Abstract
The Likoma network study (LNS) investigates the sexual networks connecting the inhabitants of Likoma, a small island of Lake Malawi with high HIV prevalence. Whereas previous studies of sexual networks and HIV/AIDS in sub-Saharan countries have focused solely on the personal networks of a small number of respondents, the LNS attempts to document the sexual networks of the entire adult population of Likoma. To do so, it uses a unique sociocentric study design, in which all members of the local population are contacted for a survey interview and are asked to nominate their five most recent sexual partners. Using these data, quasi-complete ‘maps’ of the sexual networks connecting inhabitants of the island can be constructed. These maps allow investigation of the impact of networks on HIV epidemiology and can inform mathematical models of HIV prevention. In addition to data on sexual networks, the LNS data include information on the social networks (e.g. friendship), socioeconomic characteristics and HIV status of Likoma’s residents. Baseline data were collected in 2005–06. A first follow-up was conducted in 2007–08 and a second follow-up is planned for early 2013. Access to the LNS data is contingent upon review of a short concept paper and forming collaborations with LNS investigators.
Why was the cohort set up?
Sexual networks are groups of persons who are linked to one another through direct or indirect chains of sexual relations. Theoretical models1–4 suggest that they are a key determinant of the spread of HIV and other sexually transmitted infections (STI). There are however few detailed empirical datasets on sexual networks, particularly in populations of sub-Saharan Africa (SSA) with the highest HIV prevalence levels.
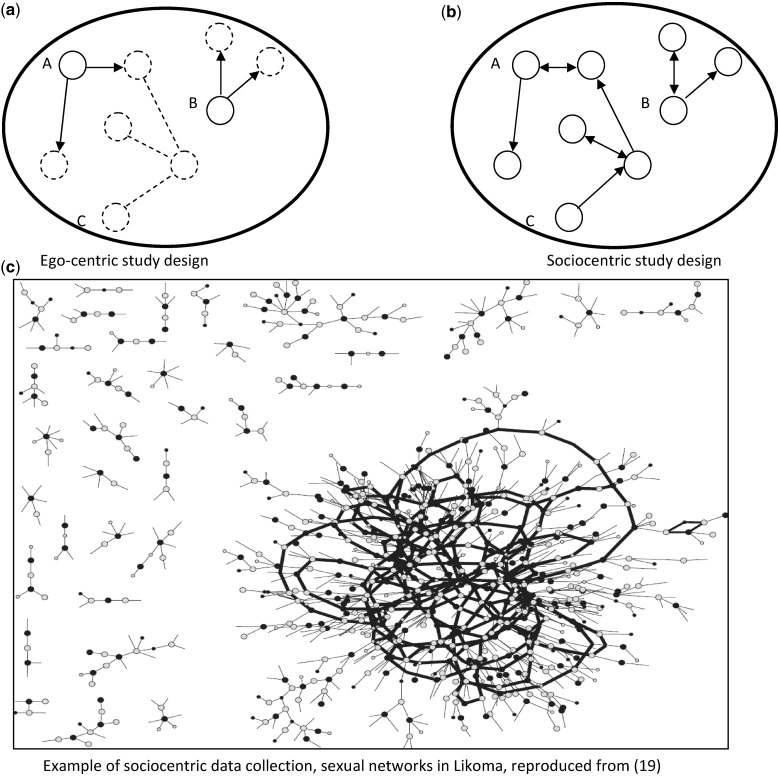
Studies of sexual networks in SSA have primarily used ‘egocentric’ study designs.5,6 These are surveys during which sampled respondents (‘egos’) are asked to enumerate and describe their own sexual partnerships (Figure 1a), but their sexual partners are not enrolled in the study. Such studies provide information on the kind of partners a respondent interacts with (e.g. whether they are younger or older). They do not permit determining, however whether connected networks emerge from these interactions and what these networks may look like.7,8
Figure 1.
Study designs available to investigate social networks empirically. Notes: each circle represents an individual, whereas lines represent relationships between these individuals. In panel a, only individuals represented by solid circles are interviewed during the study and are asked to report how many social relationships they have had. Individuals represented by dotted circles are not interviewed and connections represented by dotted lines cannot be ascertained. Arrows at the end on a line indicate that one individual (e.g., A) is the one making the nomination. Lines with arrows at both ends indicate reciprocated relationships, i.e., that both partners reported being in a relationship with each other during the survey. In the ego-centric study design (panel a), individuals A and B appear to have the same risk environment, since they both nominated two social relationships. The indirect chain of connections between A and C remains unobserved. In panel B (sociocentric design), on the other hand, all individuals within the populations are interviewed and nominate their social relationships. This study design permits ascertaining the existence of the indirect chain of connections between A and C. In panel C, we show an example of a sociocentric network ‘map’ based on data collected on Likoma. In this map, grey dots represent women and black dots represent men; lines represent sexual relationships, and thicker lines represent the portion of the networks in which multiple chains of sexual relationships exist between any two individuals. Some lines do not end with a dot, they represent relationships with a partner that could not be traced during the sociocentric study
In contrast, other types of network studies entail interviewing and determining the disease status of (some of) the partners of an individual. For example, sexual network data may become available as health departments conduct contact tracing investigations.9 During contact tracing, a health worker first elicits which partners of a patient may have been exposed to HIV (or other STIs) and obtains their name/contact information. Then he or she seeks to trace, diagnose and possibly treat these partners. Contact tracing10–14 only produces a partial picture focused on transmission networks: it does not document the indirect links between infected individuals and other susceptible individuals, nor does it include links between susceptibles.7,8 Sociocentric network studies (Figure 1b and 1c), on the other hand, aim to document the sexual networks of entire populations.6 They entail, ideally, enrolling all members of a defined population and collecting data on as many of the relationships connecting them as possible.5,8
Sociocentric studies of sexual networks have been conducted in Western countries15 and more recently in China;16 but the Likoma Network Study (LNS) is the first sociocentric study of sexual networks conducted in a sub-Saharan population affected by a generalised HIV epidemic. It was set up in 2005 in Likoma (Malawi), through collaboration between researchers at the University of Pennsylvania and at the University of Malawi College of Medicine.
