Abstract
Because of common risk factors, synchronous squamous cell carcinomas of the esophagus and head and neck are common, and their concurrent presence can significantly complicate disease eradication and survival. Here, we report the case of a patient with a history of extensive tobacco and alcohol use who was diagnosed with a localized thoracic esophageal squamous cell carcinoma, and in whom positron-emission tomography–computed tomography discovered a nearby asymptomatic localized hypopharyngeal focus that was confirmed by biopsy to also be malignant. He was treated with definitive concurrent chemoradiotherapy in a single unified radiotherapy plan, with surgery reserved for salvage treatment. He currently remains in remission without a need for surgical salvage. However, significant concern remains for both treatment failure and development of another primary because of “field cancerization.”
Keywords: Synchronous squamous cell carcinomas, esophageal cancer, hypopharyngeal cancer, chemotherapy, radiotherapy, field cancerization
1. CASE DESCRIPTION
A 55-year-old man with a 40 pack–year smoking history, heavy alcohol use, and active hemorrhoids presented to the emergency department with progressive dysphagia and odynophagia for 2 months, an unintentional 30-pound weight loss over 12 months, and progressive generalized weakness. Physical exam revealed a frail-appearing man (body mass index: 19.3) in no acute distress, with normal bowel sounds and phonation.
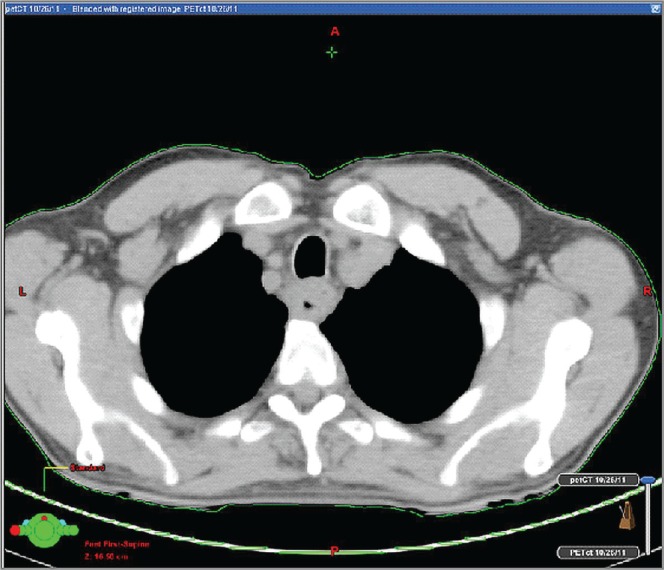
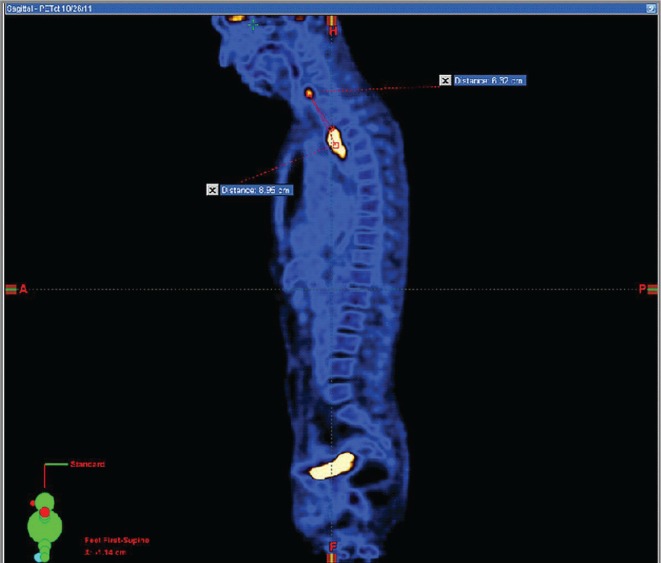
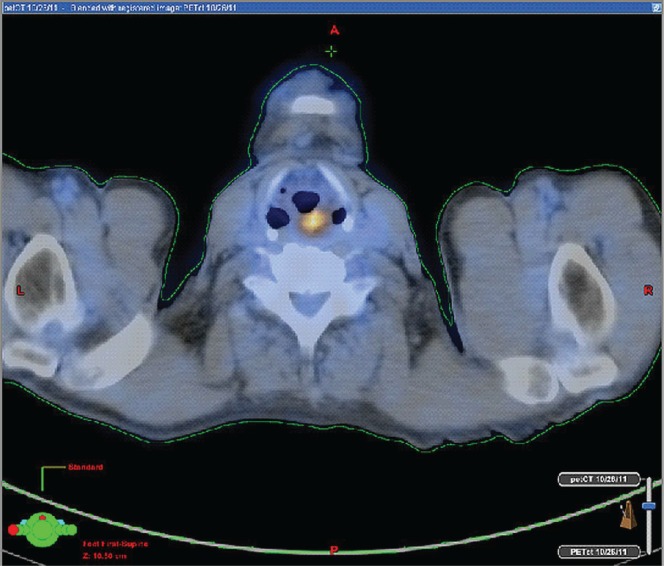
He underwent thoracic computed tomography (ct) imaging (Figure 1), which revealed an 8-cm thoracic esophageal mural thickening from the thoracic inlet to the aortic arch, without lymphadenopathy. Abdominal and pelvic ct imaging was negative, but positron-emission tomography (pet)–ct (Figures 2 and 3) revealed 3 hypermetabolic foci in the hypopharynx, thoracic esophagus, and anus.
