Abstract
Background. Natural killer (NK) cells are implicated in the pathogenesis of hepatitis C virus (HCV) infection and outcome of interferon (IFN)-α based therapy, although mechanisms remain unclear.
Methods. To evaluate NK ability to control HCV infection, we analyzed healthy donor and HCV-infected donor NK-cell cytolytic activity directed at HCV-infected target cells.
Results. HCV-infected subjects’ natural cytotoxicity receptor (NCR)–dependent NK-cell cytolytic activity directed at HCV-infected and uninfected Huh7.5 target cells was greater than that of cells from healthy donors, and this localized to the African American subset. However, IFN-α–enhanced NK cytolytic function was lower in HCV-infected subjects, again localized mainly to the African American subset. Additionally, whereas HCV-infected Huh7.5 cells were more readily targeted than uninfected cells, the selectivity of cytolytic activity for infected targets was lower during HCV infection and after IFN-α stimulation, and lower selectivity was in part attributable to greater NKp46 expression. Furthermore, cytolytic activity was associated with higher serum aspartate aminotransferase, rs12979860 IL28B genotype, and in vivo response to pegylated IFN/ribavirin therapy.
Conclusions. These data indicate that during chronic HCV infection, race-associated increase in NCR expression and IL28B-associated cytolytic activity may participate in host response to IFN-α–containing HCV therapy.
Keywords: hepatitis C, liver, natural killer, human, infection
Factors associated with response to pegylated interferon-α/ribavirin (peg-IFN/RBV) include hepatitis C virus (HCV) genotype, age, race, human immunodeficiency virus (HIV) coinfection, baseline HCV level, and IL28B gene locus polymorphism [1–7]. The combination of natural killer (NK) KIR2DL3 and its ligand (HLA-C1) are associated with resolution of acute infection and response to peg-IFN/RBV therapy [8, 9], and KIR2DS3 and KIR3DS1 expression are associated with elevated serum alanine aminotransferase (ALT) and cirrhosis, indicating that greater NK activation potential is associated with self-resolution of infection, response to HCV therapy, and liver damage [10]. Although data on NK receptor expression have been consistently shown to be altered during chronic HCV infection, data are conflicting on whether specific receptors are up- or downregulated. Reasons for discrepancy have been proposed to be the result of differences in race, sex, and stage of HCV infection, as well as analysis of fresh vs frozen samples comparing different studies. There appears, however, to be a consensus that during chronic HCV infection, NK cells have increased expression of some activating receptors (NCRs; NKG2C and DNAM-1), greater cytotoxic activity (in part mediated by NKp30 and NKp46) directed at K562 or P815-targets, yet decreased production of IFN-γ in response to K562 targets or cytokines [11–19]. At the same time, greater P815-target cell–dependent NK-cell degranulation, and expression of NKp30 and DNAM-1, negatively predict response to peg-IFN/RBV therapy [15], whereas IFN-α–enhanced NKp30, pSTAT1, TRAIL, and K562 dependent degranulation positively predict therapy response [15, 16, 20, 21]. These data together suggest that altered NCR expression and NK-cell IFN-α responsiveness are associated with therapeutic outcome.
In the HCV culture system, NK cells exhibit IFN-γ secretion and TRAIL-dependent cytolysis of HCV-infected targets [21–24]. Here we examined NK NCR expression and IFN-induced cytolytic activity directed at HCV-infected targets in relation to race, IL28B genotype, and in vivo response to peg-IFN/RBV therapy.
MATERIALS AND METHODS
Study Subjects
Twenty-five chronic HCV genotype 1–infected subjects (antibody positive ≥6 months, HCV RNA positive) naive to therapy, and 20 age-range-matched (26–62 years) healthy donors (73% male) were enrolled. Duration of HCV infection was imputed based on first year of risk factor (intravenous drug use, blood transfusion before 1992). All subjects were HIV negative, and signed Veterans Affairs Medical Center or University Hospitals Institutional Review Board informed consent. The aspartate aminotransferase (AST) to platelet (PLT) ratio index (APRI) was calculated as [(AST/upper limit of normal)/PLT] × 100 [25]. AST, ALT, PLT, and albumin levels were obtained within 3 months of immune sampling (88% <1 month, 52% same day). HCV level (branched chain method) was obtained within 3 years of immunologic sampling (91% <1 year, 76% <3 months). Thirteen subjects went on to receive HCV therapy within 1 month (5 same day) of immunologic sampling.
NKp30, NKp46, and TRAIL Expression
Freshly isolated peripheral blood mononuclear cells (PBMCs; 5 × 106) were stained or treated with 500 U/mL IFN-α2a (PBL) or media +5% human serum (Gemini) for 16 hours, stained with anti-CD3-PerCP(SK7), -CD56-PE-Cy7(B159), -CD16-APC-Cy7(3G8), -TRAIL-PE (RIK-2; BD Biosciences), -NKp30-APC (AF29-4D12; Miltenyi Biotec), and -NKp46-FITC (9E2; Santa Cruz Biotechnology) or isotype controls. Flow cytometric analysis was performed on a BD LSRII using FACSDiva software. Five hundred units per milliliter of IFN-α2a (2 ± 0.5 ng/mL IFN-α2a) was used based on pilot experiments showing linear range concentration dependence of activity, and because it is comparable to the mean serum concentration during peg-IFN-α2a therapy (8 ± 3.5 ng/mL) [26].
NK Cytolytic Activity
Huh7.5 hepatoma cells were provided by Dr C. M. Rice (Apath LLC). The pJFH1 plasmid was provided by Dr T. Wakita (Japan). Infectious JFH1 virus was prepared as described [27]. Two thousand Huh7.5 cells were infected with 1 multiplicity of infection (MOI) of JFH-1, 52 hours prior to NK-cell coculture.
