Abstract
Background
Data on real-life utilization of granulocyte colony–stimulating factors (g-csfs) in Canada are limited. The objective of the present study was to describe the reasons for, and the patterns of, g-csf use in selected outpatient oncology clinics in Ontario and Quebec.
Methods
In a retrospective longitudinal cohort study, a review of medical records from 9 Canadian oncology clinics identified patients being prescribed filgrastim (fil) and pegfilgrastim (peg). Patient characteristics, reasons for g-csf use, and treatment patterns were descriptively analyzed.
Results
Medical records of 395 patients initiating g-csf therapy between January 2008 and January 2009 were included. Of this population, 80% were women, and breast cancer was the predominant diagnosis (59%). The most commonly prescribed g-csf was fil (56% in Ontario and 98% in Quebec). The most frequent reason for g-csf use was primary prophylaxis (42% for both fil and peg), followed by secondary prophylaxis (37% fil, 41% peg). Those proportions varied by tumour type and chemotherapy regimen. Delayed g-csf administration (more than 1 day after the end of chemotherapy) was frequently observed for fil, but rarely reported for peg, and that finding was consistent across tumours and concurrent chemotherapy regimens.
Conclusions
The use of g-csf varies with the malignancy type and the provincial health care setting. The most commonly prescribed g-csf agent was fil, and most first g-csf prescriptions were for primary prophylaxis. Delays were frequently observed for patients receiving fil, but were rarely reported for those receiving peg.
Keywords: Neutropenia, prophylaxis, colony-stimulating factors, outcomes, pegfilgrastim, filgrastim
1. INTRODUCTION
Febrile neutropenia (fn) is the most serious consequence of neutropenia, and it can be associated with high medical costs, early mortality, and lengthy hospitalization, which can result in dose reductions or delays in the administration of the next cycle of chemotherapy1–5. Early recognition of individuals at risk for the development of fn, and implementation of appropriate prophylactic strategies against fn, are keys to maximizing treatment goals of systemic chemotherapy.
International guidelines from the American Society of Clinical Oncology, the European Organization for Research and Treatment of Cancer (eortc), the U.S. National Comprehensive Cancer Network, and Cancer Care Ontario6–9 recommend prophylactic use of recombinant human granulocyte colony–stimulating factor (g-csf) such as filgrastim (fil) and pegfilgrastim (peg) for adult patients with solid tumours and nonmyeloid malignancies undergoing chemotherapy when the overall risk of fn is approximately 20% or greater. Other factors taken into consideration include patient age, especially for those more than 65 years of age; prior history of chemotherapy or radiotherapy, or both; poor performance status (Eastern Cooperative Oncology Group score of 3–4); poor renal function; and liver dysfunction. Both fil and peg are indicated to lower the risk of fever and infection with myelosuppressive chemotherapies in nonmyeloid malignancies10,11.
Several clinical and observational studies have demonstrated that, for patients with severe and prolonged neutropenia or fn, efficacy is better with primary prophylaxis than with reactive strategies (that is, secondary prophylaxis)12–16. Clinical practice guidelines recommend that g-csf administration should start 24–72 hours after completion of chemotherapy7; however, that timetable is typically the case only for peg administration; fil is often started 5 days afterward17. In addition, the National Comprehensive Cancer Network guidelines recommend that fil should be continued until recovery of the post-nadir absolute neutrophil count to normal or near-normal levels7. Being rapidly cleared by the kidneys, fil requires daily subcutaneous injections for approximately 10–14 days after chemotherapy to achieve an absolute neutrophil count exceeding 10×103/μL17,18. However, recent observational studies showed an increased risk for fn in patients receiving fil rather than peg, potentially because fil was being administered for a shorter duration than recommended in the guidelines12–16,17.
The reported utilization of fil and peg is variable across jurisdictions12–17,19–21, and they are the only g-csf agents approved in Canada to reduce the incidence and severity of neutropenia and subsequent events (such as infections) for a number of indications22. Use of g-csfs can depend on a number of factors, including individual physician and patient preference, individual hospital or cancer centre care protocol, clinical guidelines, and inclusion of g-csf products on drug formularies. Although fil is available through most provincial and territorial drug benefit programs, including those of Ontario and Quebec, it can be used only for secondary prophylaxis in Ontario, subject to individual clinical review of each case23. In Quebec, fil is available for both primary and secondary prophylaxis. On the other hand, peg is eligible in only some federal and provincial drug plans, depending on limited use criteria, and is subject to individual request by the treating clinician in Ontario and Quebec. Outpatient prescriptions might be covered by private insurance or out-of-pocket by the patient. The differential funding can therefore affect access to g-csfs in Canada.
2. METHODS
2.1. Study Design
Using patient medical records from outpatient oncology clinics in Ontario and Quebec, our retrospective longitudinal cohort study set out to describe the reasons for g-csf use and to characterize the patient population and utilization patterns of g-csf. Medical records from 20 oncology clinics in Ontario and Quebec were sampled (Table i) to identify patients who had initiated a g-csf prescription at any time between January 1, 2008, and January 1, 2009. For eligible patients, the index date was the date of the initial g-csf prescription, with no prior g-csf use during the preceding 3 months. Records were abstracted for a period of 1 year after the index date. The unit of analysis was the first g-csf prescription for each unique patient during the study period. No patient was included more than once in the study.
TABLE I.
Patient characteristics
| Characteristic |
Patient groupa
|
|||||
|---|---|---|---|---|---|---|
| Filgrastim | Pegfilgrastim | Overall | ||||
|
|
|
|
||||
| (n) | (%) | (n) | (%) | (N) | (%) | |
| Patients | 286 | 109 | 395 | |||
| Mean age (years) | 55.1±14.4 | 49.0±13.1 | 53.4±14.3 | |||
| Sex | ||||||
| Men | 56 | 19.6 | 27 | 24.8 | 83 | 21.0 |
| Women | 230 | 80.4 | 82 | 75.2 | 312 | 79.0 |
| Residence | ||||||
| Home | 280 | 97.9 | 106 | 97.2 | 386 | 97.7 |
| Long-term care facility | 2 | 0.7 | 0 | 0.0 | 2 | 0.5 |
| Hospital inpatient | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| Not available | 3 | 1.0 | 3 | 2.8 | 6 | 1.5 |
| Tumour type | ||||||
| Breast cancer | 164 | 57.3 | 68 | 62.4 | 232 | 58.7 |
| Non-Hodgkin lymphoma | 38 | 13.3 | 8 | 7.3 | 46 | 11.6 |
| Colorectal cancer | 29 | 10.1 | 5 | 4.6 | 34 | 8.6 |
| Hodgkin lymphoma | 25 | 8.7 | 4 | 3.7 | 29 | 7.3 |
| Lung cancer | 8 | 2.8 | 1 | 0.9 | 9 | 2.3 |
| Other solid tumour | 7 | 2.4 | 7 | 6.4 | 14 | 3.5 |
| Testicular cancer | 6 | 2.1 | 4 | 3.7 | 10 | 2.5 |
| Other hematologic cancer | 6 | 2.1 | 1 | 0.9 | 7 | 1.8 |
| Solid bone tumour | 3 | 1.0 | 11 | 10.1 | 14 | 3.5 |
| Patients by province | ||||||
| Ontario | 134 | 46.9 | 106 | 97.2 | 240 | 60.8 |
| Quebec | 152 | 53.1 | 3 | 2.8 | 155 | 39.2 |
| Patients by centre type | ||||||
| Ontario regional cancer centre | 103 | 36.0 | 57 | 52.3 | 160 | 40.5 |
| Ontario hospital-based clinic | 31 | 10.8 | 49 | 45.0 | 80 | 20.3 |
| Quebec hospital-based clinic | 152 | 53.1 | 3 | 2.8 | 155 | 39.2 |
Percentages are calculated based on the number of patients for which the particular characteristic is known.
