Abstract
Background
In 2006, perioperative epirubicin, cisplatin, and 5-fluorouracil (ecf), compared with surgery alone, demonstrated a significant survival benefit in resectable gastroesophageal cancers. We report the results of our experience with that protocol.
Methods
The BC Cancer Agency (bcca) is a multicentre institution that treats most oncology patients for the province. Characteristics of the 83 bcca patients with localized gastric, gastroesophageal junction, or lower esophageal cancer who initiated perioperative chemotherapy either ecf or epirubicin, cisplatin, and capecitabine (ecx) from 2008 to 2011 were abstracted to an anonymous database and analyzed.
Results
Of the 83 patients in the cohort [66 men; median age: 62 years (range: 37–79 years)], 87.9% completed 3 cycles of perioperative chemotherapy, and 93.9% (n = 78) underwent an attempt at surgery (2 patients died of chemotherapy toxicities, 1 refused surgery, and 2 developed disease progression before surgery). In 11 of the surgeries (14.1%), tumours could not be resected because of unresectability (n = 1), liver metastasis (n = 1), and peritoneal carcinomatosis (n = 9). One patient died of surgical complications. The 6 patients (7.2%) who achieved a pathologic complete response are all alive and recurrence-free. Of 46 patients (55.4%) who subsequently began postoperative chemotherapy, 44.5% completed 3 cycles. Estimated median survival was 40.3 months. Weight loss was the only significant prognostic factor for worse overall survival.
Conclusions
Our multicentre experience confirmed the feasibility of the magic protocol in a real-world scenario and showed that ecx is also an adequate regimen in the perioperative setting. Weight loss was the only significant prognostic factor for worse overall survival. All patients who achieved a pathologic complete response are recurrence-free after a median follow-up of 40.3 months.
Keywords: Gastric cancer, esophageal cancer, gastroesophageal cancer, perioperative chemotherapy
1. INTRODUCTION
In Western populations, cancers originating in the esophagus, gastroesophageal junction (gej), and stomach represent a major health problem and are considered highly lethal diseases, with an overall 5-year mortality rate that ranges from 17% to 27%1. Unfortunately, most of these patients present with late-stage disease, when curative therapy is not possible1.
Although surgical resection remains the only potentially curative treatment for nonmetastatic gastroesophageal cancer, surgery alone is associated with only a modest 5-year overall survival (os) rate of about 25%–35%2–5. Several studies have evaluated the roles of adjuvant chemotherapy or radiotherapy (or both) after curative-intent surgery for gastroesophageal tumours in the hope that better outcomes could be achieved. The most well-known study in the adjuvant setting is the INT0116 trial, in which chemoradiotherapy after complete resection (compared with surgery alone) demonstrated a significant survival benefit (36 months vs. 27 months, p = 0.005), leading to adoption of that particular regimen in the United States6. The positive results of that study have nevertheless been criticized by some because of the poor extent of lymphadenectomy, which might have led to an overestimation of the adjuvant chemoradiation benefit observed. Several other studies have also investigated the role of adjuvant chemotherapy after curative resection, but most have failed to demonstrate any improvement in os or recurrence-free survival (rfs) in Western populations7–11, prompting the evaluation of neoadjuvant approaches for locally advanced gastric cancer. Moreover, according to the literature, complete resection (R0) is achieved only in approximately 70% of patients who undergo gastroesophageal cancer surgery2,3,12,13. Neoadjuvant chemotherapy with or without radiation therapy has therefore recently been added to the surgical protocol, with the aim of downstaging tumours and improving the rates of R0 resection and survival14–16.
The landmark study comparing perioperative epirubicin, cisplatin, and 5-fluorouracil (ecf) with surgery alone in patients with resectable gastroesophageal cancer (the magic trial) was published in 2006. It demonstrated a significant os benefit in favour of the combination arm14. Since then, perioperative chemotherapy with either ecf or epirubicin, cisplatin, and capecitabine (ecx) has become the standard of care for resectable gastroesophageal cancer in British Columbia. More recently, the cross trial demonstrated that, compared with surgery alone, neoadjuvant chemoradiotherapy with weekly carboplatin and paclitaxel followed by surgery also increases os for patients with esophageal or gej tumours16. The option of neoadjuvant chemoradiation with weekly carboplatin and paclitaxel has therefore also been available for locally advanced esophageal or gej tumours at our centre since 2012. After incorporation of perioperative ecx or ecf for gastroesophageal tumours into our clinical practice, a determination of whether our results are comparable to those obtained in a strictly controlled clinical trial was extremely relevant. Our retrospective study was conducted under that premise.
