Abstract
Since the early 1950s, Papanicolaou (“Pap”) cytology screening has dramatically reduced cervical cancer mortality in most high-income settings. Currently, human papillomavirus (hpv) vaccination has the greatest potential to reduce the global burden of cervical cancer and precancerous lesions. However, as the prevalence of precancerous lesions declines, maintaining cytology as the primary screening test in settings with established programs might become less efficient. A reduction in test performance (sensitivity, specificity, and positive predictive value) would lead to an increase in unnecessary colposcopy referrals. Fortunately, hpv dna testing has emerged as a suitable candidate to replace cytology. Compared with the Pap test, hpv testing is less specific but much more sensitive in detecting high-grade precancerous lesions, less prone to human error, and more reproducible across settings. Linkage of hpv vaccination and screening registries could serve the added role of monitoring vaccine efficacy. As a triage test, cytology is expected to perform with sufficient accuracy because most hpv-positive smears would contain relevant abnormalities. This approach and others—for example, hpv testing followed by genotyping—are being evaluated in large population studies and have already been recommended in some settings. Other specific biomarkers that might perform well for screening and triage include hpv E6/E7 messenger rna testing, methylation of host or viral genes, and p16INK4a staining. Considering the rapid pace of major discoveries and the anticipated arrival of a nonavalent hpv vaccine (currently in phase iii trials), the evidence base in this field has become an elusive target and will continue to be an obstacle for policymakers.
Keywords: Cervical cancer, human papillomavirus, vaccination, screening
1. INTRODUCTION
In 1941, Georgios Nicholas Papanicolaou first reported that microscopic evaluation of vaginal smears might be a useful approach for detecting uterine cancer1. His work eventually led to the establishment of the Pap smear for cervical cancer screening, which is the primary reason that most high-income countries have witnessed a major decline in cervical cancer mortality2–4. More recently, discovery of human papillomavirus (hpv) as a necessary cause of cervical cancer5 has resulted in new prevention fronts: hpv vaccination and molecular-based screening technologies.
In this article, we discuss current cervical cancer screening and prevention initiatives in North America and the need for a paradigm shift in the screening approach because of the negative impact that vaccination is expected to have on Pap screening performance. We make the argument that hpv testing alone should be adopted as the primary cervical screening test and might serve the added role of monitoring vaccine effectiveness. Ultimately, both hpv vaccination and hpv dna testing should be viewed as components along the continuum of care for cervical cancer prevention.
2. PAP CYTOLOGY SCREENING
Pap cytology screening has had remarkable success in most high-income countries, but virtually no effect in lower-resource settings. As a result, cervical cancer has now become a sentinel disease of inequality, being much more common in poor countries and in aboriginal populations in Western countries6,7. In 2008, more than 85% of cases and of deaths attributed to cervical cancer (530,000 and 270,000 respectively) occurred in developing countries8.
To be effective, cervical cancer cytology screening programs require complex and costly infrastructure to ensure adequate coverage, quality, and follow-up treatment of precancerous lesions. In the United States alone, the costs for screening and prevention programs are estimated to amount to roughly US$4 billion annually9. Although cytology has very high specificity (∼98%), its sensitivity for the detection of cervical intraepithelial neoplasia is only slightly above 50%10,11. That figure implies that roughly half the slides from women with cervical lesions will erroneously be classified as negative. Liquid-based cytology (lbc) has some advantages over conventional Pap cytology, including reduced obstruction by extraneous materials and the possibility for ancillary molecular testing; however, it is more costly and still suffers from poor sensitivity. To compensate, screening guidelines in high-income countries have traditionally recommended annual Pap testing for women starting at 18 years of age or shortly after they become sexual active. Recently, U.S. consensus guidelines (issued by the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology) and guidelines from the Canadian Task Force on Preventive Health Care were updated, recommending that cytology exams be repeated every 3 years starting at age 2112 or 2513 respectively. In women less than 25 years of age, minor cervical abnormalities are very common. However, most of those lesions will regress, and their likelihood of progressing quickly to cervical cancer is extremely low. The decision to increase the age at screening initiation and to extend the intervals between screening was therefore intended to avoid overtreatment and associated adverse outcomes in future pregnancies, and to safely reduce the burden to the health care system14,15. The latter point is particularly relevant, considering that roughly 10% of all specimens processed by cytotechnicians in the United States are flagged for abnormalities, requiring additional follow-up or treatment16.
Unfortunately, the introduction of hpv vaccination in most high-income counties is not expected to immediately improve the screening situation. Rather, in settings in which screening programs are now in place, vaccination is expected to have a major negative effect on the test’s positive predictive value (ppv), an important measure used by clinicians that provides a probabilistic value concerning action prompted by a positive test result. As the prevalence of squamous abnormalities (atypical squamous cells of undetermined significance and squamous intraepithelial lesions) attributable to hpv types 16 and 18 declines, the ppv will also substantially decrease17. In addition, the sensitivity and specificity of Pap screening might also be adversely affected because of a decrease in the “signal-to-noise” ratio (fewer true squamous abnormalities compared with cases of inflammation and reactive atypia), which could lead to less attention being paid to slides that are generally unremarkable and to more false negatives—or alternatively, to more overcalls of benign abnormalities because of fear of the false negatives, leading to unnecessary colposcopy referrals18. In fact, estimates of Pap sensitivity as low as 35% have been reported in studies conducted in low-risk settings with stringent quality-control standards (Newfoundland and Labrador, and Quebec, for example), providing evidence to support the former prediction19,20.
