or the later fetal period of functional development suggesting a broad window of risk during gestation. Brain imaging studies of MA exposed children have shown aberrant neuronal and glial development and volumetric differences in subcortical brain regions that were related to inhibitory behavior, working memory, sustained attention and visual motor integration, verbal and memory tasks [11,12,14]. A more recent study identified increased cortical thickness in perisylvian and orbital-frontal cortices; and reduced caudate nucleus volumes that were associated with deficits in attention in MA exposed children between 3 and 5 years of age. [18]
For children exposed prenatally to MA, there may be early and ongoing deficits in neurodevelopment. At present the only evidence of the long-term effects of prenatal exposure to any ATS come from an early study in Sweden that has followed the growth and development of 65 children exposed prenatally to amphetamine.[3-6,10,21,23-25]. Results using the Terman Merrill method of developmental screening showed the amphetamine exposed children at age four had lower IQ scores (103 vs 110, respectively) and more “problem children” than an unselected community sample of Swedish children.[5] However, maternal alcohol and substance abuse during pregnancy was correlated negatively with the child’s adjustment as were numbers of paternal criminal convictions, number of maternal stress factors and number of earlier children born to the mother.[6] Reports from this prospective study also found an association between the amount and duration of prenatal amphetamine exposure and aggressive behavior, social adjustment and psychometric assessments at age 8. Alcohol abuse and attitude towards the pregnancy were also significantly correlated with these outcomes. [20] [3] At age 14-15 exposed children performed more poorly on tests of Swedish language, math and physical training than their schoolmates and 15 percent were one year behind for their age.[10,22] As in earlier reports maternal alcohol abuse was correlated with one of the primary outcomes, Swedish language scores.[10] The lack of a control group and the association of other environmental factors associated with the developmental outcomes of these amphetamine exposed children make it difficult to determine whether these outcomes are due to the direct effects of prenatal exposure to amphetamine and/or alcohol, or the indirect effects of maternal factors and the postnatal environment.
Emerging evidence from the only current well-controlled, prospective, longitudinal studies of prenatal MA use, the US and NZ Infant Development, Environment and Lifestyle (IDEAL) studies, have found linkages between prenatal MA exposure and poorer quality of movement, more total stress/abstinence, physiological stress, and CNS stress in newborns of the US and NZ samples,[35,51] and more non-optimal reflexes in the NZ sample. Relevant to this report, the US IDEAL study found a lower grasping score on the Peabody Developmental Motor Scales-II (PDMS-II) at age 1, that persisted in longitudinal models through age 3 [52]. The impact of prenatal MA exposure on neurodevelopment may also be inferred from a longitudinal study of another ATS, 3,4-methylenedioxymethamine (MDMA) carried out in the United Kingdom (UK). [48] Similar to the US IDEAL findings, early reports from the UK study revealed an association between prenatal exposure to MDMA and lower quality of movement and less mature gross motor functioning at age 4 months and 1 year [48,49].
Increased psychosocial problems, multiple drug use and mental health problems are common among women who use illicit drugs [1,7,43,61,62]. Therefore, any conclusions concerning the direct effects of prenatal MA exposure on neurodevelopment must also consider the context of the home environment and a wide range of potentially confounding maternal lifestyle factors.
The specific aims of this investigation were the following: first, to describe and compare the cognitive and motor development at 1, 2 and 3 years of age in a sample of NZ children exposed prenatally to MA with a matched, non-MA exposed group of children; second, to determine the extent of change in cognitive and motor development in MA-exposed children relative to non-MA exposed children over these 3 years using longitudinal analytic techniques that control for other drug use prenatally, birth outcomes, the ensuing home environment, and maternal characteristics and lifestyle factors.
2. Methods
2.1 Sample
Recruitment occurred between 2006 and 2010 through referrals from maternity services at participating hospitals and independent midwife practices. Screening through midwives in these services determined whether the mother met the study criteria. If the mother met the criteria and agreed to learn more about the study, study staff met with her to explain the study in detail and obtain written consent to participate. Trained interviewers reviewed the study protocol again with the mother post-partum, prior to discharge, to affirm consent, collect meconium from her infant, and obtain substance use and lifestyle information. Study approval was obtained from the following: Auckland and Waitemata District Health Boards and their Māori ethics committees, and the NZ Ministry of Health’s Northern Regional Ethics Committee. Confidentiality around maternal substance use was established according to the NZ Ministry of Health Ethics Committee guidelines. Consistent with these guidelines, any evidence of child abuse or neglect would require referral to Child Youth and Family Protective Services, but there is no mandatory reporting of MA use during pregnancy.
Maternal exclusion criteria were: prenatal use of hallucinogens; history of intellectual disability; overt psychotic behavior or a documented history of psychosis; non-English speaking (except Māori); multiple gestation; newborn critically ill and unlikely to survive at birth or born with a chromosomal disorder; previous child already enrolled in the study, and mother <17.5 years of age at the infant’s birth. MA exposure was determined by maternal self-report of any MA use during the current pregnancy or MA detected in her infant’s meconium by gas chromatography-mass spectrometry. Comparison mothers required both maternal denial of MA use during pregnancy and a negative meconium result for MA or other stimulants. Comparisons were also excluded if their infant’s meconium screened positive for cocaine or opiates or if they reported use of cocaine or opiates. Of the 103 participants in the exposed group, one participant denied MA use but was identified as exposed by toxicology only; 102 participants reported amphetamine use with 92 by self report only (toxicology was negative) and 10 by self report and positive toxicology. NZ meconium samples were shipped to the United States Drug Testing Laboratory in Des Plaines, IL for analysis. Details of toxicology assays are reported elsewhere [1,16]. Eligible MA-using and comparison mothers were matched on their infant’s birth weight (<1500 g; 1500-2500 g; >2500 g), self-identified ethnicity, and level of education (achievement of 5th form certificate or not or the equivalent according to the National Certificate of Educational Achievement [NCEA] in NZ). Due to the referral process required in NZ, matching was geared to achieving equivalent ethnicity between the MA and comparison groups. Of the 421 total subjects who were identified as meeting the study criteria, only 49 refused to participate. The remainder of the subjects were either not available, not approached, or ineligible.
