Abstract
The frequency of epitope specific naïve CD4+ T cells in humans has not been extensively examined. In this study, a systematic approach was used to examine the frequency of CD4+ T cells that recognize the Protective Antigen of Bacillus anthracis in both Anthrax Vaccine Adsorbed vaccinees and non-vaccinees with HLA-DRB1*01:01 haplotypes. Three epitopes were identified that had distinct degrees of immunodominance in subjects that had received the vaccine. Average naïve precursor frequencies of T cells specific for these different epitopes in the human repertoire ranged from 0.2 to 10 per million naïve CD4+ T cells, which is comparable to precursor frequencies observed in the murine repertoire. Frequencies of protective Antigen-specific T cells were two orders of magnitude higher in immunized subjects than in nonvaccinees. The frequencies of epitope specific memory CD4+ T cells in vaccinees were directly correlated with the frequencies of precursors in the naïve repertoire. At the level of TCR usage, at least one preferred Vβ in the naïve repertoire was present in the memory repertoire. These findings implicate naïve frequencies as a crucial factor in shaping the epitope specificity of memory CD4+ T cell responses.
INTRODUCTION
The frequency of epitope specific T cells within the naïve repertoire is thought to play an important role in shaping the complexity and diversity of the memory repertoire and the specificity of adaptive immune responses to infectious pathogens or vaccinations. As such, knowledge of naïve T cell frequencies is an important research goal. Over the past 15 years, significant discoveries have been made by studies utilizing indirect assays such as adoptive transfer of TCR transgenic T cells in mouse models (1-3) or sequencing the CDR3β regions of antigen specific T cells (4). The development of MHC-tetramer reagents provided an additional tool capable of detecting antigen specific T cells in a direct fashion (5,6). Additionally, the subsequent development of an enrichment protocol for tetramer positive T cells was necessary to enable the detection of epitope specific naïve T cells, which are typically present at very low frequencies (7,8). Moon et al first used this tetramer enrichment approach to examine the frequency of epitope specific naïve CD4+ T cells in mice. Their results suggest that there are 20 to 200 naïve CD4+ T cells for specific antigenic epitopes in the mouse T cell repertoire (9). Using a similar approach, Obars et al and Kotturi et al estimated the number of epitope specific naïve CD8+ T cells to be between 15 to 1,200 cells per mouse (10,11). Most attempts to estimate the frequencies of epitope specific naïve T cells have been carried out in mice; however, two recent studies in human subjects indicated that the frequency of epitope specific CD8+ T cells ranges from 0.6 to 500 per million CD8+ T cells (12,13). Another study examined the frequency of CD45RA+ DRB1*04 restricted influenza A HA306-319 specific T cells in human subjects, and reported an average frequency of 6 per million CD4+ T cells (14). However, most human subjects have been repeatedly exposed to influenza A, and it has been suggested that memory CD4+ T cells can revert to a CD45RA+ phenotype as a consequence of repeated stimulation (15). Therefore, examining influenza A specific T cell frequencies in adults may not be the most appropriate experimental approach to assess epitope specific naïve T cell frequencies in human subjects.
In the current study, Protective Antigen (PA) of Bacillus anthracis (B. anthracis) was chosen as a model antigen for studying the frequency of epitope specific T cells in naïve subjects and vaccinees. Protective Antigen is a major component of the Anthrax Vaccine Adsorbed (AVA) (16,17). The vast majority of healthy subjects have had no exposure to this antigen. However, military personnel are typically exposed to this antigen through multiple AVA vaccinations as a precaution against the potential release of anthrax spores in an act of biological warfare (18). With blood samples from AVA vaccinees, the Tetramer Guided Epitope Mapping (TGEM) approach was utilized to identify CD4+ T cell epitopes within the Protective Antigen (PA) of B. anthracis (19,20). The PA specific tetramers were subsequently used to examine the frequency of PA specific T cells in both healthy vaccinees and non-vaccinees. Direct ex vivo tetramer staining indicated that the average frequency of naïve epitope specific T cells ranged from 0.2 to 10 per million naïve CD4+ T cells. The naïve frequencies of epitope specific CD4+ T cells also correlated with the frequency of memory T cells observed in vaccinees.
MATERIALS AND METHODS
Subject recruitment
AVA vaccinees were recruited with informed consent as part of a study approved by the Institutional Review Boards of both the Benaroya Research Institute and Madigan Army Medical Center. Subjects received the AVA vaccine during 2008, 2009, and 2010 and were recruited and sampled within one year of receiving a final vaccine dose or annual booster. Unvaccinated control subjects were recruited with informed consent from the Benaroya Research Institute. DRB1* HLA alleles were typed by PCR using Dynal Unitray SSP Kits (Invitrogen, Carlsbad, CA), and those subjects that carried DRB1*01:01 (DR0101) were selected for further study.
Protective antigen peptides and class II tetramers
A total of 94 overlapping peptides that cover the entire sequence of PA of B. anthracis were synthesized by Mimotopes (Clayton, Australia). Each peptide was 20-aa in length, with a 12-aa overlap. Peptides were divided into 19 pools, with 5 peptides in each pool. Recombinant DR0101 protein was purified from Schneider S2-transfectants using L243 affinity column chromatography. The protein was subsequently biotinylated with Bir A (Avidity, Aurora, CO) according to the manufacturer's protocol. Peptide pools at 20mg/ml were loaded onto biotinylated DR0101 monomer at 1 mg/ml for 48 hrs at pH6 in 100 mM sodium phosphate and 0.2% η-octyl-β-D-glucopyranoside (Sigma-Aldrich, St. Louis, MO). These peptide-loaded monomers were subsequently cross linked with PE-labeled streptavidin (Invitrogen, Carlsbad, CA) for at least 4 hr at room temperature to produce pooled peptide tetramers (21). Single peptide PE-labeled tetramers were produced in similar fashion by loading DR0101 monomer with each individual PA peptide from positive peptide pools. Single peptide allophycocyanin-labeled tetramers were produced with allophycocyanin-labeled streptavidin (Invitrogen).