Previously, we modelled the expected effect on the ppv of Pap cytology of a decline in lesion prevalence from as high as 50% to as low as 1%, first assuming constant conservative values for sensitivity and specificity (70% and 98% respectively) and then varying those parameters (range: 30%–70% and 95%–98% respectively)17. Under both scenarios, but especially the latter (which also took into account decreases in sensitivity and specificity), we found that the vaccine-induced reduction in lesion prevalence would create a screening scenario untenably cost-effective as ppv estimates fell to below 10%. Those projections clearly indicate that, despite the phenomenal success of Pap testing since the early 1950s, we are approaching a point at which primary screening by this approach will no longer be sustainable. Despite low vaccine uptake across the United States21, the prevalence of vaccine-targeted hpv types has already declined among young women 14–19 years of age (to 5.1% in 2007–2010 from 11.5% in 2003–2006)22. In addition, a substantial decline in the incidence of high-grade precancerous lesions has been observed in women 21–24 years of age living in Connecticut (to 688 per 100,000 women in 2011 from 834 per 100,000 women in 2008)22, reflecting the urgency of adopting an alternative screening approach. The important question now is “What will be the most efficient screening approach post-vaccination?”
3. HPV-BASED AND OTHER PROMISING SCREENING TECHNOLOGIES
In the early 1980s, with investigators well aware of the remote distal connection between sexual activity and cervical cancer, a succession of molecular epidemiologic studies were launched to evaluate the putative role of hpv as the true intermediate endpoint along that casual pathway. Over the course of two decades (that is, as polymerase chain reaction protocols for the detection of hpv dna continued to become more accurate), risk estimates rose from single to triple digits23 until investigators were eventually able to conclude with certainty that infection with hpv is necessary for the development of cervical cancer and its precursor lesions5. Immediately, scientists recognized the public health implications of that discovery and proceeded with trials comparing hpv dna testing with Pap cytology in screening for cervical cancer.
In 2007, results from the first North American trial to evaluate hpv dna testing in the context of cervical cancer screening were published, confirming the belief that this approach is much more sensitive than Pap in detecting high-grade precancerous lesions (95% vs. 55%). The only trade-off in the equation was a minor reduction in specificity (94% vs. 97%)24. It is important to note that the hpv test used in this particular trial was the Hybrid Capture 2 assay (Qiagen, Gaithersburg, MD, U.S.A.), which is capable of detecting (but not distinguishing between) 13 high-oncogenic-risk (hr) hpv genotypes from cervical specimens. All hpv screening assays detect only hr-hpv genotypes, with some, such as the Cobas test (Roche Molecular Systems, Pleasanton, CA, U.S.A.) being capable of detecting hpv types 16 and 18 individually, and a pool of 12 other hr-hpv types. In recent years, a number of other randomized and nonrandomized trials have evaluated hpv testing performance relative to Pap cytology in primary screening. Results from those studies were compiled into a meta-analysis by Cuzick et al. in 200825 and later updated by Richardson et al. in 201126, demonstrating that hpv testing has superior sensitivity (ratio: 1.29; 95% confidence interval: 1.18 to 1.39), but lower specificity (ratio: 0.94; 95% confidence interval: 0.92 to 0.96) for the detection of cervical intraepithelial neoplasia grade 2 or worse26. To reduce costs and the psychological trauma resulting from unnecessary colposcopy referrals for false-positive hpv tests, investigators are now evaluating more specific triage tests.
One test that might serve this purpose is Pap cytology. Over the years, we have argued that this algorithm (hpv testing with Pap triage) would maximize the properties of both tests while preserving a trained workforce to review smears from an “artificially enriched” hpv-positive population. That approach would also provide cytotechnicians with a smaller case load having a higher lesion prevalence (that is, a higher signal-to-noise ratio) and an overall more rewarding reviewing experience17,18,26. To evaluate that approach, a randomized controlled trial (the hpv focal trial) currently underway in British Columbia is comparing triage using hpv testing followed by lbc (Pap) with triage using lbc followed by hpv dna testing27. Results from focal, other similar comparative trials28, and demonstration projects (implementing hpv testing alone as the primary screening test) will be useful when the time comes once again to update screening guidelines. In considering alternative secondary tests for clinical management decisions after a positive hpv test, experts will be interested in selecting an approach that preserves the high sensitivity of hpv testing (maximizes benefits) and safely leads to a reduction in unnecessary colposcopy referrals (minimizes harms).
Additional biomarkers that are now being considered as triage tests for the management of hpv-positive women include hpv genotyping (hpv 16 or hpv 16 and 18)29,30, hpv E6 and E7 messenger rna testing31, methylation (and consequent silencing) of host and viral genes32,33, and novel cytologic methods that attempt to identify proliferating cells (for example, p16INK4a staining)34. With the exception of genotyping35, these molecular technologies have not been directly compared (or compared with cytology), and additional prospective studies are therefore needed to adequately assess their relative performance—for example, specificity for standard endpoints such as cervical intraepithelial neoplasia 3+. For additional information on this topic, readers are referred to a review article by Cuzick et al.36 describing the potential utility of these tests.
3.1. Role of HPV Testing in Current and Future Screening Guidelines
In addition to being more sensitive than cytology, hpv testing is also much more reproducible across settings37,38. Testing for hpv is also less prone to human error because it does not rely on human interpretation and requires only minimal quality control and technician training. Furthermore, because persistent infection with hr-hpv types is necessary for invasive cervical cancer to develop, hpv testing safely permits an extension of the screening interval (for example, to 5 years from 3), which is supported by results from a large U.S. screening study that reported identical 5- and 3-year risks for cervical intraepithelial neoplasia 3+ after either a negative hpv test or Pap exam (both 0.17%), and an only slightly lower risk after negative co-testing (5-year risk: 0.16%)39. Considering the superior sensitivity, reproducibility, and long-term safety assured by a single negative hpv test, replacing Pap cytology with hpv dna testing now seems appropriate for primary screening, and yet most professional organizations have not adopted that approach.