NK cells were isolated from PBMCs within 3 hours of phlebotomy using negative bead selection method (STEMCELL Technologies), depleting CD3/CD4/CD14/CD19/CD20/CD36/CD66b/CD123/HLA-DR/glycophorin A–expressing cells. Purity was >95%. NK cells were tested for cytolytic activity against JFH-1–infected or uninfected Huh7.5 cells using aCella-TOX (Cell Technology) [28, 29]. NK cells were stimulated with 500 U/mL of IFN-α2a or complete RPMI media for 16 hours and washed, then 6, 12, or 25 × 103 cells were added to HCV-infected or uninfected HuH 7.5 cells (10 000 at time of coculture) to achieve an effector to target ratio (E:T) ratio of 0.6, 1.2, and 2.5:1 5 hours. Coculture supernatant was analyzed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) by luminometer (VICTOR3V, PerkinElmer). Spontaneous effector and target, and maximal lytic reagent–induced target cell death was evaluated in control wells. As an independent measure of cytolytic activity, supernatants were analyzed by M30 enzyme-linked immunosorbent assay (DiaPharma), which detects Huh cell caspase-cleaved cytokeratin 18 (CK-18).
For antibody blocking experiments, freshly purified or 16-hour cultured NK cells were treated with goat polyclonal anti-NKp30, anti-NKp46 immunoglobulin (Ig) G, or isotype control (5 µg/mL, R&D Systems); 10 µg/mL NKp44 (p44-8; BioLegend), or mouse monoclonal anti-TRAIL (2E5). A mouse IgG1 isotype served as a control antibody (Enzo Life Science) at 37°C, 5% CO2 for 30 minutes and was added to target cell cultures. Cytotoxicity was calculated as [(experimental GAPDH-release–spontaneous effector cell GAPDH-release–spontaneous target cell GAPDH-release)/(maximum induced target cell GAPDH release–spontaneous target cell GAPDH release)] × 100.
NKp30 Ligand Expression
Infected and uninfected Huh7.5 cells were washed and treated with a nonenzymatic cell dissociation solution (MP Biomedicals) at 37°C, 5% CO2 for 25 minutes, optimized to maintain surface ligands. Dissociated cells were treated with FC-block (Miltenyi Biotec), stained with NKp30–human Fc-fusion protein (R&D Systems) or human IgG1 isotype conjugated to PerCP-donkey antihuman IgG (Jackson ImmunoResearch Laboratories), and analyzed by flow cytometry.
CD107a Degranulation Assay
Huh7.5 cells (1 × 104) were seeded into 96-well plates overnight, then infected with or without 1 MOI of JFH-1. Three days later, HCV-infected or uninfected cells were cocultured with freshly purified bulk NK-cells at 2.5:1 E:T in presence of anti-CD107a antibody (H4A3) or isotype control (BD Biosciences) at 37°C, 5% CO2 for 1 hour, supplemented with 4 µL of protein transport inhibitor (BD GolgiStop), then cocultured for 5 hours. Control cultures were performed with NK cells alone. NK cells were washed and stained with anti-CD3-PerCP(SK7), anti-CD56-PE-Cy7(B159), and anti-CD16-APC-Cy7 (3G8) (BD Biosciences).
IL28B Genotype
IL28B single-nucleotide polymorphism (SNP) rs12979860 was genotyped using the ABI TaqMan Allelic Discrimination Assay (Applied Biosystems). Primers and probes for rs12979860 were as described [30]. Thermal cycling was performed on an ABI 7500 HT Sequence Detector. Fluorescence data were collected and genotypes determined using SDS software (Applied Biosystems).
Statistical Analysis
Statistical analyses were performed with SPSS for Windows version 19.0 (SPSS Inc). We used Mann–Whitney U test for 2-way comparisons of continuous variables across and within groups. We used χ2 analysis to compare between APRI and IL28B genotypes between groups. Associations between continuous variables were evaluated using Spearman rank correlation coefficient. Jonckheere-Terpstra test for ordered alternatives was used to analyze ascending trends across the 3 IL28B genotypes. All tests of significance were 2-sided, and P values ≤.05 were considered significant.
RESULTS
Study Subject Clinical Characteristics
Clinical characteristics of HCV-infected subjects are shown in Table 1. We were unable to impute duration of infection for 16% of subjects. All subjects were HCV seropositive for >1 year, and 64% for >5 years. African American HCV-infected subjects had greater serum AST levels, whereas APRI did not significantly differ between groups. Although groups did not significantly differ overall for IL28B rs12979860 SNP genotype, the TT genotype associated with unfavorable IFN therapy outcome was observed in 4 African American subjects and no white subject. In those subjects who went on to receive HCV therapy (n = 13), week 4 HCV plasma levels were higher in African American subjects.
Table 1.