Tetramer Guided Epitope Mapping
PBMC were isolated from heparinized blood by Ficoll® (GE healthcare, Chalfont St. Giles, England) underlay. CD4+ T cells were isolated by ‘no touch’ CD4+ magnetic isolation (Miltenyi Biotec, Bergisch Gladbach, Germany). The CD4- fraction was used as antigen presenting cells (APC) by plating 3 × 106 APC in 1 ml complete medium (RPMI supplemented with 10% pooled human serum, 1% pen/strep, and 1% L-glutamine) in 48 well plates. Non-adherent cells were washed away and each well was seeded with 2.5 × 106 CD4+ T cells. Cells in each individual well were stimulated with a peptide pool and cultured for 14 days. Cells were fed using fresh T cell medium and 20 U/ml of IL-2 (Hemagen, Columbia, MD) as needed. On day 14, 1/10 of the cells (approximately 75,000) from each well were stained with the corresponding pooled tetramers and analyzed by flow cytometry using a FACSCalibur (BD Biosciences, San Diego, CA). Cells from tetramer positive wells were subjected to a second staining using tetramers loaded with individual peptides within the corresponding positive peptide pools (19).
Direct ex vivo staining of T cells with tetramer
The anti-PE magnetic bead enrichment protocol was used to detect and determine the frequency of PE-tetramer positive CD4+ T cells. In brief, 30 to 200 million PBMC in a volume of 200 μl to 600 μl were stained with 10 μg/ml of PE-tetramer at room temperature for 2 hr. PA713-732 epitope enrichments were carried out with 30-50 million PBMC; PA401-420 and PA505-524 enrichments were carried out with 120-200 million PBMC. During the last 20 minutes of the incubation, cells were stained with FITC-conjugated anti-CD45RA (eBioscience, San Diego, CA), or AF488-conjugated anti-CXCR3 (BD Biosciences), or FITC-conjugated Vβ13.2 (Biolegend, San Diego, CA), or FITC-conjugated Vβ13.6 (Beckman Coulter, Brea, CA), and allophycocyanin-conjugated anti-CD4 (eBioscience), and Peridinin Chlorophyll Protein (PerCP)-conjugated anti-CD14 and anti-CD19 antibodies (BD Biosciences) (gating antibodies). In some experiments, a combination of AF647-conjugated anti-CCR4 (BD Biosciences), FITC- conjugated CD4 (eBioscience) and the gating antibodies (anti-CD14 and anti-CD19) were used. Cells were then washed and incubated with anti-PE magnetic beads (Miltenyi Biotec) at 4°C for another 20 min. In some experiments, cells were stained with both PE-tetramers and allophycocyanin-tetramers and the appropriate antibodies. In other experiments, cells were stained PE-tetramers, FITC-conjugated anti-CD4 or FITC-conjugated anti-CD8, allophycocyanin-conjugated anti-CD3 (all eBiosciences) and the gating antibodies. The cells were washed again, and 1/10 of the sample was set aside (pre-column fraction) for later analysis to determine the total number of CD4+ T cells in the sample. The remainder of the cells was passed through an MS column (Miltenyi Biotec). The bound fraction was washed and eluted. This fraction inevitably contains an appreciable number of PE-negative cells. Cells in both the bound fraction and the pre-column fraction were stained with Via-Probe for 10 minutes before analysis using a FACSCalibur (BD). Lymphocytes were gated by forward and side scatter and then we gated on CD4+, CD14-, CD19- and Via-Probe- cells. The frequency was calculated as described by dividing the total number of tetramer-positive cells in the bound fraction by the total number of CD4+ T cells in the sample (10 times the number of CD4+ T cells in the pre-column fraction) and multiplying this number by one million (7,20).
Peptide binding competition assay
Various concentrations of each peptide were incubated in competition with 0.01 μM biotinylated HA306-318 peptide in wells coated with HLA-DR0101 protein essentially as previously described (22). After washing, the biotin-HA peptide was labeled using europium-conjugated streptavidin (Perkin Elmer) and quantified using a Victor2 D time resolved fluorometer (Perkin Elmer). Peptide binding curves were simulated by nonlinear regression with Prism software (Version 4.03, GraphPad Software Inc.) using a sigmoidal dose-response curve. IC50 binding values were calculated from the resulting curves as the peptide concentration needed for 50% inhibition of reference peptide binding.
TCR Vβ analysis of Anthrax reactive T cells
PA713-732 reactive T cell clones were obtained by single cell sorting DR0101/PA713-732 tetramer positive cells, followed by expansion in the presence of PHA and irradiated allogeneic-PBMC. RNA was extracted from approximately one million T cells of each clone using the RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions and eluted in 32ul DEPC-treated water (Ambion, Foster City, CA). Reverse transcription was performed with 8ul of total RNA using the SuperScript III First-Strand synthesis kit (Invitrogen) and random hexamers according to the provided protocol. To determine the identity of the TCR Vβ variable region for each clone, the synthesized cDNA was used in a multiplex PCR assay as described by Akatsuka, et al (23). The PCR reaction for each clone that contained a major band was purified using the QIAquick PCR Purification Kit (QIAGEN) according to the manufacturer's protocol. After purification, each major PCR product was subjected to full automated sequencing on the ABI Prism 3100 Genetic Analyzer using the Big Dye v3.1 DNA Sequencing Kit (Applied Biosystems, Foster City, CA). Sequencing was performed in both directions using the antisense primer from the TCR Vβ constant region and the sense primer from the TCR Vβ variable region determined by PCR band visualization as described above. To confirm the TCR Vβ identity of each major band, consensus sequences determined with the Contig Assembly Program were aligned with the known TCR Vβ sequence using the Clustal W multiple alignment program. The products from the multiplex PCR assay also include the CDR3 region.
Online Supplemental Material
Figure S1 shows the use of Tetramer Guided Epitope Mapping for the identification of DR0101 restricted PA reactive epitopes. Table S1 shows the specificity of PA reactive T cells isolated from non-vaccinees by proliferation assays. Figure S2 shows control tetramer staining of PBMC from a representative DR0101-negative individual and PBMC that had already been depleted of antigen specific cells. Figure S3 shows a representative titration experiment to illustrate the limit of detection for the PE-enrichment methodology.