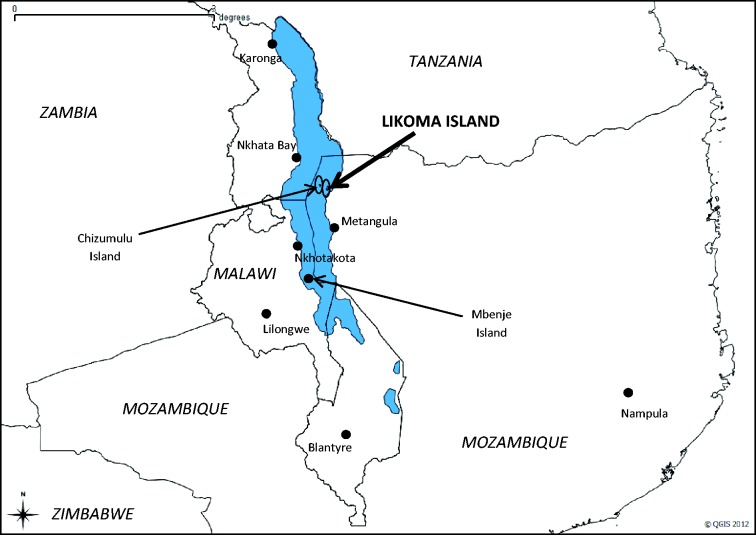
Likoma is a small island on Lake Malawi (Figure 2). It is a fishing community of approximately 7000 people. Likoma is hard to reach: it is connected to mainland Malawi by an unreliable weekly ferry service, which can take 7–20 h to reach Likoma from the coastal cities of Nkhata Bay or Nkhotakota. Inhabitants of Likoma regularly travel to mainland Malawi, however, to sell fish or engage in small-scale trading. Seasonal migration to the southern lake shore is also common: a number of men travel to Mbenje Island between April and November to fish utaka (a local cichlid). The Mozambican shore of Lake Malawi is sparsely populated and only approximately 2000 people live on neighbouring Chizumulu Island.
Figure 2.
Map of the study site
There are few visitors to the island; primarily visitors are family members residing on the mainland returning to Likoma for holidays, Mozambican residents who travel to Likoma to sell firewood or seek healthcare, soldiers (approximately 30) who are posted to the local camp of the Malawian army, and a small number of Western tourists who have limited contact with the local population. Likoma was selected as a site for the LNS because of this relative isolation and its well-defined population boundaries. We hypothesised that these features would facilitate sociocentric data collection and that Likoma would constitute a unique ‘epidemiological laboratory’17 where quasi-complete sexual networks could be conveniently observed. In establishing the LNS, our primary objectives were to describe the structure of the sexual networks through which HIV is transmitted on Likoma (Figure 1c) and to identify the characteristics of these networks that favour (or impede) HIV spread.
Who is in the cohort?
Two rounds of data collection have been completed thus far. The first round started in November 2005 and was completed in March 2006. The second round took place between August and December 2007, with clean-up visits taking place in April 2008. A third round of data collection is planned for early 2013.
In order to reconstruct the sexual networks of the population of Likoma, the LNS proceeds in three steps. First, all residents of the island are enumerated during a household listing. This establishes a roster of potential network members. Second, all adult island residents meeting eligibility criteria (see below) are visited in their homes and are invited to participate in an individual interview. During this interview, they are asked to nominate their five most recent sexual partners. Third, LNS researchers then attempt to link the lists of sexual partners reported during individual interviews to the roster of potential network members obtained during household listing. The full data collection process was described in detail in 2009.18 It is important to note that it does not involve contact tracing: study participants are contacted by the LNS solely on the basis of their residence on Likoma, not because another study participant previously nominated them as their sexual partner. If the information reported during the survey interview does not permit establishing a link between two population members, the reported relationship is left unlinked (Figure 1c).
In the LNS, households are defined as ‘a person or a group of persons, related or unrelated, who live together in the same dwelling unit, who make common provision for food and regularly take their food from the same pot or share the same grain store’. Individuals may thus be members of several households (e.g. polygamous men with multiple wives). Household listing covered all Likoma households in rounds 1 and 2. It excluded, however, the army camp located on the island because we could not obtain the necessary administrative authorisations. Members of this transient population could thus not be interviewed during the study. The frequency of sexual contact between Likoma residents and soldiers can nonetheless be ascertained using a question on the occupation of a respondent’s partner (Table 1).
Table 1.
