Abstract
Background. Staphylococcus aureus is a leading cause of superficial and invasive human disease that is often refractory to antimicrobial therapy. Vaccines have the potential to reduce the morbidity, mortality, and economic impact associated with staphylococcal infections. However, single-component vaccines targeting S. aureus have failed to show efficacy in clinical trials.
Methods. A novel glycoengineering technology for creation of a multicomponent staphylococcal vaccine is described. Genes encoding S. aureus capsular polysaccharide (CP) biosynthesis, PglB (a Campylobacter oligosaccharyl transferase), and a protein carrier (detoxified Pseudomonas aeruginosa exoprotein A or S. aureus α toxin [Hla]) were coexpressed in Escherichia coli. Recombinant proteins N-glycosylated with S. aureus serotype 5 or 8 CPs were purified from E. coli.
Results. Rabbits and mice immunized with the glycoprotein vaccines produced antibodies that were active in vitro in functional assays. Active and passive immunization strategies targeting the CPs protected mice against bacteremia, and vaccines targeting Hla protected against lethal pneumonia. The CP-Hla bioconjugate vaccine protected against both bacteremia and lethal pneumonia, providing broad-spectrum efficacy against staphylococcal invasive disease.
Conclusions. Glycoengineering technology, whereby polysaccharide and protein antigens are enzymatically linked in a simple E. coli production system, has broad applicability for use in vaccine development against encapsulated microbial pathogens.
Keywords: Staphylococcus aureus, capsular polysaccharides, bioconjugate vaccine, glycoprotein, bacteremia, pneumonia, animal infection models, glycoengineering
Staphylococcus aureus is a major cause of invasive human infections, including bacteremia, endocarditis, pneumonia, and wound infections. Methicillin-resistant S. aureus (MRSA) strains are endemic in hospitals, and community-associated MRSA strains are spreading worldwide, posing a major global challenge [1–3]. There is an urgent need for a vaccine to prevent staphylococcal disease. Several vaccines have been tested in clinical trials, but capsular polysaccharide (CP) conjugates, individual protein antigens, and monoclonal antibodies (mAbs) to lipoteichoic acid have failed at various developmental stages, underscoring the need for novel vaccines with broader efficacy [4–6]. S. aureus vaccines that elicit both humoral and cell-mediated immune responses are currently under evaluation [7], and both α toxin (Hla) and CPs are key antigens under consideration for inclusion in a multicomponent vaccine.
Serotype 5 (CP5) or serotype 8 (CP8) capsules are produced by approximately 75% of S. aureus clinical isolates, and CP antigens are critical for survival in the blood of infected animals [8, 9]. Capsular antibodies are opsonic, mediating uptake and killing of staphylococci by human neutrophils [8]. Hla is a secreted pore-forming toxin to which lymphocytes, macrophages, alveolar epithelial cells, pulmonary endothelium, and erythrocytes are susceptible [10]. A genetically detoxified protein (HlaH35L) is defective in pore formation, and antibodies to HlaH35L neutralize the lytic activity of native Hla [11]. Immunization with HlaH35L protects mice against lethal staphylococcal pneumonia, lethal peritonitis, and skin infections [12–14].
Immunization with conserved staphylococcal protein antigens glycosylated with CPs may be an elegant and efficient strategy to prevent S. aureus infections, limiting the numbers of individual vaccine components that need to be prepared and individually purified. Such an approach is feasible through the development of a novel Escherichia coli N-linked glycosylation technology [15, 16], wherein O antigens are transferred to specific sites within a protein carrier by the oligosaccharyltranferase PglB [15–17]. In contrast to chemically conjugated vaccines, bioconjugate vaccines are homogenous with a defined molecular structure, and the protein and glycan components are kept in native conformations, avoiding denaturation of essential B-cell epitopes [18]. The product contains peptide and covalently linked sugar epitopes from the same organism, thereby broadening its efficacy against numerous manifestations of microbial disease.
We have prepared glycoconjugate vaccines composed of CP5–Pseudomonas aeruginosa exoprotein A (Epa), CP8-Epa, and CP5-Hla and evaluated their protective efficacy against bacteremia and lethal pneumonia in mice. Whereas CP5-Epa and CP8-Epa significantly reduced bacteremia, the CP5-Hla bioconjugate vaccine protected against both bacteremia and lethal pneumonia.
METHODS
Expression of CP5 and CP8 in E. coli and Bioconjugate Vaccine Production
The bacterial strains, plasmid constructs, primers, and details of bioconjugate vaccine preparation are provided in the Supplementary Methods. Briefly, genes from the P. aeruginosa O11 O antigen gene cluster (wzz to wbpM) were amplified by polymerase chain reaction and cloned in a plasmid with S. aureus cap5HIJK or cap8HIJK. Recombinant plasmids were introduced into E. coli strains with mutations in lipopolysaccharide and enterobacterial antigen expression, resulting in expression of CP5 and CP8 in E. coli. Epa was modified for detoxification [19], replacement of the N-terminal signal peptide with the E. coli DsbA signal peptide, addition of 2 glycosylation consensus sequences [20], and insertion of a C-terminal hexahistidine tag. An expression plasmid for recombinant expression of HlaH35L with 1 glycosite and a signal sequence for periplasmic localization was designed on the basis of the published [11, 21] and detoxified version of S. aureus Hla.
Plasmids containing PglB and Epa or Hla were transformed into E. coli cells expressing CP5 or CP8. E. coli containing the recombinant plasmids were grown to logarithmic phase, and expression of PglB and either Epa or Hla was induced by addition of 1 mM IPTG and 0.2% arabinose. After overnight incubation at 37°C, the E. coli were harvested, and the bioconjugates were extracted by osmotic shock or high-pressure homogenization [15, 16]. The bioconjugate vaccines were purified by immobilized metal affinity, anionic exchange, hydroxyapatite, and size-exclusion chromatography as detailed in the Supplementary Methods.
Bacterial Cultures
S. aureus strains Reynolds (CP5), Reynolds (CP8), and Newman were described previously [9, 22]. S. aureus strains LAC and ST80 are community-associated MRSA isolates [23, 24]. Strains NRS 382 (USA100) and NRS 383 (USA200) are hospital-associated MRSA strains obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus program, which is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (contract HHSN272200700055C). For bacteremia studies and opsonophagocytic killing (OPK) assays, S. aureus strains were cultivated for 24 hours at 37°C on Columbia agar (Difco Laboratories) supplemented with 2% NaCl [9]. For pneumonia studies, staphylococci were harvested from tryptic soy broth (Difco) cultures grown to the logarithmic phase of growth, as described elsewhere [25].