RESULTS
Protective Antigen specific memory T cells in AVA vaccinees
Tetramer Guided Epitope Mapping was used to identify HLADRA*01:01/DRB1*01:01 (DR0101) restricted PA specific CD4+ T cell epitopes using peptide stimulated PBMC from DR0101 AVA vaccinees as described previously (19,20). Tetramer staining indicated that PA393-412, PA401-420, PA505-524 and PA713-732 contain DR0101 restricted T cell epitopes (Table I and Fig. S1). Similar experiments were carried out using samples from 2 other DR0101 AVA subjects and identical results were obtained. An in vitro binding assay indicated that these three peptides bound to DR0101 with high affinity (Table I). Given that the PA393-412 and PA401-420 peptides overlap by twelve amino acids, these regions are likely to contain a single epitope within overlapping region PA404-412. Indeed, as shown in Table I, these two peptides share the same putative core binding region as determined by the ProPred prediction algorithm (24). Based on these results, all subsequent experiments focused on PA401-420, PA505-524 and PA713-732.
Table I.
PA antigenic epitopes and their binding affinities to DR0101
| PA393-412^ | IRYVNTGTAPIYNVLPTTSL | ND |
| PA401-420 | APIYNVLPTTSLVLGKNQTL | IC50 0.39μM |
| PA505-524 | NWSEVLPQIQETTARIIFNG | IC50 0.22μM |
| PA713-732 | KLPLYISNPNYKVNVYAVT | IC50 0.20μM |
Putative MHCII binding regions are shown in bold
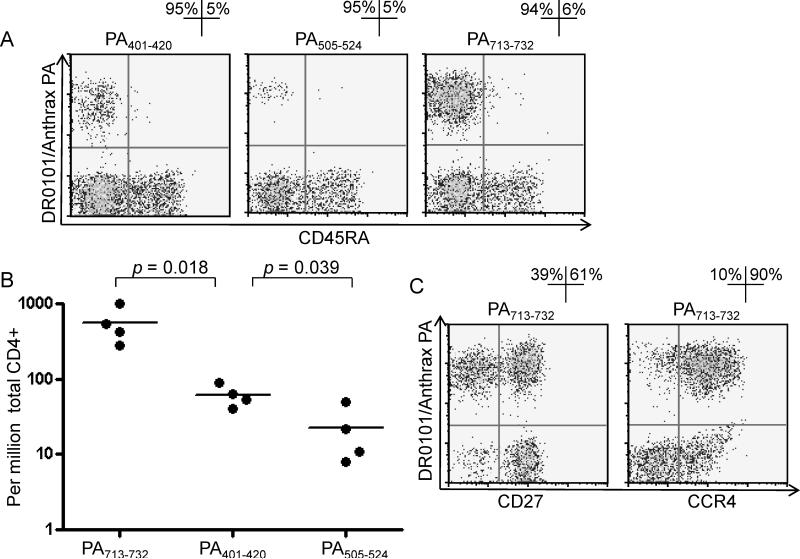
To confirm the relevance of these epitopes, DR0101/PA401-420, PA505-524 and PA713-732 tetramers were used to detect PA specific CD4+ T cells in DR0101 AVA vaccinees directly ex vivo through the anti-PE bead enrichment approach (7,8). Representative data from one subject are shown in Fig. 1A. For all three peptide specificities, PA specific CD4+ T cells exhibited a CD45RA- phenotype, indicating that these were memory T cells. The observed memory phenotype of these cells suggests that the peptides containing the epitopes are likely to be naturally processed. In this vaccinee, PA713-732 reactive CD4+ T cells were observed at a frequency of 1,000 per million total CD4+ T cells. PA401-420 and PA505-524 specific CD4+ T cells were observed at lower frequencies, 63 and 22 per million, respectively. These results indicate that PA7 -732 was the most immunodominant epitope in this subject, followed by PA401-420, and PA505-524 which could be considered subdominant epitopes (Fig. 1A). As shown in Fig. 1B, the hierarchy of frequencies observed in T cells specific for the 3 epitopes was similar in all of the DR0101 AVA vaccinees examined. Furthermore, a similar hierarchy was observed when the experiments were repeated for these four DR0101 subjects at a time point 3 months later (data not shown). Ex vivo staining with antibodies against additional surface markers of CD4+ T cells specific for the 3 epitopes indicated that most of these cells express CCR4, in agreement with a previous report (20), and that the population is heterogeneous for CD27 expression (Fig 1C and data not shown).
Figure 1.
Frequencies and phenotypes of DR0101 restricted PA reactive T cells in DR0101 AVA vaccinees. A. PBMC from a DR0101 subject were stained with DR0101/PA401-420, DR0101/PA505-524 and DR0101/PA713-732 PE-tetramers and anti-CD45RA antibody, and with gating antibodies ex vivo. The cells were subsequently incubated with anti-PE magnetic beads and enriched with a magnetic column. Frequencies of PA specific T cells were determined by dividing the number of tetramer positive cells in the enriched fraction by the total number of CD4+ T cells in the sample before fractionation, determined to be 63, 22 and 1,000 per million total CD4+ T cells for PA401-420, PA505-524 and PA713-732 epitopes, respectively. B. Frequencies of PA401-420, PA505-524 and PA713-732 T cells in four different DR0101 AVA vaccinees. Each symbol represents the epitope specific T cell frequency for a single subject. The p values were determined by using two-tailed unpaired t tests. C. CD27 and CCR4 expression of PA713-732 reactive CD4+ T cells. For each panel (A-C), we gated on CD4+, CD14-, CD19- and Via-Probe negative cells. The percentages of tetramer positive cells that expressed the surface marker are as indicated. Data as shown are representative results from two or more experiments in each of the 4 subjects.
