Abstract
Background
Prediction of prognosis is important for patients so that they can make the most of the rest of their lives. Oncologists could predict survival, but the accuracy of such predictions is unclear.
Methods
In this observational prospective cohort study, 14 oncologists treating 9 major adult solid malignancies were asked to complete questionnaires predicting survival based on performance status, oral intake, and other clinical factors when patients experienced progressive disease after standard chemotherapies. Clinically predicted survival (cps) was calculated by the oncologists from the date of progressive disease to the predicted date of death. Actual survival (as) was compared with cps using Kaplan–Meier survival curves, and factors affecting inaccurate prediction were determined by logistic regression analysis. The prediction of survival time was considered accurate when the cps/as ratio was between 0.67 and 1.33.
Results
The study cohort consisted of 75 patients. Median cps was 120 days (interquartile range: 60–180 days), and median as was 121 days (interquartile range: 40–234 days). The participating oncologists accurately predicted as within a 33% range 36% of the time; the survival time was overestimated 36% of time and underestimated 28% of the time. The factors affecting the accuracy of the survival estimate were the experience of the oncologist, patient age, and information given about the palliative care unit.
Conclusions
Prediction of cps was accurate for just slightly more than one third of all patients in this study. Additional investigation of putative prognostic factors with a larger sample size is warranted.
Keywords: Survival prediction, cancer patient survival, chemotherapy
1. INTRODUCTION
Prediction of survival is important for patients with advanced cancer so that they can make the most of the rest of their lives. Many cancer patients want to obtain information about their prognosis in a direct and honest manner1–3. Nevertheless, clinicians are often averse to predicting survival4 and divulging prognostic information5, possibly because breaking bad news to a patient can be stressful for the physician.
A physician’s level of experience in estimating survival might affect how prognosis is formulated. For example, an inexperienced physician might guess or use “intuition,” ask an “expert,” consult a textbook, search the electronic literature for prognostic studies, rely on their own judgment, or use a prognostic index6. However, even experienced oncologists find it difficult to predict survival time. Indeed, previous studies reported that clinically predicted survival (cps) by oncologists was uncertain7 and optimistic in terminally ill patients with cancer8–11. A systematic review of eight studies reported that cps for terminal ill cancer patients was accurate for only 25%, 43%, and 61% within, respectively, 1, 2, and 4 weeks of actual survival (as)11. Some studies reported that only 20%–25% of predictions were accurate (within ±33% of as); others reported that survival was overestimated in 63%–83% of terminally ill patients8–10,12. Prediction of survival for patients before terminal illness is more important than that for the terminal stage, because a prediction 1–4 weeks ahead of death might be too late for patients to make the most of their remaining life.
In previous studies, predictions of survival were limited to terminally ill patients with cancer, except in work by Stockler and colleagues7. No reports have addressed cps in patients who finished standard chemotherapies and experienced progressive disease. Predictors of prognosis have to be determined to improve the accuracy of cps estimates for such patients. In this prospective study, we examined the accuracy of cps estimates for patients who experienced progressive disease after standard chemotherapies.
2. METHODS
This single-centre prospective study was based on a questionnaire (Table i). The study was approved by the institutional review board.
TABLE I.