OPK Assays
Human blood was collected from healthy volunteers, who provided written informed consent as approved under institutional guidelines. The conventional OPK assay (0.5 mL volume) was performed with human neutrophils as described [26]. The microtiter-based OPK assay was based on that described by Burton and Nahm [27]. HL-60 cells (ATCC) were used after low passage (<3 months) and maintained in L-glutamine–containing Roswell Park Memorial Institute 1640 medium (Mediatech) supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin (Mediatech). The cells were differentiated to granulocytes with N,N-dimethylformamide as described elsewhere [27], and their phenotype was confirmed by flow cytometry [27]. The assay was performed in 96-well plates, and each well (volume, 80 µL) contained 4 × 105 HL60 cells, 103 colony-forming units (CFU) of S. aureus, rabbit immunoglobulin G (IgG) or mouse serum, and 1% guinea pig serum (Cedarlane) as a complement (C′) source. After incubation for 2 hours at 37°C with shaking, HL60 cells were lysed by the addition of 20 µL of 1% Triton X-100. The samples were plated in duplicate, and the killing effect was defined as the reduction in CFU per milliliter after 2 hours, compared with the concentration at time 0.
Hla Neutralization Assay
Hla (4 HU/mL; Toxin Technology) was incubated for 1 hour with serial 2-fold dilutions of serum from immunized mice. An equal volume of washed 2% rabbit erythrocytes was added and incubated for 60 minutes. The samples were centrifuged at 200 × g for 10 minutes, and the OD545 nm of the supernatants was measured. The percentage hemolysis of each sample was compared to that for erythrocytes lysed with 4 HU of Hla. The 50% inhibition titer was calculated using nonlinear regression for sigmoidal curves with variable slopes (by means of Prism 4 software).
Animal Infection Studies
Animal studies were conducted according to Institutional Animal Care and Use Committee guidelines. Swiss-Webster mice (purchased from Taconic Farms or Charles River Laboratories) were vaccinated on days 0, 14, and 28 by the subcutaneous route. Control animals received an irrelevant Shigella O-antigen bioconjugate vaccine (O1-Epa or 2a-Epa). EPA-conjugates were formulated with Alhydrogel, and CP5-Hla was formulated with Adju-Phos to obtain a final Al3+ concentration of 0.06%.
Blood samples were obtained from mice before each vaccination and before bacterial challenge. Sera diluted 1:100 were tested by enzyme-linked immunosorbent assay (ELISA) in microtiter plates coated with purified CPs (4 µg/mL) coupled to poly-L-lysine [28] or native Hla (1 µg/mL). Two weeks after the last vaccination, mice were inoculated by the intraperitoneal route with S. aureus, and quantitative blood cultures were performed 2 hours after challenge [9]. Weight loss and renal infection were evaluated on day 4, and culture data were analyzed by the Mann–Whitney U test. Mice were inoculated by the intranasal route for the lethal pneumonia model [25], and survival data were analyzed using the log-rank test (by means of Prism 4 software).
For passive immunization against bacteremia, mice were given rabbit IgG (300 µg–1 mg) intravenously 24 hours before challenge. Blood specimens for culture were collected from mice 1–2 hours after bacterial challenge. For passive immunization against lethal pneumonia, mice were injected intraperitoneally with 1 mg of rabbit IgG 24 hours (or 24 hours and 4 hours) before bacterial inoculation. For T-cell–depletion studies, actively immunized mice were injected intraperitoneally with either 500 µg of rat anti-mouse CD4 (clone GK 1.5) or rat anti-mouse CD8a (clone 53–6.7) mAbs 72 hours and 24 hours before intranasal challenge with S. aureus Newman. Depletion of CD4+ or CP8+ cells was verified by flow cytometric analysis of splenic lymphocytes.
RESULTS
Synthesis of S. aureus CPs in E. coli
The S. aureus CPs are assembled on the bacterial membrane carrier lipid undecaprenyl pyrophosphate by a conserved pathway that shares homology to the polymerase-dependent pathway of O polysaccharide synthesis in gram-negative bacteria [29]. O antigen assembly is initiated by the transfer of a sugar phosphate from a uridine diphosphate donor to undecaprenyl phosphate. The lipid-linked O antigen is assembled at the cytoplasmic side of the inner membrane by sequential action of different glycosyltransferases. The glycolipid is then flipped to the periplasmic space and polymerized. By replacing the O antigen ligase WaaL with the oligosaccharyltransferase PglB, the polymerized O antigen can be transferred to a protein carrier of choice rather than to the lipid A core [16, 30].