All children who were assessed at age 1, 2 or 3 years on the Bayley Scales of Infant Development, 2nd Edition[2] (BSID-II) or at 1 or 3 years on the Peabody Developmental Motor Scales, Second Edition [26] (PDMS-2) were included. There were 210 participants overall (103 children MA exposed and 107 comparison). At enrollment, all study mothers were interviewed to obtain the following: 1) demographics including age, level of education, occupation, self-identified ethnicity, marital status, and socioeconomic status (SES), calculated using the four-factor Hollingshead Index adapted to single parent and non-nuclear families,[13] with Hollingshead level V indicating low SES; and 2) obstetric history; and 3) any licit or illicit drug use during pregnancy including tobacco, alcohol and marijuana.
At each assessment mothers were given $40.00 that they could use to cover any out of pocket expenses such as babysitting for siblings. In addition, those mothers who drove to our research unit were given a $20.00 gas voucher. For those mothers who didn’t drive or own an automobile, a taxi to and from our research unit was provided. A further incentive was a $10.00 voucher that was sent to mothers who notified us when they changed their address or contact details.
2.2 Measures
NZ IDEAL uses the same measures and protocol as the US IDEAL study [63].
2.3 Substance Use
The Substance Use Inventory (SUI) was used to obtain a prenatal drug use history that included quantity and frequency across four time periods: the three months preceding, and the first, second and third trimesters of the current pregnancy. Consistent with other IDEAL studies, heavy MA use was defined as ≥ 3 days per week across pregnancy [34,35,51,52,61]. Table 1 shows the frequency of MA use across pregnancy. Over 34% continued to use MA throughout their pregnancy. Extent of prenatal exposure to other drugs was calculated as ounces of absolute alcohol per day, number of cigarettes per day and number of joints per day across pregnancy [15,53]. Postnatal use of MA, tobacco, alcohol and marijuana was also measured at ages 1, 2, and 3 years for each drug.
Table 1.
Frequency of self-reported MA use.a
| MA Use | N=101 | ||
|---|---|---|---|
|
| |||
| Trimester | |||
|
| |||
| First | Second | Third | |
|
| |||
| N (%) | N (%) | N (%) | |
| Daily | 11 (10.9%) |
5 (5.0%) | 1 (1.0%) |
| 3-6 days/wk. | 16 (15.8%) |
5 (5.0%) | 6 (5.9%) |
| 1-2 days/wk. | 19 (18.8%) |
17 (16.8%) | 6 (5.9%) |
| 1-3 days/mo. | 12 (11.9%) |
12 (11.9%) | 11 (10.9%) |
| 1-2 days/3 mos. | 23 (22.8%) |
10 (9.9%) | 16 (15.8%) |
| Not at all | 6 (5.9%) | 48 (47.2%) | 60 (59.4%) |
| Days/week (mean, SD) |
2.18(2.35) | 1.07(1.91) | 0.46(1.17) |
|
| |||
| Used all 3 trimesters | 34 (33.7%) | ||
1 of the 103 MA users in this study denied MA use but was subsequently identified as exposed by the toxicology results, and 1 further participant did not provide self-report data for frequency but did report MA use7
2.4 Maternal characteristics and home environment
Postnatal caregiver and environmental characteristics were obtained on multiple visits at 1 month, then at 1, 2, 2.5 and 3 years. At each annual visit, the current household structure and SES were obtained. The quality of the home environment (including social-emotional and cognitive support available in the home) was measured at age 2.5 using the overall summary score from the Home Observation for Measurement of the Environment (HOME) inventory [9]. Individual items are based on family interview and observations made by trained interviewers [8]. The Peabody Picture Vocabulary Test-III (PPVT-III) was also administered at age 2.5 [19]. This test measures receptive vocabulary and serves as a proxy measure of caregiver’s IQ. The standardized score reported is based on the number of correct answers.
2.5 Cognitive and motor development
Trained, certified examiners masked to group status assessed the cognitive and motor development of infants with the BSID-II at ages 1 ± 2 weeks, 2 ±4 weeks and 3 ±6 weeks. The PDMS-2 was used to measure motor development at ages 1 and 3. Age of administration was corrected for prematurity for infants born at < 37 weeks gestation. The BSID-II Psychomotor Development Index (PDI) and the PDMS-2 measures both gross and fine motor skills. The Mental Developmental Index (MDI) of the BSID-II measures cognitive development including: memory, problem solving, early number concepts, early communication and vocalization. Both the BSID-II and the PDMS-2 provide developmental quotients with a mean of 100 and a standard deviation (SD) of +/− 15. The PDMS-2 provides separate developmental quotients for fine (small muscle systems, grasping and visual-motor integration) and gross motor (large muscle systems, stationary, locomotion and object manipulation).
2.6 Statistical Analysis
The association of MA exposure with maternal and neonatal characteristics was examined using analysis of variance (ANOVA) and chi-square statistics. One-way ANOVA tested the effects of MA exposure on motor and cognitive outcomes as measured by the BSID-II and the PDMS-2. GLM tested the effect of MA exposure on BSID-II and PDMS-2 with adjustment for covariates. The level of MA use was recoded into heavy use versus some and no use to test whether heavy MA use had a greater effect on developmental outcomes.
General linear mixed models (GLMM), PROC MIXED from SAS version 9.1.3, were used to test the longitudinal effects of prenatal MA exposure on cognitive and motor outcomes, after controlling for potential covariates. GLMM can accommodate missing data and are useful for modeling repeated measures data in an unbalanced design. Separate models were conducted for BSID-II MDI and PDI scores at 1, 2, and 3 years and for PDMS-2 scores at 1 and 3 years. Figure 1 provides an overview of the participants available for follow-up across the first 3 years.