The questionnaire
| 1 | Oncologist’s name: __________________________________________________ | |||
| 2 | Malignancies | |||
| □ Lung cancer | □ Breast cancer | □ Gastric cancer | □ Colorectal cancer | |
| □ Sarcoma | □ Ovarian cancer | □ Pancreatic cancer | □ Endometrial cancer | |
| 3 | Performance status (ps) | |||
| □ 1 | □ 2 | □ 3 | □ 4 | |
| 4 | Patient’s oral intake | |||
| □ Normal | □ Moderately reduced | □ Severely reduced | ||
| 5 | Clinical prediction survival (cps), defined as the period between the date of questionnaire completion and the predicted date of death | |||
| _____ month(s) | _____ week(s)/day(s) | |||
| 6 | Main factor for cps | |||
| □ ps | □ Metastatic lesion | □ Other __________________________________________ | ||
| □ Clinical symptoms | ||||
| (□ dyspnea | □ oral intake | □ edema | □ delirium) | |
| 7 | Disclosure | |||
| Did you communicate the cps? | ||||
| □ To the patient | □ To the family | □ To neither | ||
| If you did NOT communicate the cps (“to neither”), why? | ||||
| □ Uncertainty | □ They did not ask | □ You dared not tell | ||
| □ Other ________________________________________________________________ | ||||
| 8 | Ways in which you treated the patient | |||
| □ Best supportive care | □ Chemotherapy | □ Alternative medicine | □ Second opinion | |
| □ Other | □ Surgery | □ Clinical trial | □ Palliative radiation | |
| ___________________________________________________________________________________ | ||||
| 9 | If you did NOT refer to palliative care unit (pcu), why? | |||
| □ Inappropriate time | □ You dared not suggest the pcu | □ Other | ||
| __________________________________________ | ||||
| 10 | Final decision | |||
| □ Best supportive care | □ Chemotherapy | □ Alternative medicine | □ Second opinion | |
| □ Other | □ Surgery | □ Clinical trial | □ Palliative radiation | |
| ___________________________________________________________________________________ | ||||
2.1. Patients
At the National Cancer Center Hospital in Japan, between October 2010 and October 2011, our study recruited patients with advanced unresectable cancer and patients with progressive disease after standard chemotherapies (Table ii). This observational cohort consisted of adult patients with various solid malignancies, including those of breast, lung, pancreas, colon and rectum, stomach, cervix, endometrium, and ovary, and sarcoma. The attending oncologists (n = 14) were asked to complete the cps questionnaire for patients who had acquired resistance to standard chemotherapies. The cps was estimated by the attending doctor within 7 days (at most) after the diagnosis of progressive disease after standard chemotherapy. Doctors were asked to write the predicted survival as a number of months, weeks, or days at the time of prediction. All completed questionnaires were sent to the clinical trials office. The patients were all followed until death.
TABLE II.
Development of drug resistance by malignancy
| Malignancy | Progressive disease after ... |
|---|---|
| Breast cancer | Anthracycline, taxane, or capecitabine |
| Colorectal cancer | Fluoropyrimidine, oxaliplatin, irinotecan, bevacizumab, tyrosine kinase inhibitors with wild-type egfr |
| Gastric cancer | Tegafur, gimeracil, and oteracil potassium with or without cisplatin |
| Pancreatic cancer | Gemcitabine |
| Lung cancer | Second- or third-line regimens with egfr mutation |
| Ovarian cancer | Platinum resistance |
| Cervical cancer | Cisplatin-containing regimen |
| Endometrial cancer | Doxorubicin or cisplatin |
| Sarcoma | Anthracycline- or ifosfamide-containing regimens (excluding Ewing sarcoma and rhabdomyosarcoma) |
egfr = epidermal growth factor receptor.
2.2. Definition of Terms
All primary cancers were diagnosed by pathology examination. The as was defined as the time from the date of diagnosis of progressive disease after standard chemotherapy to the date of death. The cps was defined as the time from the date the questionnaire was completed to the predicted date of death.
Oral intake was judged mainly by the attending doctor using a simple open-ended question: How do you feel about your appetite? The answer was then scored: 1, normal or good; 2, more than 50% of normal oral intake; or 3, less than 50% of normal oral intake. A palliative care unit (pcu) was defined as a place for palliation and residency without chemotherapy and included inpatient or outpatient pcus and home-based hospices. Best supportive care referred to hospice care based in a hospital or at home without any intensive chemotherapy13.
2.3. Statistical Analysis
We calculated the Spearman correlation coefficient between the as and the cps. The ratio of the cps to the as was calculated for each patient to examine the concordance between the variables. The prediction of survival time was considered accurate when the ratio was in the range 0.67–1.33 (that is, a concordance of ±33%)8. Survival analysis was performed using the Kaplan–Meier method. Using multivariate regression analysis, we assessed factors affecting the difference between as and cps. Putative factors affecting successful prediction of survival were examined using multivariate logistic regression analysis. In all statistical analyses, values of p < 0.05 were considered significant. The statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, U.S.A.).