The aims of the study were to investigate whether the results of the magic trial could be replicated in our non-experimental setting and to explore prognostic variables associated with better os in our patient population.
2. METHODS
The BC Cancer Agency is a multicentre institution that treats most oncology patients for the province of British Columbia. All patients with localized gastric, gej, or lower esophageal cancer who initiated ecx or ecf perioperative chemotherapy from March 2008 to June 2011 at our institution were identified using the pharmacy database. Patients with metastatic disease that was identified before chemotherapy commenced or who received radiation therapy as part of perioperative treatment were excluded. Baseline demographics, tumour characteristics, and treatment details were abstracted to an anonymous database and analyzed. This study was approved by the local Institutional Review Board.
Statistical analysis was performed using SPSS for Windows (version 14.0: SPSS, Chicago, IL, U.S.A.). Overall survival was calculated in months from the time of primary diagnosis to the date of death or last follow-up, and rfs was calculated from the time of primary diagnosis to the date of disease recurrence, death, or last follow-up. Kaplan–Meier curves for rfs and os were generated. The log-rank test was used to assess statistical differences between variables, with a p value less than 0.05 being considered statistically significant. Multivariable survival analyses using Cox proportional hazards models explored the effect of variables on os. Hazard ratios (hrs) and 95% confidence intervals were calculated to estimate risk of death.
3. RESULTS
3.1. Patient and Tumour Characteristics
In our cohort, 83 patients [66 men, 17 women; median age: 62 years (range: 37–79 years)] began preoperative chemotherapy with either ecx (72.3%) or ecf (27.7%). Table i summarizes patient characteristics. All patients had already undergone staging imaging by either positron-emission tomography–computerized tomography (pet-ct: 67.5%) or ct (32.5%), which showed no evidence of metastatic disease. Tumour locations included the distal esophagus (31.3%), gej (38.6%), and stomach (30.1%). The most common presenting symptom was dysphagia (n = 51), followed by weight loss of at least 5 kg (n = 45), and epigastric pain or discomfort (n = 20). Other less frequent symptoms included gastrointestinal bleeding (n = 9) and nausea (n = 8). All endoscopic biopsies were determined to be adenocarcinoma (26.5% with signet-ring cell appearance). In our cohort, 31 patients (37.3%) were never-smokers, and 22 (26.5%) had no comorbidities. No information was missing for any variable collected. Median follow-up was 40.3 months.
TABLE I.
Demographic, clinical, tumour, and treatment characteristics
| Characteristic |
Value
|
|
|---|---|---|
| (n) | (%) | |
| Patients | 83 | 100 |
| Age (years) | ||
| Median | 62 | |
| Range | 37–79 | |
| Sex | ||
| Men | 66 | 79.5 |
| Women | 17 | 20.5 |
| Cancer site | ||
| Distal esophagus | 26 | 31.3 |
| Gastroesophageal junction | 32 | 38.6 |
| Stomach | 25 | 30.1 |
| Histology | ||
| Adenocarcinoma | 83 | 100 |
| Signet-ring appearance | 22 | 26.5 |
| Symptoms | ||
| Dysphagia | 51 | 61.4 |
| Weight loss | 45 | 54.2 |
| Epigastric pain or discomfort | 20 | 24.0 |
| Gastrointestinal bleeding | 9 | 10.8 |
| Nausea | 8 | 9.6 |
| Smoking | ||
| Yes | 52 | 62.7 |