Fig. 1.
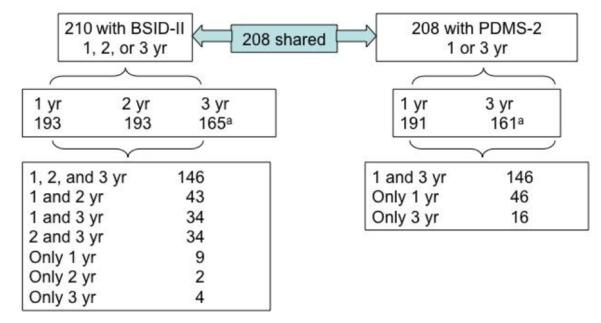
Flow chart of cohort with follow up 1 to 3 years(yr).
aData collection is ongoing
There were 38 (18.1%) missing values for the HOME Inventory and 57 (27.1%) missing values for the PPVT-III in our sample for the longitudinal analysis of the BSID-II. Multiple imputation using PROC MI in SAS was applied. Ten imputed datasets were generated for each analysis. The results were combined for the estimation of regression parameters using PROC MIANALYZE. Sensitivity analyses were performed on the data with and without imputed values for caregiver IQ and the quality of home scores. Results are reported from the imputed data to retain the full sample since the results were similar.
2.7 Covariates
Covariates were selected based on conceptual reasons, published literature, and maternal and neonatal characteristics that differed between MA exposure (P<0.05) if not highly correlated with other covariates (r>0.7). Factors unrelated to cognitive or motor outcomes are not included as covariates. Covariates included in all cross sectional and longitudinal models were any prenatal exposures to alcohol, tobacco, and marijuana, SES, gender, birth weight, and ethnicity. Quality of the home, caregiver IQ and gender were included a priori as covariates in BSID-II longitudinal analyses because they are shown to be associated with cognitive development in children. The quality of the home and caregiver IQ were not included in cross sectional analyses of BSID-II outcomes since two of three time points occurred prior to their measurement. Finally, analyses were repeated while excluding birth weight to test the possibility that birth weight was mediating the impact of MA exposure.
3. Results
Demographics and maternal and neonatal characteristics of the sample are presented in Table 2. As a result of the matching protocol there were no significant differences in level of education, or ethnicity. Relative to the comparison group, the MA-exposed group was more likely to have a lower SES, have mothers with no partner at birth and who presented later in gestation for prenatal care. The MA exposed group also had a higher percentage of mothers reporting the use of tobacco and marijuana than comparison mothers, but not alcohol (63% vs. 57%, respectively), and a higher daily use of alcohol, cigarettes and marijuana across pregnancy. Prenatal exposure to MA was also associated with lower birth weight, but no other neonatal characteristics, and postnatally, to a lower SES at the 3-year visit.
Table 2.
Sample characteristics by MA exposure
| Number (Percent) a / Mean (SD) | |||
|---|---|---|---|
|
| |||
| MA Exposed (N = 103) |
Comparison (N= 107) |
P-Value | |
|
Maternal and demographic characteristics at
birth |
|||
| Culture | 0.572 | ||
| White | 58 (56.3%) | 50 (46.7%) | |
| Maori | 33 (32.0%) | 40 (37.4%) | |
| Pacific Islander | 9 (8.7%) | 13 (12.1%) | |
| Asian | 3 (2.9%) | 3 (2.8%) | |
| Indian-Pakistani | 0 (0.0%) | 1 (0.9%) | |
| Low socioeconomic status | 50 (49.0%) | 21 (19.6%) | <0.001 |
| No partner at birth | 54 (52.4%) | 29 (27.1%) | <0.001 |
| Education <5th form | 66 (64.1%) | 55 (51.4%) | 0.063 |
| Maternal age (yr) | 26.8 (6.2) | 25.4(6.8) | 0.142 |
| GA at 1st prenatal visit, week | 15.9 (6.9) | 13.3 (5.7) | 0.004 |
| Prenatal tobacco use | 91 (88.3%) | 56 (52.3%) | <0.001 |
| Cigarettes/day across pregnancy | 8.9 (7.3) | 3.3 (5.6) | <0.001 |
| Prenatal alcohol use | 65 (63.1%) | 61 (57.0%) | 0.367 |
| Absolute alcohol/day across pregnancy | 0.32 (0.81) | 0.12 (0.28) | 0.014 |
| Prenatal marijuana use | 67 (65.0%) | 24 (22.4%) | <0.001 |
| Joints/day across pregnancy | 0.47 (0.99) | 0.18(0.66) | 0.014 |
| Neonatal characteristics | |||
| Gender (boy) | 55 (53.4%) | 58 (54.2%) | 0.907 |
| Birth Weight (g) | 3324(453) | 3492 (557) | 0.017 |
| Birth Length (cm) | 50.9 (2.3) | 51.2 (2.5) | 0.343 |
| Birth Head Circumference (cm) | 34.5 (1.7) | 34.9 (1.6) | 0.082 |
| Gestational Age (weelcs) | 39.2 (1.5) | 39.5 (1.4) | 0.080 |
| Small for Gestational Age | 9 (8.7%) | 13 (21.1%) | 0.420 |
| Low Birth (<2500) | 4 (3.9%) | 5 (4.7%) | 0.778 |
| Postnatal characteristics of the environment | |||
| Quality of the home (2.5 yr visit) | 33.3 (6.2) | 34.7 (6.2) | 0.145 |
| Caretaker IQ | 94.1 (9.6) | 90.7(13.6) | 0.081 |
| Low socioeconomic status (3 yr visit) | 46 (56.1%) | 19 (22.1%) | <0.001 |
The number of participants includes BSID-II or PDMS-2 evaluation at any visit
The growth of infants included in this report varies across the first 3 years of age (Table 3). MA exposure was associated with smaller head circumferences at 1 year, weighing less at 2 years and being shorter at 3 years.
