Abstract
Background
Islet transplantation is an alternative to pancreas transplantation to cure type 1 diabetes, but both require chronic immunosuppression, which is often accompanied by deleterious side effects. The purpose of this study was to explore prolongation of islet allograft survival by co-transplantation with myeloid-derived suppressor cells (MDSCs) without requirement of immunosuppression, and determine the role of inducible nitric oxide synthase (iNOS) produced by MDSCs in immune regulation.
Methods
Bone marrow cells were isolated from wild type (WT) or iNOS−/− mice and cultured in the presence of GM-CSF and hepatic stellate cells (HSCs), resulting in the generation of MDSCs. WT or iNOS−/− MDSCs were co-transplanted with islet allografts under the renal capsule of diabetic recipient mice.
Results
Addition of HSCs into DC culture promoted generation of MDSCs (instead of DCs). MDSCs had elevated expression of iNOS upon exposure to IFN-γ and inhibited T cell responses in an MLR culture. Co-transplantation with WT MDSCs markedly prolonged survival of islet allografts, which was associated with reduced infiltration of CD8+ T cells due to inhibited proliferative response. These effects were significantly attenuated when MDSCs were deficient in iNOS. Furthermore, iNOS−/− MDSCs largely lost their ability to protect islet allografts.
Conclusions
Co-transplantation with HSC-induced MDSCs significantly extends islet allograft survival through iNOS-mediated T cell inhibition. The results demonstrate the potential use of in vitro generated MDSCs as a novel adjunctive immunotherapy for islet transplantation.
Keywords: Immune regulation, T cell response, islet transplantation
Introduction
The loss of insulin-producing pancreatic β cells leads to the onset of type 1 diabetes. Currently, administration of exogenous insulin remains the conventional form of therapy, which is difficult to completely replace all aspects of β cell function. Presently, the cure for the loss of islet tissue can only be accomplished by the transplantation of either the whole pancreas or isolated pancreatic islets. Both require chronic immunosuppression, which is accompanied by deleterious side effects. Islet transplantation with an optimized protocol of immunosuppression stored β cell function (1,2). However, a large multicenter trial disappointingly revealed that the gain were short term (3). We have noted that outcomes of organ transplantation are superior to somatic cell transplantation. This is the case in animal models – liver transplants are often spontaneously accepted in mice (4–6), but survival of allogeneic hepatocyte transplants is limited (~10 days), obviously due to due to immunological rejection since syngeneic hepatocyte transplants can achieve long-term survival (7), suggesting that liver non-parenchymal cells (NPC) are capable of protecting the parenchymal cells from immune attack. We have tested various liver NPC and found that hepatic stellate cells (HSCs), an abundant population of liver tissue stromal cells, have very potent immunosuppressive activity (8). HSCs mixed with islet allografts and transplanted under the renal capsule effectively protect islet grafts from rejection, without requirement of immunosuppression (8). The use of HSCs for islet transplantation holds great potential for clinical application. However, one of the major challenges associated with HSCs is that they have to be patient derived. HSCs from allogeneic or third party sources cannot protect allografts as well (9). Isolation of HSCs from patients is an invasive procedure and poses risks to the patient.
