Abstract
Background: Over the past 10 years, research into environmental risk factors for autism has grown dramatically, bringing evidence that an array of non-genetic factors acting during the prenatal period may influence neurodevelopment.
Methods: This paper reviews the evidence on modifiable preconception and/or prenatal factors that have been associated, in some studies, with autism spectrum disorder (ASD), including nutrition, substance use and exposure to environmental agents. This review is restricted to human studies with at least 50 cases of ASD, having a valid comparison group, conducted within the past decade and focusing on maternal lifestyle or environmental chemicals.
Results: Higher maternal intake of certain nutrients and supplements has been associated with reduction in ASD risk, with the strongest evidence for periconceptional folic acid supplements. Although many investigations have suggested no impact of maternal smoking and alcohol use on ASD, more rigorous exposure assessment is needed. A number of studies have demonstrated significant increases in ASD risk with estimated exposure to air pollution during the prenatal period, particularly for heavy metals and particulate matter. Little research has assessed other persistent and non-persistent organic pollutants in association with ASD specifically.
Conclusions: More work is needed to examine fats, vitamins and other maternal nutrients, as well as endocrine-disrupting chemicals and pesticides, in association with ASD, given sound biological plausibility and evidence regarding other neurodevelopmental deficits. The field can be advanced by large-scale epidemiological studies, attention to critical aetiological windows and how these vary by exposure, and use of biomarkers and other means to understand underlying mechanisms.
Keywords: Autism, environmental risk factors, air pollution, environmental chemicals, maternal nutrition, maternal smoking, maternal alcohol use
Key Messages.
Research on environmental risk factors for ASD has grown dramatically over the past ∼10 years. Many factors have been examined; here we focus on modifiable preconception/prenatal maternal lifestyle factors (including substance use and diet) and exposure to chemicals (such as occur in air pollution, pesticides and household products).
Evidence to date for smoking and alcohol in association with ASD is weak, with the need for more rigorously conducted studies.
Evidence for maternal prenatal vitamins, folate and fatty acid intake is suggestive but requires replication and further study.
The literature for air pollution shows consistency, suggesting moderate increases in risk of ASD in association with heavy metals and particulate matter, though measurement accuracy and possible residual confounding need to be addressed.
Large-scale epidemiological studies that consider critical time periods and biological markers of these exposures, as well as other maternal dietary factors and chemicals, are needed given biological plausibility and suggestive results on other neurodevelopmental endpoints.
Introduction
Both genetic and environmental lines of research have led to recognition of the aetiological complexity of autism spectrum disorder (ASD). Although the contribution from the environment was originally thought to be low, based in part on remarkably high monozygotic twin concordance in earlier small studies1,2 and a limited understanding of gene-by-environment interactions, evidence currently supports a greater environmental contribution. In the largest twin study to date, Hallmayer and colleagues found that environment accounted for 55% of the variance in autism risk among twins.3 Current thinking suggests that, for most individuals, multiple causes likely operate, and these may include a number of genes and environmental factors.
Over the past decade, there has been exponential growth in the number of environmental factors studied in association with ASD. Although the field is still in its early stages, studies grounded in biologically plausible pathways and focused on critical time periods of neurodevelopment have suggested promising risk and protective factors, as well as avenues for future research. This article discusses certain environmental factors that may alter risk for ASD when exposure occurs during the preconception and prenatal periods.
The environment can be defined broadly as all non-genetic factors, from viruses to medications, from chemicals or physical agents to social and cultural influences. Due to this broad scope and the substantial body of emerging research on these environmental topics, we have focused our review specifically on modifiable maternal lifestyle factors and environmental chemicals. Potentially modifiable factors are highlighted, as these offer an opportunity for altering behaviours at the individual level or taking action at the societal level in order to change exposure and thereby improve public health through reduced incidence of ASD. Table 1 outlines criteria for inclusion in this review.
Table 1.
Inclusion criteria for studies in this review
| Criterion | Explanation |
|---|---|
| 1. Recent study of ASD | Published since 2003, in which an ASD diagnosis or a measure of ASD symptoms is the outcome. |
| 2. Valid comparison group | Case reports not included; studies must have valid control or comparison group |
| 3. Adequate sample size | Only studies with at least 50 ASD cases, to avoid severely underpowered analysis |
| 4. Focus on potentially modifiable maternal lifestyle factors and environmental chemical exposure in association with ASD | Maternal lifestyle factors (including nutrition and substance use) and exposure to environmental chemicals have strong biological plausibility and a growing number of studies that have for the most part not yet been reviewed in relation to ASD. Whereas social, cultural and family characteristics may have a substantial impact, we focus here on more biological factors. Medical factors (including illnesses, obesity, pregnancy complications) and medications have a substantial literature beyond the scope of this paper, and have also been reviewed elsewhere; similarly, the growing literature supporting the role of the maternal immune system140–142 is also not reviewed here; the reader is referred to prior reviews on these topics143 |
| 5. Exposures measured in or intended to reflect the periconceptional/prenatal period | Biological relevance of this time period for brain development: evidence of strong risk factors during the prenatal period,8,9,144 neuropathological findings in the brains of persons with autism likely to originate during fetal CNS development145 and experimental models of features of autism.9 |
| 6. Human study | We focus on human studies in which clinical or reported ASD is the outcome, although reference is made to experimental animal models where relevant for discussions of plausibility and/or mechanisms |
Environmental factors
Potential mechanisms
Environmental exposures may influence brain development at different stages, including formation and closure of the neural tube, cell differentiation and migration, formation of structures such as cortical mini-columns, synaptogenesis and myelination. Immune dysregulation or oxidative stress from xenobiotic chemicals or their metabolites, and deficiencies in nutrients or essential fatty acids may also play a pathogenic role. Further, environmental factors are likely to act in concert with susceptibility genes. Such interactions may involve epigenetics, leading to changes in gene expression and/or to a transgenerational mechanism. Additionally, genes may affect the metabolism, receptors and activity of xenobiotic chemicals, thereby altering the biochemistry of the brain. Evidence that de novo changes to DNA, i.e. alterations not inherited from parents, are associated with ASD risk suggests that environmental exposures may induce damage to the genetic code.
Methodological considerations
Many factors discussed in this article share associations with other neurodevelopmental disorders; i.e. they may not be specific to ASD but, rather, the pathways they activate can lead to various phenotypic manifestations, including autism but also attention deficits or cognitive impairments. In light of such lack of specificity, comparisons between persons with ASD and those with other developmental or mental health conditions may fail to identify causative factors. Serious biases, usually towards the null, could also arise from use of unaffected siblings or friends in case-control studies, a common strategy in psychiatric epidemiology. Ecological studies, where summary information rather than individual data is used for both exposures and outcomes (e.g. average emissions of a pollutant in relation to rates of ASD) are especially vulnerable to confounding and other biases; fortunately, cohort and case-control designs with individual-level data are becoming the norm in ASD research. Moreover, with well-defined source populations and existing exposure databases or specimen archives, some case-control studies can achieve as high quality as cohort studies.
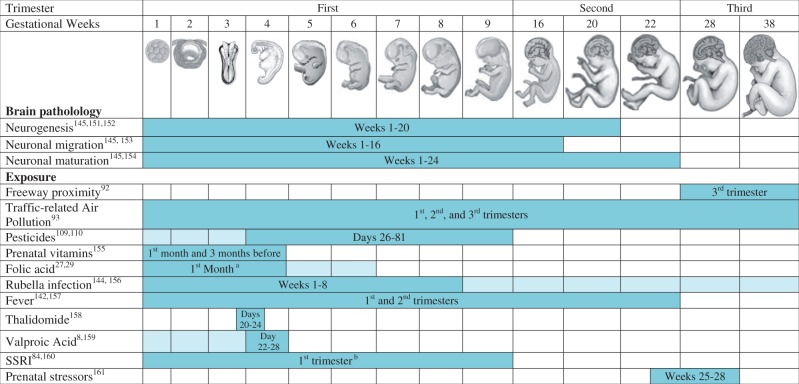
Timing is critical for neurodevelopment, and hence for studying environmental impacts. Though findings from neuroanatomical, epidemiological and animal studies support prenatal origins of ASD,4–9 the implicated vulnerable window varies by mechanism or by risk factor (Figure 1). Thus, multiple critical periods of increased susceptibility for ASD likely exist and may extend from pre-conception through the first few years of life.
Figure 1.
Critical periods of susceptibility indicated from studies of autism spectrum disorders. Neuropathology (autopsy and imaging) studies of brains of individuals with autism spectrum disorders found evidence of dysregulated neurogenesis, neuronal migration and neuronal maturation compared with brains of typically developed individuals, processes that generally occur in the first half of pregnancy. Figure shows windows of critical periods indicated by evidence from epidemiological studies of environmental factors demonstrating an association with autism spectrum disorders. Not all exposures shown in the figure are covered in this review, but they are included as exemplary of critical time windows. Time periods of higher risk within pregnancy have variable results, but tend to congregate in the first half of pregnancy. Days = fetal days after conception. For exposures with more than one study, dark blue indicates overlapping period and light blue indicates timing suggested by one but not all studies. Images adapted from those in The Developing Human: Clinically Oriented Embryology, 6th edition (1998). This material is reproduced with permission of John Wiley and Sons. aThe study by Suren et al.29 indicated that up to 6 weeks prior to (and 6 weeks after) conception (4 weeks before LMP to 8 weeks after) was important. bThe study by Rai et al.160collected information on use since becoming pregnant at the first antenatal visit which usually takes place before the end of the first trimester (median 10 weeks of gestation).
Proxies for environmental exposures
Some environmental factors may serve as markers rather than causes of higher risk. Although some proxies for environmental factors are not directly modifiable, studying them can provide aetiological clues. Season serves as a proxy variable for diverse exposures, such as influenza, other infections, sunlight/vitamin D, other nutritional factors or pesticides. Seasonality of birth or conception has been associated with increased risk of autism in several studies.10–12 Short interpregnancy interval (IPI) is another proxy strongly associated with ASD in two very large cohort studies.13,14Among other explanations (cultural, family planning or lifestyle factors), short IPI may indicate maternal depletion of essential nutrients including folate, as these become depleted during pregnancy and can remain low for up to a year postpartum.15–17
Maternal Lifestyle Factors
Background
Several lifestyle factors have been examined in relation to ASD (Table 2). Because cigarette smoking data are often available in medical and registry databases, maternal smoking was one of the first factors to be examined for associations with ASD. More recently the gap in research on lifestyle factors, such as other substance use and maternal nutrition, has begun to be addressed.
