Abstract
Patients undergoing myeloablative conditioning regimens and autologous stem-cell transplantation (asct) are at high risk of malnutrition. This randomized study aimed to determine if early nutrition support (commenced when oral intake is less than 80% of estimated requirements) compared with usual care (commenced when oral intake is less than 50% of estimated requirements) reduces weight loss in well-nourished patients undergoing high-nutritional-risk conditioning chemotherapy and asct.
In the 50 well-nourished patients who were randomized, the outcomes evaluated included changes in weight and lean body mass (mid-upper arm circumference), length of stay, time to hemopoietic engraftment, and quality of life (Memorial Symptom Assessment Scale – Short Form). On secondary analysis, after exclusion of a single extreme outlier, both groups demonstrated significant weight loss over time (p = 0.0005). Weight loss was less in the early nutrition support group at time of discharge (mean: −0.4% ± 2.9% vs. −3.4% ± 2.6% in the usual care group, p = 0.001). This difference in weight was no longer observed at 6 months after discharge (mean: −1.0% ± 6.8% vs. 1.4% ± 6.1%, p = 0.29).
In practice, an early start to nutrition support proved difficult because of patient resistance and physician preference, with 8 patients (33%) in the control group and 4 (15%) in the intervention group not commencing nutrition support when stipulated by the study protocol. No significant differences between the groups were found for other outcomes. In well-nourished patients receiving asct, early nutrition support maintained weight during admission, but did not affect other outcomes. Interpretation of results should take into consideration the difficulties encountered with intervention implementation.
Keywords: Nutrition, autologous stem-cell transplantation, malnutrition, enteral nutrition, parenteral nutrition
1. INTRODUCTION
Malnutrition is common in cancer patients and is associated with poorer patient and treatment outcomes1,2. Gastrointestinal toxicity after autologous stem-cell transplantation (asct), combined with post-transplantation infection and a catabolic state induced by the cytoreductive therapy, often results in poor dietary intake during the post-transplant period3,4. A recent pilot exploratory study in 24 patients demonstrated significant loss of lean body mass, deterioration in nutrition status, and decreased quality of life upon discharge after asct5. Nutrition support is required to prevent malnutrition as a consequence of treatment toxicities and has been established as a standard of care in many myeloablative conditioning regimens6. The conditioning regimens associated with the greatest gastrointestinal toxicity are those containing melphalan or busulphan and those incorporating total body irradiation (tbi)4,7.
Nutrition intervention studies undertaken in the asct population have focused on enteral compared with parenteral nutrition; predictors of mucositis; and the use of oral, enteral, or parenteral glutamine8,9. Evidence for nutrition interventions that are optimal in preventing malnutrition in patients undergoing asct is currently limited and largely based on expert opinion6. To date, no studies have examined whether commencing nutrition support in advance of a large deficit in oral intake improves patient outcomes.
The timing of nutrition support after asct varies between institutions4,10–12. Commonly used strategies include initiating nutrition support on a predetermined day (that is, day 1 after asct) or, alternatively, only when dietary intake declines below a specified threshold within the first week after asct. The nutrition support provided can consist of oral supplements or enteral (via nasogastric tube) or parenteral nutrition6. At our centre, nutrition support is typically initiated when oral intake declines to less than 50% of the estimated nutrition requirement.
The present study set out to determine whether initiating nutrition support early after asct in patients receiving a high-nutritional-risk conditioning regimen might result in less weight loss, less lean body mass loss, improved quality of life (qol), and shorter length of stay (los) and time to hemopoietic recovery. The primary objective was to compare weight change from day 1 of conditioning chemotherapy to day of discharge in the two study arms. Secondary objectives were to compare qol, los, mid-upper-arm muscle circumference (muamc), and time to hemopoietic recovery in the two groups. We also report on the challenges of clinical trials investigating nutrition interventions in the context of asct.
2. METHODS
Eligible patients were identified from within the Haematology service at our cancer centre between June 21, 2005, and October 8, 2008. Median follow-up was 6.8 months (range: 0.4–8.0 months), and the final patient follow-up was completed May 18, 2009. Eligible patients were those undergoing asct with a melphalan-, busulphan-, or tbi-containing conditioning regimen. Patients were admitted before commencing conditioning chemotherapy and were discharged after recovery of absolute neutrophil and platelet counts and in the absence of fever and infection. Patients were ineligible if they had pre-existing malnutrition or an acute infection requiring parenteral antibiotics at baseline, or if they were scheduled to receive reduced-dose melphalan (140 mg/m2) only.
