Abstract
αA-crystallin and αB-crystallin are members of the small heat shock protein family and function as molecular chaperones and major lens structural proteins. Although numerous studies have examined their chaperone-like activities in vitro, little is known about the proteins they protect in vivo. To elucidate the relationships between chaperone function, substrate binding, and human cataract formation, we used proteomic and mass spectrometric methods to analyze the effect of mutations associated with hereditary human cataract formation on protein abundance in αA-R49C and αB-R120G knock-in mutant lenses. Compared with age-matched wild type lenses, 2-day-old αA-R49C heterozygous lenses demonstrated the following: increased crosslinking (15-fold) and degradation (2.6-fold) of αA-crystallin; increased association between αA-crystallin and filensin, actin, or creatine kinase B; increased acidification of βB1-crystallin; increased levels of grifin; and an association between βA3/A1-crystallin and αA-crystallin. Homozygous αA-R49C mutant lenses exhibited increased associations between αA-crystallin and βB3-, βA4-, βA2-crystallins, and grifin, whereas levels of βB1-crystallin, gelsolin, and calpain 3 decreased. The amount of degraded glutamate dehydrogenase, α-enolase, and cytochrome c increased more than 50-fold in homozygous αA-R49C mutant lenses. In αB-R120G mouse lenses, our analyses identified decreased abundance of phosphoglycerate mutase, several β- and γ-crystallins, and degradation of αA- and αB-crystallin early in cataract development. Changes in the abundance of hemoglobin and histones with the loss of normal α-crystallin chaperone function suggest that these proteins also play important roles in the biochemical mechanisms of hereditary cataracts. Together, these studies offer a novel insight into the putative in vivo substrates of αA- and αB-crystallin.
Introduction
α-crystallins are major proteins of lens fiber cells that comprise approximately 35% of the water-soluble lens protein and are essential for lens transparency. Mutations in α-crystallin genes are known to cause hereditary cataracts in humans. However, the cellular functions of α-crystallin in maintaining growth, development, and transparency of the lens and the mechanisms by which loss of α-crystallin function leads to cataracts are not fully understood.
The vertebrate lens expresses two α-crystallin proteins, αA and αB, at a high concentration in lens fiber cells and at lower levels in the lens epithelium [1]–[4]. Transcription of αA and αB-crystallin genes commences early in lens development, beginning at embryonic day 10.5 and 9.5 respectively in the mouse, and continues as the lens matures [5]. In lens fiber cells, α-crystallins form heteroaggregates of αA- and αB-crystallins in a 3∶1 ratio [6]. αA- and αB-crystallins are members of the small heat shock protein family of molecular chaperones [7]. Homo-oligomers of αA-crystallin and αB-crystallin and the α-crystallin heteroaggregates possess chaperone-like activity, binding to partially unfolded or denatured proteins to suppress non-specific aggregation [7].
The molecular mechanisms by which point mutations in crystallin genes lead to hereditary human cataract formation are not completely understood [8]–[11]. Mouse models carrying naturally occurring α-crystallin mutations have provided valuable information on the functions of these mutant proteins in vivo [12]–[14].
The R49C mutation in αA-crystallin was found to be associated with nuclear cataract in four generations of a Caucasian family [15]. The mutant protein is mislocalized to the nucleus, and has reduced solubility [15], [16]. Most notably, this mutation is in the N-terminal region of αA-crystallin, a region thought to be important for aggregation interactions [16]. In mice, the R49C mutant produces a small eye/lens phenotype and severe cataracts at birth in 100% of mice homozygous for the mutation, indicating a gain in toxic function of αA-crystallin protein. Compared with homozygous mice, heterozygous αA-R49C knock-in mice, which mimic human cataract patients, develop cataracts at approximately 2 months of age and exhibit decreased protein solubility and altered cell signaling. Moreover, the R49C mutation significantly alters interactions between αA-crystallin, αB-crystallin, βB2-crystallin, γ-crystallins, and the cytoskeletal protein tubulin. The αB-R120G mutation in αB-crystallin also causes cataracts in humans [8]. αB-R120G knock-in mice have lens opacities, which are evident even in 3-week-old animals [17]. We found that 100% of heterozygous mice ranging in age from 3 weeks to 5 months had lens opacities, with severity increasing with age. Homozygous mice also developed lens opacities, but the effect did not appear to be dependent on mutant gene dosage.
Our novel studies using knock-in mouse models for these mutations have shown profound effects on the lens and eye and indicate that α-crystallins affect lens epithelial and fiber cell growth and survival, in addition to their well-known role in transparency and optical properties of the lens. Moreover, our data suggest that αA- and αB-crystallin mutations alter the structure and function of lens epithelial and fiber cells and exert toxic effects at an early stage of development, when primary fiber cell differentiation commences.
It is well established that abnormal interactions between chaperone and substrate proteins can result in increased protein aggregation and disease [8], [18]. The substrate-chaperone interaction between αB-crystallin and its substrates involves multiple interactive domains that have been extensively characterized [19], [20]. However, the in vivo substrates of αA- and αB-crystallin in the lens have not been identified. In the absence or reduction of α-crystallin chaperone function, it is likely that partially unfolded proteins will accumulate and aggregate [21], [22]. We therefore focused on determining which proteins are associated with α-crystallin chaperones with the aim of identifying proteins that are dependent on the chaperone activity of αA- and αB-crystallins to retain their native conformations in vivo. To achieve this, we analyzed the abundance of proteins in αA-R49C and αB-R120G knock-in mutant mice lenses by proteomics and mass spectrometry. We have already applied this approach to identify several proteins and enzymes not previously known to be affected by αA- or αB-crystallin loss of function [23]. This method has also been used to identify the effect of loss of function of the heat shock chaperone protein HSP90 [24].
Results
Two-day-old αA-R49C Mouse Lenses
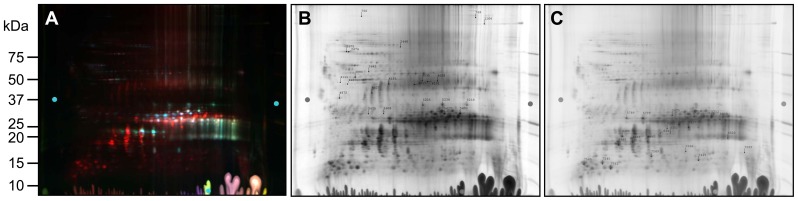
To identify proteins that showed altered abundance in mouse lenses with the R49C αA-crystallin mutation, we performed 2D-DIGE of 2-day-old WT, αA-R49C heterozygous mutant, and αA-R49C homozygous mutant lenses. Figure 1 and Fig. S1 in File S1 show 2D gels of proteins and Table 1 lists the approximately 100 protein spots that showed a change in abundance between these samples. Figure 2 shows the 3D plots for some of the spots that changed in abundance in these lenses. Compared with WT, αA-R49C heterozygous lenses had a 15-fold higher abundance of crosslinked αA-crystallin, a 3-fold higher abundance of more acidic αA-crystallin, and a 2.6-fold higher abundance of degraded αA-crystallin. The association of αA-crystallin with filensin increased 17-fold, the association of αA-crystallin with actin and creatine kinase B increased 15-fold, and the amount of actin alone increased 10.79-fold. The amount of a more acidic form of βB1-crystallin increased, whereas that of a basic form of βB1-crystallin decreased. αA-crystallin associated with βA3/A1 was more acidic and had a slightly lower apparent molecular weight than free αA-crystallin. The number of protein spots with altered abundance was much greater in the αA-R49C homozygous mutant lenses than in the heterozygous lenses. In the homozygous lenses, several proteins in the high molecular weight region (>75 kDa) were altered. A high-molecular weight crosslinked αA-crystallin associated with creatine kinase B, actin, and erlin was enhanced 15-fold. The association of αA-crystallin with α-enolase and βA3/A1 was also enhanced in homozygous lenses. In the same lenses, the amount of βB1-crystallin decreased and more acidic forms of βB1- and βB3-crystallins were associated with αA-crystallin. Among proteins in the 20-kDa region (Table 1, Fig. 1 and Fig. S1 in File S1), the amount of αA-crystallin and βA3/A1-crystallin decreased in homozygous lenses. Among the cytoskeletal proteins, the levels of more basic forms of filensin and phakinin decreased, whereas levels of more acidic forms of these proteins increased. High molecular weight forms of phakinin and actin decreased 2.9-fold in homozygous lenses. The amount of tubulin, vimentin, and microtubule associated protein RP/EB associated with αA-crystallin increased in homozygous lenses, while that of phosphoglycerate mutase decreased. The amount of hemoglobin subunit 1 complexed with γD-, αB-, γS-, γB-, βB3-, and γA-crystallins decreased in homozygous lenses and increased with age. The abundance of forms of Hsp71 increased 2.5-fold, and the amount of αA-crystallin associated with vimentin, tubulin, and T-complex protein increased 4-fold in homozygous lenses. The amount of grifin associated with αA-crystallin increased in several spots.
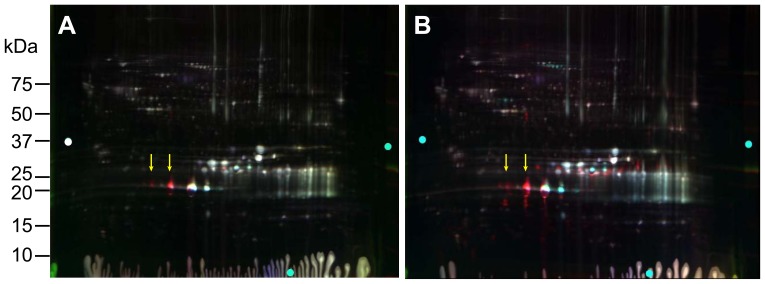
Figure 1. 2D-DIGE analysis of proteomic changes in whole lenses of 2-day-old mice with knock-in of the αA-R49C mutation.
(A) 2D gel of cyanine dye-labeled lens proteins derived from wild-type sample 1 (WT1) proteins labeled with Cy2, WT2 proteins labeled with Cy3, and αA-R49C heterozygous proteins labeled with Cy5. (B) 2D gel of cyanine dye-labeled lens proteins derived from WT1 proteins labeled with Cy2, WT2 proteins labeled with Cy3, and αA-R49C homozygous proteins labeled with Cy5. Protein spots that were selected for analysis from the gels shown in (A) and (B) are shown in Fig. S1 in File S1 and were identified by tandem mass spectrometry and Mascot searches. Quantitative image analysis and mass spectrometry data for the identified proteins are listed in Table 1. Arrows indicate the shift in position of the αA-crystallin bands (red) to a more acidic pI with the mutation.
Table 1. Single-gel analysis of proteins that showed differences in abundance between 2-day-old WT and heterozygous or homozygous αA-R49C lenses.
| Spot number | Protein | UNIPROT accession number | MW (kDa) | Number of assigned spectra | Fold change | ||
| WT1 vs. WT2 | WT1 vs. heterozygous | WT2 vs. heterozygous | |||||
| 3040 | serum albumin | P07724 | 69 | 39 | 3.67 | 17.57 | 16.1 |
| αA-crystallin | Q569M7 | 20 | 5 | ||||
| Filensin | A2AMT1 | 74 | 3 | ||||
| 4024 | αA-crystallin | Q569M7 | 20 | 11 | 1.48 | 16.89 | 14.83 |
| Actin cytoplasmic | P62737 | 42 | 5 | ||||
| Creatine kinase B | Q04447 | 43 | 4 | ||||
| Erlin-2 | Q8BFZ9 | 38 | 3 | ||||
| 4090 | αA-crystallin | Q569M7 | 20 | 16 | 1.45 | 15.56 | 11.46 |
| Actin cytoplasmic | P62737 | 42 | 5 | ||||
| Creatine kinase B | Q04447 | 43 | 3 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 1 | ||||
| 4166 | αA-crystallin | Q569M7 | 20 | 1 (99%) | 1.39 | 15.09 | 10.67 |
| 4893 | Actin cytoplasmic | P62737 | 42 | 1 | 1.39 | 10.79 | 8.77 |
| Citron Rho-interacting kinase | P49025 | 235 | 1 | ||||
| 14-3-3 protein sigma | O70456 | 28 | 1 | ||||
| Peroxiredoxin-2 | Q61171 | 22 | 1 | ||||
| Glyoxalase domain-containing protein 4 | Q9CPV4 | 20 | 1 | ||||
| 5616 | αA-crystallin | Q569M7 | 20 | 21 | 1.24 | 7.51 | 7.46 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 17 | ||||
| βA2-crystallin | Q9QXC6 | 22 | 3 | ||||
| βA4-crystallin | Q3TSJ3 | 24 | 2 | ||||
| 5816 | αA-crystallin | Q569M7 | 20 | 19 | 1.22 | 6.2 | 6.91 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 5 | ||||
| γA-crystallin | Q6PGI0 | 21 | 3 | ||||
| γD-crystallin | P04345 | 21 | 2 | ||||
| 5909 | αA-crystallin | Q569M7 | 20 | 11 | 1.17 | 6 | 6.83 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 3 | ||||
| γA-crystallin | P04345 | 21 | 2 | ||||
| γD-crystallin | Q6PGI0 | 21 | 2 | ||||
| γB-crystallin | P04344 | 21 | 2 | ||||
| Eukaryotic trans initiation factor | P63242 | 17 | 1 | ||||
| 5955 | αA-crystallin | Q569M7 | 20 | 12 | 1.12 | 5.41 | 4.76 |
| γA-crystallin | P04345 | 21 | 2 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| γD-crystallin | Q6PGI0 | 21 | 1 | ||||
| 5976 | αA-crystallin | Q569M7 | 20 | 17 | 1.08 | 4.89 | 4.57 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| γA-crystallin | P04345 | 21 | 2 | ||||
| 6006 | αA-crystallin | Q569M7 | 20 | 14 | 1.08 | 3.17 | 3.68 |
| Eukaryotic trans initiation factor | P63242 | 17 | 1 | ||||