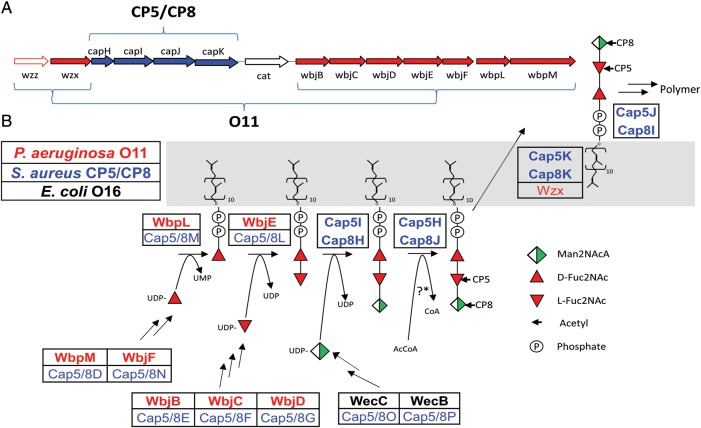
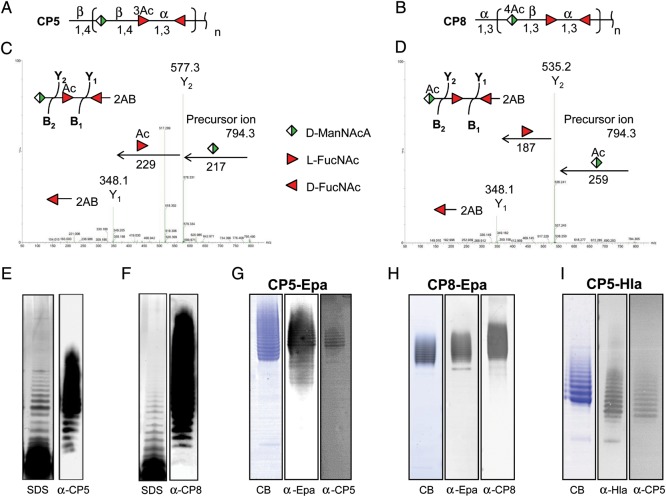
To synthesize S. aureus CP5 or CP8 in an E. coli strain deficient in O antigen production, we constructed plasmid-based chimeric genetic clusters containing genes with known function from S. aureus and P. aeruginosa (Figure 1A). The gene clusters were designed based on the similar structures of S. aureus CPs and the P. aeruginosa O11 antigen, as each contains a FucNAc disaccharide as part of the trisaccharide repeating unit [31, 32]. In combination with E. coli monosaccharide biosynthesis genes, the enzymes encoded by these genes were predicted to synthesize an undecaprenyl pyrophosphate-linked CP5 or CP8 polymer consisting of repeating trisaccharide units (Figure 1B). The recombinant plasmids were transformed into an E. coli strain with a deletion in the O-antigen ligase WaaL, and the bacterial glycolipids were extracted. The polysaccharides were released from the carrier lipid by mild acid treatment, labeled by 2-aminobenzamide (2-AB), and separated by high-performance liquid chromatography. Single repeating subunits of CP5 (Figure 2A) and CP8 (Figure 2B) were collected and characterized using MALDI-Q TOF/TOF analysis. The major ion seen at a mass-to-charge ratio of 794.3 was subjected to tandem mass spectrometry. The series of fragment ions were in agreement with the expected ions of the Na-adduct of the CP5 and CP8 single repeating units containing 2-AB at the reducing end (Figure 2C and 2D). To show that the CP5 and CP8 subunits were polymerized and transferred to lipid A, proteinase K digests of whole-cell extracts from E. coli cells expressing WaaL were prepared and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Engineered E. coli cells synthesized a CP5 or CP8 polymer visualized by silver staining and recognized on Western blots probed with S. aureus CP5- or CP8-specific antiserum, respectively (Figure 2E and 2F).
Figure 1.
Synthesis of the type 5 (CP5) and 8 capsular polysaccharides (CP8) of Staphylococcus aureus in Escherichia coli. A, Artificial gene clusters were constructed that encode enzymes responsible for the expression of CP5 or CP8 in E. coli. They comprise genes cloned from the S. aureus cap5 or cap8 locus (blue) and the Pseudomonas aeruginosa O11 LPS locus (red). B, Biosynthesis of the CP5 or CP8 polymer showing enzymatic assembly, transport, and polymerization of the uridine diphosphate–activated sugar precursors mediated by enzymes from S. aureus (blue), P. aeruginosa (red), and E. coli (black). The bolded enzymes were used to synthesize and assemble the CP5 and CP8 polymers in E. coli.
Figure 2.
Production of Staphylococcus aureus type 5 (CP5) and 8 capsular polysaccharides (CP8) in Escherichia coli. A and B, Structures of the S. aureus CP5 and CP8 repeating units are shown. Glycolipids were extracted from recombinant E. coli and labeled with 2-aminobenzamide (2-AB) after acid release from the lipid carrier; thus, the reducing end of the CP5 and CP8 repeating subunits carries 2-AB. MALDI-TOF/TOF tandem mass spectrometry analyses of the high-performance liquid chromatography elution peaks with a mass-to-charge ratio of 794.3 were performed. The fragmentation patterns of CP5 (C) and CP8 (D) are shown with arrows pointing to the corresponding monosaccharides. Proteinase K–treated whole-cell lysates from E. coli cells expressing S. aureus CPs were prepared and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). E and F, CP5 polymers (E) and CP8 polymers (F) were visualized by silver staining and Western blots probed with antibodies to CP5 or CP8. Purified bioconjugates from E. coli expressing either CP5-Epa (G), CP8-Epa (H), or CP5-Hla (I) were separated by SDS-PAGE and stained with Coomassie blue (CB). After transfer to nitrocellulose, the bioconjugates were detected by their reactivity with serum antibodies to Pseudomonas aeruginosa exoprotein A (Epa), CP5, CP8, or α toxin (Hla).
Enzymatic Synthesis of S. aureus Bioconjugate Vaccines in E. coli
To produce S. aureus vaccines in E. coli, the lipid-linked CPs were expressed in the presence of a protein carrier antigen and PglB. This allowed transfer of the polysaccharide from the carrier lipid to Asn residues within the consensus sequence D-X-N-X-S/T [20] of the protein antigen. E. coli cells expressing the CP5 or CP8 gene clusters, Epa containing 2 glycosylation consensus sequences, and PglB were cultivated. Expression of Epa and PglB was induced, and glycosylated Epa was extracted and purified. SDS-PAGE revealed a ladder of bands between 90 and 170 kDa that reacted with antibodies to Epa and CP5 (Figure 2G) or Epa and CP8 (Figure 2H), indicating variable glycosylation of Epa with S. aureus CP antigens.
A second-generation vaccine system was designed to glycosylate a staphylococcal protein antigen (Hla) in E. coli. HlaH35L containing a glycosylation consensus sequence at amino acid 130 was constructed. E. coli cells carrying genes encoding CP5 biosynthesis, HlaH35L, and PglB were cultivated. Expression of HlaH35L and PglB was induced, and CP5-HlaH35L was purified. The CP5-HlaH35L glycoconjugate subjected to SDS-PAGE revealed a ladder of bands between 55 and 70 kDa that reacted with antibodies to Hla and CP5 (Figure 2I). This finding confirms that HlaH35L can be glycosylated with CP5.