Clinical Characteristics of Hepatitis C Virus–Infected Subjects
| Characteristic | All Subjects | African American Subjects | White Subjects | P Valuea |
|---|---|---|---|---|
| No. | 25 | 13 | 12 | |
| Male sex, No. % | 23 (92) | 13 (100) | 10 (83) | |
| Age, y | 57 (43–64) | 57 (52–64) | 56 (43–64) | .54 |
| HCV plasma level, IU/ml | 1 379 190 (3111– 18 800 880) | 1 379 190 (36 893– 18 800 880) | 1 291 625 (3111– 2 968 560) | .85 |
| Genotype 1, % | 100 | 100 | 100 | |
| AST, U/L | 40 (21–162) | 45 (21–162) | 36 (25–63) | .05 |
| ALT, U/L | 47 (18–139) | 51 (18–139) | 42 (23–99) | .19 |
| PLT, K/µL | 214 (139–386) | 214 (139–386) | 215 (190–260) | .57 |
| Serum albumin, g/dL | 4.1 (3.5–4.4) | 4.1 (3.5–4.4) | 4.1 (3.5–4.4) | .94 |
| APRIb | 0.39 (0.13–2.59) | 0.46 (0.13–2.59) | 0.36 (0.26–0.64) | .08 |
| APRI <0.5, No. (%) | 19 (76) | 9 (69) | 11 (91) | .20 |
| APRI 0.5–1.5, No. (%) | 5 (20) | 3 (23) | 1 (9) | |
| APRI >1.5, No. (%) | 1 (4) | 1 (8) | 0 (0) | |
| IL28B rs12979860 CC, No. (%) | 3 (12) | 1 (7) | 2 (17) | .12 |
| IL28B rs12979860 CT, No. (%) | 9 (36) | 4 (31) | 5 (41) | |
| IL28B rs12979860 TT, No. (%) | 4 (16) | 4 (31) | 0 (0) | |
| IL28B unknown, No. (%) | 9 (36) | 4 (31) | 5 (41) | |
| Estimated duration of HCV infection, y | 36 (28–48) | 34.5 (28–41) | 36.5 (32–48) | 0.33 |
| Duration of HCV infection less certain, No. (%) | 4 (16) | 3 (23) | 1 (8) | |
| Subsequent peg-IFN/RBV therapy, No. | 13 | 6 | 7 | |
| Week 4 viral decline in those treated, IU/mL | 1 505 359 (127 952– 4 295 418) | 741 965 (127 952– 4 295 418) | 2 092 241 (170 044– 2 967 945) | .53 |
| Week 4 viral level, IU/mL | 2704 (615–255 556) | 107 209 (8550–255 556 | 1221 (615–2704) | .001 |
Data are presented as median (range) unless otherwise stated. P ≤ 0.05 are shown in bold.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; peg-IFN/RBV, pegylated interferon/ribavirin; PLT, platelet.
a African American vs white subjects.
b APRI is the AST to PLT ratio index [(AST/upper limit of normal)/PLT] × 100.
NKp30 and NKp46 Are Involved in Cytolysis of HCV-Infected and Uninfected Huh7.5 Target Cells
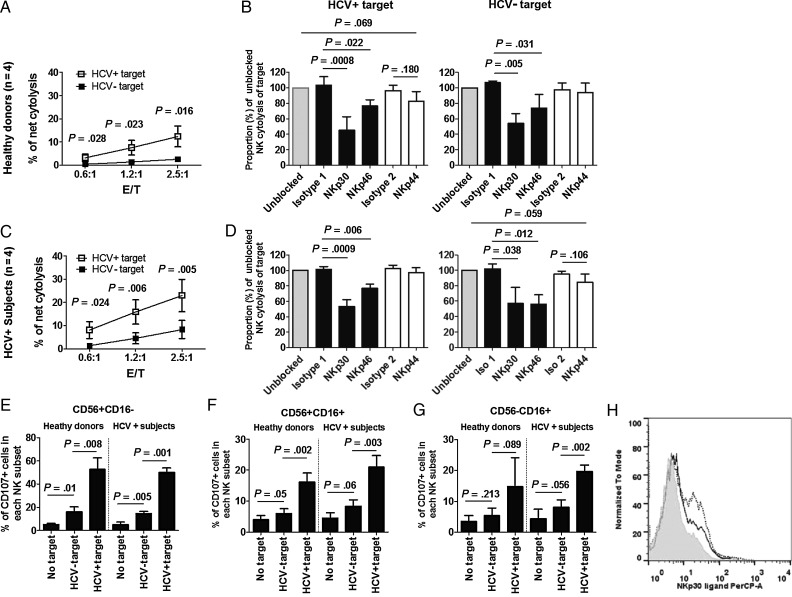
We evaluated freshly prepared unstimulated NK cells for cytolytic activity directed at HCV-infected and uninfected Huh7.5 targets for HCV-infected target selectivity and NCR dependence of cytolytic activity. Figure 1A and 1C show that both healthy donor and HCV-infected subject NK-cell anti-HCV cytolytic activity is greater than that directed at uninfected targets. There was a strong correlation between GAPDH and release of caspase-cleaved CK-18 fragment (P < .0001, data not shown), providing support for use of either method for analysis of cytolytic activity.
Figure 1.
Natural killer (NK) cell cytolytic activity targeting hepatitis C virus (HCV)–infected Huh7.5 is greater than that targeting uninfected cells, and both NKp30 and NKp46 contribute to NK-cell cytolytic activity. Negatively selected NK cells freshly prepared from 4 healthy donors (upper panels) and 4 chronic HCV–infected subjects (middle panels) were directly cocultured with targets at indicated effector: target (E:T) ratios (A and C) or 2.5:1 E:T ratio (E, F, and G), or first treated with indicated blocking antibodies or isotype controls for 0.5 hours, then cocultured with HCVcc-infected or uninfected Huh7.5 cells at 2.5:1 E:T ratio for 5 hours (B and D). Isotype 1 is isotype control for anti-NKp30 and anti-NKp46 (black bars), and isotype 2 is isotype control for anti-NKp44 (open bars). A and C, Cytolysis (y-axis) was measured for indicated E:T ratios (x-axis). B and D, Cytolytic activity is represented as a proportion (%) of cytolysis of HCV-infected and uninfected target cells in the absence of blocking antibody. E, F, and G, Degranulation activity is represented as a proportion (%) of CD107a-positive cells in each indicated NK subset after exposure to target cells or media.