Participation in the LNS by gender, study round and selected sociodemographic characteristics
| Characteristics | First contacted during round 1 (N = 1052) |
First contacted during round 2 (N = 1787) |
||||||
|---|---|---|---|---|---|---|---|---|
| Men (N = 489) |
Women (N = 563) |
Men (N = 873) |
Women (N = 914) |
|||||
| n(%)a | P-valueb | n(%)a | P-valueb | n(%)a | P-valueb | n(%)a | P-valueb | |
| Total number of respondents | 421 (86.1) | – | 502 (89.2) | – | 644 (73.8) | – | 751 (82.2) | – |
| Sociodemographic characteristics | ||||||||
| Age | <0.01 | <0.01 | 0.12 | 0.02 | ||||
| <20 years old | 75 (88.2) | 113 (87.6) | 126 (81.3) | 102 (82.3) | ||||
| 20–24 years old | 140 (89.7) | 159 (94.1) | 153 (75.0) | 154 (74.8) | ||||
| 25–29 years old | 89 (86.4) | 127 (91.4) | 89 (69.0) | 136 (82.4) | ||||
| 30–34 years old | 57 (73.1) | 65 (77.4) | 83 (69.8) | 106 (87.6) | ||||
| ≥35 years oldc | 60 (89.6) | 38 (90.5) | 193 (72.6) | 253 (84.9) | ||||
| Schoolingd | 0.14 | 0.97 | ||||||
| None | 13 (76.5) | 17 (89.5) | ||||||
| Primary school | 252 (88.4) | 311 (88.9) | ||||||
| Secondary and higher | 152 (83.1) | 171 (89.5) | ||||||
| Language spoken in householdd | 0.69 | 0.66 | ||||||
| ChiTonga | 14 (93.3) | 30 (93.8) | ||||||
| ChiChewa/Chikobwe | 370 (85.5) | 432 (88.7) | ||||||
| ChiTumbuka | 23 (85.2) | 21 (87.5) | ||||||
| Marital statusd | 0.84 | 0.15 | ||||||
| Never married | 220 (86.3) | 195 (91.6) | ||||||
| Ever married | 197 (85.7) | 305 (87.6) | ||||||
| House characteristics | ||||||||
| Roof material | 0.01 | 0.02 | 0.21 | 0.74 | ||||
| Metal sheetse | 273 (88.9) | 322 (91.0) | 311 (75.5) | 330 (81.9) | ||||
| Other | 134 (80.2) | 157 (84.4) | 304 (71.7) | 393 (82.7) | ||||
| Sanitation | 0.26 | 0.43 | 0.11 | 0.25 | ||||
| No pit latrine | 42 (91.3) | 47 (92.2) | 84 (67.7) | 103 (78.6) | ||||
| Has pit latrine | 368 (85.2) | 439 (88.5) | 556 (74.6) | 648 (82.8) | ||||
an represents the number of participants in a given category; the response rate appears in parentheses. In some instances, the total number of answers for a particular variable does not sum up to the number of respondents because of item-specific non-response.
bP-values are based on χ2 tests of association between a sociodemographic characteristic and the probability of participants in the LNS individual interview;
cDuring round 1, respondents aged >35 years were spouses of cohort members. They thus do not constitute a representative sample of the larger population of Likoma aged 35 and older.
dDuring round 2, these questions were not asked during the household listing and are thus not available for non-participants.
eMetal sheets are used as a proxy measure of the higher socioeconomic status of a household. Other roofing materials include primarily thatch.
The coverage of the individual survey interview varied between study rounds. During round 1, only seven of the island’s villages were included.18 All individuals aged 18–35 years residing in the selected villages were contacted and were asked to complete the individual interview. Older (i.e. >35 years old) spouses of respondents were also contacted in order to investigate partnership characteristics of age-discordant marriages. In total, 1052 inhabitants were contacted during round 1. Inhabitants who participated in the individual interview during round 1 form what we call the ‘young adults cohort’. During round 2, all members of the young adults cohort were re-contacted, but the study was also extended to other villages of the island. In addition, we increased the age range of eligibility to 18–49 years. In total, 1787 individuals were contacted for the first time during round 2 (Table 1).
Participation rates for the household listing were ≥99% during each round. For the individual interview, participation rates were 86.1% for men and 89.2% for women during round 1 (Table 1). They were lower for population members who were first contacted during round 2 however, with only 73.8% and 82.2% participation among men and women, respectively. This was largely explained by the timing of data collection. Round 1 was conducted in January–February when most males are present on Likoma, whereas round 2 was conducted in August–December, when a significant proportion of males engage in seasonal migration (and were thus absent) to Mbenje Island. Participation rates in the individual interview varied by age in both rounds. Among men, younger residents were generally more likely to participate in the study than older ones (respondent’s spouses aged >35 years during round 1 were an exception). Among women however, participation during round 2 was lowest among residents aged 20–24 years. Participation was highest among members of the wealthiest households (as measured by roof material) during round 1, but not among residents first contacted during round 2.
