Abstract
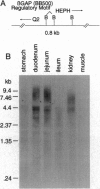
Hemochromatosis (HC) is an inherited disorder of iron absorption, mapping within the human major histocompatibility complex (MHC). We have identified a multigene system in the murine MHC that contains excellent candidates for the murine equivalent of the human HC locus and implicate nonclassical class I genes in the control of iron absorption. This gene system is characterized by multiple copies of two head-to-head genes encoded on opposite strands and driven by one common regulatory motif. This regulatory motif has a striking homology to the promoter region of the beta-globin gene, a gene obviously involved in iron metabolism and hence termed beta-globin analogous promoter (betaGAP). Upstream of the betaGAP sequence are nonclassical class I genes. At least one of these nonclassical class I genes, Q2, is expressed in the gastrointestinal tract, the primary site of iron absorption. Also expressed in the gastrointestinal tract and downstream of the betaGAP motif is a second set of putative genes, termed Hephaestus (HEPH). Based on these observations, we hypothesized that the genes that seem to be controlled by the betaGAP regulatory motifs would be responsible for the control of Fe absorption. As a test of this hypothesis, we predicted that mice which have altered expression of class I gene products, the beta2-microglobulin knockout mice, [beta2m(-/-)], would develop Fe overload. This prediction was confirmed, and these results indicate beta2m-associated proteins are involved in the control of intestinal Fe absorption.
Full text
PDF

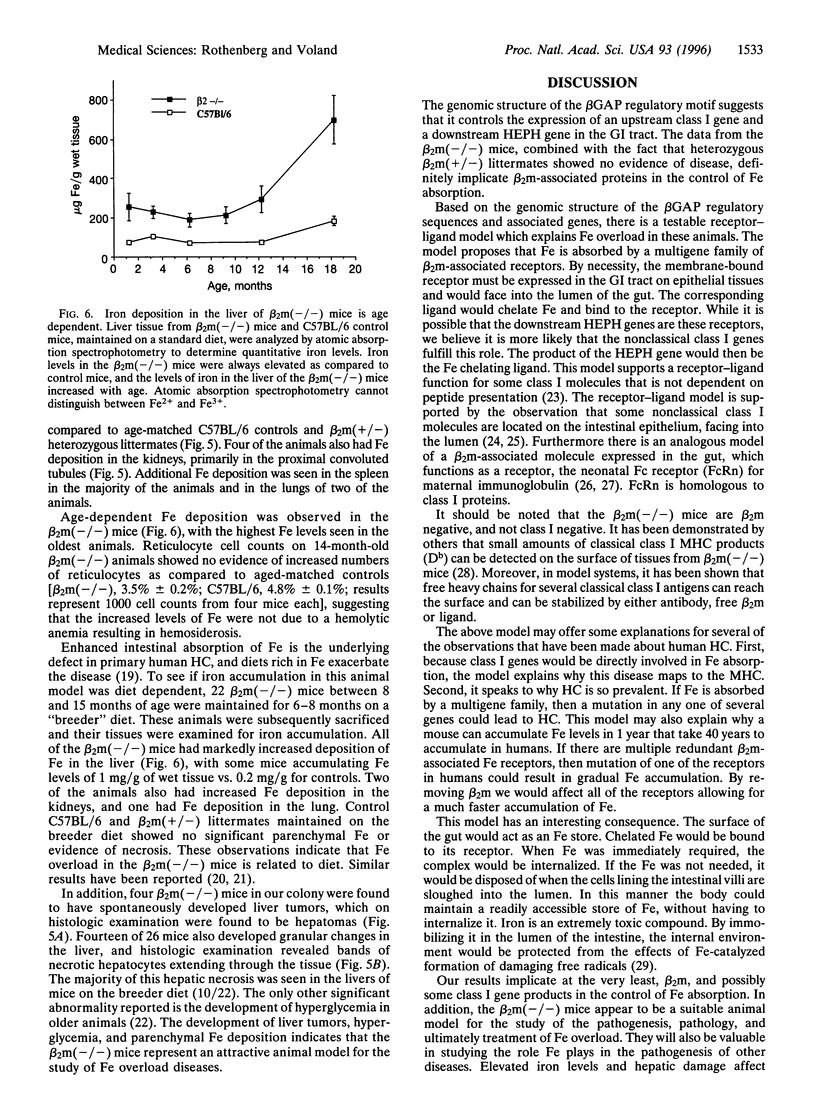

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apasov S. G., Sitkovsky M. V. Development and antigen specificity of CD8+ cytotoxic T lymphocytes in beta 2-microglobulin-negative, MHC class I-deficient mice in response to immunization with tumor cells. J Immunol. 1994 Mar 1;152(5):2087–2097. [PubMed] [Google Scholar]
- Bix M., Raulet D. Functionally conformed free class I heavy chains exist on the surface of beta 2 microglobulin negative cells. J Exp Med. 1992 Sep 1;176(3):829–834. doi: 10.1084/jem.176.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister W. P., Gastinel L. N., Simister N. E., Blum M. L., Bjorkman P. J. Crystal structure at 2.2 A resolution of the MHC-related neonatal Fc receptor. Nature. 1994 Nov 24;372(6504):336–343. doi: 10.1038/372336a0. [DOI] [PubMed] [Google Scholar]
- Cullen M. K., Lapierre L. A., Kesari K. V., Geliebter J. Identification of a recombinogenic major histocompatibility complex Q gene with diverse alleles. J Exp Med. 1993 Jun 1;177(6):1803–1807. doi: 10.1084/jem.177.6.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenerjian M. G., Kafatos F. C. Developmental specificity of a bidirectional moth chorion promoter in transgenic Drosophila. Dev Biol. 1994 Jan;161(1):37–47. doi: 10.1006/dbio.1994.1005. [DOI] [PubMed] [Google Scholar]
- Gavalas A., Dixon J. E., Brayton K. A., Zalkin H. Coexpression of two closely linked avian genes for purine nucleotide synthesis from a bidirectional promoter. Mol Cell Biol. 1993 Aug;13(8):4784–4792. doi: 10.1128/mcb.13.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordeuk V., Mukiibi J., Hasstedt S. J., Samowitz W., Edwards C. Q., West G., Ndambire S., Emmanual J., Nkanza N., Chapanduka Z. Iron overload in Africa. Interaction between a gene and dietary iron content. N Engl J Med. 1992 Jan 9;326(2):95–100. doi: 10.1056/NEJM199201093260204. [DOI] [PubMed] [Google Scholar]