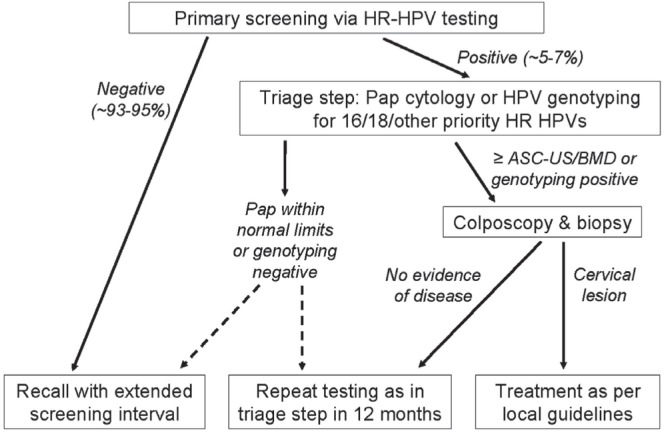
The revised U.S. consensus guidelines recommend hpv and cytology co-testing every 5 years for women over the age of 30. But for patients who are hpv-positive and cytology-negative, follow-up with co-testing is recommended after 12 months; otherwise, immediate referral for colposcopy might be recommended if the patient is positive for hpv 16 or 1812. Meanwhile, the Canadian Task Force on Preventive Health Care currently makes no recommendation concerning the use of hpv testing, concluding that evidence to suggest that hpv testing will reduce the incidence of, or mortality from, cervical cancer is still lacking, and that the marginal reduction in the risk of precancerous lesions (if used in combination with cytology for co-testing) would not be sufficient to offset the high cost, despite the lengthened screening interval13. But not all Canadian provinces have adopted the guidelines. For example, the Ontario Cervical Screening Guideline Working Group recently recommended implementation of hpv testing (with Pap cytology triage) as the primary screening approach, with repeat testing every 5 years until age 6540. In Figure 1, we present a generic screening algorithm that encompasses that and other favourable approaches—for example, hpv testing followed by genotyping for triage.
FIGURE 1.
Proposed generic algorithm for opportunistic or organized cervical cancer screening that uses human papilloma virus (hpv) testing as the primary test, followed by triage with Pap cytology or genotyping [hpv 16 and 18 and possibly other high oncogenic risk (hr) hpv types]; modified from Cuzick et al., 200825. The cytologic grade threshold and definition of extended screening interval may vary according to local preferences and be modified as new evidence from screening studies becomes available. asc-us = atypical squamous cells of undetermined significance; bmd = borderline or mild dyskaryosis.
Current guidelines present clear discrepancies with respect to the role of hpv testing in cervical screening. Although we expect that hpv testing will eventually become the primary screening approach across North America, many uncertainties still surround its implementation: for example, the most appropriate triage test; the screening interval; the management strategy for hpv-positive, Pap-negative women; and the optimal age to initiate or discontinue screening (Table i). To prevent confusion and “cherry-picking” of the recommendations to follow, policy officials should work together to evaluate the accruing evidence in an effort to harmonize future screening guidelines. Also, if we are to expect clinicians to accurately follow the guidelines, then screening algorithms should be simplified whenever possible. For instance, officials might consider adopting a universal approach that is not age-dependent—that is, an approach in which all women more than 25 years of age are tested for hpv, and cytology is reserved for triage during screening. It has already been established that screening women between the ages of 21 and 24 provides almost no benefit41 and might actually cause more harm as a result of overdiagnosis and associated treatment14. But with the advent of more specific molecular markers36 and accurate risk prediction models to guide the clinical management of patients42, officials might decide that hpv testing can safely be initiated at an earlier age. Final results are now awaited from screening trials that included women less than 30 years of age (such as the athena43 and focal27 trials) to help in determining whether primary hpv testing might effectively be combined with an appropriate triage test for all women undergoing screening. But unless experts decide to modify their recommendations, physicians across North America should, for the time being, continue to follow current guidelines and administer only Pap exams to women less than 30 years of age.
TABLE I.
Uncertainties surrounding implementation of testing for the human papilloma virus (hpv) as the primary cervical cancer screening test
| Question | Reasonable options or possibilities being explored (non-exhaustive list) |
|---|---|
| What is the best age to start screening with hpv testing?a | 25, 30,b or >30 years |
| What is the best age to stop screening with hpv testing?a | 60, 65,b 70, or >70 years |
| What triage test or tests should be used to guide colposcopy referrals after a positive hpv test?a | Pap cytology,b hpv 16 or 16 and 18 genotyping,b hpv E6/E7 messenger RNA testing, methylation (and consequent silencing) of host and viral genes, or p16INK4a staining |
| What is the most appropriate interval for hpv screening tests?a | 5,b 7, 10, >10 years |
| Should we expect hpv testing to become more affordable or cost-effective in the future? | Yes, because of market expansion and high-volume testing if adopted for primary screening |
| Is reliance on industry (hpv test manufacturers) an important concern? | Yes, because of clear commercial and financial interests that might not be in line with the public’s best interests |
Question directly related to the development of cervical cancer screening algorithms.