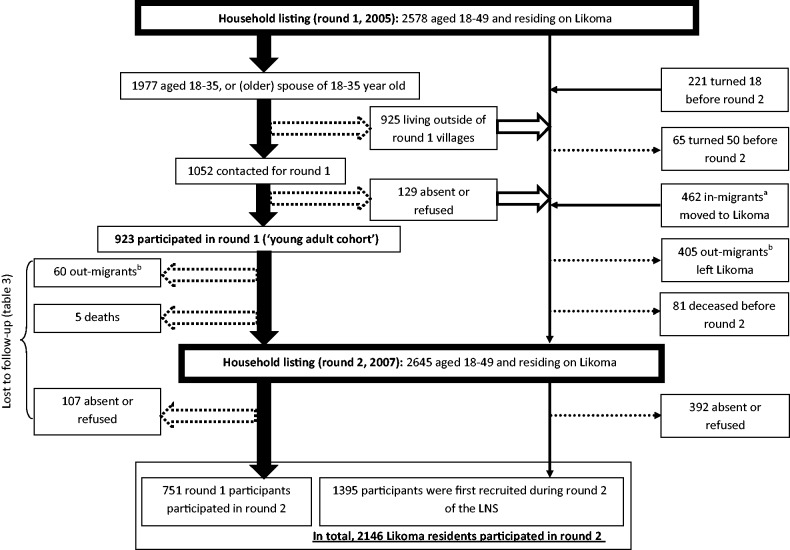
The LNS is designed as an open cohort study with individuals continuously exiting and entering the study over time. Reasons for entering the LNS cohort include reaching the age eligibility threshold (i.e. 18 years) or moving to Likoma. Between rounds 1 and 2, 221 individuals turned 18 years and 462 adult in-migrants moved to Likoma (Figure 3). In addition, individuals who were absent or refused to participate during a previous data collection (e.g. round 1) round are re-contacted for study enrolment during subsequent rounds. Reasons for leaving the cohort include reaching the age limit (i.e. 50 years), death or out-migration.
Figure 3.
LNS participant flow. Block arrows represent participant flow in the ‘young adults’ cohort enrolled during round 1. Dotted block arrows represent attrition flows from the young adult cohort; empty block arrows represent residents who did not participate in round 1 but who remained eligible for round 2. The thin arrows represent attrition (dotted arrows) and increments (solid arrows) affecting the rest of the LNS open cohort (i.e., those who were not enrolled in the young adult cohort). Participants in round 2 thus include members of the young adult cohort and other residents who were first recruited into the LNS during round 2. Notes: aIn-migrants are individuals who moved to Likoma and became resident of a Likoma household between round 1 and round 2; bout-migrants are individuals who were resident of Likoma during round 1 but who did not belong to a Likoma household at the time of round 2 and were reported as having migrated by members of their former household
Round 1 of the LNS included 1858 reports of sexual relationships, 1284 of which were with a partner who was a Likoma resident and 1068 of which were linked to the rosters obtained during household listing (83% linkage rate). The characteristics of these relationships were described in detail in 2009.18 Round 2 included 3345 reports of sexual relationships (1691 reported by men and 1654 reported by women, Table 2). Among those, 2685 were with a partner who was a Likoma resident and 2134 were linked to the household rosters (79.5% linkage rate). Women reported a higher proportion of partners residing on Likoma (77.2 vs 83.4%, Table 2), but the linkage rate did not differ by gender (78.7% vs 80.2%). During both round 118 and round 2, the linkage rate was associated with relationship type (with higher linkage rates in marriages than in non-marital relations) and relationship status (with higher linkage rates among relationships ongoing at the time of the survey).
Table 2.
Linkage rates for sexual relationships reported during the LNS survey, round 2a
| Characteristics | Reported by men (N = 1691) |
Reported by women (N = 1654) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Partner residing in Likomaa | P-value | Linked to HH listingb | P-value | Partner residing in Likomaa | P-value | Linked to HH listingb | P-value | ||
| Total number of relationship reports | 1306 (77.2) | 1028 (78.7) | 1379 (83.4) | 1106 (80.2) | |||||
| Relationship type | <0.01 | <0.01 | <0.01 | <0.01 | |||||
| Marital | 480 (92.7) | 440 (91.7) | 750 (91.4) | 674 (89.9) | |||||
| Stable partner | 296 (79.4) | 222 (75.0) | 284 (78.0) | 195 (68.7) | |||||
| Infrequent partner | 386 (68.9) | 268 (69.4) | 252 (74.3) | 171 (67.9) | |||||
| One-night stand | 138 (59.5) | 92 (66.7) | 88 (71.5) | 61 (69.3) | |||||
| Relationship statusc | <0.01 | <0.01 | <0.01 | <0.01 | |||||
| Ongoing | 556 (94.4) | 497 (89.4) | 651 (95.0) | 577 (88.6) | |||||
| Dissolved | 744 (68.0) | 525 (70.6) | 718 (75.0) | 521 (72.6) | |||||
For detailed information on linkage rates during round 1, see reference 18.
aThe residence of the partner is ascertained on the basis of two questions, asking respondents whether their partner resided on Likoma at the time of the survey and/or at the time of the relationship. If the respondent answers ‘yes’ to either of these questions, then the partner is classified as residing on Likoma. In some instances, the total number of answers for a particular variable does not sum up to the total number of relationships with a partner residing on Likoma because of item-specific non-response.
b‘Linked to the household listing’ means that the partner nominated during the ACASI interview was located in the rosters of potential network of members obtained through household listing; n represents the total number of linked partnerships, and the linkage rate appears in parentheses.
cRespondents were asked whether they still expected to have sexual intercourse with a given partner in the future; when they answered ‘yes’, a relationship was considered ‘ongoing’.
