Abstract
Background
The left and right amygdalae are key regions distinctly involved in emotion-regulation processes. Individual differences, such as personality features, may affect the implicated neurocircuits. The lateralized amygdala affective processing linked with the temperament dimension Harm Avoidance (HA) remains poorly understood. Resting state functional connectivity imaging (rsFC) may provide more insight into these neuronal processes.
Methods
In 56 drug-naive healthy female subjects, we have examined the relationship between the personality dimension HA on lateralized amygdala rsFC.
Results
Across all subjects, left and right amygdalae were connected with distinct regions mainly within the ipsilateral hemisphere. Females scoring higher on HA displayed stronger left amygdala rsFC with ventromedial prefrontal cortical (vmPFC) regions involved in affective disturbances. In high HA scorers, we also observed stronger right amygdala rsFC with the dorsomedial prefrontal cortex (dmPFC), which is implicated in negative affect regulation.
Conclusions
In healthy females, left and right amygdalae seem implicated in distinct mPFC brain networks related to HA and may represent a vulnerability marker for sensitivity to stress and anxiety (disorders).
Introduction
Emotions involve brain networks including (pre)frontal cortical and limbic areas [1], [2]. Within these emotional networks the amygdalae play a crucial role [3], [4]. Biologically oriented theories suggest specific affective information-processing roles for the left and the right amygdala [5], [6]. An emotional stimulus automatically activates the right amygdala, which is thought to play a role in dynamic emotional stimulus detection, while the left amygdala seems to be more involved in specific, sustained stimulus evaluation [7], [8]. However, how individual differences can affect left and right amygdala related neurocircuits differently remains poorly understood [9]–[12]. Trait and state anxiety has been found to modulate amygdala resting-state functional connectivity (rsFC) related to ventromedial prefrontal cortical (vmPFC), but not with the dorsomedial prefrontal cortical (dmPFC) activity [13]. Together with the amygdalae these brain regions are thought to be involved in the neuronal circuits of fear behavior, in self-referential processing and social interactions [14], [15]. Furthermore, it has been suggested that in order to stop the generation of anxious states the strength of amygdala–mPFC functional connectivity during rest represents efficient crosstalk between these brain regions [16], [17].
Only recently, researchers became interested in the relationship of amygdala rsFC with personality features [18], such as Harm Avoidance (HA). Cloningers' psychobiological theory on personality and genetic inheritance states that scoring high on the temperament factor HA is related to increased behavioral inhibition and implies a genetically determined bias towards being cautious, apprehensive and overly pessimistic [19]. Healthy individuals scoring high on HA are more at risk for developing mood- and anxiety disorders in the course of their lives [20], [21]. Based on anatomical parcellations of the amygdalae, Li and colleagues [22], have reported on sex-related amygdala rsFC differences in relation to HA. In spite that functional imaging data point to lateralization differences in amygdala emotional functioning in healthy participants, with especially the left amygdala implicated in negative affect [6], and a major topic in our line of research [12], [23]–[25], to date it remains unclear whether the temperament dimension HA may affect left or right amygdala rsFC in relation to the mPFC differently. Brain imaging approaches such as resting-state fMRI combined with HA measurements may increase our understanding of how behavioral more inhibited individuals with the tendency to be more pessimistic could be at higher risk to develop affective disorders [17], [26].
Consequently, the aim of the current study is to test the hypothesis that in a homogeneous sample of females - never documented to have suffered from neuropsychiatric illnesses – individual scores on HA are related to differential left and right amygdala - mPFC coupling. Importantly, the selection of the left and right amygdala nodes was based on brain anatomical coordinates provided by a neuroimaging study of emotion processing and emotion regulation in women resilient or susceptible to the depressogenic effects of early life stress [27]. These nodes fall within the area referred to as the Superficial Amygdala (SA), not surprisingly reported to be involved in the processing of social information [28], [29] and especially relevant to Harm Avoidance.
Across all subjects, we hypothesized the existence of rsFC differences for the connections of left and right amygdala with distinct regions in the brain. We hypothesized in high HA scoring females stronger rsFC correlations between predominantly the left amygdala seed and the mPFC. Within the mPFC, we expected in particular left amygdala rsFC-HA correlations with the vmPFC. Because amygdala lateralization differences are not consistently reported for the dmPFC, we hypothesized no lateralized amygdala rsFC-HA correlations with dmPFC areas.
Methods and Experimental Procedures
1. Participants
The study was approved by the ethics committee of our University Hospital (UZBrussel) and in accordance with the guidelines laid down in the declaration of Helsinki (2004). All participants gave written informed consent. This study was part of a larger project investigating several neuro-cognitive markers in affective disorders. After the structural MRI, all participants went through the rs-fMRI. Hereafter other psychological imaging paradigms were performed, not related to the current study.
Sixty right-handed female individuals (mean age = 21.7 y, sd = 2.5), all university students, were recruited. Right-handedness was assessed with the van Strien questionnaire [30]. Because besides gender also age may confound rsFC results, all participants were selected within a narrow age range [31]. Participants taking medication, other than birth-control pills, were excluded. None of the participants reported to have ever used psychotropic medications such as antidepressants, mood stabilizers or antipsychotics, and all were free of illicit drugs. To exclude psychiatric or neurological diseases, all volunteers were screened by the first author (C.B). Psychiatric disorders were assessed by the Dutch version of the Mini-International Neuropsychiatric Interview (MINI) [32]. Participants with a psychiatric disorder and/or a score higher than eight on the Beck Depression Inventory (BDI-II [33]) were excluded.
2. Temperament and Character Inventory
The Temperament and Character Inventory (TCI) is a 240-item questionnaire developed by Cloninger and colleagues [19], [34]. The questionnaire is based on a psychobiological model that aims to explain individual differences in personality traits [35]. The TCI consists of 4 temperament scales (Harm Avoidance (HA), Novelty seeking (NS), Reward dependence (RD), Persistence (P)), and three character scales (Cooperativeness (CO), Self-directedness (SD) and Self Transcendence (ST)) [34]. This inventory has been used in a variety of studies examining psychobiological substrates of personality, including neurobiological, neuroimaging and genetic methods [12], [36], [37]. We extracted only the temperament dimension HA for our purposes (minimum score = 0, maximum score is 36).
3. Scanning Procedure
During the resting state measurements, involving exactly five minutes of scanning, all participants were asked to stay awake with their eyes closed and to think of nothing in particular. To reduce sensory confounds as much as possible, the light in the room was dimmed during scanning. After the scan, the participants were asked to confirm that they had been awake throughout the scan and had complied with the instructions. All resting state fMRI scans were performed on Monday afternoons, between 3:00 pm and 6:00 pm.