The definition of malnutrition used at our centre during the study period was any of
more than 10% loss of body weight in the preceding 6 months,
bodyweight loss of more than 1 kg per week in the preceding 2–4 weeks, or
more than 7 days of inadequate oral intake or persistent gastrointestinal symptoms for more than 2 weeks.
The 52 patients enrolled were randomized by the study data manager; 2 patients were subsequently found to be ineligible. The study was approved by the hospital ethics committee, and all participating patients provided written informed consent.
There was no set day for the start or end of nutrition support. Instead, nutrition support was planned to start after asct as soon as oral intake fell below 80% or 50% of estimated energy requirements in the intervention group and the control group respectively. Energy requirements were expected to be higher in the first 9 days after asct because of the associated hypermetabolic state. For the first 9 days, the energy requirement was calculated to be 1.5 times the basal metabolic rate, and from day 10 onward, the energy requirement was calculated to be 1.3 times the basal metabolic rate for both groups. Those estimations are comparable with estimations used in other studies6,10. Oral intake for the preceding 24 hours was monitored daily by a dietitian using a combination of food record charts and patient recall. Once commenced, nutrition support continued until oral intake was more than 80% of the estimated energy requirement (intervention group) or more than 50% of the estimated energy requirement (control group) for 24–48 hours, or until the time of discharge. The type of nutrition support provided (oral, enteral, or parenteral) was not mandated, but instead was determined according to clinical indications—that is, nonfunctioning gut, presence of mucositis—so as to simulate the decisions made in clinical practice. The decision about the type of nutrition support provided was made by the dietitian and the treating hematologist. No adverse effects related to the study intervention were observed.
Time to commencement and cessation of nutrition support (both enteral and parenteral), duration of nutrition support, weight measured to 1 decimal place on HVL-CS scales (A&D Australasia, Kensington, Australia), and muamc (Harpenden skinfold calipers: British Indicators, Burgess Hill, U.K.) were recorded by the study dietitian. Time to hemopoietic recovery, los measured from the day of stem-cell infusion to the day of discharge, and qol measured using the validated Memorial Symptom Assessment Scale – Short Form were collected by the study data manager13. Weight, muamc, and qol were measured at day 1 of conditioning chemotherapy, the day of asct (day 0), day +10 after asct, discharge, and 3 and 6 months after discharge.
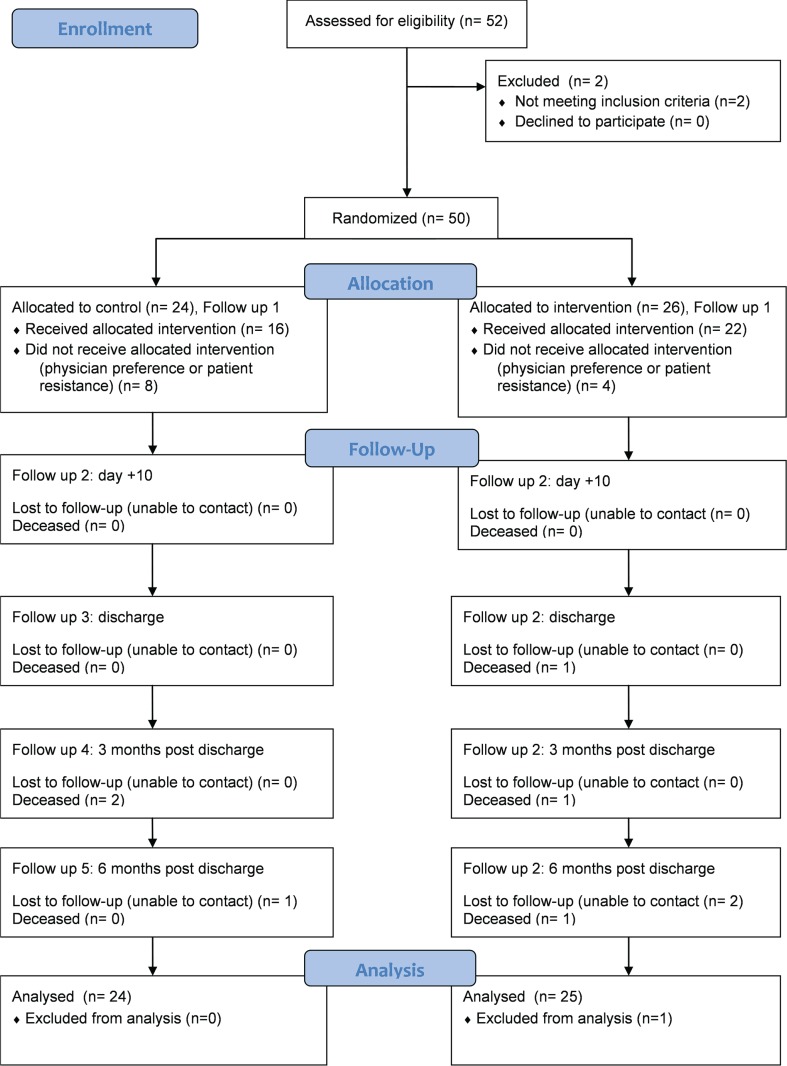