Immunogenicity of Bioconjugate Vaccines in Mice
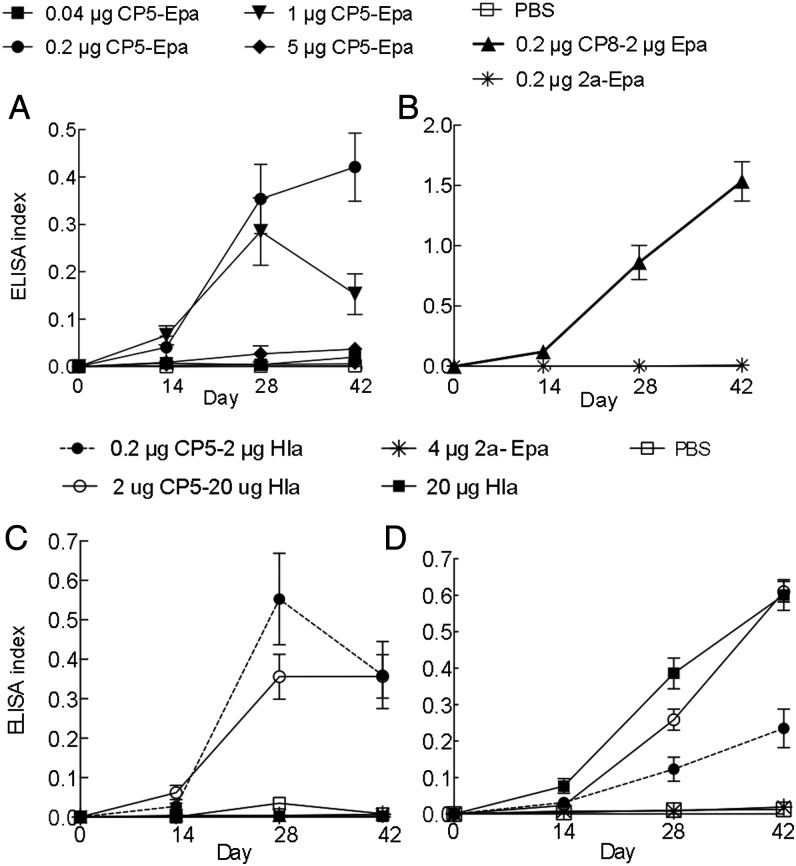
Mice were immunized with CP5-Epa, CP8-Epa or CP5-HlaH35L. The CP5-Epa vaccine showed optimal immunogenicity at 0.2–1 µg CP5/mouse (Figure 3A), and CP8-Epa showed good immunogenicity at 0.2 µg CP8 (Figure 3B). CP5-HlaH35L was immunogenic at CP5 concentrations of 0.2 and 2 µg/mouse (Figure 3C). Because the optimal HlaH35L dose was 20 µg/mouse (Figure 3D), a 2 µg CP5–20 µg HlaH35L vaccine was chosen for further evaluation.
Figure 3.
Staphylococcus aureus glycoconjugate vaccines were immunogenic in mice. Antigen-specific antibody levels in serum from mice immunized with phosphate-buffered saline (PBS), Hla, or bioconjugate vaccine formulations were determined by enzyme-linked immunosorbent assay (ELISA) at a 1:100 serum dilution. A, Antibody levels against CP5 in control mice or mice given increasing doses of bioconjugate vaccines conjugated to Epa. B, Antibody levels to CP8 in mice immunized with CP8-Epa or 2a-Epa. C, Antibody levels to CP5 in control mice, mice given CP5-Hla, and mice given Hla alone. D, Antibody levels to Hla in control mice, mice given CP5-Hla, and mice given Hla alone. The ELISA index is calculated by dividing the OD at 405 nm of the test serum (diluted 1:100) by the OD at 405 nm of a control high-titered serum that is diluted and included on every assay.
Bioconjugate Vaccines Elicit Functional Antibodies
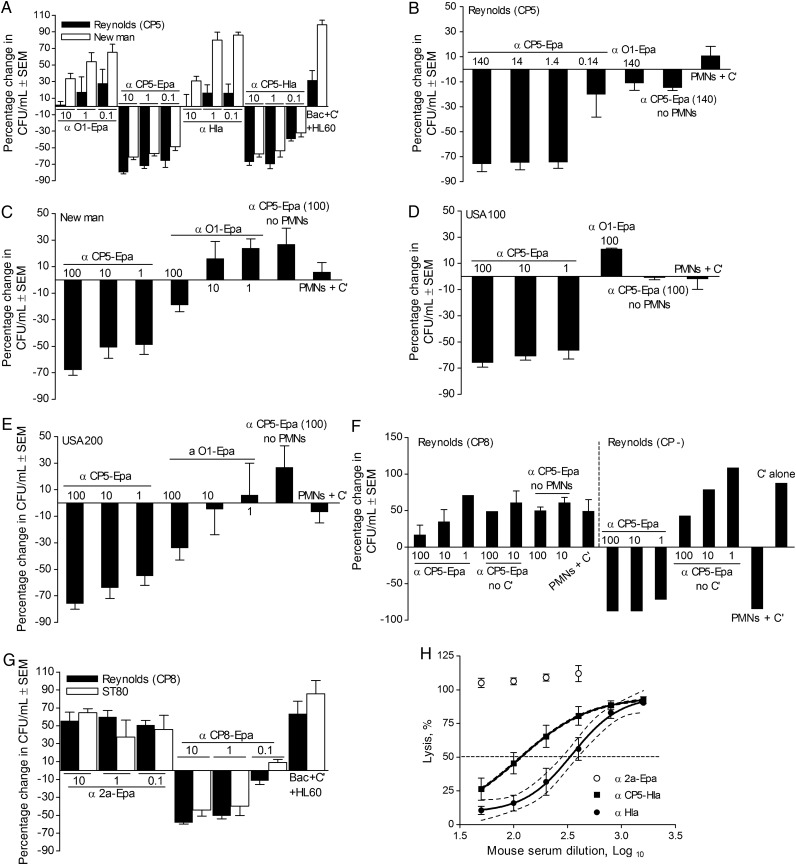
In the presence of HL60 phagocytic cells and serum complement, rabbit IgG raised to CP5-Epa or CP5-HlaH35L was opsonic for CP5+ S. aureus strains Reynolds and Newman (Figure 4A). IgG concentrations of ≥1 µg/mL resulted in ≥50% killing of the staphylococcal inoculum. Similarly, serum from mice immunized with CP5-Epa or CP5-HlaH35L showed OPK activity against CP5-producing S. aureus (Supplementary Figure 1A). Rabbit antibodies to CP5-Epa were also opsonic for S. aureus strains Reynolds (CP5) and Newman when human neutrophils were included as the phagocytic cell source (Figure 4B and 4C). MRSA strains USA100 and USA200 (both CP5+) were also killed in the presence of CP5-Epa antibodies, human neutrophils, and serum complement (Figure 4D and 4E). CP5 antibodies were not opsonic for Reynolds (CP8) (Figure 4F). The Reynolds (CP−) strain was killed by neutrophils and complement without added antibodies (Figure 4F). Rabbit CP8-Epa IgG was opsonic for S. aureus Reynolds strain (CP8) and MRSA strain ST80 (Figure 4G) at concentrations of ≥1 µg/mL. Likewise, mouse CP8-Epa antiserum showed good opsonic activity (Supplementary Figure 1B) against CP8+ S. aureus strains.