Table 2.
Summary of maternal lifestyle factors associated with autism
| Environmental factor and referencesa | Study design | Exposure assessment | Results | Strengths | Limitations | State of evidence | |
|---|---|---|---|---|---|---|---|
| Prenatal vitamins | |||||||
| Schmidt et al., 20112,6 | Case-control (429 ASD, 278 controls) | Parental report via telephone-administered questionnaire | Associated with decreased risk for ASD if taken before or near conception especially in combination with certain one-carbon metabolism genotypes | Large case-control study with clinically confirmed cases using standardized instruments. Adjustment for many important confounders | Self-reported exposure collected retrospectively, potential for recall bias | Association with decreased risk for ASD for folic acid-containing supplements taken before or near conception was replicated. Additional studies examining multivitamins or prenatal vitamins are needed. Interaction with certain one-carbon metabolism genotypes needs to be replicated | |
| Folic acid | |||||||
| Schmidt et al., 20122,7 | Case-control (429 ASD, 278 controls) | Total mean folic acid quantified from frequency of intake of supplement and cereal brands reported via telephone-administered questionnaire | Folic acid intake near conception associated with reduced risk for ASD, especially if mother or child has MTHFR 677 T-allele. Dose-response trend observed | Large case-control study with clinically confirmed cases using standardized instruments. Adjustment for important confounders | Did not assess maternal dietary folate. Exposure based on retrospective data; potential for recall bias | Supplemental folic acid intake near conception associated with reduced risk for autism/ASD in two large studies. Dose-response trend and effect modification by maternal and child MTHFR 677 T-allele and other gene variants needs replication. Dietary folate needs consideration | |
| Surén et al., 20132,9 | Birth cohort (85 176 children, 114 with autism, 56 with Asperger syndrome, 100 with PDD-NOS) | Folic acid supplement intake before conception and in early pregnancy obtained through questionnaire report at week 18 of gestation | Folic acid intake near conception (4 weeks before to 8 weeks after LMP) associated with reduced risk for autism, not Asperger syndrome or PDD-NOS | Prospective exposure collection. Subset of cases clinically confirmed. Adjustment for important confounders | Did not assess maternal dietary folate for the relevant time period. Not able to examine dose | ||
| Fish & fish oil | |||||||
| Lyall et al., 20133,9 | Nested case-control (317 mothers of children with ASD, 17 728 control mothers) | Fish intake collected using validated food frequency questionnaire | No association found for maternal fish intake | Large study with prospectively collected exposure. Adjusted for important confounders | Self-reported exposure. Limited variation in intake and few women with fish intake reported during pregnancy. Cases not clinically confirmed | Evidence suggests no association for maternal fish or fish oil in two large cohort studies with prospective exposure collection and adjustment for appropriate confounders. Both studies relied on self-report. Additional studies examining fish and fish oil intake during pregnancy are needed to replicate the findings and investigate dose effects | |
| Surén et al., 20132,9 | Birth cohort (85 176 children, 114 with autism, 56 with Asperger syndrome, 100 with PDD-NOS) | Fish oil supplement intake before conception and in early pregnancy reported on questionnaire at week 18 of gestation | No association found for maternal fish oil taken near conception (4 weeks before to 8 weeks after LMP) | Large study with prospectively collected exposure. Adjusted for important confounders. Subset of cases clinically confirmed | Self-reported exposure. Dose and frequency not evaluated | ||
| Fatty acids | |||||||
| Lyall et al., 20133,9 | Nested case-control (317 mothers of children with ASD, 17 728 control mothers). | Self-administered food frequency questionnaire for diet during the year the child was born. | Decreased ASD risk with increased maternal polyunsaturated fat intake, especially omega-6 fatty acids. Very low omega-3 intake was associated with increased ASD risk | Large study with prospectively collected exposure using validated instrument. Food and supplement sources considered | Exposure based on self-report during the year the child was born (not specifically during pregnancy). Cases not clinically confirmed | Preliminary evidence for decreased ASD risk with increased omega-6 fatty acids and very low omega-3 intake associated with increased risk. Results need to be replicated, use objective measurements, and examine timing | |
| Vitamin D | |||||||
| Whitehouse et al., 201246 | Cohort (n = 929 with a continuous score for autism-spectrum quotient, including 3 with ASD) | Serum 25(OH)-vitamin D concentrations measured at 18 weeks’ pregnancy using an enzyme immunoassay kit (a subset also had LCMS measurements). | Maternal gestational serum levels were not associated with offspring autism phenotypes (using autism-spectrum quotient); a weak association was found with the attention switching subscale | Medium-sized study with objective and prospectively measured exposure | Too few children diagnosed with autism to evaluate association with ASD diagnosis | Maternal gestational vitamin D does not appear to be associated with offspring autism phenotypes in a general population study; studies examining maternal vitamin D in relation to ASD diagnosis in the child are needed | |
| Smoking | |||||||
| Tran et al., 201358 | Population-based nested case-control study; 4019 ASD cases, 16 582 controls | Prospectively collected medical record data; self-report. | Maternal smoking during entire pregnancy associated with PDD; no association with first trimester smoking. No association with AU or Asperger’s | Controls matched 4:1 on birth date, sex, residency at birth. Adjusted for maternal age, SES and psychiatric diagnosis. Assessed timing of exposure | Adjusted for infant’s weight for gestational age, which occurs after the exposure and could be along the causal pathway. Maternally-reported smoking collected on medical records could be inaccurate. No dose or frequency information | Evidence is inconsistent, though a number of studies do not support maternal cigarette smoking as a strong risk factor for ASD. Some indication for an association with high-functioning ASD. All studies relied on self-reported smoking, often from birth records, which can be inaccurate. Few studies considered environmental cigarette smoke exposure, and those that did had other limitations. Further studies investigating high-functioning ASD and potential gene x environment interactions, and utilizing objective markers of cigarette smoking (i.e. serum cotinine levels) are needed to rule out an effect | |
| Visser et al., 201356 | Nested case-control (196 ASD, 311 typical controls) | Retrospective parent- questionnaire administered prior to diagnostic assessments | Trend towards higher prenatal tobacco use for ASD vs controls; significantly higher use in PDD-NOS vs autistic disorder | Clinically-confirmed cases and controls screened for ASD Age- and sex-matched controls | Focus was on pre- and perinatal factors; no dose, frequency or timing information assessed. No adjustment for confounders | ||
| Lee et al., 201254 | Registry-based nested case-control study of 3958 ASD cases and 38 983 controls | Self-reported information on smoking prospectively recorded by midwives at the first prenatal visit approximately 8–12 weeks after conception. | No association between maternal smoking during pregnancy and ASD | Adjusted for SES factors; assessed dose; examined association by degree of functioning; exposure data are validated and data quality is considered high; examined effects of calendar year; multi-source case-ascertainment system | Exposure not collected for all of pregnancy; no assessment of timing. Use of administrative data for outcome | ||
| Smoking, continued | |||||||
| Kalkbrenner et al., 201255 | Population-based case-cohort study; 633 989 children, including 3315 ASD | Birth certificate data. | No association with ASD or autistic disorder; modest association with ASD-NOS | Accounted for under-ascertainment bias; adjusted for SES and other confounding factors; examined case sub-groups | Birth certificate data on smoking is often inaccurate; no information on dose or frequency of smoking | ||
| Hvidtjorn et al., 201114,6 | Population-based case-control study; 3602 ASD cases, 582 694 controls | Birth record data. | Significantly higher proportion of mothers of children with ASD reported smoking | Inpatient and outpatient registry data for ASD diagnosis | Focus of study was on assisted reproduction. No adjustment for SES or other confounders; no information on dose, frequency or timing of exposure; possible case under-ascertainment | ||
| Burstyn et al., 201052 | Population-based cohort study; n = 218 890 children, including 1138 with ASD | Birth record self-reported data. | No significant association between any maternal smoking and ASD (trend in protective direction) | Adjusted for many factors including SES and found similar null association in analyses not adjusted for perinatal factors that could be in pathway | Potential inaccuracies of self-reported birth record data; no information on dose, frequency or timing | ||
| Ronald et al., 201014,7 | Population-based cohort with 13 198 twins age 7–8 years; 154 ASD cases | Parental-report at around 1.5 years old. | Significant positive correlation between number of cigarettes smoked and autistic-like features at age 7 and 8 years | Adjusted for SES and child’s general cognitive ability; included information on number of cigarettes smoked (dose). | No clinical confirmation of ASD diagnoses | ||
| Bilder et al., 200914,8 | Population-based nested case-control; 132 ASD cases, 13 200 controls | Birth record data. | No significant association; trend towards decreased risk | 100:1 controls matched to cases on sex and year of birth | No adjustment for SES or other potentially confounding variables; potential inaccuracy of smoking from birth records; no information on dose or frequency of smoking | ||
| Larsson et al., 200951 | Population-based cohort study; 4779 children, including 72 ASD cases | Parent-report collected for pregnancy when child was age 1-3 years. | Significant association with 2-fold higher risk for ASD and maternal smoking; association with paternal smoking non-significant | Exposure assessment prior to diagnoses; SES, other sociodemographic factors and bedroom flooring considered as potential confounders; also considered environmental smoke exposure in household and maternal smoking after pregnanc | Percentage of women who smoked in the overall study population was small (14%) and the number who had a child with ASD on which these associations were based was rather small (n = 21); no information on dose, frequency or specific timing during pregnancy; Tourette’s syndrome included in ASD diagnosis; parentally reported ASD (not clinically confirmed) | ||
| Smoking, continued | |||||||
| Indredavik et al., 200750 | Population-based prospective study of 84 adolescents (age 14 years) | Maternally reported at enrollment, before the 20th week of pregnancy. | Mothers, fathers, and teachers reported higher social problems score on ASEBA for the smoking exposed adolescents; ASSQ sum score for social sensitivity (as a screen for high-functioning ASD) was strongly associated with smoking exposure during pregnancy | Prospective exposure collection; assessment of sex-effects; adjusted for SES and other important maternal confounders | Not enough ASD cases to evaluate an association with diagnosis; although the information was collected, results are not reported by the number of cigarettes smoked per day; adjusted for birthweight which could be along the pathway | ||
| Maimburg et al., 200653 | Population-based case-control study; 473 cases, 4730 controls matched 10:1 on sex, year and birth county | Prospective self-report to midwives at the first antenatal visit generally at 12 weeks | No association with autism | Controls matched on sex and year of birth; adjusted for maternal and paternal age, mother’s citizenship | Adjusted for birth outcomes which could be along the pathway; no adjustment for SES or parental psychiatric conditions; no information on dose, frequency, smoking in late pregnancy or ETS; lack of outpatient ASD data | ||
| Larsson et al. 200514,9 | Population-based nested case-control study; 249 cases, 6225 controls with smoking data | Self-report at first antenatal visit | No association with infantile or atypical autism | Controls matched on sex, birth year, and age | Collected data on parental psychiatric history and SES, but did not adjust the smoking association; lack of outpatient ASD data for years smoking studied | ||
| Hultman et al., 20026 | Population-based nested case-control study; 408 autism cases, 2040 controls | Self-report to midwives at registration for antenatal care. | Significant association between daily smoking and increased risk for infantile autism | Had measure of frequency (daily or non-daily); adjusted for maternal demographic factors | Adjusted for delivery and infant factors which occur after exposure and could be along pathway; no adjustment for SES; lack of outpatient ASD data included in the national registry; no information on timing | ||
| Alcohol | |||||||
| Eliasen et al., 20106,9 | Population-based prospective cohort study (n = 80 552, including 157 with autism) | Self-reported alcohol consumption collected through telephone interview during pregnancy. | No increased risk from light to moderate maternal alcohol consumption | Large study with prospectively collected exposure information. Adjusted for important confounders. | Subject to potential inaccuracies of self-reported use. Cases not clinically confirmed. | No increased risk from light to moderate maternal alcohol consumption. Needs replication. Accurate exposure assessment difficult to achieve | |
| Visser et al., 201356 | Nested case-control (196 ASD, 311 typical controls) | Retrospective parent- questionnaire administered prior to diagnostic assessments. | ASD case parents reported significantly lower alcohol use than control parents (same for autism and PDD). | Clinically-confirmed cases and controls screened for ASD. Age- and sex-matched controls | Focus was on pre- and perinatal factors; no dose, frequency or timing information assessed. No adjustment for confounders | ||
PDD, pervasive developmental disorder; NOS, not otherwise specified; ASEBA, Achenbach System of Empirically Based Assessment; ASSQ, Autism Spectrum Screening Questionnaire; LMP, last menstrual period.