2.2. Data Source
Selection criteria for the 20 sites invited to participate included a large-enough population of patients using g-csf to provide analytic powera, the necessary resources in place to conduct the study (such as site personnel), and an ability to identify and collect data. Of the 20 invited sites, 9 participated in the study, including 4 Ontario level 1 facilities (situated at teaching institutions and administered within the Cancer Care Ontario cancer system), 2 Ontario level 2 facilities (situated at community hospitals and affiliated with the cancer system, but not directly administered within Cancer Care Ontario), and 3 Quebec university-affiliated teaching hospital–based cancer clinics. Each site received approval from its respective research ethics board.
All sites were asked to identify consecutive medical records starting at the beginning of the study period (January 1, 2008) and to select every 25th cancer clinic visit until medical records of at least 40 patients receiving g-csf treatment were identified. Table ii sets out the selection criteria for medical records. In addition to the reason for g-csf use, the clinical information extracted from the medical records included any history of neutropenia-related events and the chemotherapy-specific data for each cycle (for example, regimen received, date of first dose, and date of last dose).
TABLE II.
Study selection criteria
| Medical records were identified for inclusion if the patient initiated a prescription for granulocyte colony–stimulating factor (g-csf) any time between January 1, 2008, and January 1, 2009 (“study period”). |
Investigators identified consecutive records starting at the beginning of the study period and selected every 25th cancer clinic visita with a reported prescription for g-csf that met these inclusion criteria:
|
Each medical record was then followed forward in time (“follow-up period”) from the index date until the first occurrence of any of these events:
|
In the event that the total number of cases required at each site was not identified within the study period, selection began again at the start of the period, taking the 2nd cancer clinic visit with a reported prescription for g-csf in the study period and every 25th visit after that.
g-csf = granulocyte colony–stimulating factor.
A primary outcome of this study was to determine the reason—and timing—for the first g-csf prescription documented in the patient medical record. Primary prophylaxis was defined as the planned use of a g-csf at the beginning of the first cycle of a chemotherapy regime—that is, before a neutropenic event would have occurred within the first cycle. Secondary prophylaxis was defined as use of g-csf after a documented neutropenic event (for example, “patient had neutropenia after cycle N,” “low neutrophils after cycle N,” or “previous febrile neutropenia”). Note that, in some cases, the medical record simply stated “primary prophylaxis” or “secondary prophylaxis,” and those exact categories (rather than the definition already mentioned) were used for data abstraction and analysis. “Rescue therapy” represented initiation of treatment in response to a neutropenic or fn event, independent of primary or secondary prophylaxisb.
In addition to collecting information on the rationale and timing for the first g-csf prescription, our study also recorded any delay in g-csf administration. To maximize its efficiency, g-csf should be administered within 24 hours from the end of chemotherapy. Delay is defined as the number of days starting 24 hours after the last day of intravenous chemotherapy. Data are reported by cancer diagnosis and the most prevalent chemotherapy regimens (plus all other chemotherapy regimens combined) for the most common cancers in the study population (sample size restricted other regimen-level analyses). The delay categories used for reporting were no delay, 1–5 days’ delay, and 6 or more days’ delay.
Finally, an exploratory analysis was conducted to investigate whether fn events or fn-related hospitalizations were documented in the medical record during the study period for patients receiving g-csf therapy. The focus of the present analysis was on patients receiving treatment with fil. This probing exercise was meant to support the initiation of future research exploring potential associations between the type of chemotherapy; the timing (delay or not), duration, and type of g-csf therapy; and fn episodes in oncology patients receiving g-csf.
2.3. Analysis
Demographics and disease characteristics are reported by patient and type of g-csf administered. All analyses are descriptive. All continuous variables are summarized as mean with standard deviation, median, minimum, 25th percentile, 75th percentile, and maximum unless otherwise stated. All categorical variables are summarized as frequencies and percentages. Use of g-csf was characterized for the study population, by cancer diagnosis, by concurrent chemotherapy, by reason for use, and by province.
3. RESULTS
3.1. Patient Population
Medical records for 395 patients who met the inclusion criteria were analyzed. Most patients were female (79%). Mean age was 53.4 ± 14.3 years. Most patients (72%) received fil as the first g-csf administered; the rest received peg first (28%). Of 240 patients in Ontario with a g-csf prescription, 134 were treated with fil (56%), and 106 were treated with peg (44%). Of 155 patients from Quebec, 152 were treated with fil (98%); only 3 patients (2%) received peg (Table i).
Most patients who were treated with a g-csf had breast cancer (58.7%), and most (51.9%) had nonmetastatic disease (6.6% had metastases). The next highest use of a g-csf was in patients with non-Hodgkin lymphoma (11.6%) and then in patients with colorectal cancer (8.6%, Table iii).
TABLE III.