Table 3.
Growth measures across infancy by MA exposure
| Measurement | n | MA Exposed Mean (SD) |
n | Not Exposed Mean (SD) |
p |
|---|---|---|---|---|---|
| Length (cm) | |||||
| 1 year | 93 | 74.75 (3.47) | 100 | 75.42 (3.10) | .160 |
| 2 years | 99 | 86.68 (3.57) | 99 | 87.37(3.38) | .162 |
| 3 years | 82 | 95.34 (3.44) | 85 | 96.8 (3.78) | .009 |
| Weight (g) | |||||
| 1 year | 93 | 10288.71 (1287.04) | 99 | 10467.36(1208.33) | .323 |
| 2 years | 97 | 12859.43 (1639.11) | 98 | 13366.13 (1699.24) | .035 |
| 3 years | 82 | 15234.47 (2140.46) | 84 | 15600.30(2375.01) | .276 |
|
Head Circumference
(cm) |
|||||
| 1 year | 94 | 46.74 (1.36) | 100 | 47.15 (1.40) | .036 |
| 2 years | 98 | 49.11 (1.29) | 98 | 49.31(1.49) | .306 |
| 3 years | 82 | 50.45 (1.29) | 85 | 50.61(1.38) | .424 |
Cross-sectional analyses of motor outcomes from the PDMS-2 are reported in Table 4, unadjusted and adjusted for birth weight, gender, prenatal drug exposures, SES and ethnicity. At age 1, there were no exposure effects on gross or fine motor quotients or subtests. At age 3, prenatal MA exposure was associated with lower scores than the comparison group on the gross motor quotient (Unadjusted P<0.047), but not maintained after adjustment. Similarly, prenatal MA exposure was associated with lower stationary subtest scores (Unadjusted, P<0.027), but not after adjustment. There were no effects of heavy MA exposure on gross or fine motor quotients or subtests at 1 or 3 years (P>0.05 in all cases).
Table 4.
PDMS-2 by MA exposure
| Meant ± (SD) | ||||
|---|---|---|---|---|
|
|
||||
| Quotient/Subscales | MA Exposed | Comparison |
Unadjuste
d P-Value |
aAdjuste
d P-Value |
| 1 year | (N = 93) | (N= 99) | ||
| Gross Motor Quotient | 100.56 ± 10.61 | 102.73 ± 8.17 | 0.113 | 0.055 |
| Stationary | 10.15 ± 1.73 | 10.56 ± 1.34 | 0.070 | 0.157 |
| Locomotion | 9.67 ± 2.76 | 10.15 ± 2.43 | 0.197 | 0.058 |
| Object Manipulation | 10.69 ± 1.49 | 10.77 ± 1.39 | 0.743 | 0.467 |
| Fine Motor Quotient | 97.55 ± 10.68 | 99.24 ± 9.10 | 0.237 | 0.339 |
| Grasping | 9.88 ± 2.60 | 10.22 ± 2.20 | 0.328 | 0.592 |
| Visual-Motor Integration | 9.30 ± 1.37 | 9.53 ± 1.52 | 0.286 | 0.235 |
| 3 years | (N = 77) | (N=84) | ||
| Gross Motor Quotient | 97.38 ± 8.65 | 100.23 ± 9.35 | 0.047 | 0.137 |
| Stationary | 9.31 ± 1.95 | 9.99 ± 1.90 | 0.027 | 0.059 |
| Locomotion | 9.06 ± 1.51 | 9.40 ± 1.62 | 0.171 | 0.291 |
| Object Manipulation | 10.39 ± 1.87 | 10.73 ± 2.04 | 0.279 | 0.506 |
| Fine Motor Quotient | 96.03 ± 10.10 | 96.36 ± 10.33 | 0.838 | 0.931 |
| Grasping | 8.87 ± 2.11 | 8.79 ± 2.36 | 0.812 | 0.967 |
| Visual-Motor Integration | 9.81 ± 1.85 | 10.0 ± 1.69 | 0.486 | 0.833 |
Adjusted for birth weight, gender, prenatal drug exposures, socioeconomic status, and Maori culture
The cross-sectional results of the BSID-II MDI and PDI are reported in Table 5, unadjusted and adjusted for birth weight, gender, prenatal drug exposures, socioeconomic status and ethnicity. No effects of MA exposure on cognitive development (MDI) were found at ages 1 or 3 years. At age 2, MA exposure was related to lower MDI quotients (Unadjusted, P<0.041), but not after adjustment. However at both 1 and 2 years of age after adjustment, MA exposure was related to lower PDI scores (Adjusted P=0.035, P=0.015, respectively). No effects for exposure were found at age 3. There were no effects of heavy MA exposure on the MDI or PDI at any age (P>0.05 in all cases)
Table 5.