| 6037 | αA-crystallin | Q569M7 | 20 | 19 | −1.11 | 2.97 | 3.63 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| 6061 | αA-crystallin | Q569M7 | 20 | 37 | −1.13 | 2.62 | 2.5 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 1 | ||||
| γA-crystallin | P04345 | 21 | 1 | ||||
| 6176 | αA-crystallin | Q569M7 | 20 | 14 | −1.21 | 1.86 | 2.15 |
| Activated RNA polymerase II transcriptional coactivator p15 | P11031 | 14 | 4 | ||||
| 6218 | αA-crystallin | Q569M7 | 20 | 14 | −1.75 | 1.74 | −2.06 |
| Grifin | Q9D1U0 | 16 | 3 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| Spot number | Protein | UNIPROT accession number | MW (kDa) | Number of assigned spectra | Fold change | ||
| WT1 vs. WT2 | WT1 vs. homozygous | WT2 vs. homozygous | |||||
| 1462 | Spectrin-α | A3KGU5 | 283 | 16 | 1.09 | 10.15 | 9.34 |
| Nucleosome assembly protein 1-like 4 | Q792Z1 | 43 | 1 | ||||
| 1538 | Spectrin-α | A3KG45 | 283 | 59 | 1.21 | 8.33 | 6.91 |
| Neuronal cell adhesion molecule | Q810U4 | 139 | 8 | ||||
| αA-crystallin | Q569M7 | 20 | 5 | ||||
| Serrate RNA effector molecule homolog | Q99MR6 | 100 | 2 | ||||
| Methionine synthase | A6H5Y3 | 139 | 2 | ||||
| 2296 | Filensin | A2AMT1 | 74 | 43 | −1.07 | −5.61 | −5.19 |
| Gelsolin | P13020 | 86 | 5 | ||||
| Calpain 3 | A2AVV5 | 85 | 4 | ||||
| 3001 | 60 kDa Heat shock protein, mitochondrial | P63038 | 61 | 16 | −1.11 | 6.23 | 6.95 |
| T-complex protein 1 subunit theta | P42932 | 60 | 9 | ||||
| αA-crystallin | Q569M7 | 20 | 5 | ||||
| Tubulin alpha-1A chain | P68369 | 50 | 4 | ||||
| Tubulin beta-5 chain | P99024 | 50 | 2 | ||||
| Vimentin | P20152 | 54 | 1 | ||||
| Glutathione synthetase | P51855 | 52 | 1 | ||||
| 3084 | Ezrin | P26040 | 69 | 10 | 1.28 | 3.98 | 3.12 |
| Fascin | Q61553 | 55 | 5 | ||||
| αA-crystallin | Q569M7 | 20 | 5 | ||||
| D-3-phosphoglycerate dehydrogenase | Q61753 | 57 | 3 | ||||
| α-Enolase | P17182 | 47 | 2 | ||||
| βB1-crystallin | Q9WVJ5 | 28 | 2 | ||||
| Aspartyl-tRNA synthetase, cytoplasmic | Q922B2 | 57 | 1 | ||||
| 3295 | αA-crystallin | Q569M7 | 20 | 15 | 1.01 | −2.92 | −2.92 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 5 | ||||
| βA4-crystallin | Q9JJV0 | 22 | 4 | ||||
| γE-crystallin | QO3740 | 21 | 3 | ||||
| γB-crystallin | PO4344 | 21 | 2 | ||||
| Superoxide dismutase [Cu-Zn] | P08228 | 16 | 2 | ||||
| 3312 | αA-crystallin | Q569M7 | 20 | 23 | −1.12 | −5.51 | −4.9 |
| 3703 | αA-crystallin | Q569M7 | 20 | 13 | 1.01 | 14.68 | 14.6 |
| Filaggrin-2 | Q5D862 | 248 | 1 | ||||
| 3723 | αA-crystallin | Q569M7 | 20 | 14 | −1.58 | 2.99 | 4.75 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 3 | ||||
| βA4-crystallin | Q9JJV0 | 22 | 3 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 1 | ||||
| Superoxide dismutase [Cu-Zn] | P08228 | 16 | 1 | ||||
| 3737 | αA-crystallin | Q569M7 | 20 | 14 | 1.09 | 4.9 | 4.53 |
| 3857 | αA-crystallin | Q569M7 | 20 | 15 | 1.09 | 31.67 | 29.19 |
| Grifin | Q9D1U0 | 16 | 1 | ||||
| 4133 | αA-crystallin | Q569M7 | 20 | 11 | 1.22 | 3.93 | 3.24 |
| Grifin | Q9D1U0 | 16 | 2 | ||||
| 4545 | αA-crystallin | Q569M7 | 20 | 11 | 1.13 | 6.68 | 5.93 |
| βA4-crystallin | Q9JJV0 | 22 | 2 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| 4932 | Fatty acid binding protein epidermal | Q05816 | 20 | 18 | −1.01 | 2.97 | 3.01 |
| αA-crystallin | Q569M7 | 20 | 8 | ||||
| βA4-crystallin | Q9JJV0 | 22 | 2 | ||||
| 5163 | αA-crystallin | Q569M7 | 20 | 5 | 1.00 | 4.26 | 4.28 |
| Fatty acid binding protein epidermal | Q05816 | 20 | 3 | ||||
| 5169 | βB3-crystallin | Q9JJU9 | 24 | 6 | 1.33 | 21.6 | 16.28 |
| αA-crystallin | Q569M7 | 20 | 4 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 1 | ||||
| 5182 | αA-crystallin | Q569M7 | 20 | 5 | −1.41 | 6.57 | 9.3 |
| βB3-crystallin | Q9JJU9 | 24 | 4 | ||||
| Calpain-3 | Q64691 | 94 | 1 | ||||
| βA3/A1-crystallin | Q9QCX6 | 25 | 1 | ||||
| 5223 | αA-crystallin | Q569M7 | 20 | 6 | −1.06 | −4.42 | −4.15 |
| 5247 | αA-crystallin | Q569M7 | 20 | 4 | −1.05 | 2.69 | 2.84 |
| βB3-crystallin | Q9JJU9 | 24 | 2 | ||||
| 5257 | αA-crystallin | Q569M7 | 20 | 3 | 1.05 | 3.33 | 3.21 |
| Thymosin beta-4 | P20065 | 6 | 1 | ||||
| 5441 | αA-crystallin | Q569M7 | 20 | 4 | 1.43 | 8.35 | 5.85 |
| 5639 | αA-crystallin | Q569M7 | 20 | 5 | −1.05 | 4.83 | 5.1 |
| 5675 | αA-crystallin | Q569M7 | 20 | 5 | 1.24 | 8.29 | 6.71 |
| Thymosin beta-4 | P20065 | 6 | 2 | ||||
| 5816 | αA-crystallin | Q569M7 | 20 | 7 | −1.21 | 6.45 | 7.83 |
| βA3/A1-crystallin | Q9QCX6 | 25 | 2 | ||||
| βA4-crystallin | Q9KKV0 | 22 | 2 | ||||
| 5883 | Glutamate dehydrogenase | P26443 | 61 | 3 | 1.24 | 4.01 | 3.26 |
| 5966 | Creatine kinase B-type | Q04447 | 43 | 8 | −1.1 | 19.82 | 22.03 |
| αA-crystallin | Q569M7 | 20 | 7 | ||||
| Actin cytoplasmic 1 | P60709 | 42 | 5 | ||||
| COP9 signalosome complex subunit 4 | O88544 | 46 | 4 | ||||
| 26 S proteasome non-ATPase regulatory subunit 13 | Q9WJJ2 | 43 | 3 | ||||
| Erlin-2 | Q8BFZ9 | 38 | 2 | ||||
| Activator of 90 kDa HSP ATPase homolog 1 | Q8BK64 | 38 | 2 | ||||
| Eukaryotic initiation factor 4A-I | P60843 | 46 | 1 | ||||
| Succinyl-CoA subunit beta | Q9Z2I9 | 38 | 1 | ||||
| Farnesyl pyrophosphate synthase | Q920E5 | 50 | 1 | ||||
| Glutaredoxin-3 | Q9CQM9 | 38 | 1 | ||||
| 5976 | βB3-crystallin | Q9JJU9 | 24 | 8 | −1.17 | 14.73 | 17.35 |
| αA-crystallin | Q569M7 | 20 | 6 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 3 | ||||
| βB2-crystallin | P62696 | 23 | 2 | ||||
| Gelsolin | P13020 | 86 | 2 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 1 | ||||
| γN-crystallin | Q8VHL5 | 21 | 1 | ||||
| 6042 | αA-crystallin | Q569M7 | 20 | 5 | 1.13 | 112.49 | 100.21 |
| βB3-crystallin | Q9JJU9 | 24 | 5 | ||||
| α-Enolase | P17182 | 47 | 3 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 2 | ||||
| βB1-crystallin | Q9WVJ5 | 28 | 2 | ||||
| Poly(γC)-binding protein 1 | P60335 | 37 | 2 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 1 | ||||
| γN-crystallin | Q8VHL5 | 21 | 1 | ||||
| 6051 | Glutamate dehydrogenase mitochondrial | P26443 | 61 | 4 | −1.27 | 3.73 | 4.75 |
| Myoglobin | P04249 | 17 | 1 | ||||
| 6224 | αA-crystallin | Q569M7 | 20 | 5 | 1.34 | 23.76 | 17.83 |
| βB3-crystallin | Q9JJU9 | 24 | 4 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 2 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 1 | ||||
| 6312 | αA-crystallin | Q569M7 | 20 | 7 | 1.13 | −2.72 | −3.04 |
| βB3-crystallin | Q9JJU9 | 24 | 2 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| 6457 | βB3-crystallin | Q9JJU9 | 24 | 18 | 1.19 | 5.94 | 5.03 |
| αA-crystallin | Q569M7 | 20 | 5 | ||||
| γB-crystallin | Q6PHP7 | 21 | 3 | ||||
| γF-crystallin | Q9CXV3 | 21 | 3 | ||||
| Triosephosphate isomerase | P17751 | 27 | 1 | ||||
| αB-crystallin | P23927 | 20 | 1 | ||||
| 6465 | βB1-crystallin | Q9WVJ5 | 28 | 18 | −1.01 | 3.29 | 3.35 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 5 | ||||
| Proteasome subunit 1 type 4 | P99026 | 29 | 5 | ||||
| αA-crystallin | Q569M7 | 20 | 4 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 2 | ||||
| βA4-crystallin | Q9JJV0 | 22 | 2 | ||||
| βA2-crystalin | Q9JJV1 | 22 | 1 | ||||
| 6484 | αA-crystallin | Q569M7 | 20 | 6 | 1.34 | 6.74 | 5.05 |
| βB1-crystallin | Q9WVJ5 | 28 | 5 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 3 | ||||
| Proteasome subunit 1 type 4 | P99026 | 29 | 2 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 1 | ||||
| 6546 | αA-crystallin | Q569M7 | 20 | 10 | 1.78 | 5.92 | 3.34 |
| βB3-crystallin | Q9JJU9 | 24 | 9 | ||||
| βB1-crystallin | Q9WVJ5 | 28 | 5 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 4 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 3 | ||||
| βS-crystallin | O35486 | 21 | 1 | ||||
| 6567 | αB-crystallin | P23927 | 20 | 17 | 1.54 | 9.13 | 5.96 |
| βB3-crystallin | Q9JJU9 | 24 | 8 | ||||
| αA-crystallin | Q569M7 | 20 | 5 | ||||
| βS-crystallin | O35486 | 21 | 5 | ||||
| γF-crystallin | Q9CXV3 | 21 | 4 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 3 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 1 | ||||
| 6605 | βA3/A1-crystallin | Q9QXC6 | 25 | 15 | −1.32 | 4.39 | 5.83 |
| βB3-crystallin | Q9JJU9 | 24 | 12 | ||||
| αB-crystallin | P23927 | 20 | 7 | ||||
| αA-crystallin | Q569M7 | 20 | 5 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 5 | ||||
| βS-crystallin | O35486 | 21 | 3 | ||||
| γF-crystallin | Q9CXV3 | 21 | 2 | ||||
| 6607 | αA-crystallin | Q569M7 | 20 | 12 | −1.04 | 2.82 | 2.95 |
| γB-crystallin | Q6PHP7 | 21 | 6 | ||||
| αB-crystallin | P23927 | 20 | 4 | ||||
| βB1-crystallin | Q9WVJ5 | 28 | 4 | ||||
| Calcium-regulated heat stable protein 1 | Q9CR86 | 16 | 3 | ||||
| γF-crystallin | Q9CXV3 | 21 | 2 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 1 | ||||
| βA4-crystallin | Q9JJV0 | 22 | 1 | ||||
| γC-crystalin | Q61597 | 21 | 1 | ||||
| 6642 | αA-crystallin | Q569M7 | 20 | 5 | −1.18 | 10.22 | 12.14 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 1 | ||||
| γB-crystallin | Q6PHP7 | 21 | 1 | ||||
| 6652 | αA-crystallin | Q569M7 | 20 | 7 | 1.17 | 9.1 | 7.79 |
| 6663 | αA-crystallin | Q569M7 | 20 | 6 | −1.21 | 6.79 | 8.29 |
| 6678 | Hemoglobin subunit α | P01942 | 15 | 1 | 1.34 | 11.01 | 8.26 |
| Glyceraldehyde-3-phosphate | P25856 | 42 | 1 | ||||
| Hemoglobin subunit β | P11758 | 16 | 1 | ||||
| 6791 | αA-crystallin | Q569M7 | 20 | 5 | 1.27 | 10.07 | 7.96 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 3 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 2 | ||||
| 6920 | αA-crystallin | Q569M7 | 20 | 76 | −1.23 | 21.71 | 26.75 |
| 6981 | αA-crystallin | Q569M7 | 20 | 5 | −1.00 | 26.33 | 26.6 |
| γB-crystallin | Q6PHP7 | 21 | 1 | ||||
| Activated RNA polymerase II transcriptional coactivator P15 | P11031 | 14 | 1 | ||||
| Adenosine receptor A2B | Q60614 | 36 | 1 | ||||
| 7559 | αA-crystallin | Q569M7 | 20 | 15 | −2.29 | 8.38 | 19.32 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 5 | ||||
| Stathmin | P54227 | 17 | 4 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 3 | ||||
| βA4-crystallin | Q9JJV0 | 22 | 2 | ||||
| Superoxide dismutase [Cu-Zn] | P08228 | 16 | 1 | ||||
| Fatty acid binding protein, epidermal | Q05816 | 15 | 1 | ||||
WT, wild-type.
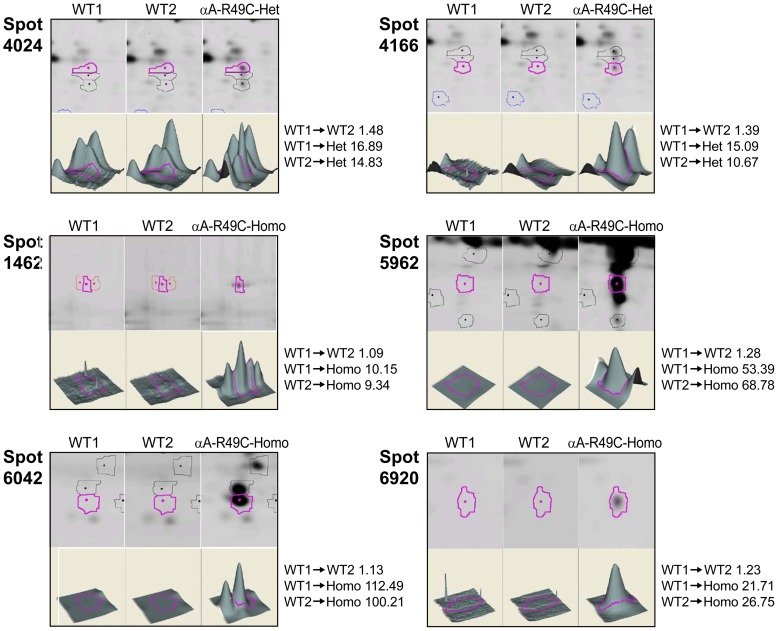
Figure 2. Quantitative analysis of abundance changes in proteins from postnatal 2-day-old WT and αA-R49C knock-in lenses by mass spectrometry.
The 3D data sets for representative proteins in two WT (WT1 and WT2) and one αA-R49C heterozygous or αA-R49C homozygous mutant sample are shown. WT1 and WT2 proteins were labeled with Cy2 and Cy3 dyes, respectively and αA-R49C mutant proteins with Cy5. Fold changes between each sample are indicated on the right. See Table 1 for the identity of proteins present in each protein spot.
There was an increase in β-globin, histone and peptidyl-prolyl cis-trans isomerase associated with αA-crystallin in homozygous lenses (Table S1). The abundance of αB-crystallin, hemoglobin, and histones also increased. A spot containing a high molecular weight form of spectrin-α and nucleosome assembly protein increased in homozygous lenses. In the high molecular weight region, the abundance of αA-crystallin and spectrin increased and that of filensin, gelsolin, and calpain 3 decreased in homozygous lenses. There was an increase in mitochondrial 60-kDa HSP associated with αA-crystallin, and many other proteins including vimentin.
Among proteins in the cytoskeletal and 20 kDa regions (Table 1, Fig. 1 and Fig. S1 in File S1), there was an increase in αA-crystallin associated with βB3-crystallin, βA4-crystallin, grifin, fatty acid binding protein, thymosin, and glutamate dehydrogenase in homozygous lenses. Surprisingly, the amount of αA-crystallin alone and in association with βA3/A1-crystallin, βA4-crystallin, γE-crystallin, and γA-crystallin in the high molecular weight region decreased in homozygous lenses.
Increased amounts of degraded proteins were detected in the low molecular weight region (<20 kDa). The amount of degraded glutamate dehydrogenase alone and in association with cytochrome c increased 4-fold and 53-fold, respectively, in homozygous lenses. The amount of more acidic forms of αA-crystallin, and more degraded forms of creatine kinase B, αA-crystallin, actin, and phakinin increased 19-fold in homozygous lenses. In the molecular weight range below 20 kDa, the amount of degraded αB-crystallin associating with βA2-crystallin, α-enolase, and other proteins increased 112-fold in homozygous lenses. The amount of other degradation products of αA-crystallin associated with β- and γ-crystallins also increased in homozygous lenses. Some of these were more basic than the original αA-crystallin. The amount of a very acidic cohort of αA-crystallin with βA3/A1-crystallin, hemoglobin subunit α, and G3PDH increased 7-fold in homozygous lenses. There was also an increase in the amount of a very low molecular weight αA-crystallin associated with stathmin and other β-crystallins in homozygous lenses.