Myeloid derived-suppressor cells (MDSCs), a heterogeneous population of myeloid cells, were initially identified in tumor settings and demonstrate remarkable immunosuppressive activity. Their functional importance in immune regulation makes them attractive candidates for use in therapy of transplantation and autoimmune diseases (10,11). Thus far, efforts to develop MDSC-based therapeutic strategies have been limited by the lack of reliable methods to generate large amounts of MDSCs (12). We have shown that HSCs are potent inducers of MDSCs. MDSCs can be generated in vitro by addition of small numbers of HSCs (either MHC matched or mismatched) into dendritic cell (DC) cultures (13), which is mediated by soluble factors produced by HSCs (14–16). Islet allografts that were co-transplanted with HSC-induced MDSCs were protected as effectively as those co-transplanted with HSCs, although the number of MDSCs that was required was 10 times greater (15). MDSCs produce key immune suppressive factors, including arginase 1 (Arg-1), inducible nitric oxide synthase (iNOS), and reactive oxygen species (17). In this study, we investigated the underlying mechanism and demonstrated that protection of islet allografts, by co-transplanted MDSCs, is dependent on iNOS mediated T cell inhibition.
Results
HSC-induced MDSCs demonstrate immune inhibitory activity in vitro
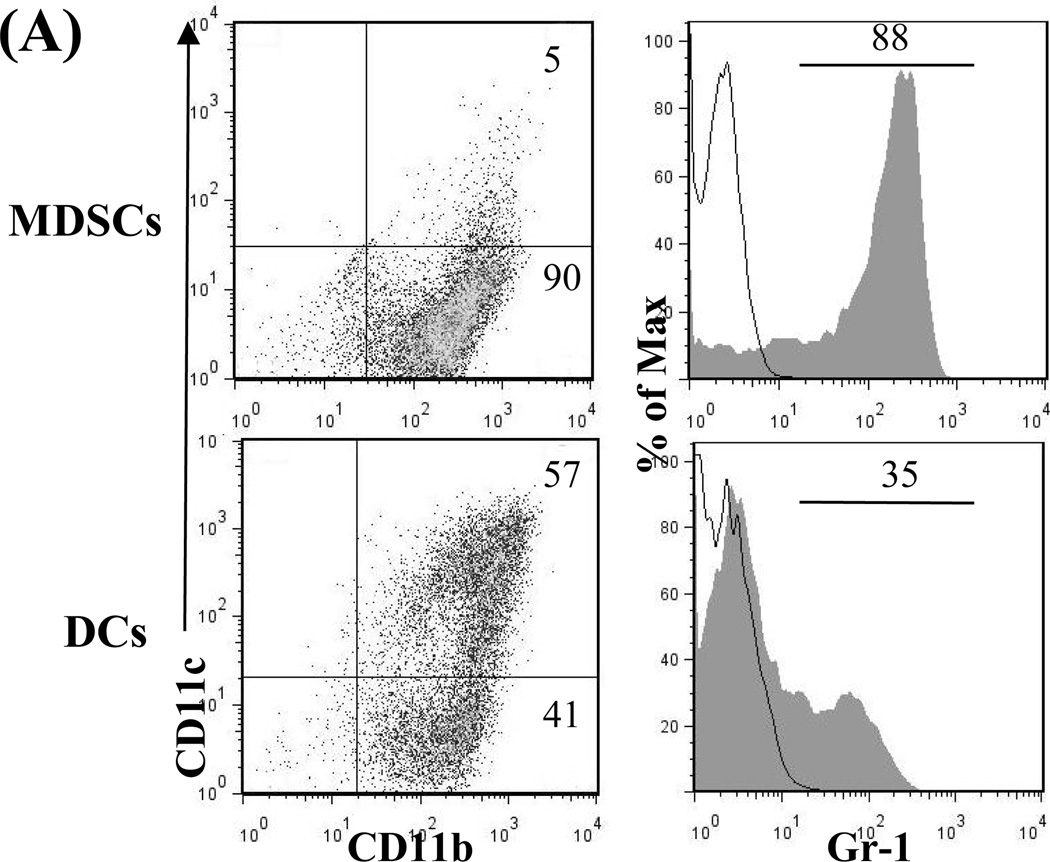
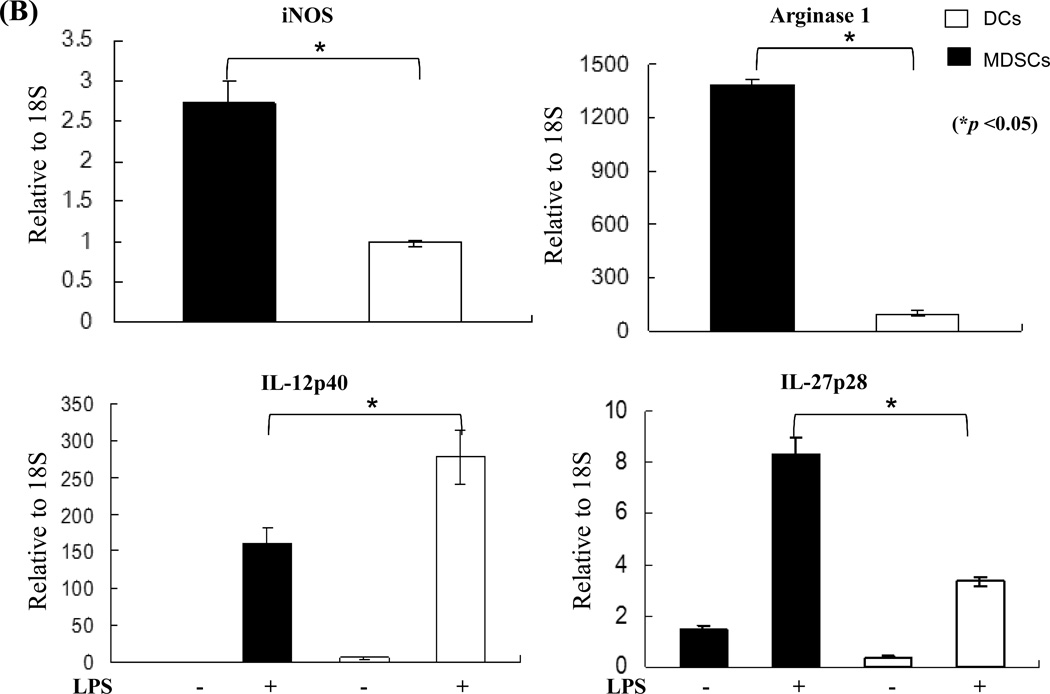
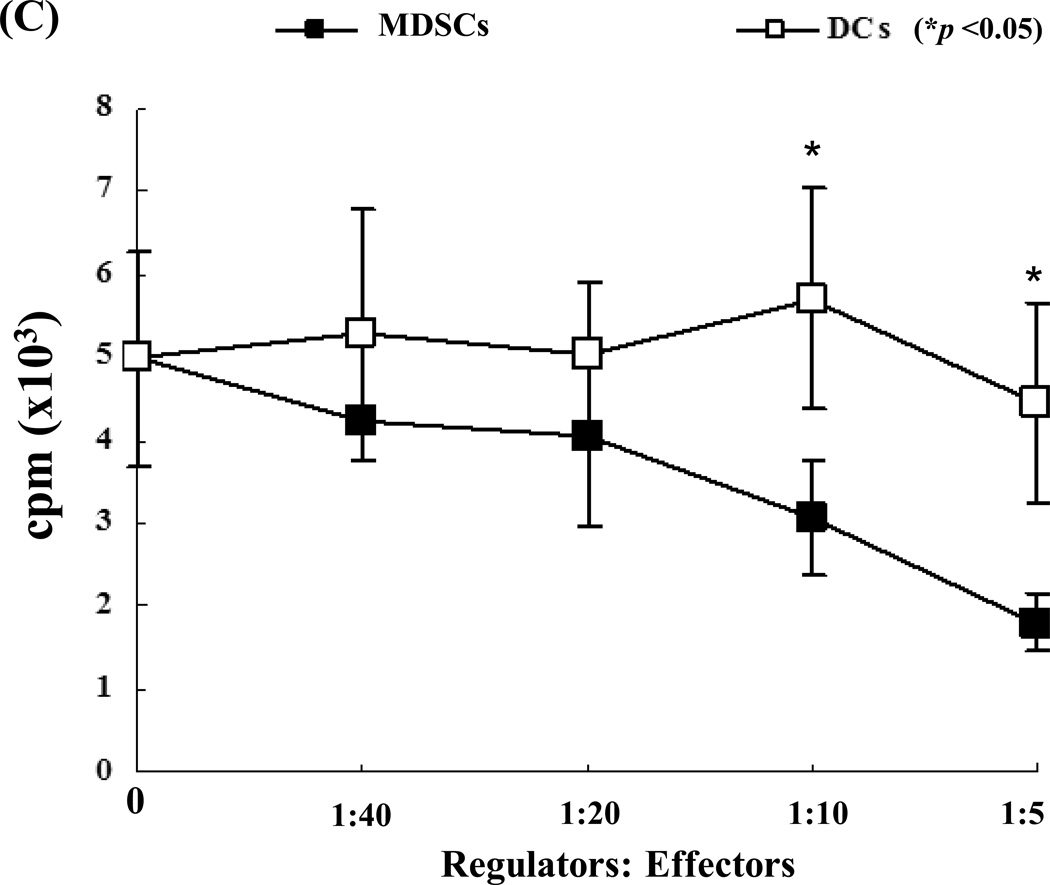
MDSCs were generated in vitro by adding B6 HSCs into a B6 bone marrow (BM) cell culture in the presence of GM-CSF and IL-4. As previously demonstrated (15), the addition of HSCs markedly inhibited generation of DCs, but promoted propagation of MDSCs. Thus, the percentage of CD11c+ cells declined from 57% (without HSC control) to 5% (with the addition of HSCs). Moreover, the percentage of CD11b+CD11c− cells increased from 41% in DC group to 90% (Fig. 1A, left panels) in the MDSC group. The phenotype the MDSCs generated by addition of HSCs into DC culture has been previously described (15). In this study, we showed that HSC-conditioned myeloid (CD11b+) cells contained markedly more Gr-1+ cells (Fig. 1A, right panels). Expression of Gr-1 has been used as a marker for MDSCs in mice (17). Furthermore, myeloid cells generated in the presence of HSCs had elevated levels of iNOS and Arg-1 mRNA, high IL-27 (p28), but low bioactive IL-12 (p40) with lipopolysaccharides (LPS) stimulation (Fig. 1B). Addition of HSC-induced MDSCs into a mixed lymphocyte reaction (MLR) culture significantly suppressed T cell proliferative response (Fig. 1C).
Figure 1. A. Quality monitoring of MDSCs used in this study.
HSCs from B6 mouse were added at the beginning of DC (B6) culture as described in Methods for 5 days. The cells cultured in the absence of HSCs were used as DCs. For further stimulation, cells were exposed to LPS at 1µg/ml for the last 18 hours of culture. A) HSC-induced MDSCs express low CD11c, high Gr-1. The floating cells harvested from the culture of MDSCs were analyzed by flow cytometry for expression of CD11b and CD11c. The percentage of positive cells in whole cell population (left panels) is indicated. Expression of Gr-1 is displayed as histograms gated on the CD11b+ cell populations. Empty areas are isotype controls. The percentage of Gr-1 positive cells in the CD11b+ cell population (right panels) is indicated. B) MDSCs express high levels of iNOS, Arg-1 and IL-27, but low IL-12. CD11b+ cells were purified by positive sorting with magnetic beads for the functional studies. The mRNA expression was determined by qPCR. C) MDSCs inhibit T cell proliferative response. Graded number of irradiated MDSCs or DCs were added at beginning into a one-way MLR culture in which B6 spleen T cells were stimulated by irradiated BALB/c DCs (T:DC = 20:1) for 3 days (n = 3). T cells proliferation was assessed by thymidine uptake.