All scans were performed on a 3T Philips Achieva MRI system (Philips, Best, The Netherlands) with an eight channel SENSE head coil. fMRI measurement was done using a SE-EPI sequence (TR/TE = 3000/70 ms; Flip angle = 90°; FOV = 230×230 mm2; resolution = 1.80×1.80 mm2; Slice thickness/gap = 4.00/1.00 mm; number of slices = 24; number of dynamics = 100; dynamic time resolution = 3000 ms). After the fMRI scan a 3D anatomical scan using a 3D T1 TFE sequence (TR/TE = 12.00/3.71 ms; Flip angle = 10°; FOV = 240×240×200 mm3; resolution = 1.00×1.00×2.00 mm3; number of slices = 100) was performed, yielding an anatomical underlay for the fMRI results.
The fMRI data were analyzed with the SPM8 software (Wellcome Department of Cognitive Neurology, London, UK). Slice-time correction was performed to correct for small differences in the time offset of consecutively measured slices. Hereafter, the images were realigned to the first volume of the time series in order to correct for head movements. Subsequently, all fMRI brain volumes were normalized to the EPI MNI template; resampled to 3-mm isotropic voxels and spatially smoothed using an 8-mm full-width half-maximum Gaussian kernel. The anatomical scans were normalized to the T1 MNI template.
Several further processing steps preceded the voxel-based correlation analysis. Data were linearly detrended and band-pass filtered (0.01–0.08 Hz). Spurious or nonspecific sources of variance were removed from the data through linear regression of: 1) the six head-motion parameters obtained in the realigning step, 2) the signal from a region in the cerebrospinal fluid, 3) the signal from a region centered in the white matter. As proposed by Murphy et al. [38] and Weissenbacher et al. [39] resting state data were processed without global signal regression. Correlation maps were obtained by extracting the BOLD time course from a seed region, then computing the correlation coefficients characterizing the correlations between that time course and the time courses from all other brain voxels. The seed regions were 6-mm-diameter spheres designed to encompass the left (MNI coordinates x = −20, y = −4, z = −15) or right amygdala (x = 22, y = −2, z = −15). These MNI coordinates were selected following the recent paper of Cisler et al [27]. To combine results across subjects and compute statistical significance, Fisher's r-to-Z transformation was used to convert these correlation maps into Z maps (maps quantifying local ‘rsFC strength’, or simply ‘rsFC’). The Z maps were submitted to a random-effects analysis in SPM8. A one-sample t-test containing age as covariate was performed for the left and right amygdala rsFC separately. To evaluate significant differences between left and right amygdala rsFC, a paired t-test was performed with age as covariate. All analyses used a cluster significance level of p<0.05, corrected for multiple comparisons (Family Wise Error (FWE)). We listed all significant clusters with a cluster extent threshold (K) of at least 50 voxels.
Concerning the influence of the temperament dimension HA on left and right amygdala rsFC separately, we calculated Pearson's correlation coefficients between the Fisher-z-transformed rsFC strength and HA scores for each voxel, producing another set of r-maps. To examine our primary research question; the influence of HA on lateralized amygdala rsFC, we calculated the Pearson's correlation coefficient between the difference of the Fisher-z-transformed rsFC strength and HA scores for each voxel left vs. right amygdala rsFC. After Fisher-z transformation on r-maps, we mapped the voxels with p-values<0.05. The anatomical labels and Montreal Neurological Institute (MNI) coordinates were obtained by the xjView MATLAB toolbox (http://www.alivelearn.net/xjview).
Results
The range in HA scores was between 2 and 30 (mean HA score = 15.52, sd = 7.04). The Shapiro-Wilk normality test showed that HA scores were normally distributed (p = .46). No volunteer stated to have fallen asleep during scanning. Due to exceeding 1.5 mm and 1.5 degree in maximum head motion, four female volunteers were removed from rs-fMRI analyses, leaving a total of 56 participants.
1. Amygdala rsFC
1.1. Left amygdala rsFC
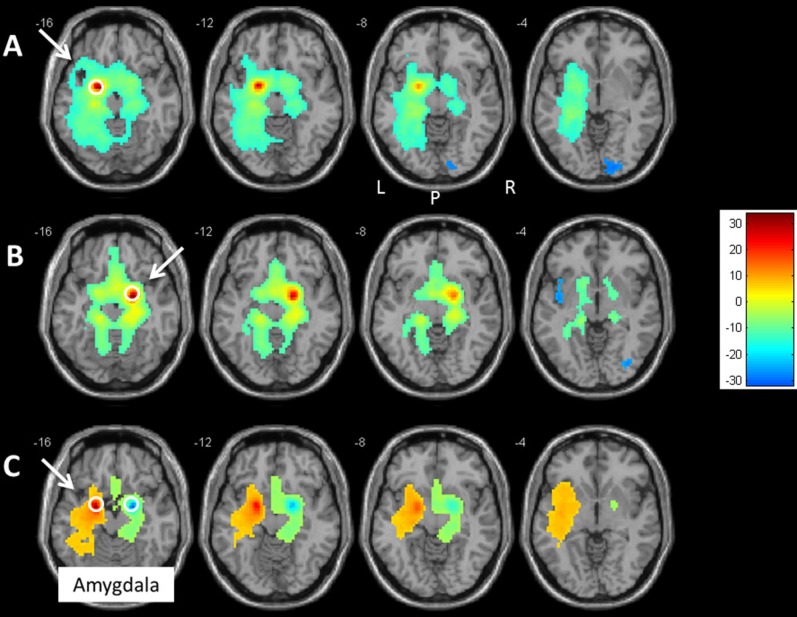
See Fig. 1 A. The result of the one-sample t-test for the left-amygdala rsFC showed one large significant cluster in the left parahippocampal gyrus (K = 6285; MNI coordinates: x = −18, y = −3, z = −15). On the left hemisphere, this rsFC region included hippocampus, insula and subgenual anterior cingulate cortex (sgACC), putamen and claustrum, fusiform gyrus and culmen. This cluster also extended to the right hippocampus, the left and right gyrus rectus, and the thalamus.
Figure 1. Left and right amygdala rsFCs.
Transversal slides displaying the results of A) the one-sample t-test for the left-amygdala rsFC and B) the one-sample t-test for the right-amygdala rsFC. Colors from yellow to red represent significantly stronger FC and colors from green to blue represent the opposite. C) the comparison of the rsFCs of left vs. right amygdala the paired t-test between the rsFCs of the left and right amygdala seeds. Colors from yellow to red represent significantly stronger FC with the left than with the right amygdala. Colors from green to blue represent the opposite: significantly stronger FC with the right amygdala than with the left. The amygdala seeds are displayed by a white circle. For an overview of all significant clusters see Table 1. P = posterior, L = left, R = right.