Previous work demonstrated that there is less insoluble protein in heterozygous lenses than in homozygous lenses [10]. To determine whether changes in protein abundance reflect this difference in solubility, equal amounts of WT, heterozygous, and homozygous mutant lens proteins were further analyzed on multiple gels using various combinations of cyanine dyes to label WT and mutant lens samples. Multi-gel analysis of WT and αA-R49C mutant proteins is shown in Table 2 and Figures 3 and 4. Biological variation analysis (BVA) of WT and αA-R49C heterozygous and homozygous lenses showed that mutant gene dosage correlated with an increase in alanyl-tRNA synthetase, αA-crystallin, the mammalian cytoplasmic chaperone TCP-1 theta, and high-molecular weight βA3/A1-crystallin. The statistical significance of the change in protein abundance of each spot is shown in Table 2. The levels of two different members of the HSP70 protein family, HSC70 and mitochondrial stress protein 70, as well as the V-type proton ATPase catalytic subunit, also increased in αA-R49C mutant lenses. Mitochondrial stress protein 70 increased in two spots (spots 928 and 948) and TCP-1 associated with αA-crystallin increased in three spots (spots 593, 1081, and 1146). High molecular weight βB1-crystallin increased slightly in a mutation- and dose-dependent manner. The abundance of βA3/A1-crystallin associated with αA-crystallin (spot 1477) and αA-crystallin alone (spot 1612) decreased. It is noteworthy that for several spots, the differences were statistically significant (p<0.05) between WT and the αA-R49C homozygous lenses only. The 79-fold increase in αA-crystallin (spot 1540) in the high molecular weight region was highly significant, suggesting increased crosslinking of αA-crystallin in αA-R49C mutant lenses. Creatine kinase B associated with αA-crystallin in the high molecular weight region increased 22-fold (spot 1519), confirming the results of the single gel analysis in Table 1. The amount of αA-crystallin associated with eukaryotic translational initiation factor increased 1.44- and 2.24-fold in heterozygous and homozygous mutant lenses, respectively. Among the proteins that showed decreased abundance in a mutation- and dosage-dependent manner were βB1-crystallin (spots 1856 and 1868) associated with eukaryotic translational initiation factor, αA-crystallin associated with histone H4, implantin, myotrophin, and more basic αA-crystallin associated with βA4- and βA3/A1-crystallins in spot 2772.
Table 2. Multi-gel analysis of proteins that showed differences in abundance between 2-day-old WT and heterozygous or homozygous αA-R49C lenses.
| Spot number | Protein | UNIPROT Accession number | MW (kDa) | Number of assigned spectra | Fold change/t-test (p-value) | ||
| WT vs. heterozygous | WT vs. homozygous | Heterozygous/ homozygous | |||||
| 593 | Alanyl-tRNA synthetase, cytoplasmic | Q8BGQ7 | 107 | 12 | 2.01/0.035 | 2.28/0.021 | −1.13/0.43 |
| αA-crystallin | Q569M7 | 20 | 2 | ||||
| T-complex protein 1 subunit theta | P42932 | 60 | 2 | ||||
| Glutathione synthetase | P51855 | 52 | 2 | ||||
| 884 | βA3/A1-crystallin | Q9QXC6 | 25 | 1 | 1.02/0.83 | 1.65/0.017 | −1.62/0.0048 |
| Putative uncharacterized protein | Q3UAF6 | 42 | 1 | ||||
| 928 | Heat shock cognate 71 kDa protein | P63017 | 71 | 28 | 1.15/0.11 | 1.42/0.018 | −1.24/0.077 |
| V-type proton ATPase catalytic subunit | P50516 | 68 | 17 | ||||
| Stress-70 protein, mitochondrial | P38647 | 74 | 5 | ||||
| Alpha-fetoprotein | P02772 | 67 | 3 | ||||
| 948 | Stress-70 protein, mitochondrial | P38647 | 74 | 6 | 1.39/0.064 | 1.79/0.017 | −1.29/0.12 |
| αA-crystallin | Q569M7 | 20 | 4 | ||||
| Heat shock cognate 71 kDa protein | P63017 | 71 | 3 | ||||
| Glutathione synthetase | P51855 | 52 | 1 | ||||
| 1081 | T-complex protein 1 subunit theta | P42932 | 60 | 16 | 1.93/0.053 | 2.03/0.044 | −1.05/0.43 |
| αA-crystallin | Q569M7 | 20 | 4 | ||||
| 1131 | Seryl-tRNA synthetase, cytoplasmic | P26638 | 58 | 2 | 1.39/0.13 | 1.62/0.051 | −1.17/0.04 |
| 1146 | αA-crystallin | Q569M7 | 20 | 9 | 7.67/0.0037 | 10.91/0.0018 | −1.42/0.075 |
| T-complex protein 1 subunit theta | P42932 | 60 | 3 | ||||
| 1190 | βB1-crystallin | Q9WVJ5 | 28 | 1 | 1.1/0.22 | 1.28/0.013 | −1.16/0.029 |
| 1351 | Filensin | A2AMT1 | 74 | 6 | 1.26/0.006 | 1.22/0.014 | 1.03/0.68 |
| Eukaryotic translation initiation factor | P60229 | 52 | 3 | ||||
| 1477 | αA-crystallin | Q569M7 | 20 | 5 | −3.53/0.063 | −4.25/0.043 | 1.2/0.47 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| 1519 | Creatine kinase B- type | Q04447 | 43 | 11 | 15.85/0.00026 | 22.33/0.0001 | −1.41/0.14 |
| αA-crystallin | Q569M7 | 20 | 9 | ||||
| Putative uncharacterized protein | Q3UAF6 | 42 | 2 | ||||
| 1540 | αA-crystallin | Q569M7 | 20 | 11 | 53.41/6.9e-005 | 79.7/5.10E-05 | −1.49/0.077 |
| 1582 | αA-crystallin | Q569M7 | 20 | 13 | 10.11/0.0025 | 14.62/0.0014 | −1.45/0.098 |
| 1612 | αA-crystallin | Q569M7 | 20 | 9 | −2.77/0.12 | −4.82/0.041 | 1.74/0.19 |
| 1625 | αA-crystallin | Q569M7 | 20 | 1 | 2.75/0.031 | −1.42/0.21 | 3.9/0.01 |
| Fructose-biphosphate aldolase A | P05064 | 36 | 1 | ||||
| 1659 | αA-crystallin | Q569M7 | 20 | 12 | 1.44/0.0027 | 2.24/0.0069 | −1.56/0.12 |
| Eukaryotic translation initiation factor | Q9QZD9 | 36 | 2 | ||||
| Putative uncharacterized protein | Q3UAF6 | 42 | 1 | ||||
| 1767 | Glyceraldehyde-3-phosphate dehydrogenase | P16858 | 36 | 2 | −2.11/0.085 | −1.78/0.069 | −1.19/0.12 |
| Heterogeneous nuclear ribonucleoproteins A2/B1 | O88569 | 37 | 2 | ||||
| 1786 | Heterogeneous ribonucleoprotein A1 | P49312 | 34 | 5 | −1.93/0.053 | −1.16/0.42 | −1.67/0.088 |
| 1856 | βB1-crystallin | Q9WVJ5 | 28 | 8 | 1.08/0.54 | −1.65/0.089 | 1.77/0.0045 |
| 1865 | βB1-crystallin | Q9WVJ5 | 28 | 13 | −2.14/0.0017 | −1.42/0.075 | −1.51/0.057 |
| 1868 | βB1-crystallin | Q9WVJ5 | 28 | 8 | −1.04/0.47 | −1.48/0.049 | 1.42/0.12 |
| Eukaryotic translation initiation factor 4H | Q9WUK2 | 27 | 2 | ||||
| 1902 | βB1-crystallin | Q9WVJ5 | 28 | 12 | 1.06/0.50 | −2/0.0075 | 2.13/0.0022 |
| 2640 | αA-crystallin | Q569M7 | 20 | 9 | −2.15/0.00096 | −2.66/0.00022 | 1.24/0.036 |
| Histone H4 | P62806 | 11 | 2 | ||||
| Implantin (fragment) | P83891 | 2 | 1 | ||||
| 2661 | Regulating synaptic membrane exocytosis protein 2 | Q9EQZ7 | 173 | 1 | −2.38/0.039 | 1.00/0.95 | −2.39/0.021 |
| 2684 | Myotrophin | P62774 | 13 | 2 | −1.32/0.11 | −1.57/0.033 | 1.19/0.16 |
| 2772 | αA-crystallin | Q569M7 | 20 | 8 | −3.65/0.074 | −7.3/0.03 | 2/0.18 |
| βA4-crystallin | Q9JJV0 | 22 | 3 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| 1923 | βB1-crystallin | Q9WVJ5 | 28 | 2 | 1.43/0.053 | −1.54/0.019 | 2.2/0.011 |
| 2018 | αA-crystallin | Q569M7 | 20 | 8 | −1.4/0.021 | −1.13/0.35 | −1.24/0.059 |
| Implantin (fragment) | P83891 | 2 | 2 | ||||
| Coactosin-like protein | Q9CQI6 | 16 | 2 | ||||
| 2109 | βA3/A1-crystallin | Q9QXC6 | 25 | 12 | −1.16/0.18 | −1.49/0.034 | 1.28/0.15 |
| αA-crystallin | Q569M7 | 20 | 6 | ||||
| βA4-crystallin | Q9JJV0 | 22 | 5 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 2 | ||||
| 2115 | βA3/A1-crystallin | Q9QXC6 | 25 | 9 | −3.14/0.059 | −3.92/0.034 | 1.25/0.48 |
| αB-crystallin | P23296 | 20 | 9 | ||||
| βB2-crystallin | P62696 | 23 | 9 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 8 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 8 | ||||
| αA-crystallin | Q569M7 | 20 | 6 | ||||
| 2123 | αA-crystallin | Q569M7 | 20 | 17 | −4.44/0.029 | −8.57/0.016 | 1.93/0.17 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 8 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 8 | ||||
| βB2-crystallin | P62696 | 23 | 6 | ||||
| αB-crystallin | P23296 | 20 | 3 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 2 | ||||
| 2154 | αA-crystallin | Q569M7 | 20 | 12 | −1.44/0.0024 | −1.26/0.0094 | −1.14/0.13 |
| 2233 | αB-crystallin | P23296 | 20 | 19 | −1.59/0.084 | −1.8/0.053 | 1.13/0.35 |
| γD-crystallin | Q6PGI0 | 20 | 16 | ||||
| γA-crystallin | P04345 | 21 | 7 | ||||
| γF-crystallin | Q9CXV3 | 21 | 4 | ||||
| γB-crystallin | Q6PHP7 | 21 | 3 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| Plectin | Q9QXS1 | 534 | 2 | ||||
| 2261 | γA-crystallin | P04345 | 21 | 2 | 1.65/0.074 | −1.53/0.11 | 2.52/0.022 |
| αB-crystallin | P23296 | 20 | 2 | ||||
| 2294 | αA-crystallin | Q569M7 | 20 | 16 | 4.16/0.020 | 5.64/0.012 | −1.36/0.054 |
| 2317 | αA-crystallin | Q569M7 | 20 | 14 | −2.83/0.095 | −5.09/0.032 | 1.8/0.17 |
| 2320 | αA-crystallin | Q569M7 | 20 | 21 | −4.85/0.11 | −9.28/0.035 | 1.91/0.14 |
| 2337 | βA3/A1-crystallin | Q9QXC6 | 25 | 8 | −2.28/0.024 | −3.49/0.06 | 1.53/0.23 |
| αA-crystallin | Q569M7 | 20 | 8 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 2 | ||||
| 2351 | αA-crystallin | Q569M7 | 20 | 12 | 4.61/0.0057 | 10.45/0.0049 | −2.27/0.14 |
| 2388 | αA-crystallin | Q569M7 | 20 | 10 | 1.48/0.27 | 5.71/0.038 | −3.85/0.074 |
| 2413 | γD-crystallin | Q6PGI0 | 20 | 10 | −1.14/0.86 | −3.8/0.046 | 3.33/0.007 |
| Peptidyl-prolyl cis-trans isomerase | P17742 | 18 | 6 | ||||
| γA-crystallin | P04345 | 21 | 6 | ||||
| γB-crystallin | Q6PHP7 | 21 | 4 | ||||
| γC-crystallin | Q61597 | 21 | 4 | ||||
| 2417 | αA-crystallin | Q569M7 | 20 | 15 | 7.51/0.0023 | 10.53/0.0015 | −1.4/0.15 |
| 2454 | Nucleoside diphosphate kinase | E9PZF0 | 30 | 6 | −1.25/0.016 | −1.47/0.0067 | 1.18/0.11 |
| Peptidyl-prolyl cis-trans isomerase | P17742 | 18 | 4 | ||||
| γD-crystallin | Q6PGI0 | 20 | 3 | ||||
| αA-crystallin | Q569M7 | 20 | 3 | ||||
| γB-crystallin | Q6PHP7 | 21 | 2 | ||||
| 2462 | αA-crystallin | Q569M7 | 20 | 3 | −1.48/0.12 | −2.08/0.017 | 1.41/0.021 |
| γN-crystallin | Q8VHL5 | 21 | 2 | ||||
| 2469 | Nucleoside diphosphate kinase | E9PZF0 | 30 | 6 | −1.37/0.052 | −1.56/0.023 | 1.14/0.17 |
| αA-crystallin | Q569M7 | 20 | 3 | ||||
| 2501 | γC-crystallin | Q61597 | 21 | 2 | −1.55 | −2.11/0.012 | 1.36 |
| 2533 | αA-crystallin | Q569M7 | 20 | 3 | −1.82/0.10 | −2.44/0.027 | 1.34/0.26 |
| 2538 | αA-crystallin | Q569M7 | 20 | 3 | −2.41/0.021 | −2.32/0.067 | −1.04/0.88 |
| 40S ribosomal protein S12 | P63323 | 15 | 2 | ||||
| 2553 | Fatty acid-binding protein epidermal | Q05816 | 15 | 12 | −1.96/0.67 | −3.48/0.2 | 1.77/0.19 |
| αA-crystallin | Q569M7 | 20 | 7 | ||||
| 2631 | αA-crystallin | Q569M7 | 20 | 2 | −2.58/0.13 | −2.92/0.038 | 1.13/0.079 |
WT, wild-type.
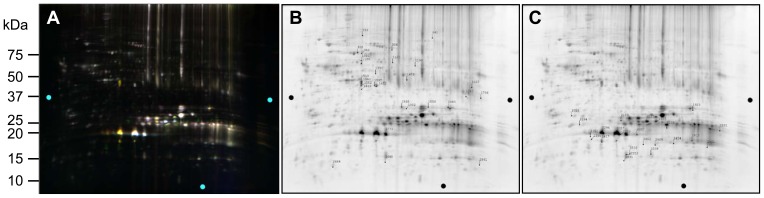
Figure 3. 2D-DIGE analysis of proteomic changes in whole lenses of WT, αA-R49C heterozygous, and αA-R49C homozygous mutant lenses using a pool-based analysis.
(A) WT samples were labeled with Cy2, a pool of all samples (containing WT, αA-R49C heterozygous and homozygous proteins) was labeled with Cy3, and the αA-R49C heterozygous mutant sample was labeled with Cy5. The pool sample was a common comparator for each sample. (B, C) Spots that were selected based on analysis of the gels are shown. Quantitative image analysis by biological variation analysis was performed across several samples, and mass spectrometry data for the identified proteins from these gels are listed in Table 3.