Disease characteristics by patient group
| Characteristic |
Patient group
|
|||||
|---|---|---|---|---|---|---|
| Filgrastim (n=286) | Pegfilgrastim (n=109) | Overall (N=395) | ||||
|
|
|
|
||||
| (n) | (%) | (n) | (%) | (n) | (%) | |
| Breast cancer | 164 | 57.3 | 68 | 62.4 | 232 | 58.7 |
| Metastatic | 21 | 7.3 | 5 | 4.6 | 26 | 6.6 |
| Nonmetastatic | 142 | 49.7 | 63 | 57.8 | 205 | 51.9 |
| Not available | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| Colorectal cancer | 29 | 10.1 | 5 | 4.6 | 34 | 8.6 |
| Metastatic | 11 | 3.8 | 1 | 0.9 | 12 | 3.0 |
| Nonmetastatic | 18 | 6.3 | 4 | 3.7 | 22 | 5.6 |
| Lung cancer | 8 | 2.8 | 1 | 0.9 | 9 | 2.3 |
| Metastatic | 3 | 1.0 | 0 | 0.0 | 3 | 0.8 |
| Nonmetastatic | 4 | 1.4 | 0 | 0.0 | 4 | 1.0 |
| Extensive | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| Localized | 0 | 0.0 | 1 | 0.9 | 1 | 0.3 |
| Hodgkin lymphoma | 25 | 8.7 | 4 | 3.7 | 29 | 7.3 |
| B Symptoms | 11 | 3.8 | 0 | 0.0 | 11 | 2.8 |
| A No B symptoms | 13 | 4.5 | 2 | 1.8 | 15 | 3.8 |
| Not applicable | 1 | 0.3 | 2 | 1.8 | 3 | 0.8 |
| Non-Hodgkin lymphoma | 38 | 13.3 | 8 | 7.3 | 46 | 11.6 |
| Metastatic | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| B Symptoms | 18 | 6.3 | 4 | 3.7 | 22 | 5.6 |
| A No B symptoms | 19 | 6.6 | 4 | 3.7 | 23 | 5.8 |
| Testicular cancer | 6 | 2.1 | 4 | 3.7 | 10 | 2.5 |
| Metastatic | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| Nonmetastatic | 5 | 1.7 | 4 | 3.7 | 9 | 2.3 |
| Solid bone tumour | 3 | 1.0 | 11 | 10.1 | 14 | 3.5 |
| Metastatic | 3 | 1.0 | 4 | 3.7 | 7 | 1.8 |
| Nonmetastatic | 0 | 0.0 | 7 | 6.4 | 7 | 1.8 |
| Other hematologic cancer | 6 | 2.1 | 1 | 0.9 | 7 | 1.8 |
| Metastatic | 2 | 0.7 | 0 | 0.0 | 2 | 0.5 |
| Nonmetastatic | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| Recurrent | 1 | 0.3 | 0 | 0.0 | 1 | 0.3 |
| Not applicable | 0 | 0.0 | 1 | 0.9 | 1 | 0.3 |
| Missing | 2 | 0.7 | 0 | 0.0 | 2 | 0.5 |
| Other solid tumour | 7 | 2.4 | 7 | 6.4 | 14 | 3.5 |
| Metastatic | 2 | 0.7 | 6 | 5.5 | 8 | 2.0 |
| Nonmetastatic | 5 | 1.7 | 0 | 0.0 | 5 | 1.3 |
| Missing | 0 | 0.0 | 1 | 0.9 | 1 | 0.3 |
Table iv lists the most common chemotherapy regimens, organized by disease site. For the common cancers, the most frequently prescribed chemotherapy regimens were fec-d (fluorouracil–epirubicin–cyclophosphamide, followed by docetaxel) for breast cancer (35%), folfox (folinic acid–fluorouracil–oxaliplatin) for colorectal cancer (39%), cisplatin–vinblastine for lung cancer (44%), abvd (doxorubicin–bleomycin–vinblastine–dacarbazine) for Hodgkin lymphoma (93%), and chop-r [cyclophosphamide–hydroxydaunorubicin (doxorubicin)–vincristine–prednisone, and then rituximab] for non-Hodgkin lymphoma [nhl (80%)].
TABLE IV.
Most common chemotherapy regimens by cancer and g-csf administered
| Regimena |
Patient groupb
|
|||||
|---|---|---|---|---|---|---|
| Filgrastim (n=286) | Pegfilgrastim (n=109) | Overall (N=395) | ||||
|
|
|
|
||||
| (n) | (%) | (n) | (%) | (n) | (%) | |
| Breast cancer | 163 | 100.0 | 68 | 100.0 | 231 | 100.0 |
| fec-d | 54 | 33.1 | 26 | 38.2 | 80 | 34.6 |
| tc | 36 | 22.1 | 12 | 17.6 | 48 | 20.8 |
| fec | 13 | 8.0 | 10 | 14.7 | 23 | 10.0 |
| tch | 12 | 7.4 | 1 | 1.5 | 13 | 5.6 |
| tac | 13 | 8.0 | 0 | 0.0 | 13 | 5.6 |
| Colorectal cancer | 26 | 100.0 | 5 | 100.0 | 31 | 100.0 |
| folfox6 | 9 | 34.6 | 3 | 60.0 | 12 | 38.7 |
| folfox4 | 8 | 30.8 | 1 | 20.0 | 9 | 29.0 |
| folfox | 3 | 11.5 | 0 | 0.0 | 3 | 9.7 |
| folfiri | 2 | 7.7 | 4 | 80.0 | 6 | 19.4 |
| Lung cancer | 8 | 100.0 | 1 | 100.0 | 9 | 100.0 |
| Cisplatin–vinblastine | 4 | 50.0 | 0 | 0.0 | 4 | 44.4 |
| Carboplatin–epirubicin | 2 | 25.0 | 0 | 0.0 | 2 | 22.2 |
| Gemcitabine–carboplatin | 2 | 25.0 | 0 | 0.0 | 2 | 22.2 |
| Epirubicin–cisplatin | 0 | 0.0 | 1 | 100.0 | 1 | 11.1 |
| Hodgkin lymphoma | 23 | 100.0 | 4 | 100.0 | 27 | 100.0 |
| abvd | 21 | 91.3 | 4 | 100.0 | 25 | 92.6 |
| gdp | 1 | 4.3 | 0 | 0.0 | 1 | 3.7 |
| chop-r | 1 | 4.3 | 0 | 0.0 | 1 | 3.7 |
| Non-Hodgkin lymphoma | 36 | 100.0 | 8 | 100.0 | 44 | 100.0 |
| chop-r | 28 | 77.8 | 7 | 87.5 | 35 | 79.5 |
| chop | 2 | 5.6 | 1 | 12.5 | 3 | 6.8 |
| dhap | 2 | 5.6 | 0 | 0.0 | 2 | 4.5 |
| cvp-r | 2 | 5.6 | 0 | 0.0 | 2 | 4.5 |
| Testicular cancer | 6 | 100.0 | 4 | 100.0 | 10 | 100.0 |
| bep | 6 | 100.0 | 3 | 75.0 | 9 | 90.0 |
| Epirubicin–cisplatin | 0 | 0.0 | 1 | 25.0 | 1 | 10.0 |
| Solid bone tumour | 3 | 100.0 | 10 | 100.0 | 13 | 100.0 |
| Cisplatin–doxorubicin–dexamethasone | 0 | 0.0 | 2 | 20.0 | 2 | 15.4 |
| Ifosfamide–mitozantrone–dexamethasone | 0 | 0.0 | 2 | 20.0 | 2 | 15.4 |
| Ifosfamide–doxorubicin, mesna | 0 | 0.0 | 2 | 20.0 | 2 | 15.4 |
| Other solid tumour | 6 | 100.0 | 7 | 100.0 | 13 | 100.0 |
| Gemcitabine–docetaxel | 1 | 16.7 | 3 | 42.9 | 4 | 30.8 |
| Paclitaxel–carboplatin | 2 | 33.3 | 1 | 14.3 | 3 | 23.1 |
| Other hematologic cancer | 3 | 100.0 | 0 | 0.0 | 3 | 100.0 |
| Fludarabine (oral) | 1 | 33.3 | 0 | 0.0 | 1 | 33.3 |
| Fludarabine plus cytarabine | 1 | 33.3 | 0 | 0.0 | 1 | 33.3 |
| cvp-r | 1 | 33.3 | 0 | 0.0 | 1 | 33.3 |
For each cancer type, only the top 5 regimens, in order of frequency, are included.