BSID-II by MA exposure
| Meant ± (SD) | ||||
|---|---|---|---|---|
|
|
||||
| Standard scores |
MA Exposed | Comparison | Unadjusted P-Value |
aAdjusted P-Value |
| 12 months | (N = 94) | (N= 99) | ||
| MDI | 93.16 ± 12.73 | 95.82 ± 13.28 | 0.158 | 0.139 |
| (N=93) | (N=99) | |||
| PDI | 87.03 ± 17.45 | 89.13 ± 16.79 | 0.397 | 0.035 |
| 24 months | (N = 97) | (N= 96) | ||
| MDI | 85.78 ± 15.76 | 90.21 ± 14.11 | 0.041 | 0.156 |
| PDI | 87.14 ± 17.71 | 94.33 ± 14.88 | 0.003 | 0.015 |
| 36 months | (N = 79) | (N= 86) | ||
| MDI | 87.75 ± 12.75 | 88.52 ± 12.58 | 0.695 | 0.412 |
| PDI | 90.86 ± 14.30 | 93.53 ± 14.51 | 0.235 | 0.657 |
Adjusted for birth weight, gender, prenatal drug exposures, socioeconomic status, and culture
GLMM, were used to examine the effects of prenatal MA exposure on longitudinal scores from the BSID-II and the PDMS-2 with adjustment for prenatal exposures to alcohol, tobacco, and marijuana, SES, gender, birth weight and ethnicity. Overall, there were no effects of prenatal MA exposure on the MDI (Table 6). However, Māori scored 4.4 points lower on the MDI (P=.010) and boys scored 5.3 points lower than girls (P=<0.001). Notable was the finding that MA-exposed children scored 4.3 points lower on the PDI relative to children with no exposure (P=0.032). For each 100g increment in birth weight, PDI increases by 0.33 (P=0.048). Overall gross motor quotient scores were 3.2 points lower in the MA-exposed group versus the comparison group (P=.027). There were no significant effects of prenatal MA exposure on the fine motor quotient scores of the PDMS-2. However, boys scored significantly lower than girls on both the fine motor quotient score and grasping subtest (P=0.004). There were no significant effects of heavy MA exposure on the BSID-II indices or PDMS-2 quotients or subtests. Birth weight was also tested, but there were no significant mediating effects (P>0.05).
Table 6.
Selected repeated measures coefficients from fixed-effect mixed models.
| Outcome | Parameter | Estimate | SE | p |
|---|---|---|---|---|
| PDMS-2 | ||||
| Gross motor | Prenatal MA | −3.22 | 1.44 | 0.027 |
| Stationary | Prenatal MA | −0.59 | 0.25 | 0.021 |
| Locomotion | Prenatal MA | −0.63 | 0.32 | 0.055 |
| Object manipulation | Prenatal MA | −0.27 | 0.29 | 0.346 |
| Fine motor | Prenatal MA | −0.66 | 1.44 | 0.648 |
| Gender (Boy) | −3.51 | 1.19 | 0.004 | |
| Grasping | Prenatal MA | −0.10 | 0.32 | 0.751 |
| Gender (Boy) | −0.76 | 0.26 | 0.004 | |
| Visual-motor integration | Prenatal MA | −0.13 | 0.25 | 0.621 |
| BSID-II | ||||
| MDI | Prenatal MA | −1.32 | 1.67 | 0.428 |
| Maori | −4.40 | 1.72 | 0.010 | |
| Gender (Boy) | −5.25 | 1.42 | <0.001 | |
| PDI | Prenatal MA | −4.28 | 1.99 | 0.032 |
| Birth weighta | 0.33 | 0.02 | 0.048 |
Notes: Non-significant findings for prenatal exposure to alcohol, tobacco, and marijuana, low socioeconomic status, birth weight, culture, IQ, and quality of the home are not presented. Time trends were significantly decreasing in all analyses.
The increment for birth weight for parameter estimates is 100g
4.0 Discussion
We found evidence for poorer motor development among children exposed prenatally to MA. Relative to non-MA exposed children MA-exposed children had poorer psychomotor scores on the BSID-II at 1 and 2 years of age. Longitudinal models employed to examine the relative effect of prenatal MA exposure over the first three years of development revealed lower scores on composite measures of psychomotor performance (PDI) and gross motor performance on the PDMS-2. These findings persisted after controlling for maternal characteristics, other prenatal drug exposures, and SES. However, birth weight was independently associated with better performance on the PDI.
No associations were found between MA exposure and cognitive scores on the BSID-II or fine motor development on the PDMS-2. Rather, poorer cognitive performances over time were associated with being male and Māori, and poorer fine motor and grasping skills with being male.
The finding that prenatal exposure to MA is associated with poorer motor outcomes is consistent with reports that have raised concerns about the neurodevelopmental outcomes of infants exposed prenatally to cocaine [44] MDMA[48,49], and a recent report of prenatal MA exposure from US IDEAL [52]. Findings from US IDEAL found heavy MA exposure was associated with one aspect of fine motor development, lower scores on the grasping subscale at ages 1 and 3 of the PDMS-2 [52]. Prenatal MDMA exposure was associated with lower quality of movement and less mature gross motor functioning at 4 months of age and at 1 year in heavily exposed infants [48,49].
Our findings extend the literature in two ways. First, despite the range of prenatal MA exposure in this sample and the finding that nearly one third of the mothers continued to use MA across all three trimesters, poorer motor outcomes in this study were not restricted to the most heavily MA exposed children. Second, we found MA exposure in longitudinal models were associated with poorer motor performance on two measures of motor development, the PDI that measures aspects of fine and gross motor development and the gross motor quotient of the PDMS-2. Taken together these findings suggest robust associations between MA exposure and motor outcomes over the first 3 years.
Notable also were the findings that males of Māori descent exhibited poorer performances on cognitive and fine motor tasks overtime. Numerous reports document the disparity in childhood illness and unmet health needs between Māori children and NZ non-Māori children [37]. Of particular concern are reports that Māori children are three times more likely to be hospitalized for bronchiolitis, rheumatic fever and bronchiectasis and have significantly higher rates of middle ear infections. However, this is the first published evidence to our knowledge that Māori males are more at risk of cognitive and fine motor delay during infancy and toddlerhood than Māori girls or other non-Māori NZ children.