Approach recommended in screening guidelines or currently the most widely accepted.
4. HPV VACCINATION: A NEW PARADIGM IN CERVICAL CANCER PREVENTION
The International Agency for Research on Cancer has now classified 13 hpv genotypes as definite or probable carcinogens44. However, only 2 of them (hpv 16 and 18) are responsible for approximately 70% of the cervical cancer burden worldwide and are targeted by current vaccines45,46. Both Cervarix (GlaxoSmithKline, London, U.K.) and Gardasil (Merck and Co., Whitehouse Station, NJ, U.S.A.) were evaluated in randomized controlled trials and found to be nearly 100% effective in preventing new infections from the target types—that is, hpv 16 and 18, or hpv 6, 11, 16, and 18 respectively—and from precancer associated with the two oncogenic types47,48. Gardasil was also 100% effective in preventing genital warts, which are normally caused by hpv 6 and 1148. Because both vaccines are exclusively prophylactic, routine vaccination is recommended for pre-teen girls before sexual debut (ages 9–12 years), with “catch-up” vaccination for women 13–26 years of age49,50. However, considering that most government-funded hpv immunization programs target only pre-teen girls and that the latency between hr-hpv infection and development of invasive cancer is long, we expect that more than a decade will pass before a reduction in mortality is observed in most countries7. A notable exception might become evident in countries with successful “catch-up” programs in place. For example, Australia was the first country to introduce a fully-funded national immunization program for women up to the age of 26, and as a result of high vaccine uptake in the “catch-up” age range, it was also the first country to report a significant decline in the rate of high-grade precancerous lesions51. Recently, a decline in the rate of high-grade cervical lesions was also observed in the state of Connecticut52, which represents the first report of a reduction in cervical neoplasia in North America since vaccines were introduced. Connecticut also happens to be among the leaders in vaccine coverage, with reports estimating that 61% of adolescent girls (13–17 years) received at least 1 dose of the vaccine in 201153. Although uptake among young women more than 17 years of age is still very low across North America54, British Columbia became, in 2013, the first province to offer free vaccination to women up to age 26, which is expected to lead to higher uptake and a reduction in cervical cancer mortality even sooner in that province.
Despite our expectation that hpv vaccination will eventually lead to declines in cervical cancer incidence and mortality, the quick approval and rollout of the vaccines has prompted considerable criticism55. Aside from reservations related to cost and the need for hpv vaccination in settings with low cervical cancer mortality already, some policy analysts are concerned about the duration of hpv protection, the possibility of type replacement, and the safety of the vaccines in general. Fortunately, among vaccinated individuals, the rates of reported adverse events have been comparable to rates among placebo recipients and within expected background rates in the general population56. In addition, the latest trial results indicate that protection has endured unabated for nearly a decade (for licensed hpv vaccines) and longer (approximately 13 years for the prototype hpv 16 vaccine) without any indication of waning antibodies57–59. Finally, epidemiology studies investigating the potential for hpv type replacement (that is, the scenario in which other hpv types take over the niches vacated by the eradication of vaccine target types) have so far provided no strong evidence of natural type competition, which is considered a requirement for type replacement to occur in vaccinated populations60. Meanwhile, evidence of cross-type protection (primarily for phylogenetically related types 31, 33, and 45) suggests that the benefit of vaccination might ultimately be greater than expected61,62.
Following from the decision to invest in hpv vaccination programs, officials have to decide which vaccine to purchase, the required number of doses, the age groups to target, and whether young men should also be included. Despite the large amount of literature addressing those issues, such decisions will ultimately depend on the objectives and available resources of each program. For instance, if an important objective is to reduce the prevalence of anogenital warts, then the quadrivalent vaccine would be the best choice; however, if prevention of cervical cancer and high-grade lesions is the main priority, then either the bivalent or the quadrivalent vaccine might be equally suitable63. With regard to number of doses, current evidence suggests administering 2 rather than the recommended 3 immunizations might be sufficient, and that protocol has already been implemented in some settings, including Quebec64. Furthermore, according to a recent trial comparing alternative dosing schedules, nonadherence with the manufacturers’ recommended schedule of 0, 2, and 6 months is not expected to have a major effect on vaccine efficacy65. There is also now strong evidence that supports vaccination for the prevention of anogenital hpv infection and related lesions in men66, which has led the U.S. Advisory Committee on Immunization Practices to update their recommendations to include routine use of the quadrivalent vaccine for boys 11–12 years of age, with “catch-up” vaccination up to age 2167. However, according to recent modelling studies, the most cost-effective approach to reduce the burden of hpv in both sexes is to attain high vaccine coverage among girls 9–13 years of age and to rely on herd immunity to protect heterosexual boys and men68,69.
Unfortunately, rates of hpv vaccination coverage are still very low across the United States and in some parts of Canada. Although coverage rates among Grade 8 girls have steadily increased in Ontario since introduction of the vaccine in 2007, Ontario still had the lowest coverage rate in Canada: 59% as of 201070. Meanwhile, among girls targeted across Eastern Canada and Quebec, coverage rates are generally above 85%, which represents a huge contrast with the situation in the United States, where only 32% of girls 13–17 years of age received the vaccine in 201021. As expected, the lowest coverage rates in the United States were among the uninsured (just 14% nationally) and in poorer areas where cervical cancer rates are the highest and Pap testing prevalence is the lowest—that is, the regions that could benefit the most from vaccination. In addition to the high cost, one of the major obstacles that countries now face in implementing wide-scale hpv vaccination is anti-vaccine activism, propagated mainly through the Internet by illegitimate Web sites posing as authoritative sources. To help dispel the myths or misperceptions spread by those sites concerning vaccination safety and importance, health care providers should be armed with correct information that they can use to educate their patients71.