A sample size of 38 was required to detect a difference of 5% weight change between the control and intervention groups with a power of 90% and a significance level of 0.05. We recruited 50 patients to allow for dropouts because of death before discharge. Patients were stratified by conditioning regimen with or without tbi because of the potential for the small number of patients treated with tbi to bias the results. One stratum consisted of all conditioning regimens including tbi; the other consisted of all regimens excluding tbi. Computer-generated randomization charts were prepared for each stratum, printed and accessible only to the study data manager. The method of group assignment was based on an adaptive biased coin procedure using a restricted randomization method. Figure 1 presents the study flow diagram.
FIGURE 1.
consort flow diagram.
Mixed models, using sex, randomization arm, and interaction between time and arm were used for the longitudinal analysis of changes in anthropometry. The t-test was used to compare means when the assumption of normality was met; otherwise, a Wilcoxon test was used. Kaplan–Meier estimates were used for time-to-event analyses. A log-rank test was used to examine differences between the randomization arms.
3. RESULTS
Results are presented as an intention-to-treat analysis, with the exclusion of 1 patient in the intervention arm because of an exceptionally long and complicated post-asct admission of 162 days compared with the average of 14.5 days in the rest of the sample. Table i describes patient characteristics. No participant received tbi.
TABLE I.
Patient characteristics
| Variable | Control | Intervention |
|---|---|---|
| Patients (n) | 24 | 26 |
| Sex [n (%) men] | 11 (46) | 23 (88) |
| Age (years) | ||
| Median | 56 | 56.3 |
| Range | 30–72 | 36–70 |
| Mean | 56±8.6 | 56.5±9.6 |
| Mean weight (kg) | 77.3±19.5 | 84.0±10.9 |
| Diagnosis [n (%)] | ||
| Non-Hodgkin lymphoma | 7 (29.1) | 6 (23.1) |
| Multiple myeloma | 15 (62.5) | 18 (69.2) |
| Acute myeloid leukemia | 1 (4.2) | 0 (0) |
| Breast cancer | 1 (4.2) | 0 (0) |
| Other | 0 (0) | 2 (7.7) |
| Conditioning regimen [n (%)] | ||
| Melphalan 200 mg/m2 | 16 (66.7) | 19 (73.1) |
| beama | 5 (20.8) | 2 (7.7) |
| Busulphan–melphalan | 2 (8.3) | 5 (19.2) |
| Other | 1 (4.2) | 0 (0) |
Carmustine, etoposide, cytarabine, melphalan.
Both patient groups experienced significant weight loss over time (p = 0.0005). The intervention group experienced less weight loss during admission (mean: −0.4% ± 2.9% vs −3.4% ± 2.6%, p = 0.001). That difference was not sustained at 3 months (mean: −1.8% ± 4.8 vs. −0.9% ± 4.0%, p = 0.61) or 6 months (–1.0% ± 6.8% vs. 1.4% ± 6.1, p = 0.29) after discharge. Both groups showed a trend toward a decrease in muamc over time (p = 0.05) without a significant difference between the groups (Table ii). We observed no difference between the groups in time to hemopoietic recovery (11.3 days control vs. 11.7 days intervention, p = 0.40) or los (16 days control vs. 14.5 days intervention, p = 0.41). There was no difference in qol score between the groups from admission to 6 months after discharge (p = 0.61).