FIGURE 1.
Computed tomography of the thorax reveals abnormal thoracic esophageal thickening.
FIGURE 2.
Sagittal-view positron-emission tomography–computed tomography demonstrates three abnormal foci in the hypopharynx, thoracic esophagus, and anus, with the centres of the first two foci about 9 cm apart.
FIGURE 3.
Axial-view positron-emission tomography–computed tomography demonstrates the hypermetabolic hypopharyngeal mass.
An esophagoduodenogastroscopy performed two days later demonstrated that the mass began at 22 cm from the incisors. Biopsy later revealed moderately differentiated squamous cell carcinoma (scc). By esophagoduodenogastroscopy (no endoscopic ultrasonography was performed), the gastroenterologist estimated the lesion to be T2–3; the confirmed esophageal malignancy was therefore T2–3N0M0, stage iib. A hypopharyngeal biopsy by Otolaryngology revealed scc, and surgical evaluation and imaging revealing the mass to be T2N0M0, stage ii.
Pathology was not able to differentiate the two biopsy specimens either morphologically or by immunohistochemical staining, but the clinical picture was far more consistent with two separate primary malignancies rather than either being a metastatic focus. With respect to the third hypermetabolic site, the patient refused anoscopy, but a thorough digital anal examination by three independent physicians revealed no masses, nodularity, or other suspicious findings.
An interdisciplinary decision was made with the patient to place a percutaneous endoscopic gastrotomy for nutritional prophylaxis, and then to proceed to definitive concurrent chemoradiotherapy to the thoracic esophagus and hypopharynx, with laryngopharyngoesophagectomy reserved for salvage treatment.