Previous reports of this sample of children found prenatal MA exposure resulted in early motor disturbances at birth and 1 month [35]. These results along with the current findings suggest an early perturbation of motor development with ongoing consequences for motor performance. One explanation for the variability in the linkages we, and others have found between prenatal MA exposure and poorer motor performance is that prenatal or perinatal stressors may bring about a different setting of the monoaminergic systems. The monoaminergic systems are widespread systems involved in the modulation of behavior. These changes have been associated with impairments in the development of somatosensory processes associated with atypical motor development [28,29]. This impaired development may reflect a situation where a child may have a typical range of movements but has difficulties in selecting the best strategy to complete a motor task in a novel situation due to deficits in processing of sensory information. As a result of this inability to vary motor behavior to meet task-specific requirements, a child may exhibit more variable behavior during motor tests. In this instance, the child may require more time and/or more practice to learn new motor skills [30,31].
Despite the number of studies that have identified delayed motor development in children exposed prenatally to a range of recreational drugs, few intervention studies specifically targeting motor development exist. Rather the focus of intervention research has been to reduce the environmental risks associated with maternal substance abuse [17,61] thereby improving parenting and in turn fostering more optimal child development. However the effectiveness of these programs has been variable and few have reported positive effects for motor development. Given that we found delayed motor development in MA exposed children persisted after adjusting for numerous environmental risks, it would seem interventions are needed that are more directly child focused and include strategies to improve early motor development[38].
The major strengths of this study include a non-clinical representative sample, a very high retention for this high-risk population after 3 years (91%); and a database that provides multiple data sources and a rich history of contextual and maternal characteristics. The main limitation is the reliance mostly on maternal self-report to determine the extent of MA and other drug use. However, women in NZ are likely to be more forthcoming with reports of their drug use during pregnancy as there is no legal mandate for health professionals to report them to child protection services [63].
In conclusion, the findings from this report suggest prenatal MA exposure is associated with ongoing perturbations of motor development during early development. However, further longitudinal research is needed to determine whether these motor disturbances will persist, and the impact they may have on the development of more complex motor responses required for early and ongoing cognitive and social emotional competencies.
Longitudinal study of early neurodevelopment in New Zealand children exposed prenatally to methamphetamine
Methamphetamine exposure was associated with lower motor scores on two standardized measures of motor development
No significant association was found between methamphetamine exposure and cognitive outcomes
Males of Maori descent performed more poorly on cognitive tasks, and males in general on fine motor tasks
Acknowledgements
Funding and Support: this work is part of the US and NZ Infant Development, Environment and Lifestyle Study funded by NIH grants: National Institutes on Drug Abuse, 2RO1DA014948 and RO1DA021757. The NZ IDEAL study had additional support from the Auckland Medical Research Foundation. None of the funders were involved in the design, collection, analysis or interpretation of the data, the writing of the report or the decision to submit this report.
NZ IDEAL Study team: Jenny Rogers, BSc (Nursing), PGCert Child & Adolescent Mental Health, Josephine Cliffe, MPH, Suzanne Cumming, BSW, Heather Stewart, RN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
None of the authors have a financial association with any product associated with this research or other conflict of interest. There were no commercial sponsors involved in the above matters. Dr. Trecia Wouldes wrote the first draft of the manuscript and there was no honorarium, grant, or other form of payment given to anyone to produce this manuscript.
5.0 REFERENCES
- 1.Arria AM, Derauf C, LaGasse LL, Grant P, Shah R, Smith L, Haning W, Huestis M, Strauss A, Della Grotta S. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Maternal & Child Health Journal. 10(2006):293–302. doi: 10.1007/s10995-005-0052-0. others. [DOI] [PubMed] [Google Scholar]
- 2.Bayley N. Bayley Scales of Infant Development. 2nd The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 3.Billing L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse & Neglect. 18(1994):3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 4.Billing L, Eriksson M, Larsson G, Zetterstrom R. Amphetamine addiction and pregnancy. III. One year follow-up of the children. Psychosocial and pediatric aspects. Acta Paediatr. 85(1980):204–208. doi: 10.1111/j.1651-2227.1980.tb07342.x. [DOI] [PubMed] [Google Scholar]
- 5.Billing L, Eriksson M, Steneroth G, Zetterstrom R. Pre-school children of amphetamine-addicted mothers. I. Somatic and psychomotor development. Acta Paediatr. 74(1985):179–184. doi: 10.1111/j.1651-2227.1985.tb10946.x. [DOI] [PubMed] [Google Scholar]
- 6.Billing L, Eriksson M, Steneroth G, Zetterstrom R. Predictive indicators for adjustment in 4-year old children whose mothers used amphtetamine during pregnancy. Child Abuse & Neglect. 12(1988):503–507. doi: 10.1016/0145-2134(88)90067-1. [DOI] [PubMed] [Google Scholar]
- 7.Bjorn B, Kesmodel US. Prenatal alcohol exposure--a systematic review of the effects on child motor function. Acta Obstet Gynecol Scand. 90(2011):210–226. doi: 10.1111/j.1600-0412.2010.01039.x. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell BM, Bradley RH. Administration manual (revised edition): home observation for measurement of the environment. University of Arkansas; Little Rock; 1984. [Google Scholar]