Separate HIV testing and counselling (HTC) visits were conducted during each round after all other study components were completed.19 In round 1, only individuals who completed the individual interview were asked to participate in HTC.19 In round 2, HTC counsellors visited all adults aged 18–49 in their homes, regardless of their prior participation in the individual interview. Patterns of participation in HTC during the LNS were described in detail in 2009 for round 11,9 and in 2012 for round 2.20
How often have they been followed up?
Only the 923 members of the young adult cohort have been followed up so far. Among those, 60 had migrated out of Likoma and 5 had died before round 2; 104 were absent; 3 refused to participate; and 751 (81.4%) were re-interviewed during round 2 (Figure 3). Men were significantly more likely to be lost to follow-up than women (24.5% vs 13.8%, Table 3). Attrition rates were associated with age, education, religion and symptoms of STI at round 1 among women. Attrition rates were not associated with sociodemographic characteristics or HIV-related behaviours among men (Table 3).
Table 3.
Determinants of loss to follow-up during the LNS among respondents contacted during rounds 1 and 2 (young adults cohort), by gender
| Characteristics | Men (N = 421) |
Women (N = 502) |
||
|---|---|---|---|---|
| n(%)b | P-valuec | n(%)b | P-valuec | |
| Total number lost to follow-upa | 103 (24.5) | 69 (13.8) | ||
| Sociodemographic characteristicsd | ||||
| Age | 0.66 | 0.01 | ||
| <20 years old | 16 (21.3) | 25 (22.1) | ||
| 20–24 years old | 36 (25.7) | 14 (8.8) | ||
| 25–29 years old | 18 (20.2) | 13 (10.2) | ||
| 30–34 years old | 17 (29.8) | 12 (18.5) | ||
| ≥35 years old | 16 (26.7) | 5 (13.2) | ||
| Schooling | 0.99 | 0.10 | ||
| Did not complete primary school | 48 (24.5) | 23 (10.8) | ||
| Completed primary school | 55 (24.4) | 46 (15.9) | ||
| Marital status | 0.23 | 0.27 | ||
| Never married | 57 (25.9) | 31 (16.8) | ||
| Currently married | 41 (21.7) | 30 (11.5) | ||
| Divorced/widowed/separated | 5 (41.7) | 8 (14.8) | ||
| Household income | 0.62 | 0.25 | ||
| Bottom quartile | 20 (22.5) | 14 (10.8) | ||
| Upper quartiles | 83 (25.0) | 55 (14.8) | ||
| Religion | 0.73 | 0.08 | ||
| Anglican | 86 (24.2) | 52 (12.5) | ||
| Other religions | 17 (26.2) | 17 (19.8) | ||
| HIV-related behaviours and attitudesd | ||||
| HIV testing | 0.67 | 0.42 | ||
| Never tested for HIV | 73 (23.9) | 48 (13.1) | ||
| Ever tested for HIV | 24 (26.1) | 17 (16.2) | ||
| HIV risk perception | 0.68 | 0.55 | ||
| Not worried about HIV | 73 (25.7) | 38 (12.5) | ||
| Worried a little | 14 (20.6) | 17 (13.3) | ||
| Worried a lot | 10 (24.4) | 8 (18.6) | ||
| Self-reported STI symptoms | 0.32 | 0.07 | ||
| None | 77 (23.3) | 59 (14.9) | ||
| At least one | 20 (29.0) | 6 (7.4) | ||
aMembers of the young adult cohort lost to follow-up are round 1 respondents who either migrated out of Likoma prior to round 2, died prior to round 2, were absent at the time of round 2 or refused to participate in round 2.
bn represents the number lost to follow-up with a particular sociodemographic characteristic; the attrition rate appears in parentheses.
cP-values are based on χ2 tests of association between a sociodemographic characteristic and the probability of attrition among round 1 participants.
dAll variables included in this table were measured at round 1.
What has been measured?
The LNS contains data collected at multiple levels: household, individual and relationship (Table 4).
Table 4.
Content of LNS datasets, by study round
| Data collection level | Round 1 | Round 2 |
|---|---|---|
| Households | Name of household head; house characteristics including roof material, presence of pit latrine; GPS coordinates | Name of household head; house characteristics including roof material, presence of pit latrine, electricity in the household, whether house is owned or rented, wall material; GPS coordinates |
| List of household members including names, nicknames, father’s name, relationship to household head, ethnicity, marital status, educational level | ||
| Update list of household members, including members who died, were born, left or joined the household | ||
| For each household member: names, nicknames, father’s name | ||
| Individuals |
Written interview:
|
Written interview:
|
| Relationships |
Sexual networks:
|
Sexual networks:
|
| HIV status | HIV antibodies (Determine and Unigold rapid tests) | HIV antibodies (Determine and Unigold rapid tests) Dried blood spots for HIV genetic sequencing and HLA-typing |
Emboldened fields indicate variables that have been collected during both rounds.
At the household level, key informants described attributes of the house they lived in, enumerated the individuals who were members of their household and the relationship between each household member and the household head. Handheld global positioning system (GPS) devices were used to map major roads and paths, key landmarks (e.g. health facilities) and household locations.18
At the individual level, data collection first comprised a traditional paper-and-pencil interview, focused on background characteristics. This included information on residence, religion, education and socioeconomic status (SES), as well as a detailed marital history (round 1). After the pen-and-pencil component, respondents were asked to complete an audio computer-assisted self-interviewing (ACASI) component,21 which covered more sensitive health topics as well as sexual and social relationships. ACASI interviews used an automated process during which respondents listened to pre-recorded questions through a headset and answered these questions either through a colour-coded keypad or by speaking through a microphone (for nominations of sexual partners). Individual-level data collected during the ACASI component included data on general health, use of HIV services (e.g. HTC), and factors that affect an individual HIV susceptibility/infectivity (male circumcision, presence of STI symptoms).