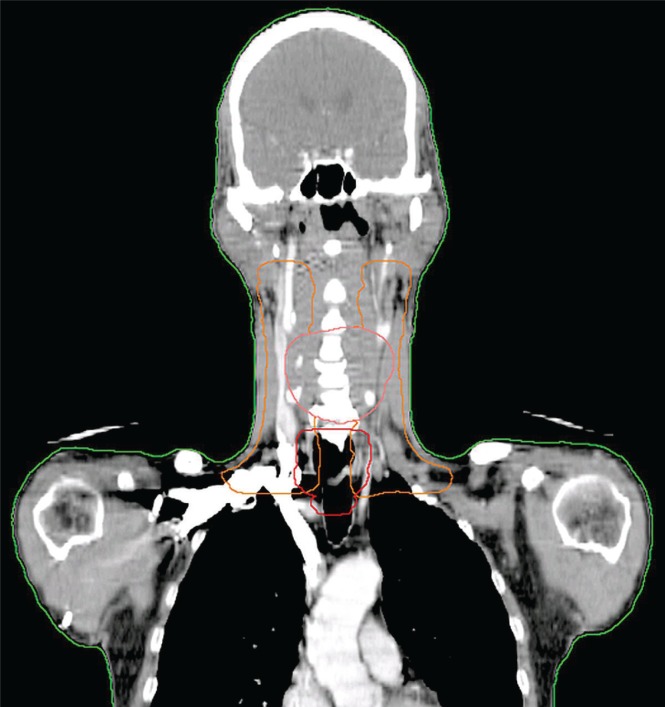
Chemotherapy consisted of weekly cisplatin 25 mg/m2 and docetaxel 25 mg/m2 1 day per week for 8 cycles. Radiotherapy included 50 Gy intensity-modulated radiation therapy with a simultaneously integrated boost to the esophageal mass, 54.1 Gy intensity-modulated radiation therapy with a simultaneously integrated boost to the bilateral cervical lymph nodes levels ii–v, plus a boost to 70 Gy to the hypopharyngeal mass (Figure 4).
FIGURE 4.
Coronal reconstruction image of the planning target volume (ptv) 50 (red), ptv 54.1 (orange), and ptv 70 (pink) demonstrates the overlap of the hypopharyngeal and esophageal treatment targets.
The patient quit smoking and alcohol before the start of chemoradiotherapy. Midway through treatment, he was hospitalized because of weight loss and hypotension, necessitating rehydration, nutritional supplementation, and a 6-day treatment break; ultimately, he lost approximately 10% of his weight over the treatment course. He also developed grade 2 (Radiation Therapy Oncology Group toxicity scale) dermatitis and nausea without vomiting, plus grade 1 esophagitis, dysphagia, xerostomia, hoarseness, and mucositis. He was treated with liquid morphine, silver sulfadiazine, and Aquaphor (Beiersdorf Canada, Saint-Laurent, QC).
The patient has been evaluated multiple times in follow-up since completing treatment and underwent surveillance imaging (pet–ct and diagnostic ct) at 2, 5, 12, and 15 months. At his 2-month follow-up, the patient said that his side effects were significantly improving and that he was bothered mostly by dysphagia, although he soon thereafter had the percutaneous endoscopic gastrotomy removed and proceeded to weekly esophageal dilatations. However, the pet–ct imaging revealed mildly increased metabolic activity (standardized uptake value: 2.5–5.3) in the posterior cricoid region, for which the ct had no soft-tissue correlate. On flexible laryngoscopy at 5 and 10 months, no recurrent disease was evident, and the increased activity was deemed to be attributable to his esophageal dilatations.
The pet–ct at 15 months revealed that the posterior larynx hypermetabolism had increased to a standardized uptake value of 5.7, without any soft-tissue correlate. The patient presented to Otolaryngology several days later, with flexible laryngoscopy revealing no evidence of recurrence. The patient presented to Otolaryngology again at 18 months, and flexible laryngoscopy revealed no evidence of disease. Therefore, at the time of writing, he remained in remission, with only grade 1 dysphagia.
2. DISCUSSION AND CONCLUSIONS
Approximately 16,980 people in the United States were diagnosed with esophageal cancer in 2011, with a 5-year survival rate of only 16.8%1. This malignancy carries a very poor prognosis mainly because of late presentation, only 22% of cases being localized when found1. Reasons for early spread include the rich lymphatic network of the esophagus and its lack of a serosal covering; moreover, its expansile nature often requires circumferential tumour involvement or significant luminal penetration to cause pain. When the malignancy is discovered in its localized state, the 5-year survival rate is more than 90%2; however, that encouraging statistic might be influenced by lead-time bias. In North America and western Europe, alcohol and tobacco are the major risk factors for esophageal scc, accounting for 80%–90% of cases, with high synchronicity with head-and-neck cancers3–5.