TABLE II.
Change in weight loss and mid-upper-arm muscle circumference (muamc)
| Time point | Compared with day 1 conditioning | |||||
|---|---|---|---|---|---|---|
| Mean weight change (%) | Mean muamc change (%) | |||||
|
|
|
|||||
| Control | Intervention | p Value | Control | Intervention | p Value | |
| Day 0 | −0.2±2.5 | 0.6±2.2 | 0.26 | −0.4±2.1 | 0.5±2.5 | 0.19 |
| Day +10 | 1.9±4.7 | 1.1±4.4 | 0.57 | −1.5±4.1 | −0.6±3.7 | 0.49 |
| At discharge | −3.4±2.6 | −0.4±2.9 | 0.001 | −2.3±3.9 | −1.3±3.2 | 0.40 |
| After discharge | ||||||
| 3 Months | −0.9±4.0 | −1.8±4.8 | 0.61 | −1.0±3.7 | −0.2±5.3 | 0.61 |
| 6 Months | 1.4±6.1 | −1.0±6.8 | 0.29 | 0.9±6.8 | −0.7±0.51 | 0.51 |
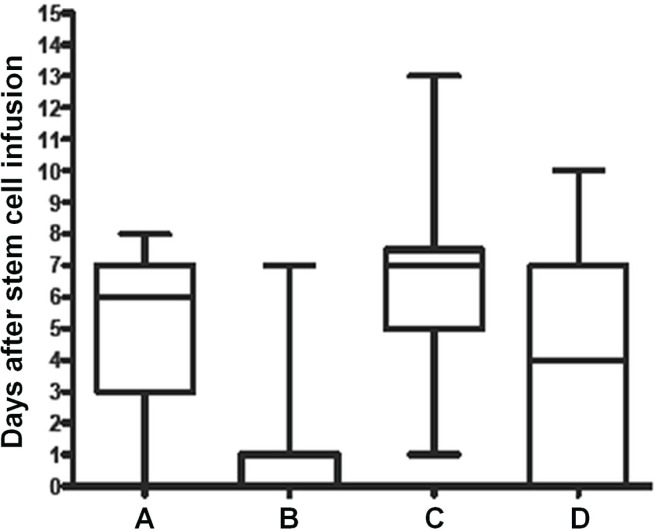
Of the 24 patients in the control group, 13 (54%) started nutrition support, with 9 (38%) receiving it parenterally. In the intervention group, 21 of the 26 patients (81%) started nutrition support, with 18 (69%) receiving it parenterally. Nutrition support was not indicated in 3 patients (13%) in the control group and in 1 patient (3.8%) in the intervention group because dietary intake remained above 50% or 80% of requirement respectively. However, despite fulfilling the study protocol definition of requiring nutrition support, 8 patients (33%) in the control group and in 4 patients (15%) in the intervention group never received nutrition support. Figure 2 illustrates the median time to commencement of nutrition support and shows the actual and planned timing per study protocol for both groups. It demonstrates that nutrition support was, on average, commenced earlier in the intervention group. The intervention group showed a trend toward a median longer duration of nutrition support (intervention: 5 days; range: 0–20 days; control: 2 days; range: 0–17 days; p = 0.09). The median difference in time to cessation of nutrition support was 1 day (control: 13 days; range: 8–37 days; intervention: 12 days; range: 2–22 days; p = 0.04).
FIGURE 2.
Planned and actual median time to commencement of nutrition support. (A) Actual day nutrition support started (intervention arm). (B) Day nutrition support was planned to start per the protocol (intervention arm). (C) Actual day nutrition support started (control arm). (D) Day nutrition support was planned to start per the protocol (control arm). Each box marks the middle 50% of the data. The horizontal line within each box represents the median, and the whiskers extend to the 5th and 95th percentiles.
4. DISCUSSION
Nutrition research in stem-cell transplantation has focused largely on three areas: the feasibility of enteral compared with parenteral nutrition8, predictors of mucositis4,7,11, and the role of glutamine8. In the present study, we investigated whether early provision of nutrition support, before a large deficit in oral intake emerged, improved clinical outcomes.
