Abstract
Objectives
We studied survival and associated risk factors in an Italian nationwide cohort of HIV-infected individuals after an AIDS-defining cancer (ADC) or non-AIDS-defining cancer (NADC) diagnosis in the modern cART era.
Methods
Multi-center, retrospective, observational study of HIV patients included in the MASTER Italian Cohort with a cancer diagnosis from January 1998 to September 2012. Malignancies were divided into ADC or NADC on the basis of the Centre for Disease Control-1993 classification. Recurrence of cancer and metastases were excluded. Survivals were estimated according to the Kaplan-Meier method and compared according to the log-rank test. Statistically significant variables at univariate analysis were entered in a multivariate Cox regression model.
Results
Eight hundred and sixty-six cancer diagnoses were recorded among 13,388 subjects in the MASTER Database after 1998: 435 (51%) were ADCs and 431 (49%) were NADCs. Survival was more favorable after an ADC diagnosis than a NADC diagnosis (10-year survival: 62.7%±2.9% vs. 46%±4.2%; p = 0.017). Non-Hodgkin lymphoma had lower survival rates than patients with Kaposi sarcoma or cervical cancer (10-year survival: 48.2%±4.3% vs. 72.8%±4.0% vs. 78.5%±9.9%; p<0.001). Regarding NADCs, breast cancer showed better survival (10-year survival: 65.1%±14%) than lung cancer (1-year survival: 28%±8.7%), liver cancer (5-year survival: 31.9%±6.4%) or Hodgkin lymphoma (10-year survival: 24.8%±11.2%). Lower CD4+ count and intravenous drug use were significantly associated with decreased survival after ADCs or NADCs diagnosis. Exposure to cART was found to be associated with prolonged survival only in the case of ADCs.
Conclusions
cART has improved survival in patients with an ADC diagnosis, whereas the prognosis after a diagnosis of NADCs is poor. Low CD4+ counts and intravenous drug use are risk factors for survival following a diagnosis of ADCs and Hodgkin lymphoma in the NADC group.
Introduction
Several studies have shown that cancers are an increasingly important cause of illness and death in people with HIV [1]–[3]. Despite the link between HIV infection and cancer incidence, the question of whether HIV affects cancer prognosis in infected individuals has not been adequately addressed to date. Cancer prognosis in HIV is of interest for physicians because it can support decision-making and communication on therapeutic and palliative treatment decisions, management of co-morbid conditions, palliative care, and decisions regarding prioritization of management of other chronic conditions and/or HIV. Trends in relative incidence rates of AIDS-defining cancers (ADCs) and non-AIDS-defining cancers (NADCs) have been well characterized [4]–[9], but little is known about survival after a diagnosis of cancer in the setting of HIV infection- with the exception of non Hodgkin lymphoma (NHL), Kaposi sarcoma (KS), and anal cancer [10]–[13]. Since the introduction of combination antiretroviral therapy (cART), there has been a dramatic decrease in the incidence of AIDS-related morbidity and mortality in HIV-positive patients [14]. The cART has also improved the short- and medium-term survival in HIV-infected patients with ADCs [15]–[16] and some types of NADCs [17]. A recent Italian study has analyzed the long term survival in HIV-infected patients after malignancies [18] but few studies have investigated possible factors associated with survival in the era of cART and data in the literature are often limited to specific cancers [10]–[13]. The aim of the present work is to investigate survival after diagnosis with either ADCs or NADCs in HIV-infected patients engaged in routine care at five sites across Italy from 1998 by September 2012 and to explore possible predictors of mortality after a diagnosis of cancer in this population. The proposed study will add further information in estimates of cancers (ADCs and NADCs), their characteristics, and prognosis in the setting of HIV and cART, to better understand the challenges that this booming population poses to oncologic and infectious health services in the near future.
Materials and Methods
MASTER cohort and study population
The MASTER (MAnagement Standardizzato di TERapia antiretrovirale) cohort started in 1997 in several HIV outpatient clinics across Italy (Brescia, Bergamo, Monza, Cremona, Ferrara, Firenze, Roma, Bari), with the following aims (i) to create a population-based nationwide database of HIV patients for scientific projects, (ii) to monitor the spread and demographic characteristics of the HIV epidemic in Italy, (iii) to monitor and compare the effects of antiretroviral strategies at a national level. Patients medical records were retrospectively traced back to 1986 and prospectively followed up until now. (http://www.mastercohort.it). Enrollment in MASTER is independent of the stage of disease, the degree of immune suppression, or whether the individual is receiving antiretroviral therapy. Comorbid conditions (i.e. diabetes mellitus, hypertension, cardiovascular disease, cancer diagnosis, substance abuse, liver and renal conditions) and causes of death are accurately reported. Laboratory data, including CD4 T-cell count and HIV viral load, are collected at each patient's visit. Data are recorded over a standardized time-scale every three/four months in a common electronic chart (NetCare or Health&Notes). Data merging and cleaning are performed at a central level every six months.
Here, we conducted a retrospective cohort study from January 1998 to December 2012 on HIV-infected patients with a diagnosis of cancer, either naïve or experienced to antiretroviral therapy, with the following available records: date of first HIV positive test or cohort-entry, date of death (for patients who died during the study period), date of last visit (for patients still alive or lost to follow-up), and at least one record of CD4+ T-cell count available. A follow-up period of at least 1 day was required. Patients without any data recorded during 1 year or longer have been considered lost to follow-up. Measures for CD4+ T-cell count performed within 3 months before or after of the date of cancer diagnosis were reported as referred to the time of cancer diagnosis.
Ethics Statement
At first visit, patients provide written informed consent to include their clinical and biological data in the MASTER database for scientific purpose. The data was anonymized before it was provided and the database is hosted in Fondazione MISI's headquarter in compliance with current regulations.
The study was approved by the Ethical Committee of the Hospital Spedali Civili, Brescia (Coordinating Centre) and those of the following Institutions: University Hospital of Ferrara, Ferrara; AO Papa Giovanni XXIII, Bergamo; University of Bari, Bari; San Gerardo de' Tintori" Hospital, Monza; Hospital of Cremona, Cremona; “Santa Maria Annunziata” Hospital, Firenze; University of Sacred Heart, Roma.
Cancer diagnoses and deaths
Malignant cancer diagnoses were collected from medical records and verified through a standardized process, including detailed record abstraction and adjudication of malignancies. Only incident cancer events that occurred during the follow-up were included in the analysis. Recurrence of cancer and metastases were excluded. Cancer type or cancer site were coded according to the WHO classification [19] and malignancies were defined as ADCs (Non-Hodgkin lymphoma, Kaposi sarcoma and invasive cervical carcinoma) and NADCs (all other types of cancers). Primary central nervous system lymphoma (PCNSL) and systemic non-Hodgkin lymphomas were grouped together. Death and date of death were ascertained by centers through chart review and in some, cross-checks with mortality registers.
Statistical analysis
Values are reported as medians (interquartile range, IQR) or frequencies (%), as appropriate. Quantitative variables were compared by the non-parametric Mann-Whitney rank-sum test; the χ2test was used to assess independence between qualitative variables.
For each patient included in the study, person-years at risk have been calculated starting from the date of cancer diagnosis. The observation period ended on December 31st, 2012, or last follow-up visit, or death, whichever occurred first. According to cancer occurrence patients were classified in 2 groups: i) patients who developed ADC; ii) patients who developed NADC. Multiple primaries, i.e. cancers of a different type occurring in the same subject, were included in the analysis and each cancer was considered as a single case. Cancer incidence rates (IRs) were computed for ADC and NADC, dividing observed cases by the corresponding person-years at risk, and standardized for sex and age using the direct method with the European population as the standard and truncated at 65 years-old. The rates were expressed per 1,000 person-years. To compare the incidence of specific cancers in our HIV-infected patients with that observed in the Italian general population, the standardized incidence ratios (SIRs) and their corresponding 95% confidence intervals (with the Byar's approximation of Poisson model) were calculated using the number of expected cases based on the general population gender- and age-specific rates for Italy provided by 5 Italian Cancer Provincial Registries (Torino, Varese, Ferrara, Latina, Ragusa). SIRs were calculated until 2007, since these registries are updated until this year.