Antibody blockade showed that both HCV-infected and healthy donor sample cytolysis of HCV-infected and uninfected targets was dependent on NKp30 and NKp46, but not NKp44 (Figure 1B and 1D). As CD107a is a degranulation marker associated with NK cytolytic function [31], we tested CD107a expression on HCV-infected and uninfected target exposed NK cells. Flow cytometric gating is shown in Supplementary Figure 1. CD107a expression was greater on NK cells (CD16+56−, CD16+56+, and CD16−56+ subsets) exposed to HCV-infected targets compared to uninfected targets (Figure 1E–G). JFH-1 infection has been found to upregulate NKp46 ligand expression on Huh7.5 cells [32], although NKp30 ligand expression on Huh7.5 cells has not been described. Therefore, we measured NKp30 ligand expression on JFH-1–infected and uninfected Huh7.5 cells. Surface expression of NKp30 ligand on JFH-1 infected Huh7.5 cells was higher than that on uninfected cells (Figure 1H). Upregulation of NKp30 and NKp46 ligands on virally infected cells may therefore contribute to preferential recognition of JFH-1–infected Huh7.5 cells.
NK Cells From African American Subjects Have Greater Cytolytic Activity Directed at HCV-Infected and Uninfected Targets, but Lower IFN-α–Enhanced Cytolytic Activity Directed at HCV-Infected Targets Compared to Those From White Subjects
In our prior study, we observed that NCR expression and IFN-α–induced pSTAT1 differed between African American and white subjects, and this correlated with magnitude of HCV decline in vivo during IFN-α therapy [16]. We therefore evaluated NK cytolytic activity by race. We observed that both media-treated and IFN-treated NK cells had greater cytolytic activity directed at both HCV-infected and uninfected targets in samples from HCV-infected subjects compared to healthy donors, and this difference was localized to African American subjects (Figure 2A, 2B, 2D, and 2E). In contrast, IFN-enhanced cytolytic activity directed at HCV-infected targets was lower in samples from HCV-infected subjects than controls, again localized to the African American subjects’ samples (Figure 2C). Uninfected target directed IFN-enhanced activity was also lower in samples from HCV-infected subjects compared to uninfected controls (P = .001), but in this case both African American (P = .04) and white (P = .02) subjects’ samples had lower activity (not shown). This indicates that NK cells of HCV-infected subjects, especially African American subjects, have greater activity directed at HCV-infected and uninfected HUH targets, while at the same time having lower IFN-enhanced activity directed at HCV-infected targets.
Figure 2.
Natural killer (NK) cells in African American subjects have greater cytolytic activity directed at both hepatitis C virus (HCV)–infected and uninfected targets, but lower interferon (IFN)-α enhanced cytolytic activity directed at HCV-infected targets compared to NK cells from white subjects. Negatively selected NK cells from 20 healthy donors (HD), 9 African American subjects (AA), and 11 white Americans and 25 chronic HCV–infected (HCV) subjects (13 AA and 12 white) were cultured in absence (A and D) or presence (B and E) of 500 U/mL of IFN-α for 16 hours, then washed and cocultured with HCV-uninfected or infected Huh7.5 cells for 5 hours. Cytolytic activity of media (A and D) or IFN-α–treated (B and E) NK cells at 2.5:1 E:T ratio are shown with HCV+ (HCVcc infected) and HCV– (uninfected) target cells. IFN-α–dependent killing (C) is represented as fold enhancement of cytolysis comparing treatment with IFN-α to media at 2.5:1 E:T.
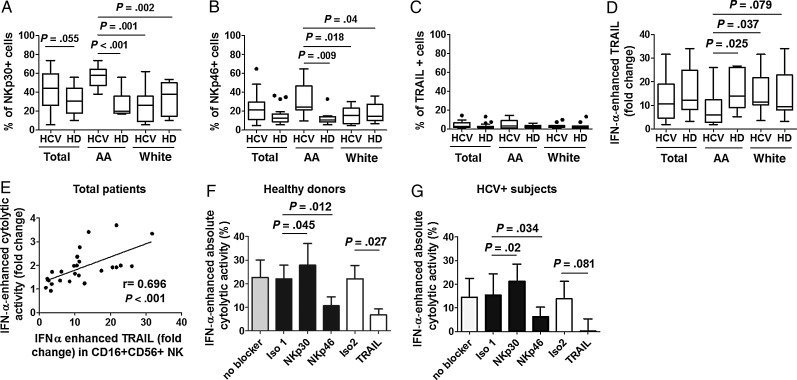
During Chronic HCV Infection, African Americans’ CD16+56+ NK Cells Express Greater NKp30 and NKp46 Directly Ex Vivo, but Lower TRAIL in Response to IFN-α Than Do White Subjects’ Samples
Because NK cytolytic function is dependent on NKp30 and NKp46 in this system, and activity differs by race, we measured the expression of NKp30 and NKp46. TRAIL expression was also measured, as TRAIL-mediated cytolysis of HCV-infected Huh7.5 target cells has been shown [21]. The gating strategy is shown in Supplementary Figure 2. We observed greater NKp30 (Figure 3A) and NKp46 (Figure 3B), but not TRAIL (Figure 3C) expression on CD16+56+ NK cells from African American HCV-infected subjects compared to those of healthy donors. Similar findings were observed within the CD16-56hi subset for NKp30 expression (not shown). Treatment of NK cells with IFN-α–enhanced TRAIL expression on CD16+56+ NK cells (Figure 3D), and degree of enhancement was lower for NK cells derived from African American subjects. Although TRAIL expression was enhanced with IFN-α in CD16+56− and CD16−56+ NK cells as well, there were no significant differences in degree of enhancement comparing groups within these other NK subsets (not shown). Additionally, degree of HCV-infected subjects’ IFN-α–enhanced CD16+56+ NK TRAIL correlated with IFN-α–enhanced bulk NK cytolytic activity directed at HCV-infected targets (Figure 3E), and this correlation held within African American and white subject subgroups (P = .01 and P = .02). Because of this correlation, we next evaluated NCR and TRAIL dependence of IFN-enhanced cytolytic activity directed at HCV-infected target cells. We observed IFN-α–enhanced cytolytic activity to depend upon TRAIL and NKp46, but not NKp30, in both uninfected control and HCV-infected subjects’ samples (Figure 3F and 3G). These data indicate that while NKp30 is required for cytolytic activity directed at HCV-infected targets when NK cells are not stimulated (Figure 1), both NKp46 and TRAIL contribute to IFN-dependent cytolysis, the latter differing by race.