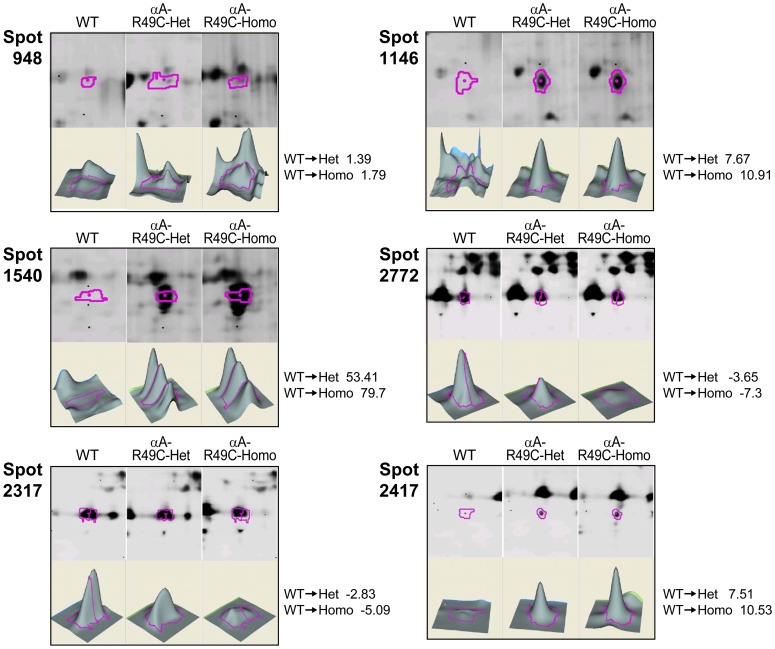
Figure 4. Pool-based quantitative analysis of changes in abundance of postnatal 2-day-old lens proteins from WT and αA-R49C knock-in lenses by mass spectrometry.
The 3D data sets for representative proteins in one WT, one pool, and one αA-R49C heterozygous or αA-R49C homozygous mutant sample are shown. WT proteins were labeled with Cy2, pool proteins with Cy3, and αA-R49C heterozygous mutant proteins with Cy5. Fold changes between each sample are indicated on the right. See Table 2 for the identity of proteins present in each protein spot.
Additional proteins that decreased in abundance relative to wild type (Fig. 4 and Table 2) were βB1-crystallin (in homozygous lenses only), and a mutation- and dose-dependent decrease in βA3/A1-, βA4-, βA2-crystallins associated with αA-crystallin (spot 2109), αB-crystallin, and βB2-crystallin (spots 2115 and 2123 showed a 8.57-fold decrease in homozygous lenses relative to WT). The abundance of γD-crystallin, peptidyl-prolyl cis-trans isomerase, γA-crystallin, γB-crystallin, and γC-crystallin also decreased (spot 2413). Other spots that decreased in abundance in a mutation- and dose-dependent manner were nucleoside diphosphate kinase, peptidyl-prolyl cis-trans isomerase, and γD-crystallin (spot 2454), fatty acid-binding protein and αA-crystallin (spot 2553). A more acidic form of αA-crystallin increased 4- and 5-fold in heterozygous and homozygous lenses (spot 2294). In contrast, spot 2317 decreased 4.8- and 9.2-fold in heterozygous and homozygous mutant lenses, respectively. Spot 2351 increased in a mutation- and dose-dependent manner with 4.6- and 10.4-fold increases in heterozygous and homozygous lenses, respectively. Spots 2317 and 2351 contained only αA-crystallin at its normal molecular weight, but spot 2351 was more acidic, suggesting a decrease in the pI of αA-crystallin by the R49C mutation. Spot 2417, containing only a lower-than normal molecular weight αA-crystallin also increased 7.5- and 10.5-fold in αA-R49C mutant lenses relative to WT, but two additional spots containing only αA-crystallin decreased (spots 2533 and 2631). The abundance of epidermal fatty acid binding protein and 40S ribosomal protein S12 also decreased in association with αA-crystallin, but these changes were not mutation- and dose-dependent.
Two-week Old αA-R49C Mouse Lenses
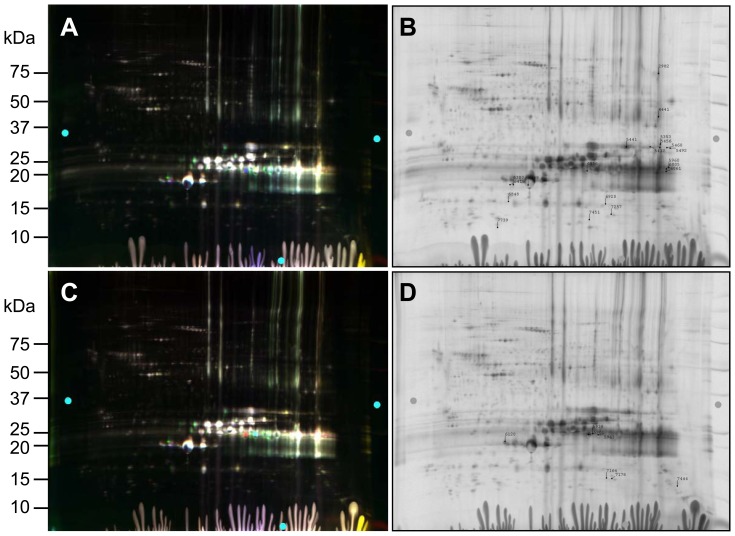
Figure 5 shows 2D gels for 14-day-old WT and mutant proteins of αA-R49C knock-in mice. Table 3 shows the approximately 50 protein spots that showed a change in abundance between WT and αA-R49C mutant in 14-day-old lenses. The abundance of the high molecular weight cytoskeletal protein spectrin-α and its acidic forms decreased in αA-R49C lenses (spots 700 and 769). Acidic forms of filensin increased 4-fold (spot 2675), whereas basic forms decreased 15-fold (spot 2448). Hsp70 also increased 3- to 6-fold in three spots. High molecular weight phakinin decreased 10-fold, while acidic and low molecular weight phakinin increased 8-fold.
Figure 5. 2D-DIGE analysis of proteomic changes in whole lenses of 14-day-old mice induced by knock-in of the αA-R49C mutation.
(A) A 2D gel of lens proteins labeled with cyanine dyes derived from WT1 proteins labeled with Cy2, WT2 proteins labeled with Cy3, and αA-R49C homozygous lens proteins labeled with Cy5. (B, C) Protein spots that were selected for analysis from the gel in (A) are shown. Proteins were identified by tandem mass spectrometry and Mascot searches of spots that were selected from the gels. Quantitative image analysis and mass spectrometry data for the identified proteins from these gels are listed in Table 3.
Table 3. Protein spots that showed a change in abundance between 2-week-old WT and αA-R49C homozygous mutant lenses.
| Spot number | Protein | Uniprot accession number | MW (kDa) | Number of assigned spectra | Fold change | ||
| WT1 vs. WT2 | WT1 vs. homozygous | WT2 vs. homozygous | |||||
| 700 | Spectrin α-2 | A3KGU5 | 283 | 47 | −1.6 | −13.77 | −8.51 |
| 769 | Spectrin α-2 | A3KGU5 | 283 | 20 | 1.77 | −8.19 | −14.35 |
| γA-crystallin | 21 | 2 | |||||
| 1164 | Adult male sm. intestine cDNA | Q9CPN9 | 26 | 1 | −2.56 | −7.87 | −3.05 |
| 2448 | Filensin | A2AMT1 | 74 | 39 | −2.05 | −15.14 | −7.32 |
| 2675 | Filensin | A2AMT1 | 74 | 8 | −1.89 | 3.81 | 7.27 |
| 78 kDa glucose-regulated protein precursor | P20029 | 72 | 4 | ||||
| GTP binding protein Di- Ras 1 | Q91Z61 | 22 | 1 | ||||
| Collagen α2v chain precursor | Q3U962 | 145 | 1 | ||||
| 2676 | 78 kDa glucose-regulated protein precursor | P20029 | 72 | 12 | −1.87 | 3.4 | 6.42 |
| 3641 | Phakinin | Q6NVD9 | 46 | 24 | −1.59 | −10.22 | −6.38 |
| 3880 | Phakinin | Q6NVD9 | 46 | 15 | 1.23 | 8.33 | 6.82 |
| 16 days embryo kidney cDNA | Q3TGJ9 | 81 | 7 | ||||
| 4035 | αA-crystallin | Q569M7 | 20 | 8 | 1.00 | 7.71 | 7.76 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 4 | ||||
| βB1-crystallin | Q9WVJ5 | 28 | 2 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 2 | ||||
| 4058 | βA3/A1-crystallin | Q9QXC6 | 25 | 7 | 1.77 | 3.79 | 2.16 |
| αA-crystallin | Q569M7 | 20 | 4 | ||||
| βB1-crystallin | Q9WVJ5 | 28 | 1 | ||||
| Fructose-bisphosphate aldolase | A6ZI44 | 45 | 1 | ||||
| 4101 | αA-crystallin | Q569M7 | 20 | 8 | 1.2 | 3.41 | 2.86 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 6 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 2 | ||||
| βB1-crystallin | Q9WVJ5 | 28 | 1 | ||||
| 4111 | αA-crystallin | Q569M7 | 20 | 3 | −1.49 | −10.27 | −6.85 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 3 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 2 | ||||
| 40S ribosomal protein SA(Laminin receptor 1) | P14206 | 33 | 1 | ||||
| 4191 | αA-crystallin | Q569M7 | 20 | 7 | −1.07 | 26.32 | 28.36 |
| βB1-crystallin | Q9WVJ5 | 28 | 2 | ||||
| 4207 | Phakinin | Q6NVD9 | 46 | 8 | 2.02 | −9.58 | −19.19 |
| αA-crystallin | Q569M7 | 20 | 1 | ||||
| 4217 | αA-crystallin | Q569M7 | 20 | 10 | 1.36 | 5.67 | 4.21 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 7 | ||||
| βB1-crystallin | Q9WVJ5 | 28 | 2 | ||||
| Phakinin | Q6NVD9 | 46 | 2 | ||||
| 4627 | αA-crystallin | Q569M7 | 20 | 8 | 1.21 | 22.44 | 18.67 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| Adult male stomach cDNA | Q3Y2E0 | 27 | 2 | ||||
| Adult male sm. intestine cDNA | Q9CPN9 | 26 | 1 | ||||
| WD repeat-containing protein 81 | Q5ND34 | 96 | 1 | ||||
| NOD-derived CD11c positive dendritic cells cDNA | Q5ND34 | 17 | 1 | ||||
| 4872 | αA-crystallin | Q569M7 | 20 | 5 | −1.31 | 3.99 | 5.26 |
| Annexin A5 | ANXA5 | 36 | 5 | ||||
| Adult male stomach cDNA | Q3Y2E0 | 27 | 2 | ||||
| Adult male small intestine cDNA | Q9CPN9 | 26 | 1 | ||||
| SRC kinase signaling inhibitor 1 | Q9QWI6 | 135 | 1 | ||||
| Filamin-B | Q80X90 | 278 | 1 | ||||
| Probable E3 ubiquitin-protein ligase HERC2 | Q4U2R1 | 528 | 1 | ||||
| 5218 | βB1-crystallin | Q9WVJ5 | 28 | 13 | 1.76 | −24.55 | −42.86 |
| 5225 | βB1-crystallin | Q9WVJ5 | 28 | 20 | −1.05 | −6.09 | −5.72 |
| 5236 | βB1-crystallin | Q9WVJ5 | 28 | 20 | −1.27 | −8.76 | −6.82 |
| Complement factor C2 | Q792Q3 | 53 | 1 | ||||
| 5608 | βB1-crystallin | Q9WVJ5 | 28 | 15 | −1.23 | −8.75 | −7.05 |
| αA-crystallin | Q569M7 | 20 | 1 | ||||
| 5609 | βB3-crystallin | Q9JJU9 | 24 | 19 | 1.43 | 21.18 | 14.97 |
| Glutathione S-transferase µ | A2AE89 | 24 | 5 | ||||
| βB2-crystallin | P62696 | 23 | 3 | ||||
| βB1-crystallin | Q9WVJ5 | 28 | 1 | ||||
| Complement factor C2 | Q792Q3 | 53 | 1 | ||||
| Adult male sm. intestine cDNA | Q9CPN9 | 26 | 1 | ||||
| 5617 | βB2-crystallin | P62696 | 23 | 34 | −1.55 | −8.44 | −5.38 |
| βB3-crystallin | Q9JJU9 | 24 | 12 | ||||
| Glutathione S-transferase µ | A2AE89 | 24 | 5 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| βB1-crystallin | Q9WVJ5 | 28 | 1 | ||||
| 5625 | βB2-crystallin | P62696 | 23 | 17 | −1.38 | −18.58 | −13.36 |
| βB3-crystallin | Q9JJU9 | 24 | 6 | ||||
| Heat shock protein B1 (HspB1) | P14602 | 23 | 2 | ||||
| Glutathione S-transferase µ | A2AE89 | 24 | 2 | ||||
| NOD-derived Cd11c-positive dendritic cells cDNA | Q3TCH2 | 25 | 2 | ||||
| 5695 | βA3/A1-crystallin | Q9QXC6 | 25 | 66 | −1.53 | −20.2 | −13.04 |
| βB2-crystallin | P62696 | 23 | 56 | ||||
| βB3-crystallin | CRBB3 | 24 | 13 | ||||
| START domain containing 9 | AZAKH9 | 408 | 1 | ||||
| 5719 | βB2-crystallin | P62696 | 23 | 46 | 1.23 | 8.38 | 6.87 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 36 | ||||
| βB3-crystallin | CRBB3 | 24 | 32 | ||||
| βB1-crystallin | CRBB1 | 28 | 10 | ||||
| αA-crystallin | Q569M7 | 20 | 4 | ||||
| 5753 | βB3-crystallin | CRBB3 | 24 | 67 | 1.49 | 9.82 | 6.67 |
| βB2-crystallin | P62696 | 23 | 29 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 9 | ||||
| 5789 | βB3-crystallin | CRBB3 | 24 | 40 | 1.02 | 17.66 | 17.52 |
| βB1-crystallin | CRBB1 | 28 | 21 | ||||
| βB2-crystallin | P62696 | 23 | 6 | ||||
| αA-crystallin | Q569M7 | 20 | 4 | ||||
| Development and differentiation enhancing factor 2 | Q7SIG6 | 107 | 3 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 2 | ||||
| 5799 | βB1-crystallin | CRBB1 | 28 | 38 | −1.1 | 13.89 | 15.37 |
| βA3/A1-crystallin | Q9QXC6 | 25 | 11 | ||||
| Proteasome subunit beta type 4 | P99026 | 29 | 6 | ||||
| αA-crystallin | Q569M7 | 20 | 3 | ||||
| βA4-crystallin | Q9JJV0 | 22 | 3 | ||||
| Development and differentiation enhancing factor 2 | Q7SIG6 | 107 | 2 | ||||
| Adult male small intestine cDNA | Q9CPN9 | 26 | 2 | ||||
| Prickle3 protein | Q8CIL5 | 72 | 1 | ||||
| βB3-crystallin | CRBB3 | 24 | 1 | ||||
| Cardiotrophin-like cytokine factor 1 precursor | Q9QZM3 | 25 | 1 | ||||
| βA2-crystallin | CRBA2 | 22 | 1 | ||||
| UTP14, U3 small nucleolar ribonucleoprotein homolog A yeast | Q4QY64 | 87 | 1 | ||||
| Leucine-rich repeat-containing protein 33 precursor | Q8BMT4 | 77 | 1 | ||||
| Adult male testis cDNA RIKEN | Q9D9G7 | 28 | 1 | ||||
| GTP-binding protein Di-Ras1 | Q91Z61 | 22 | 1 | ||||
| NOD-derived CD11c positive dendritic cells cDNA | Q8VDD8 | 55 | 1 | ||||
| 16 days neonate thymus cDNA | Q3TRH2 | 48 | 1 | ||||
| Bone marrow macrophage cDNA | Q3UAB1_MOUSE | 50 | 1 | ||||
| DNA polymerase epsilon subunit 2 | DPOE2_MOUSE | 59 | 1 | ||||
| 5830 | βB1-crystallin | CRBB1 | 28 | 29 | 1.19 | −8.59 | −10.09 |
| βS (γS-crystallin) | O35486 | 21 | 15 | ||||
| γC-crystallin | Q61597 | 21 | 14 | ||||
| γB-crystallin | Q6PHP7 | 21 | 10 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 4 | ||||
| γD-crystallin | Q6PGI0 | 21 | 4 | ||||
| Glutathione S-transferase P1 | P19157 | 24 | 3 | ||||
| αA-crystallin | Q569M7 | 20 | 2 | ||||
| 6012 | αB-crystallin | P23927 | 20 | 42 | 1.07 | 14.08 | 13.3 |
| γB-crystallin | Q6PHP7 | 21 | 29 | ||||
| βA2-crystallin | CRBA2 | 22 | 25 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 23 | ||||
| βS (γS-crystallin) | O35486 | 21 | 20 | ||||
| γN-crystallin | Q8VHL5 | 21 | 18 | ||||