Survival was determined from the date of diagnosis of cancer to the end of the follow up, corresponding to the end of observation period. The probabilities of survival were estimated at 1, 5 and 10 years according to Kaplan-Meier with Greenwood standard error (SE) for total ADCs, total NADCs and specific cancers. Relative survival and expected survival were estimated according to the Ederer II method from life-tables for all-cause mortality by age, gender and calendar year. In the case of patients who had both ADC and NADC diagnosis, survival analysis was performed on the first cancer diagnosed. The factors associated with all-cause mortality were identified using the log–rank test for univariate analyses. Furthermore, the same variables were tested by multivariate analysis using Cox proporzional harzards models. First, fully adjusted models were fitted including the following variables: age, gender, cART, year of cancer diagnosis (categorized as 1998–2002, 2003–2007, 2008–2012), HIV viral loads and CD4 counts at cancer diagnosis, previous AIDS event, and risk factor for HIV acquisition. Afterwards, a selection of variables was performed with a stepwise backward procedure to generate parsimonious models. Age and gender were included as possible confounders, regardless of statistical significance. Results are shown with estimated hazards ratios (HR), 95% CIs, and P values (according to the Wald test). The proportional hazard assumption was assessed for each variable either graphically (by examining the log-log survival plot and the comparison of “observed” with “expected” survival curves) or by the goodness of fit approach. All the statistical tests were two-sided, assumed a level of significance of 0.05 and were performed using the STATA 12 software (STATA Statistics/Data Analysis 12.0 - STATA Corporation, College Station, TX, USA).
Results
Characteristics of Master Cohort and cancer incidence
From January 1998, a total of 13,388 patients have been included in the Master database and followed during 96,228 person-years (PY). Over this time a total of 900 cancer diagnosis were recorded: 454 ADCs (Incidence rate [IR]: 4.2/1000 PY, 95% confidence interval [CI] [3.7–4.8]) and 446 NADCs (IR: 4.6/1000 PY, 95%CI 3.9–5.3). A total of 27 patients had multiple cancer diagnosis. IRs were also calculated after stratification by calendar period (1998–2002; 2003–2007; 2008–2012). As expected, in our cohort the incidence rates in ADC had decreased in the last years (IR 1998–2002: 6.6/1000 PY, 95%CI 4.7–8.4; IR 2003–2007:5.3/1000 PY, 95%CI 4.0–6.7; IR 2008–2012: 3.4/1000 PY, 95%CI 2.5–4.2) whereas the incidence rates in NADC remained almost stable over time (IR 1998–2002: 5.7/1000 PY, 95%CI 3.0–8.5; IR 2003–2007:4.4/1000 PY, 95%CI3.6–5.3; IR 2008–2012: 4.4/1000 PY, 95%CI 3.4–5.4). Although the global incidence for NADCs resulted comparable to that detected in the Italian general population (SIR 1.1 [95%CI 1.0–1.2]), we observed higher SIRs for liver cancer (SIR 15.1 [95%CI 11.4–19.6]), Hodgkin lymphoma (SIR 14.2 [95%CI 10.1–19.4]), and a two-fold higher incidence also for lung cancer (SIR 2.2 [95%CI 1.5–3.2]). Breast cancer incidence was similar to the general population (SIR 1.3 [95%CI 0.8–1.9]).
Survival following ADC and NADC diagnosis
For the survival analysis we excluded 34 patients with a post-mortem cancer diagnosis or with a follow-up after a cancer diagnosis <1 day. Therefore 866 patients with malignancies were included in the analysis: 435 (51%) patients with an ADC and 431 (49%) with a NADC diagnosis. Table 1 summarizes the patient's characteristics at cancer diagnosis.
Table 1. Patients' characteristics at cancer diagnosis.
| Variable | Categories | ADCs (n = 435) | NADCs (n = 431) | Overall (n = 866) | P-value |
| Death, n (%) | 142 (33) | 166 (38) | 308 (36) | 0.071 | |
| Gender, n (%) | 0.372 | ||||
| Male | 345 (79) | 331 (77) | 676 (78) | ||
| Female | 90 (21) | 100 (23) | 190 (22) | ||
| Age at cancer diagnosis, years, n (%) | <0.001 | ||||
| 18–34 | 82 (19) | 36 (8) | 118 (14) | ||
| 35–49 | 258 (59) | 251 (58) | 509 (59) | ||
| ≥50 | 95 (22) | 144 (33) | 239 (27) | ||
| HIV risk factor, n (%) | 0.001 | ||||
| IVDU | 140 (32) | 185 (43) | 325 (38) | ||
| other | 295 (68) | 246 (57) | 541 (62) | ||
| HCV or HBV co-infection, n (%) | 183 (42) | 238 (55) | 421 (49) | <0.001 | |
| Previous AIDS event, n (%) | 184 (42) | 183 (42) | 367 (42) | 0.962 | |
| CD4 cell count at cancer diagnosis, cell/mm3 | <0.001 | ||||
| ≥200 | 203 (46) | 310 (72) | 513 (59) | ||
| <200 | 181 (42) | 103 (24) | 284 (33) | ||
| missing | 51 (12) | 18 (4) | 69 (8) | ||
| Nadir Cd4, cell/mm3, n(%) | <0.001 | ||||
| ≥200 | 113 (26) | 176 (41) | 289 (33) | ||
| 100–199 | 187 (43) | 170 (39) | 357 (41) | ||
| <50 | 135 (31) | 85 (20) | 220 (26) | ||
| Antiretroviral therapy, n (%) | 0.007 | ||||
| No cART | 80 (18) | 51 (12) | 131 (15) | ||
| cART | 355 (82) | 380 (88) | 735 (85) | ||
| HIVRNA, copies/mL, n (%) | <0.001 | ||||
| Undetectable | 107 (25) | 198 (46) | 305 (36) | ||
| Positive | 267 (61) | 213 (49) | 480 (55) | ||
| missing | 61 (14) | 20 (5) | 81 (9) | ||
| Median age at diagnosis (IQR) | 42 (36–48) | 48 (41–54) | 44 (38–50) | <0.001 | |
| Median CD4 cell count (IQR) | 214(85–409) | 349 (200–498) | 291 (133–457) | <0.001 | |
| Median HIVRNA, copies/mL | 657 (37–21000) | 70 (37–4300) | 301 (37–9400) | <0.001 |
Note: ADCs: AIDS-defining cancers, NADCs: Non-AIDS-defining cancers, IVDU: injection drug users, HBV: hepatitis B virus, HCV: hepatitis C virus, cART: combined antiretroviral therapy.
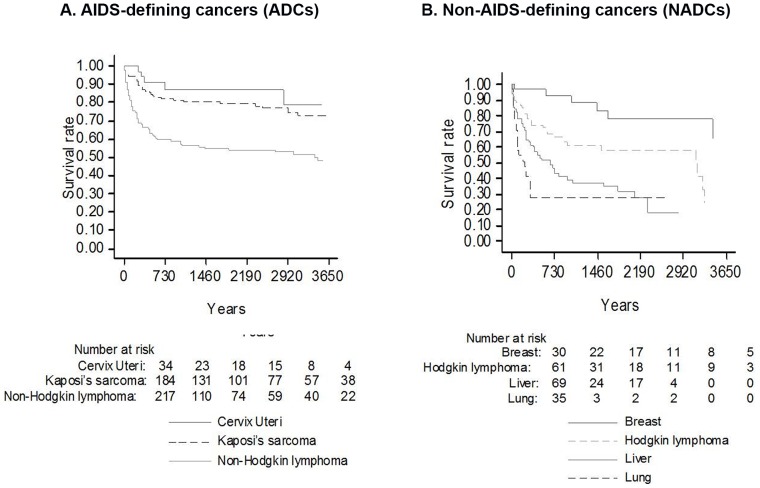
Among ADCs, 184 (42%) patients were diagnosed with Kaposi sarcoma, 34 (8%) patients with cervical cancer and 217 (50%) patients with Non-Hodgkin lymphoma (NHL). Most of the patients (77%) diagnosed with cervical cancer had a CD4+ T-cell count >200 cells/mm3 and almost half of them (44%) had a nadir CD4+ count >200 cell/mm3. At the time of analysis, patients with non-Hodgkin lymphoma had a higher number of deaths (n = 97, 45%) compared to those with Kaposi sarcoma (n = 40, 22%) and invasive cervical carcinoma (n = 5, 15%, p<0.001).