Figure 3.
During chronic hepatitis C virus (HCV) infection, CD16+56+ natural killer (NK) cells in African American (AA) subjects express greater levels of NKp30 and NKp46, yet lower levels of TRAIL, in response to in vitro–administered interferon (IFN)-α than do samples from white subjects, and both NKP46 and TRAIL contribute to IFN-dependent activity. Peripheral blood mononuclear cells isolated from the same healthy donors (HD) and 25 chronic HCV–infected subjects represented in Figure 1 were immediately stained or treated with 500 U/mL of IFN-α or media for 16 hours, then stained with CD3, CD16, CD56, TRAIL, NKp30, and NKp46 monoclonal antibodies vs isotype control. A–D, Baseline expression of NKp30 (A), NKp46 (B), and TRAIL (C) and IFN-α enhanced fold change of TRAIL (D) on CD16+CD56+ NK cells was analyzed. E, Correlation of IFN-α enhanced fold change of TRAIL on CD16+CD56+ NK cells and NK cytolytic activity toward HCV-infected targets at E:T 2.5:1 are shown for 25 HCV-infected subjects. F–G, NK cells from 4 healthy donors and 4 chronic HCV–infected subjects were treated with media or 500 U/mL of IFN-α for 16 hours, washed, blocked with each indicated blocker or isotype control for 30 minutes, then cocultured with HCV-infected targets at 2.5:1 E:T ratio for 5 hours. Isotype 1 is isotype control for anti-NKp30 and anti-NKp46 (black bars), and isotype 2 is isotype control for anti-TRAIL (open bars). IFN-α enhanced absolute cytolytic activity by each indicated antibody or isotype control preblocked NK cells from healthy donors (F) and HCV-infected subjects (G) are shown. Baseline expression is represented as proportion (%) positive, based upon isotype control. IFN-α–enhanced fold change of TRAIL is represented as the ratio of TRAIL expression (%) in presence of IFN-α to media alone. IFN-α enhanced fold change and absolute change of cytolytic activity are represented as the quotient and the remainder of cytolysis of HCVcc-infected targets by NK cells treated with IFN-α to media, respectively.
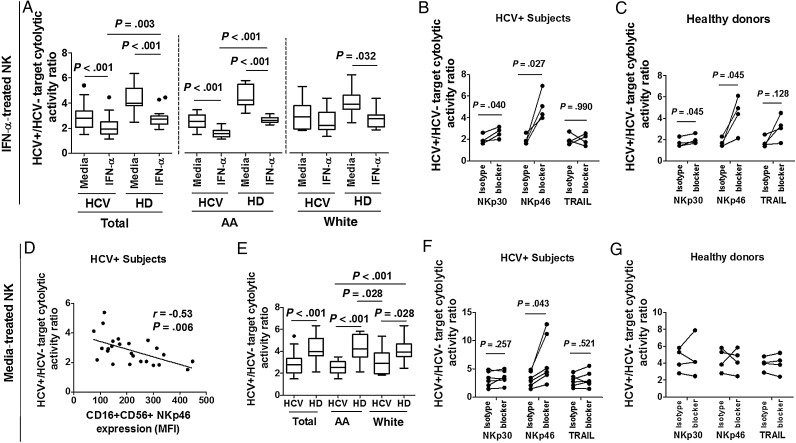
HCV-Infected/Uninfected Target Selectivity of NK Cytolytic Activity Is Diminished After IFN-α Treatment of NK Cells Derived From Uninfected Controls and African American HCV-Infected Subjects, and Greater NKp46 Expression Contributes to Lower Selectivity
To gain insight into mechanisms governing liver damage, we evaluated selectivity of cytolytic activity by comparing the ratio of activity directed at HCV-infected to uninfected targets in the presence vs absence of IFN-α pretreatment. We observed infected target selectivity to diminish after IFN-α treatment of uninfected control and HCV-infected subject cells (Figure 4A), and this difference was also observed in both African American HCV-infected and healthy donor subgroups. NKp46 expression negatively correlated with selectivity of cytolytic activity (Figure 4D), whereas NKp30 and TRAIL expression did not (data not shown). We therefore analyzed HCV-infected target selectivity in the presence vs absence of NKp46 blockade. NKp46 blockade restored IFN-α treated NK-cell selectivity of healthy donors and HCV-infected subjects, as well as media-treated NK cells of HCV-infected subjects (Figure 4B, 4C, 4F and 4G). In contrast, NKp30 or TRAIL blockade had little effect on cytolytic selectivity.
Figure 4.