Co-transplantation with HSC-induced MDSCs effectively protects islet allografts from rejection
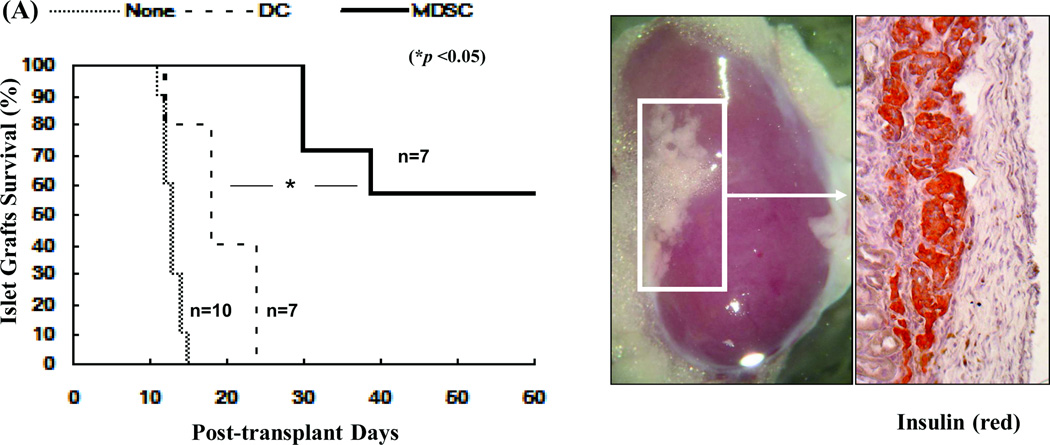
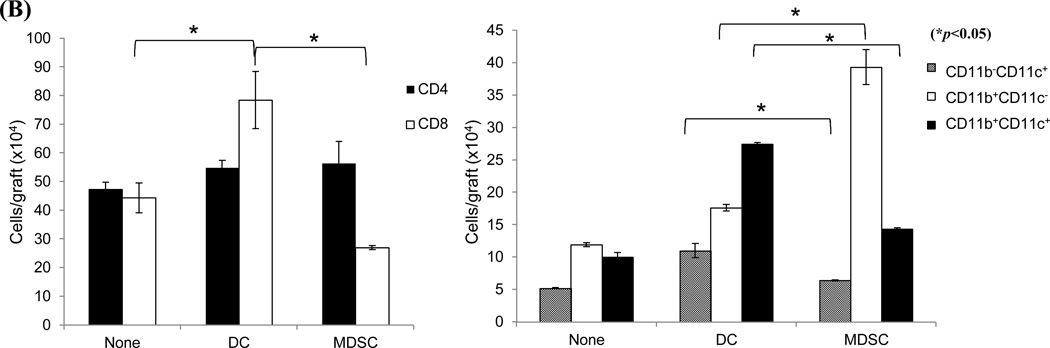
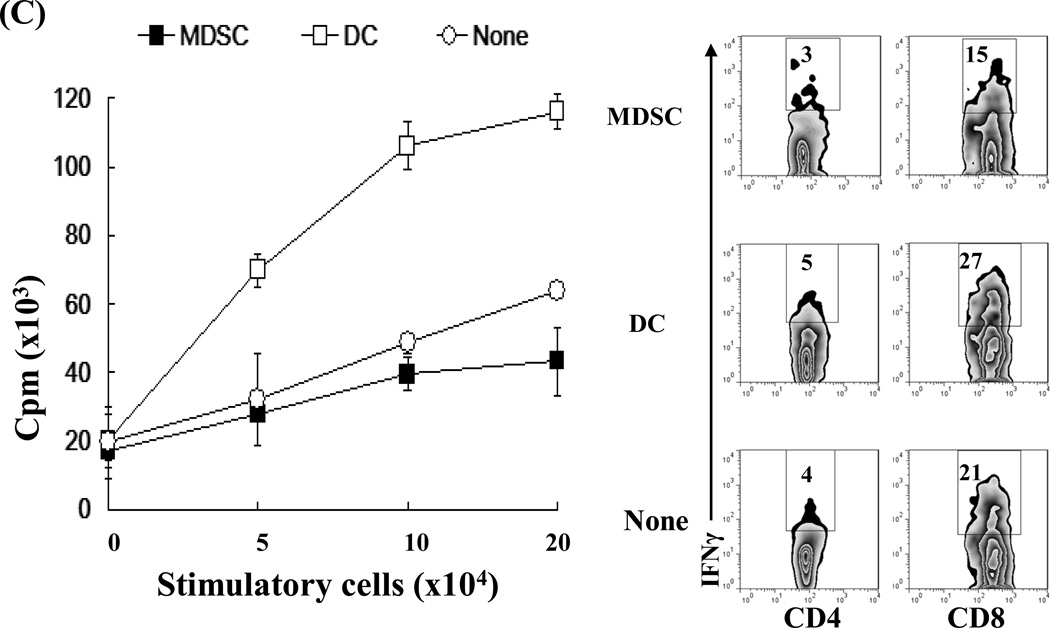
The in vivo immunoregulatory activity of the MDSCs was tested in an islet allograft transplantation model. MDSCs (2×106) were mixed with 300 BALB/c islets and transplanted under the renal capsule of diabetic recipients (B6). Survival of the islet grafts was monitored by the non-fasting blood glucose levels. ~55% of islet grafts in the MDSC-treatment group survived >60 days. None of the islet grafts in the control group (no-treatment) or DC co-transplantation group survived more than 25 days (Fig. 2A, left panel). The kidneys from recipients bearing long-term surviving islet grafts (>60 days) were removed and stained for insulin to confirm the presence of islet grafts (Fig. 2A, right panel). To clarify the mechanisms associated with improved graft survival with MDSC co-transplantation, recipients were sacrificed on post-operative day (POD) 10. The leukocytes isolated from islet allografts were stained with anti-CD4, -CD8, -CD11b and –CD11c mAbs and analyzed by flow cytometry. MDSC co-transplantation was associated with reduced infiltration of CD8+ T cells (Fig. 2B, left panel). As expected, islet/MDSC grafts contained significantly more CD11b+CD11c− cells (15), while islet/DC grafts exhibited an accumulation of CD11c+ cells (including both mature (CD11b−CD11c+) and immature DC (CD11b+CD11c+) (Fig. 2B, right panel). To address the effect of MDSC co-transplantation on T cell response, T cells were isolated from the spleen of recipients and restimulated by graded numbers of irradiated BALB/c (donor) splenocytes in vitro. While T cells from the DC-treated group elicited a strong proliferative response and had a high frequency of interferon (IFN)-γ producing CD8+ T cells, those isolated from MDSC-treated recipients generated a significantly lower proliferative response (Fig. 2C, left panel) and had fewer IFN -γ producing CD8+ T cells (Fig. 2C, right panels), suggesting induction of T cell hyporesponsiveness. Taken together, these results suggest that MDSC co-transplantation effectively protects islet allograft via inhibition of T cell response.
Figure 2. Co-transplantation with MDSCs prolongs survival of islet allografts.