Protective Antigen specific naïve T cells in non-AVA vaccinees
To interrogate the naïve repertoire, the anti-PE bead enrichment protocol was applied to determine the frequency and phenotype of PA401-420, PA505-524 and PA713-732 specific CD4+ T cells in PBMC obtained from DR0101 non-AVA vaccinees. Initial experiments focused on PA713-732, the most immunodominant CD4+ T cell epitope in vaccinated subjects. Representative staining for one of these unvaccinated subjects is shown in Fig. 2. Tetramer staining in the CD3+ cell population was specific for CD4+ cells, with no non-specific staining detected in the CD8+ population (Fig. 2A and B). The frequency of PA713-732-specific T cells for this subject was determined to be 6 per million total CD4+ T cells. To confirm the specificity of tetramer staining, PBMC from unvaccinated subjects were co-stained with the PE-conjugated tetramer and with a tetramer that is labeled with a dye not used for enrichment (allophycocyanin). While the observed frequency of PA713-732 specific CD4+ T cells in this particular staining was slightly lower (3 per million total CD4+ T cells), more than 85% of the PE-tetramer labeled cells were also allophycocyanin-tetramer positive (Fig. 2C). In addition, PMBC from non-DR0101 subjects and previously depleted PBMC from DR0101 subjects were stained to further validate the sensitivity and specificity of ex vivo tetramer staining (Figure S2). The surface phenotype of PA713-732 specific T cells in non-AVA vaccinees was also characterized by direct ex vivo staining. Typically, at least 80% of the tetramer positive cells were CD45RA+. Because these subjects were neither vaccinated with AVA nor naturally exposed to Bacillus anthracis, the surface phenotype suggests that these epitope specific CD4+ T cells are naïve. In contrast to the anthrax-specific memory T cells from vaccinees (Fig. 1C), PA713-732 specific cells in non-AVA vaccines were exclusively CD27+ positive and predominantly CCR4 - (Fig. 2E and F). Antigen specificity of PA713-732 tetramer positive T cells was confirmed by single cell sorting PE-tetramer positive T cells from PBMC of 3 different subjects. After non-specific expansion, the clones were stained with DR0101/PA713-732 tetramer. Out of 96 T cell clones examined, 90 could be clearly stained by tetramer following expansion (data not shown). Antigen specificity for 20 of these clones was further corroborated with proliferation assays using DR0101 PBMC as antigen presenting cells. Eighteen of 20 clones showed specific proliferation in response to PA713-732 peptide (Table S1).
Figure 2.
Frequencies and phenotypes of PA713-732 reactive T cells in non-AVA vaccines. A. PBMC from a DR0101 subject were stained with DR0101/PA713-732 PE-tetramers, CD4 antibody, and gating antibodies (anti-CD3, anti-CD14 and anti-CD19) ex vivo. B. PBMC were stained with DR0101/PA713-732 PE-tetramers, CD8 antibody, and gating antibodies ex vivo. Samples from A and B were obtained from the same subject and the frequency was determined to be 6 per million total CD4+ T cells. C. PBMC from a second subject were co-stained with DR0101/PA713-732 tetramers labeled with either PE or allophycocyanin and stained with gating antibodies (anti-CD4, anti-CD14 and anti-CD19) ex vivo. The frequency was determined to be 3 per million total CD4+ T cells. D. E and F. PBMC from DR0101 subjects were stained with DR0101/PA713-732 PE-tetramers, gating antibodies and anti-CD45RA, anti-CCR4 and anti-CD27 antibodies ex vivo. For each panel (A-F), we enriched using anti-PE magnetic beads and examined tetramer positive cells ex vivo, gating on forward and side scattering, and then on cells that were Via-Probe-, CD3+ or CD4+ , CD14- and CD19-. The percentages of tetramer positive cells that expressed the surface marker are as indicated. Data shown for each panel are representative results of more than 4 independent experiments.
In addition to CD4+ T cells specific for PA713-732, PA401-420 specific T cells could also be detected in PBMC from unvaccinated subjects. The specificity of this staining was confirmed by double staining T cells with both PE conjugated and allophycocyanin conjugated DR0101/ PA401-420 tetramers (Fig. 3A). The frequency of DR0101/ PA401-420 specific CD4+ T cells in the representative subject shown was determined to be 1.3 per million total CD4+ T cells. The majority of the tetramer positive T cells were also CD45RA positive (Fig. 3B). As observed for PA713-732 specific naïve T cells, the vast majority of PA401-420 specific cells were CD27+ and CCR4- (data not shown). Detecting DR0101/PA505-524 specific T cells was more difficult because the signal was relatively weak and infrequent. In spite of their low frequency, DR0101/ PA505-524 specific T cells could be double stained with PE conjugated and allophycocyanin conjugated tetramers, confirming the specificity of the staining. Based on this staining, the frequency of DR0101/ PA505-524 specific T cells of the subject as shown was determined to be 0.2 per million total CD4+ T cells (Fig. 3C). However, this staining is close to the limit of detection for our methodology, which (based on empirical observations) is estimated to be 0.2 per million total CD4+ T cells with 200 million PBMC (see figure S3). Therefore, these frequencies should be considered to be approximate. Because of the limited number of events, we were unable to determine the surface phenotype of DR0101/ PA505-524 specific T cells.
Figure 3.
Frequencies of DR0101 restricted PA401-420 reactive T cells and PA505-524 reactive T cells in DR0101 non-AVA vaccinees. A. PBMC from a DR0101 subject were stained with DR0101/PA401-420 PE-tetramers, DR0101/PA401-420 allophycocyanin -tetramers and gating antibodies ex vivo followed by enrichment with anti-PE magnetic beads. The frequency of PA401-420 specific T cells was determined to be 1.3 per million total CD4+ T cells. B. PBMC were stained with DR0101/PA401-420 PE-tetramers and CD45RA antibody, and gating antibodies ex vivo then enriched. The frequency of PA401-420 specific T cells was determined to be 0.5 per million total CD4+ T cells. C. PBMC from a DR0101 subject were stained with DR0101/PA505-524 PE-tetramers, DR0101/PA505-524 allophycocyanin-tetramers and gating antibodies ex vivo then enriched. The frequency of PA401-420 specific T cells was determined to be 0.2 per million total CD4+ T cells. Each plot represents staining from a different DR0101 subject. For each panel (A-C), we gated on forward and side scattering and then on cells that were Via-Probe-, CD4+ , CD14- and CD19-. Data shown in A and B are representative of more than 11 experiments in 11 subjects, and the data shown in C are representative of data from 7 different subjects.