Percentages are calculated based on the number of patients with the particular cancer type.
fec-d = fluorouracil–epirubicin–cyclophosphamide, docetaxel; tc = docetaxel–cyclophosphamide; fec = fluorouracil–epirubicin–cyclophosphamide; tch = docetaxel–carboplatin–trastuzumab; tac = docetaxel–doxorubicin–cyclophosphamide; folfox = folinic acid–fluorouracil–oxaliplatin; folfiri = folinic acid–fluorouracil–irinotecan; abvd = doxorubicin–bleomycin–vinblastine–dacarbazine; gdp = gemcitabine–dexamethasone–cisplatin; chop-r = cyclophosphamide–hydroxydaunorubicin (doxorubicin)–vincristine–prednisone, rituximab; chop = cyclophosphamide–hydroxydaunorubicin (doxorubicin)–vincristine–prednisone; dhap = dexamethasone–cytarabine–cisplatin; cvp-r = cyclophosphamide–vincristine–prednisone, rituximab; bep = bleomycin–etoposide–cisplatin; cvp-r = cyclophosphamide–vincristine–prednisone, rituximab.
3.2. Reason for G-CSF Use
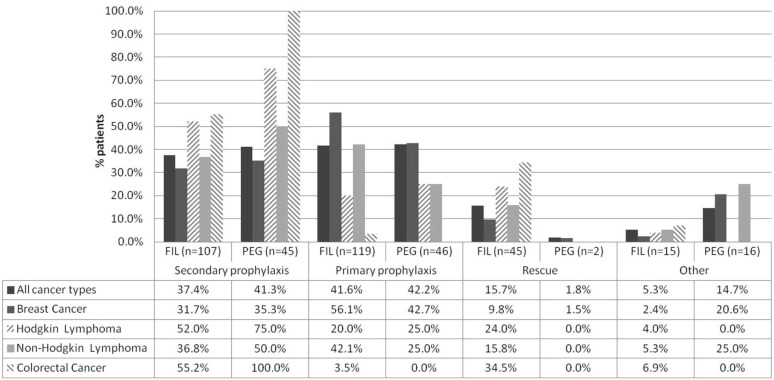
Among patients who received fil (n = 286 of 395), the most frequently documented reason was primary prophylaxis (42%), followed by secondary prophylaxis (37%) and rescue treatment (16%). In the remaining medical records, the reason for fil administration either was not available (1%) or was documented using another reason (4%) (Figure 1). For patients prescribed peg (n = 109), there was a near-equal split between primary prophylaxis (42%) and secondary prophylaxis (41%); rescue treatment accounted for only 2% of prescriptions. In the remaining patient charts, the reason for peg administration was not available (11%) or other reasons were listed (4%) (Figure 1). Tables v and vi present the reasons for g-csf use summarized by cancer diagnosis and concurrent chemotherapy regimen.
FIGURE 1.
Reasons for administration of filgrastim (fil) or pegfilgrastim (peg). The graph represents only patients diagnosed with the most common cancers. For the complete data set, including the reasons for use of granulocyte colony–stimulating factor (g-csf) when added to the most prevalent chemotherapy regimens, see Tables iii and iv. Percentages refer to the proportion of patients with a given diagnosis receiving g-csf for the given reason at first administration.
TABLE V.
Charted reasons for filgrastim use in 286 patients
| Cancer type and regimen | Reasona | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Prophylaxis | Rescueb | Otherc | Not available | Overall | ||||||||
|
|
|
|
|
|
||||||||
| Secondary | Primary | (n) | (%) | (n) | (%) | (n) | (%) | (n) | (%) | |||
|
|
|
|||||||||||
| (n) | (%) | (n) | (%) | |||||||||
| Total group | 107 | 37.41 | 119 | 41.61 | 45 | 15.73 | 11 | 3.85 | 4 | 1.40 | 286 | 100.00 |
| Breast cancer | 52 | 31.71 | 92 | 56.10 | 16 | 9.76 | 3 | 1.83 | 1 | 0.61 | 164 | 100.00 |
| fec-d | 27 | 50.00 | 18 | 33.33 | 7 | 12.96 | 2 | 3.70 | 0 | 0.00 | 54 | 100.00 |
| All other treatments | 24 | 22.02 | 74 | 67.89 | 9 | 8.26 | 1 | 0.92 | 1 | 0.92 | 109 | 100.00 |
| Regimen nr | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 100.00 |
| Hodgkin lymphoma | 13 | 52.00 | 5 | 20.00 | 6 | 24.00 | 0 | 0.00 | 1 | 4.00 | 25 | 100.00 |
| abvd | 12 | 57.14 | 3 | 14.29 | 5 | 23.81 | 0 | 0.00 | 1 | 4.76 | 21 | 100.00 |
| All other treatments | 1 | 50.00 | 1 | 50.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 100.00 |
| Regimen nr | 0 | 0.00 | 1 | 50.00 | 1 | 50.00 | 0 | 0.00 | 0 | 0.00 | 2 | 100.00 |
| Non-Hodgkin lymphoma | 14 | 36.84 | 16 | 42.11 | 6 | 15.79 | 2 | 5.26 | 0 | 0.00 | 38 | 100.00 |
| chop-r | 12 | 42.86 | 13 | 46.43 | 3 | 10.71 | 0 | 0.00 | 0 | 0.00 | 28 | 100.00 |
| All other treatments | 2 | 25.00 | 3 | 37.50 | 2 | 25.00 | 1 | 12.50 | 0 | 0.00 | 8 | 100.00 |
| Regimen nr | 0 | 0.00 | 0 | 0.00 | 1 | 50.00 | 1 | 50.00 | 0 | 0.00 | 2 | 100.00 |
| Colorectal cancer | 16 | 55.17 | 1 | 3.45 | 10 | 34.48 | 1 | 3.45 | 1 | 3.45 | 29 | 100.00 |
| folfox6 | 7 | 77.78 | 0 | 0.00 | 2 | 22.22 | 0 | 0.00 | 0 | 0.00 | 9 | 100.00 |
| All other treatments | 7 | 41.18 | 0 | 0.00 | 8 | 47.06 | 1 | 5.88 | 1 | 5.88 | 17 | 100.00 |
| Regimen nr | 2 | 66.67 | 1 | 33.33 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 3 | 100.00 |
| Other hematologic cancer | 2 | 33.33 | 0 | 0.00 | 2 | 33.33 | 2 | 33.33 | 0 | 0.00 | 6 | 100.00 |
| Lung cancer | 5 | 62.50 | 0 | 0.00 | 1 | 12.50 | 1 | 12.50 | 1 | 12.50 | 8 | 100.00 |
| Other solid tumour | 3 | 42.86 | 2 | 28.57 | 1 | 14.29 | 1 | 14.29 | 0 | 0.00 | 7 | 100.00 |
| Solid bone tumour | 1 | 33.33 | 0 | 0.00 | 2 | 66.67 | 0 | 0.00 | 0 | 0.00 | 3 | 100.00 |
| Testicular cancer | 1 | 16.67 | 3 | 50.00 | 1 | 16.67 | 1 | 16.67 | 0 | 0.00 | 6 | 100.00 |
Charted reasons were categorized by clinical adjudication. Percentages were calculated based on the number of instances for each cancer type and regimen.