- Heikkilä P., Soininen R., Tryggvason K. Directional regulatory activity of cis-acting elements in the bidirectional alpha 1(IV) and alpha 2(IV) collagen gene promoter. J Biol Chem. 1993 Nov 25;268(33):24677–24682. [PubMed] [Google Scholar]
- Hershberg R., Eghtesady P., Sydora B., Brorson K., Cheroutre H., Modlin R., Kronenberg M. Expression of the thymus leukemia antigen in mouse intestinal epithelium. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9727–9731. doi: 10.1073/pnas.87.24.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Leggett B. A., Brown N. N., Bryant S. J., Duplock L., Powell L. W., Halliday J. W. Factors affecting the concentrations of ferritin in serum in a healthy Australian population. Clin Chem. 1990 Jul;36(7):1350–1355. [PubMed] [Google Scholar]
- Lennard A. C., Fried M. The bidirectional promoter of the divergently transcribed mouse Surf-1 and Surf-2 genes. Mol Cell Biol. 1991 Mar;11(3):1281–1294. doi: 10.1128/mcb.11.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot K., Maltby L., Duarte R., Veale R., Segev O. Conserved cis-elements bind a protein complex that regulates Drosophila ras2/rop bidirectional expression. Br J Cancer. 1994 Feb;69(2):264–273. doi: 10.1038/bjc.1994.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg B. E. The self recognition concept: an active function for the molecules of major histocompatibility complex based on the complementary interaction of protein and carbohydrate. Dev Comp Immunol. 1978 Feb;2(1):23–37. doi: 10.1016/s0145-305x(78)80022-6. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Simon M., Brissot P. The genetics of haemochromatosis. J Hepatol. 1988 Feb;6(1):116–124. doi: 10.1016/s0168-8278(88)80471-9. [DOI] [PubMed] [Google Scholar]
- Stuve L. L., Myers R. M. A directly repeated sequence in the beta-globin promoter regulates transcription in murine erythroleukemia cells. Mol Cell Biol. 1990 Mar;10(3):972–981. doi: 10.1128/mcb.10.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Geliebter J., Tonkonogy S., Flaherty L. Expression of the Q2 gene of the MHC in thymus and intestinal epithelial cells. Immunogenetics. 1993;38(5):370–372. doi: 10.1007/BF00210481. [DOI] [PubMed] [Google Scholar]
- Watts S., Davis A. C., Gaut B., Wheeler C., Hill L., Goodenow R. S. Organization and structure of the Qa genes of the major histocompatibility complex of the C3H mouse: implications for Qa function and class I evolution. EMBO J. 1989 Jun;8(6):1749–1759. doi: 10.1002/j.1460-2075.1989.tb03568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., van Kaer L., Itohara S., Tonegawa S. Highly restricted expression of the thymus leukemia antigens on intestinal epithelial cells. J Exp Med. 1991 Jul 1;174(1):213–218. doi: 10.1084/jem.174.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Doolittle R. F. Presence of a vertebrate fibrinogen-like sequence in an echinoderm. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2097–2101. doi: 10.1073/pnas.87.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- de Sousa M., Reimão R., Lacerda R., Hugo P., Kaufmann S. H., Porto G. Iron overload in beta 2-microglobulin-deficient mice. Immunol Lett. 1994 Feb;39(2):105–111. doi: 10.1016/0165-2478(94)90094-9. [DOI] [PubMed] [Google Scholar]