Hepatitis C virus (HCV)–infected/uninfected target selectivity of natural killer (NK) cytolytic activity is diminished after interferon (IFN)-α pretreatment of NK cells derived from healthy donors and African American HCV-infected subjects, and NKP46 contributes to NK cytolytic selectivity. Negatively selected NK cells from 20 healthy donors and 25 chronic HCV–infected subjects were cultured in absence or presence of 500 U/mL of IFN-α for 16 hours, then washed and cocultured with HCV-uninfected or infected Huh7.5 cells for 5 hours. A and E, HCV+ to HCV– target selectivity is compared between media and IFN-α–activated NK cells or between media-treated NK cells of healthy donors and HCV-infected subjects at 2.5:1 E:T ratio. D, Correlation between expression of NKp46 on resting CD16+CD56+ NK cells and HCV-infected/uninfected target cytolytic ratio at 2.5:1 E:T ratio is shown for 25 HCV-infected subject samples. NK cells from 4 healthy donors and 4 chronic HCV-infected subjects were treated with media or 500 U/mL IFN-α for 16 hours, washed, blocked with each indicated blocker or isotype control for 30 minutes, then cocultured with HCV-infected targets at a 2.5:1 E:T ratio for 5 hours. The role of NKp30, NKp46, and TRAIL in selective cytolysis of HCV-infected targets by IFN-α pretreated (B and C) or media pretreated (F and G) NK cells from HCV-infected subjects and healthy donors were evaluated by antibody blockade. Two additional NK cell samples from HCV-infected subjects were treated by media alone, blocked by specific or isotype antibodies, then cocultured with HCV-infected or uninfected Huh7.5 cells at a 2.5:1 E:T ratio. The HCV-infected/uninfected target cytolytic ratio of these 2 additional samples was also shown in (F). HCV+/HCV− target cytolytic activity ratio is represented by the ratio of percentage of cytolysis of HCV-infected to uninfected target cells by NK cells.
During Chronic HCV Infection, Serum AST Correlates With In Vitro NK Cytolytic Activity and Ex Vivo NCR Expression, and IFN-α–Enhanced Cytolytic Activity Directed at HCV-Infected Targets Is Associated With In Vivo Peg-IFN-α/RBV–Induced Week 4 Viral Decline
To evaluate the relation between NK-cell phenotype, function, liver disease, and response to IFN-based therapy, we compared in vitro cytolytic activity and ex vivo NCR expression to serum AST and ALT and in vivo peg-IFN-α/RBV therapy–induced viral decline. NK cell cytolytic activity directed at HCV-infected and uninfected targets both positively correlated with serum AST (Figure 5A, HCV-infected targets, P < .001 for uninfected targets, data not shown), although not significantly so for ALT (P = .1 and P = .09, not shown). Additionally, both NKp46 and NKp30 expression on CD16+56+ NK cells positively correlated with serum AST. Magnitude of viral decline at 4 weeks of peg-IFN/RBV therapy negatively correlated with baseline NK-cell cytolytic activity directed at HCV-infected targets, but positively correlated with baseline IFN-enhanced cytolytic activity directed at HCV-infected targets (Figure 5B and 5C). This relation did not hold for uninfected target-directed activity (P = .131 and P = .138, data not shown). Additionally, week 4 AST and ALT positively correlated with baseline AST and ALT, baseline CD16+56+ NK-cell NKP30 expression, baseline NK cytolytic activity against HCV-infected targets (r = 0.59, P = .03 and r = 0.65, P = .02), week 4 HCV level (r = 0.86, P = .001 and r = 0.87, P < .001), and negatively correlated with baseline IFN-α–induced NK cytolytic activity against HCV-infected targets (r = −0.60, P = .03 and r = −0.58, P = .04). These data indicate that during chronic HCV infection, greater NK cytolytic activity and NCR expression are associated with liver inflammation. At the same time, IFN-α–enhanced NK activity directed at HCV-infected targets is associated with in vivo response to IFN therapy.
Figure 5.
During chronic hepatitis C virus (HCV) infection, serum aspartate aminotransferase (AST) correlates with in vitro natural killer (NK) cytolytic activity, and interferon (IFN)-α–enhanced cytolytic activity directed at HCV-infected targets is associated with week 4 virological response to in vivo pegylated IFN-α/ribavirin (peg-IFNα/RBV) administration. A–C, Correlations between serum AST and media-treated NK cell cytolysis of HCV-infected Huh7.5 targets (A), CD16+56+ NKp46 expression (B), and CD16+56+ NKp30 expression (C) is shown for 25 HCV-infected subjects. D and E, Correlation between media-treated NK cell cytolysis of HCV-infected Huh7.5 targets (D) or IFN-α–enhanced fold change of cytolysis of HCV-infected targets (E) and HCV decline magnitude during the first 4 weeks after therapy are shown for 13 HCV-infected subjects who are undergoing IFN-α/RBV therapy. Baseline net cytolysis is represented as cytolysis of targets by media-treated resting NK cells before the peg-IFN-α/RBV therapy. IFN-α–enhanced fold change of cytolytic activity is represented as the ratio of cytolysis of HCV-infected targets by NK cells treated with IFN-α to media alone.
IL28B Genotype Is Associated With NK Cytolytic Activity Against HCV-Infected Target Cells
IL28B genotype is associated with magnitude of HCV decline in the setting of IFN-α–based therapy for HCV [5]. We were able to perform IL28B rs12979860 genotype analysis on samples from 16 subjects in this data set (10 of whom went on to receive peg-IFN-α2a/RBV therapy). As expected, IL28B rs12979860 genotype was associated with week 4 HCV level in these genotype 1–infected individuals (r = 0.69, P = .03), and this correlation held for the African American subgroup (P = .008, n = 6). Only 4 treated white subjects were genotyped for IL28B, so it is difficult to determine whether an association held for the white subgroup. IL28B rs12979860 genotype also correlated with NK cytolytic activity directed at HCV-infected target cells, and nearly with IFN-enhanced cytolytic activity against HCV-infected target cells (Figure 6), indicating host genotype is associated with HCV-directed NK cytolytic activity.
Figure 6.