5. SUMMARY AND OTHER ISSUES TO CONSIDER
Discovery of hpv as the necessary cause of cervical cancer and subsequent studies to evaluate hpv vaccination and molecular-based screening technologies are among the best examples of multi- and interdisciplinary research on cancer causes and prevention. Vaccination against hpv now holds tremendous potential to reduce global mortality, and yet many challenges for equitable implementation in high- and low-resource settings remain. Also, because current vaccines protect against only a fraction of the oncogenic hpv types, cervical cancer screening will continue to be needed in the post-vaccination era. As we have discussed here, the effect of vaccination on the prevalence of precancerous cervical lesions means that the current paradigm for screening must change. Fortunately, sensitive hpv-based technologies have emerged, and we expect that policymakers will soon decide to implement hpv testing as the sole primary screening test based on favourable results from ongoing demonstration projects and cost-effectiveness analyses revealing improved performance and safety, and based on projected lowered costs because of lengthened screening intervals, market expansion, and high-volume hpv testing18. However, adoption of hpv testing will result in many more women becoming aware of their hpv status, which could result in psychological trauma. It is therefore important for clinicians to educate their patients about hpv and to inform them that although most infections will clear on their own, additional follow-up care (monitoring, testing, and treatment) might be required to reduce their risk of cervical cancer.
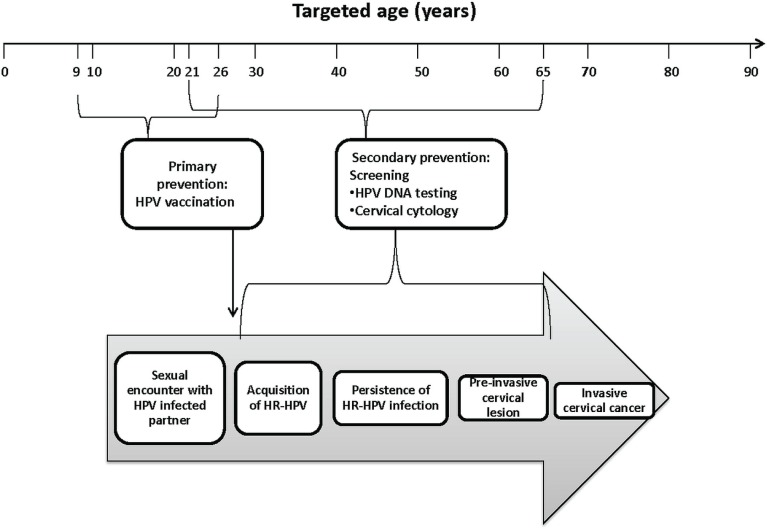
In the post-vaccination era, linkage of vaccine registries with hpv screening and other disease registries (for example, using medical records) might provide a low-cost method of monitoring vaccine efficacy, including type replacement, cross-protection, and protection duration. Such linkages might also help in the evaluation of long-term safety or adverse events that are too rare to be evaluated in clinical trial populations. Ultimately, integration of primary and secondary prevention strategies inherently lends itself to acting as a single prevention strategy through record linkage and shared resources. By adopting this sort of life-course approach to cervical cancer prevention, young women or their parents might also be able to easily identify where they or their child fit into the continuum and to understand why they are being targeted for vaccination or screening (Figure 2).
FIGURE 2.
Opportunities for prevention at various ages and times along the pathway from exposure, acquisition, and persistence of the human papilloma virus (hpv) to development of preinvasive lesions. The boxes within the large arrow depict the natural history of cervical carcinogenesis. At each step during this process, most cases will regress. In the absence of screening, only a very small fraction would be expected to progress to invasive cervical cancer. Targeted age groups for vaccination (9–26 years, including “catch-up”) and screening (21–65 years) presented here are based on current recommendations in the United States. In Canada, the only difference is that women less than 25 years of age are not advised to initiate screening, according to guidelines from the Canadian Task Force on Preventive Health Care. hr-hpv = high oncogenic risk hpv.
Another important issue that we have not touched on is the possibility that hpv vaccination might affect the behaviour of young women by conveying a false sense of security and promoting risky sexual behaviour. This sort of “risk compensation”72 could result in an increase in other hpv genotypes not targeted by vaccination60 and in other sexually transmitted infections. This concern is shared by many parents and health officials, but fortunately, studies from the United States and the United Kingdom focussing on this topic have not found any association between hpv vaccination and increased risky sexual behaviour—for example, a higher number of sexual partners or lesser condom use73,74.
As policy officials struggle to address unresolved issues surrounding implementation of hpv vaccination and screening, it will be important, because of obvious commercial interests, to monitor the involvement of biotechnology and pharmaceutical companies in influencing policy decisions. For instance, recommending vaccination to broader age groups of men and women, and reducing screening intervals in redesigned algorithms would certainly be in the interest of industry, but maybe not of the public. The necessary monitoring might be challenging, because until recently, no commercial interests had been involved in the policy process. The only screening method available was conventional Pap cytology (a technology in the public domain), and therefore countries simply needed to decide whether they were going to assign the resources necessary to attain adequate quality and coverage75. Some practical issues also surround the introduction of hpv testing in primary screening; those issues must be addressed before wide-scale implementation. For example, delays from notification of hpv results to appropriate triage testing might pose a serious threat if they last long enough to allow precancerous lesions to progress. To avoid that possibility, officials should consider specimen co-collection to permit hr-hpv t esting w ith reflex cytology, u sing banked cervical specimens collected at the initial screening visit. Adopting this approach might also help to preserve another advantage of hpv testing over conventional cytology—that is, increased coverage of women in remote areas by self-collection of specimens outside of clinic, and use of the same specimen for both hpv testing and lbc for triage of hpv-positive samples.