Figure 4.
Staphylococcus aureus glycoconjugate vaccines elicited functional antibodies. A, Rabbit immunoglobulin G (IgG; 10, 1, or 0.1 µg/mL) to either CP5-Epa or CP5-HlaH35L was opsonic for serotype 5 S. aureus strains Reynolds (CP5) and Newman in HL-60 opsonophagocytic killing (OPK) assays (n = 3), whereas antibodies to Hla and Shigella O1-Epa were nonopsonic. B–E, OPK assays performed with human neutrophils revealed that rabbit antibodies to CP5-Epa were opsonic for Reynolds (CP5; B), Newman (C), USA100 (D), and USA200 (E) at concentrations as low a 1 µg/mL. Rabbit antibodies to the control bioconjugate Shigella O1-Epa vaccine lacked opsonic activity. OPK assays were performed as described (26) with CP5-Epa (concentrations shown are µg/mL), human polymorphonuclear leukocytes (PMNs), and guinea pig serum as a complement (C′) source. F, Specificity of the assay was shown by the inability of CP5-Epa IgG to opsonize a serotype 8 strain (Reynolds [CP8]) for phagocytic killing. The capsule-negative Reynolds (CP–) strains were killed by PMNs + C′ alone (with or without capsular antibodies). G, Rabbit IgG (≥1 µg/mL) to CP8-Epa was opsonic for serotype 8 S. aureus strains Reynolds (CP8) and methicillin-resistant S. aureus strain ST80. No bacterial killing was observed in samples lacking antibodies or with Shigella 2a-Epa IgG. H, Sera from mice (n = 7–16) immunized with 20 µg HlaH35L or 2 µg CP5-20 µg HlaH35L neutralized the lytic activity of Hla toward rabbit erythrocytes. The percentage hemolysis was calculated in comparison to 100% lysis of erythrocytes with 4 U/mL of native Hla. Sera from mice immunized with HlaH35L showed greater in vitro neutralization activity than sera from mice given CP5-HlaH35L. Lack of overlap in the 95% confidence intervals between the 50% lysis titers indicates differences significant at a P value of <.05. Bars represent standard errors of the mean (SEM). Abbreviation: CFU, colony-forming units.
Functional antibodies to Hla neutralize its lytic activity. As shown in Figure 4H, mouse sera against either HlaH35L or CP5-HlaH35L neutralized the in vitro lytic activity of native Hla. Mice immunized with 20 µg HlaH35L showed higher neutralizing titers than mice given 2 µg CP5-20 µg HlaH35L.
Bioconjugate Vaccines Protect Mice Against S. aureus Bacteremia
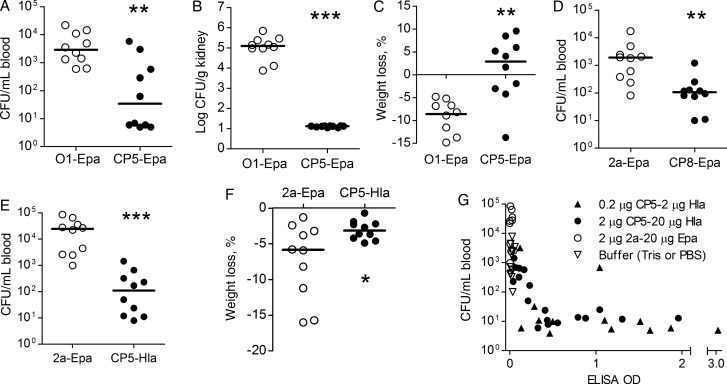
Mice vaccinated with CP5-Epa or the Shigella O1-Epa vaccine were challenged intraperitoneally with S. aureus Reynolds (CP5). Mice given CP5-Epa were protected against bacteremia (Figure 5A), renal abscess formation (Figure 5B), and weight loss (Figure 5C), compared with mice given the irrelevant Shigella vaccine. Similarly, animals immunized with CP8-Epa and challenged with Reynolds (CP8) showed a significant reduction in bacteremia (Figure 5D).
Figure 5.
Active immunization with Staphylococcus aureus glycoconjugate vaccines protects mice against bacteremia, weight loss, and renal abscesses. A–C, Mice immunized on days 0, 14, and 28 with CP5-Epa and challenged by the intraperitoneal route with approximately 107 colony-forming units (CFU) of S. aureus Reynolds (CP5) had fewer bacteria recovered from the blood (A; P = .0029) and kidneys (B; P < .0001) and showed reduced weight loss (C; P = .005), compared with mice immunized with Shigella O1-Epa. D, Mice immunized with 0.2 µg CP8-Epa and challenged with 3.9 × 107 CFU Reynolds (CP8) showed reduced bacteremia compared to control mice (P = .0015). E and F, Mice immunized with 0.2 µg CP5-20 µg HlaH35L were protected against bacteremia (E) and weight loss (F) provoked by challenge with approximately 107 CFU strain Reynolds (CP5). Horizontal lines represent group medians. Statistical analysis was performed with a Mann–Whitney U test (*P < .05, **P < .01, and ***P < .0001). G, Inverse correlation (P < .001 by Spearman nonparametric analysis) between mouse CP5 antibody levels and bacteremia levels in mice actively immunized with CP5-HlaH35L, 2a-Epa, or buffer. Mouse sera obtained 1 day before bacterial challenge were diluted 1:100 and tested by a CP5-specific enzyme-linked immunosorbent assay. Mice were challenged intraperitoneally with 1 × 107 CFU of S. aureus Reynolds, and quantitative blood cultures were performed after 2 hours. Each dot represents a sample from 1 mouse, and the lower limit of detection for bacteremia levels ranged from 5 to 20 CFU/mL.
The second-generation bioconjugate vaccine contained a staphylococcal toxoid (HlaH35L) glycosylated with CP5. CP5-HlaH35L was protective against bacteremia (Figure 5E) and associated weight loss (Figure 5F) provoked by challenge with Reynolds (CP5). There was an inverse correlation between CP5 antibody levels and bacteremia levels in individual mice (Figure 5G), confirming that protection was mediated by vaccine-induced antibodies.
CP5-HlaH35L Protects Against Lethal Pneumonia Caused by CP5+ and CP8+ S. aureus.