| No | 31 | 37.3 |
| Comorbidities | ||
| Yes | 61 | 73.5 |
| No | 22 | 26.5 |
| Type of chemotherapy | ||
| Epirubicin–cisplatin–5-fluorouracil | 23 | 27.7 |
| Epirubicin–cisplatin–capecitabine | 60 | 72.3 |
| Chemotherapy cycles | ||
| Preoperative | ||
| 1 | 5 | 6.0 |
| 2 | 5 | 6.0 |
| 3 | 73 | 88.0 |
| Postoperative | ||
| 0 | 35 | 42.2 |
| 1 | 5 | 6.0 |
| 2 | 6 | 7.2 |
| 3 | 37 | 44.6 |
| Surgery | ||
| Yes | 78 | 94.0 |
| No | 5 | 6.0 |
| R0 resection | ||
| Yes | 59 | 71 |
| No | 24 | 29 |
3.2. Treatment
Of the 83 patients, 73 (87.9%) completed 3 preoperative cycles of either ecx or ecf. The response rate among patients who underwent imaging before surgery was 49.3% (response defined by the radiologist). Chemotherapy toxicities caused the death of 2 patients from febrile neutropenia, 1 patient refused surgery, and 2 patients developed disease progression before surgery. Surgery was attempted in the remaining 78 patients (93.9%), but in 11 of the surgeries (14.1%), the tumour could not be resected because of unresectability (n = 1), liver metastasis (n = 1), or peritoneal carcinomatosis (n = 9). Only 1 patient died of surgical complications. In 59 patients (71%), complete resection (R0) was achieved. In those 59 patients, the median number of lymph nodes examined was 10 (range: 2–41), and the median number of lymph nodes with metastatic involvement was 1 (range: 0–21). The 6 patients who achieved a pathologic complete response were all alive and recurrence-free at the time of writing. Interestingly, all 6 patients with a pathologic complete response received 3 cycles of preoperative chemotherapy, and in imaging before surgery, 5 showed a marked response to that chemotherapy. (In 1 patient, no imaging was performed to evaluate response.)
Among patients who achieved an R0 resection, a trend toward worse os was evident in the group having 3 or more lymph nodes with metastatic involvement than in the group having 2 or fewer lymph nodes with metastatic involvement (p = 0.23). Compared with patients in whom 10 or more lymph nodes were examined, patients having fewer than 10 lymph nodes resected also showed a trend toward worse os (p = 0.24). Overall survival tended to be better in patients who received 3 preoperative cycles of chemotherapy than in patients who could not complete the 3 planned cycles (p = 0.24).
Of the 59 patients with an R0 resection, 46 (55.4% of the total cohort) subsequently began postoperative chemotherapy, and 37 (44.6%) completed 3 cycles. The reasons for not embarking on postoperative chemotherapy were patient refusal (n = 2), postoperative complications (n = 5), toxicity during preoperative chemotherapy (n = 5), and postoperative death (n = 1).
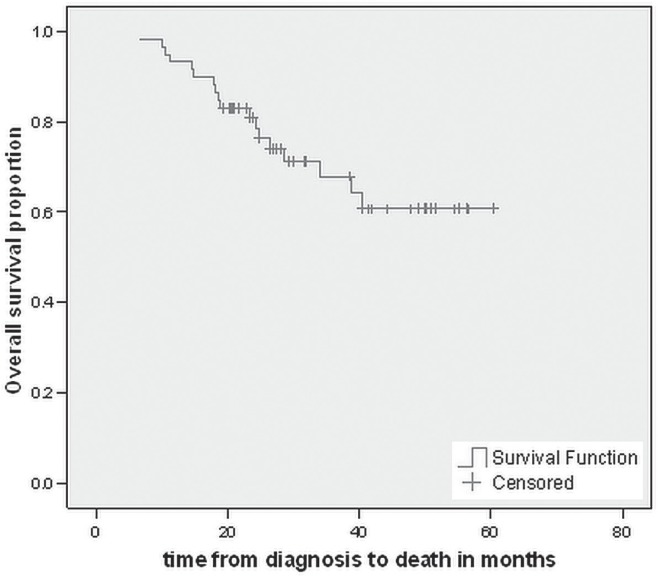
On multivariate analysis, no statistical significance was observed for number of lymph nodes involved (hr: 1.13; p = 0.058), number of lymph nodes examined (hr: 0.95; p = 0.164), n umber of preoperative chemotherapy cycles (hr: 0.80; p = 0.62), or number of postoperative cycles (hr: 0.80; p = 0.29) in the cohort of patients who received a curative resection. However, a trend for worse os was again observed depending on the number of involved lymph nodes. The median os time for patients who achieved an R0 resection has not yet been reached (Figure 1).
FIGURE 1.
Kaplan–Meir curve for overall survival in patients achieving an R0 resection.