Because of the rapid pace of technological change and new discoveries, the evidence base in the area of cervical cancer prevention remains an elusive target, making it difficult to predict what lies ahead. Inability to predict will continue to be an obstacle for policymaking in this field. For example, if a second generation of hpv vaccines that extend protection to other hr-hpv types comes to market, policymakers might once again need to readjust screening guidelines76, reinforcing the need for an innovative risk-assessment strategy that is flexible with respect to the growth of the new technologies42. Recently, ground-breaking discoveries have also occurred in the field of hpv microbicides and therapeutic vaccines research that might once again transform the landscape of cervical cancer prevention and control strategies to include novel treatment options for hpv and associated malignancies76– 78. Since introduction of the first clinical hpv tests roughly 20 years ago, the field of hpv research has experienced tremendous growth (reflected by a 400% increase in the annual number of articles on papillomavirus in Medline’s PubMed database over that time period) marked by all the key events outlined here and ultimately capped with a Nobel prize to Dr. zur Hausen in 2008 for his pioneering role in establishing hpv as the main causal agent in cervical cancer75. We expect that the next 20 years will continue to be an important period for discovery for this field, but with a more concerted effort placed on evaluation of novel hpv prevention technologies and policy decisions surrounding their implementation.
6. CONFLICT OF INTEREST DISCLOSURES
The HPV and Cervical Cancer Research Program at McGill University’s Division of Cancer Epidemiology has been funded by the Canadian Institutes of Health Research, the U.S. National Institutes of Health, the Cancer Research Society, Fonds de la recherche en santé du Québec, and the National Cancer Institute of Canada. The Division has also received unconditional funding from Merck for investigator-initiated projects.
ELF holds a James McGill Chair. He has served as occasional consultant to companies involved with hpv vaccines (Merck, GlaxoSmithKline), hpv diagnostics (Roche, Gen-Probe, Qiagen, Becton Dickinson), and cervical cytology (Cytyc, Ikonisys). The other authors have no industry involvement to disclose.
7. REFERENCES
- 1.Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. Am J Obstet Gynecol. 1941;42:193–206. [PubMed] [Google Scholar]
- 2.Quinn M, Babb P, Jones J, Allen E. Effect of screening on incidence of and mortality from cancer of cervix in England: evaluation based on routinely collected statistics. BMJ. 1999;318:904–8. doi: 10.1136/bmj.318.7188.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbyn M, Raifu AO, Weiderpass E, Bray F, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. Eur J Cancer. 2009;45:2640–8. doi: 10.1016/j.ejca.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS One. 2010;5:e9584. doi: 10.1371/journal.pone.0009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Franco EL, Duarte–Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. CMAJ. 2001;164:1017–25. [PMC free article] [PubMed] [Google Scholar]
- 7.Tota JE, Chevarie–Davis M, Richardson LA, Devries M, Franco EL. Epidemiology and burden of hpv infection and related diseases: implications for prevention strategies. Prev Med. 2011;53(suppl 1):S12–21. doi: 10.1016/j.ypmed.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Arbyn M, Castellsague X, de Sanjose S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–86. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 9.Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus–related disease in the U.S.: analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107–22. doi: 10.2165/00019053-200523110-00004. [DOI] [PubMed] [Google Scholar]
- 10.Nanda K, McCrory DC, Myers ER, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810–19. doi: 10.7326/0003-4819-132-10-200005160-00009. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on hpv testing in primary cervical cancer screening. Int J Cancer. 2006;119:1095–101. doi: 10.1002/ijc.21955. [DOI] [PubMed] [Google Scholar]
- 12.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–72. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollock S, Dunfield L, Shane A, et al. on behalf of the Canadian Task Force on Preventive Health Care Recommendations on screening for cervical cancer. CMAJ. 2013;185:35–45. doi: 10.1503/cmaj.121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol. 2000;96:219–23. doi: 10.1016/S0029-7844(00)00882-6. [DOI] [PubMed] [Google Scholar]
- 16.Franco EL, Duarte–Franco E, Ferenczy A. Prospects for controlling cervical cancer at the turn of the century. Salud Publica Mex. 2003;45(suppl 3):S367–75. doi: 10.1590/S0036-36342003000900011. [DOI] [PubMed] [Google Scholar]
- 17.Franco EL, Mahmud SM, Tota J, Ferenczy A, Coutlee F. The expected impact of hpv vaccination on the accuracy of cervical cancer screening: the need for a paradigm change. Arch Med Res. 2009;40:478–85. doi: 10.1016/j.arcmed.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Tota J, Mahmud SM, Ferenczy A, Coutlee F, Franco EL. Promising strategies for cervical cancer screening in the post–human papillomavirus vaccination era. Sex Health. 2010;7:376–82. doi: 10.1071/SH10022. [DOI] [PubMed] [Google Scholar]
- 19.Ratnam S, Franco EL, Ferenczy A. Human papillomavirus testing for primary screening of cervical cancer precursors. Cancer Epidemiol Biomarkers Prev. 2000;9:945–51. [PubMed] [Google Scholar]
- 20.Mayrand MH, Duarte–Franco E, Coutlee F, et al. Randomized controlled trial of human papillomavirus testing versus Pap cytology in the primary screening for cervical cancer precursors: design, methods and preliminary accrual results of the Canadian cervical cancer screening trial (cccast) Int J Cancer. 2006;119:615–23. doi: 10.1002/ijc.21897. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (hpv)–associated cancers and hpv vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (hpv) prevalence among young women following hpv vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis. 2013;208:385–93. doi: 10.1093/infdis/jit192. [DOI] [PubMed] [Google Scholar]