In addition, the one-sample t-test showed a significant inverse correlation between the left amygdala and the right middle occipital gyrus (BA 18: K = 392; MNI coordinates: x = 15, y = −87, z = 6).
1.2. Right amygdala rsFC
The result of the one-sample t-test for the right-amygdala rsFC showed two significant clusters. One large cluster was situated in the right parahippocampal gyrus (K = 3376; MNI coordinates: x = 18, y = −3, z = −15). A second cluster was located in the right anterior cingulate cortex (BA 24; K = 148; x = 3, y = 27, z = 18). See also Fig. 1 B.
The one-sample t-test revealed an inverse correlation between the right amygdala and the right fusiform gyrus (K = 89; x = 51, y = −18, z = −30), the left insula (BA 13; K = 62; x = −42, y = −6, z = 0), the left middle frontal gyrus (BA 9; K = 410; x = −33, y = 33, z = 42), and the right (K = 222; x = 48, y = −66, z = 6) and left middle temporal gyrus (K = 186; x = −45, y = −69, z = 9).
1.3. Comparison between the left and right amygdala rsFCs
See also Fig. 1 C. The paired t-test revealed that the contrast (left amygdala rsFC>right amygdala rsFC) yielded a significant cluster in the left parahippocampal gyrus, with the maximum peak in the left amygdala (K = 2889; MNI coordinates: x = −21, y = −3, z = −15). Other peak areas were located at the left side: in the hippocampus, insula (BA 13), putamen, fusiform gyrus, and pons.
The paired t-test for the contrast (right amygdala rsFC>left amygdala rsFC) revealed a significant cluster one in the right parahippocampal gyrus with the maximum peak in the right amygdala (K = 578; x = 21, y = −3, z = −15), hippocampus, culmen, and lingual gyrus (BA 17).
2. Amygdala rsFC-HA correlation analyses
2.1. Left amygdala
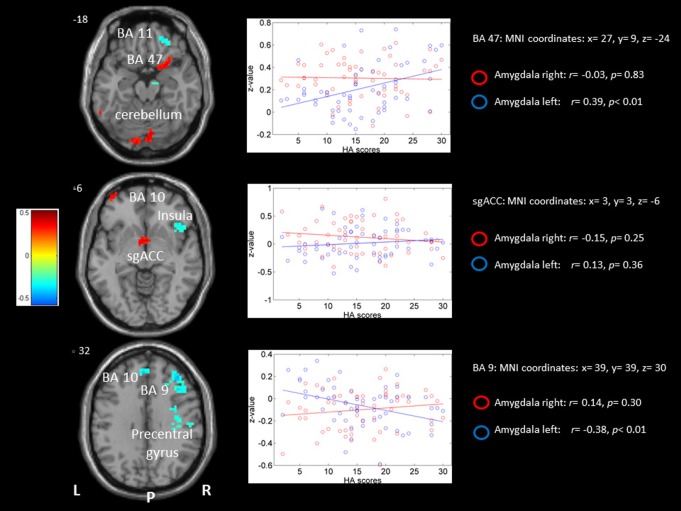
The results of the rsFC-HA correlation analysis for the left amygdala seed (MNI coordinates x = −20, y = −4, z = −15) yielded a positive association in the left occipital gyrus and the sgACC (BA 25), the right amygdala and larger parts of the cerebellar regions. Negative correlations were observed in the left cerebellum and the right superior frontal gyrus (BA 9). See Table 1 and Fig. 2.
Table 1. Results for the correlation between the individual scores on Harm Avoidance and the rsFC of the left amygdala seed.
| Seed | Correlation | Hemisphere | Cluster size | Anatomical region | BA | Z-value | Peak coordinates (x,y,z) (mm) |
| Left Amygdala | |||||||
| Positive correlation | |||||||
| Left | 242 | Inferior occipital Gyrus | - | 0.53 | −24 −93 −12 | ||
| 126 | Middle occipital gyrus | - | 0.50 | −51 −66 −15 | |||
| 60 | Gyrus Rectus | 25 | 0.43 | −3 12 −21 | |||
| Right | 236 | Cerebellum posterior lobe | - | 0.50 | 18 −48 −36 | ||
| 106 | Inferior frontal gyrus | 45 | 0.45 | 63 12 24 | |||
| 91 | Limbic lobe | amygdala | 0.49 | 24 6 −21 | |||
| 71 | Parietal lobe | 39 | 0.47 | 45 −69 30 | |||
| Negative correlation | |||||||
| Left | 59 | Cerebellum | - | −0.42 | −36 −66 −24 | ||
| Right | 64 | Superior Frontal Gyrus | 9 | −0.42 | 18 48 39 |
For each cluster, we reported the Z-value and MNI coordinates at the position of the maximum, the cluster size (K) and the corresponding Brodmann area (BA).
Figure 2. Amygdala rsFC -HA correlation analysis.
Transversal slide exhibiting positive (yellow to red) and negative (green to blue) correlation clusters for the correlation between the rsFC of the left amygdala seed (Left crosshair on white sphere; MNI coordinates: x = −20, y = −4, z = −15), the right amygdala seed (Right crosshair on white sphere; MNI coordinates: x = 22, y = −2, z = −15), and HA. For an overview of all significant clusters see Table 1 and Table 2. P = posterior, L = left, R = right, BA = Brodmann area.
2.2. Right amygdala
The rsFC-HA correlation analysis for the right amygdala seed (MNI coordinates: x = 22, y = −2, z = −15) showed a positive association with the left inferior frontal gyrus (BA 10) and the post cinglulate gyrus (BA 31). Strong correlations were also observed in the right middle frontal gyrus (BA 9 as well as BA 11), the right parietal lobe (BA 7), and cerebellar regions. Bilateral positive correlations were observed for the parietal and occipital cortex and thalamus. Negative correlations were found in the left ACC (BA 24), the right parahippocampus and cerebellum bilaterally. See Table 2 and Fig. 2.