At the relationship level, we have collected data on sexual and social networks during both rounds of the LNS. Sexual partnerships were defined as a relationship between two individuals having involved vaginal penetrative intercourse. Same-sex relationships were not elicited during the LNS. Sexual network data included descriptions of (i) the individual characteristics of each partner (including name and residence to enable linkages), (ii) the context and timing of the relationship between a respondent and his/her partner, and (iii) the presence of HIV risk factors during the relationship (e.g. condom use). During round 1, data collection on social networks included information on friendship and support networks: respondents were asked to nominate their four closest friends on Likoma, as well as five persons they would turn for help in case of unexpected hardship. In round 2, we restricted the collection of data on social networks to nominations of the respondent’s best friend. Finally, during round 1, we also collected data on individual participation in community organisations and attendance at local events/places. These data were collected to measure social networks through co-memberships and affiliations.6,22–24
Data on the HIV status of individuals collected during HTC included HIV antibody testing, as determined by two rapid tests (Unigold and Determine). During round 2, we also collected blood samples on filter paper from all HTC participants in order to conduct genetic sequencing of HIV isolates and HLA-typing of all HIV-infected respondents.25
What has it found? Key findings and publications
The key contributions of the LNS concern the field of sexual networks research. We have first shown the feasibility of sociocentric data collection in SSA, and we have extensively documented our approach to collecting such data.8,18 We have then provided the first ‘map’ (Figure 1c) of a sexual network through which an HIV epidemic has become generalised.26 It showed that sexual networks emerged through decentralised chains of sexual relationships in which individuals had at most three to four sexual partners over a 3-year period, rather than through contacts with high-risk groups such as commercial sex workers.27–30 This robust network structure creates multiple pathways through which HIV can be transmitted within the population and greatly amplifies epidemic potential.31–32 We have shown an association between partnership concurrency and HIV-serodiscordance in couples.33 We have also contributed to a finer understanding of the role of migrations in HIV epidemics: in Likoma, HIV risk was not only associated with circular out-migration as previously investigated34 but also with sexual contacts with temporary in-migrants to the island.35
In sociocentric studies, both partners in a sexual relationship are potentially interviewed and their reports of risk behaviours can thus be compared.36 We have used this opportunity to assess the reliability of the data on sexual behaviours reported by survey respondents. This suggested that measures of the prevalence of partnership concurrency recommended by UNAIDS37 were highly unreliable.38 In addition, we have found that women generally under-estimated the age of their partners during survey interviews. The proportion of HIV infections attributable to age-mixing in SSA populations may thus be significantly larger than previously thought.39
Finally, studies of participation in HTC conducted as part of the LNS have shown that offering home-based HTC services could represent an effective approach to scaling up HTC rates, particularly among the poorest.19
What are the main strengths and weaknesses?
The main strength of the LNS rests in its sociocentric design. It permits observing indirect connections between members of a population, which have been linked to the transmission of infectious diseases and the adoption of healthy behaviours.40 In addition, the sociocentric networks were followed longitudinally, thus enabling the study of network evolution over time. Prior sociocentric studies of sexual networks have been limited to cross-sectional assessments.15 Finally, the LNS data may serve as a benchmark against which to evaluate innovations in network research methodology, including new approaches to network sampling.
There are several limitations to the LNS. First, Likoma is an island and its sexual networks may not be representative of sexual networks transmitting HIV in mainland populations. Second, the LNS has only elicited a subset of the sexual relationships within which island inhabitants are potentially involved. For example, we only asked respondents to nominate their five most recent sexual partners and we did not include same-sex relationships. Third, the LNS has thus far not included inhabitants aged less than 18 years. This is potentially problematic because youth represent a key focus of HIV prevention programmes. Fourth, because of non-response and sexual networking with mainland populations, the LNS does not provide a truly complete picture of the sexual networks within which Likoma residents are embedded and which puts them at risk of contracting/transmitting HIV. Finally, the LNS has only included a crude assessment of the HIV status of participants based on antibody testing. It does not permit identifying acute HIV infections, which may contribute disproportionately to HIV transmission in this setting.41–43
Can I get hold of the data? Where can I find out more?
Full dataset documentation is available upon request. It includes the definition of variables and the questionnaires which were used for data collection during each round. Researchers interested in using the LNS data should submit a two-page proposal (including an analysis plan) to the study investigators (sh2813@columbia.edu or hpkohler@pop.upenn.edu). If this is deemed scientifically sound and not overlapping with ongoing LNS work, they will then be asked to sign a data use agreement to be able to access the data. All analyses of the LNS are conducted in collaboration with members of the LNS team.
Funding
This research received support through the National Institute of Child Health and Development (grants no. RO1 HD044228 and RO1 HD/MH41713), National Institute on Aging (grant no. P30 AG12836), the Boettner Center for Pensions and Retirement Security at the University of Pennsylvania, and the National Institute of Child Health and Development Population Research Infrastructure Program (grant no. R24 HD-044964), all at the University of Pennsylvania; as well as through National Institute of Child Health and Development (grant no. R03HD071122) to Columbia University.