aReferences meeting study inclusion criteria (Table 1).
Maternal nutrition
Despite a growing number of studies investigating diet and nutrition among children affected with ASD, very little research has directly explored maternal nutrition in association with risk of ASD in the offspring. However, maternal nutrition is essential to fetal brain development, and maternal nutrient deprivation has been associated with strong increases in risk of schizophrenia,18 neural tube defects19,20 and other adverse neurodevelopmental outcomes.21–23 Nutritional deficiencies are particularly common during pregnancy due to increased metabolic demands imposed by a growing placenta, fetus and maternal tissues,24,25 and have been shown to influence brain development in terms of structure and function. There is strong biological plausibility, therefore, that maternal nutrition might influence ASD risk as well.
Prenatal vitamins and folic acid
In a large population-based case-control study, consumption of prenatal vitamin supplements near the time of conception was associated with about 40% reduction in risk for ASD.26 This significant finding was adjusted for maternal education and child’s birth year. The study also reported significant gene-environment interactions, suggesting even stronger protection from prenatal vitamins that contain high levels of folic acid when the children or their mothers carried gene variants leading to less efficient folate-dependent one-carbon metabolism. In further work, Schmidt and colleagues (2012) reported significantly lower mean total folic acid intake (quantified from vitamins, other supplements and breakfast cereals) in the first month of pregnancy for mothers of children with ASD (n = 429) than for mothers of typically developing children (n = 278). The study also found a significant trend of decreasing ASD risk as mean daily folic acid intake increased (Ptrend = 0.001)27 Reported intake of ≥600 ug of folic acid (the recommended dietary allowance for pregnant women in the USA28) was associated with protection only when either the mother or the child carried a common variant in the methylenetetrahydrofolate reductase gene, MTHFR 677 C>T, which leads to less efficient folate metabolism. A Norwegian cohort study of 85 176 children, of whom 270 were diagnosed with ASD,29 tightly replicated these folic acid supplement findings in a country where cereal grains are not fortified with folic acid (as they are in the USA). Risk for autistic disorder was 40% lower among those whose mothers prospectively reported at 18 weeks of gestation having taken folic acid supplements periconceptionally (∼6 weeks before and after conception).
These studies are among the first to suggest modifiable factors that could reduce risk of autism. Periconceptional folic acid improves other neurodevelopmental and behavioural outcomes, including reduced risk for neural tube defects, hyperactivity and severe language delay, as well as improved attention, social competence, and verbal and executive function.30–34 Around the time of implantation, when the epidemiological data suggest folic acid to be most important, mammalian embryos undergo extensive DNA demethylation, followed by reestablishment of methylation patterns.35 Because folate provides a major source of one-carbon (methyl) groups, mechanisms involving methylation—and hence expression or activity of genes, proteins and neurotransmitters—may link folic acid to ASD.27 Impaired methylation capacity36 and altered DNA methylation have been implicated in ASD aetiology.37
Fish and fish oil supplements
Maternal fish intake may also be relevant to neurodevelopment and ASD, both as a source of fatty acids and vitamin D (which may confer protective effects) and as a potential source of mercury (which is deleterious to fetal brain development). As reviewed by Oken and Bellinger,38 the majority of studies examining maternal fish intake and child neurodevelopmental outcomes have suggested that higher maternal fish consumption is associated with higher child development scores, though type of fish and mercury levels need to be taken into account. The only study to date to examine maternal fish intake in association with ASD specifically did not find any relationship.39 The study was limited, however, by low variation in fish intake and few women with fish intake reported during pregnancy. The Norwegian cohort study on folic acid supplements also found no association between autism and prenatal fish oil supplements.29
Fatty acids
Developing fetuses require maternal stores of omega-3 fatty acids for optimal brain development.40,41 The only study to date that has examined maternal fat and fatty acid intake during pregnancy in association with ASD39 used prospectively collected dietary data from validated food frequency questionnaires in a subgroup of a large US cohort, the Nurses’ Health Study II. Children of mothers with higher intake of polyunsaturated fatty acids (PUFA) before and during pregnancy had reduced risk of ASD relative to children of mothers with the lowest PUFA intake. In addition, women with very low intakes (the lowest 5% of the distribution) of omega-3 fatty acids had a significantly increased risk of having offspring with ASD relative to those in the middle 90% of the distribution.
Vitamin D
Low maternal (and thus fetal) vitamin D levels have been hypothesized as risk factors for ASD,42 based on reports of increased rates of ASD among children of dark-skinned immigrant mothers who moved to high latitudes43 and among children born or conceived in certain seasons.12,44 No significant differences were observed in serum 25-hydroxyvitamin D, comparing mothers of children with ASD to mothers of children without ASD among Somalis or Swedes.45 However, the study had a small sample size and lacked vitamin D measurements during gestation.
Another study found maternal serum 25-hydroxyvitamin D concentrations from around the 18th week pregnancy did not predict the child’s total Autism-Spectrum Quotient (AQ) scores in early adulthood.46 However, the study was too small to examine ASD diagnoses. No study to date has examined the relationship between maternal or gestational vitamin D and ASD diagnosis.
Despite a lack of epidemiological evidence for a maternal vitamin D effect on ASD risk, vitamin D influences neuronal differentiation, metabolism of neurotrophic factors and neurotoxins, protection from brain inflammation, endocrine functions and fetal brain growth, providing ample biological plausibility for a relationship. Rats born to vitamin D-deficient dams have profound alterations in the brain,47 and human maternal vitamin D insufficiency has been linked with impaired language development of the child at ages 5 and 10 years.46
Substance use
Cigarette smoking
A number of investigations have assessed maternal smoking in association with ASD, each with limitations, and overall producing inconsistent findings.6,7,48–58 Earlier work lacked adjustment for socioeconomic factors that likely confounded associations6,7,48,49 or adjusted for potentially mediating variables (i.e. birthweight52,53) which could have masked an elevated risk. A large registry-based study of 3958 ASD cases and 38 983 controls in Sweden found no association after adjustment for family sociodemographic factors.54 The two most recent studies, a population-based study of 633 989 US children57 and a Finnish nested case-control study,58 found no association between maternal smoking and ASD, but did find an association with high-functioning autism or pervasive development disorder. Nevertheless, collecting accurate data on smoking can be challenging, and under-reporting in medical records, by self-report, or from birth certificates57 in these studies could have led to exposure misclassification and bias towards the null.
Maternal smoking could influence neurodevelopment and risk for ASD through mechanisms such as placental insufficiency, reduced blood flow and oxygen deprivation in the brain,59 changes in fetal brain gene expression,60 altered nicotinic receptors,61 persistent changes in neurotransmitter activity and turnover62,63 and increased intrauterine testosterone.64 Prenatal smoking in some mothers may also be an indicator of underlying psychological problems that themselves could influence risk in the offspring.65
Despite numerous studies, evidence for maternal cigarette smoking as a risk factor for ASD remains contradictory. Though the latest studies do not support a strong effect of maternal smoking on autism risk, it is premature to draw conclusions, given: (i) limitations of exposure assessment in studies; (ii) evidence for a harmful effect for higher-functioning autism in well-conducted investigations; (iii) strong biological plausibility; and (iv) evidence for effects on social problems and other psychiatric disorders.66 Because smoking has shown a propensity for gene by environment interaction effects in relation to other birth outcomes,67,68 investigation of maternal smoking in combination with genetic susceptibility, and potential epigenetic effects of cigarette smoke components, may yield more definitive answers.