Table 2. Results for the correlation between the individual scores on Harm Avoidance and the rsFC of the right amygdala seed.
| Seed | Correlation | Hemisphere | Cluster size | Anatomical region | BA | Z-value | Peak coordinates (x,y,z) (mm) |
| Right Amygdala | |||||||
| Positive correlation | |||||||
| Left | 232 | Middle Occipital Gyrus | 18 | 0.48 | −45 −72 −12 | ||
| 232 | Parietal Lobe | Precuneus | 0.43 | −27 −69 42 | |||
| 57 | Parietal Lobe | 42 | 0.39 | −60 −27 12 | |||
| 53 | Parietal lobe | 40 | 0.34 | −27 −24 57 | |||
| 136 | Thalamus | - | 0.37 | −15 −18 15 | |||
| 71 | Post cingulate gyrus | 31 | 0.43 | 0 −54 30 | |||
| 66 | Inferior Frontal Gyrus | 10 | 0.47 | −45 30 3 | |||
| 58 | Parahippocampal gyrus | Amygdala | 0.49 | −9 0 −24 | |||
| Right | 307 | Occipital lobe | 19 | 0.48 | 24 −72 −6 | ||
| 306 | Cerebellum posterior lobe | - | 0.51 | 15 −57 −45 | |||
| 256 | Postcentral Gyrus | - | 0.54 | 33 −21 39 | |||
| 136 | Parietal Lobe | 7 | 0.47 | 24 −54 60 | |||
| 132 | Inferior temporal lobe (Fusiform gyrus) | - | 0.47 | 54 −18 −24 | |||
| 117 | Thalamus | - | 0.47 | 18 −24 12 | |||
| 84 | Limbic lobe | 30 | 0.44 | 21 −51 0 | |||
| 79 | Middle Frontal Gyrus | 9 | 0.39 | 45 21 36 | |||
| 76 | Middle frontal gyrus | 11 | 0.41 | 27 33 −15 | |||
| 72 | Superior temporal gyrus | 38 (amygdala) | 0.36 | 42 3 −24 | |||
| Negative correlation | |||||||
| Left | 23 | Cuneus | - | −0.32 | −12 −78 6 | ||
| 49 | Anterior Cingulate Cortex | 24 | −0.41 | −6 21 27 | |||
| Right | 77 | Parahippocampal gyrus | 30 | 0.41 | 9 −39 −3 | ||
| 45 | Cerebellum | - | −0.39 | 3 −81 −21 |
For each significant cluster, we reported the Z-value and MNI coordinates at the position of the maximum, cluster size (K) and the corresponding Brodmann area (BA).
2.3. Left vs. right amygdala confined the entire sample
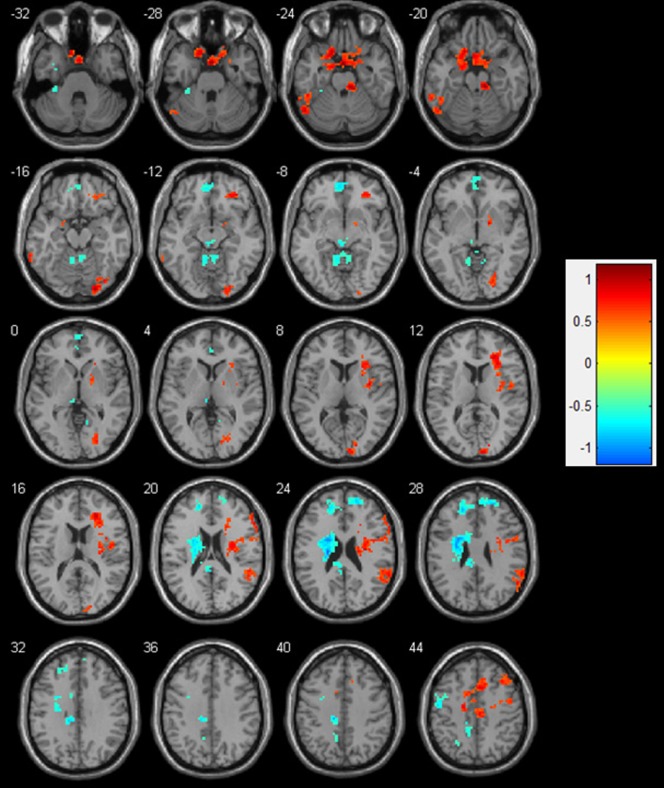
rsFC-HA correlation analyses revealed that the high scorers on HA displayed stronger left compared to right amygdala rsFC within some clusters situated around the left premotor cortex (BA 6) and supplementary motor area (SMA). Further, healthy females scoring higher on HA displayed stronger left vs. right amygdala rsFC in the right inferior frontal gyrus and the right sgACC (BA 25). See Table 3 and Fig. 3.
Table 3. Results for the correlation between the individual scores on Harm Avoidance and the rsFC of the left vs. right amygdala seed.
| Seed | Hemisphere | Cluster size | Anatomical region | BA | Z-value | Peak coordinates (x,y,z) (mm) |
| Left>Right Amygdala Correlation | ||||||
| Left | 81 | Cerebellum posterior | - | 0.40 | −12 −81 −21 | |
| 55 | Precentral gyrus | 6 | 0.52 | −18 −12 48 | ||
| 35 | Precentral gyrus | 6 | 0.45 | −12 −21 75 | ||
| 21 | Supplementary motor area | 6 | 0.42 | −15 −3 66 | ||
| 22 | Middle frontal gyrus | 10 | 0.34 | −39 60 −6 | ||
| Right | 63 | Inferior frontal gyrus (Parahippocampus) | 47 | 0.49 | 27 9 −24 | |
| 21 | Anterior cingulate | 25 | 0.37 | 3 3 −6 | ||
| 21 | Inferior frontal gyrus | 45 | 0.36 | 60 24 24 | ||
| Right>Left Amygdala Correlation | ||||||
| Left | 47 | Posterior cingulate | 30 | 0.38 | −3 −54 12 | |
| 30 | Medial frontal gyrus | 10 | 0.40 | −9 51 15 | ||
| Right | 228 | Middle frontal gyrus | 9 | 0.52 | 39 39 30 | |
| 162 | Precentral gyrus | - | 0.57 | 33 −21 42 | ||
| 49 | Inferior frontal gyrus | Insula | 0.36 | 42 24 −6 | ||
| 29 | Fronterior superior orbital gyrus | 11 | 0.39 | 24 42 −15 | ||
| 25 | Parahippocampal gyrus | - | 0.35 | 18 −12 −24 |
For each cluster, we reported the Z-value and MNI coordinates at the position of the maximum, the cluster size (K) and the corresponding Brodmann area (BA).
Figure 3. Left vs right amygdala rsFC -HA correlation analysis.