2 × 106 MDSCs or DCs were propagated from B6 BM cells in the presence or absence of HSCs, mixed with BALB/c islets (300), and transplanted under the renal capsule of B6 diabetic recipients. Recipients without co-transplantation (none) were used as the control. A) Co-transplantation with MDSCs markedly prolongs survival of islet allografts (left panel). Kidneys bearing long-term surviving islet grafts (>60 days) were removed for visual examination and insulin staining to determine the function of the islet graft. The pictures in the right panels are nephrectomy specimens at day 60. B) MDSC co-transplantation inhibits infiltration of CD8+ T cells in islet graft. Leukocytes isolated from the islet graft on POD 10 were stained for CD4, CD8, CD11b and CD11c, and analyzed by flow cytometry. The absolute numbers of CD4, CD8, CD11b+CD11c−, CD11b+CD11c+, CD11b−CD11c+ cells in each graft was calculated based on the flow analysis data (n = 3). C) Co-transplantation with MDSCs is associated with T cell hyporesponsiveness. T cells isolated from spleen of the recipients were restimulated in vitro by irradiated BALB/c splenocytes at a ratio of 1:1 for 3 days. T cell proliferative response was assessed by thymidine uptake (left panel, n = 3). Production of IFN-γ was determined by intracellular staining and expressed as percentage of IFN-γ positive cells in CD4+ and CD8+ T cell populations (right panels). The data are representative of three separate experiments.
T cell inhibition by MDSC co-transplantation is mediated by iNOS
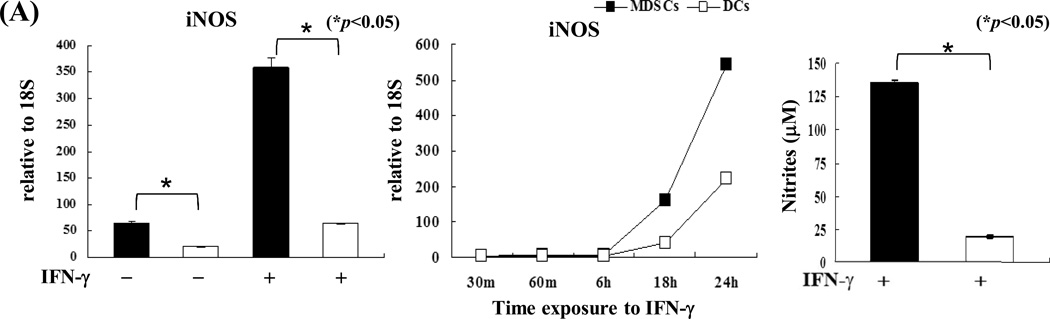
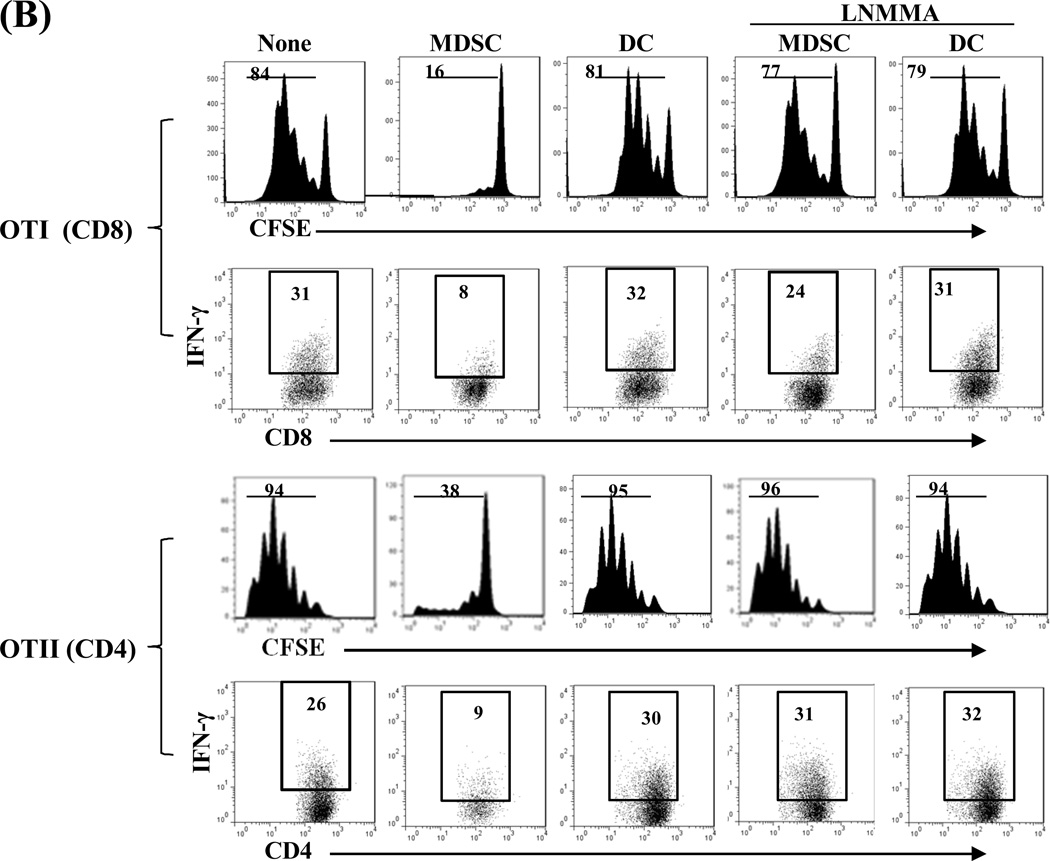
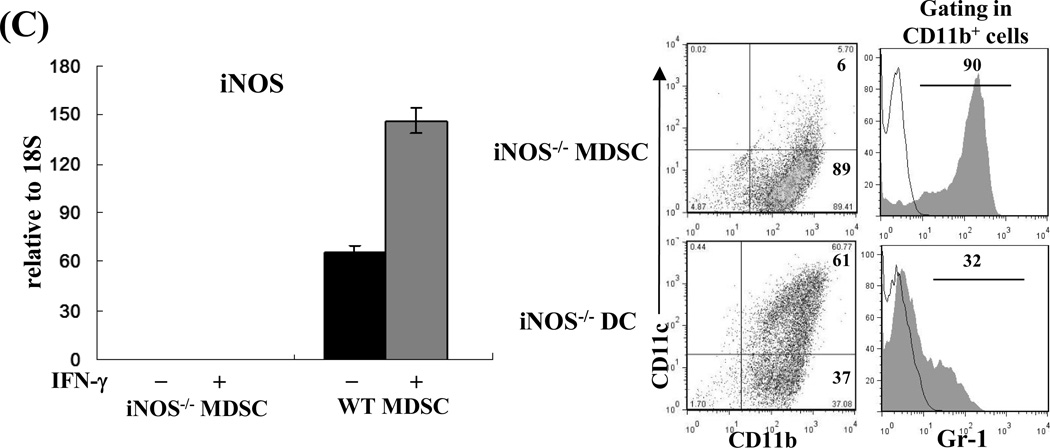
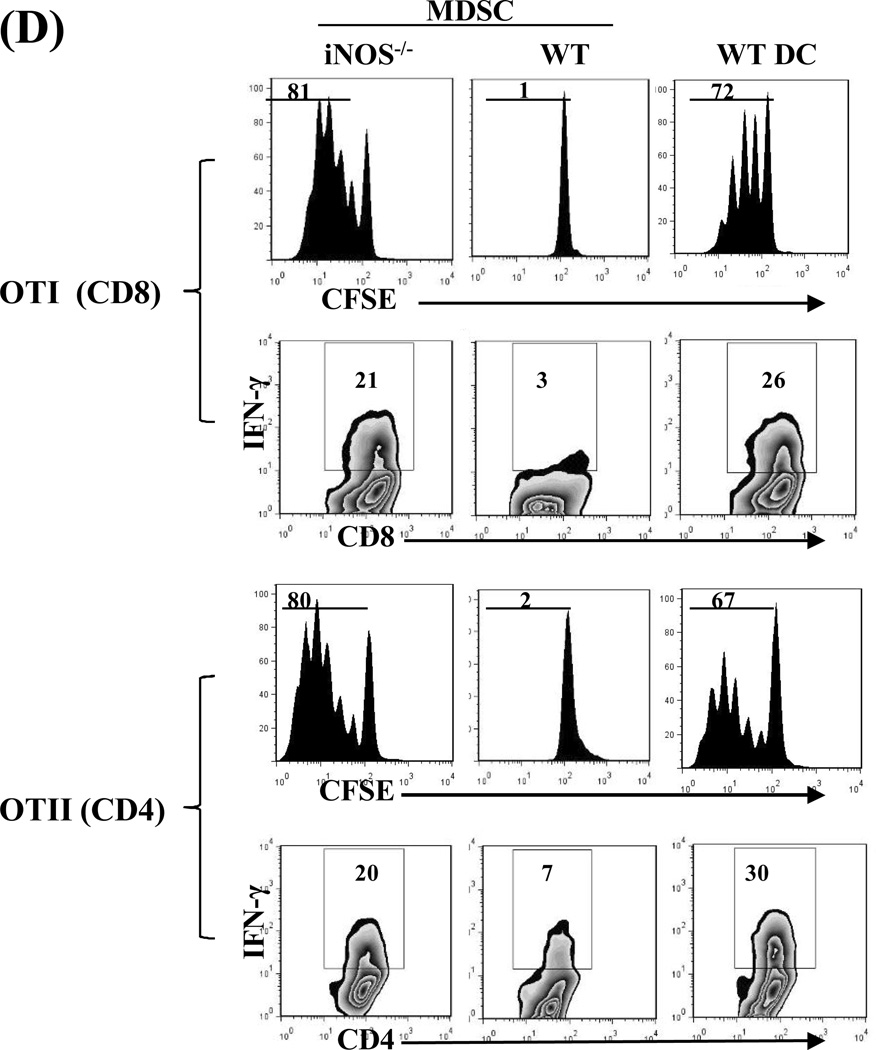
Compared to DCs, MDSCs had higher expression of iNOS, which was markedly enhanced following exposure to IFN-γ (Fig. 3A, left panel) in a time-dependent manner (Fig. 3A middle panel) and was associated with an increase in nitrite production (Fig. 3A, right panel). We tested the role of iNOS in the inhibitory activity of MDSCs in an experimental system in which the chicken ovalbumin (OVA)-specific T cells isolated from TCR transgenic mice OT-I (CD8) and OT-II (CD4) were stimulated by OVA plus DCs (as APCs). Addition of MDSCs markedly inhibited the proliferative response and production of IFN-γ in both CD4 and CD8 OVA-specific T cells (Fig. 3B). Addition of the iNOS inhibitor L-NMMA (10 µM) into the co-cultures reduced the suppressive capacity of MDSCs (Fig. 3B), providing in vitro evidence that blocking iNOS activity impedes the ability of MDSCs to inhibit T