Alcohol
Maternal alcohol consumption can be teratogenic; high prenatal alcohol exposure impairs neurodevelopment in humans,69–71 and in animal studies produces social avoidance72 and structural brain anomalies congruent with those observed in children with ASD.73 However, surprisingly few rigorous studies have examined maternal alcohol use and ASD risk. A pedigree analysis of families that have a child with ASD suggested an association with reported alcoholism in females among first- and second-degree relatives,74 whereas a nested case-control study in Sweden found no association between ASD and alcohol and drug addiction/misuse.75 Other results on the relationship of maternal alcoholism to ASD are also conflicting.74–79
In the largest study of prenatal alcohol exposure and ASD risk, Eliasen and colleagues examined a population-based cohort of 80 552 Danish children and their mothers.69 Average self-reported alcohol consumption was not associated with either ASD or infantile autism. A probably spurious association was found between a single binge episode and lower ASD risk. Selection bias arising from low participation in the study (∼30%) and reporting bias may have distorted the association, but the prospective design and large size suggest that this is the best evidence to date on maternal alcohol intake and ASD.
Other substances
Maternal misuse of other drugs for recreational purposes, such as marijuana and cocaine, has not been investigated in studies meeting criteria for this review (case reports only). Similar to alcohol and smoking, there is biological plausibility for such exposures to interfere with brain development80–82 and increased susceptibility to ASD, but assessment tends to be difficult and prone to inaccuracies and bias. As noted previously, maternal use of pharmaceutical medications, which have been studied more extensively,83–86 is outside the scope of this review, as are herbal preparations.
Environmental Chemicals
Background
Despite historical evidence of reproductive and neurodevelopmental aberrations with exposure to chemicals like lead or pesticides, federal requirements that substances be tested for long-term behavioural consequences have been slow to evolve. Tens of thousands of compounds present in consumer products and other settings remain unregulated at the US federal or state level,87 with many of these likely to have stronger impact on fetuses and children, as compared with adults.
The list of suspected aetiological agents for ASD begins with known neurotoxins and neurodevelopmental toxins, but also extends to compounds that operate via mechanisms initiated outside the central nervous system (CNS), such as immune dysregulation, altered lipid metabolism and mitochondrial dysfunction, among others. Both persistent and non-persistent organic chemicals may influence ASD. Finally, chemicals having widespread contact with human populations deserve close scrutiny as highly prevalent exposures, even when their effects are modest on an individual basis, can greatly impact public health. Environmental chemicals that have been examined in studies meeting our inclusion criteria are summarized in Table 3.
Table 3.
Summary of environmental chemicals potentially associated with autisma
| Environmental factor and referencesa | Study design | Exposure assessment | Results | Strengths | Limitations | State of the evidence |
|---|---|---|---|---|---|---|
| Air pollution | ||||||
| Metals, Solvents, PAHs | Consistent elevated risk with metals and some solvents (methylene chloride) in two of three studies. One study observed high OR for methylene chloride in urban areas, but unlike all other air pollution studies, used speech- and language-impaired as control group. Associations not consistent across studies for lead and vinyl chloride. Available studies used same exposure database, which represents only select years of modelling. A problematic discrepancy is with tobacco smoke, which contains many of the same constitutents [e.g. metals, PAHs, as well as particles (below)], but has not been consistently associated with ASD | |||||
| Windham et al., 200694 | Retrospective nested case-control, (284 ASD cases; 657 population controls with no known developmental disability) | US EPA-modelled estimates (in 2nd year of life) assigned to Census tract of home. Location: San Francisco | Census tract residence in top quartile of metal exposures (collectively and individually for cadmium, mercury and nickel), chlorinated (but not aromatic) solvents, at higher risk for ASD | First analysis of autism using individual-level data on air pollution exposures. Large study, objective measure of a wide range of a priori-selected pollutants. Adjusted for child race, maternal age, maternal education | Exposure estimates pertained to 2nd year of life; assumed to be equivalent to prenatal period | |
| Roberts et al., 201391 | Cohort (325 ASD cases; 22 101 population controls without ASD; Nurses’ Health Study II) | US EPA-modelled estimates for year nearest birth year assigned to Census tract of birth home. Location: across the USA | Census tract residence in top quintile of metal exposures (collectively and individually for mercury, lead, manganese and nickel) at higher risk for ASD. Dose-response observed | Large study, objective measure of wide range of pollutants. Adjusted for maternal education, maternal age, child’s year of birth and Census tract SES variables. Adjusted for multiple comparisons | Exposure estimated for years that ranged from 0 to 3 years before or after delivery | |
| Kalkbrenner et al., 20108,9 | Case-control (374 ASD cases; 3177 controls with speech or language impairment) | US EPA-modelled estimates for 1996 (births were in years 1992, 1994 and 1996). Location: North Carolina and West Virginia | Quinoline and styrene associated with elevated risk. Methylene chloride also elevated, particularly in urban areas, though not with precision | Large study, objective measure of wide range of pollutants. Used semi-Bayes analysis to adjust simultaneously for all pollutants. Adjusted for child’s race, maternal age, education, smoking, marital status and Census tract SES variables. | Control group had speech or language impairments, may not represent exposures of population; hence results could be biased, not comparable with other studies. Exposure estimated for periods 0 to 4 years after delivery. Some exposures lower than in California | |
| Particles, ozone, and oxides of nitrogen (also known as ‘criteria pollutants’) | Results for diesel particles are not consistent, although different control groups may explain the differences. Two distinct studies implicate criteria pollutants including NO2, PM2.5. A single study examined ozone and found consistent associations across various models adjusted for a second pollutant. Although all studies adjusted for multiple socioeconomic and demographic factors, possible residual confounding cannot be precluded. Additionally, the lack of consistent evidence for an association with tobacco smoke, which contains metals, NO2, PM2.5, PM10, as well as volatile and semi-volatile compounds, raises concerns about coherence of findings | |||||
| Windham et al., 200694 | See above | See above | Census tract residence in top quartile of diesel particles at higher risk for ASD | See above | See above | |
| Kalkbrenner et al., 20108,9 | See above | See above | No association with diesel particles | See above | See above | |
| Volk et al., 201192 | Case-control (304 ASD cases; 259 typically developing population controls); CHARGE study | Proximity of home to nearest freeway. Location: California | Higher risk for those residing <309 m from nearest freeway. No association with living near other major roads | Confirmation of cases and controls developmental status. Adjustment for child sex and race/ethnicity, maternal age and smoking, parental education. Exposure surrogate (proximity to freeway) was for residence at time of delivery | Exposure is global measure of traffic-related air pollution. Individual pollutants therefore not analysed | |
| Volk et al., 201393 | Case-control (279 ASD cases; 245 typically developing population controls); CHARGE study | Estimated levels of NO2, PM2.5 and PM10 at geocoded home address by time period (gestation and first year of life) from LINE-1 dispersion model and regional air monitoring programmes. Location: California | NO2, PM2.5 and PM10 each associated with approximate doubling of risk from gestational exposures; somewhat greater risk from 1st year of life exposures. Both traffic-related and regional pollutant estimates were robust when one was adjusted for a different pollutant in other group | Same as above, plus exposure measure provides more specific information based on a validated model of traffic pollutant dispersion, and regional pollutant measurements. Analysed pairs of a traffic-related air pollutant estimate and a regional pollutant estimate in same model | Because of correlations across individual pollutants, had limited power to tease apart specific pollutant actors (i.e. among traffic-related; or among regional pollutants) | |
| Particles, ozone, NOx (continued) | ||||||
| Becerra et al., 201388 | Nested case-control (7603 cases, 76 782 population controls with no known ASD diagnosis) | Estimated daily NO2, PM2.5, PM10 levels at geocoded home address based on monitoring data and land use regression. Location: Los Angeles County | Ozone from monitoring and NO2 estimated from land-use regression were associated with elevated risk in single and two-pollutant models. Similarly for ozone and PM2.5, both estimated from air monitoring | Very large population-based study. Adjusted for maternal age, education, race/ethnicity, birthplace, parity, insurance type and child’s gestational age at delivery and birth year | Exposure estimates were in different units and hence results cannot be compared quantitatively with other studies | |
| Endocrine disrupting chemicals (EDCs) | ||||||
| Phthalates | ||||||
| Larsson et al., 200951 | Cohort (n = 4779, including 79 cases); Dampness in Buildings and Health Study. | Maternal report of household flooring material via questionnaire when children were 1-3 years of age. | Association of ASD with vinyl flooring in bedrooms, a major indoor contributor to phthalates. | Unbiased, population-based sampling; exposure reporting unlikely to be influenced by case status. | Retrospective reporting of household flooring type; inclusion of Tourette’s syndrome in case definition; all information maternally-reported rather than measured. | Suggestive risk for ASD, but further studies needed, with appropriate attention to timing of exposure information. Need to consider the possibility that different phthalates may have different effects, and hence multiple sources for human exposures. |