Equivalent ex vivo tetramer staining experiments were completed for a total of 11 unvaccinated DR0101 subjects. PA713-732 and PA401-420 specific T cells were detected in every subject examined. Because PA505-524 staining required a much larger sample volume for analysis (200 million PBMC), ex vivo tetramer staining was repeated for a total of 7 subjects. For these, detectable staining was observed in only 3 subjects, as staining was below the threshold of detection for the other 4 subjects. As summarized in Figure 4, PA713-732 specific T cells were most frequent, averaging 5 per million total CD4+ T cells or approximately 10 per million naïve CD4+ T cells, followed by PA401-420 specific T cells, averaging around 1 per million total CD4+ T cells or approximately 2 per million naïve CD4+ T cells, and PA505-524 specific T cells were seen at no more than 0.4 cells per million naïve CD4+ T cells. These results suggest a hierarchy of frequencies for these three epitopes in the naïve T cell repertoire which corresponded to the relative immunodominance of these specificities in the expanded CD4+ T cell repertoire of DR0101 AVA vaccinees.
Figure 4.
Frequencies of DR0101 restricted PA reactive T cells in DR0101 non-AVA vaccinees. Each symbol within each PA epitope represents staining for a different subject. Frequencies less than 0.1 per million were below the threshold of detection. The p values were determined by using two-tailed unpaired t tests.
T cell receptor V beta usages of naïve and memory PA713-732 specific T cells
PA713-732 reactive T cell clones were isolated from three unvaccinated subjects by tetramer staining and single cell sorting. The TCR Vβ regions of these naïve PA713-732 reactive T cells were sequenced and analyzed as described in the Methods section (Table II). TCR Vβ sequencing indicated that each of the T cell clones has a different clonotype. Although a limited number of clones were sequenced, the data indicated that TCR Vβ 13.6 and Vβ 15 genes were preferentially selected for the recognition of DR0101/PA713-732 (seen in 6/23 and 5/23 clones respectively) among the naïve repertoire of these subjects. Similarly, the TCR Vβ gene usage of memory PA713-732 specific CD4+ T cells from three DR0101 AVA vaccinees was examined by co-staining PA713-732 tetramer positive T cells with TCR Vβ 13.2 and TCR Vβ 13.6 antibodies. TCR Vβ 15 was not included in this analysis because Vβ 15 antibody is not currently available. Antibody staining results are summarized in Table III. Like the naïve population, these PA713-732 reactive memory T cells in vaccinees were enriched for TCR Vβ 13.6 compared to the total CD4+ T cell population (a paired student t test indicated a p value of 0.04). While the average percentage of PA713-732 reactive memory T cells which utilized Vβ 13.6 in AVA vaccinees (7%) was lower than the average percentage of Vβ 13.6 PA713-732 reactive naïve T cells in unvaccinated subjects (26%), this discrepancy could be due in part to differences in the sensitivity of antibody-based measurement versus PCR-based quantitation.
Table II.
TCR Vβ sequences from different PA713-732 T cell clones derived from non-vaccinees.
| Individual/clone# | TCR V-beta type | TCR V-beta subtype | CDR3 sequence |
|---|---|---|---|
| 835#8 | V3 | S1 | AAPPRVIGRSTEAFFGQGTRLTVV |
| 835#13 | V3 | S1 | ASTPRGWGYTFGSGTRLTVV |
| 835#19 | V6 | S3 | ASSPLRSESSYNSPLHFGNGTRLTVT |
| 835#18 | V11 | S1 | ASSDDRAGETQYFGPGTRLLVL |
| 835#4 | V13 | S6 | ASSYSTGQGLDTQYFGPGTRLTVL |
| 835#5 | V13 | S2 | ASLRDRGYNEQFFGPGTRLTVL |
| 835#16 | V13 | S6 | ASSYSDSNQPQHFGDGTRLSIL |
| 835#24 | V13 | S6 | ASSYSTTGSDQPQHFGDGTRLSIL |
| 835#12 | V15 | S1 | ATSDRGDSGANVLTFGAGSRLTVL |
| 835#15 | V15 | S1 | ATSDSGRDLDEQFFGPGTRLTVL |
| 835#22 | V15 | S1 | ATSDRTGVNTGELFFGEGSRLTVL |
| 835#25 | V15 | S1 | ATSDLTQQFFGPGTRLTVL |
| 835#7 | V23 | S1 | ASSSSGRADNEQFFGPGTRLTVL |
| 835#23 | V17 | S1 | ASSTRDRASTDTQYFGPGTRLTVL |
| 410#12 | V5 | S4 | ASSLGRWGGVFQETQYFGPGTRLLVL |
| 410#1 | V6 | S3 | ASSTLRGQVEQFFGPGTRLTVL |
| 410#11 | V8 | S1/2 | ASSPRVTGELFFGEGSRLTVL |
| 410#3 | V13 | S6 | ASSYSIAGINYGYTFGSGTRLTVV |
| 410#6 | V13 | S6 | ASSYSTGFLGSPLHFGNGTRLTVT |
| 410#4 | V23 | S1 | ASSSPGMNTEAFFGQGTRLTVV |
| 765#38 | V13 | S2 | ASSKETANSPLHFGNGTRLTVT |
| 765#31 | V13 | S6 | ASSYSSSSGSETQYFGPGTRLLVL |
| 765#13 | V15 | S1 | ATSDRGLAAVNEQFFGPGTRLTVL |
Table III.
Vβ13.2 and Vβ13.6 usage in total CD4+ T cells and DR0101 restricted PA713-732 specific memory T cells.
| % Vβ 13.2 | % Vβ 13.6 | |||
|---|---|---|---|---|
| Total CD4+ T cells | PA713-732 T cells | Total CD4+ T cells | PA713-732 T cells | |
| Subject | ||||
| #1 | 2% | 0% | 2% | 14% |
| #1a | 2% | 0% | 2% | 13% |
| #2 | 2% | 0% | 0.7% | 4% |
| #2a | 2% | 0% | 0.5% | 3% |
| #3 | 2% | 5% | 0.5% | 4% |
Experiment was performed with PBMC from the same subject 3 months after the first experiment.