| αA-crystallin | Q569M7 | 20 | 15 | ||||
| γA-crystallin | P04345 | 21 | 8 | ||||
| γD-crystallin | Q6PGI0 | 21 | 7 | ||||
| γC-crystallin | Q61597 | 21 | 3 | ||||
| 6117 | αB-crystallin | P23927 | 20 | 43 | −1.14 | 4.51 | 5.17 |
| γB-crystallin | Q6PHP7 | 21 | 24 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 17 | ||||
| βA2-crystallin | CRBA2 | 22 | 14 | ||||
| γA-crystallin | P04345 | 21 | 11 | ||||
| γD-crystallin | Q6PGI0 | 21 | 10 | ||||
| γS-crystallin | O35486 | 21 | 7 | ||||
| αA-crystallin | Q569M7 | 20 | 4 | ||||
| γN-crystallin | Q8VHL5 | 21 | 4 | ||||
| γC-crystallin | Q61597 | 21 | 3 | ||||
| γE-crystallin | A2RTH4 | 21 | 3 | ||||
| Sodium channel voltage-gated Type III alpha | A2ASI5 | 221 | 1 | ||||
| 6161 | αB-crystallin | P23927 | 20 | 30 | 1.61 | 10.3 | 6.45 |
| αA-crystallin | Q569M7 | 20 | 19 | ||||
| γB-crystallin | Q6PHP7 | 21 | 6 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 6 | ||||
| γD-crystallin | Q6PGI0 | 21 | 5 | ||||
| βA2-crystallin | CRBA2 | 22 | 4 | ||||
| γA-crystallin | P04345 | 21 | 3 | ||||
| Tenascin precursor | Q80YX1 | 232 | 2 | ||||
| Mark 1 protein | Q14DQ3 | 88 | 1 | ||||
| Tenascin precursor | Q80YX1 | 232 | 1 | ||||
| 6341 | αA-crystallin | Q569M7 | 20 | 31 | 1.2 | −60.51 | −71.83 |
| αB-crystallin | P23927 | 20 | 9 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 6 | ||||
| βA2-crystallin | CRBA2 | 22 | 5 | ||||
| γN-crystallin | Q8VHL5 | 21 | 5 | ||||
| βB1-crystallin | CRBB1 | 28 | 4 | ||||
| βA4-crystallin | Q9JJV0 | 22 | 3 | ||||
| γA-crystallin | P04345 | 21 | 2 | ||||
| 6352 | αA-crystallin | Q569M7 | 20 | 25 | 1.2 | −13.31 | −15.81 |
| αB-crystallin | P23927 | 20 | 14 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 7 | ||||
| Adult male small intestine cDNA | Q9CPN9 | 26 | 3 | ||||
| Cardiotrophin-like cytokine factor 1 precursor | Q9QZM3 | 25 | 3 | ||||
| Leucine-rich repeat-containing protein 33 precursor | Q8BMT4 | 77 | 2 | ||||
| ELMO domain-containing protein 1 | Q3V1U8 | 38 | 2 | ||||
| γA-crystallin | P04345 | 21 | 1 | ||||
| UTP14, U3 small nucleolar ribonucleoprotein homolog A yeast | Q4QY64 | 87 | 1 | ||||
| βA2-crystallin | CRBA2 | 22 | 1 | ||||
| Adult male testis cDNA RIKEN | Q9D9G7 | 28 | 1 | ||||
| GTP-binding protein Di-Ras 1 | Q91Z61 | 22 | 1 | ||||
| Regulator of nonsense transcripts 1 | Q9EPU0 | 124 | 1 | ||||
| 6485 | αA-crystallin | Q569M7 | 20 | 26 | 1.35 | 34.08 | 25.47 |
| Adult male small intestine cDNA | Q9CPN9 | 26 | 2 | ||||
| αB-crystallin | P23927 | 20 | 2 | ||||
| Adult male testis cDNA RIKEN | Q9D9G7 | 28 | 2 | ||||
| γA-crystallin | P04345 | 21 | 1 | ||||
| Pyruvate carboxylase, mitochondrial | Q05920 | 130 | 1 | ||||
| GTP-binding protein Di-Ras 1 | Q91Z61 | 22 | 1 | ||||
| ATPase family AAA domain-containing protein 5 (chromosome fragility-associated gene 1 protein | Q4QY64 | 204 | 1 | ||||
| MKIAA0327 splice variant | Q8CHE5 | 102 | 1 | ||||
| 6520 | γA-crystallin | P04345 | 21 | 32 | 1.22 | −5.96 | −7.23 |
| γB-crystallin | Q6PHP7 | 21 | 22 | ||||
| γC-crystallin | A3RLD4 | 21 | 19 | ||||
| γD-crystallin | Q6PGI0 | 21 | 8 | ||||
| Adult male small intestine cDNA | Q9CPN9 | 26 | 1 | ||||
| 6663 | αA-crystallin | Q569M7 | 20 | 35 | 1.75 | 15.24 | 8.8 |
| γC-crystallin | A3RLD4 | 21 | 11 | ||||
| γA-crystallin | P04345 | 21 | 8 | ||||
| γB-crystallin | Q6PHP7 | 21 | 7 | ||||
| 2 days neonate thymus thymic cells cDNA | Q7TNP7 | 69 | 3 | ||||
| Adult male small intestine cDNA | Q9CPN9 | 26 | 2 | ||||
| γD-crystallin | Q6PGI0 | 21 | 2 | ||||
| Nuclear protein localization protein 4 homolog | P60670 | 68 | 2 | ||||
| 6668 | αA-crystallin | Q569M7 | 20 | 28 | 1.72 | 73.82 | 43.31 |
| Adult male small intestine cDNA | Q9CPN9 | 26 | 2 | ||||
| 2 days neonate thymus thymic cells cDNA | Q7TNP7 | 69 | 2 | ||||
| Nuclear protein localization protein 4 homolog | P60670 | 68 | 2 | ||||
| Adult male testis cDNA RIKEN | Q9D9G7 | 28 | 2 | ||||
| ELMO domain-containing protein 1 | Q3V1U8 | 38 | 2 | ||||
| Prickle3 protein | Q8CIL5 | 72 | 2 | ||||
| 6788 | αA-crystallin and many others | Q569M7 | 20 | 30 | 2.57 | 498.57 | 195.82 |
| Nuclear protein localization protein 4 homolog | P60670 | 68 | 3 | ||||
| 2 days neonate thymus thymic cells cDNA | Q7TNP7 | 69 | 2 | ||||
| 7068 | αA-crystallin | Q569M7 | 20 | 27 | −1.05 | −21.14 | −20.03 |
| 2 days neonate thymus thymic cells cDNA | Q7TNP7 | 69 | 2 | ||||
| Prickle3 protein | Q8CIL5 | 72 | 2 | ||||
| γA-crystallin | P04345 | 21 | 2 | ||||
| 7089 | γS-crystallin | O35486 | 21 | 14 | 1.76 | −8.63 | −15.01 |
| γC-crystallin | A3RLD4 | 21 | 8 | ||||
| γB-crystallin | Q6PHP7 | 21 | 3 | ||||
| αA-crystallin | Q569M7 | 20 | 2 | ||||
| γD-crystallin | Q6PGI0 | 21 | 1 | ||||
| 7269 | αA-crystallin | Q569M7 | 20 | 22 | 1.1 | 55.72 | 51.25 |
| UTP14, U3 small nucleolar ribonucleoprotein homolog A yeast | Q4QY64 | 87 | 2 | ||||
| Palmitoyl protein thioesterase-like protein | Q8R2F8 | 16 | 1 | ||||
| 7419 | αA-crystallin | Q569M7 | 20 | 18 | −2.33 | 14.04 | 32.99 |
| UTP14, U3 small nucleolar ribonucleoprotein homolog A yeast | Q4QY64 | 87 | 2 | ||||
| 2 days neonate thymus thymic cells cDNA | Q7TNP7 | 69 | 2 | ||||
| Adult male small intestine cDNA | Q9CPN9 | 26 | 2 | ||||
| 7540 | αA-crystallin | Q569M7 | 20 | 20 | 1.02 | 91.8 | 91.26 |
| Ceruloplasmin precursor | Q61147 | 121 | 8 | ||||
| Heparin cofactor 2 precursor | HEP2_mouse | 54 | 4 | ||||
| Plexin-A4 precursor | Q80UG2 | 213 | 3 | ||||
| Attractin | Q9WU60 | 158 | 3 | ||||
| Serum Amyloid-P component | Q4JFI8 | 26 | 3 | ||||
| Lumican precursor | P51885 | 38 | 2 | ||||
| Gelsolin | P13020 | 86 | 2 | ||||
| UTP14, U3 small nucleolar ribonucleoprotein homolog A yeast | Q4QY64 | 87 | 2 | ||||
| ELMO domain-containing protein 1 | Q3V1U8 | 38 | 2 | ||||
| Complement factor I precursor | Q61129 | 67 | 2 | ||||
| 7568 | αA-crystallin | Q569M7 | 20 | 17 | −1.45 | 134.39 | 197.02 |
| Ceruloplasmin precursor | Q61147 | 121 | 9 | ||||
| Gelsolin | P13020 | 86 | 4 | ||||
| Lumican precursor | P51885 | 38 | 4 | ||||
| Pyruvate carboxylase, mitochondrial | Q05920 | 130 | 3 | ||||
| Plexin-A4 precursor | Q80UG2 | 213 | 3 | ||||
| ELMO domain-containing protein 1 | Q3V1U8 | 38 | 3 | ||||
| Complement factor I precursor | Q61129 | 67 | 3 | ||||
| Cardiotrophin-like cytokine factor 1 precursor | Q9QZM3 | 25 | 2 | ||||
| GTP-binding protein Di-Ras 1 | Q91Z61 | 22 | 2 | ||||
| Myoferlin | Q69ZN7 | 233 | 2 | ||||
| 7751 | αA-crystallin | Q569M7 | 20 | 9 | 1.15 | 160.65 | 141.07 |
| Ceruloplasmin precursor | Q61147 | 121 | 8 | ||||
| Lumican precursor | P51885 | 38 | 4 | ||||
| ELMO domain-containing protein 1 | Q3V1U8 | 38 | 2 | ||||
| Cardiotrophin-like cytokine factor 1 precursor | Q9QZM3 | 25 | 2 | ||||
| 8192 | αA-crystallin | Q569M7 | 20 | 7 | 1.24 | 75.08 | 61.08 |
| Ceruloplasmin precursor | Q61147 | 121 | 7 | ||||
| Lumican precursor | P51885 | 38 | 3 | ||||
| ELMO domain-containing protein 1 | Q3V1U8 | 38 | 3 | ||||
| Adult male testis cDNA RIKEN | Q9D9G7 | 28 | 3 | ||||
| Adult male small intestine cDNA | Q9CPN9 | 26 | 2 | ||||
| UTP14, U3 small nucleolar ribonucleoprotein homolog A yeast | Q4QY64 | 87 | 2 | ||||
WT, wild-type.
Among the crystallins, the amount of αA-crystallin that was crosslinked and associated with βA3/A1-crystallin increased in four spots, and αA-crystallin associated with annexin increased 3-fold in one spot (spot 4872). Normal and basic forms of βB1-crystallin decreased 6- to 25-fold in three spots. More basic forms of βB2- and βB3-crystallins in association with glutathione S-transferase-µ (GST-µ) increased 8-21-fold in two spots. Very acidic forms of βB2-crystallin, βB3-crystallin, and GST-µ decreased 18-fold in spot 5625.
αB-crystallin that was degraded and associated with β- and γ-crystallins increased in two spots. The amount of αA-crystallin slightly larger than 20 kDa decreased 60- to 71-fold (spot 6341), and 13-fold when associated with β- and γ-crystallins (spot 6352). Acidic and degraded αA-crystallin increased 34-fold (spot 6485). Spots containing γA-, γB-, γC-, and γD-crystallins decreased 6-fold. Degraded αA-crystallin associated with γC-, γA-, and γB- crystallins increased 15-fold. Nine spots containing degraded αA-crystallin increased in mutant lenses, whereas degraded but more basic forms than the original αA-crystallin decreased in abundance (spots 7068, 7089, and 7419). Wild type and αA-R49C homozygous lenses were further analyzed (Fig. S2 in File S1 and Table S1). There was a large change in βB2-crystallin expression with age of the wild type lenses (from 2 days to 2 weeks). Spots 5446 and 5466 (Table S1) show an increase in βB2-crystallin in wild type mouse lenses confirming the results of a previous study [23].
Two-week Old αB-R120G Mouse Lenses
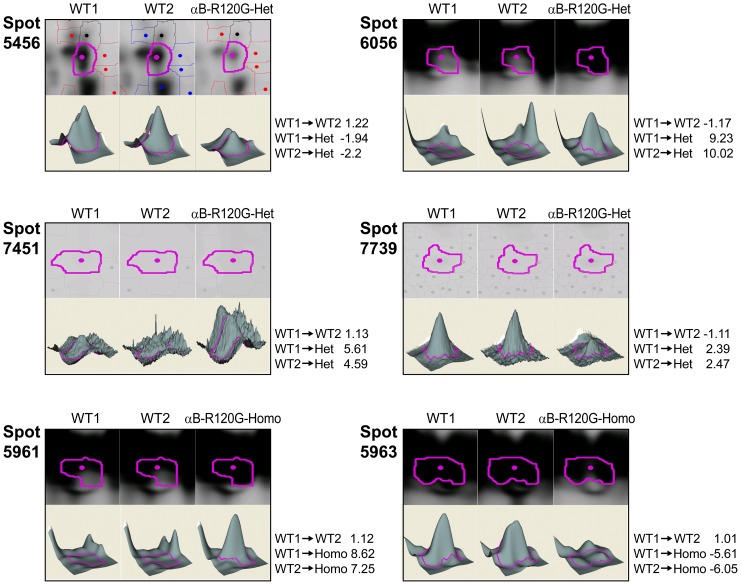
Figure 6 shows 2D gels for 14-day-old WT and mutant proteins of αB-R120G knock-in mice. Table 4 shows the approximately 50 protein spots that showed a change in abundance between WT and mutant spots in the 14-day-old lenses. Figure 7 shows 3D plots for some of the protein spots that changed in abundance in the αB-R120G mutant lenses. Heterozygous αB-R120G lenses showed several spots with decreased abundance of phosphoglycerate mutase (spots 5353, 5441, 5456 and 5468). Phosphoglycerate mutase was the only protein in spots 5353 and 5468 but was associated with βB1-crystallin in spots 5441 and 5456. αA- and αB-crystallins decreased in a very basic high molecular weight spot (spot 2982). The abundance of αA-crystallin increased 2.8- to 10- fold in spot 6415, and was slightly degraded and more acidic than normal αA-crystallin. In the same region, spots 6449 and 6848 (αA-crystallin associated with grifin) increased 12-fold and 2.5 fold, respectively. Degraded and more basic forms of αA-crystallin alone (spots 6920 and 7257) or with αB-crystallin and βB3-crystallin (spot 7451) also increased in abundance in heterozygous lenses. A spot containing αA-, γA-, γB-, γC-, and γD-crystallins also decreased 2.7-fold in heterozygous lenses.
Figure 6. 2D-DIGE analysis of proteomic changes in whole lenses of 14-day-old mice induced by knock-in of the αB-R120G mutation.
(A) 2D gel of lens proteins labeled with cyanine dyes derived from WT1 proteins labeled with Cy5, WT2 proteins labeled with Cy3, and αB-R120G heterozygous lens proteins labeled with Cy2. (B) 2D gel of lens proteins labeled with cyanine dyes derived from WT1 proteins labeled with Cy2, WT2 proteins labeled with Cy3, and αB-R120G homozygous proteins labeled with Cy5. (C, D) Protein spots that were selected for analysis from the gel shown in (A) and (B) are shown in (C) and (D), respectively. Proteins were identified by tandem mass spectrometry and Mascot searches of spots that were selected from analysis of the gels. Quantitative image analysis and mass spectrometry data for the identified proteins from these gels are listed in Table 4.