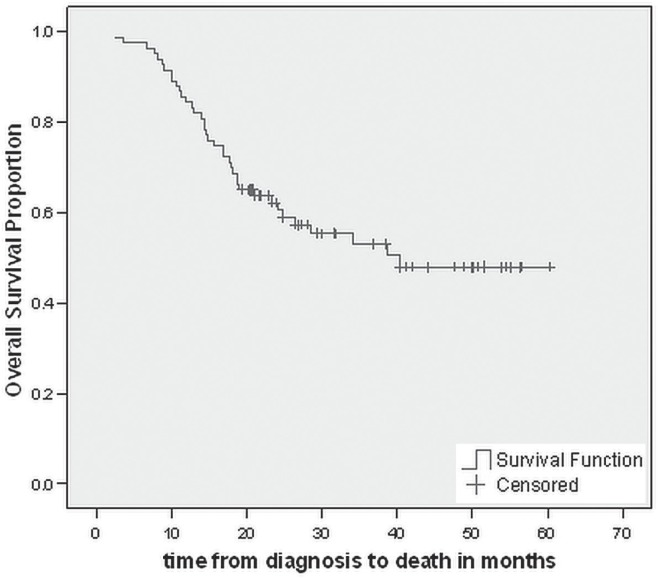
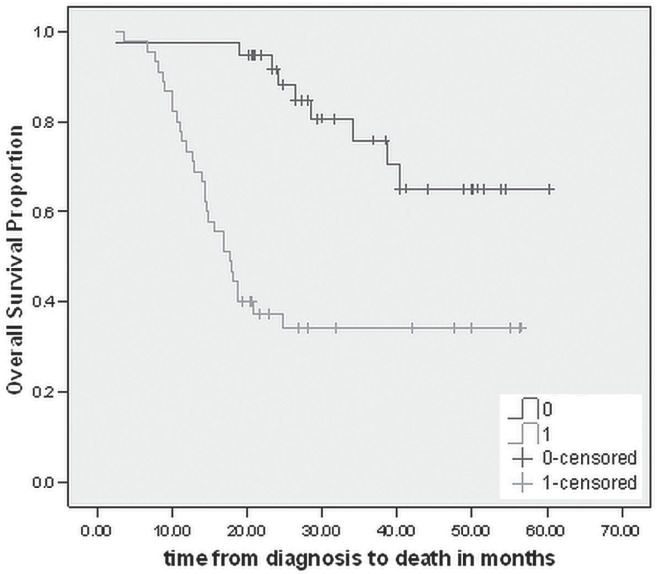
At December 2012, 39 patients (47%) had died. The estimated median os for the entire cohort was 40.3 months (Figure 2). On univariate analyses, initial presentation with weight loss was associated with worse os (p < 0.001), the median os being 17.6 months for those who presented with a weight loss of at least 5 kg and not yet reached for the patients with no history of weight loss (Figure 3). Age, sex, prior history of smoking, comorbidities, number of preoperative chemotherapy cycles, and number of postoperative chemotherapy cycles were not independent prognostic factors for os. On multivariate analyses, only presentation with weight loss was significantly associated with worse os (hr: 0.196; p < 0.001).
FIGURE 2.
Kaplan–Meir curve for overall survival in the entire study cohort.
FIGURE 3.
Kaplan–Meir curve for overall survival in patients with weight loss at presentation.
4. DISCUSSION
Comparing the results of our retrospective study with those of the magic trial (Table ii), we observed similarities in terms both of median age at diagnosis and of male predominance. However, we also observed an important discrepancy with respect to tumour location: esophageal and gej tumours constituted a much higher proportion of the disease in our patients. That finding is not surprising because the magic trial initially included stomach cancers only; in 1999, the protocol was modified to include adenocarcinomas of the esophagus. Another important consideration is that all perioperative chemotherapy administered in the magic trial consisted of ecf. Given the noninferiority with ecx in the metastatic setting and the easier administration in that regimen, we incorporated ecx into the perioperative scenario as an alternative17. Therefore, compared with 100% of patients in the magic trial, only 27.7% of our patients received perioperative ecf. Despite the difference in the chemotherapy combinations used, the proportion of patients who completed 3 preoperative and 3 postoperative cycles were similar in both studies, as were the proportions of patients who underwent surgery and who achieved an R0 resection.