Left column) Transversal slides displaying the results of the rsFC-HA correlation analyses for the left vs. right amygdala rsFC seeds. Colors from yellow to red represent significantly stronger FC with the left compared to the right amygdala. Colors from green to blue represent the opposite: significantly stronger FC for the right compared to the left amygdala. For an overview of all significant clusters see Table 3. P = posterior, L = left, R = right, BA = Brodmann area. Right column) Scatter plots representing left vs. right amygdala rsFC-HA correlations with their respective correlation coefficients. The red circles represent more left vs. right amygdala rsFC; the blue circles represent the reverse: more right vs. left amygdala rsFC.
On the other hand, when comparing right vs. left amygdala rsFC, higher scores on HA showed stronger rsFC-HA correlation within the right parahippocampal gyrus and prefrontal cortex, including the middle frontal gyrus (BA 9), insula, and orbitofrontal cortex (BA 11). In this contrast stronger left hemispheric rsFC-HA correlations were found in left posterior cingulate (BA 30) and medial prefrontal gyri (BA 10).
2.4. Left vs. right amygdala confined to high HA scorers
To evaluate possible involvement of HA in the development of affective disorders, we selected those females scoring high on HA according Dutch and Flemish normative data set (n = 1041) (The Netherlands are a neighboring country closely related to Flanders, Belgium). According this data set, which provide normative TCI data for males and females separately [40], from our 56 female participants 18 scored high or very high on the temperament dimension HA. For an overview see Table 4 and Fig. 4.
Table 4. Results for the correlation between the selected individual scores high on Harm Avoidance (n = 18) and the rsFC of the left vs. right amygdala seed.
| Seed | Hemisphere | Cluster size | Anatomical region | BA | Z-value | Peak coordinates (x,y,z) (mm) |
| Left>Right Amygdala Correlation | ||||||
| Left | 286 | Limbic lobe | - | 0.08 | 0 0 −24 | |
| 47 | Cerebellum | - | 2.17 | −51 −63 −24 | ||
| 30 | Inferior temporal gyrus | 0.98 | −66 −45 −18 | |||
| Right | 195 | Medial frontal gyrus | 6 | 1.56 | 48 0 51 | |
| 185 | Insula | 13 | −1.35 | 36 −3 9 | ||
| 181 | Medial frontal gyrus | 32/24 | 1.26 | 6 15 45 | ||
| 127 | Insula | 13 | 0.77 | 27 33 15 | ||
| 90 | Occipital lobe | 18 | −4.41 | 9 −96 12 | ||
| 79 | Parietal lobe | 40 | −2.83 | 63 −48 24 | ||
| 63 | Occipital lobe | 18 | −3.09 | 24 −90 −15 | ||
| 37 | Middle frontal gyrus | 11 | −1.28 | 36 39 −12 | ||
| 36 | Middle frontal gyrus | 8 | 2.00 | 36 24 45 | ||
| 33 | Cerebellum | 1.09 | 12 −30 −21 | |||
| 31 | Inferior frontal gyrus | 45 | −2.94 | 60 24 21 | ||
| 26 | Lentiform nucleus | - | −0.60 | 18 0 −3 | ||
| 20 | Cerebellum | - | 1.09 | 30 −66 −42 | ||
| Right>Left Amygdala Correlation | ||||||
| Left | 252 | Caudate nucleus | - | 1.32 | −21 −15 24 | |
| 97 | Cerebellum | - | 3.32 | −6 −51 −12 | ||
| 92 | Superior frontal gyrus | 9 | −0.28 | −18 42 27 | ||
| 89 | Medial frontal gyrus | 10/11 | −0.003 | −3 54 −9 | ||
| 76 | Cingulate gyrus | 31 | −0.30 | −12 −27 39 | ||
| 44 | Precentral gyrus | 6 | 1.22 | −51 −9 45 | ||
| 30 | Precuneus | - | 3.46 | −12 −42 45 | ||
| 28 | Thalamus | - | −1.51 | −3 −27 −9 | ||
| 25 | Parietal lobe | 7 | 1.05 | −24 −60 48 | ||
| 21 | Culmen | - | 3.36 | −33 −33 −30 | ||
| 20 | Inferior temporal gyrus | - | 2.31 | −33 3 −36 | ||
| Right | 66 | Superior frontal gyrus | 10 | −2.34 | 24 51 27 | |
| 24 | Supplemental motor area | 6 | 0.39 | 3 −12 78 |
For each cluster, we reported the Z-value and MNI coordinates at the position of the maximum, the cluster size (K) and the corresponding Brodmann area (BA).
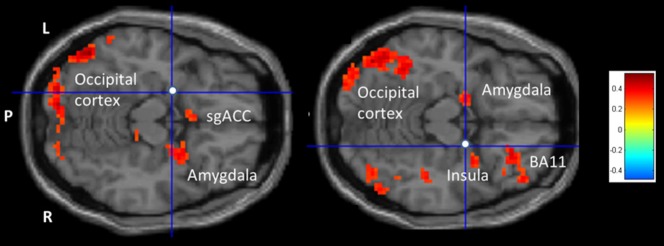
Figure 4. Left vs right amygdala rsFC -HA correlation analysis confined to high HA scoring females.
Transversal slides displaying the results of the rsFC-HA correlation analyses for the left vs. right amygdala rsFC seeds. Colors from yellow to red represent significantly stronger FC with the left compared to the right amygdala. Colors from green to blue represent the opposite: significantly stronger FC for the right compared to the left amygdala. For an overview of all significant clusters see Table 4.
In short, left vs. right amygdala rsFC showed stronger rsFC-HA correlations with the right hemisphere, such as the right insula, but importantly also with a larger cluster within the vmPFC, comprising both amygdalae and the sgACC, the inferior frontal and rectal gyrus, extending to both parahippocampi. This contrast also revealed significant rsFC-HA correlation with the dmPFC, more in particular the medial prefrontal gyrus (BA 32/24).
On the other hand, the right vs. left amygdala rsFC showed stronger rsFC-HA correlations with the left hemisphere, including the basal ganglia, the left superior frontal (BA 9) and bilateral medial frontal (BA 10) gyri.
Discussion
Although not the main scope of the current research, our overall amygdala rsFC observations without the inclusion of the HA scores are in line with the findings of Roy and colleagues [41] where spontaneous activities in the amygdalae predicted spontaneous activity in similar parahippocampal and prefrontal regions, the thalamus, and occipital cortex. Our general rsFC results point to distinct functional network connections largely within the same hemisphere for left or right amygdala seed separately. Further, the amygdala rsFC-HA correlations showed mostly positive associations with temporal, parietal, occipital and cerebellar cortices. These areas play critical roles in the perceptual processing of socially and emotionally relevant visual information, especially in non-clinical samples with higher trait anxiety [42]–[46].