Liver cancer was the most frequent NADCs (n = 69, 16%), followed by Hodgkin lymphoma (n = 61, 14%), lung cancer (n = 35, 8%) and breast cancer (n = 30, 7%). Patients with Hodgkin lymphoma and breast cancer tended to be younger at diagnosis than those with other cancer (median age: 42 years vs. 47 and 52 years for liver and lung cancer, respectively). At the time of NADC diagnosis, 75% of the patients had a CD4+ T-cell count >200 cells/mm3. Fewer number of deaths were observed at the time of analysis among the patients diagnosed with breast cancer (n = 6, 20%) compared to the patients with Hodgkin lymphoma (n = 26, 43%) and those with liver or lung cancers (n = 44, 64% and n = 22, 63%, respectively, p<0.001).
Figure 1 shows the 10-years survival analysis after an ADC or NADC diagnosis. Although in the first 2 years after the cancer diagnosis no statistically significant difference in survival between ADC and NADC was found (p = 0.474), patients with an ADC diagnosis had a significantly higher long-term survival probability compared to those with a NADC diagnosis (10-year survival: 62.7%±2.9% vs. 46%±4.2%; p = 0.017). One year survival was also analyzed in relation to the time of cancer diagnosis. HIV-infected patients with a more recent NADC diagnosis had a statistically significant higher survival rate: one-year survival in patients with NADC diagnosis after 2008 was 80% vs. 76% and 65% in patients with NADC diagnosis between the period 2002–2007 and before 2002, respectively (P = 0.047).
Figure 1. Survival probabilities according to cancer classification.
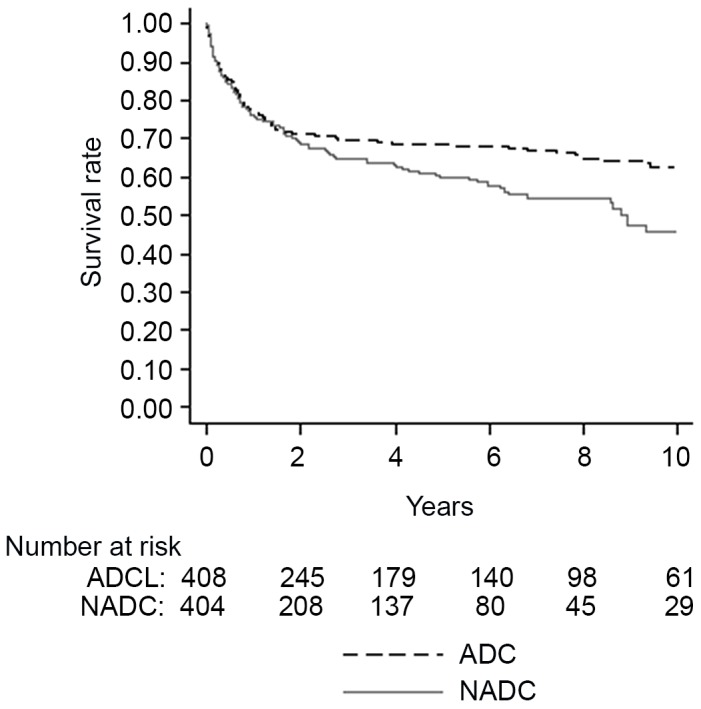
ADC, AIDS-defining cancer. NADC, non-AIDS-defining cancer.
Survival in patients diagnosed with the different types of ADCs and with the most representative NADCs are showed in Figure 2 (Panel A and Panel B).
Figure 2. Survival curves according to specific cancer types.
Panel (A) Survival probabilities according to AIDS-defining cancer (ADC) diagnosis. Overall, the median survival time from diagnosis of all ADC was 3.4 years; more specifically, the median survival time of NHL was 2.1 years, Kaposi sarcoma 4.7 years, and cervical cancer 5.1 years. Panel (B) Survival probabilities according to non-AIDS-defining cancer (NADC) diagnosis. The overall median survival time of NADC was 1.6 years; more specifically, the median survival time of liver cancer was 441 days, lung cancer 113 days, breast cancer 1624 days, and Hodgkin lymphoma 795 days.
Predictors of mortality
Risk factors for poorer survival after ADCs in univariate analysis were, as expected, viro-immunological variables (lower nadir- and CD4+ T-cell count at diagnosis, detectable HIVRNA, previous AIDS event) and IDVU mode of infection (Table 2). Notably, after a cervical cancer diagnosis only lower CD4+ T-cell count was associated with decreased survival rates (Table 2).
Table 2. Rate of overall survival ADCs by patients' characteristics.
| Variables | Categories | ADCs (n = 435) | P-value | CERVICAL CA (n = 34) | P-value | KAPOSI SARCOMA (n = 184) | P-value | NON HODGKIN LYMPHOMA (n = 217) | P-value | ||||||||
| 1y | 5y | 10y | 1y | 5y | 10y | 1y | 5y | 10y | 1y | 5y | 10y | ||||||
| Total | 77.0±2.0 | 67.7±2.4 | 61.1±2.9 | 90.9±5.0 | 87.3±6.0 | 78.5±9.9 | 86.9±2.5 | 79.1±3.2 | 72.8±4.0 | 66.6±3.2 | 55.0±3.5 | 48.2±4.3 | |||||
| Gender | 0.613 | NE* | 0.498 | 0.874 | |||||||||||||
| Male | 77.8±2.3 | 66.7±2.7 | 60.5±3.2 | - | - | - | 87.7±2.6 | 79.2±3.3 | 73.5±4.1 | 66.9±3.6 | 54.6±4.0 | 47.4±4.9 | |||||
| Female | 76.8±4.5 | 71.7±4.9 | 63.8±6.1 | 90.9±5.0 | 87.3±6.0 | 78.5±9.9 | 76.9±11.7 | 76.9±11.7 | 65.9±14.3 | 65.1±7.6 | 57.0±8.0 | 51.3±9.0 | |||||
| Age, years | 0.372 | 0.751 | 0.911 | 0.619 | |||||||||||||
| 18–34 | 82.4±4.3 | 76.2±5.0 | 65.1±6.7 | 100 | 100 | 66.7±2.7 | 89.6±4.9 | 83.7±6.1 | 69.6±9.0 | 67.1±8.5 | 57.8±9.7 | 57.8±9.7 | |||||
| 35–49 | 74.9±2.8 | 65.7±3.1 | 59.8±3.7 | 85.7±7.6 | 80.4±8.8 | 80.4±8.8 | 84.7±3.8 | 76.0±4.6 | 73.6±5.0 | 66.4±4.0 | 56.1±4.4 | 46.4±5.5 | |||||
| 50-max | 77.8±4.3 | 65.6±5.1 | 61.5±6.2 | 100 | - | - | 89.0±4.6 | 81.5±6.0 | 74.1±8.9 | 66.7±6.8 | 49.7±7.6 | 49.7±7.6 | |||||
| IDVU | <0.001 | 0.060 | 0.002 | 0.276 | |||||||||||||
| No | 80.7±2.3 | 73.2±2.7 | 67.7±3.5 | 91.3±5.9 | 91.3±5.9 | 73.0±17.0 | 90.0±2.5 | 83.4±3.3 | 77.8±4.4 | 68.0±4.2 | 58.1±4.7 | 54.6±5.5 | |||||
| Yes | 69.3±3.9 | 56.4±4.3 | 48.6±4.8 | 90.0±9.5 | 80.0±12.6 | 80.0±12.6 | 74.8±7.3 | 62.5±8.3 | 53.8±9.1 | 64.7±5.0 | 51.4±5.4 | 42.0±6.2 | |||||
| AIDS event | 0.024 | 0.273 | 0.162 | 0.022 | |||||||||||||
| No | 79.0±2.6 | 73.4±2.9 | 65.4±4.0 | 90.5±6.4 | 90.5±6.4 | 90.5±6.4 | 88.8±3.3 | 86.3±3.7 | 74.8±6.3 | 70.5±4.0 | 61.8±4.3 | 55.5±5.8 | |||||
| Yes | 74.3±3.3 | 60.4±3.8 | 55.2±4.1 | 91.7±8.0 | 81.5±11.9 | 61.1±19.8 | 85.0±3.8 | 72.2±5.0 | 70.2±5.2 | 60.2±5.5 | 44.4±5.8 | 37.8±6.1 | |||||