DISCUSSION
The primary objective of this study was to examine the frequency of epitope specific naïve CD4+ T cells in the human T cell repertoire. Because most healthy subjects in the US have not been exposed to B. anthracis, the PA protein of B. anthracis provided an ideal model antigen for studying epitope specific naïve CD4+ T cells in the general population. First, the TGEM approach was used to identify DR0101 restricted PA epitopes using samples from military personnel who had received the AVA vaccine. A total of three epitopes were consistently present in the T cell repertoire of these subjects. Among these, PA713-732 was identified as the most immunodominant CD4+ T cell epitope, with CD4+ T cells specific for this epitope occurring at markedly higher frequencies (up to 1000 per million total CD4+ T cells). T cell frequencies for the other two specificities (PA401-420 and PA505-524) were lower (average of 62 and 23 per million total CD4+ T cells respectively) in the subjects examined. However, all of these frequencies were comparable to those observed for prevalent influenza specific CD4+ T cells, which typically range from 30 to 1600 per million CD4+ T cells (25). The surface phenotype of PA-specific T cells detected in AVA vaccinees was consistently CD45RA-, CCR4+ and CD27 heterogeneous, suggesting that these are central memory T cells (26). The ex vivo detection of these memory T cells in all subjects tested verifies their importance in the PA-specific response elicited by AVA vaccination.
These same tetramers allowed the visualization of PA-reactive T cells in healthy DR0101 non-AVA vaccinated subjects. While the majority of PA reactive T cells in these non-exposed subjects were naïve (CD45RA+), approximately 20% were observed in the CD45RA- population. One possible explanation for this, based on a study of the murine naïve repertoire, is that naïve T cells that undergo homeostatic proliferation can acquire a CD45RA- phenotype (27). A more likely explanation is that existing memory T cells in these unexposed subjects can cross-recognize PA epitopes presented by DR0101. The existence of cross-reactive memory T cells in non-exposed subjects is intriguing, since this implies that such cells could play a protective role, augmenting the naïve response. This would suggest that individuals with less developed memory repertoires could have reduced protection against novel pathogens. As shown in Table 4, average naïve T cell frequencies for all three epitopes were less than ten per million total CD4+ T cells, roughly two orders of magnitude lower than the stable frequencies of memory T cells in vaccinated subjects. It is possible that expansion of PA specific T cells could have been even more robust if measured soon after vaccination. However, to draw firm conclusions about expansion kinetics it would be necessary to assay longitudinal samples from subjects at multiple time points before and after immunization. As in subjects exposed to the vaccine, naïve T cells specific for the immunodominant PA713-732 epitope were most frequent, followed by PA401-420 and PA505-524.
Table IV.
Average frequency of PA-specific T cells in vaccinated and unvaccinated subjects.
| Epitope | Vaccinated | Unvaccinated | Approximate expansion |
|---|---|---|---|
| PA713-732 | 500 per million* | 5 per million | 100-fold |
| PA401-420 | 60 per million | 1 per million | 60-fold |
| PA505-524 | 22 per million | 0.1 per million | 200-fold |
Frequency per million total CD4+ T cells.
Average naïve precursor frequencies of PA specific T cells in the human repertoire ranged from 0.2 to 10 per million naïve CD4+ T cells. Frequencies as high as 20 per million CD4+ T cells were observed for the three PA epitopes combined. A recent study concluded that low affinity (tetramer-negative) CD4+ T cells can be prevalent during polyclonal responses, comprising up to half of the effector T cell population (28). Because the proportion of low affinity naïve CD4+ T cells that are of low avidity is not clear, it is possible that tetramers underestimate the overall frequency by failing to detect low affinity cells. However, Geiger et al. described an approach for examining the diversity of the human naïve CD4+ T cell repertoire using polyclonal, expanded naïve CD4+ T cells and determined frequencies of PA specific T cells to be in the range of 10 to 26 per million naïve CD4+ T cells (29). Because their assay did not distinguish between T cells which recognize different epitopes, these results probably reflect responses to multiple PA epitopes restricted by any of the 3 to 8 MHC II HLA alleles within each subject. Therefore, the total frequencies observed in our study and those observed by Geiger et al are comparable, suggesting the tetramer approach does not underestimate the frequency of epitope specific CD4+ T cells.
Our observed frequency estimates for epitope specific naïve CD4+ T cells in humans are also consistent with those reported in mice. Moon et al estimated that the number of naïve antigen specific CD4+ T cells ranged from 20 to 200 cells per mouse. Given that a mouse has roughly 30 million naïve CD4+ T cells (9,30), these estimates correspond to frequencies ranging from 0.7 to 7 specific cells per million. To some degree, this similarity is surprising as the human TCR repertoire is known to be at least 10 times more diverse than that of mice (31-33). Based on frequency observations in humans, we project that the frequency of most antigenic epitope specific naïve CD4+ T cells, similar to naïve PA401-420 specific cells, is approximately 2 per million naïve CD4+ T cells. We speculate that 10 to 20 cells per million may represent an upper range limit for the frequency of epitope specific CD4+ T cells in the naïve T cell repertoire. Detection of antigen specific T cells which are present at very low frequency still presents a technical challenge. As shown in figure S3, we estimated that the limit of detection for our assay is 0.2 per million total CD4+ T cells. It is likely this sensitivity of detection is both HLA and epitope dependent, and that some of the observed background staining could be authentic allo-recognition rather than non-specific staining. Therefore, possible differences in tetramer sensitivity should be taken into consideration when tetramers are being used to estimate the frequency of antigen specific CD4+ cells that are present at low frequencies.
Others have estimated the precursor frequency of naïve CD8+ T cells in humans to be between 0.6 and 500 per million (12,13). Thus, the frequency of naïve CD8+ T cells can be up to 50-fold higher than that of naïve CD4+ T cells for a given antigenic epitope specificity. It is well documented that the magnitude of CD8+ immune responses is stronger than that of CD4+ T cells (34,35). This has been partly attributed to the fact that CD4+ T cells require continuous antigen exposure for expansion, while CD8+ T cells can expand in the absence of antigen (36-38). The observed differences in frequency imply that the lower number of naïve CD4+ T cells (per epitope) compared to CD8+ T cells may also contribute to the lower magnitude of CD4+ T cell responses.