- 23.Franco EL, Tota J. Invited commentary: human papillomavirus infection and risk of cervical precancer—using the right methods to answer the right questions. Am J Epidemiol. 2010;171:164–8. doi: 10.1093/aje/kwp392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayrand MH, Duarte–Franco E, Rodrigues I, et al. Human papillomavirus dna versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–88. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 25.Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus–based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(suppl 10):K29–41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Richardson LA, Tota J, Franco EL. Optimizing technology for cervical cancer screening in high-resource settings. Expert Rev Obstet Gynecol. 2011;6:343–53. doi: 10.1586/eog.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (hpv) testing for cervical cancer screening: trial design and preliminary results (hpv focal trial) BMC Cancer. 2010;10:111. doi: 10.1186/1471-2407-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leinonen M, Nieminen P, Kotaniemi–Talonen L, et al. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J Natl Cancer Inst. 2009;101:1612–23. doi: 10.1093/jnci/djp367. [DOI] [PubMed] [Google Scholar]
- 29.Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (hpv) type 16 or 18 and the possible utility of type-specific hpv testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–9. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 30.Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus–positive, cytologically normal women over a 10-year period. Cancer Res. 2006;66:10630–6. doi: 10.1158/0008-5472.CAN-06-1057. [DOI] [PubMed] [Google Scholar]
- 31.Schweizer J, Lu PS, Mahoney CW, et al. Feasibility study of a human papillomavirus E6 oncoprotein test for diagnosis of cervical precancer and cancer. J Clin Microbiol. 2010;48:4646–8. doi: 10.1128/JCM.01315-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wentzensen N, Sherman ME, Schiffman M, Wang SS. Utility of methylation markers in cervical cancer early detection: appraisal of the state-of-the-science. Gynecol Oncol. 2009;112:293–9. doi: 10.1016/j.ygyno.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wentzensen N, Sun C, Ghosh A, et al. Methylation of HPV18, HPV31, and HPV45 genomes and cervical intraepithelial neoplasia grade 3. J Natl Cancer Inst. 2012;104:1738–49. doi: 10.1093/jnci/djs425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carozzi F, Confortini M, Dalla Palma P, et al. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the ntcc randomised controlled trial. Lancet Oncol. 2008;9:937–45. doi: 10.1016/S1470-2045(08)70208-0. [DOI] [PubMed] [Google Scholar]
- 35.Cox JT, Castle PE, Behrens CM, Sharma A, Wright TC, Jr, Cuzick J on behalf of the athena hpv Study Group Comparison of cervical cancer screening strategies incorporating different combinations of cytology, hpv testing, and genotyping for hpv 16/18: results from the athena hpv study. Am J Obstet Gynecol. 2012;208:184.e1–11. doi: 10.1016/j.ajog.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Cuzick J, Bergeron C, von Knebel Doeberitz M, et al. New technologies and procedures for cervical cancer screening. Vaccine. 2012;30(suppl 5):F107–16. doi: 10.1016/j.vaccine.2012.05.088. [DOI] [PubMed] [Google Scholar]
- 37.Castle PE, Lorincz AT, Mielzynska–Lohnas I, et al. Results of human papillomavirus dna testing with the Hybrid Capture 2 assay are reproducible. J Clin Microbiol. 2002;40:1088–90. doi: 10.1128/JCM.40.3.1088-1090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carozzi FM, Del Mistro A, Confortini M, et al. Reproducibility of hpv dna testing by Hybrid Capture 2 in a screening setting. Am J Clin Pathol. 2005;124:716–21. doi: 10.1309/84E5WHJQHK83BGQD. [DOI] [PubMed] [Google Scholar]
- 39.Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663–72. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy J, Kennedy EB, Dunn S, et al. on behalf of the Ontario Cervical Screening Program and the Program in Evidence-Based Care Cervical screening: a guideline for clinical practice in Ontario. J Obstet Gynaecol Can. 2012;34:453–8. doi: 10.1016/S1701-2163(16)35242-2. [DOI] [PubMed] [Google Scholar]
- 41.Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ. 2009;339:b2968. doi: 10.1136/bmj.b2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. J Low Genit Tract Dis. 2008;12:1–7. doi: 10.1097/lgt.0b013e31815ea58b. [DOI] [PubMed] [Google Scholar]
- 43.Wright TC, Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The athena human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206:46.e1–11. doi: 10.1016/j.ajog.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer. 2009;4:8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 46.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 47.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 48.Garland SM, Hernandez–Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 49.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER, on behalf of the Centers for Disease Control and Prevention (cdc) and the Advisory Committee on Immunization Practices (acip) Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (acip) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention (cdc) fda licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated hpv vaccination recommendations from the Advisory Committee on Immunization Practices (acip) MMWR Morb Mortal Wkly Rep. 2010;59:626–9. [PubMed] [Google Scholar]
- 51.Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the hpv vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet. 2011;377:2085–92. doi: 10.1016/S0140-6736(11)60551-5. [DOI] [PubMed] [Google Scholar]