About 2500–3000 cases of hypopharyngeal cancer are diagnosed annually in the United States, with a 5-year survival rate of only 30%–33%6,7. Interestingly, according to the Surveillance Epidemiology and End Results database, the percentage of cases diagnosed in a localized stage decreased to 9.3% from 23.0% during 1974–1999; distant-stage presentation decreased to 18.6% from 25.7%7. Compared with the dismal overall 5-year survival rate of one third for hypopharyngeal cancer, localized disease has a survival rate of 56.0%7. In addition to human papilloma virus exposure, smoking is very strongly associated with the development of hypopharyngeal cancer, and although alcohol alone has a minimal effect or no effect on development of the malignancy, it can potentiate the effects of tobacco8.
Because of the risk factors commonly associated with the development of head-and-neck and esophageal scc, it is common to see synchronous and metachronous tumours develop in this region, and the concept of “field cancerization” has become widely accepted. Estimates suggest that, among head-and-neck cancer patients diagnosed with a first primary tumour, about 2.4% have synchronous second malignancies9. Second malignancies in the hypopharynx and esophagus appear to have a very strong correlation. A Japanese study reported 104 patients with hypopharyngeal cancer who were simultaneously examined for esophageal cancer in both the pre- and postoperative periods10. At a mean follow-up of 6.7 years, 7 patients had synchronous esophageal cancer (diagnosed at the same time or within 6 months), 4 patients had metachronous esophageal cancer, and 1 patient had metachronous hypopharyngeal cancer. Notably, 40 patients died of their primary cancer within 3 years without a second cancer.
Head-and-neck plus esophageal scc survivors carry an elevated risk of second primary malignancies to the head and neck, esophagus, and lungs, and when deliberate efforts are made to screen at-risk patients, secondary malignancies can be treated in the early and potentially curable stages11.
Our patient had two synchronous malignancies that usually have poor prognoses (in large part because they remain occult until more advanced at symptomatic presentation). With increasing adoption of pet–ct for head-and-neck and esophageal cancer workup, discovery of the hypopharyngeal focus (second primary cancer) was possible at an early stage in the absence of any symptoms from the site. In this case, both tumours were detected at a localized stage, giving the patient a far better potential for survival. At 18 months, he remained in remission.
Among the various published reports of synchronous esophageal and hypopharyngeal sccs, all known treatments involved surgery. Because of the proximity of our patient’s lesions (6.3 cm at the closest distance), an interdisciplinary decision was made with the patient to proceed with definitive concurrent chemoradiotherapy in a single unified radiotherapy treatment plan, with surgery reserved for salvage. This case therefore represents the first known treatment of synchronous esophageal and hypopharyngeal scc entirely with chemoradiation, enabling organ preservation. Although our patient’s plan included a very long treatment field (about 25 cm), he tolerated the treatment to its completion with percutaneous endoscopic gastrotomy insertion and one treatment break, and he experienced only mild long-term toxicity. However, this option may not be feasible for many head-and-neck or esophageal synchronous tumours, because in the present case, the separation of the two primaries was relatively small, ensuring contiguity of the radiation fields and thus lesser irradiation of normal tissues. Also, the patient quit smoking, which if maintained, would have greatly enhanced the toxicity12.
The chemotherapy regimen used cisplatin as the backbone for the treatment, because cisplatin has a proven survival advantage when administered with 5-fluorouracil or paclitaxel for esophageal cancer chemoradiation and as a mono-agent for head-and-neck chemoradiation13–15. Cisplatin–taxane combination therapy was chosen based on the recent cross trial and patient preference to avoid a venous access catheter (which would be needed with 5-fluorouracil) 14; docetaxel was substituted for paclitaxel because of the more extensive data and experience with the former taxane in treating head-and-neck cancers.
