Abstract
It is unclear how tumor-associated macrophages (TAMs) contribute to the initiation of oncogenesis and how they are regulated at the molecular level. By using a lineage-specific deletion strategy, we found that heat shock protein 90kDa β (Grp94), member 1 (HSP90B1), a master chaperone for Toll-like receptors and integrins also known as GP96, critically endows TAMs with the ability to promote genotoxic stress and colitis-associated colon cancer.
Keywords: colon cancer, grp94, gp96, inflammation, TLR, tumor-associated macrophages
The discovery of “phagocytes” by Ilya Mechnikov in the late 19th century was made in the context of the host defense against infectious agents. As one of the best-known professional phagocytes, macrophages have been recognized to underpin an evolutionarily ancient homeostatic system that plays essential roles in development, tissue repair, angiogenesis, metabolic regulation, as well as host defenses against microbial challenges.1 Unfortunately, the very same activities of macrophages that are required for the survival of the host can be hijacked by transformed cells to support invasion and metastasis.2,3 To which extent macrophages contribute to oncogenesis and how this is regulated at the molecular level is unclear.
We have recently demonstrated that the oncogenic functions of tumor-associated macrophages (TAMs) are controlled conspiratorially by heat shock protein 90kDa β, member 1 (HSP90B1), also known as glycoprotein 96 (GP96) or glucose-regulated protein 94 (GRP94), a molecular chaperone.4 Chaperones have evolved for maintaining other proteins soluble in the crowded intracellular microenvironment and to facilitate the conformational maturation of nascent polypeptide chains. GP96 operates in the lumen of the endoplasmic reticulum (ER) and its role in protein folding was hypothesized soon after its discovery, mostly because GP96 is upregulated in conditions that cause the accumulation of misfolded proteins in the ER, such as glucose starvation, oxidative stress, acidosis, perturbations of Ca2+ homeostasis and other types of metabolic stress.5 These conditions of metabolic stress are often, if not always, found in the tumor microenvironment. Two key observations suggest that GP96 might have a unique and significant impact on the biology of TAMs and thus drive oncogenesis. First, by a transgenic fate mapping strategy, it was found that the transcriptional activity at the HSP90B1 promoter is significantly increased in TAMs.6 Second, GP96 was unexpectedly found to be an obligate chaperone for Toll-like receptors (TLRs), as well as for multiple integrins.7 The TLR/integrin-chaperone function of GP96 cannot be compensated by any other molecular chaperones of the ER including HSPA5 (best known as GRP78) and calreticulin, fueling the speculation that GP96 is a specialized in immunological functions.
To tease out the roles of GP96 in TAMs, we conditionally deleted Hsp90b1 in macrophages.4 Mice bearing a macrophage-specific Hsp90b1 knockout (KO) exhibit a significant loss (but not a complete absence) of GP96, particularly among gut-associated macrophages, which prompted us to examine the impact of the Hsp90b1 deletion in a colitis-associated colon cancer model. The first surprise was to observe that KO mice are more resistant to dextran sodium sulfate (DSS)-induced colitis than their wild-type (WT) counterparts, even though dendritic cells and CD4+ T cells do not appear to differ in these animals. In response to one dose of the carcinogen azoxymethane (AOM) combined with the chronic administration of DSS, KO mice developed fewer and less advanced colon tumors than WT mice, exhibiting decreased levels of a variety of cytokines including interleukin (IL)-6, IL-17, IL-23 and tumor necrosis factor α (TNFα) systemically and/or in the tumor microenvironment. Strikingly, when mucosal tissues from an identical anatomic site (i.e., the distal colon) of KO and WT mice were compared, a majority of the latter (but not of the former) displayed mutations in the regulatory domain of β-catenin that contains glycogen synthase kinase 3β (GSK3β) phosphorylation sites, demonstrating that TAMs can promote genetic instability and oncogenesis in a GP96-dependent manner.
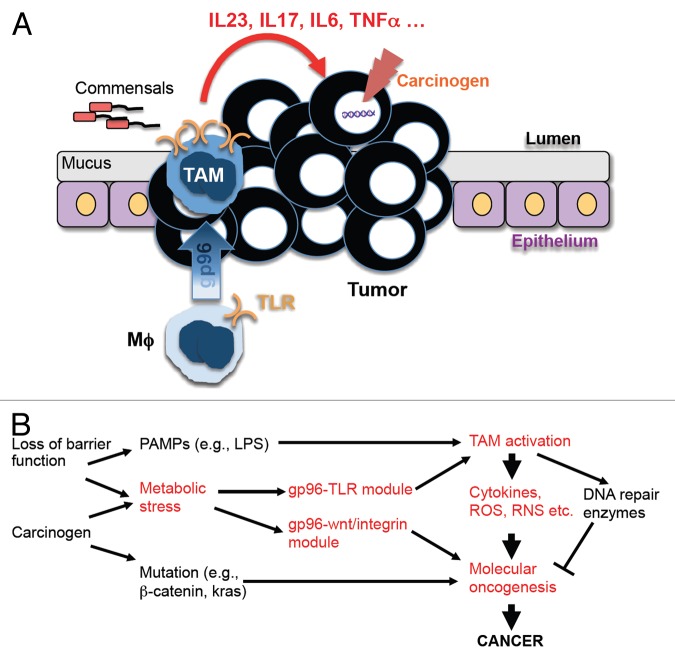
Thus, the molecular chaperone GP96 appears to be linked to ability of TAMs to drive inflammation-associated colon cancer (Fig. 1). Intriguingly, GP96 is expressed by both malignant cells and tumor-infiltrating immune cells. In 2 separate studies,8,9 GP96 was found to constitute a critical chaperone for low-density lipoprotein receptor-related protein 6 (LRP6), a co-receptor for WNT/β-catenin signaling pathway. Thus, GP96 plays at least a dual role in oncogenesis: (1) it promotes oncogenesis in a cancer cell-intrinsic fashion via WNT and integrin signaling; and (2) it enhances tumor-related inflammation in a cancer cell-extrinsic manner by hijacking the functions of macrophages. Thus, GP96 stands out as an attractive target for cancer therapy.