Table 4. Quantitative analysis of protein abundance in 14-day-old WT, αB-R120G lenses heterozygous and homozygous.
| Spot number | Protein | UNIPROT accession number | MW (kDa) | Number of assigned spectra | WT1 vs. WT2 | WT1 vs. heterozygous | WT2 vs. heterozygous |
| 2982 | αB-crystallin | P23927 | 20 | 2 | 1.19 | −1.82 | −2.34 |
| αA-crystallin | Q569M7 | 20 | 1 | ||||
| RIKEN cDNA 2210010C04, isoform CRA_b | Q9CPN9 | 26 | 1 | ||||
| Probable peptide chain release factor C12orf65 homolog, mitochondrial | Q80VP5 | 21 | 1 | ||||
| 4441 | αA-crystallin | Q569M7 | 20 | 1 | 1.13 | −2.03 | −2.48 |
| 5353 | Phosphoglycerate mutase | O70250 | 29 | 2 | 1.15 | −2.06 | −2.55 |
| 5432 | βB1-crystallin | Q9WVJ5 | 28 | 17 | 1.06 | −1.81 | −2.06 |
| βB3-crystallin | Q9JJU9 | 24 | 5 | ||||
| αA-crystallin | Q569M7 | 20 | 1 | ||||
| αB-crystallin | P23927 | 20 | 1 | ||||
| 5441 | βB1-crystallin | Q9WVJ5 | 28 | 2 | 1.06 | −1.93 | −2.2 |
| Phosphoglycerate mutase | O70250 | 29 | 2 | ||||
| 5456 | βB1-crystallin | Q9WVJ5 | 28 | 5 | 1.22 | −1.94 | −2.56 |
| Phosphoglycerate mutase | O70250 | 29 | 4 | ||||
| γC-crystallin | Q61597 | 21 | 1 | ||||
| γB-crystallin | Q6PHP7 | 21 | 1 | ||||
| 5468 | Phosphoglycerate mutase | O70250 | 29 | 2 | −1.03 | −2.4 | −2.52 |
| 5492 | βB1-crystallin | Q9WVJ5 | 28 | 1 | 1.17 | −2.95 | −3.72 |
| Riken cDNA2210010C04 isoformCRA-b | Q9CPN9 | 26 | 1 | ||||
| 5960 | Putative uncharacterized protein | Q8C2C1 | 20 | 1 | 1.3 | −2.52 | −3.52 |
| γB-crystallin | Q6PHP7 | 21 | 1 | ||||
| Riken cDNA2210010C04 isoformCRA-b | Q9CPN9 | 26 | 1 | ||||
| 6005 | γC-crystallin | Q61597 | 21 | 1 | 1.54 | −1.81 | −2.99 |
| 6056 | αB-crystallin | P23927 | 20 | 25 | −1.17 | 9.23 | 10.02 |
| βB2-crystallin | P62696 | 23 | 10 | ||||
| βA3/A1-crystallin | Q9QXC6 | 25 | 9 | ||||
| αA-crystallin | Q569M7 | 20 | 7 | ||||
| βS-crystallin | O35486 | 21 | 4 | ||||
| γD-crystallin | Q6PGI0 | 21 | 4 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 4 | ||||
| γB-crystallin | Q6PHP7 | 21 | 2 | ||||
| βA2-crystallin | Q9JJV1 | 22 | 2 | ||||
| 6415 | αA-crystallin | Q569M7 | 20 | 7 | 3.28 | 10.01 | 2.83 |
| 6449 | αA-crystallin | Q569M7 | 20 | 53 | 2 | 12.67 | 5.87 |
| 6848 | Grifin | Q9D1U0 | 16 | 5 | −1.04 | 2.42 | 2.33 |
| αA-crystallin | Q569M7 | 20 | 5 | ||||
| 6920 | αA-crystallin | Q569M7 | 20 | 4 | −1.55 | 1.89 | 2.71 |
| 7257 | αA-crystallin | Q569M7 | 20 | 6 | −1.13 | 2.7 | 2.84 |
| 7451 | αB-crystallin | P23927 | 20 | 6 | 1.13 | 5.61 | 4.59 |
| αA-crystallin | Q569M7 | 20 | 5 | ||||
| βB3-crystallin | Q9JJU9 | 24 | 2 | ||||
| 7739 | αA-crystallin | Q569M7 | 20 | 5 | −1.11 | 2.39 | 2.47 |
| Riken cDNA2210010C04 isoform CRA-b | Q9CPN9 | 26 | 1 | ||||
| 6061 | γC-crystallin | Q61597 | 21 | 2 | −1.04 | −2.63 | −2.73 |
| αA-crystallin | Q569M7 | 20 | 2 | ||||
| γB-crystallin | Q6PHP7 | 21 | 2 | ||||
| γD-crystallin | Q6PGI0 | 21 | 1 | ||||
| γA-crystallin | P04345 | 21 | 1 |
WT, wild-type.
Figure 7. Quantitative analysis of the changes in abundance of proteins in postnatal 14-day-old lens from WT and αB-R120G knock-in mice by mass spectrometry.
The 3D data sets for representative proteins in two WT (WT1 and WT2) and one αB-R120G mutant sample are shown. (A) WT1 and WT2 proteins were labeled with Cy3 and Cy5 dyes, respectively, and αB-R120G heterozygous mutant lenses with Cy2. (B) WT1 and WT2 proteins were labeled with Cy2 and Cy3 dyes, respectively, and αB-R120G homozygous mutant lenses with Cy5. Fold changes between each sample are indicated on the right. See Table 4 for the identity of proteins present in each protein spot.
Homozygous αB-R120G lenses showed an 8-fold increase in the abundance of a more acidic spot (5961) containing αB- and other crystallins, whereas the more basic spot 5963 decreased 5.6-fold. Spot 6120 containing αA-, αB-, and γB-crystallins also increased in abundance in homozygous lenses. This spot was more acidic than the other αB-crystallin spots and was located near the αA-crystallin position. Spot 5938, which was very close to spot 5963 but slightly more acidic, also decreased in abundance. Spots 7164 increased in abundance by 2.0-fold in αB-R120G homozygous lenses relative to WT. It contained both αA- and αB-crystallins, which were more degraded and basic than the original proteins. Overall, a few unique spots changed in abundance in αB-R120G homozygous lenses than in αB-R120G heterozygous lenses.
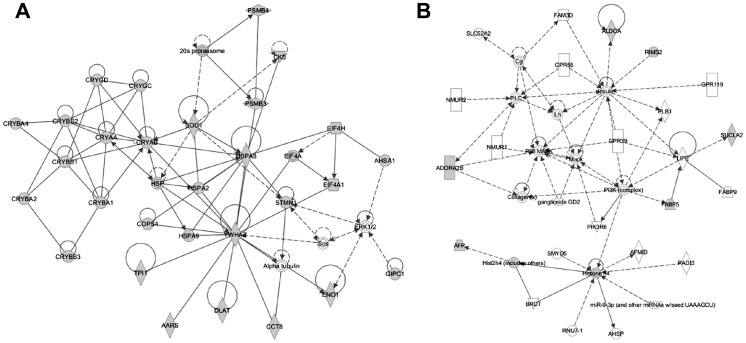
To obtain a general perspective of cellular systems affected in the αA-R49C and αB-R120G mutant lenses, we mapped the proteins identified by mass spectrometric analysis to existing networks. These networks represent interactions known to occur among the proteins identified in our analysis. The interactions shown in these networks did not originate from lens tissue in our study. Ingenuity Pathway software analysis generated eight different networks for the proteins identified in the αA-R49C mutant lenses, two of which are shown in Figure 8, with additional networks shown in Fig. S3 in File S1. One network generated by this approach included the chaperones HSPA8 and HSPA2 which interact with αB-crystallin. A second network included histone H4 which has been shown to interact with the PI3kinase complex. Four different protein networks were generated by this method in the αB-R120G lenses including one in which the ubiquitin proteasome was at the hub (Figure S4). An interaction between the lens-specific protein grifin and the transcription factor IKZF1 was evident in both αA-R49C and αB-R120G mutant lenses (Figs. S3, S4 in File S1, and Table S2).
Figure 8. Ingenuity Pathways analysis of lens proteins identified in αA-R49C knock-in mutant lenses.
Analysis of altered protein networks by Ingenuity Pathway software. Biological networks and pathways generated from input data (Wild-type vs. αA-R49C, Tables 1-3 and Table S1) indicate proteins with altered abundance in gray. (A) A network with HSPA8 at the hub. (B) A second network highlights Histone H4 at the hub of the protein connectivity map. Additional networks are shown in Fig. S3 in File S1.
Discussion
Several mechanisms can cause hereditary cataracts, including increases in protein mass, aggregation, insolubility, and light scattering. In the present study, we characterized changes in protein abundance at an early postnatal age in mouse lenses with knock-in mutations of αA- or αB-crystallins. We also investigated proteins that showed increased association with αA- or αB-crystallins in mutant lenses, defined by an increase in the level of urea-resistant protein in the same spot.
Several important assumptions of this study require further discussion. The present study identified changes in abundance of many spots in which αA- or αB-crystallin was present together with other proteins. This association indicates similar pI and molecular weights of the ancillary proteins and the α-crystallin in these spots. We cannot speculate on the mechanism by which the proteins are associated with α-crystallins. Our evidence from 2D-gel analysis is suggestive of an association, but is not conclusive. Since this association was observed in multiple gels of wild type and knock-in mutant lenses, the presence of αA- and/or αB-crystallin with specific proteins in the same spots is suggestive of a true association. Previous studies suggest that mutant α-crystallins may exert a gain-of-toxic function on the lens [25]. Thus, it is possible that the differences in protein abundances between normal and knock-in mouse lenses may not be directly due to incompetent chaperones per se, although a previous study with the αA and αB-crystallin DKO mouse lenses strengthens the conclusions of the present work [23]. Nevertheless, a toxic gain-of function by the mutant α-crystallins could be a potential factor in the observed results.
There was a significant decrease in the abundance of actin (15.6-fold), filensin (17.5-fold), βA3/A1-crystallin, γD-crystallin (6-fold), and grifin (1.74-fold). We also observed degradation of glutamate dehydrogenase, which was associated with cytochrome c in some spots. Because the abundance of these proteins changed at a young age, even in the heterozygous mutant αA-R49C lens, with no apparent change in lens morphology, it is very likely that they are in vivo substrates of α-crystallin. Our analysis also suggests that enzymes involved in lens metabolism, such as creatine kinase B and phosphoglycerate mutase, and the detoxification enzyme GST-µ are in vivo substrates of αA- and αB-crystallins. These proteins may be structurally labile and might interact with αA- and αB-crystallins for conformational maintenance during the early stages of lens growth but become more stably associated with the protein when the chaperone is mutated. Structural analysis of these enzymes is necessary to reveal any common structural domains. These findings suggest that key metabolic pathways are involved in the mechanism of cataract formation by the αA-R49C or αB-R120G mutations. The decrease in phosphoglycerate mutase levels in the postnatal αB-R120G knock-in mouse lens suggests that mutation of the chaperone protein in the lens affects lens metabolism even before the opacification process becomes evident.
The association of histones with αA-crystallin increased in the mutant lenses. The possibility that histones are protected by α-crystallins is particularly important because histones are critical and long-lived proteins [26]. The R49C mutant of αA-crystallin exhibits increased apoptosis and aberrant accumulation of nuclei in the lens, suggesting a possible explanation for the increased abundance of histones [15], [27]. We previously reported an increased abundance of histones in αA/αB double knock-out (DKO) lenses [23], and in lens cells expressing another human cataract-related mutant of αA-crystallin in which the arginine 116 residue is replaced by cysteine [28]. Therefore it seems likely that histones may be protected by αA- and αB-crystallins in the lens. In 2-week-old αA-R49C mutant lenses there was an increase in αA-crystallin associated with annexins. These proteins are associated with apoptosis, which has been observed in the αA-R49C mouse. Interestingly, phosphoglycerate mutase, α-enolase, and peptidyl-prolyl cis-trans isomerase are oxidized and have reduced enzyme activities in Alzheimer's disease, another disease associated with protein aggregation [29].
An intriguing observation of the present study was the presence of albumin in the 2-day-old lens (Table 1). Extracellular albumin, an abundant protein in the aqueous humor, becomes internalized in the lens in vivo [30]. It has been suggested that albumin is a carrier for lipids and other metabolites, and could be essential for normal lens physiology [31], [32]. A decrease in plasma albumin has been linked with an increased risk of human cataract [33]. The abundance of the spot containing albumin, αA-crystallin and filensin showed a 3.6-fold variation between the two biological replicates of the WT mouse lens, and increased 16- to 17-fold in the αA-R49C heterozygous lenses. Further studies will be necessary to understand the significance of these observations. We detected increased αA-crystallin in protein spots containing cytoskeletal proteins, and increased abundance of degraded and more acidic cytoskeletal proteins including spectrin-α, filensin, phakinin, tubulin, vimentin, and microtubule-associated protein RP/EB in the αA-R49C mutant knock-in lenses. The abundance of filensin and phakinin decreased in αA-R49C mutant lenses, suggesting that these proteins are in vivo substrates for αA-crystallin. The spectrin-actin membrane skeleton contributes significantly to lens fiber cell organization and is functionally linked to the phakinin-filensin network [34]. Disruption of the spectrin-actin membrane cytoskeletal complexes may therefore be responsible for the morphological changes observed in αA-R49C homozygous mutant lenses at an early age [27], [35]. There was also an increase in the amount of degraded and more acidic grifin, a protein whose interaction with αA-crystallin has been demonstrated previously [36], and the abundance of αA-R49C associated with grifin increased 16-fold in homozygous mutant lenses. The amount of hemoglobin subunit α decreased in αA-R49C homozygous mutant lenses indicating that it is a likely substrate for αA-crystallin. Previous studies support the possibility that destabilized forms of hemoglobin show increased binding to αB-crystallin in vitro [37].
We found an increase in β-crystallin isoforms with more acidic pI in the mutant lenses. Decreases in more basic forms of βB1- and βB3-crystallins and increases in more acidic forms indicate that αA-crystallin is a chaperone for these two crystallins. Furthermore, αA- and αB-crystallins were increasingly associated with β-crystallins in the mutant lenses, suggesting that they may have formed heteromeric complexes. Previous studies have identified covalent multimers of crystallins in aging human lenses [38]. Recently, the crosslinks between β-crystallin isoforms have been identified by mass spectrometry [39]. Deamidation of βB2-crystallin has been proposed to disrupt normal crystallin structure and short-range order necessary for lens transparency [40]. Deamidation has been shown to lower the temperature necessary for βB2-crystallin unfolding and aggregation, suggesting decreased βB2-crystallin stability, although its 3D dimeric structure was not significantly altered [41]. Interestingly, the nature and amount of the destabilized β-crystallin intermediate is important for recognition by the chaperone [42]. Decreased amounts of βB1-crystallin were detected in five spots and in an additional four spots containing other β-crystallin polypeptides. αA-crystallin was associated with β-crystallins in these spots. The decrease in βB1-crystallin was noteworthy because βB1-crystallin has a unique role in promoting higher order crystallin association in the lens, and any change in this order could result in increased light scattering and loss of transparency [43]–[45]. The amount of αA and αB-crystallins associating with βA3/A1-, βA2-, and βA4-crystallins increased significantly in homozygous 2-day-old lenses. Our studies also demonstrated a decrease in γ-crystallins in homozygous lenses at a young age. Many of these changes occurred in a mutation- and dose-dependent manner; i.e., changes in the amounts of certain proteins were greater in the complete absence of a WT αA-crystallin gene (homozygous mutant) than with only one copy of the WT gene (heterozygous mutant). Examples are shown in Tables 1-3 for the αA-R49C protein. The effect of developmental age was investigated using 2- and 14-day-old R49C mutant lenses (Fig. S2 in File S1 and Table S1). The increased abundance of several proteins and the degradation of αA-crystallin previously observed in 2-day-old homozygous mutant lenses were confirmed at 14 days.
An important conclusion of the present study is that the αB-R120G mutation causes specific in vivo changes in protein abundance. Protein changes in the αB-R120G lenses were distinctly different from those in αA-R49C mutant lenses. The main changes in the αB-R120G mutant lens included altered abundance of β- and γ-crystallins, increased degradation of αA-, αB-, and γ-crystallins, and degradation of phosphoglycerate mutase, a glycolytic enzyme that is very important in metabolism but has not been studied in the lens in detail [46]–[49]. There was also a 12-fold increase in the amount of αA-crystallin associated with grifin in these lenses.