| Coinfection | 0.075 | 0.957 | 0.129 | 0.895 | |||||||||||||
| No | 78.6±2.6 | 70.8±3.0 | 67.4±3.5 | 85.7±9.3 | 85.7±9.3 | 85.7±9.3 | 88.8±2.8 | 82.1±3.6 | 76.5±4.6 | 65.5±4.7 | 55.5±5.0 | 55.5±5.0 | |||||
| Yes | 75.0±3.2 | 63.61±3.7 | 53.7±4.5 | 94.7±5.1 | 88.8±7.5 | 76.1±13.4 | 82.8±5.2 | 72.5±6.3 | 64.7±7.7 | 67.7±4.5 | 54.8±5.0 | 44.2±6.0 | |||||
| cART | 0.001 | 0.660 | 0.007 | 0.024 | |||||||||||||
| No | 63.7±5.5 | 51.7±5.9 | 49.6±6.0 | 90.0±9.5 | 90.0±9.5 | 90.0±9.5 | 67.4±9.5 | 58.9±10.0 | 58.9±10.0 | 55.5±7.7 | 39.2±7.8 | 35.9±7.8 | |||||
| Yes | 80.0±2.2 | 71.3±2.5 | 63.6±3.2 | 91.3±5.9 | 86.5±7.3 | 74.1±13.0 | 90.0±2.4 | 82.4±3.2 | 75.0±4.3 | 69.4±3.5 | 59.3±3.9 | 51.7±4.9 | |||||
| CD4+ count, cell/mm3 | <0.001 | 0.003 | 0.001 | 0.001 | |||||||||||||
| 200-max | 84.3±2.6 | 76.6±3.2 | 70.6±4.2 | 96.1±3.8 | 96.1±3.8 | 96.1±3.8 | 94.1±2.9 | 89.6±4.1 | 86.2±5.2 | 74.6±4.3 | 62.9±5.0 | 53.2±6.9 | |||||
| 0–199 | 64.3±3.6 | 53.7±3.9 | 49.3±4.1 | 71.4±17.1 | 57.1±18.7 | 42.9±18.7 | 78.8±4.6 | 69.1±5.3 | 64.3±5.9 | 50.8±5.4 | 39.5±5.3 | 36.9±5.6 | |||||
| Nadir CD4+ count, cell/mm3 | <0.001 | 0.085 | 0.001 | 0.033 | |||||||||||||
| ≥200 | 87.4±3.2 | 81.3±3.8 | 77.9±4.9 | 100 | 100 | 100 | 97.9±2.1 | 93.0±3.9 | 85.9±7.8 | 73.3±6.3 | 63.9±7.1 | 63.9±7.1 | |||||
| 50–199 | 69.3±3.5 | 77.7±3.1 | 61.4±4.4 | 75.0±15.3 | 62.5±17.1 | 62.5±17.1 | 88.8±3.7 | 84.0±4.5 | 75.6±6.1 | 69.8±4.6 | 59.2±5.1 | 49.9±6.6 | |||||
| <50 | 67.5±4.1 | 54.5±4.5 | 48.8±4.9 | 90.0±9.5 | 90.0±9.5 | 90.0±9.5 | 75.5±5.7 | 61.9±6.6 | 58.8±7.0 | 56.8±6.2 | 42.8±6.3 | 37.1±6.8 | |||||
| HIVRNA, copies/mL | 0.020 | 0.841 | 0.015 | 0.032 | |||||||||||||
| Undetectable | 84.8±3.5 | 77.8±4.3 | 72.6±5.3 | 91.7±7.9 | 91.7±7.9 | 61.1±25.5 | 97.6±2.3 | 91.2±5.0 | 86.1±6.8 | 73.2±6.1 | 64.4±6.8 | 64.4±6.8 | |||||
| Positive | 70.8±2.8 | 60.0±3.2 | 54.8±3.6 | 90.5±6.4 | 84.8±8.1 | 84.8±8.1 | 80.4±3.9 | 71.7±4.6 | 67.5±5.2 | 60.2±4.2 | 47.1±4.5 | 40.2±5.1 | |||||
Note: Plus minus are value standard error, P value are from long rank test. - The sample size was too small for Kaplan Meier analysis.
*NE, not evaluable. ADCs: AIDS-defining cancers, IVDU: Intravenous Drug Use, cART: combined antiretroviral therapy; CA: cancer.
In univariate analyses, poorer survival after NADC diagnosis was associated with intravenous drug use (IVDU) mode of infection, lower CD4+ T-cell count at NADC diagnosis and previous AIDS event (Table 3). Analyses of risk factors for mortality after diagnosis of specific NADC were limited due to the relatively small number of patients experiencing these events. However, male gender, age between 35–49, previous AIDS event, lower CD4+cell count at diagnosis and lower nadir CD4+ T-cell count were associated with mortality after Hodgkin lymphoma. No association was observed between type of NADCs and epidemiologic and clinical variable among the patients with lung, liver or breast cancers (Table 3).
Table 3. Rate of overall survival NADCs by patients' characteristics.
| Variables | Categories | NADCs (n = 431) | P-value | LIVER CA (n = 69) | P-value | LUNG CA (n = 35) | P-value | BREAST CA (n = 30) | P-value | HODGKIN LYMPHOMA (n = 61) | P-value | |||||||||
| 1y | 5y | 10y | 1y | 5y | 10y | 6month | 1y | 3y | 5y | 10y | 1y | 5y | 10y | |||||||
| Total | 76.1±2.1 | 59.2±2.7 | 45.0±4.1 | 59.8±6.0 | 31.9±6.4 | - | 54.4±8.7 | 28.0±8.6 | 96.7±3.3 | 78.1±8.9 | 65.1±14.0 | 74.2±5.7 | 57.8±7.2 | 24.8±11.2 | ||||||
| Gender | 0.152 | 0.380 | NE* | 0.089 | 0.003 | |||||||||||||||
| Male | 74.7±2.4 | 56.8±3.1 | 40.9±4.8 | 56.0±6.5 | 27.4±6.8 | - | 58.3±9.3 | 32.0±9.6 | 80.0±17.9 | 53.3±24.8 | 26.7±22.6 | 77.0±5.8 | 63.0±7.5 | 27.1±12.2 | ||||||
| Female | 80.4±4.0 | 67.4±5.1 | 58.6±6.5 | 87.5±11.7 | 62.5±17.1 | - | - | - | 90.3±6.6 | 83.9±8.7 | 83.9±8.7 | 50.0±20.4 | - | - | ||||||
| Age, years | 0.331 | 0.096 | 0.094 | 0.108 | 0.045 | |||||||||||||||
| 18–34 | 82.3±6.6 | 68.4±8.4 | 62.1±9.6 | - | - | - | - | 66.7±2.7 | 33.3±2.7 | 33.3±2.7 | 83.1±11.0 | 71.2±14.5 | 71.2±14.5 | |||||||
| 35–49 | 76.2±2.7 | 58.1±3.5 | 42.4±5.3 | 67.9±6.8 | 33.2±7.5 | - | 42.8±13.2 | 14.3±9.3 | 88.2±7.9 | 80.9±10.1 | 53.9±23.0 | 68.3±7.6 | 49.5±8.6 | 9.9±9.0 | ||||||
| 50-max | 74.2±3.8 | 59.4±5.0 | 44.5±8.1 | 40.9±11.1 | 35.1±10.9 | - | 81.6±5.3 | 71.1±6.4 | - | - | - | 88.9±10.5 | 88.9±10.5 | 88.9±10.5 | ||||||
| IVDU | 0.007 | 0.753 | 0.606 | 0.292 | 0.686 | |||||||||||||||
| No | 81.7±2.5 | 63.1±3.7 | 53.5±5.4 | 60.5±13.8 | 32.3±16.7 | - | 57.9±12.2 | 30.4±13.4 | 86.5±7.3 | 80.8±8.8 | 80.8±8.8 | 78.1±7.3 | 60.2±9.8 | 60.2±9.8 | ||||||
| Yes | 68.7±3.5 | 54.1±4.0 | 37.2±5.5 | 59.6±6.7 | 31.8±6.7 | - | 50.9±12.5 | 25.5±11.0 | 100 | 66.7±2.7 | - | 70.0±8.9 | 56.1±10.2 | 18.7±11.3 | ||||||