3. RESULTS
3.1. Patient and Physician Characteristics
The study enrolled 75 patients who met the eligibility criteria. Oncologists were stratified according to professional experience: less than 10 years’ experience (n = 7) and 10 or more years’ experience (n = 7). Table iii shows patient and oncologist characteristics, and Table iv shows the decisions of the oncologists based on patient factors. Nearly 70% of the study patients had a performance status of 0–1 at the time of progressive disease. At that time, approximately 60% were considered preferable for best supportive care by their oncologist, but 70% of all patients did not receive any information about their prognosis. The main reason for that information not being conveyed was uncertainty about the survival prediction or lack of a request for the information. Although the patients had experienced progressive disease after standard chemotherapy, 35% were supposed to receive further chemotherapy. In cases of progressive disease after standard chemotherapies, 80% of patients were referred to hospice before death, and 80% of referred patients died at hospice.
TABLE III.
Oncologist and patient characteristics
| Characteristic | Value |
|---|---|
| Oncologists (n) | 14 |
| Career [n (%)] | |
| >10 Years | 7 (50) |
| <10 Years | 7 (50) |
| Patients (n) | 75 |
| Age (years) | |
| Median | 60 |
| Range | 26–78 |
| Sex [n (%)] | |
| Men | 26 (35) |
| Women | 49 (65) |
| Site of malignancy [n (%)] | |
| Breast | 11 (15) |
| Colorectum | 15 (20) |
| Stomach | 4 (5) |
| Pancreas | 15 (20) |
| Lung | 11 (15) |
| Ovary | 5 (7) |
| Cervix | 3 (4) |
| Endometrium | 3 (4) |
| Sarcoma | 8 (11) |
| Performance status | |
| 0–1 | 51 (68) |
| ≥2 | 24 (32) |
| Oral intake | |
| Normal | 41 (55) |
| Moderately reduced | 25 (33) |
| Severely reduced | 9 (12) |
TABLE IV.
Oncologist decisions about patient factors
| Factor | Responses (n) | Decision | Value [n (%)] |
|---|---|---|---|
| Communicate information about cps | 75 | No | 54 (72) |
| Yes (to patients) | 13 (17) | ||
| Yes (to family only) | 8 (11) | ||
| Reason for not communicating cps | 54 | Uncertainty | 31 (57) |
| Information not requested | 20 (37) | ||
| Apprehensive about communicating cps | 1 (2) | ||
| Other | 2 (3) | ||
| Main factor in cps | 75 | Performance status | 29 (39) |
| Metastatic lesion | 39 (52) | ||
| Dyspnea | 2 (2) | ||
| Other | 5 (7) | ||
| Final treatment | 75 | Best supportive care | 45 (60) |
| Chemotherapy | 26 (35) | ||
| Clinical trial | 3 (4) | ||
| Alternative medicine | 1 (1) |
cps = clinical prediction of survival.
3.2. Survival Estimates
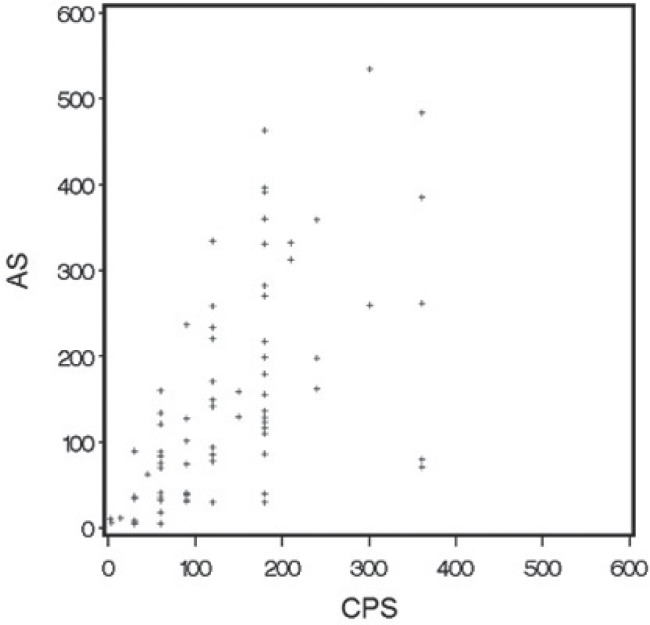
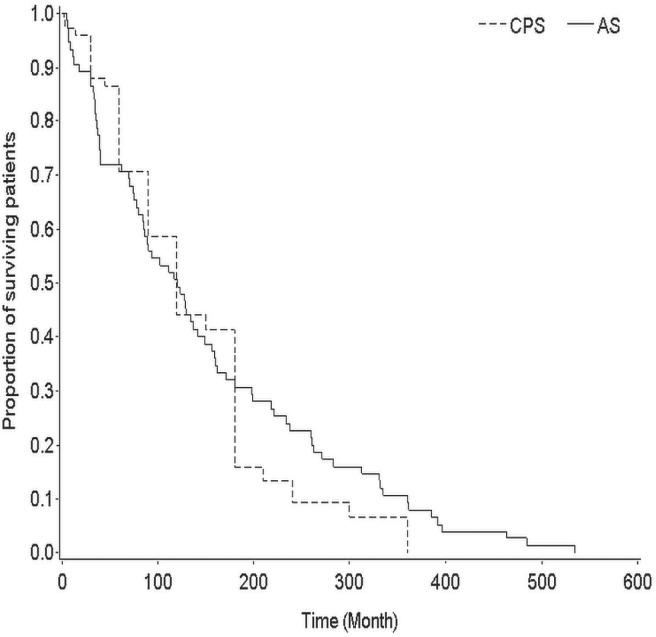
In the 75 patients, median cps was 120 days (interquartile range: 60–180 days), and the median as was 121 days (interquartile range: 40–234 days). Figure 1 shows the Kaplan–Meier curves for cps and as. The median difference between cps and as (cps − as) was −5 days (interquartile range: −74 to 43 days). The Spearman correlation coefficient indicated a highly significant correlation between cps and as (0.70, p < 0.001, Figure 2.).