As hypothesized, left vs. right amygdala rsFC-HA correlation analyses showed that females scoring higher on HA displayed stronger left amygdala FC within mPFC regions. Within the vmPFC, more in particular the sgACC, this area is related to arousal processes and implicated in a corticolimbic neurocircuit associated with ‘visceromotor’ functions playing an important role in modulating affect, such as sadness activation and ruminative thought patterns [47]. This functional amygdala - sgACC coupling has also been reported in female anxiety patients scoring higher than controls on HA [48]. However, our findings seem to be in disagreement with the study of Kim and colleagues [13] where reverse amygdala –vmPFC FC results were reported in relation to higher scores on the State and Trait Anxiety Inventory self-report questionnaires (STAI-S, STAI-T [49]). Of note, albeit higher HA scorers may display higher anxiety levels, HA and STAI scales do not measure the same construct [20] (Cloninger et al., 2006). Further, although Kim et al [13] defined the sgACC as part of the vmPFC, on a functional level it may be that not the sgACC but the more ventral-rostral portions of the ACC and vmPFC are involved in regulating strong emotional responses [50]–[52]. Indeed, in the selected group of High HA scorers in particular the left amygdala and the right medial frontal gyrus (BA 32/24) were functionally connected, the latter pregenual anterior cingulate cortex (pACC) documented to control emotional neuronal processes, such as self-conscious emotion [53], [54].
The left vs. right amygdala rsFC-HA correlation analyses showed positive right amygdala rsFC-HA with predominantly the right hippocampus and insula, the dorsolateral prefrontal cortex (DLPFC) and orbitofrontal cortex (OFC). The latter are part of the more dorsal parts of the mPFC [17]. See also Table 3. In spite that we did not hypothesize lateralized amygdalae rsFC-HA correlations with dmPFC areas, these findings concur with a right prefrontal involvement in the regulation of negative affect. Besides that the DLPFC and the amygdala are indirectly implicated in top-down/bottom-up emotion-regulation processes [55], the right DLPFC in particular seems to be implied in behavioral inhibition, negative affect regulation, increased vigilance and sustained attention, uncertainty and ambiguity [56] (Shackman et al., 2009). Of interest, the right OFC shows co-activations with insular parts associated with interoception and gustation [57]. Interoceptive information such as visceral sensations implicated in processes of awareness and experiences of aversive responses are thought to be channeled into in the right anterior insula [58]–[60]. Indeed, the neurobiological modulation of stress responses has been reported to be lateralized to the right prefrontal cortex [61], [62]. Interestingly, in the selected group of high HA scorers, also the left amygdala showed significant FC with the right insular regions. Furthermore, the right DLPFC, OFC and insula were found to be activated in anticipation to withdrawal-related emotional experiences [63]. Our current results further provide insight that in behavioral more inhibited and cautious individuals, not only the right amygdala may play a key role in regulating these processes, but in more stress sensitive individuals both amygdalae seem to be involved. In addition, rsFC-HA correlations showed that the both amygdalae were significantly stronger functionally correlated with the medial frontal gyrus (BA 10) also part of the dmPFC area. These rostral parts of the dmPFC are associated with emotion regulation, sustained attention, memory, and mentalizing processes [64]–[67]. Being part of the DMN, the BA 10 is implicated when individuals make self-relevant affective decisions [68], [69]. Importantly, right amygdala also correlated with the posterior cingulate gyrus, part of the DMN as well, and together with the mPFC and hippocampus is thought to be implicated in the processing of autobiographical memory, past self-relevant stimuli and future prospection [70]. As this DMN is especially implicated when at risk for clinical depression, it is tempting to speculate that this right amygdala rsFC-HA dmPFC association may represent a ‘neuronal network vulnerability’ for the development of mood disorders in a later stage of life.
Finally, for the higher HA scorers bilateral amygdala rsFC-HA correlations extended from the vmPFC to the basal ganglia. This is an important observation because the involvement of dopaminergic nuclei is not surprising. Besides that the amygdala, hippocampus, and these ventromedial prefrontal cortical areas are key brain regions that not only modulate emotions and cognition but also the response to stress itself - resulting in hypertrophy of dendritic arborization and increases in spine density [71]–[73] - the mentioned vmPFC areas are consistently involved in positive and negative reward processing (for an overview see Liu et al. [74]). This is of particular importance in more behavioral inhibited and pessimistic individuals. These dopaminergic neurons coming from the ventral tegmental area (VTA) are crucial for the recognition of rewards and their consumption [75]. Again, as the selected amygdala nodes fall within the area referred to as the Superficial Amygdala (SA) which has been shown to be specialized in the processing of social information [28]–[29], our results add to the assumption that individuals scoring high on HA not only display more behavioral inhibition and pessimism, but may also be more vulnerable to stressful interpersonal experiences.
Although the selection of psychopathology-free female subjects can be considered a major advantage of the study, including only healthy women within a certain age range means that we cannot generalize our findings to other populations. Because no cardiac and respiratory data were collected during rs-fMRI, this should be noted as a limitation of our study. As it has been reported that the different subnuclei of the amygdalae may have specific functional connections with distinct parts of the brain [41], [76], by not examining dedicated seeds in these subnuclei, important information could have been missed. However, our main research objective was to examine rsFC differences in relation to specific left and right-sided amygdalar nodes which were documented to be involved in emotion regulation brain networks among individuals resilient or susceptible to the depressogenic effects of early life stress [27]. This makes the choice of these selected nodes particularly relevant in relation to personality features such as harm avoidance. And again these nodes fall within the area referred to as the Superficial Amygdala involved in social information processing [28]–[29]. Nevertheless, future research examining amygdala rsFC in relation to personality features may do well to include a larger number of seeds, comprising the different amygdalar subnuclei.
In conclusion, amygdala rsFC analyses in relation to individual differences in HA may prove to be a valid method to investigate behavioural inhibition and pessimism, possible risk factors for mental illness development. Our rsFC-HA results confirm the right amygdala's key role in right anterior hemisphere cross-talk in females who are likely more stress-sensitive. Furthermore, the combination of enhanced left amygdala –vmPFC and right amygdala-dmPFC coupling may represent a vulnerability marker for females with an elevated risk to develop mood and anxiety disorders. Longitudinal follow-up studies in both genders are needed to substantiate such hypotheses and to demonstrate whether or not such amygdala rsFC-HA patterns within the medial prefrontal cortex are of use to predict the development of mood and anxiety disorders.