| AIDS event | 0.008 | 0.683 | 0.074 | 0.480 | 0.021 | |||||||||||||||
| No | 78.5±2.7 | 67.4±3.3 | 48.6±6.0 | 56.8±7.9 | 40.5±8.4 | - | 56.7±10.9 | 39.2±11.4 | 92.9±6.8 | 85.1±9.7 | 63.8±19.8 | 82.2±7.3 | 74.8±9.7 | 24.9±20.6 | ||||||
| Yes | 72.8±3.4 | 48.6±4.3 | 39.7±5.4 | 64.3±9.1 | 22.7±9.3 | - | 50.3±14.4 | - | 82.5±11.3 | 68.7±15.7 | 68.7±15.7 | 65.5±8.8 | 41.3±9.7 | 20.7±11.4 | ||||||
| Coinfection | 0.146 | NE* | 0.295 | 0.850 | 0.699 | |||||||||||||||
| No | 78.1±3.1 | 62.2±4.1 | 52.1±6.1 | - | - | 63.6±11.1 | 33.9±12.6 | 83.5±10.8 | 74.3±13.0 | 74.3±13.0 | 64.5±9.1 | 59.6±9.7 | 59.6±9.7 | |||||||
| Yes | 74.5±2.9 | 56.9±3.6 | 40.6±5.2 | 59.8±6.0 | 31.9±6.4 | 42.9±13.2 | 21.4±11.0 | 92.9±6.9 | 79.6±13.6 | 39.8±28.9 | 83.3±6.8 | 59.0±9.9 | 19.7±11.8 | |||||||
| cART | 0.152 | 0.572 | NE* | NE* | 0.532 | |||||||||||||||
| No | 71.9±6.4 | 47.2±7.4 | 43.9±7.6 | 57.1±16.4 | 45.7±16.6 | - | - | - | - | - | - | 80.0±17.9 | 40.0±21.9 | - | ||||||
| Yes | 76.7±2.2 | 61.0±2.9 | 45.1±4.5 | 60.2±6.5 | 29.0±7.0 | - | 57.0±9.4 | 34.1±9.9 | 96.7±3.3 | 78.1±8.9 | 65.1±14.0 | 73.7±6.1 | 60.4±7.5 | 25.9±11.7 | ||||||
| CD4+ T-cell count, cell/mm3 | 0.014 | 0.225 | NE* | 0.270 | 0.006 | |||||||||||||||
| 200-max | 78.1±2.4 | 62.4±3.2 | 48.7±5.1 | 61.6±6.9 | 38.8±8.2 | - | 61.7±9.6 | 32.1±10.4 | 91.7±8.0 | 81.5±11.9 | 81.5±11.9 | 83.2±5.8 | 63.3±8.6 | 25.3±14.3 | ||||||
| 0–199 | 68.7±4.7 | 48.8±5.3 | 32.7±7.0 | 59.2±11.9 | 17.8±9.3 | - | - | - | 80.0±12.6 | 68.6±15.1 | 51.4±18.7 | 50.0±13.4 | 40.0±13.4 | - | ||||||
| Nadir CD4+ counts, cell/mm3 | 0.337 | 0.891 | 0.427 | 0.433 | 0.083 | |||||||||||||||
| ≥200 | 76.2±3.3 | 62.9±4.3 | 51.6±6.8 | 53.1±9.9 | 31.9±12.1 | - | 64.0±10.9 | 33.9±11.5 | 90.9±8.7 | 90.9±8.7 | 90.9±8.7 | 81.9±9.5 | 51.6±14.0 | - | ||||||
| 50–199 | 78.4±3.2 | 57.9±4.3 | 42.9±6.0 | 61.0±8.5 | 30.5±8.3 | - | 57.1±16.4 | 21.4±17.5 | 85.7±13.2 | 64.3±2.1 | 64.3±2.1 | 78.6±7.7 | 50.6±8.4 | 40.7±16.0 | ||||||
| <50 | 71.1±5.0 | 54.4±6.2 | 39.5±8.8 | 75.0±15.3 | 37.5±17.1 | - | - | - | 87.5±11.7 | 72.9±16.5 | 48.6±22.7 | 52.7±14.1 | 44.0±14.3 | - | ||||||
| HIVRNA, copies/mL | 0.110 | 0.149 | NE* | 0.668 | 0.233 | |||||||||||||||
| Undetectable | 79.8±2.9 | 60.0±4.6 | 48.5±7.6 | 69.9±7.6 | 35.9±9.9 | - | 72.3±10.6 | 47.9±13.7 | 100 | 53.3±24.8 | - | 85.0±7.9 | 67.7±13.2 | - | ||||||
| Positive | 71.7±3.2 | 55.8±3.7 | 41.7±4.9 | 49.0±9.3 | 26.4±8.4 | - | - | - | 81.7±9.6 | 81.7±9.6 | 81.7±9.6 | 68.7±7.9 | 55.1±8.8 | 22.0±12.6 | ||||||
Note: Plus minus are value standard error, P value are from long rank test. - The sample size was too small for Kaplan Meier analysis.
*NE, not evaluable.
NADCs: Non-AIDS-defining cancers, IVDU: Intravenous Drug Use, cART: combined antiretroviral therapy; CA: cancer.
In multivariable analyses, predictors of lower survival probability after both ADCs and NADCs diagnosis were lower CD4+ T-cell count and IVDU as mode of HIV infection (Table 4). Being on cART at cancer diagnosis was associated with improved survival after ADCs. A previous AIDS event was no longer associated with survival after both ADCs and NADCs when imputed with the other covariates in the multivariable model. For ADC, the association between the HIV RNA serum levels at the time of cancer diagnosis and mortality risk was next to threshold for statistical significance (HR: 1.6, 95%CI 0.99–2.53, p = 0.060). Multivariate analyses of risk factors for mortality after diagnosis of specific ADC or NADC cancers are shown in Table 5.
Table 4. Cox regression multivariate models for ADCs and NADCs.
| Variable | Categories | ADCs | NADCs | ||
| HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| Gender | Male vs Female | 1.4 (0.86–2.20) | 0.179 | 1.2 (0.78–1.78) | 0.443 |
| Age, years | |||||
| 18–34Rif | 1Rif | 1Rif | - | ||
| 35–49 | 1.3 (0.77–2.15) | 0.338 | 1.2 (0.62–2.39) | 0.576 | |
| 50-max | 1.7 (0.94–3.19) | 0.077 | 1.8 (0.91–3.68) | 0.092 | |
| Years of Diagnosis | |||||
| 1998–2002 | 1Rif | 1Rif | - | ||
| 2003–2007 | 1.15 (0.77–1.71) | 0.487 | 1.2 (0.81–1.82) | 0.354 | |
| 2008–2012 | 0.88 (0.51–1.51) | 0.641 | 0.8 (0.49–1.32) | 0.398 | |
| cART Therapy | Yes vs No | 0.5 (0.34–0.82) | 0.004 | 0.8 (0.52–1.33) | 0.452 |
| CD4+ count at diagnosis, cell/mm3 | 0–199 vs 200-max | 2.4 (1.59–3.49) | <0.001 | 1.5 (1.05–2.08) | 0.025 |
| IVDU | Yes vs No | 1.9 (1.30–2.77) | 0.001 | 1.6 (1.13–2.34) | 0.008 |
| HIV RNA at diagnosis | Positive vs. undetectable | 1.6 (0.99–2.53) | 0.060 | 1.2 (0.85–1.74) | 0.281 |
| Previous AIDS event | Yes vs No | 1.0 (0.70–1.46) | 0.944 | 1.2 (0.91–1.74) | 0.168 |
Note: ADCs: AIDS-defining cancers, NADCs: Non-AIDS-defining cancers, IVDU: injection drug users, cART: combined antiretroviral therapy; HR: Hazard ratio; CI: confidence interval.