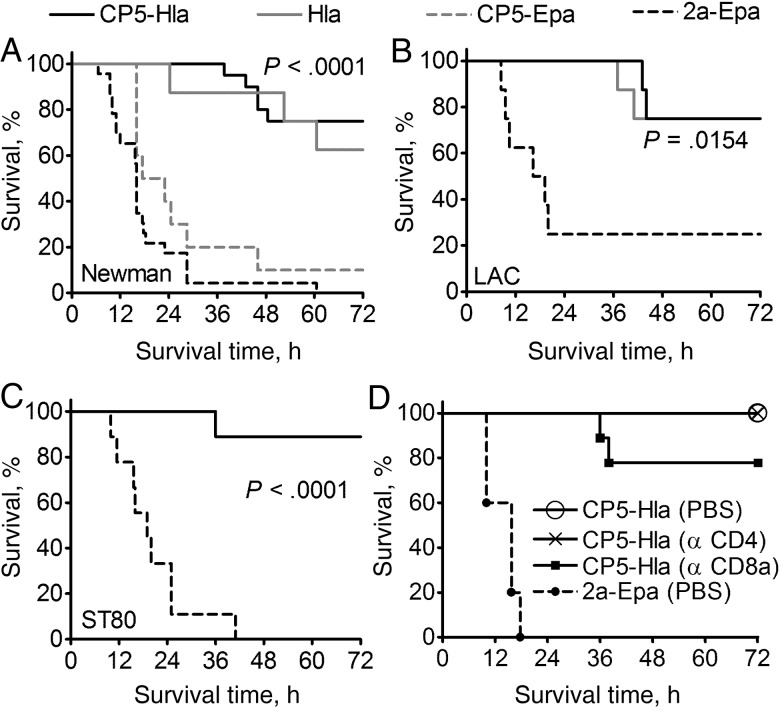
Mice were vaccinated with HlaH35L, CP5-HlaH35L, CP5-Epa, or Shigella 2a-Epa and challenged intranasally with CP5+ strain Newman. Mice immunized with HlaH35L or CP5-HlaH35L were protected against lethal pneumonia. CP5 antibodies did not mediate protection, since mice vaccinated with CP5-Epa succumbed to the infection, similar to the mice given Shigella 2a-Epa (Figure 6A). The HlaH35L and CP5-HlaH35L vaccines also protected against lethal pneumonia induced by the CP– strain USA300 LAC (Figure 6B) and the CP8+ MRSA strain ST80 (Figure 6C). Despite the fact that HlaH35L elicited a higher neutralizing antibody response to Hla than CP5-HlaH35L (Figure 4H), protection against pneumonia elicited by the 2 vaccines was equivalent. Thus, CP5 glycosylation of S. aureus Hla broadened the protective effect of the bioconjugate vaccine, such that it protected mice against both bacteremia (Figure 5E) and lethal pneumonia (Figure 6A–C).
Figure 6.
Immunization with CP5-HlaH35L protected mice against lethal pneumonia provoked by diverse Staphylococcus aureus isolates. A–C, Mice immunized with 2 µg CP5-20 µg HlaH35L or 20 µg HlaH35L were protected from lethal pneumonia resulting from intranasal challenge with 1.4 × 109 colony-forming units (CFU) of S. aureus Newman (A; n = 15–18), 1.6 × 109 CFU of LAC (B; n = 8), or 6.0 × 108 CFU of ST80 (C; n = 9). D, Mice were immunized with bioconjugate vaccines on days 0, 14, and 28. Mice were injected with monoclonal antibodies to CD4 or CD8a 72 hours and 24 hours before challenge with S. aureus Newman. Control animals were given phosphate-buffered saline (PBS). CP5-HlaH35L–immunized mice (with or without T-cell depletion) showed similar survival curves, whereas mice immunized with the Shigella 2a-EPA bioconjugate died within 24 hours of bacterial challenge. Data were analyzed with the log-rank test.
T Cells Are Not Critical for CP5-HlaH35L–Mediated Protection Against Lethal Pneumonia
Our passive immunization experiments (described below) and previous reports [13, 33] suggest that Hla antibodies alone are protective against S. aureus lethal pneumonia. To address a possible role for T cells in immunity to staphylococcal pneumonia, we vaccinated mice with CP5-HlaH35L or Shigella 2a-Epa. The animals were given 500 µg of rat anti-mouse CD4 (clone GK 1.5) or rat anti-mouse CD8a (clone 53–6.7) mAbs 72 hours and 24 hours before challenge with S. aureus Newman. Control mice were given phosphate-buffered saline. Depletion of CD4+ or CP8+ T cells was verified by flow cytometric analysis of splenocytes from treated animals. Control CP5-HlaH35L immunized mice and those subjected to T-cell depletion showed similar survival in the lethal pneumonia model, whereas mice vaccinated with Shigella 2a-Epa succumbed to the infection (Figure 6D). These results indicate that T cells are not critical for CP5-HlaH35L–mediated protection against lethal S. aureus pneumonia.
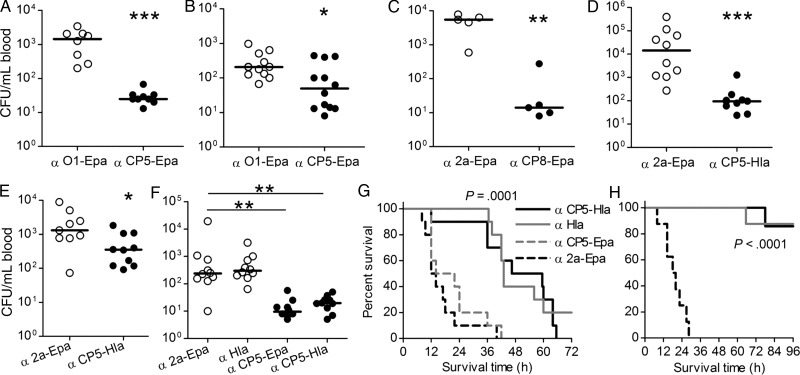
Antibodies to Bioconjugate Vaccines Protected Against Bacteremia and Lethal Pneumonia
One day before intraperitoneal challenge with S. aureus, mice were passively immunized intravenously with rabbit IgG to CP5-Epa, CP8-Epa, or CP5-HlaH35L. Compared with Shigella O1-Epa antibodies, CP5-Epa antibodies were protective against bacteremia induced by Reynolds (CP5; Figure 7A) and by MRSA strain USA200 (Figure 7B). Similarly, Reynolds (CP8) bacteremia was significantly reduced by antibodies to CP8-Epa (Figure 7C). Antibodies to CP5-HlaH35L reduced bacteremia induced by MRSA strain USA100 (Figure 7D), Newman (Figure 7E), and Reynolds (CP5; Figure 7F). The protection was CP specific because antibodies to either CP5-Epa or CP5-HlaH35L reduced bacteremia, whereas antibodies to S. aureus HlaH35L or Shigella 2a-Epa provided no protection (Figure 7F).