Funding Statement
This research was supported by a grant from the Scientific Fund W. Gepts UZBrussel and supported by the Ghent University Multidisciplinary Research Partnership “The integrative neuroscience of behavioral control”. Preparation of this paper was also supported by Grant BOF10/GOA/014 for a Concerted Research Action of Ghent University (awarded to RDR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, et al. (2002) Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry 52: 478–502. [DOI] [PubMed] [Google Scholar]
- 2. Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF (2012) The brain basis of emotion: a meta-analytic review. Journal of Behavioral and Brain Science 35: 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, et al. (2000) Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience 3: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 4. Sergerie K, Chochol C, Armony JL (2008) The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews 32: 811–830. [DOI] [PubMed] [Google Scholar]
- 5. Zald DH (2003) The human amygdala and the emotional evaluation of sensory stimuli. Brain Researc. Brain Research Reviews 41: 88–123. [DOI] [PubMed] [Google Scholar]
- 6. Baas D, Aleman A, Kahn RS (2004) Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research Brain Research Reviews 45: 96–103. [DOI] [PubMed] [Google Scholar]
- 7. Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, et al. (2001) Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. NeuroReport 12: 379–383. [DOI] [PubMed] [Google Scholar]
- 8. Glascher J, Adolphs R (2003) Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala,. Journal of Neuroscience 23: 10274–11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Canli T (2004) Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. Journal of Personality 72: 1105–1132. [DOI] [PubMed] [Google Scholar]
- 10. Mather M, Canli T, English T, Whitfield S, Wais P, et al. (2004) Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science 15: 259–263. [DOI] [PubMed] [Google Scholar]
- 11. Wager TD, Ochsner KN (2005) Sex differences in the emotional brain. Neuroreport 16: 85–87. [DOI] [PubMed] [Google Scholar]
- 12. Baeken C, De Raedt R, Ramsey N, Van Schuerbeek P, Hermes D, et al. (2009) Amygdala responses to positively and negatively valenced baby faces in healthy female volunteers: influences of individual differences in harm avoidance. Brain Research 1296: 94–103. [DOI] [PubMed] [Google Scholar]
- 13. Kim JM, Gee DG, Loucks RA, Davis FC, Whalen PJ (2011a) Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex 21: 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fossati P (2012) Neural correlates of emotion processing: from emotional to social brain. Eur Neuropsychopharmacol 22 Suppl 3: S487–491. [DOI] [PubMed] [Google Scholar]
- 15. Courtin J, Bienvenu TC, Einarsson EÖ, Herry C (2013) Medial prefrontal cortex neuronal circuits in fear behavior. Neuroscience 240: 219–242. [DOI] [PubMed] [Google Scholar]
- 16. Kim MJ, Whalen PJ (2009) The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience 29: 11614–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, et al. (2011b) The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioral Brain Research 223: 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vaidya CJ, Gordon EM (2013) Phenotypic variability in resting-state functional connectivity: current status. Brain Connectivity 3: 99–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cloninger CR (1987) A systematic method for clinical description and classification of personality variants. A proposal. Archives of General Psychiatry 44: 573–588. [DOI] [PubMed] [Google Scholar]
- 20. Cloninger CR, Svrakic DM, Przybeck TR (2006) Can personality assessment predict future depression? A twelve-month follow-up of 631 subjects. Journal of Affective Disorders 92: 35–44. [DOI] [PubMed] [Google Scholar]
- 21. Kampman O, Poutanen O (2011) Can onset and recovery in depression be predicted by temperament? A systematic review and meta-analysis. Journal of Affective Disorders 135: 20–27. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Qin W, Jiang T, Zhang Y, Yu C (2012) Sex-dependent correlations between the personality dimension of harm avoidance and the resting-state functional connectivity of amygdala subregions. Public Library of Science One 7: e35925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baeken C, Van Schuerbeek P, De Raedt R, Bossuyt A, Vanderhasselt MA, et al. (2010) Passively viewing negatively valenced baby faces attenuates left amygdala activity in healthy females scoring high on ‘Harm Avoidance’. Neuroscience Letters 478: 97–101. a. [DOI] [PubMed] [Google Scholar]
- 24. Baeken C, De Raedt R, Van Schuerbeek P, Vanderhasselt MA, De Mey J, et al. (2010) Right prefrontal HF-rTMS attenuates right amygdala processing of negatively valenced emotional stimuli in healthy females. Behav Brain Res 214: 450–455. b. [DOI] [PubMed] [Google Scholar]
- 25. Baeken C, Van Schuerbeek P, De Raedt R, Vanderhasselt MA, De Mey J, et al. (2012) Stress sensitive healthy females show less left amygdala activation in response to withdrawal-related visual stimuli under passive viewing conditions. Brain & Cognition 80: 230–236. [DOI] [PubMed] [Google Scholar]
- 26. Wang L, Hermans DF, Hickie IB, Lagopoulos J (2012) A systematic review of resting-state functional-MRI studies in major depression. Journal of Affective Disorders 142: 6–12. [DOI] [PubMed] [Google Scholar]
- 27. Cisler JM, James GA, Tripathi S, Mletzko T, Heim C, et al. (2013) Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychological Medicine 43: 507–518. [DOI] [PubMed] [Google Scholar]
- 28. Goossens L, Kukolja J, Onur OA, Fink GR, Maier W, et al. (2009) Selective processing of social stimuli in the superficial amygdala. Hum Brain Mapp 30: 3332–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB (2013) An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp 34: 3247–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Strien JW, Van Beek S (2000) Ratings of emotion in laterally presented faces: Sex and handedness effects. Brain and Cognition 44: 645–652. [DOI] [PubMed] [Google Scholar]
- 31. Ferreira LK, Busatto GF (2013) Resting-state functional connectivity in normal brain aging. Neuroscience & Biobehavioral Reviews 37: 384–400. [DOI] [PubMed] [Google Scholar]
- 32. Sheehan D, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59 Suppl 20: 22–33. [PubMed] [Google Scholar]
- 33.Beck AT, Steer RA, Brown GK (1996) Beck Depression Inventory Manual (2nd ed). San Antonio: The Psychological Corporation. [Google Scholar]
- 34.Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD (1994) The Temperament and Character Inventory (TCI): A guide to its development and use. Center for Psychobiology of Personality, Washington University, St Louis, MO. [Google Scholar]
- 35. Cloninger CR, Svrakic DM, Przybeck TR (1993) A psychobiological model of temperament and character. Archives of General Psychiatry 50: 975–990. [DOI] [PubMed] [Google Scholar]