cells. This was re-examined using iNOS−/− mice. MDSCs propagated from iNOS−/− mice did not express iNOS mRNA prior to and following exposure to IFN-γ (Fig. 3C). Deficiency in iNOS appeared not to affect the expression of the key molecules on MDSCs, including CD11b, CD11c and Gr-1 (Fig. 3C, right panels). However, in contrast to wide type (WT) controls, iNOS−/− MDSCs failed to inhibit proliferative response and IFN-γ production in OVA-specific T cells (Fig. 3D), indicating that iNOS is critical for MDSCs to exert immune regulatory activity.
Figure 3. iNOS in MDSCs is critical for exerting inhibitory activity.
A) iNOS expression in MDSCs is markedly upregulated by exposure to IFN-γ. MDSCs or DCs were exposed to IFN-γ (100U/ml, overnight, 18 hours) and expression of iNOS was determined by qPCR (left panel). Expression of iNOS was dynamically analyzed following exposure to IFN-γ (100U/ml) (middle panel). The concentrations of nitrites in the supernatants were measured by Griess assay (right panel). B) Blockade of iNOS activity impedes inhibitory activity of MDSCs in vitro. The iNOS inhibitor L-NMMA (10 µM) was added at the beginning into a one-way MLR culture, in which the proliferative response of OT-I (CD8+) or OT-II (CD4+) OVA specific T cells was inhibited by addition of MDSCs (DCs served as the control), as described above. T cell proliferation was analyzed by CFSE-dilution assay. The percentage of dividing T cells is indicated. Production of IFN-γ was determined by intracellular staining. The percentage of IFN-γ positive cells is indicated. C) Deficiency in iNOS gene expression does not affect generation of MDSCs. qPCR assay results confirmed the absence of iNOS in MDSCs propagated from iNOS−/− mice with or without IFN-γ stimulation (left panel). The expression patterns of CD11c, CD11b and Gr-1 on MDSCs and DCs propagated from iNOS−/− mice (right panels) were similar to those propagated from WT mice (compared to Fig. 1A). The percentage of positive cells is indicated. D) MDSCs deficient in iNOS lose their ability to inhibit T cell response in vitro. iNOS−/− or WT MDSCs were added at the beginning into a one-way MLR culture in which the proliferative response of OT-I (CD8+) or OT-II (CD4+) OVA specific T cells was elicited by OVA and syngeneic DCs (addition of WT DCs was used as control). The percentage of dividing cells is indicated. IFN-γ expression was analyzed by flow cytometry following intracellular mAb staining. The percentage of IFN-γ positive cells is indicated. The data are representative of three separate experiments.