FIGURE 1.
The Spearman rank correlation coefficient for clinically predicted survival (cps) compared with actual survival (as) was 0.70 (p < 0.001), indicating a highly significant association.
FIGURE 2.
Kaplan–Meier curves for clinical predicted survival (cps) and actual survival (as). The median difference (cps – as) was −5 days (interquartile range: −74 to 43 days).
The survival estimation was accurate (cps within ±33% of as) in 36.0% of patients [95% confidence interval (ci): 25.2% to 47.9%], overestimated in another 36.0% of patients (95% ci: 25.2% to 47.9%), and underestimated in 28.0% of patients (95% ci: 18.2% to 39.6%).
3.3. Multivariate Analyses
We examined independent factors correlated with the difference between cps and as (Table v). These variables were significant in multivariate regression analysis for inaccurate survival estimations:
Oncologists with less than 10 years’ experience tended to estimate shorter survival times (72.2 days; 95% ci: 8.4 to 136.0 days; p = 0.027).
In patients more than 65 years of age, oncologists tended to underestimate survival times (54.7 days; 95% ci: 6.9 to 102.4 days; p = 0.025).
In patients who did not receive information about pcus, oncologists overestimated survival times (78.6 days; 95% ci: 15.7 to 141.4 days; p = 0.014).
TABLE V.
Factors independently correlated with differences between clinical predicted survival (cps) and actual survival in multivariate analysis
| Factor |
Difference in days
|
p Value | |
|---|---|---|---|
| Estimate | 95% ci | ||
| Oncologist’s professional experience | |||
| >10 Years | Reference | ||
| <10 Years | 72.2 | 8.4 to 136.0 | 0.027 |
| Site of malignancy | |||
| Breast | Reference | ||
| Colorectum | −92.5 | −188.6 to 3.7 | 0.060 |
| Stomach | −38.0 | −149.1 to 73.1 | 0.503 |
| Pancreas | 10.2 | −69.9 to 90.3 | 0.803 |
| Lung | 37.5 | −52.7 to 127.7 | 0.415 |
| Ovary | 4.5 | −96.2 to 105.2 | 0.930 |
| Cervix | −13.5 | −135.9 to 108.9 | 0.829 |
| Endometrium | 57.2 | −65.2 to 179.5 | 0.360 |
| Sarcoma | −14.6 | −104.3 to 75.1 | 0.750 |
| Age (years) | |||
| <65 Years | Reference | ||
| >65 Years | −54.7 | −102.4 to −6.9 | 0.025 |
| Performance status | |||
| 0–1 | Reference | ||
| ≥2 | 0.5 | −69.0 to 70.1 | 0.988 |
| Oral intake | |||
| Normal | Reference | ||
| Moderately reduced | −30.0 | −96.9 to 37.0 | 0.380 |
| Severely reduced | −72.1 | −198.0 to 53.9 | 0.262 |
| Main factor for cps | |||
| PS | Reference | ||
| Metastatic lesion | −36.3 | −85.3 to 12.7 | 0.146 |
| Dyspnea | −14.8 | −153.5 to 124.0 | 0.835 |
| Other | −14.6 | −103.5 to 74.3 | 0.748 |
| Final treatment | |||
| Best supportive care | Reference | ||
| Chemotherapy | 0.6 | −62.9 to 64.0 | 0.986 |
| Clinical trial | −36.1 | −155.6 to 83.3 | 0.553 |
| Alternative medicine | 52.1 | −131.6 to 235.7 | 0.579 |
| Referral to palliative care uni | |||
| Yes | Reference | ||
| No | 78.6 | 15.7 to 141.4 | 0.014 |
ci = confidence interval.