Table 5. Cox regression multivariate models for ADC and NADC specific cancers.
| Variable | Categories | ADCs | NADCs | ||||||||||||
| CERVICAL CA | KS | NHL | LIVER CA | LUNG CA | BREAST CA | HL | |||||||||
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | HR 95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| Gender | Male vs Female | .. | .. | 0.8 (0.26–2.54) | 0.725 | 1.2 (0.68–2.10) | 0.525 | 1.2 (0.81–1.84) | 0.340 | .. | .. | 1.9 (0.13–27.75) | 0.634 | 0.4 (0.11–1.09) | 0.072 |
| Age, years | |||||||||||||||
| 18–34 | 1Rif | - | 1Rif | - | 1Rif | - | .. | - | 1Rif | - | 1Rif | - | 1Rif | - | |
| 35–49 | 1.2 (0.13–11.23) | 0.874 | 1.1 (0.48–2.75) | 0.746 | 1.4 (0.70–2.78) | 0.337 | 1Rif | 0.4 (0.05–3.69) | 0.450 | 0.3 (0.02–3.41) | 0.327 | 1.6 (0.44–5.56) | 0.486 | ||
| 50-max | .. | .. | 1.50 (0.51–4.46) | 0.462 | 1.4 (0.66–3.09) | 0.365 | 2.1 (1.05–4.21) | 0.036 | 0.2(0.02–1.61) | 0.120 | .. | .. | 0.4 (0.04–4.18) | 0.458 | |
| CARTTherapy | Yes vs No | .. | .. | 0.3 (0.15–0.78) | 0.011 | 0.6 (0.39–1.06) | 0.086 | 1.5 (0.59–3.66) | 0.398 | .. | .. | .. | .. | 0.5 (0.12–1.74) | 0.258 |
| CD4+ count at diagnosis, cell/mm3 | 0–199 vs 200-max | 13.0 (1.36–124.10) | 0.026 | 4.0 (1.73–9.42) | 0.001 | 2.2 (1.31–3.10) | 0.001 | 2.0 (0.99–3.96) | 0.050 | .. | .. | 0.8 (0.06–8.95) | 0.821 | 2.6 (1.01–6.44) | 0.046 |
| IVDU | Yes vs No | .. | .. | 3.2 (1.53–6.55) | 0.002 | .. | .. | .. | 1.6 (1.13–2.33) | 0.008 | 2.1 (0.18–24.64) | 0.559 | .. | .. | |
| HIV RNA at diagnosis | Positive vs Negative | .. | .. | .. | .. | 1.7 (0.96–3.00) | 0.071 | .. | .. | .. | .. | .. | .. | ||
Note: ADCs: AIDS-defining cancers, NADCs: Non-AIDS-defining cancers, IVDU: injection drug users, cART: combined antiretroviral therapy, CA: cancer, KS: Kaposi Sarcoma; NHL: non Hodgkin lymphoma; HL: Hodgkin lymphoma; HR: Hazard ratio; CI: confidence interval.
HRs and 95%CIs from the most parsimonious Cox models for ADC and NADC specific cancers. We used the stepwise backward procedure to eliminate non-significant variables. The “..” denotes the variables that were eliminated from the models in the process of stepwise backwards elimination, and factors that were not evaluable for some cancers.
Discussion
Our results, from a large, multicenter Italian HIV-infected cohort, describe survival after cancer diagnoses in patients from 1998–2012. Cancer occurrence has increasingly contributed to overall mortality among HIV-infected populations [20]–[22]. We found that around 6% of HIV-infected patients developed malignancies (900 malignancies) and almost 40% of these patients were dead at the time of analysis.
Although no differences in survival were observed between ADC and NADC categories during the first 2 years after cancer diagnosis, with a mortality rate close to 30% for both of them, the overall survival after a NADC diagnosis was poorer than after an ADC diagnosis and varied substantially depending on the type of NADC. Moreover, only 45% of patients with NADC were alive 10 years after cancer diagnosis, compared with 60% of those with ADC. Notably, patients with NADCs had a better immunological status than patients with ADCs at the time of cancer diagnosis with statistically significant higher nadir and CD4+ T-cell counts, indicating that patients with NADC appeared to have inferior survival despite better immunity.
Considering the year of diagnosis, we observed that earlier period of NADC diagnosis was associated with a poorer prognosis. This probably reflects the extended use of cancer therapy in HIV-infected people in recent years or improved screening of these patients leading to a diagnosis of early-stage disease (data about cancer screening and cancer staging are not available in the Master cohort). Similar association was reported in other studies [22]–[23]. On contrary, we found no differences in survival after ADCs depending on period of diagnosis (p = 0.218). Since the introduction of effective HIV treatment, there has been an improvement in the control of HIV replication and greater CD4+ T-cell count increases. Therefore patients with ADCs have a better prognosis, although a more advanced immunodeficiency status is still the dominant risk factor for death [24]. Recently, a study within the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) cohort described that persons with HIV infection are not fully immune reconstituted until CD4 counts increased to >750 cells/mm3. Therefore they remain at AIDS events risk, although values >500 cells/mm3 give them a good immunity [25]. This improvement in viro-immunological markers and the younger age of patients at ADC diagnosis could in part explain the higher survival rates we observed in patients after an ADC diagnosis. As previously described, after a cancer diagnosis the mortality increases with age [20]. A better control of HIV-related factors is therefore a key factor to continue improving survival of patients who develop ADCs even in the era of cART.
Moreover, our results show variations in survival among types of cancer within the two categories, NADCs and ADCs. In particular, we found that NHL for ADC and lung cancer for NADC had the poorest prognosis. Consistent with other studies, we observed a five-year survival of approximately 50% for NHL [15], [26] and a one-year survival of just 30% for lung cancer [27]. The shift toward less biologically favorable and curable lymphomas could in part explain the poor survival after NHL even in the cART era. Indeed, a reversal in incidence of different lymphomas during the last years have been reported: incidence of Burkitt's lymphoma increased, whereas diffuse large B-cell lymphoma and primary central nervous system lymphoma decreased [28]. For lung cancer, as in HIV-negative cases, the clinical stage of cancer is highly predictive of survival, and long-term overall survival can only be achieved at the limited stages [27].
Liver cancer is mainly driven by hepatitis coinfection in the HIV-infected patient as a late complication of liver cirrhosis [30]. Indeed, all patients diagnosed with liver cancer in our study were coinfected with hepatitis virus. Potent cART has improved the survival of HIV-infected individuals long enough to allow liver cancer to emerge in patients with known risk factors for liver cancer, such as prolonged ethanol consumption or chronic viral hepatitis [30]. Moreover, the management of liver cirrhosis by clinicians attending HIV-infected patients has probably improved in the last decade, resulting in longer survival of HIV-infected patients with cirrhosis. Compared to the data of the Italian cancer registries (the Italian Association of Cancer Registries AIRTUM) [29], we observed an increased 5 years survival for HIV patients with liver cancer (relative survival rate: 15% vs 32%, respectively). Improvements in liver cancer prognosis could be due to a combination of diagnostic anticipation and better control of disease progression. On contrary, we observed a poorer survival for HIV-infected patients with HL (5-years relative survival rate: 58%) respect to the general population with HL (5-years relative survival rate: 83%). This result could be in part explained by the differences in clinicopathological characteristics of HIV-related HL (HIV-HL from those of HL in HIV-uninfected population [31]. In fact, HIV-HL is characterized by a more aggressive clinical presentation, with an unfavorable histological subtype as opposed to the subtype observed in HIV-negative young adults. The five-year relative survival (age and gender-standardized) in the Italian general population for the cancers diagnosed between 2000 and 2004, and the 5-years relative survival in the master cohort for the cancers diagnosed in the period 1998–2012 are reported in the Table S1.