TABLE II.
Comparison with the perioperative group from the magic trial
| Variable | Present study | magic trial |
|---|---|---|
| Median age (years) | 62 | 62 |
| Sex (%) | ||
| Men | 79.5 | 82 |
| Women | 20.5 | 18 |
| Location (%) | ||
| Distal esophagus | 31.3 | 11.2 |
| Gastroesophageal junction | 38.6 | 14.8 |
| Stomach | 30.1 | 74 |
| Type of chemotherapy (%) | ||
| Epirubicin–cisplatin–5-fluorouracil | 27.7 | 100 |
| Epirubicin–cisplatin–capecitabine | 72.3 | 0 |
| Completed chemotherapy | ||
| Preoperative | 88 | 86 |
| Postoperative | 41.6 | 44.5 |
| Surgery | ||
| Yes | 94.0 | 91.6 |
| No | 6.0 | 6.1 |
| R0 resection | ||
| Yes | 71.1 | 69.3 |
| No | 28.9 | 30.7 |
| Median overall survival (months) | 40.3 | 26a |
Estimated from the Kaplan–Meier curve, because the median overall survival was not reported.
It is noteworthy that our estimated median os is 40.3 months, when in the magic trial, it was only about 26 months (estimated from the Kaplan–Meier curve because the relevant data were not provided). One potential reason for the observed difference might be the use of pet-ct imaging for staging in 67.5% of our cases; pet-ct was not performed in the magic trial. Accuracy in preoperative staging is known to be higher with pet-ct than with ct imaging (68% vs. 53%); pet-ct also identifies more clinically occult metastatic disease18. The selection bias implied by preventing more patients with incurable disease from receiving perioperative treatment could explain the improved os seen in our cohort. Perhaps more importantly, a high proportion of our patients received ecx (72.3%) instead of ecf. At least in the metastatic setting, the real-2 trial showed noninferiority for ecx compared with ecf17, and a recent meta-analysis demonstrated superior os for patients treated with capecitabine combinations compared with patients receiving 5-fluorouracil combinations19. Whether capecitabine use also translates into better os for non-metastatic disease remains unknown. The inclusion of a higher proportion of patients with esophageal and gej tumours might be another potential explanation for the better os in our study; however, univariate and multivariate analyses showed no difference in os according to tumour location. Lastly, the time period of the studies might also represent an advantage for our cohort, because staging and surgical procedures tend to improve over time.
Although the extent of lymph node dissection remains controversial, a recent study in 1377 patients showed that lymphadenectomy leads to improved outcomes20. The magic trial reported a 42.5% rate of D2 lymphadenectomy without mentioning the number of lymph nodes examined. By contrast, we could not determine the type of lymphadenectomy performed, but our median number of lymph nodes examined was 10.
In our study, the only statistically significant prognostic factor for worse os was weight loss, which was also previously reported by other authors21–25. For the subset of patients who achieved R0 resection, a trend toward worse os was observed for the group with 3 or more lymph nodes having metastatic involvement. Since 1999, response to neoadjuvant chemotherapy has been well established to be predictive of survival in patients with resectable gastric cancer26,27. Another more recent study conducted at Memorial Sloan–Kettering Cancer Center showed that 3-year disease-specific survival was significantly higher for patients achieving a better than 50% pathologic response to preoperative chemotherapy than for those achieving a lesser histologic response (69% vs. 44%)28. Currently, identifying the patients who would best respond to neoadjuvant therapy remains a challenge; no demographic variable or tumour characteristic can, as yet, identify responders a priori.
In the best-case scenario, preoperative treatment can induce a complete pathologic response, which is well known to be associated with improved outcomes in numerous malignancies, such as those of breast29–32, lung33, rectum34, and esophagus35–38. However, the prognostic value of a pathologic complete response after neoadjuvant treatment for gastric cancer is still a matter of debate39 and deserves further analyses. Unfortunately, the magic trial has not reported a rate of complete pathologic response. In our cohort of patients, all who achieved a complete histologic response (7.2%) remained alive and had not experienced disease recurrence after a median follow-up of 40.3 months. That finding emphasizes the importance of complete pathologic response as a prognostic factor after administration of neoadjuvant chemotherapy and raises the question of whether intensifying preoperative chemotherapy could translate into higher rates of cure.