- 52.Niccolai LM, Julian PJ, Meek JI, McBride V, Hadler JL, Sosa LE. Declining rates of high-grade cervical lesions in young women in Connecticut, 2008–2011. Cancer Epidemiol Biomarkers Prev. 2013;22:1446–50. doi: 10.1158/1055-9965.EPI-13-0272. [DOI] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention (cdc) National and state vaccination coverage among adolescents aged 13–17 years—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–7. [Erratum in: MMWR Morb Mortal Wkly Rep 2012;61:844] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention (cdc) Adult vaccination coverage—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:66–72. [PubMed] [Google Scholar]
- 55.Porta M. The improbable plunge. What facts refute reasons to expect that the effectiveness of hpv vaccination programs to prevent cervical cancer could be low? Prev Med. 2009;48:407–10. doi: 10.1016/j.ypmed.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750–7. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- 57.Dillner J, Kjaer SK, Wheeler CM, et al. on behalf of the future i/ii Study Group Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;341:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehtinen M, Paavonen J, Wheeler CM, et al. on behalf of the hpv patricia Study Group Overall efficacy of hpv-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind patricia trial. Lancet Oncol. 2012;13:89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 59.Roteli–Martins CM, Naud P, De Borba P, et al. Sustained immunogenicity and efficacy of the hpv-16/18 AS04-adjuvanted vaccine: up to 8.4 years of follow-up. Hum Vaccin Immunother. 2012;8:390–7. doi: 10.4161/hv.18865. [DOI] [PubMed] [Google Scholar]
- 60.Tota JE, Ramanakumar AV, Jiang M, et al. Epidemiological approach to evaluate the potential for human papillomavirus type replacement post-vaccination. Am J Epidemiol. 2013;178:625–34. doi: 10.1093/aje/kwt018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (hpv; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine hpv types in generally hpv-naive women aged 16–26 years. J Infect Dis. 2009;199:926–35. doi: 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 62.Wheeler CM, Castellsague X, Garland SM, et al. on behalf of the hpv patricia Study Group Cross-protective efficacy of hpv-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic hpv types: 4-year end-of-study analysis of the randomised, double-blind patricia trial. Lancet Oncol. 2012;13:100–10. doi: 10.1016/S1470-2045(11)70287-X. [DOI] [PubMed] [Google Scholar]
- 63.Van de Velde N, Boily MC, Drolet M, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104:1712–23. doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- 64.Kreimer AR, Rodriguez AC, Hildesheim A, et al. on behalf of the cvt Vaccine Group Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent hpv16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–51. doi: 10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neuzil KM, Canh do G, Thiem VD, et al. Immunogenicity and reactogenicity of alternative schedules of hpv vaccine in Vietnam: a cluster randomized noninferiority trial. JAMA. 2011;305:1424–31. doi: 10.1001/jama.2011.407. [DOI] [PubMed] [Google Scholar]
- 66.Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent hpv vaccine against hpv infection and disease in males. N Engl J Med. 2011;364:401–11. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Centers for Disease Control and Prevention (cdc) Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (acip), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–8. [PubMed] [Google Scholar]
- 68.Brisson M, van de Velde N, Franco EL, Drolet M, Boily MC. Incremental impact of adding boys to current human papillomavirus vaccination programs: role of herd immunity. J Infect Dis. 2011;204:372–6. doi: 10.1093/infdis/jir285. [DOI] [PubMed] [Google Scholar]
- 69.Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male hpv vaccination in the United States. Vaccine. 2011;29:8443–50. doi: 10.1016/j.vaccine.2011.07.096. [DOI] [PubMed] [Google Scholar]
- 70.Wilson SE, Harris T, Sethi P, Fediurek J, Macdonald L, Deeks SL. Coverage from Ontario, Canada’s school-based hpv vaccine program: the first three years. Vaccine. 2013;31:757–62. doi: 10.1016/j.vaccine.2012.11.090. [DOI] [PubMed] [Google Scholar]
- 71.Poland GA, Jacobson RM. The clinician’s guide to the anti-vaccinationists’ galaxy. Hum Immunol. 2012;73:859–66. doi: 10.1016/j.humimm.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 72.Hedlund J. Risky business: safety regulations, risks compensation, and individual behavior. Inj Prev. 2000;6:82–90. doi: 10.1136/ip.6.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Forster AS, Marlow LA, Stephenson J, Wardle J, Waller J. Human papillomavirus vaccination and sexual behaviour: cross-sectional and longitudinal surveys conducted in England. Vaccine. 2012;30:4939–44. doi: 10.1016/j.vaccine.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 74.Liddon NC, Leichliter JS, Markowitz LE. Human papillomavirus vaccine and sexual behavior among adolescent and young women. Am J Prev Med. 2012;42:44–52. doi: 10.1016/j.amepre.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 75.Franco EL, de Sanjose S, Broker TR, et al. Human papillomavirus and cancer prevention: gaps in knowledge and prospects for research, policy, and advocacy. Vaccine. 2012;30(suppl 5):F175–82. doi: 10.1016/j.vaccine.2012.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peres J. For cancers caused by hpv, two vaccines were just the beginning. J Natl Cancer Inst. 2011;103:360–2. doi: 10.1093/jnci/djr053. [DOI] [PubMed] [Google Scholar]
- 77.Bagarazzi ML, Yan J, Morrow MP, et al. Immunotherapy against hpv16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012;4:155ra38. doi: 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barrios K, Celis E. TriVax-hpv: an improved peptide-based therapeutic vaccination strategy against human papillomavirus–induced cancers. Cancer Immunol Immunother. 2012;61:1307–17. doi: 10.1007/s00262-012-1259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]