4. DISCUSSION
In the present study, we investigated the accuracy of cps estimates in patients with advanced cancer who had experienced progressive disease after standard chemotherapy. Survival was accurately predicted in only 36% of cases, although the cps estimate was highly correlated with as overall. The professional experience of the oncologist, patient age, and referral to a pcu were independent factors for a difference between cps and as.
Giving information as needed to patients, including expected survival, is important even though patients might not ask doctors for that information. In the present study, more than half the patients had a performance status of 0 or 1 at detection of progressive disease after standard chemotherapies. Prediction of survival might have been more difficult for doctors in that setting than in the terminally ill setting. Previous studies reported that only 20%–25% of predictions are accurate in terminally ill cancer patients8,9. In our study, 36% of the predictions were in the accurate range, and more than 80% of the predictions were based either on performance status or metastatic lesions (Table iv). As seen in earlier studies, survival predictions for the near future were more accurate than those for more than 6 months into the future (Figure 1 and 2). A report on the association between the professional experience of the oncologist and prediction shows that prognostic accuracy increases with the experience of the doctor8; however, another study reported contradictory findings10. Of the oncologists who did not disclose the cps to their patients because the patient did not request that information, 80% had been practicing for less than 10 years. Less-experienced oncologists might tend to build strong doctor–patient relationships, and they might therefore be overly optimistic and unwilling to accept the imminent death of their patients. Alternatively, they might be trying not to scare patients14. However, an optimistic cps can result in late referral to a pcu8. Indeed, our study findings indicated that patients who were not referred to a pcu had optimistic cps estimates, although the observed relation between pcu referral and cps is preliminary because of the small sample size. Patients should be given enough time to prepare for a pcu and should be in appropriate physical and psychological condition for referral. In addition, patients who have no information about pcus tend to receive aggressive chemotherapy near the end of life, which can contribute to poor quality of life13.
Predicting survival time is difficult, and disclosing the prediction to patients is therefore also difficult. In the present study, the cps was disclosed in only 28% of cases. Many articles suggest that most patients with incurable cancer are keen on receiving information regarding their prognosis1–3,15,16. Most patients would like to know their predicted survival, although physician and patient predictions are largely discordant17. Nevertheless, most physicians remain unwilling to disclose prognosis estimates to patients with incurable cancer. In previous studies, physicians favoured providing frank survival estimates in only 37% of cases18. Although disclosing the estimated survival time to a patient is not always necessary, doctors should make a considerable effort to communicate with their patients and to help them decide how they wish to live the remainder of their life19–21.
This study has some limitations. First, because of the small sample size, we might have missed some factors affecting the survival prediction other than experience as an oncologist, patient age, and pcu information given. A larger sample would be required to adequately identify other factors. Second, predictive factors that might improve the accuracy of cps estimates could not be clarified because of variations in patient characteristics and the professional experience of the oncologists. Third, patients might have been told their cps after the questionnaire was completed, which might have affected subsequent care choices.
5. CONCLUSIONS
Although it is difficult to accurately estimate survival for patients who acquire resistance to standard chemotherapies, an earnest attempt should be made to provide as accurate a cps as possible for patients who wish to have this information so that they can improve their quality of life. Well-planned studies to identify predictive factors that can assist in making an accurate assessment of cps and to determine how best to deliver that information are warranted.