In our trial, both groups demonstrated significant weight loss over time, as expected and consistent with the findings of other studies12,14. However, patients in the intervention group maintained their weight during admission for asct, and members of the control group lost an average of 3.4% of body weight. The mean change of 0.4% in body weight in our intervention group is less weight loss than the 2% observed in the intervention group in the study by Roberts et al.12, but the change is difficult to compare to results from other studies because those studies used actual weight change in kilograms rather than percentage change15–17. The difference was not sustained after discharge: on average, all patients were close to their pre-transplantation body weight at 6 months after discharge. We observed a trend toward loss of lean body mass over time in both study groups, but no differences between the groups were observed. However, our results should be interpreted with caution in view of the difficulty in implementing the intervention.
We acknowledge several limitations of this study in view of the protocol deviations. In practice, starting nutrition support once oral intake had declined to less than 80% of nutritional requirements proved difficult. Although nutrition support began earlier for patients in the intervention group than in the control group, support was not actually instituted per the protocol-prescribed intervention. Observed reasons for the deviation included patient resistance, physician preference, and practical limitations. Although patients had consented to the study, once faced with the start of nutrition support at a time when oral intake was still relatively good (that is, less than 80% but more than 50% of nutritional requirements), they were resistant, particularly to insertion of a nasogastric tube. Physician preference was also a factor both for the timing and the type of nutrition support. It has been suggested that routine insertion of central lines in this patient group enables relatively easy delivery of parenteral nutrition and leads to resistance to enteral nutrition, which might be seen as more invasive8. In addition, the time between the planned intervention (nutrition support starting when intake had declined to less than 80% of requirements) and usual care (nutrition support starting when intake had declined to less than 50% of requirements) was often minimal, so that using this parameter to define time to the start of nutrition support was not always practical.
5. CONCLUSIONS
Compared with usual care, early nutrition support maintained weight during admission, but did not affect other outcomes. Well-nourished patients undergoing conditioning chemotherapy with a high risk of gastrointestinal toxicities should be monitored closely, and nutrition support should be commenced if the patient’s nutrition status is deteriorating or if oral intake is expected to be suboptimal for an extended period. These results may not be applicable to patients receiving allogeneic stem-cell transplantation, because of their higher risk of gastrointestinal toxicity and graft-versus-host disease. Future studies should investigate the effect of early nutrition support specifically in malnourished patients undergoing asct with high-nutritional-risk conditioning chemotherapy and should take into consideration the practical difficulties of early intervention.
6. ACKNOWLEDGMENTS
Thanks go to Deborah Cruickshank for data management and to Kally Yuen for statistical support; to Vivian Kong and Belinda Gleeson for data collection and patient management; and to the Peter MacCallum Cancer Centre Nutrition Department and Haematology Service for their support of the research. This study was undertaken with financial support from the Peter MacCallum Cancer Centre Haematology Service.
7. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts to disclose.