Several factors could account for the relative immunodominance of PA specific CD4+ T cells. For example, MHC binding and antigen processing can explain some differences. To test this possibility, we examined the binding affinities of the three PA peptides, PA401-420, PA505-524 and PA713-732 to DR0101 using a competition assay. These results indicated that all three peptides bound with similar affinities (Table I). The role of antigen processing is subtle and more difficult to study directly. PA505-524-specific T cells were sub-dominant in the DR0101-restricted repertoire. Therefore it might be expected that this epitope is not efficiently processed. However, our previous study demonstrated that PA505-524 is an immunodominant epitope in DR0701 vaccinees. This implies that the PA505-524 peptide can be processed and presented effectively in that context (20). Thus, neither MHC binding nor antigen processing appear to account for the hierarchy of immunodominance observed for the three DR0101 restricted PA epitopes in the repertoire of DR0101 AVA vaccinees. La Gruta et al have suggested the extent of naïve T cell recruitment and subsequent clonal expansion are critical in determining immunodominance (39). These factors are dependent on MHC/peptide loading on the surface of APC, which is, in turn, dependent on antigen processing, affinity of the peptide for MHC, and antigen availability. In the current study, we focused on epitopes within a single antigen. For multiple epitopes derived from a single antigen, antigen availability and naïve T cell recruitment can be expected to be similar for epitopes with similar processing efficiency. Thus, binding affinity of the peptides, efficiency of processing, and antigen availability and recruitment fail to adequately explain the relative immunodominance seen for these three PA epitopes. Rather, this hierarchy is best accounted for by differences in the relative frequencies of cells specific for these epitopes in the naïve T cell repertoire. Table 4 summarizes average naïve frequencies (in unvaccinated subjects) and memory frequencies (in vaccinated subjects) for PA-specific T cells. Although these frequencies were measured in different groups of subjects, these observations provide an estimate of the expansion of naïve PA-specific T cells in response to vaccination. The change in frequency between the two groups implies roughly a 60 to 200-fold expansion in response to AVA vaccination (Table 4). These conclusions are supported by similar findings in murine studies (9,10).
Healthy individuals have approximately 1 × 1011 naïve CD4+ T lymphocytes (40,41). Given estimates that the human TCR gene has up to 108 possible rearrangements (32,32,33), assuming an even distribution it follows that there are roughly 1,000 naïve T cells per TCR clonotype. The average frequency of epitope specific naïve CD4+ T cells appears to be around 2 per million. Therefore, each individual should have an average about of 2 × 105 naïve T cells for a typical epitope, and up to 1 x 106 naïve cells for immunodominant epitopes. Epitope specific naive T cells for a specific MHC/peptide complex can be expected to consist of 200 to 1,000 distinct TCR clonotypes for the average and more abundant epitopes. Our limited TCR Vβ sequencing data indicated that PA713-732-specific clones utilized multiple TCR Vβ types. Interestingly, although the CDR3 regions within each TCR sequence were 100% diverse, naïve DR0101 restricted PA713-732 reactive cells were enriched for Vβ13.6 and Vβ15. The PA713-732 reactive T cells in the memory repertoire were also enriched for Vβ13.6. However, the percentage of PA713-732 CD4+ T cells with Vβ13.6 in the memory repertoire was lower than in the naïve repertoire. Previous studies have documented that T cells with high affinity TCR were selectively favored to expand (3,42,43). Therefore, given their relative lack of expansion, it could be argued that PA713-732 CD4+ T cells that utilize Vβ13.6 may have lower avidities for DR0101/ PA713-732 complexes than PA713-732-specific T cells which utilize other Vβ. We did not analyze Vβ15 usage in memory PA713-732-specific T cells because a Vβ15 antibody was not available. Thus it remains unclear whether T cells that utilize Vβ15 were preferentially expanded.
In summary, we utilized direct ex vivo tetramer analysis to estimate the frequency of epitope specific naïve CD4+ T cells in human PBMC. The observed frequencies for PA-specific naïve T cells ranged from 2 to 10 per million naïve CD4+ cells for the more dominant epitopes but were 0.2 per million or lower for the subdominant epitope. Based on the consistent hierarchy observed, these data also strongly suggest that frequencies of naïve T cells play a crucial role in determining the immunodominant hierarchy in the memory repertoire after antigen exposure. PA-specific T cells expand two orders of magnitude following immunization. At the level of TCR usage, at least one preferred Vβ in the naïve repertoire were preserved in the memory repertoire. Given the correspondence between anthrax-specific frequencies and those other model antigens such as influenza, it is likely that these observations will hold true in other settings.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the assistance of Diana Sorus in the preparation of this manuscript.
ABBREVIATIONS
- AVA
Anthrax Vaccine Adsorbed
- PA
Protective Antigen
- TGEM
Tetramer Guided Epitope Mapping
Footnotes
This study was supported by NIH contracts HHSN266200400028C and HHSN272200900043C.