| Miodovnik et al., 2011150 | Cohort (n = 404 with continuous measure on the Social Responsiveness Scales (SRS)) | Maternal 3rd trimester urine samples. | Low molecular weight phthalate metabolite concentrations associated with poorer Social Responsiveness Scale scores (Social Cognition, Communication, Awareness domains). | Measurement of phthalates during pregnancy, which thereby accounts for multiple sources of the low molecular weight compounds. | Outcomes assessed were continuous scales (SRS domain scores) rather than ASD diagnosis. Single measure-ments of phthalates may not be representative of long-term exposures. | |
| PCBs | ||||||
| Cheslack-Postava et al., 2013101 | Pilot nested case-control (75 each) | Archived maternal pregnancy serum samples | No significant associations, though elevated OR (1.91) for sum of PCBs above 90th percentile of control levels | Well-defined study population, use of archived samples with pregnancy-period exposure assessment | Small sample size/lack of power. | Insufficient to draw conclusions on risk for ASD. |
| Pesticides | ||||||
| Organophosphates (OP's) | ||||||
| Rauh et al., 2006111 | Prospective cohort (n = 228 with continuous measure of symptoms of Pervasive Developmental Delay; <5% PDD cases) | Measures of chlorpyrifos in plasma (cord or maternal) | Highest chlorpyrifos exposure group had greater risk for PDD as defined by scores on Child Behavior Checklist (CBCL). | Measured concentrations at time of delivery; adjusted for wide range of potential confounders, including gestational age, race/ethnicity, sex, maternal education and IQ, and HOME score | Relatively small sample. Chlorpyrifos levels were below the limit of detection for 1/3 of samples. PDD not based on ADI-R, ADOS or clinician judgment, the accepted gold standards | Available studies suggest potential association between an organophosphate pesticide and ASD or related symptoms. Error in studies to date likely to be nondifferential. However, use of one or two isolated measurements may not provide valid surrogates for overall prenatal or infant exposures. Further research with confirmation of diagnoses using gold standard protocols and better measures of individual-level exposures over time is needed |
| Eskenazi et al., 2007110 | CHAMACOS prospective cohort (n = 355 with continuous measure of symptoms of Pervasive Developmental Delay; 51 PDD cases) | Prenatal and child organophosphate urinary metabolite levels | Prenatal and postnatal dialkylphosphate (DAP) metabolites associated with more than 2-fold higher risk for PDD as defined by scores on Child Behavior Checklist | Measured levels at two time points; prospective exposure information available; adjusted for sex, age, breastfeeding duration, parity, income, maternal IQ and HOME score | PDD not based on gold standard. High prevalence of PDD calls into question validity of instrument in this linguistic/cultural group | |
| Roberts E. et al., 2007109 | Case-control (n = 465 cases and 6975 matched controls) | Proximity to agricultural applications of organophosphates. | ASD community diagnosis modestly associated with organophosphate applications within 250 m, during gestation | Strong data linkage methods and localization of pesticide applications both geographically and temporally, relatively large n | Potential exposure misclassification due to address changes, time away from home, and drift due to wind conditions. | |
| Other pesticides | ||||||
| Roberts E. et al., 2007109 | (see above) | Proximity to agricultural applications of organochlorine insecticides, or pyrethroid insecticides | ASD community diagnosis strongly associated with residential proximity to organochlorine applications during 1st trimester, and moderately for the pyrethroid, bifenthrin, during the overall gestation | (see above). Additionally, organochlorine analyses adjusted for multiple tests | (see above) | Analyses from one report suggesting a strong association of ASD with organochlorines and a moderate one with a pyrethroid require confirmation in independent samples, preferably with gold standard diagnoses |
Air pollution
A growing body of research has emerged on air pollution, or proxies for it, in relation to ASD.88–94 Though methods of exposure assessment and definition have varied across studies, many reports have linked household addresses to US Environmental Protection Agency (EPA) models to derive exposure information. Most studies have suggested modest increases in risk of ASD, with odds ratios (ORs) around 1.5 to 2, for those individuals with higher estimated exposure to air pollution. The first such analysis (n = 284 cases and 657 controls), conducted in California, examined 19 hazardous air pollutants (HAPs) from US EPA-derived census tract-based estimates.95 Elevated risk for ASD was found in adjusted analyses of the top quartile of exposure to chlorinated solvents, heavy metals, diesel particles and other individual compounds. A limitation, however, was that exposures were based on modelled estimates derived for residences 2 years after the birth, and thus may not have adequately captured prenatal exposure.
The first investigation not restricted to one region of the country was an analysis of Nurses’ Health Study II, including 325 maternally-reported cases and 22 000 controls from across the USA. These authors also found significantly elevated risk of ASD from air pollutants,91 using US EPA-modelled levels of HAPs for the year of birth. ORs were remarkably similar to the Windham et al. study, ranging from 1.5 (overall metals) to 2.0 (diesel and mercury), and with statistically significant positive linear trends. In addition, associations were stronger for male children for most pollutants. A study of children from North Carolina and West Virginia also found elevated risk of ASD for numerous air pollutants, but not after adjusting for demographic factors.89 However, variability in pollutant concentrations was low, and the referent group consisted of children with speech and language impairments, a group which could be linked to the same exposures.
Volk and colleagues conducted two studies on the topic, using data from a large population-based case control study in California, the CHildhood Autism Risks from Genetics and the Environment (CHARGE) study.96 The first of these (n = 304 cases and 259 controls) examined proximity to a major freeway as a proxy for air pollution.92 Residence within 309 meters (m) of a freeway at time of delivery (as compared with >1419 m) was linked, in analyses adjusted for sociodemographics, with nearly a doubling in odds of having a child with ASD. This association strengthened somewhat when examining third-trimester exposure. Intermediate distances were not associated with ASD, nor was proximity to smaller roadways, which is consistent with the established high concentration of pollutants near major freeways and a decline in particulate matter to background levels beyond 300 m from a major freeway.97 In further analyses, these authors examined specific traffic-related air pollutants using EPA models (similar to previous studies), but with improved specificity on timing of exposure.93 Risk was highest with gestational and first year of life exposures for overall traffic-related air pollution, nitrogen dioxide and particulate matter less than 2.5 and less than10 μm in diameter (PM 2.5 and PM 10).
The largest study to date of air pollution and ASD (n = 7603 cases and 10 matched controls per case) was conducted in Los Angeles County and used geocoded residences at the time of delivery linked to air pollutant monitoring data supplemented with land-use regression data for nitrogen dioxide (NO2) and nitrogen monoxide (NO).88 Risk for ASD was elevated for higher estimated ozone and NO2 exposure during the entire pregnancy, in models adjusted for a range of sociodemographic confounders. Associations increased when two-pollutant models were used, and for those in the lowest educational stratum.
Thus, a rapidly growing literature suggests potential associations between air pollutants and ASD risk. Because air pollution is a highly complex and variable mixture of different compounds that are highly correlated, determining the impact of specific chemicals is difficult. Additionally, although most analyses adjusted for sociodemographic factors such as parental education, socioeconomic status (SES) or income, race/ethnicity and/or parental age, residual confounding could still be present, particularly by SES factors that influence the likelihood of a diagnosis. Confounding by other factors that may affect child outcomes, e.g. noise pollution through sleep disturbances, should also be considered. Bias could also be introduced by misclassification or missingness of exposure assessments. Nevertheless, a number of potential biological pathways might explain observed associations, including direct effects on CNS development, oxidative stress, dysregulation of repair mechanisms or compromise of the blood-brain barrier. Animal studies showing alterations in blood-brain barrier signalling following exposure to diesel particulates,98 and alterations in neural circuitry and behavioural deficits following prenatal exposure to HAPs,99,100 lend support to these hypothesized pathways.
Persistent organic pollutants
Since the 1960s, a number of organic compounds have been identified that are persistent, widely distributed throughout the environment and toxic to both wildlife and human health. These compounds, POPs (Persistent Organic Pollutants), targeted for reduction and/or elimination (http://chm.pops.int/Convention/ConventionText/tabid/2232/Default.aspx), include certain pesticides, industrial chemicals and by-products of industrial processes. Some POPs have demonstrated adverse effects on neurodevelopment.
Several POPs are endocrine-disrupting chemicals (EDCs). One such class of chemicals is polychlorinated biphenyls (PCBs). These have only been studied in association with ASD in one investigation to date.101 This small pilot investigation found a non-significant elevation of odds for ASD in association with measured concentrations of PCBs from maternal samples taken at delivery (Table 3).
Despite little research, mechanistic considerations suggest the potential importance of EDCs. The fetus depends on maternal thyroid hormones (T3 and T4) during early gestation, as they regulate neuronal growth, cell migration and differentiation in the hippocampus, cerebral cortex and cerebellum.102 The only human study on ASD and thyroid hormones found an association with very low levels at birth.103 In mouse models, changes in thyroid hormone levels affected dendritic development of Purkinje cells,104 notable given Purkinje cell loss is one of the most highly replicated findings in ASD.105 For these reasons, disruption of thyroid hormones during pregnancy has been hypothesized as a potential aetiological pathway for autism.106 The strong male:female ratio in autism of over four has been hypothesized to indicate that steroid hormones or their targets107 or early sexual differentiation of the brain might play a role in aetiology, and both testosterone and estradiol influence fetal brain development. EDCs may also act in combination with genes to influence ASD risk.
Some, but not all, of the pesticides discussed below are classified as POPs.