- 9.Caldwell BM, Bradley RH. HOME inventory administration manual. Third University of Arkansas; Little Rock:AR: 2001. [Google Scholar]
- 10.Cernerud L, Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. Amphetamine addiction during pregnancy: 14-year follow-up of growth and school performance. Acta Paediatrica. 85(1996):204–8. doi: 10.1111/j.1651-2227.1996.tb13993.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, Hedemark B, Smith LM, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine. Neuroimage`. 48(2009):391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Research. 132(2)(2004):95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 9(2002):145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- 14.Cloak CC, Ernst T, Fujii L, Hedermark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 72(2009):2068–2075. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day NL, Leech SL, Goldschmidt L. The effects of prenatal marijuana exposure on delinquent behaviors are mediated by neurocognitive functioning. Neurotoxicol Teratol. 33(2011):129–136. doi: 10.1016/j.ntt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Della Grotta S, LaGasse LL, Arria A, Derauf C, Grant P, Smith LM, Shah R, Huestis M, Liu J, Lester BM. Patterns of methamphetamine use during pregnancy: results from the Infant Development. Environment, and Lifestyle (IDEAL) Study, Maternal & Child Health Journal. 14(2009):519–527. doi: 10.1007/s10995-009-0491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derauf C, LaGasse LL, Smith LM, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S. Demographic and psychosocial characteristics of mothers using methamphetamine during pregnancy: Preliminary results of the Infant Development, Environment, and Lifestyle Study (IDEAL) The American Journal of Drug and Alcohol Abuse. 33(2007):281–289. doi: 10.1080/00952990601175029. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derauf C, Lester BM, Neyzi N, Kekatpure M, Gracia L, Davis J, Kallianpur K, Efird JT, Kosofsky B. Subcortical and cortical structural central nervous system changes and attention processing deficits in preschool-aged children with prenatal methamphetamine and tobacco exposure. Developmental Neuroscience. 34(2012):327–341. doi: 10.1159/000341119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn LM, Dunn LM. Peabody picture vocabulary test. Third edition American Guidance Service, Circle Pines; MN: 1997. [Google Scholar]
- 20.Eriksson M, Billing I, Steneroth G, Zetterstrom R. Health and development of 8-year-old children whose mothers abused amphetamines during pregnancy. Acta Paediatrica Scandinavica. 78(1989):944–949. doi: 10.1111/j.1651-2227.1989.tb11179.x. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson M, Jonsson B, Steneroth G, Zetterstrom R. Cross-sectional growth of children whose mothers abused amphetamines during pregnancy. Acta Paediatrica. 83(1994):612–7. doi: 10.1111/j.1651-2227.1994.tb13091.x. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson M, Jonsson B, Zetterstrom R. Children of mothers abusing amphetamine: head circumference during infancy and psychosocial development until 14 years of age. Acta Paediatrica. 89(2000):1474–8. doi: 10.1080/080352500456679. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson M, Larsson G, Winbladh B, Zetterstrom R. The influence of amphetamine addiction on pregnancy and the newborn infant. Acta Paediatrica Scandinavica. 67(1978):95–9. doi: 10.1111/j.1651-2227.1978.tb16283.x. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson M, Larsson G, Zetterstrom R. Abuse of alcohol. drugs and tobacco during pregnancy--consequences for the child, Paediatrician. 8(1979):228–42. [PubMed] [Google Scholar]
- 25.Eriksson M, Larsson G, Zetterstrom R. Amphetamine addiction and pregnancy. II. Pregnancy, delivery and the neonatal period. Socio-medical aspects. Acta Obstet Gynecol Scand. 60(1981):253–259. doi: 10.3109/00016348109158127. [DOI] [PubMed] [Google Scholar]
- 26.Folio R, Fewell RR. Peabody Developmental Motor Scales. Second Pro-Ed; Austin, TX: 2000. [Google Scholar]
- 27.Fuller R, Hemrick-Luecke S. Further studies on the long-term depletion of striatal dopamine in iprindole-treated rats by amphetamine. Neuropharmacology. 21(1992):433–438. doi: 10.1016/0028-3908(82)90027-2. [DOI] [PubMed] [Google Scholar]
- 28.Hadders-Algra M. The neuronal group selection theory: an attractive framework to explain variation in normal motor development. Dev Med Child Neurol. 42(2000):566–572. doi: 10.1017/s0012162200001067. [DOI] [PubMed] [Google Scholar]
- 29.Hadders-Algra M. Variation and variability: Key words in human motor development. Phys Ther. 90(2010):1823–1837. doi: 10.2522/ptj.20100006. [DOI] [PubMed] [Google Scholar]
- 30.Hadders-Algra M, Brogren E, Katz-Salamon M, Forssberg H. Periventricular leukomalacia and preterm birth have different detrimental effects on postural adjustments. Brain. 1999;122:727–740. doi: 10.1093/brain/122.4.727. pt4. [DOI] [PubMed] [Google Scholar]
- 31.Heineman KR, La Bastide-van Gemert S, Fidler V, Middelburg KJ, Bos AF, Hadders-Algra M. Construct validity of the Infant Motor Profile: relation with prenatal, perinatal and neonatal risk factors. Dev Med Child Neurol. 2010;52:e209–e215. doi: 10.1111/j.1469-8749.2010.03667.x. [DOI] [PubMed] [Google Scholar]
- 32.Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plusmaze for dissociation of amnesic and behavioral effects of drugs in mice. Eur J Pharmacol. 194(1991):71–76. doi: 10.1016/0014-2999(91)90125-a. [DOI] [PubMed] [Google Scholar]
- 33.Jeng W, Wong AW, Ting AK, Wells PG. Methamphetamine-enhanced embryonic oxidative DNA damage andneurodevelopmental deficits. Free Radic Biol Med. 39(2005):317–326. doi: 10.1016/j.freeradbiomed.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 34.LaGasse LL, Derauf C, Smith LM, Newman E, Shah R, Neal C, Arria A, Huestis M, Della Grotta S, Lin H. Prenatal methamphetamine exposure and childhood behavior problems at 3 and 5 years of age. Pediatrics. 129(2012):681–688. doi: 10.1542/peds.2011-2209. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaGasse LL, Wouldes T, Newman E, Smith LM, Shah RZ, Derauf C, Huestis MA, Arria AM, Grotta SD, Wilcox T. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicol Teratol. 33(2011):166–175. doi: 10.1016/j.ntt.2010.06.009. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Developmental Neuroscience. 2009(2009):23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 37.Ministry of Health . The Health of New Zealand Children: Key Findings of the New Zealand Health Survey 2011/2012. Ministry of Health; Wellington, New Zealand: 2012. [Google Scholar]