HIV-related immunosuppression is a well-accepted, strong biological risk factor for the virus-associated cancers of AIDS-defining malignancies. As expected, among ADCs, viro-immunological variables and HIV treatment at cancer diagnosis influenced the prognosis of HIV-positive patients diagnosed with Kaposi sarcoma and NHL. Many important cancer sites have benefited from screening [32]–[33]. In our study, cervical cancer and breast cancer, both malignancies in which screening programs have been introduced in general population and more specifically during the follow-up for HIV infection, are the cancers with the better prognosis. Survival rates after cervical cancer were statistically significantly lower in patients with CD4+ T-cell count less than 200 cell/mm3 at cancer diagnosis, although in our cohort the proportion of patients with CD4 counts above 200 cell/mm3 at cervical cancer diagnosis was higher compared with patients with other AIDS-related malignancies (p = 0.005). Different studies showed that women with AIDS related cervical cancer differ from women with other HIV related malignancies in two ways: they had less immune suppression and the cause of death was more likely to be attributed to cancer than to opportunistic infections [34]–[35]. Moreover, data in the literature have shown that the clinical course of cervical cancer becomes more aggressive when the CD4+ T-cell count is low [36]. Differently from the other ADCs, in our study cervical cancer survival seemed to be unaffected by the use of cART.
Importantly, low CD4+ T-cell count at NADC diagnosis was strongly associated with poor survival for all NADC combined and in particular for Hodgkin lymphoma, emphasizing the need for timely HIV treatment. For liver cancer, the association between the CD4 count at the time of cancer diagnosis and mortality risk was next to threshold for statistical significance (p = 0.05). For breast and lung cancers we found no association between HIV clinical variables (cART, CD4 count, HIVRNA) and subsequent mortality, indicating that HIV may have no effect on these malignancies.
The cumulative survival probabilities after a malignancy- both ADCs and NADCs- were worse in patients with a history of IVDU. Notably, a history of IVDU was an important risk factor for survival after lung cancer diagnosis. Indeed, HIV transmission mode is an important predictor of prognosis in HIV-infected persons and different studies [25], [37]–[38] showed that IDVU is strongly associated to AIDS events even with CD4 counts >500 mm3. IVDU may be also a marker for other lifestyle habits (e.g. smoking habits, alcohol intake) that can influence the risk of death. Moreover, adherence to antiretroviral therapy among IVDU is often suboptimal. Finally, we found that this factor had a more negative impact on survival rates of HIV-infected patients respect to the absence of cART at cancer diagnosis. Interestingly, in our study cART at the time of cancer diagnosis seemed to influence ADC but not NADC prognosis.
Our study has several limitations. First, the data were retrospectively collected and so it is possible that the number of cancer diagnoses and death were underestimated. Second, information regarding the life-style of patients (tobacco exposure, alcohol abuse), cancer stage and treatment, and causes of death were not available in this study. However, it is important to underline that the assessment of cancer as cause of death among subjects with HIV/AIDS is complicated, as they frequently show several concomitant serious medical condition (immunodeficiency secondary to chemotherapy, interruption of cART due to chemotherapy interaction or increased of secondary effects, etc.). The survival analysis was not performed for any type of NHL although they have different prognosis. However, our data are consistent with those reported in other cohort study that evaluate survival for all NHL combined [15] and for diffuse large-cell lymphoma [26]. The small number of cases for some cancers also is a limitation; in particular, the study included around 30 patients with cervical, breast or lung cancer. Finally, just a few patients reached a follow-up ≥10 years (median follow-up was 4.58 years for patients with an ADC diagnosis and 3.35 years for those with a NADC diagnosis) and it was too small/short to determine the related mid- or long term survival probabilities. Despite these limitations, a strength of our work is the long-term follow-up, including both person/years and median years of follow-up.
Taken together, findings from this large prospective study of survival in HIV-infected patients suggest that maintaining higher CD4+ T-cell counts is a key factor to improve prognosis after both ADCs and NADCs, in particular when considering Kaposi sarcoma and the lymphomas. On contrary CD4 The survival after diagnosis of NADCs is poorer than after ADCs but has shown an improvement in the last years. Importantly, patients with a history of injection drug use represent the population with the worst survival after a cancer diagnosis and therefore these patients should be targeted with screening and preventative strategies. Survival studies on cancer in HIV-infected patients can help to describe an important phenomenon, giving an indication on overall access to early diagnosis and diffusion of screening interventions, and no less importantly, quality, equity and response to cancer treatments with respect to HIV-uninfected patients.
Supporting Information
The five-year relative survival (age and gender-standardized) in the Italian general population for the cancers diagnosed between 2000 and 2004, and the 5-years relative survival in the Master cohort for the cancers diagnosed in the period 1998–2012.
(DOCX)
Acknowledgments
Part of the data included in this manuscript has been presented as a poster presentation at EACS, Brussels, Belgium October 2013.
Contributors
Members of the Master Cohort Group are:
F. Castelli (University Division of Infectious and Tropical Diseases, University of Brescia, Brescia, Italy), C. Torti (University Division of Infectious and Tropical Diseases, University of Brescia, Brescia, Italy, and Unit of Infectious Diseases, Department of Medical and Surgical Sciences, University “Magna Graecia”, Catanzaro, Italy), S. Casari (Hospital Division of Infectious and Tropical Diseases, Spedali Civili Hospital, Brescia, Italy), P. Nasta (University Division of Infectious and Tropical Diseases, University of Brescia, Brescia, Italy), F. Castelnuovo (Hospital Division of Infectious and Tropical Diseases, Spedali Civili Hospital, Brescia, Italy), I. El Hamad (Hospital Division of Infectious and Tropical Diseases, Spedali Civili Hospital, Brescia, Italy), A. Saracino (Clinic of Infectious Diseases, University of Bari, Bari, Italy), L. Monno (Clinic of Infectious Diseases, University of Bari, Bari, Italy), R. Cauda (Institute of Clinical Infectious Diseases, Catholic University of Sacred Heart, Roma, Italy), S. Di Giambenedetto (Institute of Clinical Infectious Diseases, Catholic University of Sacred Heart, Roma, Italy), M. Colafigli (Institute of Clinical Infectious Diseases, Catholic University of Sacred Heart, Roma, Italy), F. Mazzotta (Clinic of Infectious Diseases, “Santa Maria Annunziata” Hospital, Firenze, Italy), S. Lo Caputo (Clinic of Infectious Diseases, “Santa Maria Annunziata” Hospital, Firenze, Italy), P. Pierotti (Clinic of Infectious Diseases, “Santa Maria Annunziata” Hospital, Firenze, Italy), M. Di Pietro (Clinic of Infectious Diseases, “Santa Maria Annunziata” Hospital, Firenze, Italy), C. Ble (Clinic of Infectious Diseases, “Santa Maria Annunziata” Hospital, Firenze, Italy), G. Carnevale (Clinic of Infectious Diseases, Hospital of Cremona, Cremona, Italy), A. Gori (Clinic of Infectious Diseases, Hospital of Cremona, Cremona, Italy), S. Costarelli (Clinic of Infectious Diseases, Hospital of Cremona, Cremona, Italy).
Funding Statement
The authors have no support or funding to report.