This case study should re-enforce the scc field cancerization that tobacco and alcohol carcinogens can regionally produce, such that patients with a history of scc of the esophagus or of the head and neck should also be considered for surveillance of the other site through pan-endoscopy. By extension, patients who already have synchronous tumours are at risk for additional (metachronous) sccs within the cancerous field. Furthermore, the area at risk because of tobacco also includes the lungs, because lung cancers in both men and women are overwhelmingly caused by smoking.
Our patient might be a long-term survivor because of the early stage of each primary malignancy, but he remains at very high risk for both recurrence and metachronous malignancies within the cancerous field, and so we have tailored special surveillance for his follow-up appointments (including maintenance of smoking cessation, routine panendoscopies, and routine pet–ct) to examine not only for esophageal and hypopharyngeal treatment failure, but also for new head-and-neck, esophageal, and lung primary tumours.
3. CONFLICT OF INTEREST DISCLOSURES
The authors declare no conflicts of interest, and no grants or other financial support were provided.
4. REFERENCES
- 1.United States National Institutes of Health National Cancer Institute(nci), Surveillance Epidemiology and End Results Program . SEER Stat Fact Sheets: Esophageal Cancer [Web page] Bethesda, MD: NCI; 2011. [Current version available at: http://seer.cancer.gov/statfacts/html/esoph.html; cited February 10, 2014] [Google Scholar]
- 2.Wang VS, Hornick JL, Sepulveda JA, Mauer R, Poneros JM. Low prevalence of submucosal invasive carcinoma at esophagectomy for high-grade dysplasia or intramucosal adenocarcinoma in Barrett’s esophagus: a 20-year experience. Gastrointest Endosc. 2009;6:777–83. doi: 10.1016/j.gie.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Schottenfield D. Epidemiology of cancer of the esophagus. Semin Oncol. 1984;11:92–100. [PubMed] [Google Scholar]
- 4.Wang WL, Lee CT, Lee YC, et al. Risk factors for developing synchronous esophageal neoplasia in patients with head and neck cancer. Head Neck. 2011;33:77–81. doi: 10.1002/hed.21397. [DOI] [PubMed] [Google Scholar]
- 5.Fukuzawa K, Noguchi Y, Yoshikawa T, et al. High incidence of synchronous cancer of the oral cavity and the upper gastrointestinal tract. Cancer Lett. 1999;144:145–51. doi: 10.1016/S0304-3835(99)00223-2. [DOI] [PubMed] [Google Scholar]
- 6.Ries LAG, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Review, 1975–2002. Bethesda, MD: National Cancer Institute; 2005. [Google Scholar]
- 7.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the seer database. Int J Cancer. 2005;114:806–16. doi: 10.1002/ijc.20740. [DOI] [PubMed] [Google Scholar]
- 8.Anantharaman D, Marron M, Lagiou P, et al. Population attributable risk of tobacco and alcohol for upper aerodigestive tract cancer. Oral Oncology. 2011;47:725–31. doi: 10.1016/j.oraloncology.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Rennemo E, Zätterström U, Boysen M. Synchronous second primary tumors in 2,016 head and neck cancer patients: role of symptom-directed panendoscopy. Laryngoscope. 2011;121:304–9. doi: 10.1002/lary.21385. [DOI] [PubMed] [Google Scholar]
- 10.Kohmura T, Hasegawa Y, Matsuura H, Terada A, Takahashi M, Nakashima T. Clinical analysis of multiple primary malignancies of the hypopharynx and esophagus. Am J Otolaryngol. 2001;22:107–10. doi: 10.1053/ajot.2001.22566. [DOI] [PubMed] [Google Scholar]
- 11.Jéqu J, Binder–Foucard F, Borel C, Velten M. Trends over three decades of the risk of second primary cancer among patients with head and neck cancer. Oral Oncol. 2013;49:9–14. doi: 10.1016/j.oraloncology.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Chen AM, Chen LM, Vaughan A, et al. Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys. 2011;79:414–19. doi: 10.1016/j.ijrobp.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (rtog 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 14.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 15.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]