6. ACKNOWLEDGMENTS
We thank all the physicians who took part in this study project. We are also grateful to Ms. Nao Nakamura for secretarial support.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
8. REFERENCES
- 1.Robinson TM, Alexander SC, Hays M, et al. Patient–oncologist communication in advanced cancer: predictors of patient perception of prognosis. Support Care Cancer. 2008;16:1049–57. doi: 10.1007/s00520-007-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagerty RG, Butow PN, Ellis PA, et al. Cancer patient preferences for communication of prognosis in the metastatic setting. J Clin Oncol. 2004;22:1721–30. doi: 10.1200/JCO.2004.04.095. [DOI] [PubMed] [Google Scholar]
- 3.Butow PN, Dowsett S, Hagerty R, Tattersall MH. Communicating prognosis to patients with metastatic disease: what do they really want to know? Support Care Cancer. 2002;10:161–8. doi: 10.1007/s005200100290. [DOI] [PubMed] [Google Scholar]
- 4.Glare P, Sinclair C, Downing M, Stone P, Maltoni M, Vigano A. Predicting survival in patients with advanced disease. Eur J Cancer. 2008;44:1146–56. doi: 10.1016/j.ejca.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Mack JW, Smith TJ. Reasons why physicians do not have discussions about poor prognosis, why it matters, and what can be improved. J Clin Oncol. 2012;30:2715–17. doi: 10.1200/JCO.2012.42.4564. [DOI] [PubMed] [Google Scholar]
- 6.Glare P. Clinical predictors of survival in advanced cancer. J Support Oncol. 2005;3:331–9. [PubMed] [Google Scholar]
- 7.Stockler MR, Tattersall MH, Boyer MJ, Clarke SJ, Beale PJ, Simes RJ. Disarming the guarded prognosis: predicting survival in newly referred patients with incurable cancer. Br J Cancer. 2006;94:208–12. doi: 10.1038/sj.bjc.6602908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christakis NA, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–72. doi: 10.1136/bmj.320.7233.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Llobera J, Esteva M, Rifa J, et al. Terminal cancer: duration and prediction of survival time. Eur J Cancer. 2000;36:2036–43. doi: 10.1016/S0959-8049(00)00291-4. [DOI] [PubMed] [Google Scholar]
- 10.Gripp S, Moeller S, Bolke E, et al. Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol. 2007;25:3313–20. doi: 10.1200/JCO.2006.10.5411. [DOI] [PubMed] [Google Scholar]
- 11.Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. Br Med J. 2003;327:195–8. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkes CM. Accuracy of predictions of survival in later stages of cancer. Br Med J. 1972;2:29–31. doi: 10.1136/bmj.2.5804.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto K, Yonemori K, Katsumata N, et al. Factors that affect the duration of the interval between the completion of palliative chemotherapy and death. Oncologist. 2009;14:752–9. doi: 10.1634/theoncologist.2008-0257. [DOI] [PubMed] [Google Scholar]
- 14.Smith JL. Why do physicians overestimate life expectancy of a person who is terminally ill? Commentary. West J Med. 2000;172:313–14. doi: 10.1136/ewjm.172.5.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helft PR. Necessary collusion: prognostic communication with advanced cancer patients. J Clin Oncol. 2005;23:3146–50. doi: 10.1200/JCO.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Miyashita M, Hashimoto S, Kawa M, et al. Attitudes toward disease and prognosis disclosure and decision making for terminally ill patients in Japan, based on a nationwide random sampling survey of the general population and medical practitioners. Palliat Support Care. 2006;4:389–98. doi: 10.1017/S1478951506060482. [DOI] [PubMed] [Google Scholar]
- 17.Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–14. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 18.Lamont EB, Christakis NA. Prognostic disclosure to patients with cancer near the end of life. Ann Intern Med. 2001;134:1096–105. doi: 10.7326/0003-4819-134-12-200106190-00009. [DOI] [PubMed] [Google Scholar]
- 19.Costantini M, Morasso G, Montella M, et al. Diagnosis and prognosis disclosure among cancer patients. Results from an Italian mortality follow-back survey. Ann Oncol. 2006;17:853–9. doi: 10.1093/annonc/mdl028. [DOI] [PubMed] [Google Scholar]
- 20.Lamont EB, Christakis NA. Complexities in prognostication in advanced cancer: “to help them live their lives the way they want to”. JAMA. 2003;290:98–104. doi: 10.1001/jama.290.1.98. [DOI] [PubMed] [Google Scholar]
- 21.Kodish E, Post SG. Oncology and hope. J Clin Oncol. 1995;13:1817. doi: 10.1200/JCO.1995.13.7.1817. [DOI] [PubMed] [Google Scholar]