Treatment of neutropenia or febrile neutropenia.
Prophylaxis of neutropenia, avoid dose delay, avoid or prevent neutropenia or febrile neutropenia, cycle written beneath end date, increase hemoglobin and reduce need for transfusion, preparation for transplantation, stem-cell collection, avoid dose delay, avoid neutropenia and dose delay, prevent febrile neutropenia, and prevent neutropenia.
fec-d = fluorouracil–epirubicin–cyclophosphamide, docetaxel; nr = not reported; abvd = doxorubicin–bleomycin–vinblastine–dacarbazine; chop-r = cyclophosphamide–hydroxydaunorubicin (doxorubicin)–vincristine–prednisone, rituximab; folfox = folinic acid–fluorouracil–oxaliplatin.
TABLE VI.
Charted reasons for pegfilgrastim use in 109 patients
| Cancer type and regimen | Reasona | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Prophylaxis | Not available | Otherb | Rescuec | Overall | ||||||||
|
|
|
|
|
|||||||||
| Secondary | Primary | (n) | (%) | (n) | (%) | (n) | (%) | |||||
|
|
|
|
||||||||||
| (n) | (%) | (n) | (%) | (n) | (%) | |||||||
| Total group | 45 | 41.28 | 46 | 42.20 | 12 | 11.01 | 4 | 3.67 | 2 | 1.83 | 109 | 100.00 |
| Breast cancer | 24 | 35.29 | 29 | 42.65 | 11 | 16.18 | 3 | 4.41 | 1 | 1.47 | 68 | 100.00 |
| fec-d | 11 | 42.31 | 9 | 34.62 | 6 | 23.08 | 0 | 0.00 | 0 | 0.00 | 26 | 100.00 |
| All other treatments | 13 | 30.95 | 20 | 47.62 | 5 | 11.90 | 3 | 7.14 | 1 | 2.38 | 42 | 100.00 |
| Colorectal cancer | 5 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 5 | 100.00 |
| folfox6 | 3 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 3 | 100.00 |
| All other treatments | 2 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 2 | 100.00 |
| Non-Hodgkin lymphoma | 4 | 50.00 | 2 | 25.00 | 1 | 12.50 | 1 | 12.50 | 0 | 0.00 | 8 | 100.00 |
| chop-r | 3 | 42.86 | 2 | 28.57 | 1 | 14.29 | 1 | 14.29 | 0 | 0.00 | 7 | 100.00 |
| All other treatments | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 100.00 |
| Hodgkin lymphoma | 3 | 75.00 | 1 | 25.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 4 | 100.00 |
| abvd | 3 | 75.00 | 1 | 25.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 4 | 100.00 |
| All other treatments | 0 | — | 0 | — | 0 | — | 0 | — | 0 | — | 0 | 100.00 |
| Solid bone tumour | 2 | 18.18 | 8 | 72.73 | 0 | 0.00 | 0 | 0.00 | 1 | 9.09 | 11 | 100.00 |
| Other solid tumour | 3 | 42.86 | 4 | 57.14 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 7 | 100.00 |
| Testicular cancer | 3 | 75.00 | 1 | 25.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 4 | 100.00 |
| Lung cancer | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 100.00 |
| Other hematologic cancer | 0 | 0.00 | 1 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 100.00 |
Charted reasons were categorized by clinical adjudication. Percentages are calculated based on the number of instances for each cancer type and regimen.
Prophylaxis of neutropenia, avoid dose delay, avoid or prevent neutropenia or febrile neutropenia, cycle written beneath end date, increase hemoglobin and reduce need for transfusion, preparation for transplantation, stem-cell collection, avoid dose delay, avoid neutropenia and dose delay, prevent febrile neutropenia, and prevent neutropenia.
Treatment of neutropenia or febrile neutropenia.
fec-d = fluorouracil–epirubicin–cyclophosphamide, docetaxel; folfox = folinic acid–fluorouracil–oxaliplatin; chop-r = cyclophosphamide–hydroxydaunorubicin (doxorubicin)–vincristine–prednisone, rituximab; abvd = doxorubicin–bleomycin–vinblastine–dacarbazine.
3.3. Treatment Patterns
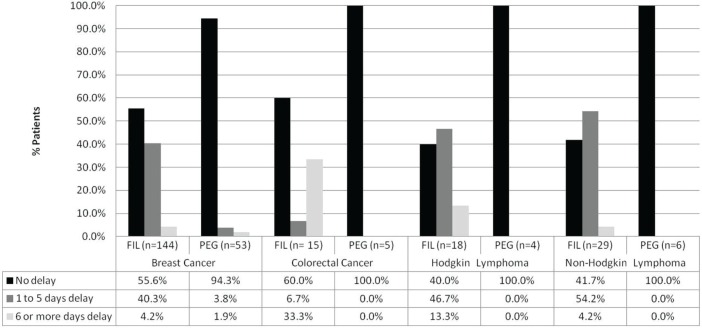
At the start of the first cycle, delays in fil administration were commonly observed for all tumour types, but patients receiving peg had no documentation of a delay in any of the cancer groups except for breast cancer, where approximately 6% of patients experienced a delay in peg administration (Figure 2). Tables vii and viii present the frequency of delays in g-csf administration by cancer and concurrent chemotherapy.