IL28B genotype is associated with natural killer (NK) cytolytic activity against hepatitis C virus (HCV)–infected target cells. A, Media-treated NK cytolytic activity directed at HCV-infected target cells at E:T 2:5:1 is shown by IL28B genotype. B, IFN-α–enhanced NK cytolytic activity directed at HCV-infected targets is shown by IL28B genotype.
DISCUSSION
In the present study, we demonstrate that during chronic HCV infection, overall Huh7.5-directed NK-cell cytolytic activity is enhanced, mainly localized to the African American subset of the HCV-infected cohort. At the same time, responsiveness to IFN-α is impaired during HCV infection, again mainly in the African American subset. In vitro IFN treatment was found to diminish selectivity of cytolytic activity for HCV-infected targets, and NKp46 negatively affected selectivity. Additionally we observed greater NKp30 expression on NK cells of African American subjects compared to those of white subjects. Because NKp30 expression is required for NK cytolytic function in the absence of IFN exposure, and expression of NKp30 ligand is upregulated on HCV-infected target cells, NKp30 may directly contribute to the observed enhanced cytolytic function in this group. Additionally, cytolytic function and NCR (NKp30 and NKp46) expression on NK cells are both associated with in vivo liver inflammation. The latter is consistent with a model where NK activity contributes to liver inflammation and damage. Furthermore, IFN-enhanced cytolytic activity directed at HCV-infected, but not uninfected, targets is directly correlated with in vivo response to IFN therapy, indicating a potential causal role. Finally, IL28B genotype correlates with NK cytolytic activity directed at HCV-infected targets, providing support for a model where genetic factors directly or indirectly influence NK activity that can contribute to liver damage and HCV clearance.
NKp30 and NKp46 have been observed to mediate greater NK-cell degranulation activity to P815 cells comparing samples from chronic HCV-infected subjects to those of healthy donors [33]. However, the role of NCRs in the selective recognition of HCV-infected hepatocytes has been unclear. We found that NKp30 and NKp46, but not NKp44, contribute to NK-cell–mediated cytolysis of HCV-infected and uninfected targets.
Enhanced NK cytolytic activity observed here during HCV infection is in agreement with recent studies showing greater NK cytotoxicity and higher expression of activating receptors, including NKp30 and NKp46 [14, 15, 17–19, 33] . Both cytolytic activity and NCR expression (NKp30 and NKp46) on NK cells are associated with AST. One interpretation would be that NCR-dependent NK cytolytic activity directed at HCV-infected and uninfected hepatic cells, modeled in this in vitro system, contributes to liver inflammation in vivo. Consistent with this concept, greater NKp46 expression on intrahepatic NK cells, which correlates with peripheral blood NKp46 expression, is associated with degree of liver inflammation [34].
Genetic variations in the IL28B gene (rs12979860 and rs8099917) are associated with peg-IFN/RBV therapy response during genotype 1 HCV infection [5]. Race is also associated with response [6]. IL28B SNP is thought to account for a substantial portion of racially based variability in peg-IFN/RBV therapy response [5, 7, 35–37]. IL28B SNP may be associated with variable levels of IFN-λ messenger RNA expression [36, 37], although the mechanism accounting for the IL28B SNP link to IFN-α therapy response is not known [38]. We find NK cytolytic activity to associate with both race and IL28B genotype, providing one plausible mechanistic link between race and IFN-α response that merits further investigation. In our prior data set, we identified an association between IL28B genotype and CD16+56− NK-subset IFN-αR expression [16]. Notably, these associations do not necessarily indicate a direct effect of IL28B on NK cells. NK-cell IL28B receptor expression has been observed, but without identified function [39]. In fact, we have been unable to find any direct and consistent activating or inhibitory effect of IL28B on isolated NK cells during in vitro cell culture performed in the presence or absence of IFN-α, interleukin 12, or interleukin 15 (not shown). Accessory cells may play a role.
Weaknesses of this study include the relatively small sample size; a variable time differential between in vivo HCV viral level analysis and immunologic sampling, limiting ability to interpret relation between immune parameters and in vivo HCV level in absence of HCV therapy; the absence of direct analysis of intrahepatic cell populations, limiting our ability to comment on this compartment; and inherent limitations of analysis of cells after removal from the host. Strengths include direct ex vivo analysis of freshly prepared cells within 3 hours of phlebotomy, analysis of HCV-infected hepatoma cell target lysis that provides a plausible model assay for in vivo function, and a well-characterized study population.
Findings here are consistent with a model where in vivo NK-cell activation results in overall enhanced cytolytic activity, but at the same time results in reduced infected target selectivity and IFN-α responsiveness. The former may contribute to bystander liver damage, and may in part be mediated by enhanced NKp46 expression, while the latter may contribute to impaired IFN-α therapy response. Mechanisms underlying the observed correlation between IL28B genotype and NK activity are currently unclear, although this association supports a model where host genotype contributes to NK activation state, disposition to liver disease progression, and responsiveness to IFN-α during chronic HCV infection. Further investigation of mechanisms underlying the endogenous IFN and NK-cell–dependent response to HCV are anticipated to clarify pathways to both improve treatment response in IFN-free direct-acting antiviral therapy–resistant patient subgroups, and guide strategies aimed at limiting liver disease progression.