Figure 1. A pivital role of GP96 in driving inflammation and inflammation-associated colonic carcinogenesis. (A and B) The loss of barrier functions leads to bacterial translocation across the intestinal wall, which activates macrophages through Toll-like receptor (TLR) ligands such as lipopolysaccharide (LPS). Chronic inflammation coupled with carcinogens triggers the upregulation of glycoprotein 96 (GP96) and the functional conversion of macrophages (Mϕs) into tumor-associated macrophages (TAMs), which fuel oncogenesis by producing cytokines, reactive oxygen species (ROS), reactive nitrogen species (RNS), etc. The metabolic stress conditions that characterize the tumor microenvironment also stimulate the increased expression of GP96 in (pre)neoplastic cells, promoting malignant transformation through multiple signaling modules including the WNT and integrin pathways.
Many unresolved issues remain. For example, it is unclear whether the GP96-dependent oncogenic function of TAMs is generalizable to other cancers. It is known that colon carcinogenesis can be driven by microbes. In cancer types that are not associated with such an etiological components, like breast carcinoma, TAMs may participate in oncogenesis via entirely different mechanisms that do not depend on GP96. Second, as GP96 can be translocated onto the cell surface in response to stress, it is unclear how specific pool of GP96 (the soluble one in the lumen of the ER and that exposed on the outer leaflet of the plasma membrane) underlies the oncogenic activity of TAMs. Third, GP96 is ubiquitously expressed. It is therefore a challenge to design a therapeutic strategy that selectively targets GP96 in the tumor microenvironment, even though both peptide-based inhibitors10 and purine scaffold inhibitors9 of GP96 have already emerged. Finally, is there a prima molecule that switches on the tumor-promoting functions of TAMs and, if so, which one is it? Could it be a molecular chaperone that responds to all flavors of oncogenic stress and is responsible for the folding of critical innate immune receptors? Further investigation into these questions will undoubtedly propel the study of TAMs to a new era of opportunities.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by NIH grants (to Z.L.). We thank Joseph Bennett for his editorial assistance.
Glossary
Abbreviations:
- ER
endoplasmic reticulum
- LPS
lipopolysaccharide
- Mϕ
macrophage
- PAMP
pathogen-associated molecular pattern
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TAM
tumor-associated macrophage
- TLR
Toll-like receptor
Citation: Hong F, Wu BX, Li Z. Molecular regulation of macrophages in unleashing cancer-related inflammation. OncoImmunology 2013; 2:e27659; 10.4161/onci.27659
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27659
References
- 1.Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37(Suppl 1):S9–17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- 2.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Morales C, Rachidi S, Hong F, Sun S, Ouyang X, Wallace C, Zhang Y, Garret-Mayer E, Wu J, Liu B, et al. Immune chaperone gp96 drives the contributions of macrophages to inflammatory colon tumorigenesis. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-1677. Forthcoming; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Li Z. Roles of heat shock protein gp96 in the ER quality control: redundant or unique function? Mol Cells. 2005;20:173–82. [PubMed] [Google Scholar]
- 6.Reddy RK, Dubeau L, Kleiner H, Parr T, Nichols P, Ko B, Dong D, Ko H, Mao C, DiGiovanni J, et al. Cancer-inducible transgene expression by the Grp94 promoter: spontaneous activation in tumors of various origins and cancer-associated macrophages. Cancer Res. 2002;62:7207–12. [PubMed] [Google Scholar]
- 7.Liu B, Yang Y, Qiu Z, Staron M, Hong F, Li Y, Wu S, Li Y, Hao B, Bona R, et al. Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun. 2010;1:79. doi: 10.1038/ncomms1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Staron M, Hong F, Wu BX, Sun S, Morales C, Crosson CE, Tomlinson S, Kim I, Wu D, et al. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc Natl Acad Sci U S A. 2013;110:6877–82. doi: 10.1073/pnas.1302933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua Y, White-Gilbertson S, Kellner J, Rachidi S, Usmani SZ, Chiosis G, Depinho R, Li Z, Liu B. Molecular chaperone gp96 is a novel therapeutic target of multiple myeloma. Clin Cancer Res. 2013;19:6242–51. doi: 10.1158/1078-0432.CCR-13-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S, Hong F, Gewirth D, Guo B, Liu B, Li Z. The molecular chaperone gp96/GRP94 interacts with Toll-like receptors and integrins via its C-terminal hydrophobic domain. J Biol Chem. 2012;287:6735–42. doi: 10.1074/jbc.M111.309526. [DOI] [PMC free article] [PubMed] [Google Scholar]