FIGURE 2.
Delay of filgrastim (fil) or pegfilgrastim (peg) administration after the first chemotherapy cycle. The graph represents only patients diagnosed with the most common cancers, and all data are based on documentation of primary or secondary prophylaxis in the patient charts. For the complete data set, including delays in the administration of granulocyte colony–stimulating factor when added to the most prevalent chemotherapy regimens, please see Tables v and vi.
TABLE VII.
Delay in filgrastim prophylaxis for patients with the most common cancersa
| Cancer type | Regimen |
Delayb at start of 1st cycle [n (%)]c
|
||||
|---|---|---|---|---|---|---|
| Overall | Missing or unknown | None | 1–5 Days | ≥6 Days | ||
| Breast cancer | fec-d | 45 (100) | 1 (2.2) | 23 (51.1) | 21 (46.7) | 0 (0) |
| Other | 98 (100) | 0 (0) | 57 (58.2) | 36 (36.7) | 5 (5.1) | |
| Regimen nr | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 0 (0) | |
| Colorectal cancer | folfox6 | 7 (100) | 0 (0) | 3 (42.9) | 0 (0) | 4 (57.1) |
| Other | 7 (100) | 0 (0) | 5 (71.4) | 1 (14.3) | 1 (14.3) | |
| Regimen nr | 3 (100) | 0 (0) | 1 (33.3) | 0 (0) | 2 (66.7) | |
| Hodgkin lymphoma | abvd | 15 (100) | 0 (0) | 6 (40) | 7 (46.7) | 2 (13.3) |
| Other | 2 (100) | 0 (0) | 1 (50) | 0 (0) | 1 (50) | |
| Regimen nr | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | |
| Non-Hodgkin lymphoma | chop-r | 25 (100) | 0 (0) | 10 (40) | 14 (56) | 1 (4) |
| Other | 5 (100) | 0 (0) | 2 (40) | 2 (40) | 1 (20) | |
| Regimen nr | 0 | — | — | — | — | |
Includes only the uses adjudicated to be primary or secondary prophylaxis.
Delay at the start of a cycle is the number of days between the last day of intravenous chemotherapy and the day that granulocyte colony–stimulating factor (g-csf) was started (start date of g-csf, minus end date of chemotherapy, minus 1). No delay—that is, a zero result—indicates that g-csf was begun the day after the last dose of intravenous chemotherapy.
Percentages are calculated based on the number of delays for each cancer type and regimen.
fec-d = fluorouracil–epirubicin–cyclophosphamide, docetaxel; nr = not reported; folfox = folinic acid–fluorouracil–oxaliplatin; abvd = doxorubicin–bleomycin–vinblastine–dacarbazine; chop-r = cyclophosphamide–hydroxydaunorubicin (doxorubicin)–vincristine–prednisone, rituximab.
TABLE VIII.
Delay in pegfilgrastim prophylaxis for patients with the most common cancersa
| Cancer type | Regimen |
Delayb at start of 1st cycle [n (%)]c
|
||||
|---|---|---|---|---|---|---|
| Overall | Missing or unknown | None | 1–5 Days | ≥6 Days | ||
| Breast cancer | fec-d | 20 (100) | 0 (0) | 19 (95) | 1 (5) | 0 (0) |
| Other | 33 (100) | 0 (0) | 31 (93.9) | 1 (3) | 1 (3) | |
| Regimen nr | 0 | — | — | — | — | |
| Colorectal cancer | folfox6 | 3 (100) | 0 (0) | 3 (100) | 0 (0) | 0 (0) |
| Other | 2 (100) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | |
| Regimen nr | 0 | — | — | — | — | |
| Hodgkin lymphoma | abvd | 4 (100) | 0 (0) | 4 (100) | 0 (0) | 0 (0) |
| Other | 0 | — | — | — | — | |
| Regimen nr | 0 | — | — | — | — | |
| Non-Hodgkin lymphoma | chop-r | 5 (100) | 0 (0) | 5 (100) | 0 (0) | 0 (0) |
| Other | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 0 (0) | |
| Regimen nr | 0 | — | — | — | — | |
Includes only the uses adjudicated to be primary or secondary prophylaxis.
Delay at the start of a cycle is the number of days between the last day of intravenous chemotherapy and the day that granulocyte colony–stimulating factor (g-csf) was started (start date of g-csf, minus end date of chemotherapy, minus 1). No delay—that is, a zero result—indicates that g-csf was begun the day after the last dose of intravenous chemotherapy.
Percentages are calculated based on the number of delays for each cancer type and regimen.
3.4. Documentation of FN Events
An exploratory analysis was conducted to determine if fn events and fn-related hospitalizations were documented in the medical records of study patients who received fil (n = 286, 72%). The analysis found that 18% of those patients (n = 51) experienced at least 1 fn event, with 90% of them (n = 46) being hospitalized for fn during the study period.
4. DISCUSSION
For patients with nonmyeloid malignancies such as breast cancer, lymphoma, or lung cancer who are receiving myelosuppressive chemotherapy, g-csf is recommended to reduce the incidence of fn and the duration of severe neutropenia, allowing continuation of full-dose chemotherapy4,24.
Most international guidelines (American Society of Clinical Oncology, eortc, National Comprehensive Cancer Network, and Cancer Care Ontario)6–9 currently recommend the use of g-csf from the first cycle of chemotherapy when the overall risk of fn is approximately 20% or greater (compared with the previous threshold of 40%). The eortc8 and American Society of Clinical Oncology6 guidelines both present good evidence that prophylactic g-csf lowers the incidence of dose reductions and delays in chemotherapy. In addition, both guidelines advocate the use of primary prophylaxis with g-csf to maintain the intended dose intensity of chemotherapy when a survival benefit is expected, such as in patients with breast cancer or nhl6,8.