- 36. Soloff PH, Price JC, Mason NS, Becker C, Meltzer CC (2010) Gender, personality, and serotonin-2A receptor binding in healthy subjects. Psychiatry Research 181: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Schuerbeek P, Baeken C, De Raedt R, De Mey J, Luypaert R (2011) Individual differences in local gray and white matter volumes reflect differences in temperament and character: a voxel-based morphometry study in healthy young females. Brain Research 1371: 32–42. [DOI] [PubMed] [Google Scholar]
- 38. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA (2009) The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44: 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, et al. (2009) Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage 47: 1408–1416. [DOI] [PubMed] [Google Scholar]
- 40. Duijsens IJ, Spinhoven Ph, Goekoop JG, Spermon T, Eurelings-Bontekoe EHM (2000) The Dutch temperament and character inventory (TCI): dimensional structure, reliability and validity in a normal and psychiatric outpatient sample. Personality and Individual Differences 28: 487–499. [Google Scholar]
- 41. Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, et al. (2009) Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45: 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, et al. (1998) Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology 35: 199–210. [PubMed] [Google Scholar]
- 43. Adolphs R (2002) Neural systems for recognizing emotion Current Opinion in Neurobiology. 12: 169–177. [DOI] [PubMed] [Google Scholar]
- 44. Pujol J, Harrison BJ, Ortiz H, Deus J, Soriano-Mas C, et al. (2009) Influence of the fusiform gyrus on amygdala response to emotional faces in the non-clinical range of social anxiety. Psychological Medicine 39: 1177–1187. [DOI] [PubMed] [Google Scholar]
- 45. Pannekoek JN, Veer IM, van Tol MJ, van der Werff SJ, Demenescu LR, et al. (2013) Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. European Neuropsychopharmacology 23: 186–195. [DOI] [PubMed] [Google Scholar]
- 46. Wang D, Buckner RL, Liu H (2013) Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. Journal of Neurophysiology 109: 46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith R, Fadok RA, Purcell M, Liu S, Stonnington C, et al. (2011) Localizing sadness activation within the subgenual cingulate in individuals: a novel functional MRI paradigm for detecting individual differences in the neural circuitry underlying depression. Brain Imaging and Behavior 5: 229–239. [DOI] [PubMed] [Google Scholar]
- 48. Hillert L, Jovanovic H, Åhs F, Savic I (2013) Women with multiple chemical sensitivity have increased harm avoidance and reduced 5-HT(1A) receptor binding potential in the anterior cingulate and amygdala. Public Library of Science One 8: e54781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spielberger CD, Gorsuch RL, Lushene RE (1988) STAI-Manual for the State Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press. [Google Scholar]
- 50. Ochsner KN, Gross JJ (2005) The cognitive control of emotion. Trends in Cognitive Sciences 9: 242–249. [DOI] [PubMed] [Google Scholar]
- 51. Amting JM, Greening SG, Mitchell DG (2010) Multiple mechanisms of consciousness: the neural correlates of emotional awareness. Journal of Neuroscience 30: 10039–10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Etkin A, Egner T, Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Science 15: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Price JL, Drevets WC (2012) Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences 16: 61–71. [DOI] [PubMed] [Google Scholar]
- 54. Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, et al. (2013) Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Social Cognitive and Affective Neuroscience 8: 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dolcos F, Iordan AD, Dolcos S (2011) Neural correlates of emotion-cognition interactions: A review of evidence from brain imaging investigations. Journal of Cognitive Psychology 23: 669–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ (2009) Right dorsolateral prefrontal cortical activity and behavioral inhibition. Psychological Science 20: 1500–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ray RD, Zald DH (2012) Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience & Biobehavioral Reviews 36: 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience 3: 655–666. [DOI] [PubMed] [Google Scholar]
- 59. Craig AD (2011) Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences 1225: 72–82. [DOI] [PubMed] [Google Scholar]
- 60. Harrison NA, Gray MA, Gianaros PJ, Critchley HD (2010) The embodiment of emotional feelings in the brain. Journal of Neuroscience 30: 12878–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sullivan RM, Gratton A (2002) Prefrontal cortical regulation of hypothalamic–pituitary–adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology 27: 99–114. [DOI] [PubMed] [Google Scholar]
- 62. Cerqueira JJ, Almeida OF, Sousa N (2008) The stressed prefrontal cortex. Left? Right! Brain, Behavior, and Immunity 22: 630–638. [DOI] [PubMed] [Google Scholar]
- 63. Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ (2006) Functional neuroanatomy of aversion and its anticipation. Neuroimage 29: 106–116. [DOI] [PubMed] [Google Scholar]
- 64. Ramnani N, Owen AM (2004) Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience 5: 184–194. [DOI] [PubMed] [Google Scholar]
- 65. Amodio DM, Frith CD (2006) Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience 7: 268–277. [DOI] [PubMed] [Google Scholar]
- 66. Burgess PW, Gilbert SJ, Dumontheil I (2007 a) Function and localization within rostral prefrontal cortex (area 10). Philosophical Transactions of the Royal Society B: Biological Sciences 362: 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Burgess PW, Dumontheil I, Gilbert SJ (2007 b) The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences 11: 290–298. [DOI] [PubMed] [Google Scholar]
- 68. Wendelken C, Nakhabenko D, Donohu SE, Carter CS, Bunge SA (2008) “Brain is to thought as stomach is to ??”: investigating the role of rostrolateral prefrontal cortex in relational reasoning. Journal of Cognitive Neuroscience 20: 682–693. [DOI] [PubMed] [Google Scholar]
- 69. Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010) Functional-anatomic fractionation of the brain's default network. Neuron 65: 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Marchetti I, Koster EH, Sonuga-Barke EJ, De Raedt R (2012) The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev 22: 229–251. [DOI] [PubMed] [Google Scholar]
- 71. Seamans JK, Yang CR (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in Neurobiology 74: 1–58. [DOI] [PubMed] [Google Scholar]
- 72. Holroyd CB, Yeung N (2012) Motivation of extended behaviors by anterior cingulate cortex. Trends in Cognitive Sciences 16: 122–128. [DOI] [PubMed] [Google Scholar]
- 73. Lucassen PJ, Pruessner J, Sousa N, Almeida OF, Van Dam AM, et al. (2014) Neuropathology of stress. Acta Neuropathologica 127: 109–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu X, Hairston J, Schrier M, Fan J (2011) Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews 35: 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Russo SJ, Nestler EJ (2013) The brain reward circuitry in mood disorders. Nature Reviews Neuroscience 14: 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD (2009) Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry 66: 1361–1372. [DOI] [PubMed] [Google Scholar]