Pesticides
Pesticides are typically designed to damage the nervous systems of the targeted species, and often act on neurotransmission. Several pesticides have been classified as POPs (http://chm.pops.int/Convention/ThePOPs/tabid/673/Default.aspx) and/or have endocrine-disrupting properties.108 Western countries without endemic malaria have reduced use of most organochlorine pesticides, due to their persistence and adverse effects on wildlife, but some continue to be used for other purposes, such as head lice. In one study, ASD risk was elevated in children from households located near agricultural applications of organochlorines during the first trimester.109
Organophosphate insecticides, which degrade rapidly, replaced organochlorines for many uses, and have been widely applied both agriculturally and residentially, though production of residential products was banned by the US EPA in 2001. In a cohort study conducted in a farm worker community, poorer scores on a subscale of the Child Behavior Checklist measuring symptoms for pervasive developmental disorder (PDD) were associated with higher levels of organophosphate pesticide metabolites in urine samples from pregnancy.110 Similarly, higher umbilical cord blood plasma concentrations of a common orgaphophosphate, chlorpyrifos, were also associated with PDD symptoms in early childhood in a New York City cohort.111 Other investigations found organophosphates related to deficits in motor coordination, visuospatial performance and memory,112 and decrements in cognitive development,113 with evidence that a gene involved in metabolizing these chemicals, paraoxonase 1 (PON1), may be a susceptibility factor. Moreover, a neuroimaging study by Rauh and colleagues demonstrated that children with higher chlorpyrifos concentrations had altered volume in cortical regions of the brain that have been associated with attention, receptive language processing, social cognition and inhibitory control.114
Since the ban on organophosphates for household products, other pesticides have become increasingly common115 but have not been reported in relation to ASD. As of 2009, over 3500 products registered in the USA contained synthetic pyrethroids or their naturally derived counterparts, pyrethrins.116 Despite relatively short half-lives in humans, pyrethroid metabolites were found in over 70% of adults in the USA,117 reflecting common usage and ubiquitous presence in household products. Other pesticides of concern include imidacloprid and fipronil, both used in insecticide gels, flea sprays and other products to eliminate pests. These products might influence neurodevelopment through a variety of mechanisms (reviewed by Shelton and colleagues118): interference with establishment of serotonergic systems; altered monoamine oxidase or acetylcholinesterase activity or GABA function; reduced expression of GABA receptors; mitochondrial dysfunction; and endocrine disruption.106 Pesticides may also affect calcium signalling.119,120
Non-persistent organic pollutants
Not all compounds deleterious to neurodevelopment are persistent; some are short-lived. Phthalates, a class of EDC that may influence ASD, are used in cosmetics, lotions, fragrances and building materials, and have anti-androgenic properties.121 In children, prospective research found associations of some phthalate metabolites with body size,122 conduct disorder or attention problems ,123 and social deficits.124 In particular, phthalate metabolites measured in third-trimester maternal urine were associated with poorer scores on several subscales of the Social Responsiveness Scale.124 Also, a recent Swedish study investigating indoor environmental factors found an unexpected doubling of risk for ASD in children whose homes had vinyl (PVC) flooring in the child and parent bedrooms;51 PVC flooring is a significant source of airborne phthalates. Both BPA125 and phthalates126 have also been linked to thyroid dysfunction, discussed above. Continued investigation of these short-lived endocrine-disrupting chemicals in association with neurodevelopment and autism is needed.
Suggested chemicals for future research
Above, we have highlighted environmental chemicals and dietary factors for which little research has yet been conducted, despite animal or in vitro evidence supporting biological plausibility. Here we mention a few others that deserve attention. Although PCBs have shown associations with cognitive impairment or behavioural problems,127,128 other candidate EDCs include dioxins, polybrominated diphenyl ethers (PBDEs),129 bisphenol A (BPA) and perfluorinated compounds. These have demonstrated a wide range of toxic effects. For instance, PBDEs (used as flame-retardants in textiles, including children’s clothing, and plastic cases for electronics) easily cross the placenta130 and produce hyperactivity and altered motor behaviour and development in rodents131,132 and in humans, deficits in mental and psychomotor development133 and increased attention problems at school age. Highest levels are found in the youngest individuals.134 Perinatal treatment of Mecp2 mice with one of the most abundant PBDEs, BDE-47, resulted in hypomethylation of adult brain DNA, with accompanying reduced sociability that was independent of genotype.135 This demonstration of perinatal PBDE effects on social behaviours in genetically predisposed mice suggests potential relevance to ASD in humans carrying certain genetic susceptibilities. To date, little research has addressed the influence of specific environmental factors on epigenetics, on gene expression relevant to neurodevelopment, or in genetically susceptible subsets of the population.
As another example, BPA, a plasticizer found in products including plastic food wrappings, can linings, plastic drink and baby bottles, is a non-persistent organic pollutant with estrogenic properties, and has been linked to obesity136 and diabetes137 which, in pregnant women, have been associated with increased risk for ASD in offspring.138 A prospective study found maternal BPA metabolites were associated with an increase in externalizing problem behaviours,139 suggesting relevance to ASD and need for investigation.
Summary and Future Directions
Recently, considerable progress has been made in uncovering clues about environmental contributions to autism. Rapidly emerging evidence supports potential roles for preconception or prenatal maternal nutrition, lifestyle and exposures to environmental chemicals found in air pollution or pesticides. Other environmental factors acting during this period but not covered in this article, including infections, medications and pregnancy complications, also have considerable support for an association with ASD. It is clear that there is no single or universal cause of autism; rather, many environmental and genetic factors are likely involved, and the specific subsets of factors that are operating will vary across different individuals. Evidence regarding a protective association of maternal nutrition with ASD, particularly folic acid, is strong, and appears unlikely to be explained by confounding. The literature on air pollution shows remarkable consistency, though measurement accuracy and possible residual confounding need to be addressed; also, the lack of solid links with cigarette smoking raises questions about the coherence of this work, given similar constituents in the two. At present, a few studies support a potential role for organophosphate pesticides in relation to ASD, as well as phthalate exposures. For most other modifiable environmental factors in the periconception and prenatal periods, the literature is inconsistent and/or of insufficient quality and quantity.
As noted, many of the factors reviewed here have associations with a broader class of neurodevelopmental or psychiatric conditions, and therefore may not be unique risk factors for autism. Genetic factors or critical time periods may influence how these xenobiotics or non-inherited conditions alter brain connectivity and determine whether the exposure results in autism as opposed to other deficits. Large gene-by-environment studies are one approach to capture the complexity of this disorder. Other research gaps include determination of critical aetiological windows for environmental exposures and how these vary by type of exposure (see Figure 1), along with continued investigation into maternal nutritional, obstetric, metabolic and other factors during the pre-conception, prenatal and perinatal periods, and disentangling the role of maternal and paternal influences. Although the preconception and prenatal periods likely have the strongest impact, continued plasticity of the central nervous system implies that further insults or protective factors in the first year or two of life may also contribute to the phenotypic development of the child and concomitant risk for ASD.
Funding
This work was supported by the following grants: National Institutes of Health (NIH) R01-ES015359, NIH R01-ES020392, NIH P01 ES11269, NIH K12HD051958 and U.S. Environmental Protection Agency (EPA) STAR #R829388 & R833292. The authors have no financial relationships relevant to this article.
Conflict of interest: None declared.
References
- 1.Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry 1977;18:297–321 [DOI] [PubMed] [Google Scholar]
- 2.Steffenburg S, Gillberg C, Hellgren L, et al. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry 1989;30:405–16 [DOI] [PubMed] [Google Scholar]
- 3.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011;68:1095–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med 1988;318:1349–54 [DOI] [PubMed] [Google Scholar]
- 5.Herbert MR, Ziegler DA, Deutsch CK, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain 2005;128(Pt 1):213–26 [DOI] [PubMed] [Google Scholar]
- 6.Hultman CM, Sparen P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology 2002;13:417–23 [DOI] [PubMed] [Google Scholar]
- 7.Larsson HJ, Eaton WW, Madsen KM, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol 2005;161:916–25; discussion 26–28 [DOI] [PubMed] [Google Scholar]
- 8.Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol 1996;370:247–61 [DOI] [PubMed] [Google Scholar]
- 9.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci 2003;23:297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert KJ, Miller LL, Joinson CJ. Association of autistic spectrum disorder with season of birth and conception in a UK cohort. Autism Res 2010;3:185–90 [DOI] [PubMed] [Google Scholar]
- 11.Kolevzon A, Weiser M, Gross R, et al. Effects of season of birth on autism spectrum disorders: fact or fiction? Am J Psychiatry 2006;163:1288–90 [DOI] [PubMed] [Google Scholar]
- 12.Zerbo O, Iosif AM, Delwiche L, Walker C, Hertz-Picciotto I. Month of conception and risk of autism. Epidemiology 2011;22:469–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheslack-Postava K, Liu K, Bearman PS. Closely spaced pregnancies are associated with increased odds of autism in California sibling births. Pediatrics 2011;127:246–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodds L, Fell DB, Shea S, Armson BA, Allen AC, Bryson S. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord 2011;41:891–902 [DOI] [PubMed] [Google Scholar]
- 15.Smits LJ, Essed GG. Short interpregnancy intervals and unfavourable pregnancy outcome: role of folate depletion. Lancet 2001;358:2074–77 [DOI] [PubMed] [Google Scholar]
- 16.van Eijsden M, Smits LJ, van der Wal MF, Bonsel GJ. Association between short interpregnancy intervals and term birth weight: the role of folate depletion. Am J Clin Nutr 2008;88:147–53 [DOI] [PubMed] [Google Scholar]
- 17.O'Rourke KM, Redlinger TE, Waller DK. Declining levels of erythrocyte folate during the postpartum period among Hispanic women living on the Texas-Mexico border. J Womens Health Gend Based Med 2000;9:397–403 [DOI] [PubMed] [Google Scholar]