- 38.Nair P, Schuler ME, Black MM, Kettinger L, Harrington D. Cumulative environmental risk in substance abusing women: early intervention, parenting stress, child abuse potential and child development. Child Abuse Negl. 27(2003):993–995. doi: 10.1016/s0145-2134(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 39.Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: A review. J Neuropsychiatry Clin Neurosci. 15(2003):317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- 40.Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, Barr AM. Methamphetamine use: A comprehensive review of molecular. preclinical and clinical findings, Drug Alcohol Depend. 129(2013):167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Pu C, Voorhees CV. Developmental dissociation of methamphetamine-induced depletion of dopaminergic terminals and astrocyte reaction in rat striatum. Brain Res Dev Brain Res. 72(1993):325–328. doi: 10.1016/0165-3806(93)90201-k. [DOI] [PubMed] [Google Scholar]
- 42.Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMS toxicity. The AAPS Journal. 2006;8:E337–347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson GA, Day NL, Goldschmidt L. Prenatal alcohol, marijuana, and tobacco use: Infant mental and motor development. Neurotoxicol Teratol. 17(1995):479–487. doi: 10.1016/0892-0362(95)00006-d. [DOI] [PubMed] [Google Scholar]
- 44.Richardson GA, Goldschmidt L, Willford J. The effects of prenatal cocaine use on infant development. Neurotoxicol Teratol. 30(2008):96–106. doi: 10.1016/j.ntt.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 39(2001):32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Salisbury AL, Ponder KL, Padbury JF, Lester BM. Fetal effects of psychoactive drugs. Clinics in Perinatology. 36(2009):595–619. doi: 10.1016/j.clp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott JC, Woods SP, Matt GE, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychol Rev. 17(2007):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- 48.Singer LT, Moore DG, Fulton S, Goodwin J, Turner JJD, Min MO, Parrott AC. Neurobehavioral outcomes of infants exposed to MDMA (Ecstasy) and other recreational drugs during pregnancy. Neurotoxicol Teratol. 34(2012):303–310. doi: 10.1016/j.ntt.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singer LT, Moore DG, Min MO, Goodwin J, Turner JJD, Fulton S, Parrott AC. One-year outcomes of prenatal exposure to MDMA and other recreational drugs. Pediatrics. 130(2012):407–415. doi: 10.1542/peds.2012-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slamberova R, Pometlova M, Syllabova L, Mancuskova M. Learning in the place navigation task, not the new-learning task, is altered by prenatal methamphetamine exposure. Developmental Brain Research. 157(2005):217–219. doi: 10.1016/j.devbrainres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 30(2008):20–28. doi: 10.1016/j.ntt.2007.09.005. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith LM, LaGasse LL, Derauf C, Newman E, Shah R, Haning W, Arria A, Huestis M, Strauss A, Della Grotta S. Motor and cognitive outcomes through three years of age in children exposed to prenatal methamphetamine. Neurotoxicol Teratol. 33(2011):176–184. doi: 10.1016/j.ntt.2010.10.004. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sood B, Delaney-Black V, Covington C, Nordstrom-Klee B, Ager J, Templin T, Janisse J, Martier S, Sokol RJ. Prenatal alcohol exposure and childhood behavior at age 6 to 7 years: I. dose-response effect. Pediatrics. 2001;108 doi: 10.1542/peds.108.2.e34. http://pediatrics.aappublications.org/content/108/2/e34.full.html [DOI] [PubMed] [Google Scholar]
- 54.Stek A, Fisher BK, Baker RS, Lang U, Tseng C, Clark KE. Maternal and fetal cardiovascular responses to methamphetamine in the pregnant sheep. Amercian Journal of Obstetrics and Gynecology. 169(1993):888–897. doi: 10.1016/0002-9378(93)90022-b. [DOI] [PubMed] [Google Scholar]
- 55.Substance Abuse and Mental Health Services Administration . Treatment Episode Data Set (TEDS). 1999-2009. National Admissions to Substance Abuse Treatment Services, DASIS Series: S-56. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. HHS Publication No (SMA) 11-4646. [Google Scholar]
- 56.UNODC . World Drug Report 2010. United Nations Office on Drugs and Crime; Vienna: 2010. [Google Scholar]
- 57.UNODC . World Drug Report 2011. United Nations Office on Drugs and Crime; Vienna: 2011. [Google Scholar]
- 58.UNODC . In: World Drug Report 2012. Crime U.N.O.o.D.a., editor. World Drug Report, United Nations Office on Drugs and Crime; Vienna: 2012. [Google Scholar]
- 59.Wallace TL, Gudelsky GA, Voorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity. stereotypic behavior, and stimulated dopamine release in the rat, J Neurosci. 19(1999):9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wouldes TA, LaGasse L, Sheridan J, Lester BM. Maternal methamphetamine use during pregnancy and child outcome: what do we know? The New Zealand Medical Journal. 2004 [PubMed] [Google Scholar]
- 61.Wouldes TA, LaGasse LL, Derauf C, Newman E, Shah R, Smith LM, Arria A, Huestis M, Della Grotta S, Wilcox T. Co-morbidity of substance use disorder and psychopathology in women who use methamphetamine during pregnancy in the US and New Zealand. Drug Alcohol Depend. 127(2013):101–107. doi: 10.1016/j.drugalcdep.2012.06.016. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wouldes TA, Woodward LJ. Maternal methadone dose during pregnancy and infant clinical outcome. Neurotoxicol Teratol. 32(2010):406–413. doi: 10.1016/j.ntt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Wu M, LaGasse LL, Wouldes TA, Arria AM, Wilcox T, Derauf C, Newman E, Shah R, Smith LM, Neal CR. Predictors of inadequate prenatal care in methamphetamine-using mothers in New Zealand and the United States. Maternal & Child Health Journal. 2013 doi: 10.1007/s10995-012-1033-8. others. [DOI] [PMC free article] [PubMed] [Google Scholar]