The present study describes g-csf utilization in clinical practice at multiple sites in Ontario and Quebec where most of the population consisted of women with a diagnosis of breast cancer. In general, the most frequent reason for g-csf use was primary prophylaxis (fil and peg, both 42%) followed closely by secondary prophylaxis (fil 37%, peg 41%); however, the results varied by cancer diagnosis and concurrent chemotherapy regimen. Our study also found that delays in the administration of fil (number of days starting 24 hours after the last day of intravenous chemotherapy) were common for all cancer types, but that delays in peg administration were rarely reported. Finally, an exploratory analysis showed that fn events were charted at least once for 18% of the patients receiving fil, with 90% of those charts also showing an fn-related hospitalization during the study period.
A retrospective study by Scott et al.19 examined medical records from the U.S. Oncology practice patterns study (1991–1999) of patients with intermediate-grade nhl treated with first-line chop chemotherapy and prophylactic fil. The study reported that only 37% of the nhl patients received g-csf as primary prophylaxis. In contrast, a recent, though small, Canadian study by Zhu et al.25 reported data from the medical records of 36 patients from a hospital cancer centre in Ontario. Of those 36 patient records, 86% showed receipt of adjuvant treatment, and 14%, receipt of neoadjuvant treatment for early-stage breast cancer. The study found that 81% of the patients (n = 29) received g-csf for primary prophylaxis, and it reported high levels of fil use (n = 34, 94%). Our study found that 42% of patients received g-csf as primary prophylaxis, which is closer to the rate reported by Scott et al. (37%). The higher rate in the present study might be attributable to the observation period. The study by Scott and colleagues reviewed records between 1991 and 1999; our study looked at medical charts a decade later (2008–2009), after additional guidance had been published recommending the use of g-csf for primary prophylaxis6,8. The difference in the rates of primary prophylaxis between the present study (42%) and that of Zhu et al. (81%) might be attributable to the relatively small sample size in the Zhu study25 (n = 39) compared with the present study (n = 231 breast cancer patients) and to the fact that our study included oncology clinics from Quebec, potentially accounting for the differences in prescribing patterns.
The present study observed frequent delays in the administration of fil, which has also been demonstrated in other chart review studies17 and might lead to an increase in fn events. In our study, an exploratory analysis was also conducted, which showed that 18% of patients receiving fil prophylaxis experienced a fn event, and of those patients, 90% had an fn-related hospitalization during the 1-year follow-up period. Further study is required to investigate whether the delays in g-csf administration and the occurrence of fn are correlated. Interestingly, a study by Gerlier et al.26 reported a fn event for 31% of breast cancer patients who did not receive g-csf primary prophylaxis, and another recent study (Rajan et al.27) reported that primary prophylaxis with a g-csf led to a 16% reduction in hospitalizations within the first 3 months of chemotherapy initiation. Together, those studies demonstrate the benefit of primary prophylaxis with g-csf in preventing fn and fn-related hospitalization; the potential impact on medical resources is sizeable. In a Canadian inpatient setting, Lathia et al.28 reported a mean overall cost of CA$6,324 per fn episode, based on a sample of 46 patients with mostly hematologic malignancies.
The present study has a few limitations that should be noted. First, it assumed that all prescribed g-csf doses (as recorded in the medical charts) were administered, regardless of the administration route (subcutaneous self-administration or intravenous infusion in the health care centre). Second, the reason for the g-csf selection (fil vs. peg), the rationale for immediate or delayed use of g-csf, and the duration of g-csf administration are not always systematically documented in patient medical records. Third, only the index administration was reported at the patient level, and therefore any change in the g-csf agent (for example, fil used initially, with a subsequent switch to peg in another cycle, or vice versa) is not reported. Fourth, the sample in our study is based on a small number of outpatient cancer clinics in Ontario and Quebec, which might affect the representativeness of the results and the interpretation for some of the tumour types. In Quebec, peg is not covered by provincial health insurance and therefore only patients with private insurance are able to access it, which explains the low use of peg in that province. In addition, to be able to conduct the study with a large enough sample of patients, only sites with high g-csf use, based on a site qualification questionnaire, were selected; the results will therefore be generalizable only to such high-usage sites. Fifth, the data related to fn events and fn-related hospitalizations for patients receiving fil prophylaxis reflect only an exploratory analysis; future studies to confirm those findings are needed. Finally, the use of g-csf in daily practice does not always conform to clinical practice guidelines, and reasons for such deviations are not always evident in a chart review. Furthermore, the delay categories chosen for the present study were based on the product monographs, which state that g-csf administration should occur at least 24 hours after chemotherapy. The observed percentage of patients experiencing a delay in g-csf administration might have been lower if the definition of “no delay” were to be expanded (for example, within 48 hours after chemotherapy); however, the substantial differences between patients receiving delayed administration of fil compared with those receiving delayed administration of peg are unlikely to change. Future studies could investigate the impact of using different time frames for the definition of delay in g-csf administration.
5. CONCLUSIONS
Our study shows how the use of g-csfs in actual clinical practice varies from clinical guideline recommendations. In the present study, fil was the g-csf more commonly prescribed in a sample of medical records from two provinces (particularly in Quebec, where peg is not available under provincial insurance coverage), and most first g-csf prescriptions were for primary prophylaxis. In the records of patients that showed fil administration, fn and fn-related hospitalizations were observed, but further studies are required to investigate the impact of delayed administration on such events.
6. ACKNOWLEDGMENTS
The authors thank the research coordinators at each site who were responsible for extracting data from the medical records. The authors also acknowledge the writing assistance of Tara Cowling, of Medlior Health Outcomes Research Ltd.
Footnotes
An initial list was provided by Amgen Canada. Sites identified by Amgen received a “qualification questionnaire” to determine whether the patient population using g-csf was large enough to provide a sufficient sample size. One of the questions was: “Please indicate if 40 cases (between 01-Jan-2008 and 01-Jan-2009) can be identified at your centre?”
The reason for g-csf administration was recorded in the chart and abstracted verbatim. The reasons were then adjudicated by the clinical advisors as fitting into one of the study categories: primary prophylaxis, secondary prophylaxis, rescue therapy, and other.
7. CONFLICT OF INTEREST DISCLOSURES
Unrestricted funding for this project was provided by Amgen Canada. NM has received fellowship funding and consultancy funding from Amgen Canada.