- 18.Susser E, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry 1996;53:25–31 [DOI] [PubMed] [Google Scholar]
- 19.Brown AS, Susser ES. Sex differences in prevalence of congenital neural defects after periconceptional famine exposure. Epidemiology 1997;8:55–58 [DOI] [PubMed] [Google Scholar]
- 20.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–35 [DOI] [PubMed] [Google Scholar]
- 21.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr 2003;23:41–58 [DOI] [PubMed] [Google Scholar]
- 22.Black MM. Micronutrient deficiencies and cognitive functioning. J Nutr 2003;133(11 Suppl 2):3927S–31S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Black MM. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr Bull 2008;29(Suppl 2):S126–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute of Medicine. Nutrition During Pregnancy 1990. Washington, DC: National Academy Press, 1990 [Google Scholar]
- 25.Picciano MF. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr 2003;133:1997S–2002S [DOI] [PubMed] [Google Scholar]
- 26.Schmidt RJ, Hansen RL, Hartiala J, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 2011;22:476–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr 2012;96:80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline . Washington, DC: National Academy Press, 2000 [PubMed] [Google Scholar]
- 29.Suren P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA 2013;309:570–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Julvez J, Fortuny J, Mendez M, Torrent M, Ribas-Fito N, Sunyer J. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr Perinat Epidemiol 2009;23:199–206 [DOI] [PubMed] [Google Scholar]
- 31.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 1991;338:131–37 [PubMed] [Google Scholar]
- 32.Roza SJ, van Batenburg-Eddes T, Steegers EA, et al. Maternal folic acid supplement use in early pregnancy and child behavioural problems: The Generation R Study. Br J Nutr 2010;103:445–52 [DOI] [PubMed] [Google Scholar]
- 33.Schlotz W, Jones A, Phillips DI, Gale CR, Robinson SM, Godfrey KM. Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J Child Psychol Psychiatry 2010;51:594–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth C, Magnus P, Schjolberg S, et al. Folic acid supplements in pregnancy and severe language delay in children. JAMA 2011;306:1566–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reik W, Walter J. Genomic imprinting: parental influence on the genome. Nat Rev Genet 2001;2:21–32 [DOI] [PubMed] [Google Scholar]
- 36.James SJ, Melnyk S, Jernigan S, et al. A functional polymorphism in the reduced folate carrier gene and DNA hypomethylation in mothers of children with autism. Am J Med Genet B Neuropsychiatr Genet 2010;153B:1209–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet 2006;15:R138–50 [DOI] [PubMed] [Google Scholar]
- 38.Oken E, Bellinger DC. Fish consumption, methylmercury and child neurodevelopment. Curr Opin Pediatr 2008;20:178–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyall K, Munger KL, O'Reilly EJ, Santangelo SL, Ascherio A. Maternal dietary fat intake in association with autism spectrum disorders. Am J Epidemiol 2013;178:209–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids 2006;75:329–49 [DOI] [PubMed] [Google Scholar]
- 41.Rombaldi Bernardi J, de Souza Escobar R, Ferreira CF, Pelufo Silveira P. Fetal and neonatal levels of omega-3: effects on neurodevelopment, nutrition, and growth. ScientificWorldJournal 2012;2012:202473 Epub 2012/11/06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grant WB, Soles CM. Epidemiologic evidence supporting the role of maternal vitamin D deficiency as a risk factor for the development of infantile autism. Dermato-endocrinology 2009;1:223–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dealberto MJ. Prevalence of autism according to maternal immigrant status and ethnic origin. Acta Psychiatr Scand 2011;123:339–48 [DOI] [PubMed] [Google Scholar]
- 44.Bolton P, Pickles A, Harrington R, Macdonald H, Rutter M. Season of birth: issues, approaches and findings for autism. J Child Psychol Psychiatry 1992;33:509–30 [DOI] [PubMed] [Google Scholar]
- 45.Fernell E, Barnevik-Olsson M, Bagenholm G, Gillberg C, Gustafsson S, Saaf M. Serum levels of 25-hydroxyvitamin D in mothers of Swedish and of Somali origin who have children with and without autism. Acta Paediatr 2010;99:743–4 [DOI] [PubMed] [Google Scholar]
- 46.Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Hart PH, Kusel MM. Maternal vitamin D levels and the autism phenotype among offspring. J Autism Dev Disord 2013;43:1495–504 [DOI] [PubMed] [Google Scholar]
- 47.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience 2003;118:641–53 [DOI] [PubMed] [Google Scholar]
- 48.Hvidtjorn D, Grove J, Schendel D, et al. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. J Epidemiol Community Health 2011;65:497–502 [DOI] [PubMed] [Google Scholar]
- 49.Williams G, Oliver JM, Allard A, Sears L. Autism and associated medical and familial factors: a case control study. J Dev Phys Disabil 2003;15:335–49 [Google Scholar]
- 50.Indredavik MS, Brubakk AM, Romundstad P, Vik T. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr 2007;96:377–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6-8 years of age. Neurotoxicology 2009;30:822–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can 2010;30:125–34 [PubMed] [Google Scholar]
- 53.Maimburg RD, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatr Scand 2006;114:257–64 [DOI] [PubMed] [Google Scholar]
- 54.Lee BK, Gardner RM, Dal H, et al. Brief report: maternal smoking during pregnancy and autism spectrum disorders. J Autism Dev Disord 2012;42:2000–05 [DOI] [PubMed] [Google Scholar]
- 55.Kalkbrenner AE, Braun JM, Durkin MS, et al. Maternal smoking during pregnancy and the prevalence of autism spectrum disorders, using data from the autism and developmental disabilities monitoring network. Environ Health Perspect 2012;120:1042–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Visser JC, Rommelse N, Vink L, et al. Narrowly versus broadly defined autism spectrum disorders: differences in pre- and perinatal risk factors. J Autism Dev Disord 2013;43:1505–16 [DOI] [PubMed] [Google Scholar]
- 57.Land TG, Landau AS, Manning SE, et al. Who underreports smoking on birth records: a Monte Carlo predictive model with validation. PloS One 2012;7:e34853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tran PL, Lehti V, Lampi KM, et al. Smoking during pregnancy and risk of autism spectrum disorder in a Finnish National Birth Cohort. Paediatr Perinat Epidemiol 2013;27:266–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albuquerque CA, Smith KR, Johnson C, Chao R, Harding R. Influence of maternal tobacco smoking during pregnancy on uterine, umbilical and fetal cerebral artery blood flows. Early Hum Dev 2004;80:31–42 [DOI] [PubMed] [Google Scholar]
- 60.Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther 1985;8:384–95 [DOI] [PubMed] [Google Scholar]
- 61.Williams GM, O'Callaghan M, Najman JM, et al. Maternal cigarette smoking and child psychiatric morbidity: a longitudinal study. Pediatrics 1998;102:e11. [DOI] [PubMed] [Google Scholar]
- 62.Muneoka K, Ogawa T, Kamei K, Mimura Y, Kato H, Takigawa M. Nicotine exposure during pregnancy is a factor which influences serotonin transporter density in the rat brain. Eur J Pharmacol 2001;411:279–82 [DOI] [PubMed] [Google Scholar]
- 63.Roy TS, Sabherwal U. Effects of gestational nicotine exposure on hippocampal morphology. Neurotoxicol Teratol 1998;20:465–73 [DOI] [PubMed] [Google Scholar]
- 64.James WH. Potential explanation of the reported association between maternal smoking and autism. Environ Health Perspect 2013;121:a42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Arch Gen Psychiatry 1998;55:721–27 [DOI] [PubMed] [Google Scholar]
- 66.Button TM, Maughan B, McGuffin P. The relationship of maternal smoking to psychological problems in the offspring. Early Hum Dev 2007;83:727–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Honein MA, Paulozzi LJ, Moore CA. Family history, maternal smoking, and clubfoot: an indication of a gene-environment interaction. Am J Epidemiol 2000;152:658–65 [DOI] [PubMed] [Google Scholar]
- 68.van Rooij IA, Wegerif MJ, Roelofs HM, et al. Smoking, genetic polymorphisms in biotransformation enzymes, and nonsyndromic oral clefting: a gene-environment interaction. Epidemiology 2001;12:502–07 [DOI] [PubMed] [Google Scholar]
- 69.Eliasen M, Tolstrup JS, Nybo Andersen AM, Gronbaek M, Olsen J, Strandberg-Larsen K. Prenatal alcohol exposure and autistic spectrum disorders—a populatio n-based prospective study of 80,552 children and their mothers. Int J Epidemiol 2010;39:1074–81 [DOI] [PubMed] [Google Scholar]
- 70.Jacobson JL, Jacobson SW. Effects of prenatal alcohol exposure on child development. Alcohol Res Health 2002;26:282–86 [PMC free article] [PubMed] [Google Scholar]
- 71.Lebel C, Mattson SN, Riley EP, et al. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci 2012;32:15243–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Middleton FA, Varlinskaya EI, Mooney SM. Molecular substrates of social avoidance seen following prenatal ethanol exposure and its reversal by social enrichment. Dev Neurosci 2012;34:115–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Casanova MF. The neuropathology of autism. Brain Pathol 2007;17:422–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miles JH, Takahashi TN, Haber A, Hadden L. Autism families with a high incidence of alcoholism. J Autism Dev Disord 2003;33:403–15 [DOI] [PubMed] [Google Scholar]
- 75.Daniels JL, Forssen U, Hultman CM, et al. Parental psychiatric disorders associated with autism spectrum disorders in the offspring. Pediatrics.2008;121:e1357–62 [DOI] [PubMed] [Google Scholar]
- 76.Bolton PF, Pickles A, Murphy M, Rutter M. Autism, affective and other psychiatric disorders: patterns of familial aggregation. Psychol Med 1998;28:385–95 [DOI] [PubMed] [Google Scholar]
- 77.DeLong GR, Dwyer JT. Correlation of family history with specific autistic subgroups: Asperger's syndrome and bipolar affective disease. J Autism Dev Disord 1988;18:593–600 [DOI] [PubMed] [Google Scholar]
- 78.Lobascher ME, Kingerlee PE, Gubbay SS. Childhood autism: an investigation of aetiological factors in twenty-five cases. Br J Psychiatry 1970;117:525–29 [DOI] [PubMed] [Google Scholar]
- 79.Piven J, Chase GA, Landa R, et al. Psychiatric disorders in the parents of autistic individuals. J Am Acad Child Adolesc Psychiatry 1991;30:471–78 [DOI] [PubMed] [Google Scholar]
- 80.Irner TB. Substance exposure in utero and developmental consequences in adolescence: a systematic review. Child Neuropsychol 2012;18:521–49 [DOI] [PubMed] [Google Scholar]
- 81.Jutras-Aswad D, DiNieri JA, Harkany T, Hurd YL. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur Arch Psychiatry Clin Neurosci 2009;259:395–412 [DOI] [PubMed] [Google Scholar]
- 82.Liu J, Lester BM, Neyzi N, et al. Regional brain morphometry and impulsivity in adolescents following prenatal exposure to cocaine and tobacco. JAMA Pediatr 2013;167:348–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christensen J, Gronborg TK, Sorensen MJ, et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013;309:1696–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–12 [DOI] [PubMed] [Google Scholar]
- 85.Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry 2009;195:7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda ML. Association of autism with induced or augmented childbirth in north Carolina birth record (1990-1998) and education research (1997-2007) databases. JAMA Pediatr 2013;167:959–66 [DOI] [PubMed] [Google Scholar]
- 87.Landrigan PJ, Garg A. Chronic effects of toxic environmental exposures on children's health. J Toxicol Clin Toxicol 2002;40:449–56 [DOI] [PubMed] [Google Scholar]