MDSCs deficient in iNOS lose their ability to protect islet allografts
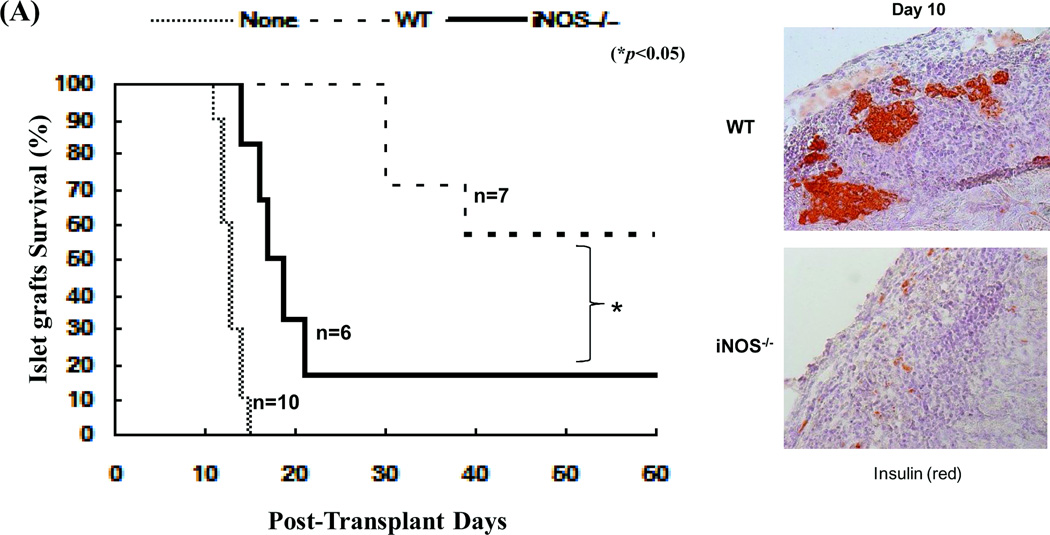
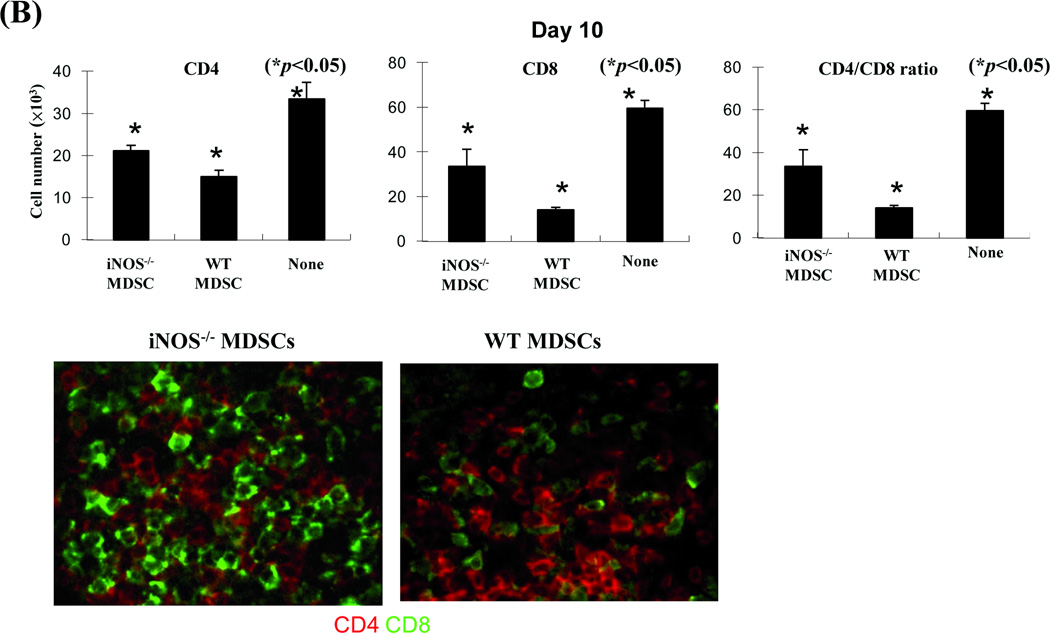
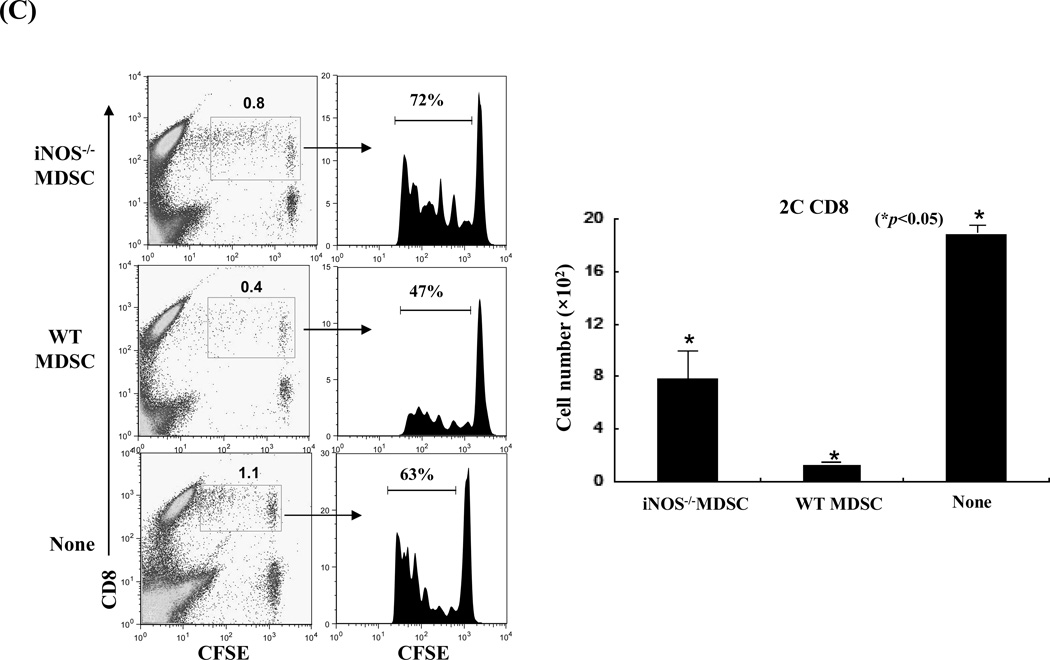
To gain in vivo evidence, MDSCs propagated from iNOS−/− mice were co-transplanted with islet allografts as described above. Compared to WT controls, iNOS−/− MDSCs largely lost their ability to protect islet allografts (Fig. 4A). To understand the underlying mechanisms, the recipients were sacrificed on POD 10. Analyses of the kidneys by immunohistochemistry showed that the WT MDSC group retained healthy islets, while very few functional islets were identified in iNOS−/− MDSC treatment group (Fig. 4A, right panels). Flow analysis revealed greater CD8+ T cell infiltration in iNOS−/− MDSC co-transplanted islet grafts compared to the WT controls (Fig. 4B, upper panels). Immunohistochemical staining performed in parallel, confirmed these observation, showing increased infiltration in islet grafts contransplanted with iNOS−/− MDSC (Fig. 4B, lower panels). The influence of co-transplantation with iNOS−/− MDSCs on donor specific CD8+ T cell responses was examined by adoptive transfer of CSFE-labeled CD8+ 2C T cells (Ld specific, B6 background) into the recipients. Three days later, the graft infiltrating lymphocytes were isolated for flow analysis. Co-transplantation with WT MDSCs was associated with fewer graft infiltrating CD8+ 2C T cells with low proliferative activity according to CFSE dilution analysis, while co-transplantation with MDSCs deficient in iNOS largely lost their ability to inhibit 2C T cell responses (Fig. 4E). These results provide in vivo evidence that inhibition of T cells and protection of islet allografts by MDSCs is dependent on iNOS expression and NO mediated effector functions.
Figure 4. MDSCs deficient in iNOS lose their ability to protect islet allografts.
MDSCs (2 × 106) propagated from iNOS−/− or WT B6 mice were mixed with BALB/c islet grafts (300), and transplanted under renal capsule of B6 diabetic recipients. Recipients without MDSC co-transplantation served as the control (none). A) Survival of islet allografts was determined by monitoring blood glucose levels as described in Methods (p<0.05, WT vs. iNOS−/− MDSC group). B) Co-transplantation with iNOS−/− MDSCs was associated greater numbers of infiltrating CD8+ T cells in islet grafts compared to WT controls. The recipients (n = 3) were sacrificed on POD 10 and the lymphocytes were isolated from the islet grafts and stained with anti-CD4 and CD8 mAbs. The CD4/CD8 ratio and CD4+ and CD8+ T cell numbers were calculated based on flow cytometric analysis (upper panels, p<0.05, compared between groups). The cryostat sections of islet graft were fluorescently stained for CD4 (red) and CD8 (green) and visualized under the microscope (lower panels). C) Co-transplantation with iNOS−/− MDSCs is associated with enhanced infiltration of donor specific CD8+ T cells in the grafts compared to WT controls. On POD 3, recipients were injected (i.v.) with 1.5 × 107 CFSE-labeled T cells from 2C TCR transgenic mice (B6 background). Three days later, lymphocytes were isolated from islet grafts and stained with anti-CD8 mAb for flow analysis. The CFSE+CD8+ cells were identified as Ld-specific CD8+ T cells. Left panels: CFSE dilution assays; the percentage indicates CFSE+CD8+ T cells in the whole cell population. Middle panels: CFSE dilution assays gated on CFSE+CD8+ T cells, expressed as histograms. The percentage of dividing CD8+ 2C T cells is indicated. The data are representative of three separate experiments. Right panel: absolute numbers of CD8+ 2C T cells in islet grafts were calculated based on the flow analysis data (n=3, p<0.05, compared between groups).