Our study has some limitations inherent to all retrospective analyses, especially the potential for selection bias associated with information obtained from chart reviews. Given its retrospective nature, our study could not determine whether the surgery that was performed included a D1 or D2 lymphadenectomy, because that description was not always available in the final pathology report. Moreover, the adverse events related to perioperative chemotherapy, which would otherwise increase the strength of the present study, could not be reviewed.
5. CONCLUSIONS
In summary, our multicentre experience confirms the feasibility of the magic protocol in the real-world context and shows that ecx is also an adequate regimen in the perioperative setting. Initial presentation with weight loss was the only significant prognostic factor for worse os. All patients who achieved a pathologic complete response had received 3 cycles of preoperative chemotherapy and were recurrence-free at the time of writing. Given the prognostic value of a complete response after neoadjuvant therapy, future studies should explore the utility of intensifying preoperative chemotherapy to determine whether intensification might contribute to even better outcomes.
6. CONFLICT OF INTEREST DISCLOSURES
The authors report no financial conflicts of interest.
7. REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Institutes of Health, National Cancer Institute; 2012. [Current version available at: http://seer.cancer.gov/csr/1975_2010; cited November 2012] [Google Scholar]
- 2.Siewert JR, Böttcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–61. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofstetter W, Swisher SG, Correa AM, et al. Treatment outcomes of resected esophageal cancer. Ann Surg. 2002;236:76–84. doi: 10.1097/00000658-200209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246:363–72. doi: 10.1097/SLA.0b013e31814697f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–14. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 7.De Vita F, Giuliani F, Orditura M, et al. Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: a randomized phase iii trial by the Gruppo Oncologico Italia Meridionale (goim 9602 Study) Ann Oncol. 2007;18:1354–8. doi: 10.1093/annonc/mdm128. [DOI] [PubMed] [Google Scholar]
- 8.Di Costanzo F, Gasperoni S, Manzione L, et al. Adjuvant chemotherapy in completely resected gastric cancer: a randomized phase iii trial conducted by goirc. J Natl Cancer Inst. 2008;100:388–98. doi: 10.1093/jnci/djn054. [DOI] [PubMed] [Google Scholar]
- 9.Kulig J, Kolodziejczyk P, Sierzega M, et al. Adjuvant chemotherapy with etoposide, Adriamycin and cisplatin compared with surgery alone in the treatment of gastric cancer: a phase iii randomized, multicenter, clinical trial. Oncology. 2010;78:54–61. doi: 10.1159/000292360. [DOI] [PubMed] [Google Scholar]
- 10.Bouché O, Ychou M, Burtin P, et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the ffcd randomized phase iii trial (8801) Ann Oncol. 2005;16:1488–97. doi: 10.1093/annonc/mdi270. [DOI] [PubMed] [Google Scholar]
- 11.Nitti D, Wils J, Dos Santos JG, et al. on behalf of the eortc gi Group and the International Collaborative Cancer Group (iccg) Randomized phase iii trials of adjuvant famtx or femtx compared with surgery alone in resected gastric cancer. A combined analysis of the eortc gi Group and the iccg. Ann Oncol. 2006;17:262–9. doi: 10.1093/annonc/mdj077. [DOI] [PubMed] [Google Scholar]
- 12.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662–9. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 13.Sauvanet A, Mariette C, Thomas P, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg. 2005;201:253–62. doi: 10.1016/j.jamcollsurg.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham D, Allum WH, Stenning SP, et al. on behalf of the magic trial participants Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 15.Stahl M, Walz MK, Stuschke M, et al. Phase iii comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851–6. doi: 10.1200/JCO.2008.17.0506. [DOI] [PubMed] [Google Scholar]
- 16.van Hagen P, Hulshof MC, van Lanschot JJ, et al. on behalf of the cross Group Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham D, Starling N, Rao S, et al. on behalf of the Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum SJ, Stergar H, Antoch G, Veit P, Bockisch A, Kühl H. Staging and follow-up of gastrointestinal tumors with pet/ct. Abdom Imaging. 2006;31:25–35. doi: 10.1007/s00261-005-0031-3. [DOI] [PubMed] [Google Scholar]