- 88.Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect 2013;121:380–86 Epub 2012/12/20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology 2010;21:631–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palmer RF, Blanchard S, Wood R. Proximity to point sources of environmental mercury release as a predictor of autism prevalence. Health Place 2009;15:18–24 [DOI] [PubMed] [Google Scholar]
- 91.Roberts A, Lyall K, Hart JE, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect 2013;121:978–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect 2011;119:873–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry 2013;70:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environ Health Perspect 2006;114:1438–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Windham G, Zhang L, Gunier R, Croen L, Grether J. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environ Health Perspect 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect 2006;114:1119–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc 2002;52:1032–42 [DOI] [PubMed] [Google Scholar]
- 98.Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J 2008;22:2723–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bouayed J, Desor F, Rammal H, et al. Effects of lactational exposure to benzo[alpha]pyrene (B[alpha]P) on postnatal neurodevelopment, neuronal receptor gene expression and behaviour in mice. Toxicology 2009;259:97–106 [DOI] [PubMed] [Google Scholar]
- 100.Brown LA, Khousbouei H, Goodwin JS, et al. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology 2007;28:965–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheslack-Postava K, Rantakokko PV, Hinkka-Yli-Salomaki S, et al. Maternal serum persistent organic pollutants in the Finnish Prenatal Study of Autism: A pilot study. Neurotoxicol Teratol 2013;38:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol 2012;355:240–48 [DOI] [PubMed] [Google Scholar]
- 103.Hoshiko S, Grether JK, Windham GC, Smith D, Fessel K. Are thyroid hormone concentrations at birth associated with subsequent autism diagnosis? Autism Res 2011;4:456–63 [DOI] [PubMed] [Google Scholar]
- 104.Kimura-Kuroda J, Nagata I, Kuroda Y. Disrupting effects of hydroxy-polychlorinated biphenyl (PCB) congeners on neuronal development of cerebellar Purkinje cells: a possible causal factor for developmental brain disorders? Chemosphere 2007;67:S412–20 [DOI] [PubMed] [Google Scholar]
- 105.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci .2008;31:137–45 [DOI] [PubMed] [Google Scholar]
- 106.Roman GC. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J Neurol Sci 2007;262:15–26 [DOI] [PubMed] [Google Scholar]
- 107.Hu VW. Is retinoic acid-related orphan receptor-alpha (RORA) a target for gene-environment interactions contributing to autism? Neurotoxicology 2012;33:1434–35 [DOI] [PubMed] [Google Scholar]
- 108.Korrick SA, Sagiv SK. Polychlorinated biphenyls, organochlorine pesticides and neurodevelopment. Curr Opin Pediatr 2008;20:198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect 2007;115:1482–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eskenazi B, Marks AR, Bradman A, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect 2007;115:792–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rauh VA, Garfinkel R, Perera FP, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 2006;118:e1845–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harari R, Julvez J, Murata K, et al. Neurobehavioral deficits and increased blood pressure in school-age children prenatally exposed to pesticides. Environ Health Perspect 2010;118:890–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Engel SM, Wetmur J, Chen J, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect 2011;119:1182–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rauh VA, Perera FP, Horton MK, et al. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc Natl Acad Sci U S A 2012;109:7871–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Williams MK, Rundle A, Holmes D, et al. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000-2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ Health Perspect 2008;116:1681–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.U.S. Environmental Protection Agency. Reevaluation: Pyrethrins and Pyrethroids. 2010. http://www.epa.gov/oppsrrd1/reevaluation/pyrethroids-pyrethrins.html [Google Scholar]
- 117.Barr DB, Olsson AO, Wong LY, et al. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and Nutrition Examination Survey 1999-2002. Environ Health Perspect 2010;118:742–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shelton JF, Hertz-Picciotto I, Pessah IN. Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environ Health Perspect 2012;120:944–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lawrence LJ, Casida JE. Stereospecific action of pyrethroid insecticides on the gamma-aminobutyric acid receptor-ionophore complex. Science 1983;221:1399–401 [DOI] [PubMed] [Google Scholar]
- 120.Malaviya M, Husain R, Seth PK. Perinatal effects of two pyrethroid insecticides on brain neurotransmitter function in the neonatal rat. Vet Hum Toxicol 1993;35:119–22 [PubMed] [Google Scholar]
- 121.Sharpe RM. “Additional” effects of phthalate mixtures on fetal testosterone production. Toxicol Sci 2008;105:1–4 [DOI] [PubMed] [Google Scholar]
- 122.Teitelbaum SL, Mervish N, Moshier EL, et al. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environ Res 2012;112:186–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Engel SM, Miodovnik A, Canfield RL, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect 2010;118:565–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miodovnik A, Engel SM, Zhu CB, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011;32:261–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chevrier J, Gunier RB, Bradman A, et al. Maternal urinary bisphenol A during pregnancy and maternal and neonatal thyroid function in the CHAMACOS Study. Environ Health Perspect 2013;121:138–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ Health Perspect 2011;119:1396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Newman J, Gallo MV, Schell LM, DeCaprio AP, Denham M, Deane GD. Analysis of PCB congeners related to cognitive functioning in adolescents. Neurotoxicology 2009;30:686–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Park HY, Park JS, Sovcikova E, et al. Exposure to hydroxylated polychlorinated biphenyls (OH-PCBs) in the prenatal period and subsequent neurodevelopment in eastern Slovakia. Environ Health Perspect 2009;117:1600–06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ren XM, Guo LH. Assessment of the binding of hydroxylated polybrominated diphenyl ethers to thyroid hormone transport proteins using a site-specific fluorescence probe. Environ Sci Technol 2012;46:4633–40 [DOI] [PubMed] [Google Scholar]
- 130.Frederiksen M, Vorkamp K, Mathiesen L, Mose T, Knudsen LE. Placental transfer of the polybrominated diphenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: an experimental study. Environ Health 2010;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gee JR, Moser VC. Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol Teratol 2008;30:79–87 [DOI] [PubMed] [Google Scholar]
- 132.Suvorov A, Girard S, Lachapelle S, Abdelouahab N, Sebire G, Takser L. Perinatal exposure to low-dose BDE-47, an emergent environmental contaminant, causes hyperactivity in rat offspring. Neonatology 2009;95:203–09 [DOI] [PubMed] [Google Scholar]
- 133.Herbstman JB, Sjodin A, Kurzon M, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect 2010;118:712–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rose M, Bennett DH, Bergman A, Fangstrom B, Pessah IN, Hertz-Picciotto I. PBDEs in 2-5 year-old children from California and associations with diet and indoor environment. Environ Sci Technol 2010;44:2648–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Woods R, Vallero RO, Golub MS, et al. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2308 mutation. Hum Mol Genet 2012;21:2399–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA 2012;308:1113–21 [DOI] [PubMed] [Google Scholar]
- 137.Silver MK, O'Neill MS, Sowers MR, Park SK. Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003-2008. PloS One 2011;6:e26868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Krakowiak P, Walker CK, Bremer AA, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 2012;129:e1121–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Braun JM, Yolton K, Dietrich KN, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 2009;117:1945–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Atladottir HO, Thorsen P, Schendel DE, Ostergaard L, Lemcke S, Parner ET. Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: a Danish cohort study. Arch Pediatr Adolesc Med 2010;164:470–7 [DOI] [PubMed] [Google Scholar]
- 141.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 2009;204:313–21 [DOI] [PubMed] [Google Scholar]
- 142.Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) Study. J Autism Dev Disord 2013;43:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goines P, Van de Water J. The immune system's role in the biology of autism. Curr Opin Neurol 2010;23:111–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chess S. Autism in children with congenital rubella. J Autism Child Schizophr 1971;1:33–47 [DOI] [PubMed] [Google Scholar]
- 145.Wegiel J, Kuchna I, Nowicki K, et al. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol 2010;119:755–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hvidtjørn D, Grove J, Schendel D, Schieve LA, Sværke C, Ernst E, Thorsen P. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. 2011;65:497–502 [DOI] [PubMed] [Google Scholar]
- 147.Ronald A, Happe F, Dworzynski K, Bolton P, Plomin R. Exploring the relation between prenatal and neonatal complications and later autistic-like features in a representative community sample of twins. Child Dev 2010;81:166–82 [DOI] [PubMed] [Google Scholar]
- 148.Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics 2009;123:1293–300 [DOI] [PubMed] [Google Scholar]
- 149.Larsson HJ, Eaton WW, Madsen KM, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol 2005;161:916–25; discussion 926–28 [DOI] [PubMed] [Google Scholar]
- 150.Miodovnik A, Engel SM, Zhu C, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011;32:261–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Samuelsen GB, Larsen KB, Bogdanovic N, et al. The changing number of cells in the human fetal forebrain and its subdivisions: a stereological analysis. Cereb Cortex 2003;13:115–22 [DOI] [PubMed] [Google Scholar]
- 152.de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early Hum Dev 2006;82:257–66 [DOI] [PubMed] [Google Scholar]
- 153.Sidman RL, Rakic P. Neuronal migration, with special reference to developing human brain: a review. Brain Res 1973;62:1–35 [DOI] [PubMed] [Google Scholar]
- 154.Sarnat HB, Nochlin D, Born DE. Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev 1998;20:88–94 [DOI] [PubMed] [Google Scholar]
- 155.Schmidt RJ, Hansen RL, Hartiala J, et al. Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology 2011;22:476–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Arndt TL, Stodgell CJ, Rodier PM. The teratology of autism. Int J Dev Neurosci 2005;23:189–99 [DOI] [PubMed] [Google Scholar]
- 157.Atladottir HO, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics 2012;130:e1447–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stromland K, Nordin V, Miller M, Akerstrom B, Gillberg C. Autism in thalidomide embryopathy: a population study. Dev Med Child Neurol 1994;36:351–56 [DOI] [PubMed] [Google Scholar]
- 159.Moore SJ, Turnpenny P, Quinn A, et al. A clinical study of 57 children with fetal anticonvulsant syndromes. J Med Genet 2000;37:489–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ 2013;346:f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Beversdorf DQ, Manning SE, Hillier A, et al. Timing of prenatal stressors and autism. J Autism Dev Disord 2005;35:471–78 [DOI] [PubMed] [Google Scholar]