8. REFERENCES
- 1.Arends J, Bodoky G, Bozzetti F, et al. on behalf of espen (European Society for Parenteral and Enteral Nutrition) espen guidelines on enteral nutrition: non-surgical oncology. Clin Nutr. 2006;25:249–59. doi: 10.1016/j.clnu.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Bozzetti F, Arends J, Lundholm K, Mickelwright A, Zurcher G, Muscaritoli M, on behalf of espen espen guidelines on parenteral nutrition: non-surgical oncology. Clin Nutr. 2009;28:445–54. doi: 10.1016/j.clnu.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Sonis ST, Oster G, Fuchs H, et al. Oral mucositis and the clinical and economic outcomes of haematopoietic stem-cell transplantation. J Clin Oncol. 2001;19:2201–5. doi: 10.1200/JCO.2001.19.8.2201. [DOI] [PubMed] [Google Scholar]
- 4.Wardley AM, Jayson GC, Swindell R, et al. Prospective evaluation of oral mucositis in patients receiving myeloblative conditioning regimens and haemopoietic progenitor rescue. Br J Haematol. 2000;110:292–9. doi: 10.1046/j.1365-2141.2000.02202.x. [DOI] [PubMed] [Google Scholar]
- 5.Hung YC, Bauer J, Horsley P, Waterhouse M, Bashford J, Isenring E. Changes in nutritional status, body composition, quality of life, and physical activity levels of cancer patients undergoing autologous peripheral blood stem cell transplantation. Support Care Cancer. 2013;21:1579–86. doi: 10.1007/s00520-012-1698-y. [DOI] [PubMed] [Google Scholar]
- 6.Raynard B, Nitenberg G, Gory–Delabaere G, et al. Summary of the standards, options and recommendations for nutritional support in patients undergoing bone marrow transplantation (2002) Br J Cancer. 2003;89:S101–6. doi: 10.1038/sj.bjc.6601091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robien K, Schubert MM, Bruemmer B, Lloid ME, Potter JD, Ulrich CM. Predictors of oral mucositis in patients receiving hematopoietic cell transplants for chronic myelogenous leukemia. J Clin Oncol. 2004;22:1268–75. doi: 10.1200/JCO.2004.05.147. [DOI] [PubMed] [Google Scholar]
- 8.Murray SM, Pindoria S. Nutrition support for bone marrow transplant patients. Cochrane Database Syst Rev. 2009:CD002920. doi: 10.1002/14651858.CD002920. [DOI] [PubMed] [Google Scholar]
- 9.Guieze R, Lemal R, Cabrespine A, et al. Enteral versus parenteral nutritional support in allogeneic haematopoietic stem-cell transplantation. Clin Nutr. 2013 doi: 10.1016/j.clnu.2013.07.012. S0261–5614(13)00203–3. [DOI] [PubMed] [Google Scholar]
- 10.Iestra JA, Fibbe WE, Zwinderman AH, Romijn JA, Kromhout D. Parenteral nutrition following intensive cytotoxic therapy: an exploratory study on the need for parenteral nutrition after various treatment approaches for haematological malignancies. Bone Marrow Transplant. 1999;23:933–9. doi: 10.1038/sj.bmt.1701747. [DOI] [PubMed] [Google Scholar]
- 11.Rapoport AP, Miller Watelet LF, Linder T, et al. Analysis of factors that correlate with mucositis in recipients of autologous and allogeneic stem-cell transplants. J Clin Oncol. 1999;17:2446–53. doi: 10.1200/JCO.1999.17.8.2446. [DOI] [PubMed] [Google Scholar]
- 12.Roberts S, Miller J, Pineiro L, Jennings L. Total parenteral nutrition vs oral diet in autologous hematopoietic cell transplant recipients. Bone Marrow Transplant. 2003;32:715–21. doi: 10.1038/sj.bmt.1704204. [DOI] [PubMed] [Google Scholar]
- 13.Chang VT, Hwang SS, Feuerman M, Kasimis BS, Thaler HT. The Memorial Symptom Assessment Scale Short Form (msas-sf) Cancer. 2000;89:1162–71. doi: 10.1002/1097-0142(20000901)89:5<1162::AID-CNCR26>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Schulte C, Reinhardt W, Beelen D, Mann K, Schaefer U. Low T3-syndrome and nutritional status as prognostic factors in patients undergoing bone marrow transplantation. Bone Marrow Transplant. 1998;22:1171–8. doi: 10.1038/sj.bmt.1701502. [DOI] [PubMed] [Google Scholar]
- 15.Weisdorf SA, Lysne J, Wind D, et al. Positive effect of prophylactic total parenteral nutrition on long-term outcome of bone marrow transplantation. Transplantation. 1987;43:833–8. doi: 10.1097/00007890-198743060-00012. [DOI] [PubMed] [Google Scholar]
- 16.Skop A, Kolarzyk E, Skotnicki AB. Importance of parenteral nutrition in patients undergoing hemapoietic stem cell transplantation procedures in the autologous system. JPEN J Parenter Enteral Nutr. 2005;29:241–7. doi: 10.1177/0148607105029004241. [DOI] [PubMed] [Google Scholar]
- 17.Mousavi M, Hayatshahi A, Sarayani A, et al. Impact of clinical pharmacist-based parenteral nutrition service for bone marrow transplantation patients: a randomized clinical trial. Support Care Cancer. 2013;21:3441–8. doi: 10.1007/s00520-013-1920-6. [DOI] [PubMed] [Google Scholar]