REFERENCES
- 1.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitmire JK, Benning N, Whitton JL. Precursor frequency, nonlinear proliferation, and functional maturation of virus-specific CD4+ T cells. J. Immunol. 2006;176:3028–3036. doi: 10.4049/jimmunol.176.5.3028. [DOI] [PubMed] [Google Scholar]
- 4.McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- 5.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 6.Stetson DB, Mohrs M, Mallet-Designe V, Teyton L, Locksley RM. Rapid expansion and IL-4 expression by Leishmania-specific naive helper T cells in vivo. Immunity. 2002;17:191–200. doi: 10.1016/s1074-7613(02)00363-1. [DOI] [PubMed] [Google Scholar]
- 7.Day CL, Seth NP, Lucas M, Appel H, Gauthier L, Lauer GM, Robbins GK, Szczepiorkowski ZM, Casson DR, Chung RT, Bell S, Harcourt G, Walker BD, Klenerman P, Wucherpfennig KW. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J. Clin. Invest. 2003;112:831–842. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes E, Ward SM, Kasprowicz VO, Dusheiko G, Klenerman P, Lucas M. Ultra-sensitive class I tetramer analysis reveals previously undetectable populations of antiviral CD8+ T cells. Eur. J. Immunol. 2004;34:1570–1577. doi: 10.1002/eji.200424898. [DOI] [PubMed] [Google Scholar]
- 9.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotturi MF, Scott I, Wolfe T, Peters B, Sidney J, Cheroutre H, von Herrath MG, Buchmeier MJ, Grey H, Sette A. Naive precursor frequencies and MHC binding rather than the degree of epitope diversity shape CD8+ T cell immunodominance. J. Immunol. 2008;181:2124–2133. doi: 10.4049/jimmunol.181.3.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alanio C, Lemaitre F, Law HK, Hasan M, Albert ML. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood. 2010;115:3718–3725. doi: 10.1182/blood-2009-10-251124. [DOI] [PubMed] [Google Scholar]
- 13.Legoux F, Debeaupuis E, Echasserieau K, De La Salle H, Saulquin X, Bonneville M. Impact of TCR reactivity and HLA phenotype on naive CD8 T cell frequency in humans. J. Immunol. 2010;184:6731–6738. doi: 10.4049/jimmunol.1000295. [DOI] [PubMed] [Google Scholar]
- 14.Chu HH, Moon JJ, Takada K, Pepper M, Molitor JA, Schacker TW, Hogquist KA, Jameson SC, Jenkins MK. Positive selection optimizes the number and function of MHCII-restricted CD4+ T cell clones in the naive polyclonal repertoire. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11241–11245. doi: 10.1073/pnas.0902015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weekes MP, Wills MR, Sissons JG, Carmichael AJ. Long-term stable expanded human CD4+ T cell clones specific for human cytomegalovirus are distributed in both CD45RAhigh and CD45ROhigh populations. J. Immunol. 2004;173:5843–5851. doi: 10.4049/jimmunol.173.9.5843. [DOI] [PubMed] [Google Scholar]
- 16.Scorpio A, Blank TE, Day WA, Chabot DJ. Anthrax vaccines: Pasteur to the present. Cell Mol. Life Sci. 2006;63:2237–2248. doi: 10.1007/s00018-006-6312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bienek DR, Loomis LJ, Biagini RE. The anthrax vaccine: no new tricks for an old dog. Hum. Vaccin. 2009;5:184–189. doi: 10.4161/hv.5.3.7308. [DOI] [PubMed] [Google Scholar]
- 18.Wright JG, Quinn CP, Shadomy S, Messonnier N. Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm. Rep. 2010;59:1–30. [PubMed] [Google Scholar]
- 19.Novak EJ, Liu AW, Gebe JA, Falk BA, Nepom GT, Koelle DM, Kwok WW. Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J. Immunol. 2001;166:6665–6670. doi: 10.4049/jimmunol.166.11.6665. [DOI] [PubMed] [Google Scholar]
- 20.Kwok WW, Yang J, James E, Bui J, Huston L, Wiesen AR, Roti M. The anthrax vaccine adsorbed vaccine generates protective antigen (PA)- Specific CD4+ T cells with a phenotype distinct from that of naive PA T cells. Infect. Immun. 2008;76:4538–4545. doi: 10.1128/IAI.00324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J. Clin. Invest. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ettinger RA, Kwok WW. A peptide binding motif for HLA DQA1*0102/DQB1*0602, the class II MHC molecule associated with dominant protection in insulin-dependent diabetes mellitus. J. Immunol. 1998;160:2365–2373. [PubMed] [Google Scholar]
- 23.Akatsuka Y, Martin EG, Madonik A, Barsoukov AA, Hansen JA. Rapid screening of T-cell receptor (TCR) variable gene usage by multiplex PCR: application for assessment of clonal composition. Tissue Antigens. 1999;53:122–134. doi: 10.1034/j.1399-0039.1999.530202.x. [DOI] [PubMed] [Google Scholar]
- 24.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236–1237. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- 25.Danke NA, Kwok WW. HLA class II-restricted CD4+ T cell responses directed against influenza viral antigens postinfluenza vaccination. J. Immunol. 2003;171:3163–3169. doi: 10.4049/jimmunol.171.6.3163. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 27.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J. Exp. Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabatino JJ, Jr., Huang J, Zhu C, Evavold BD. High prevalence of low affinity peptide-MHC II tetramer-negative effectors during polyclonal CD4+ T cell responses. J. Exp. Med. 2011;208:81–90. doi: 10.1084/jem.20101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geiger R, Duhen T, Lanzavecchia A, Sallusto F. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J. Exp. Med. 2009;206:1525–1534. doi: 10.1084/jem.20090504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu. Rev. Immunol. 2010;28:275–294. doi: 10.1146/annurev-immunol-030409-101253. [DOI] [PubMed] [Google Scholar]
- 31.Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J. Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- 32.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 33.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J. Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 34.Whitmire JK, Murali-Krishna K, Altman J, Ahmed R. Antiviral CD4 and CD8 T-cell memory: differences in the size of the response and activation requirements. Philos. Trans. R. Soc. Lond B Biol. Sci. 2000;355:373–379. doi: 10.1098/rstb.2000.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 36.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J. Exp. Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J. Exp. Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.La Gruta NL, Rothwell WT, Cukalac T, Swan NG, Valkenburg SA, Kedzierska K, Thomas PG, Doherty PC, Turner SJ. Primary CTL response magnitude in mice is determined by the extent of naive T cell recruitment and subsequent clonal expansion. J. Clin. Invest. 2010;120:1885–1894. doi: 10.1172/JCI41538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganusov VV, De Boer RJ. Do most lymphocytes in humans really reside in the gut? Trends Immunol. 2007;28:514–518. doi: 10.1016/j.it.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Bains I, Antia R, Callard R, Yates AJ. Quantifying the development of the peripheral naive CD4+ T-cell pool in humans. Blood. 2009;113:5480–5487. doi: 10.1182/blood-2008-10-184184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 43.Standifer NE, Burwell EA, Gersuk VH, Greenbaum CJ, Nepom GT. Changes in autoreactive T cell avidity during type 1 diabetes development. Clin. Immunol. 2009;132:312–320. doi: 10.1016/j.clim.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.