Discussion
MDSCs were initially described in cancer patients and tumor-bearing mice and participate in the inhibition of the cell-mediated immune response against the tumor. MDSCs are absent under physiological conditions, however their expansion is triggered under conditions of autoimmunity, inflammation and infection (18), suggesting that MDSCs play as a crucial regulatory component of the immune system. Adoptive cellular therapy with MDSCs is an attractive strategy to inhibit immune response in settings of organ transplantation and autoimmune diseases. The importance of MDSCs in transplantation settings was first demonstrated in a rat allogeneic kidney model, in which recipients were administered anti-CD28 mAb. A population of CD3− class II− CD11b+ CD80/86+ cells, which were characterized as MDSCs, were prevalent in the peripheral blood and grafts of tolerogenic recipients (19,20). Based on these findings, we were interested in utilizing MDSCs for induction of hyporesponsiveness in transplantation. However, the main challenge was identifying a reliable approach to generate sufficient amounts of MDSCs. We have shown that HSCs are potent inducers of MDSCs. Addition of small amounts of HSCs into DC culture skews differentiation of MDSCs instead of conventional DCs, which is mediated by soluble factors released from HSCs, including complement 3 (C3) (15,16). Importantly, co-transplantation of HSC-induced MDSCs can prolong survival of islet allografts as effectively as HSCs, although the number of MDSCs required to achieve the same outcome is greater (9). However, neither HSC or MDSC cotransplantation achieves long-term survival in all islet grafts, reflecting deviated immunoregulatory activity of the used HSCs and MDSCs. We have shown that only activated HSCs demonstrate immune regulatory activity (9), which is currently determined by expression of α-smooth muscle actin (SMA), while expression of α-SMA may not correlated with immunoregulatory activity of HSCs. MDSCs contain heterogeous cell populations which renders quality control more difficult.
Results of the present study highlighted the importance of iNOS in exerting immune regulatory activity in an allogeneic transplantation setting. MDSCs deficient in iNOS were significantly compromised in their ability to inhibit T cell responses in vitro and were not as effective in protecting islet allografts in vivo. iNOS has been implicated as an effector molecule in MDSC-mediated immune suppression. T cell proliferation and activation is dependent on the availability of L-arginine. L-arginine, a nonessential amino acid, is a substrate for two enzymes, iNOS and Arg-1. MDSCs express both enzymes at high levels (17). The increased activity of Arg- 1 and iNOS in MDSCs leads to enhanced L-arginine catabolism, which results in a reduction or depletion of L-arginine in the microenvironment. Limitations in the availability of L-arginine inhibit T cell function (21,22). In addition, enhanced iNOS activity leads to increased production of NO (23,24). NO is a powerful modulator of inflammation and can regulate T cells by interfering with the activation of several important signaling molecules in T cells, including Janus-activated kinase 1 (JAK1), JAK3, signal transducer and activator of transcription 5 (STAT5), extracellular signal-regulated kinase (ERK), and AKT (25). NO can also induce T cell apoptosis (17,26). However, compared to the islet transplantation alone group, graft survival in the iNOS−/− MDSCs group was modestly prolonged, suggesting that there may be some other mechanisms involved in the regulation of immune responses against islet allografts. For example, we observed that iNOS−/− MDSCs, like WT MDSCs, were still able to induce CD4+CD25+Foxp3+ regulatory T cells (Tregs) in vivo (data not shown).
The data presented in this study showed that the expression of iNOS in MDSCs was induced by IFN-γ, a proinflammatory cytokine mainly produced by immune effector cells, including T and NK cells, etc. suggesting that MDSCs actively participate in the regulation of immune responses, triggered by inflammation itself and through the expression of the effector molecule NO. This observation was in agreement with previous studies on other cell types and in other experimental settings. For example, one report found that production of iNOS in APCs was dependent on Th1-cell-derived IFN-γ (27). MDSCs exhibited increased expression of iNOS in many tumors and other pathological conditions (17). However, IFN-γ might not be the only stimulus for iNOS. A recent study revealed that lymphocytes could still induce iNOS expression in the intestinal epithelium in response to parasite infection in mice lacking IFN-γ, suggesting the involvement of other regulatory factors.
In summary, HSCs are powerful non-tumor-associated inducers of MDSCs. Co-transplantation with HSC-induced MDSCs can significantly prolong survival of islet allografts by iNOS-mediated T cell inhibition. These results suggest the potential use of the in vitro generated MDSCs as novel adjunctive immunotherapy for islet transplantation.
Materials and Methods
Mice
Male C57BL/6J (B6, H2b), BALB/c (H2d) and iNOS−/− mice (H2b), OTI and OTII (H2b) transgenic mice that expressed OVA specific TCR on CD8+ or CD4+ T cells, respectively, were purchased from the Jackson Laboratory (Bar Harbor, ME). 2C (H2b) transgenic mice expressed Ld specific TCR on CD8+ T cells. All mice were used in accordance with the guidelines of National Institutes of Health.
Preparation of HSCs
HSCs were isolated from the mouse liver and prepared as previously described (9,14). Briefly, the liver was perfused with collagenase IV (Sigma-Aldrich, St. Louis, MO) solution (1.0 mg/mL) via the portal vein. After 30 minutes of digestion at 37°C, the isolated cells were filtered through a nylon mesh (200 microns mesh size). The HSCs were enriched by Percoll density gradient centrifugation (Sigma-Aldrich; 1.130 relative density for 10 min at 39,000g) and cultured in RPMI 1640 complete medium supplemented with 20% fetal bovine serum for 14 to 21 days. The purity of HSCs exceeded 90% determined by desmin immunostaining.
Generation of MDSCs
As previously described (15), BM cells (2 × 106/well) were isolated from tibias and femurs of B6 mice and cultured in RPMI-1640 medium containing 10% FCS and mouse granulocyte-macrophage colony-stimulating factor (GM-CSF, 8 ng/ml) and IL-4 (1000 U/mL, both from Schering-Plough, Kenilworth, NJ) for 5 days. HSCs from B6 mice were added at the beginning of DC culture (HSC:BM cell ratio = 1:50) to generate MDSCs. CD11b+ cells were purified by positive sorting with MACS micro-beads (Miltenyi Biotec, Auburn, CA). The cells cultured in the absence of HSCs were used as DCs. For further stimulation, cells were exposed to LPS (1 µg/ml, Sigma-Aldrich) or IFN-γ (100U/ml, R & D Systems, Minneapolis, MN) for the last 18 hours of culture.
Flow Cytometric Analysis
Anti-CD4, -CD8, -CD11b, -CD11c, -CD40, -CD45.1, -CD45.2, -CD86, -B7-H1, -H2Kb, -IAb, -Gr-1 and -IFN-γ mAbs were purchased from BD Biosciences (San Diego, CA). The appropriate isotype-matched irrelevant antibodies were used as controls. Flow analysis was performed on a FACSCalibur flow cytometer (BD Biosciences). For CFSE labeling, T cells suspended in PBS (1×107 cells/mL) were incubated with 2 µM CFSE (Molecular Probes, Eugene, OR) for 10 minutes at 37°C and washed with complete medium. To perform the intracellular staining, cells were permeabilized with 0.1% saponin, followed by staining with specific mAb.