Our studies demonstrated that 2-week-old αA-R49C homozygous lenses contained a high abundance of low molecular weight proteins (<14 kDa) indicating that the absence of WT αA-crystallin leads to protein instability, greater susceptibility to proteolysis, and protein degradation. This occurred as a primary event at an early postnatal stage. Previous studies have identified lens protein truncation with age in human lenses [50], [51]. In future work, we intend to identify the common structural features that make the proteins more labile to proteolysis, which will provide critical information needed to develop a model of in vivo cataract formation. Our previous studies involving molecular weight measurements of the αA-R49C homozygous lenses by light scattering also demonstrated an increase in low molecular weight proteins (∼15 kDa) in these lenses [10]. We first examined the presence of low molecular weight proteins in the homozygous lenses, and then compared WT, heterozygous, and homozygous lenses. We subsequently identified the low molecular weight proteins as αA-crystallin associated with other crystallins, gelsolin and degraded ceruloplasmin, that were absent from WT mouse lenses but abundant in 2-week-old αA-R49C homozygous lenses (Table 3).
αA- and αB-crystallins were degraded in both αA-R49C and αB-R120G mutant lenses at a young age, suggesting that the mutations make these proteins less stable. Decreased stability was associated with increased crosslinking of αA-crystallin, as shown by the 15-fold increase in crosslinking of αA-crystallin to form a higher molecular weight form of approximately 40 kDa that corresponded to a crosslinked dimer. We detected increased crosslinking of αA-crystallin very early, even in lenses of 2-day-old postnatal αA-R49C heterozygous mice. Previous studies have shown that increased crosslinking can reduce the chaperone activity of α-crystallin [52]. We previously used immunoblot analysis to show an increase in the amount of water-insoluble αB-crystallin in 6-week-old αB-R120G mutant lenses [17]. We now demonstrate the presence of high molecular weight αB-crystallin in postnatal αB-R120G heterozygous and homozygous lenses, indicating that they appear early during postnatal development and consistent with their important role in opacification of αB-R120G heterozygous and homozygous lenses.
In previous studies we investigated the effect of αA/B double knock-out. The expression of βB2-crystallin increased 40-fold in 6-week-old αA/B DKO lens epithelial cells; however, the upregulation of βB2-crystallin protein was not observed in 2-day-old DKO lenses, indicating that this was not a physiological stress-induced effect but probably developmental. Surprisingly, in 6-week-old DKO mouse lenses we did not observe an increase of lower molecular weight (<14 kDa) proteins as seen in the knock-in lenses. This was the major difference between αA/B DKO lenses and αA-R49C homozygous lenses although there were other distinct differences between the proteins altered in knock-out versus knock-in αA-R49C mutant and αB-R120G mutant lenses. For example, the following effects were observed only in knock-in mutant lenses: increased abundance of creatine kinase B associated with αA-crystallin (only in αA-R49C mutant lenses); decreased abundance of phosphoglycerate mutase; changes in grifin associated with αA-crystallin; association of chaperones of the HSP70 and TCP-1 families with αA-crystallin (only in the αA-R49C mutant lenses); decreased abundance of in γ-crystallins; increased abundance of the apoptotic protein annexin. In contrast, degradation of titin, β1-catenin, and a decrease in serine threonine protein kinase were observed only in αA/αB DKO lenses. However, common features in our analyses of αA/αB-knock-out lenses and the αA-R49C and αB-R120G mutant knock-in lenses included changes in histones, hemoglobin, glutamate dehydrogenase, GST-µ, and βB1-crystallin. An increase in βB1-crystallin crosslinking and degradation was observed in the knock-in mutant lenses, but only its crosslinking increased in the knock-out lenses. Crosslinking of vimentin, tubulin, and actin increased and their abundance decreased in both knock-out and knock-in lenses. These differences in protein abundance and degradation among the three model systems indicate that specific cellular conditions dictate the substrates for α-crystallins during the early stages of lens development. This reveals variable substrate recognition by α-crystallins, which when fully understood may provide insights into how to limit the damage resulting from protein unfolding in cataracts and could implicate use of the aggregation-preventing properties of α-crystallins to control damage due to stress and disease.
It has been proposed that a combination of interaction sites could be key in substrate recognition by αA-crystallin [53]. The interaction of α-crystallins with substrate proteins is non-covalent in nature, and hydrophobic interactions need only a subtle change on the protein surface of the target proteins. Hydrophobic interactions are probably more common than previously believed because proteins are dynamic systems. A very small area might become exposed and bind to a hydrophobic surface on the chaperone protein even though the particle size may not change sufficiently to cause light scattering. Moreover, changes in the pI of proteins can occur without a stability change. Surface anisotropy can change many times in response to unidentified factors in the environment of cells. There is no change in protein size in many hereditary cataracts caused by γ-crystallin mutations, instead the cataract is formed by increased electrostatic interaction between the positively charged E107A γD-crystallin and the negatively charged α-crystallins, which increases the amount of light scattering [54], [55]. This may also occur in αA-R49C and αB-R120G mutant proteins in which the negative charge on arginine is lost when it is replaced by cysteine or glycine, respectively, and the proteins have a more acidic pI, resulting in an increase in light scattering. Thurston et al. showed that the strength of the interaction between native γ- and α-crystallins is essentially optimal for lens transparency, and that a small increase in this interaction can increase light scattering and lead to cataract [56], [57]. Further studies are needed to elucidate the hierarchy in the interaction of αA- and αB-crystallins with different proteins and the interactive sequences involved.
In summary, our studies demonstrate that characterization of changes in protein abundance in postnatal lenses is an effective way to identify in vivo substrates of αA- and αB-crystallins. Proteins that showed the greatest change in abundance at an early age are very likely to be in vivo substrates of the α-crystallins. Further quantitative studies are required to define the relationship(s) between binding of αA- and αB-crystallins and polymerization and subcellular distribution of the substrates identified in this study. This will provide new information into protein abundance changes that may occur in cataracts, even before the opacification process becomes obvious. Our approach could therefore characterize the in vivo state at the beginning of cataract development in the mouse lens, providing information necessary to develop interventional strategies to prevent future lens opacities.
Materials and Methods
Animals and Lenses
αA-R49C knock-in mice and αB-R120G knock-in mice were generated by stem cell-based techniques as described previously [17]. Mice were converted to the C57 background using speed congenics. Wild type (WT), heterozygous mutant, and homozygous mutant mice used in this study were genotyped by PCR-based methods. All procedures involving mice were performed by trained veterinary staff at the Mouse Genetics Core at Washington University. All protocols and animal procedures were approved by the Washington University Animal Studies Committee (protocol number 20110258). Lenses from two different age groups of αA-R49C knock-in mice (2-day old and 2-week-old) were analyzed by mass spectrometry (2-4 mice in each replicate set of WT1, WT2, and αA-R49C heterozygous mice and WT1, WT2, and αA-R49C homozygous mice). WT and αA-R49C knock-in mutant lenses were subjected to two-dimensional difference gel electrophoresis (2D-DIGE). Lenses from 2-week-old αB-R120G heterozygous and homozygous mice were also analyzed by 2D-DIGE.
Mass Spectrometric Analysis
Lenses were dissected and placed in lysis buffer containing 30 mM Tris-HCl (BioRad, Hercules, CA), 2 M thiourea (Sigma-Aldrich, St. Louis, MO), 7 M urea (BioRad), 4% CHAPS (BioRad), and 1× complete protease inhibitor cocktail tablets (Roche, Indianapolis, IN), pH 8.5. Lens proteins (50 µg) were labeled with 400 pmol Cy2, Cy3, or Cy5. Pools were prepared by mixing equal quantities of protein from each sample after dye labeling [58]. 2D-DIGE was performed at the Proteomics Core Laboratory according to published methods [59]. Briefly, samples were equilibrated onto immobilized pH gradient strips at 100 V and subjected to isoelectric focusing using a maximum of 10,000 focusing volts (PROTEAN IEF cell: BioRad). After focusing, proteins were reduced with Tris(2-carboxyethyl) phosphine hydrochloride (TCEP, 10 mM) and alkylated with iodoacetamide (20 mM). The strip was then layered on a 10-20% polyacrylamide gel, and proteins were separated by SDS-PAGE. Samples were imaged with a Typhoon 9400 Imager (GE Healthcare, Piscataway, New Jersey) using specific excitation and emission wavelengths for Cy2 (488 and 522 nm), Cy3 (520 and 580 nm), and Cy5 (620 and 670 nm). Control and experimental samples were labeled with blue or red fluorescent dyes and run on the same 2D gel [60], [61]. Image analysis was performed to assess differences between WT and homozygous/heterozygous mutant lenses. Individual protein spots that showed differential intensities were excised from the gel and analyzed by mass spectroscopy. Fold changes represented proteins with increased (positive numbers) or decreased (negative numbers) expression in mutant versus WT samples.
Single or multi-gel analyses were used to determine changes in protein abundance between WT and knock-in mouse lenses. Single gel analysis was performed to compare the following conditions: WT and αA-R49C heterozygous and homozygous mutant lenses (Tables 1, 3, Figs. 1 and 2, Table S1), and WT and αB-R120G heterozygous and homozygous mutant lenses (Table 4, Figs. 6 and 7). In addition, multi-gel analysis was performed with a pooled internal standard. This approach was used to compare 2-day-old WT, αA-R49C heterozygous, and homozygous mutant lenses (Table 2 and Figs. 3 and 4). Multi-gel comparisons were performed using different combinations of sample sets. The WT sample was labeled with Cy2 and mutant samples were labeled with Cy5. A pool of all samples was labeled with Cy3 and served as a standard that was common to each gel. The pooled standard, the control, and one test sample were combined and run on each gel. Images were generated and compared within each 2D gel using DeCyder v.6.5 image analysis software (GE Healthcare). Differential in-gel analysis (DIA) was used to normalize and compare quantitative differences between images from each gel. Image analysis using DeCyder software generates a relative value for the abundance of the spot in different samples, but there is no mechanism to determine the statistical significance of the differences. We therefore performed analysis of combined biological replicates for the different genotypes. In addition, we used Biological Variation Analysis (BVA) for the 2-day-old αA-R49C knock-in mouse lenses to obtain statistical significance as described below [59].
Analysis of Pool-Based Data
Pool-based studies involved a pool of proteins from all samples in the experiment, providing a common comparator for each sample. Because the pool is identical on each gel, the fold change “difference” for a spot in the pool image is 1.0 (representing no change) when comparing pool images from any two gels. This designation allowed us to compare protein amounts for spots of WT or αA-R49C heterozygous lens samples to the pool on the same gel to determine relative amounts of protein. Although WT and mutant samples were resolved on different gels, their fold changes were determined in comparison to the pooled sample, which was also run on each gel. Because the pool from one gel is identical to the pool from another, the WT and mutant fold change values could be directly compared. Pairwise analysis of proteins across different physical gels was performed using the BVA module to quantify relative differences between the samples [59]. BVA compares the quantitative value of the spot as it is represented among different samples. BVA data generates t-test and assigns p value to identify statistical significance. p < 0.05 denotes statistical significance (Table 2).
Database Searching
The mass spectra were acquired using nano-LC-MS as previously described [62]. All tandem mass spectrometry samples were analyzed using Mascot (Matrix Science, London, UK; version 2.1.1.0) as previously described [23]. Mascot was set to search the Uniprot mouse database (downloaded 12/28/2010, 135387 entries) using trypsin as the digestion enzyme, with a fragment ion mass tolerance of 0.80 Da and a parent ion tolerance of 50 ppm for data from the LTQ FT mass spectrometer. The QSTAR data were searched using a parent and fragment tolerance of 0.1 Da. The iodoacetamide derivative of cysteine was specified in Mascot as a fixed modification and methionine as a variable modification. Scaffold software (v. 3.6.1) was used to display proteomic data. Additional data processing details have been previously described [59].
Criteria for Protein Identification
Scaffold (version Scaffold_3_01_00, Proteome Software Inc., Portland, OR) was used to qualify MS/MS-based peptide and protein identifications [63]. Protein identification was accepted if identity could be established at >95.0% probability and involved at least one identified peptide. Protein probabilities were assigned using the Protein Prophet algorithm ([64] AI et al 2003). Proteins that contained similar peptides but were not differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Mass spectra for all the proteins identified in this study are shown in Table S4.
Knowledge-based Network Analysis
After false positive analysis (Protein Prophet) and removal of contaminants (e.g., keratins), proteins listed in Tables 1-4 and S1 (identified by UNIPROT accession numbers) were entered into Ingenuity Pathways (www.ingenuity.com) (IPA, version 8.8, Redwood City, CA) as a *.xls file. The software mapped 99 of 118 Gi numbers, corresponding to 99 gene symbols. Duplicate names corresponding to the same gene were eliminated. Ingenuity was set to generate up to 25 networks containing up to 35 members each, with no additional restrictions. Biological networks and pathways were generated from the input data (‘focus genes”) and gene objects in the Ingenuity Pathways Knowledge Base (IPKB). Interaction networks generated using this method showed proteins present in our samples as shaded in grey and other interacting proteins not identified from these gels as unshaded.
Supporting Information
Analysis of proteins that showed differences in abundance between 2-day-old WT, 14-day-old WT and 2-day-old αA-R49C homozygous mouse lenses. WT, Wild-type.
(DOC)
Ingenuity Pathway Analysis (IPA) molecules table for proteins affected by αA-crystallin R49C mutation in the mouse lens.
(XLS)
Ingenuity Pathway Analysis (IPA) molecules table for proteins affected by αB-crystallin R120G mutation in the mouse lens.
(XLS)
Mass spectrometry and database search results for proteins identified in this study.
(XLSX)
Supplementary figures. Figure S1, 2D-DIGE analysis of proteomic changes in whole lenses of 2-day-old mice with knock-in of the αA-R49C mutation. Protein spots that were picked for analysis from the 2D gels of WT and αA-R49C heterozygous (A) and WT and αA-R49C homozygous lenses (B-D) shown in Figure 1. Quantitative image analysis and mass spectrometry data for identified proteins from these gels are listed in Table 1. Figure S2, 2D-DIGE analysis of proteomic changes in whole lenses of 2-day-old and 14-day-old mice induced by knock-in of the αA-R49C mutation. (A) A 2D gel of lens proteins labeled with cyanine dyes derived from 2-day-old WT proteins labeled with Cy3, 14-day-old WT proteins labeled with Cy5, and αA-R49C homozygous lens proteins labeled with Cy2. (B, C) Protein spots that were selected for analysis from the gel shown in (A). Proteins were identified by tandem mass spectrometry and Mascot searches of spots that were selected from the gels. Quantitative image analysis and mass spectrometry data for the identified proteins from these gels are listed in Table S1. Figure S3, Protein connectivity networks identified by Ingenuity Pathway analysis of lens proteins in αA-R49C knock-in mutant lenses. Analysis of altered protein networks by Ingenuity Pathway software. Biological networks and pathways generated from input data (Wild type vs. αA-R49C, Tables 1–3 and S1) indicate proteins with changed abundance in gray. (A) A network with GAPDH at the hub. (B) A second network with F-actin at the hub. (C) A third network highlights NPM1 at the hub of the protein connectivity map. (D) A fourth network with TGFB1 at the hub. (E) A fifth network indicates the interaction between grifin and IKZF1. (F) A sixth network shows Gm5409 at the hub. Note that two additional networks are shown in Figure 8. Figure S4, Networks revealed by Ingenuity Pathway analysis of lens proteins that changed in amount in WT vs. αB-R120G knock-in lenses. Biological networks and pathways generated from input data (Wild type vs. αB-R120G, Table 4) indicate proteins with changed abundance in gray. (A) A network with MAF at the hub. (B) A second network with UBC at the hub. (C) A third network shows the interactions between grifin and IKZF1. (D) A fourth network highlights CTRB2 at the hub of the protein connectivity map.