Supplementary Material
Notes
Acknowledgments. We thank the study participants at the Cleveland VA Medical Center and University Hospitals of Cleveland for making this work possible.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National, and Department of Veterans Affairs Institute of Allergy and Infectious Diseases, National Institutes of Health (grant numbers R01 DK068361, R21 AI100809-01, VA Merit 110BX001894-01, and R01 AI069195).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Carrat F, Bani-Sadr F, Pol S, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–48. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 2.Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–9. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 4.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–50. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien TR. Interferon-alfa, interferon-lambda and hepatitis C. Nat Genet. 2009;41:1048–50. doi: 10.1038/ng.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–9. doi: 10.1053/j.gastro.2010.04.013. e18. [DOI] [PubMed] [Google Scholar]
- 7.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 8.Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 9.Vidal-Castineira JR, Lopez-Vazquez A, Diaz-Pena R, et al. Effect of killer immunoglobulin-like receptors in the response to combined treatment in patients with chronic hepatitis C virus infection. J Virol. 2010;84:475–81. doi: 10.1128/JVI.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paladino N, Flores AC, Marcos CY, et al. Increased frequencies of activating natural killer receptors are associated with liver injury in individuals who do not eliminate hepatitis C virus. Tissue Antigens. 2007;69(suppl 1):109–11. doi: 10.1111/j.1399-0039.2006.762_7.x. [DOI] [PubMed] [Google Scholar]
- 11.Corado J, Toro F, Rivera H, Bianco NE, Deibis L, De Sanctis JB. Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin Exp Immunol. 1997;109:451–7. doi: 10.1046/j.1365-2249.1997.4581355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier UC, Owen RE, Taylor E, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–74. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–77. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahlenstiel G, Titerence RH, Koh C, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325–35. doi: 10.1053/j.gastro.2009.08.066. e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozzano F, Picciotto A, Costa P, et al. Activating NK cell receptor expression/function (NKp30,NKp46,DNAM-1) during chronic viraemic HCV infection is associated with the outcome of combined treatment. Eur J Immunol. 2011;41:2905–14. doi: 10.1002/eji.201041361. [DOI] [PubMed] [Google Scholar]
- 16.Conry SJ, Meng Q, Hardy G, et al. Genetically associated CD16(+)56(-) natural killer cell interferon (IFN)-alphaR expression regulates signaling and is implicated in IFN-alpha-induced hepatitis C virus decline. J Infect Dis. 2012;205:1131–41. doi: 10.1093/infdis/jis027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Maria A, Fogli M, Mazza S, et al. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–55. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 18.Dessouki O, Kamiya Y, Nagahama H, et al. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: reversion by anti-viral treatment. Biochem Biophys Res Commun. 2010;393:331–7. doi: 10.1016/j.bbrc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Golden-Mason L, Madrigal-Estebas L, McGrath E, et al. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121–8. doi: 10.1136/gut.2007.130963. [DOI] [PubMed] [Google Scholar]
- 20.Ahlenstiel G, Edlich B, Hogdal LJ, et al. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231–9. doi: 10.1053/j.gastro.2011.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stegmann KA, Bjorkstrom NK, Veber H, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–97. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 22.Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crotta S, Brazzoli M, Piccioli D, Valiante NM, Wack A. Hepatitis C virions subvert natural killer cell activation to generate a cytokine environment permissive for infection. J Hepatol. 2010;52:183–90. doi: 10.1016/j.jhep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang SH, Huang CX, Ye L, et al. Natural killer cells suppress full cycle HCV infection of human hepatocytes. J Viral Hepat. 2008;15:855–64. doi: 10.1111/j.1365-2893.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 26.Dahari H, Affonso de Araujo ES, Haagmans BL, et al. Pharmacodynamics of peg-IFN-alpha-2a in HIV/HCV co-infected patients: implications for treatment outcomes. J Hepatol. 2010;53:460–7. doi: 10.1016/j.jhep.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang H, Russell RS, Yonkers NL, et al. Differential effects of hepatitis C virus JFH1 on human myeloid and plasmacytoid dendritic cells. J Virol. 2009;83:5693–707. doi: 10.1128/JVI.02671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogbomo H, Hahn A, Geiler J, Michaelis M, Doerr HW, Cinatl J., Jr NK sensitivity of neuroblastoma cells determined by a highly sensitive coupled luminescent method. Biochem Biophys Res Commun. 2006;339:375–9. doi: 10.1016/j.bbrc.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Ogbomo H, Michaelis M, Kreuter J, Doerr HW, Cinatl J., Jr Histone deacetylase inhibitors suppress natural killer cell cytolytic activity. FEBS Lett. 2007;581:1317–22. doi: 10.1016/j.febslet.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 30.Urban TJ, Thompson AJ, Bradrick SS, et al. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology. 2010;52:1888–96. doi: 10.1002/hep.23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Golden-Mason L, Stone AE, Bambha KM, Cheng L, Rosen HR. Race- and gender-related variation in natural killer p46 expression associated with differential anti-hepatitis C virus immunity. Hepatology. 2012;56:1214–22. doi: 10.1002/hep.25771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliviero B, Varchetta S, Paudice E, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–60. doi: 10.1053/j.gastro.2009.05.047. 1160.e1–7. [DOI] [PubMed] [Google Scholar]
- 34.Pembroke T, Christian A, Jones E, et al. The paradox of NKp46+ natural killer cells: drivers of severe hepatitis C virus-induced pathology but in-vivo resistance to interferon alpha treatment. Gut. 2013 doi: 10.1136/gutjnl-2013-304472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balagopal A, Thomas DL, Thio CL. IL28B and the control of hepatitis C virus infection. Gastroenterology. 2010;139:1865–76. doi: 10.1053/j.gastro.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–4. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–9. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 38.Wapner J. Pharmacogenomics. Gene variants affect hepatitis C treatment, but link is elusive. Science. 2010;330:579. doi: 10.1126/science.330.6004.579. [DOI] [PubMed] [Google Scholar]
- 39.Witte K, Gruetz G, Volk HD, et al. Despite IFN-lambda receptor expression, blood immune cells, but not keratinocytes or melanocytes, have an impaired response to type III interferons: implications for therapeutic applications of these cytokines. Genes Immun. 2009;10:702–14. doi: 10.1038/gene.2009.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.