Real-time quantitative (q) PCR
Total RNA was extracted with TRIzol Reagent (Life Tech, Carlsbad, CA). Complementary DNA was synthesized with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA). The primers were TGGCCACCTTGTTCAGCTACG/GCCAAGGCCAAACACAGCATAC for iNOS, CACGGCAGTGGCTTTAACCT/TGGCGCATTCACAGTCACTT for arginase 1, and CACCCTTGCCCTCCTAAAC/CACCTGGCAGGTCCAGAG for IL-12p40 and ACCCATTGAAGCCACGACTT/AGCTGACACCTGGATGCAATACT for IL-27p28. The messenger RNAs were quantified using Applied Biosystems 7500 Fast PCR System in duplicates. The expression levels were normalized to 18S messenger RNA.
Mixed Lymphocyte Reaction (MLR)
One-way MLR culture was performed in triplicates in 96-well, round-bottom microculture plates (Corning, NY). Nylon wool-eluted splenic T cells (2 × 105/ well) from B6 mice were used as responders. T cell proliferation was elicited by graded doses of γ-irradiated 20Gy; (X-ray source) indicated stimulator cells. Cultures were maintained in RPMI-1640 complete medium, for 3 days in 5% CO2. T cell proliferative response was determined by either thymidine uptake or flow CFSE dilution assay. IFN-γ was measured by intracellular mAb staining. To examine the suppressive effect, the irradiated regulator cells were added the MLR culture at the indicated ratio of regulators:effectors.
Measurement of nitrites
Nitrites produced by MDSCs or DCs were measured by the Griess reagent kit (G2930, Promega, Madison, WI) as recommended by the manufacturers. Spectrophotometric absorbance was measured at 550 nm after 4 min at room temperature.
Islet Transplantation
As previously described (9,14,15), diabetes was induced in recipient mice (B6) through a single intraperitoneal injection of streptozotocin (STZ, Sigma-Aldrich). Only mice with non-fasting blood glucose ≥350 mg/dL were used as recipients. 300 islets were isolated from the pancreases of donor (BALB/c) mice, alone or mixed with 2×106 MDSCs or DCs, and transplanted under the renal capsule of the diabetic recipient without immunosuppression. Islet transplantation was considered successful when recipient’s blood glucose level was ≤150 mg/dL after transplantation. Rejection was determined by two consecutive blood measurements (glucose level ≥250 mg/dL).
Immunohistochemistry
Insulin in the islet graft was stained by anti-insulin mAb (Santa Cruz Biotechnology, Santa Cruz, CA), and color was developed using 3-amino-9-ethylcarbazole. CD4+ and CD8+ T cells in the graft were identified by fluorescent staining using the specific mAbs (BD PharMingen) and fluorochrome-conjugated avidin-biotin system.
Statistics
Graft survival between groups of transplanted animals was compared using the log-rank test. The parametric data were analyzed by Student’s t-test (2-tailed). Values of p<0.05 were considered statistically significant.
Acknowledgements
The authors thank Kathleen Brown for performing immunohistochemistry.
This study was partially supported by NIH grants: DK084192 (LL) and AI090468 (SQ).
Footnotes
Y. A. performed most of the experiments, analyzed the data, and wrote the manuscript; J. Q. performed part of the cellular immunology experiments; H-S. C. performed part of the in vivo experiments, L.W. performed molecular biology experiments; D. S and A. G were consultants for iNOS studies; J.J.F. served as the senior advisor and contributed to the experimental design; L.L. and S.Q. participated in research design and data analyses, wrote and finalized the manuscript, and sponsored the project.
The authors declare no financial conflicts of interest.
References
- 1.Shapiro AM. State of the art of clinical islet transplantation and novel protocols of immunosuppression. Curr Diab Rep. 2011;11:3453–3454. doi: 10.1007/s11892-011-0217-8. [DOI] [PubMed] [Google Scholar]
- 2.Gibly RF, Graham JG, Luo X, Lowe WL, Jr, Hering BJ, Shea LD. Advancing islet transplantation: from engraftment to the immune response. Diabetologia. 2011;54:2494–2505. doi: 10.1007/s00125-011-2243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood K. Outlook for longer-lasting islets. Nat Med. 2008;14:1156–1157. doi: 10.1038/nm1108-1156. [DOI] [PubMed] [Google Scholar]
- 4.Calne RY, Sell RA, Pena JR, Davis DR, Millard PR, Binns RM, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;233:472–474. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 5.Kamada N, Brons G, Davies HS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29:429–431. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology. 1994;19:916–923. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bumgardner GL, Orosz CG. Unsual patterns of alloimmunity evoked by allogeneic liver parenchymal cells. Immunol Rev. 2000;175:260–279. doi: 10.1034/j.1600-0528.2002.017409.x. [DOI] [PubMed] [Google Scholar]
- 8.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 9.Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 10.Boros P, Ochando JC, Chen SH, Bromberg JS. Myeloid-derived suppressor cells: natural regulators for transplant tolerance. Human Immunol. 2010;71:1061–1066. doi: 10.1016/j.humimm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochando JC, Chen AH. Myeloid-derived suppressor cells in transplantation and cancer. Immunol Res. 2012;54:275–285. doi: 10.1007/s12026-012-8335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Ishigaki H, Ishida H, Itoh Y, Noda Y, Ogasawara K. Analysis of splenic Gr-1int immature myeloid cells in tumor-bearing mice. Microbiol Immunol. 2008;52:47–53. doi: 10.1111/j.1348-0421.2008.00009.x. [DOI] [PubMed] [Google Scholar]
- 13.Chou HS, Hsieh CC, Charles R, Wang L, Wagner T, Fung JJ, et al. Myeloid-derived suppressor cells protect islet transplants by B7-H1 mediated enhancement of T regulatory cells. Transplantation. 2012;93:272–382. doi: 10.1097/TP.0b013e31823ffd39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang HR, Chou HS, Gu X, Wang L, Brown KE, Fung JJ, et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-gamma signaling. Hepatology. 2009;50:1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou HS, Hsieh CC, Yang HR, Wang L, Arakawa Y, Brown K, et al. Hepatic stellate cells regulate immune response by way of induction of myeloid suppressor cells in mice. Hepatology. 2011;53:1007–1019. doi: 10.1002/hep.24162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh C-C, Chou HS, Yang H-R, Lin F, Bhatt S, Wang L, et al. The Role of Complement Component 3 C3) in Differentiation of Myeloid-Derived Suppressor Cells. Blood. 2013;121:1760–1768. doi: 10.1182/blood-2012-06-440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochando JC, Chen SH. Mteloid-derived suppressor cells in transplantation and cancer. Immunol Res. 2012;54:275–285. doi: 10.1007/s12026-012-8335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lees JR, Azimzadeh AM, Bromberg JS. Myeloid derived suppressor cells in transplantation. Curr Opin Immunol. 2011;23:692–697. doi: 10.1016/j.coi.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12:417–30. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 21.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clinic Cancer Res. 2007;13:721s–725s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez PC, Ernstoff MS, Hernandez C. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer K, Gottfried E, Kreutz M, Mackensen A. Suppression of T-cell responses by tumor metabolites. Cancer Immunol Immunother. 2011;60:425–431. doi: 10.1007/s00262-010-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, et al. Myeloid suppressor lines inhibit T cell responses by a NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 26.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;1828:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Veen RC. Nitric oxide and T helper cell immunity. Int Immunopharmacol. 2001;18:1491–500. doi: 10.1016/s1567-5769(01)00093-5. [DOI] [PubMed] [Google Scholar]