Figure 7.
Passive immunization with rabbit immunoglobulin G (IgG) specific for Staphylococcus aureus bioconjugate vaccines protected mice against bacteremia and lethal pneumonia. A and B, Mice administered CP5-Epa IgG and challenged intraperitoneally with 6 × 106 colony-forming unit (CFU) of S. aureus Reynolds (CP5; A) or 7 × 107 CFU USA200 (B) had significantly fewer bacteria recovered from the blood 2 hours after challenge, compared with mice given Shigella O1-Epa IgG. C, Mice given CP8-Epa IgG were protected against bacteremia induced by 4 × 107 CFU of S. aureus Reynolds (CP8). D and E, Mice administered CP5-Hla IgG showed reduced bacteremia provoked by S. aureus USA100 (8 × 107 CFU per mouse; D) or Newman (9 × 107 CFU/mouse; E). F, Mice passively immunized with 300 µg CP5-Epa or CP5-HlaH35L IgG showed similar reductions in bacteremia after challenge with strain Reynolds (CP5; 4 × 106 CFU). Antibodies to Hla had no effect on bacteremia levels. Horizontal lines represent group medians, and P values (*<.05, **<.01 and ***<.0001) were determined with the Mann–Whitney U test. G, Mice (10/group) passively immunized with a single 1 mg dose of CP5-HlaH35L IgG or Hla IgG survived longer (P = .0001) than mice given CP5-Epa IgG or O1-Epa IgG 24 hours before intranasal challenge with 6.1 × 108 CFU of S. aureus Newman. H, Mice given 1 mg of Hla IgG or CP5-HlaH35L IgG 4 hours and 24 hours before bacterial challenge with 9.7 × 108 CFU of strain Newman were protected (P < .0001) against lethal pneumonia. P values for the survival studies were determined with the log-rank test.
Passive immunization with CP5-HlaH35L IgG administered intraperitoneally 24 hours before intranasal bacterial challenge provided limited protection against lethal pneumonia (Figure 7G) induced by strain Newman. However, when a second dose of IgG was given 4 hours before bacterial inoculation, 90% of the mice given CP5-HlaH35L survived a lethal inoculum (Figure 7H).
DISCUSSION
The increasing prevalence of S. aureus infection in the hospital and community and the expanding resistance of S. aureus to antibiotics has emphasized the need for a preventive vaccine against this microbe. However, development of an effective staphylococcal vaccine has remained elusive. Vaccination with single-component vaccines based on CPs or S. aureus iron surface determinant B failed to protect patients from invasive disease in phase 3 clinical trials [6, 7, 34, 35]. Similarly, passive immunization strategies targeting clumping factor A or lipoteichoic acid did not prevent staphylococcal sepsis in premature neonates [7]. Current endeavors are focused on the preparation of vaccines that target multiple staphylococcal virulence factors. In addition to the importance of antibodies in mediating toxin neutralization and opsonophagocytic killing by neutrophils, T-cell–based immunity has recently been shown in animal infection models to be critical for vaccine-mediated protection induced by certain protein antigens [7, 36–38].
Because opsonophagocytic killing by neutrophils is a key component for host clearance of S. aureus, we have targeted staphylococcal CPs, which elicit opsonic antibodies. Production of glycoconjugate vaccines is complex and expensive, requiring the preparation of recombinant proteins and extraction and purification of complex polysaccharides. Because of the nonspecific nature of chemical conjugations, chemically conjugated vaccines are heterogenous, variable from batch to batch, and often produced in low yield. Moreover, conventional conjugation of polysaccharides to protein antigens requires denaturing chemicals that may affect the protein carrier or certain labile polysaccharides, resulting in alteration of critical epitopes.
We have developed a novel technology that allows the conjugation of an S. aureus CP to a relevant S. aureus protein without the risk of protein denaturation. The Campylobacter oligosaccharyl transferase PglB is able to transfer an oligosaccharide to a specific protein consensus sequence [20], thereby allowing the production of glycoproteins in bacterial cells. This protein glycosylation system has been functionally transferred into E. coli [15]. By using this glycosylation machinery, a variety of polysaccharides can be transferred to recombinant proteins, allowing the production of bioconjugates that can be exploited as novel vaccines. Bioconjugate vaccine lots are homogenous, and no free polysaccharide is present during the production to inhibit T-cell–dependent immune responses. Because the bioconjugate is produced in E. coli, growth of toxic organisms for polysaccharide extraction is not required.
In this study, we demonstrated production, purification, and efficacy of CP5-Epa and CP8-Epa bioconjugate vaccines. In addition, we showed that the disaccharide intermediate of P. aeruginosa O11 antigen can serve as substrate for S. aureus glycosyltransferases, showing for the first time that glycosyltransferases of gram-positive and gram-negative bacteria can be combined. The bioconjugate vaccines elicited opsonic antibodies in mice and rabbits, and active and passive immunization strategies protected mice against experimental bacteremia. The second-generation bioconjugate vaccine (CP5-HlaH35L) was an important proof-of-concept product to show the potential of covalently linking protein and polysaccharide antigens from the same microbe. Like animals given CP5-Epa, mice immunized with CP5-HlaH35L were protected against bacteremia provoked by several CP5+ S. aureus isolates. Importantly, the CP5-HlaH35L vaccine also protected mice against lethal pneumonia induced by serotype 5, serotype 8, or capsule-negative S. aureus strains. Thus, the CP5-HlaH35L bioconjugate vaccine showed protective efficacy against bacteremia (mediated by CP5 antibodies) and lethal pneumonia (mediated by HlaH35L antibodies).