- 19.Okines AF, Norman AR, McCloud P, Kang YK, Cunningham D. Meta-analysis of the real-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil–based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol. 2009;20:1529–34. doi: 10.1093/annonc/mdp047. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol. 2007;14:317–28. doi: 10.1245/s10434-006-9218-2. [DOI] [PubMed] [Google Scholar]
- 21.Stephens MR, Lewis WG, White S, et al. Prognostic significance of alarm symptoms in patients with gastric cancer. Br J Surg. 2005;92:840–6. doi: 10.1002/bjs.4984. [DOI] [PubMed] [Google Scholar]
- 22.Maconi G, Kurihara H, Panizzo V, et al. Gastric cancer in young patients with no alarm symptoms: focus on delay in diagnosis, stage of neoplasm and survival. Scand J Gastroenterol. 2003;38:1249–55. doi: 10.1080/00365520310006360. [DOI] [PubMed] [Google Scholar]
- 23.Bowrey DJ, Griffin SM, Wayman J, Karat D, Hayes N, Raimes SA. Use of alarm symptoms to select dyspeptics for endoscopy causes patients with curable esophagogastric cancer to be overlooked. Surg Endosc. 2006;20:1725–8. doi: 10.1007/s00464-005-0679-3. [DOI] [PubMed] [Google Scholar]
- 24.Fein R, Kelsen DP, Geller N, Bains M, McCormack P, Brennan MF. Adenocarcinoma of the esophagus and gastroesophageal junction. Prognostic factors and results of therapy. Cancer. 1985;56:2512–18. doi: 10.1002/1097-0142(19851115)56:10<2512::AID-CNCR2820561032>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Costa ML, de Cássia Braga Ribeiro K, Machado MA, Costa AC, Montagnini AL. Prognostic score in gastric cancer: the importance of a conjoint analysis of clinical, pathologic, and therapeutic factors. Ann Surg Oncol. 2006;13:843–50. doi: 10.1245/ASO.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 26.Lowy AM, Mansfield PF, Leach SD, Pazdur R, Dumas P, Ajani JA. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229:303–8. doi: 10.1097/00000658-199903000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521–30. doi: 10.1002/cncr.11660. [DOI] [PubMed] [Google Scholar]
- 28.Mansour JC, Tang L, Shah M, et al. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Ann Surg Oncol. 2007;14:3412–18. doi: 10.1245/s10434-007-9574-6. [DOI] [PubMed] [Google Scholar]
- 29.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. [DOI] [PubMed] [Google Scholar]
- 30.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 31.Adams S, Chakravarthy AB, Donach M, et al. Preoperative concurrent paclitaxel–radiation in locally advanced breast cancer: pathologic response correlates with five-year overall survival. Breast Cancer Res Treat. 2010;124:723–32. doi: 10.1007/s10549-010-1181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavez–Macgregor M, Litton J, Chen H, et al. Pathologic complete response in breast cancer patients receiving anthracycline- and taxane-based neoadjuvant chemotherapy: evaluating the effect of race/ethnicity. Cancer. 2010;116:4168–77. doi: 10.1002/cncr.25296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen AM, Jahan TM, Jablons DM, Garcia J, Larson DA. Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced nonsmall-cell lung cancer: clinical implications for the subsequent management of the brain. Cancer. 2007;109:1668–75. doi: 10.1002/cncr.22565. [DOI] [PubMed] [Google Scholar]
- 34.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 35.Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–7. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Chao YK, Chan SC, Liu YH, et al. Pretreatment T3–4 stage is an adverse prognostic factor in patients with esophageal squamous cell carcinoma who achieve pathological complete response following preoperative chemoradiotherapy. Ann Surg. 2009;249:392–6. doi: 10.1097/SLA.0b013e3181949e9f. [DOI] [PubMed] [Google Scholar]
- 37.Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg. 2009;87:392–8. doi: 10.1016/j.athoracsur.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JW, Kim JH, Choi EK, et al. Prognosis of esophageal cancer patients with pathologic complete response after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol. 2011;81:691–7. doi: 10.1016/j.ijrobp.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 39.Reed VK, Krishnan S, Mansfield PF, et al. Incidence, natural history, and patterns of locoregional recurrence in gastric cancer patients treated with preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2008;71:741–7. doi: 10.1016/j.ijrobp.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]