8. REFERENCES
- 1.Caggiano V, Weiss RV, Rickert TS, Linde–Zwirble WT. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103:1916–24. doi: 10.1002/cncr.20983. [DOI] [PubMed] [Google Scholar]
- 2.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100:228–37. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Kuderer N, Greene J, Balducci L. The economics of febrile neutropenia: implications for the use of colony-stimulating factors. Eur J Cancer. 1998;34:1857–64. doi: 10.1016/S0959-8049(98)00222-6. [DOI] [PubMed] [Google Scholar]
- 4.Lyman GH, Kuderer NM. Filgrastim in patients with neutropenia: potential effects on quality of life. Drugs. 2002;62(suppl 1):65–78. doi: 10.2165/00003495-200262001-00005. [DOI] [PubMed] [Google Scholar]
- 5.Daniel DB, Crawford J, Kuderer NM, Dale DC, Lyman GH, on behalf of the anc Study Group Risk and mortality associated with febrile neutropenia in lung cancer patients [abstract 7223] J Clin Oncol. 2004;22 [Available online at: http://meeting.ascopubs.org/cgi/content/abstract/22/14_suppl/7223; cited February 14, 2014] [Google Scholar]
- 6.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Myeloid Growth Factors. Fort Washington, PA: NCCN; 2012. Ver. 1.2012. [Google Scholar]
- 8.Aapro MS, Bohlius J, Cameron DA, et al. on behalf of the European Organisation for Research and Treatment of Cancer 2010 Update of eortc guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Campbell C, Bramwell V, Charette M, Oliver T, on behalf of the Systemic Treatment Disease Site Group . The Role of Colony-Stimulating Factor (csf) in Patients Receiving Myelosuppressive Chemotherapy for the Treatment of Cancer. Toronto, ON: Cancer Care Ontario, Program in Evidence-Based Care; 2003. Practice guideline report 12-2. [Google Scholar]
- 10.Amgen Canada . Neupogen (Filgrastim): Sterile Solution for Injection [product monograph] Mississauga, ON: Amgen Canada; 2007. [Google Scholar]
- 11.Amgen Inc. Neulasta (Pegfilgrastim) Injection for Subcutaneous Use [U.S. package insert] Thousand Oaks, CA: Amgen; 2007. [Google Scholar]
- 12.Weycker D, Malin J, Kim J, et al. Risk of hospitalization for neutropenic complications of chemotherapy in patients with primary solid tumors receiving pegfilgrastim or filgrastim prophylaxis: a retrospective cohort study. Clin Ther. 2009;31:1069–81. doi: 10.1016/j.clinthera.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40:402–7. doi: 10.1345/aph.1G516. [DOI] [PubMed] [Google Scholar]
- 14.Weycker D, Malin J, Barron R, Edelsberg J, Kartashov A, Oster G. Comparative effectiveness of filgrastim, pegfilgrastim, and sargramostim as prophylaxis against hospitalization for neutropenic complications in patients with cancer receiving chemotherapy. Am J Clin Oncol. 2012;35:267–74. doi: 10.1097/COC.0b013e31820dc075. [DOI] [PubMed] [Google Scholar]
- 15.Tan H, Tomic K, Hurley D, Daniel G, Barron R, Malin J. Comparative effectiveness of colony-stimulating factors for febrile neutropenia: a retrospective study. Curr Med Res Opin. 2011;27:79–86. doi: 10.1185/03007995.2010.536527. [DOI] [PubMed] [Google Scholar]
- 16.von Minckwitz G, Kümmel S, du Bois A, et al. on behalf of the German Breast Group Pegfilgrastim +/– ciprofloxacin for primary prophylaxis with tac (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the gepartrio study. Ann Oncol. 2008;19:292–8. doi: 10.1093/annonc/mdm438. [DOI] [PubMed] [Google Scholar]
- 17.Morrison VA, Wong M, Hershman D, Campos LT, Ding B, Malin J. Observational study of the prevalence of febrile neutropenia in patients who received filgrastim or pegfilgrastim associated with 3–4 week chemotherapy regimens in community oncology practices. J Manag Care Pharm. 2007;13:337–48. doi: 10.18553/jmcp.2007.13.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes FA, O’Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage ii or stage iii/iv breast cancer. J Clin Oncol. 2002;20:727–31. doi: 10.1200/JCO.20.3.727. [DOI] [PubMed] [Google Scholar]
- 19.Scott SD, Chrischilles EA, Link BK, Delgado DJ, Fridman M, Stolshek BS. Days of prophylactic filgrastim use to reduce febrile neutropenia in patients with non-Hodgkin’s lymphoma treated with chemotherapy. J Manag Care Pharm. 2003;9(suppl):15–21. doi: 10.18553/jmcp.2003.9.s2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson G, Bergstrom K, Stump E, Miyahara T, Herfindal ET. Growth factor usage patterns and outcomes in the community setting: collection through a practice-based computerized clinical information system. J Clin Oncol. 2000;18:1764–70. doi: 10.1200/JCO.2000.18.8.1764. [DOI] [PubMed] [Google Scholar]
- 21.Fortner BV, Mao Q, Stolshek BS, Schwartzberg LS. Association of IV antibiotic use and hospitalization with filgrastim (f) and pegfilgrastim (pegf) utilization in a community oncology clinic sample [abstract 2139] Am J Health Syst Pharm. 2003;60 [Google Scholar]
- 22.Kouroukis CT, Chia S, Verma S, et al. Canadian supportive care recommendations for the management of neutropenia in patients with cancer. Curr Oncol. 2008;15:9–23. doi: 10.3747/co.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ontario, Ministry of Health and Long-Term Care (mohltc) EAP Reimbursement Criteria for Frequently Requested Drugs and Indications. Toronto, ON: MOHLTC; 2012. [Google Scholar]
- 24.Vose JM, Crump M, Lazarus H, et al. Randomized, multi-center, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J Clin Oncol. 2003;21:514–19. doi: 10.1200/JCO.2003.03.040. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Bouganim N, Vandermeer L, Dent SF, Dranitsaris G, Clemons MJ. Use and delivery of granulocyte colony–stimulating factor in breast cancer patients receiving neoadjuvant or adjuvant chemotherapy—single-centre experience. Curr Oncol. 2012;19:e239–43. doi: 10.3747/co.19.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerlier L, Lamotte M, Awada A, et al. The use of chemotherapy regimens carrying a moderate or high risk of febrile neutropenia and the corresponding management of febrile neutropenia: an expert survey in breast cancer and non-Hodgkin’s lymphoma. BMC Cancer. 2010;10:642. doi: 10.1186/1471-2407-10-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rajan SS, Lyman GH, Stearns SC, Carpenter WR. Effect of primary prophylactic granulocyte-colony stimulating factor use on incidence of neutropenia hospitalizations for elderly early-stage breast cancer patients receiving chemotherapy. Med Care. 2011;49:649–57. doi: 10.1097/MLR.0b013e318215c42e. [DOI] [PubMed] [Google Scholar]
- 28.Lathia N, Mittmann N, DeAngelis C, et al. Evaluation of direct medical costs of hospitalization for febrile neutropenia. Cancer. 2010;116:742–8. doi: 10.1002/cncr.24773. [DOI] [PubMed] [Google Scholar]