(DOC)
Funding Statement
This work is supported by the National Institutes of Health (NIH) Grant R01EY05681-29 to U.P.A.; Core Grant EY02687; Research to Prevent Blindness grants to the Department of Ophthalmology and Visual Sciences at Washington University School of Medicine, Washington University Institute of Clinical and Translational Sciences; Grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the NIH; grants from the National Center for Research Resources (5P41RR000954-35); and a grant from the National Institute of General Medical Sciences (8 P41 GM103422-35). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Andley UP (2007) Crystallins in the eye: Function and pathology. Prog Retin Eye Res 26: 78–98. [DOI] [PubMed] [Google Scholar]
- 2. Andley UP (2008) The lens epithelium: focus on the expression and function of the alpha-crystallin chaperones. Int J Biochem Cell Biol 40: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhat S (2002) The ocular lens epithelium. Bioscience Reports 21: 537–563. [DOI] [PubMed] [Google Scholar]
- 4. Wang X, Garcia CM, Shui YB, Beebe DC (2004) Expression and regulation of alpha-, beta-, and gamma-crystallins in mammalian lens epithelial cells. Invest Ophthalmol Vis Sci 45: 3608–3619. [DOI] [PubMed] [Google Scholar]
- 5. Robinson ML, Overbeek PA (1996) Differential expression of alpha A- and alpha B-crystallin during murine ocular development. Invest Ophthalmol Vis Sci 37: 2276–2284. [PubMed] [Google Scholar]
- 6. Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, et al. (2004) Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol 86: 407–485. [DOI] [PubMed] [Google Scholar]
- 7. Horwitz J (1992) Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A 89: 10449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, et al. (1998) A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20: 92–95. [DOI] [PubMed] [Google Scholar]
- 9. Andley UP (2006) Crystallins and hereditary cataracts: molecular mechanisms and potential for therapy. Expert Rev Mol Med 8: 1–19. [DOI] [PubMed] [Google Scholar]
- 10. Andley UP, Hamilton PD, Ravi N (2008) Mechanism of insolubilization by a single-point mutation in alphaA-crystallin linked with hereditary human cataracts. Biochemistry 47: 9697–9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pande A, Zhang, J, Shekhtman, A, Pande, J. A high resolution NMR study of human gammaD crystallin and its cataract-linked P23T mutant: Small conformational changes in P23T explain its low and retrograde solubility.; 2008. pp. 284.
- 12. Xia CH, Liu H, Chang B, Cheng C, Cheung D, et al. (2006) Arginine 54 and Tyrosine 118 residues of {alpha}A-crystallin are crucial for lens formation and transparency. Invest Ophthalmol Vis Sci 47: 3004–3010. [DOI] [PubMed] [Google Scholar]
- 13. Hsu CD, Kymes S, Petrash JM (2006) A transgenic mouse model for human autosomal dominant cataract. Invest Ophthalmol Vis Sci 47: 2036–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang Q, Ding L, Phan KB, Cheng C, Xia CH, et al. (2009) Mechanism of cataract formation in alphaA-crystallin Y118D mutation. Invest Ophthalmol Vis Sci 50: 2919–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mackay DS, Andley UP, Shiels A (2003) Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet 11: 784–793. [DOI] [PubMed] [Google Scholar]
- 16. Koteiche HA, McHaourab HS (1999) Folding pattern of the alpha-crystallin domain in alphaA-crystallin determined by site-directed spin labeling. J Mol Biol 294: 561–577. [DOI] [PubMed] [Google Scholar]
- 17. Andley UP, Hamilton PD, Ravi N, Weihl CC (2011) A knock-in mouse model for the R120G mutation of alphaB-crystallin recapitulates human hereditary myopathy and cataracts. PLoS One 6: e17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Y, Zhang X, Luo L, Wu M, Zeng R, et al. (2006) A Novel {alpha}B-Crystallin Mutation Associated with Autosomal Dominant Congenital Lamellar Cataract. Invest Ophthalmol Vis Sci 47: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghosh JG, Estrada MR, Clark JI (2005) Interactive domains for chaperone activity in the small heat shock protein, human alphaB crystallin. Biochemistry 44: 14854–14869. [DOI] [PubMed] [Google Scholar]
- 20. Ghosh JG, Shenoy AK Jr, Clark JI (2007) Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry 46: 6308–6317. [DOI] [PubMed] [Google Scholar]
- 21. Bates GP (2006) BIOMEDICINE: One Misfolded Protein Allows Others to Sneak By. Science 311: 1385–1386. [DOI] [PubMed] [Google Scholar]
- 22. Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI (2006) Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science 311: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 23. Andley UP, Malone JP, Hamilton PD, Ravi N, Townsend RR (2013) Comparative proteomic analysis identifies age-dependent increases in the abundance of specific proteins after deletion of the small heat shock proteins alphaA- and alphaB-crystallin. Biochemistry 52: 2933–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma K, Vabulas RM, Macek B, Pinkert S, Cox J, et al. (2012) Quantitative proteomics reveals that Hsp90 inhibition preferentially targets kinases and the DNA damage response. Mol Cell Proteomics 11 : M111 014654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koteiche HA, McHaourab HS (2006) Mechanism of a hereditary cataract phenotype. Mutations in alphaA-crystallin activate substrate binding. J Biol Chem 281: 14273–14279. [DOI] [PubMed] [Google Scholar]
- 26. Toyama BH, Hetzer MW (2013) Protein homeostasis: live long, won't prosper. Nat Rev Mol Cell Biol 14: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xi JH, Bai F, Gross J, Townsend RR, Menko AS, et al. (2008) Mechanism of small heat shock protein function in vivo: a knock-in mouse model demonstrates that the R49C mutation in alpha A-crystallin enhances protein insolubility and cell death. J Biol Chem 283: 5801–5814. [DOI] [PubMed] [Google Scholar]
- 28. Andley UP, Patel HC, Xi JH (2002) The R116C mutation in alpha A-crystallin diminishes its protective ability against stress-induced lens epithelial cell apoptosis. J Biol Chem 277: 10178–10186. [DOI] [PubMed] [Google Scholar]
- 29. Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, et al. (2006) Redox proteomics identification of oxidized proteins in Alzheimer's disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol Aging 27: 1564–1576. [DOI] [PubMed] [Google Scholar]
- 30. Sabah JR, Davidson H, McConkey EN, Takemoto L (2004) In vivo passage of albumin from the aqueous humor into the lens. Mol Vis 10: 254–259. [PubMed] [Google Scholar]
- 31. Sabah J, McConkey E, Welti R, Albin K, Takemoto LJ (2005) Role of albumin as a fatty acid carrier for biosynthesis of lens lipids. Exp Eye Res 80: 31–36. [DOI] [PubMed] [Google Scholar]
- 32. Sabah JR, Schultz BD, Brown ZW, Nguyen AT, Reddan J, et al. (2007) Transcytotic passage of albumin through lens epithelial cells. Invest Ophthalmol Vis Sci 48: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 33. Delcourt C, Dupuy AM, Carriere I, Lacroux A, Cristol JP, et al. (2005) Albumin and transthyretin as risk factors for cataract: the POLA study. Arch Ophthalmol 123: 225–232. [DOI] [PubMed] [Google Scholar]
- 34. Gokhin DS, Nowak RB, Kim NE, Arnett EE, Chen AC, et al. (2012) Tmod1 and CP49 synergize to control the fiber cell geometry, transparency, and mechanical stiffness of the mouse lens. PLoS One 7: e48734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andley UP, Reilly MA (2010) In vivo lens deficiency of the R49C alphaA-crystallin mutant. Experimental Eye Research 90: 699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barton KA, Hsu CD, Petrash JM (2009) Interactions between small heat shock protein alpha-crystallin and galectin-related interfiber protein (GRIFIN) in the ocular lens. Biochemistry 48: 3956–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clark TJ, Houck SA, Clark JI (2012) Hemoglobin interactions with alphaB crystallin: a direct test of sensitivity to protein instability. PLoS One 7: e40486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Srivastava OP, Kirk MC, Srivastava K (2004) Characterization of covalent multimers of crystallins in aging human lenses. J Biol Chem 279: 10901–10909. [DOI] [PubMed] [Google Scholar]
- 39. Wang Z, Lyons B, Truscott RJ, Schey KL (2014) Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell 13: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lampi KJ, Amyx KK, Ahmann P, Steel EA (2006) Deamidation in human lens betaB2–crystallin destabilizes the dimer. Biochemistry 45: 3146–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michiel M, Duprat E, Skouri-Panet F, Lampi JA, Tardieu A, et al. (2010) Aggregation of deamidated human betaB2-crystallin and incomplete rescue by alpha-crystallin chaperone. Exp Eye Res 90: 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Putilina T, Skouri-Panet F, Prat K, Lubsen NH, Tardieu A (2003) Subunit exchange demonstrates a differential chaperone activity of calf alpha-crystallin toward beta LOW- and individual gamma-crystallins. J Biol Chem 278: 13747–13756. [DOI] [PubMed] [Google Scholar]
- 43. Ajaz MS, Ma Z, Smith DL, Smith JB (1997) Size of human lens beta-crystallin aggregates are distinguished by N-terminal truncation of betaB1. J Biol Chem 272: 11250–11255. [DOI] [PubMed] [Google Scholar]
- 44. McHaourab HS, Kumar MS, Koteiche HA (2007) Specificity of alphaA-crystallin binding to destabilized mutants of betaB1-crystallin. FEBS Lett 581: 1939–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dolinska MB, Sergeev YV, Chan MP, Palmer I, Wingfield PT (2009) N-terminal extension of beta B1-crystallin: identification of a critical region that modulates protein interaction with beta A3-crystallin. Biochemistry 48: 9684–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ohrloff C, Berdjis H, Hockwin O, Bours J (1983) Age-related changes of glyceraldehyde-3-phosphate-dehydrogenase (E.C. 1.2.1.12), 3-phosphoglyceratekinase (E.C. 2.7.2.3), phosphoglyceratemutase (E.C. 2.7.5.3), and enolase (E.C. 4.2.1.11) in bovine lenses. Ophthalmic Res 15: 293–299. [DOI] [PubMed] [Google Scholar]
- 47. Takata T, Shimo-Oka T, Miki K, Fujii N (2005) Characterization of new D-beta-aspartate-containing proteins in a lens-derived cell line. Biochem Biophys Res Commun 334: 1022–1031. [DOI] [PubMed] [Google Scholar]
- 48. Hoehenwarter W, Kumar NM, Wacker M, Zimny-Arndt U, Klose J, et al. (2005) Eye lens proteomics: from global approach to detailed information about phakinin and gamma E and F crystallin genes. Proteomics 5: 245–257. [DOI] [PubMed] [Google Scholar]
- 49. St-Amand J, Okamura K, Matsumoto K, Shimizu S, Sogawa Y (2001) Characterization of control and immobilized skeletal muscle: an overview from genetic engineering. FASEB J 15: 684–692. [DOI] [PubMed] [Google Scholar]
- 50. Grey AC, Schey KL (2009) Age-related changes in the spatial distribution of human lens alpha-crystallin products by MALDI imaging mass spectrometry. Invest Ophthalmol Vis Sci 50: 4319–4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lampi KJ, Ma Z, Hanson SR, Azuma M, Shih M, et al. (1998) Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res 67: 31–43. [DOI] [PubMed] [Google Scholar]
- 52. Bhattacharyya J, Shipova EV, Santhoshkumar P, Sharma KK, Ortwerth BJ (2007) Effect of a single AGE modification on the structure and chaperone activity of human alphaB-crystallin. Biochemistry 46: 14682–14692. [DOI] [PubMed] [Google Scholar]
- 53. Ghosh JG, Houck SA, Clark JI (2008) Interactive sequences in the molecular chaperone, human alphaB crystallin modulate the fibrillation of amyloidogenic proteins. Int J Biochem Cell Biol 40: 954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Banerjee PR, Pande A, Patrosz J, Thurston GM, Pande J (2011) Cataract-associated mutant E107A of human gammaD-crystallin shows increased attraction to alpha-crystallin and enhanced light scattering. Proc Natl Acad Sci U S A 108: 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Asherie N (2011) Blind attraction: the mechanism of an inherited congenital cataract. Proc Natl Acad Sci U S A 108: 437–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dorsaz N, Thurston GM, Stradner A, Schurtenberger P, Foffi G (2009) Colloidal characterization and thermodynamic stability of binary eye lens protein mixtures. J Phys Chem B 113: 1693–1709. [DOI] [PubMed] [Google Scholar]
- 57. Stradner A, Foffi G, Dorsaz N, Thurston G, Schurtenberger P (2007) New insight into cataract formation: enhanced stability through mutual attraction. Phys Rev Lett 99: 198103. [DOI] [PubMed] [Google Scholar]
- 58. Andley UP, Malone JP, Townsend RR (2011) Inhibition of Lens Photodamage by UV-Absorbing Contact Lenses. Investigative Ophthalmology and Visual Science 52: 8330–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mendelsohn BA, Malone JP, Townsend RR, Gitlin JD (2009) Proteomic analysis of anoxia tolerance in the developing zebrafish embryo. Comp Biochem Physiol Part D Genomics Proteomics 4: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hu Y, Malone JP, Fagan AM, Townsend RR, Holtzman DM (2005) Comparative proteomic analysis of intra- and interindividual variation in human cerebrospinal fluid. Mol Cell Proteomics 4: 2000–2009. [DOI] [PubMed] [Google Scholar]
- 61. Amacher DE, Adler R, Herath A, Townsend RR (2005) Use of proteomic methods to identify serum biomarkers associated with rat liver toxicity or hypertrophy. Clin Chem 51: 1796–1803. [DOI] [PubMed] [Google Scholar]
- 62. Marionneau C, LeDuc RD, Rohrs HW, Link AJ, Townsend RR, et al. (2009) Proteomic analyses of native brain K(V)4.2 channel complexes. Channels (Austin) 3: 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keller A, Eng J, Zhang N, Li XJ, Aebersold R (2005) A uniform proteomics MS/MS analysis platform utilizing open XML file formats. Mol Syst Biol 1 : 2005 0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nesvizhskii AI, Keller A, Kolker E, Aebersold R (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of proteins that showed differences in abundance between 2-day-old WT, 14-day-old WT and 2-day-old αA-R49C homozygous mouse lenses. WT, Wild-type.
(DOC)
Ingenuity Pathway Analysis (IPA) molecules table for proteins affected by αA-crystallin R49C mutation in the mouse lens.
(XLS)
Ingenuity Pathway Analysis (IPA) molecules table for proteins affected by αB-crystallin R120G mutation in the mouse lens.
(XLS)
Mass spectrometry and database search results for proteins identified in this study.
(XLSX)
Supplementary figures. Figure S1, 2D-DIGE analysis of proteomic changes in whole lenses of 2-day-old mice with knock-in of the αA-R49C mutation. Protein spots that were picked for analysis from the 2D gels of WT and αA-R49C heterozygous (A) and WT and αA-R49C homozygous lenses (B-D) shown in Figure 1. Quantitative image analysis and mass spectrometry data for identified proteins from these gels are listed in Table 1. Figure S2, 2D-DIGE analysis of proteomic changes in whole lenses of 2-day-old and 14-day-old mice induced by knock-in of the αA-R49C mutation. (A) A 2D gel of lens proteins labeled with cyanine dyes derived from 2-day-old WT proteins labeled with Cy3, 14-day-old WT proteins labeled with Cy5, and αA-R49C homozygous lens proteins labeled with Cy2. (B, C) Protein spots that were selected for analysis from the gel shown in (A). Proteins were identified by tandem mass spectrometry and Mascot searches of spots that were selected from the gels. Quantitative image analysis and mass spectrometry data for the identified proteins from these gels are listed in Table S1. Figure S3, Protein connectivity networks identified by Ingenuity Pathway analysis of lens proteins in αA-R49C knock-in mutant lenses. Analysis of altered protein networks by Ingenuity Pathway software. Biological networks and pathways generated from input data (Wild type vs. αA-R49C, Tables 1–3 and S1) indicate proteins with changed abundance in gray. (A) A network with GAPDH at the hub. (B) A second network with F-actin at the hub. (C) A third network highlights NPM1 at the hub of the protein connectivity map. (D) A fourth network with TGFB1 at the hub. (E) A fifth network indicates the interaction between grifin and IKZF1. (F) A sixth network shows Gm5409 at the hub. Note that two additional networks are shown in Figure 8. Figure S4, Networks revealed by Ingenuity Pathway analysis of lens proteins that changed in amount in WT vs. αB-R120G knock-in lenses. Biological networks and pathways generated from input data (Wild type vs. αB-R120G, Table 4) indicate proteins with changed abundance in gray. (A) A network with MAF at the hub. (B) A second network with UBC at the hub. (C) A third network shows the interactions between grifin and IKZF1. (D) A fourth network highlights CTRB2 at the hub of the protein connectivity map.
(DOC)