References
- 2. Bonnet F, Burty C, Lewden C, Costagliola D, May T, et al. (2009) Changes in cancer mortality among HIV-infected patients: the Mortalitè 2000 and 2005 Survey. Clin Infect Dis 48: 633–639. [DOI] [PubMed] [Google Scholar]
- 3.Simard EP, Engels EA (2010) Cancer as a cause of death among people with AIDS in the United States. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 51(8): : 957-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, et al. (2008) Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med 148: 728–736. [DOI] [PubMed] [Google Scholar]
- 5. Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, et al. (2006) Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS 20: 1645–1654. [DOI] [PubMed] [Google Scholar]
- 6. Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, et al. (2009) HIV infection and the risk of cancers with and without a known infectious cause. AIDS 23: 2337–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crum-Cianflone N, Hullsiek KH, Marconi V, Weintrob A, Ganesan A, et al. (2009) Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS 23: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shiels MS, Cole SR, Kirk GD, Poole C (2009) A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune DeficSyndr 52: 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albini L, Calabresi A, Gotti D, Ferraresi A, Festa A, et al. (2013) Burden of Non-AIDS-Defining and Non-Virus-Related Cancers Among HIV-Infected Patients in the Combined Antiretroviral Therapy Era. AIDS Res Hum Retroviruses 29(8): 1097–1104. [DOI] [PubMed] [Google Scholar]
- 10. Vaccher E, Spina M, Talamini R, Zanetti M, di Gennaro G, et al. (2003) Improvement of systemic human immunodeficiency virus-related non Hodgkin lymphoma outcome in the era of highly active antiretroviral therapy. Clin Infect Dis 37: 1556–1564. [DOI] [PubMed] [Google Scholar]
- 11. Lim ST, Karim R, Nathwani BN, Tulpule A, Espina B, et al. (2005) AIDS-related Burkitt's lymphoma versus diffuse large cell lymphoma in the pre-highly active antiretroviral therapy (HAART) and HAART eras: significant differences in survival with standard chemotherapy. J Clin Oncol 23: 4430–4438. [DOI] [PubMed] [Google Scholar]
- 12. Alfa-Wali M, Allen-Mersh T, Antoniou A, Tait D, Newsom-Davis T, et al. (2012) Chemoradiotherapy for anal cancer in HIV-patients causes prolonged CD4 cell count suppression. Ann Oncol 23(1): 141–147. [DOI] [PubMed] [Google Scholar]
- 13. Rengan R, Mitra N, Liao K, Armstrong K, Vachani A (2012) Effect of HIV on survival in patients with non-small-cell lung cancer in the era of highly active antiretroviral therapy: a population-based study. The Lancet Oncology 13(12): 1203–1209. [DOI] [PubMed] [Google Scholar]
- 14. Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, et al. (2003) Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet 362: 22–29. [DOI] [PubMed] [Google Scholar]
- 15. Hoffmann C, Wolf E, Fatkenheuer G, Buhk T, Stoehr A, et al. (2003) Response to highly active antiretroviral therapy strongly predicts outcome in patients with AIDS related lymphoma. AIDS 17: 1521–1529. [DOI] [PubMed] [Google Scholar]
- 16. Dupont C, Vasser E, Beauchet A, Aegert P, Berthe' H, et al. (2000) Long-term efficacy on Kaposi sarcoma of highly active antiretroviral therapy in a cohort of HIVpositive patients. AIDS 14: 987–993. [DOI] [PubMed] [Google Scholar]
- 17. Hentrich M, Maretta L, Chow KU, Bogner JR, Schurmann D, et al. (2006) Highly active antiretroviral therapy (HAART) improves survival in HIV-associated Hodgkin's disease: results of a multicenter study. Ann Oncol 17: 914–919. [DOI] [PubMed] [Google Scholar]
- 18. Spagnuolo V, Galli L, Salpietro S, Gianotti N, Guffanti M, et al. (2012) Ten-year survival among HIV-1-infected subjects with AIDS or non-AIDS-defining malignancies. Int J Cancer 130: 2990–2996. [DOI] [PubMed] [Google Scholar]
- 19.International Classification of Disease (ICD)-10 version (2010) World Health Organization. Available: http://www.who.int/classifications/icd/en/. Accessed 2013 Jun 25.
- 20. Galli L, Spagnuolo V, Salpietro S, Gianotti N, Cossarini F, et al. (2012) Mortality of HIV-infected patients with or without cancer: comparison with the general population in Italy. Antivir Ther 17(3): 447–458. [DOI] [PubMed] [Google Scholar]
- 21. Simard EP, Engels EA (2010) Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis 51: 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Achenbach CJ, Cole SR, Kitahata MM, Casper C, Willig JH, et al. (2011) Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS 25: 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Long JL, Engels EA, Moore RD, Gebo KA (2008) Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS 22: 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biggar RJ, Engels EA, Ly S, Kahn A, Schymura MJ, et al. (2005) Survival after cancer diagnosis in persons with AIDS. J Acquir Immune DeficSyndr 39(3): 293–299. [DOI] [PubMed] [Google Scholar]
- 25. Mocroft A, Furrer HJ, Miro JM, Reiss P, Mussini C, et al. (2013) The Incidence of AIDS-Defining Illnesses at a Current CD4 Count > = 200 Cells/µL in the Post-Combination Antiretroviral Therapy Era. Clin Infect Dis 57(7): 1038–1047. [DOI] [PubMed] [Google Scholar]
- 26. Lim ST, Karim R, Tulpule A, Nathwani BN, Levine AM (2005) Prognostic factors in HIV-related diffuse large-cell lymphoma: Before versus after highly active antiretroviral therapy. J Clin Oncol 23: 8477–8482. [DOI] [PubMed] [Google Scholar]
- 27. Lavolé A, Chouaïd C, Baudrin L, Wislez M, Raguin G, et al. (2009) Effect of highly active antiretroviral therapy on survival of HIV infected patients with non-small-cell lung cancer. Lung Cancer 65(3): 345–350. [DOI] [PubMed] [Google Scholar]
- 28. Gopal S, Patel MR, Yanik EL, Cole SR, Achenbach CJ, et al. (2013) Temporal trends in presentation and survival for HIV-associated lymphoma in the antiretroviral therapy era. J Natl Cancer Inst 105(16): 1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Italian association of medical oncology (AIOM)-AIRTUM Working Group (2012) I numeri del Cancro. Intermedia Editore. 129 p. [Google Scholar]
- 30. Sulkowski M (2009) Hepatocellular carcinoma in HIV-infected patients comes of age: The convergence of epidemiology and treatment effectiveness. J Hepatol 50: 655–658. [DOI] [PubMed] [Google Scholar]
- 31. Vaccher E, Spina M, Tirelli U (2001) Clinical aspects and management of Hodgkin's disease and other tumours in HIVinfected individuals. Eur J Cancer 37: 1306–1315. [DOI] [PubMed] [Google Scholar]
- 32. Mani D, Aboulafia DM (2013) Screening guidelines for non-AIDS defining cancers in HIV-infected individuals. Curr Opin Oncol 25(5): 518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoppenot C, Stampler K, Dunton C (2012) Cervical cancer screening in high- and low-resource countries: implications and new developments. Obstet Gynecol Surv 67(10): 658–67. [DOI] [PubMed] [Google Scholar]
- 34. Maiman M, Fruchter RG, Clark M, Arrastia CD, Matthews R, et al. (1997) Cervical cancer as an AIDS-defining illness. Obstet Gynecol 89: 76–80. [DOI] [PubMed] [Google Scholar]
- 35. Clarke B, Chetty R (2002) Postmodern cancer: the role of human immunodeficiency virus in uterine cervical cancer. MolPathol 55(1): 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spano JP, Atlan D, Breau JL, Farge D (2002) AIDS and non-AIDS-related malignancies: a new vexing challenge in HIV-positive patients. Part 11. Cervical and anal squamous epithelial lesions, lung cancer, testicular germ cell cancers, and skin cancers. Eur J Intern Med 13: 227–232. [DOI] [PubMed] [Google Scholar]
- 37. Reekie J, Gatell JM, Yust I, Bakowska E, Rakhmanova A, et al. (2011) Fatal and nonfatal AIDS and non-AIDS events in HIV-1-positive individuals with high CD4 cell counts according to viral load strata. AIDS 25: 2259–2268. [DOI] [PubMed] [Google Scholar]
- 38. May M, Sterne JA, Sabin C, Costagliola D, Justice AC, et al. (2007) Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of pro-spective studies. AIDS 21: 1185–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The five-year relative survival (age and gender-standardized) in the Italian general population for the cancers diagnosed between 2000 and 2004, and the 5-years relative survival in the Master cohort for the cancers diagnosed in the period 1998–2012.
(DOCX)