Conflict of interest: None declared.
KEY MESSAGES.
The Likoma Network Study (LNS) is the first cohort study to document the sexual networks of an entire sub-Saharan population with high HIV prevalence (Likoma, Malawi).
It has shown that dense and robust sexual networks can emerge even when network members engaged in limited risk behaviours; this helps explain the spread of HIV beyond high-risk groups in sub-Saharan populations.
It has also contributed to a better assessment of the extent of biases in survey reports of sexual behaviours and HIV risk factors
Data from the LNS can be used as input for the calibration of mathematical models used to plan HIV prevention policies; they also constitute a resource for the development of new methodologies to study sexual networks empirically.
References
- 1.Kretzschmar M, Morris M. Measures of concurrency in networks and the spread of infectious disease. Math Biosci. 1996;133:165–95. doi: 10.1016/0025-5564(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 2.Morris M. Sexual networks and HIV. AIDS. 1997;11(Suppl A):S209–16. [PubMed] [Google Scholar]
- 3.Newman ME. Spread of epidemic disease on networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;66(1 Pt 2):016128. doi: 10.1103/PhysRevE.66.016128. [DOI] [PubMed] [Google Scholar]
- 4.Eames KT, Keeling MJ. Monogamous networks and the spread of sexually transmitted diseases. Math Biosci. 2004;189:11530. doi: 10.1016/j.mbs.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Morris M. Overview of network survey designs. In: Morris M, editor. Network Epidemiology. Oxford: Oxford University Press; 2004. [Google Scholar]
- 6.Valente TW. Social Networks and Health: Models, Methods, and Applications. Oxford and New York: Oxford University Press; 2010. [Google Scholar]
- 7.Doherty IA, Padian NS, Marlow C, Aral SO. Determinants and consequences of sexual networks as they affect the spread of sexually transmitted infections. J Infect Dis. 2005;191(Suppl 1):S42–54. doi: 10.1086/425277. [DOI] [PubMed] [Google Scholar]
- 8.Doherty IA. Sexual networks and sexually transmitted infections: innovations and findings. Curr Opin Infect Dis. 2011;24:70–77. doi: 10.1097/QCO.0b013e3283422647. [DOI] [PubMed] [Google Scholar]
- 9.Brewer DD. Case-finding effectiveness of partner notification and cluster investigation for sexually transmitted diseases/HIV. Sex Transm Dis. 2005;32:78–83. doi: 10.1097/01.olq.0000153574.38764.0e. [DOI] [PubMed] [Google Scholar]
- 10.Klovdahl AS, Potterat JJ, Woodhouse DE, Muth JB, Muth SQ, Darrow WW. Social networks and infectious disease: the Colorado Springs Study. Soc Sci Med. 1994;38:79–88. doi: 10.1016/0277-9536(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 11.Woodhouse DE, Rothenberg RB, Potterat JJ, et al. Mapping a social network of heterosexuals at high risk for HIV infection. AIDS. 1994;8:1331–36. doi: 10.1097/00002030-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Fichtenberg CM, Muth SQ, Brown B, Padian NS, Glass TA, Ellen JM. Sexual network structure among a household sample of urban African American adolescents in an endemic sexually transmitted infection setting. Sex Transm Dis. 2009;36:41–48. doi: 10.1097/OLQ.0b013e3181860711. [DOI] [PubMed] [Google Scholar]
- 13.Auerbach DM, Darrow WW, Jaffe HW, Curran JW. Cluster of cases of the acquired immune deficiency syndrome. Patients linked by sexual contact. Am J Med. 1984;76:487–92. doi: 10.1016/0002-9343(84)90668-5. [DOI] [PubMed] [Google Scholar]
- 14.Hurt CB, Beagle S, Leone PA, et al. Investigating a sexual network of Black men who have sex with men: Implications for transmission and prevention of HIV infection in the United States. J Acquir Immune Defic Syndr. 2012;61:515–21. doi: 10.1097/QAI.0b013e31827076a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bearman PS, Moody J, Stovel K. Chains of affection: The structure of adolescent romantic and sexual networks. Am J Sociol. 2004;110:44–91. [Google Scholar]
- 16.Fu Z, He N, Duan S, et al. HIV infection, sexual behaviors, sexual networks, and drug use among rural residents in Yunnan Province, China. AIDS Behav. 2011;15:1017–25. doi: 10.1007/s10461-010-9797-6. [DOI] [PubMed] [Google Scholar]
- 17.Cliff AD, Haggett P, Smallman-Raynor M. Island Epidemics. Oxford and New York: Oxford University Press; 2000. [Google Scholar]
- 18.Helleringer S, Kohler HP, Chimbiri A, Chatonda P, Mkandawire J. The Likoma Network Study: Context, data collection and initial results. Demogr Res. 2009;21:427–68. doi: 10.4054/DemRes.2009.21.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helleringer S, Kohler HP, Frimpong JA, Mkandawire J. Increasing uptake of HIV testing and counseling among the poorest in sub-Saharan countries through home-based service provision. J Acquir Immune Defic Syndr. 2009;51:185–93. doi: 10.1097/QAI.0b013e31819c1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helleringer S, Mkandawire J, Reniers G, Kalilani-Phiri L, Kohler H-P. Should home-based HIV testing and counseling services be offered periodically in programs of ARV treatment as prevention? A case study in Likoma (Malawi) AIDS Behav. 2012;9:3288–309. doi: 10.1007/s10461-012-0365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mensch BS, Hewett PC, Erulkar AS. The reporting of sensitive behavior by adolescents: a methodological experiment in Kenya. Demography. 2003;40:247–68. doi: 10.1353/dem.2003.0017. [DOI] [PubMed] [Google Scholar]
- 22.Savage A, Russell LA. Tangled in a web of affiliation: social support networks of dually diagnosed women who are trauma survivors. J Behav Health Serv Res. 2005;32:199–214. [PubMed] [Google Scholar]
- 23.Field S, Frank KA, Schiller K, Riegle-Crumb C, Muller C. Identifying positions from affiliation networks: Preserving the duality of people and events. Soc Networks. 2006;28:97–123. doi: 10.1016/j.socnet.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frost SD. Using sexual affiliation networks to describe the sexual structure of a population. Sex Transm Infect. 2007;83(Suppl 1):i37–42. doi: 10.1136/sti.2006.023580. [DOI] [PubMed] [Google Scholar]
- 25.Yang OO, Lewis MJ, Reed EF, et al. Human leukocyte antigen class I haplotypes of human immunodeficiency virus-1-infected persons on Likoma Island, Malawi. Hum Immunol. 2011;72:877–80. doi: 10.1016/j.humimm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helleringer S, Kohler HP. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS. 2007;21:2323–32. doi: 10.1097/QAD.0b013e328285df98. [DOI] [PubMed] [Google Scholar]
- 27.Pickering H, Okongo M, Nnalusiba B, Bwanika K, Whitworth J. Sexual networks in Uganda: casual and commercial sex in a trading town. AIDS Care. 1997;9:199–207. doi: 10.1080/09540129750125217. [DOI] [PubMed] [Google Scholar]
- 28.Morison L, Weiss HA, Buve A, et al. Commercial sex and the spread of HIV in four cities in sub-Saharan Africa. AIDS. 2001;15(Suppl 4):S61–69. doi: 10.1097/00002030-200108004-00007. [DOI] [PubMed] [Google Scholar]
- 29.Schneeberger A, Mercer CH, Gregson SA, et al. Scale-free networks and sexually transmitted diseases: a description of observed patterns of sexual contacts in Britain and Zimbabwe. Sex Transm Dis. 2004;31:380–87. doi: 10.1097/00007435-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Latora V, Nyamba A, Simpore J, et al. Network of sexual contacts and sexually transmitted HIV infection in Burkina Faso. J Med Virol. 2006;78:724–29. doi: 10.1002/jmv.20614. [DOI] [PubMed] [Google Scholar]
- 31.Moody J. The importance of relationship timing for diffusion. Social Forces. 2002;81:25–56. [Google Scholar]
- 32.Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S. Concurrent partnerships and HIV prevalence disparities by race: linking science and public health practice. Am J Public Health. 2009;99:1023–31. doi: 10.2105/AJPH.2008.147835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helleringer S, Kohler HP, Kalilani-Phiri L. The association of HIV serodiscordance and partnership concurrency in Likoma Island (Malawi) AIDS. 2009;23:1285–87. doi: 10.1097/QAD.0b013e32832aa85c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lurie MN, Williams BG, Zuma K, et al. Who infects whom? HIV-1 concordance and discordance among migrant and non-migrant couples in South Africa. AIDS. 2003;17:2245–52. doi: 10.1097/00002030-200310170-00013. [DOI] [PubMed] [Google Scholar]
- 35.Helleringer S, Kohler HP, Chimbiri A. Characteristics of external/bridge relationships by partner type and location where sexual relationship took place. AIDS. 2007;21:2560–61. doi: 10.1097/QAD.0b013e3282f112bd. [DOI] [PubMed] [Google Scholar]
- 36.Adams J, Moody J. To tell the truth: Measuring concordance in multiply reported network data. Social Networks. 2007;29:44–58. [Google Scholar]
- 37.UNAIDS. HIV: consensus indicators are needed for concurrency. Lancet. 2010;375:621–22. doi: 10.1016/S0140-6736(09)62040-7. [DOI] [PubMed] [Google Scholar]
- 38.Helleringer S, Kohler HP, Kalilani-Phiri L, Mkandawire J, Armbruster B. The reliability of sexual partnership histories: implications for the measurement of partnership concurrency during surveys. AIDS. 2011;25:503–11. doi: 10.1097/QAD.0b013e3283434485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helleringer S, Kohler H-P, Mkandawire J. Women under-estimate the age of their sexual partners during survey interviews: implications for HIV risk associated with age mixing in Northern Malawi. Sexually Transmitted Diseases. 2011;38:1030–35. doi: 10.1097/OLQ.0b013e318227a486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith KP, Christakis NA. Social networks and health. Ann Rev Sociol. 2008;34:405–29. [Google Scholar]
- 41.Novitsky V, Woldegabriel E, Wester C, et al. Identification of primary HIV-1C infection in Botswana. AIDS Care. 2008;20:806–11. doi: 10.1080/09540120701694055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothenberg R. HIV transmission networks. Curr Opin HIV AIDS. 2009;4:260–65. doi: 10.1097/COH.0b013e32832c7cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–68. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]