This novel glycoengineering approach to conjugate vaccine development could revolutionize the industry. The trivalent S. aureus vaccine candidate (described herein) composed of CP5, CP8, and HlaH35L elicits functional antibodies and broadly protects in different animal models. Glycosylation of an S. aureus surface protein with CP8 has been accomplished, and it is currently undergoing production and testing. Glycoengineering technology enables the development of well-defined, novel, and effective vaccines against microbial pathogens such as S. aureus, for which protein or polysaccharide antigens alone are not sufficient to provide broad protection. In addition, conjugation of capsular antigens to protein antigens allows the reduction of components to be injected compared to a vaccine that contains separate capsular conjugate and protein components.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Shannon Brennan, Justin Owumi, Patrick Tavares, Sacha Keller, Fabian Meyer, Stefanie Balada, Micha Tobler, and Markus Müller, for technical assistance; Reka Szathmary, for initial work toward vaccine synthesis; and Skip Waechter, for critically reading the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by GlycoVaxyn (agreement A201436 to J. C. L.), the National Institutes of Health (grant R01 AI088754 to J. C. L.), and the Chinese Scholarship Council (to L. W.).
Potential conflicts of interest. M. W., M. K., G. L., A. F., C. A., M. B., J. Q., V. G., P. C., and M. S. are employees of GlycoVaxyn, which has secured exclusive, worldwide rights from the Swiss Federal Institute of Technology to its proprietary technology and has submitted patent applications in the United States on capsular gram-positive bacteria bioconjugate vaccines. J. C. L. is a paid consultant on vaccine development for Sanofi Pasteur and Pfizer and receives research support from Centegen. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bassetti M, Nicco E, Mikulska M. Why is community-associated MRSA spreading across the world and how will it change clinical practice? Int J Antimicrob Agents. 2009;34(Suppl 1):S15–9. doi: 10.1016/S0924-8579(09)70544-8. [DOI] [PubMed] [Google Scholar]
- 2.Bradley SF. Staphylococcus aureus pneumonia: emergence of MRSA in the community. Semin Respir Crit Care Med. 2005;26:643–9. doi: 10.1055/s-2005-925528. [DOI] [PubMed] [Google Scholar]
- 3.Chambers HF. Community-associated MRSA--resistance and virulence converge. N Engl J Med. 2005;352:1485–7. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 4.Shinefield H, Black S, Fattom A, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 5.Broughan J, Anderson R, Anderson AS. Strategies for and advances in the development of Staphylococcus aureus prophylactic vaccines. Expert Rev Vaccines. 2011;10:695–708. doi: 10.1586/erv.11.54. [DOI] [PubMed] [Google Scholar]
- 6.Fowler VG, Allen KB, Moreira ED, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA. 2013;309:1368–78. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 7.Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54:1179–86. doi: 10.1093/cid/cis033. [DOI] [PubMed] [Google Scholar]
- 8.Thakker M, Park J-S, Carey V, Lee JC. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun. 1998;66:5183–9. doi: 10.1128/iai.66.11.5183-5189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts A, Ke D, Wang Q, Pillay A, Nicholson-Weller A, Lee JC. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect Immun. 2005;73:3502–11. doi: 10.1128/IAI.73.6.3502-3511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–51. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menzies BE, Kernodle DS. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect Immun. 1994;62:1843–7. doi: 10.1128/iai.62.5.1843-1847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–8. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wardenburg JB, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–94. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauch S, DeDent AC, Kim HK, Bubeck Wardenburg J, Missiakas DM, Schneewind O. Abscess formation and alpha-hemolysin induced toxicity in a mouse model of Staphylococcus aureus peritoneal infection. Infect Immun. 2012;80:3721–32. doi: 10.1128/IAI.00442-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wacker M, Linton D, Hitchen PG, et al. N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science. 2002;298:1790–3. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- 16.Feldman MF, Wacker M, Hernandez M, et al. Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc Natl Acad Sci U S A. 2005;102:3016–21. doi: 10.1073/pnas.0500044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetter M, Kowarik M, Steffen M, Carranza P, Corradin G, Wacker M. Engineering, conjugation, and immunogenicity assessment of Escherichia coli O121 O antigen for its potential use as a typhoid vaccine component. Glycoconj J. 2013;30:511–22. doi: 10.1007/s10719-012-9451-9. [DOI] [PubMed] [Google Scholar]
- 18.Ihssen J, Kowarik M, Dilettoso S, Tanner C, Wacker M, Thony-Meyer L. Production of glycoprotein vaccines in Escherichia coli. Microb Cell Fact. 2010;9:61. doi: 10.1186/1475-2859-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killeen KP, Collier RJ. Conformational integrity of a recombinant toxoid of Pseudomonas aeruginosa exotoxin A containing a deletion of glutamic acid-553. Biochim Biophys Acta. 1992;1138:162–6. doi: 10.1016/0925-4439(92)90057-t. [DOI] [PubMed] [Google Scholar]
- 20.Kowarik M, Young NM, Numao S, et al. Definition of the bacterial N-glycosylation site consensus sequence. EMBO J. 2006;25:1957–66. doi: 10.1038/sj.emboj.7601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jursch R, Hildebrand A, Hobom G, et al. Histidine residues near the N terminus of staphylococcal alpha-toxin as reporters of regions that are critical for oligomerization and pore formation. Infect Immun. 1994;62:2249–56. doi: 10.1128/iai.62.6.2249-2256.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 23.Linde H, Wagenlehner F, Strommenger B, et al. Healthcare-associated outbreaks and community-acquired infections due to MRSA carrying the Panton-Valentine leucocidin gene in southeastern Germany. Eur J Clin Microbiol Infect Dis. 2005;24:419–22. doi: 10.1007/s10096-005-1341-7. [DOI] [PubMed] [Google Scholar]
- 24.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–70. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 25.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–70. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S, Kelley KA, Vinogradov E, et al. Characterization of the structure and biological functions of a capsular polysaccharide produced by Staphylococcus saprophyticus. J Bacteriol. 2010;192:4618–26. doi: 10.1128/JB.00104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton RL, Nahm MH. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in Streptococcus pneumoniae serotype 20. Clin Vaccine Immunol. 2012;19:835–41. doi: 10.1128/CVI.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray BM. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol. 1979;28:187–92. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- 29.Bugg TD, Brandish PE. From peptidoglycan to glycoproteins: common features of lipid-linked oligosaccharide biosynthesis. FEMS Microbiol Lett. 1994;119:255–62. doi: 10.1111/j.1574-6968.1994.tb06898.x. [DOI] [PubMed] [Google Scholar]
- 30.Wacker M, Casimiro DR. Synthesizing vaccines with microbes. In: von Gabain A, Klade C, editors. Development of novel vaccines. Wien: Springer-Verlag; 2012. pp. 125–45. [Google Scholar]
- 31.Dean CR, Franklund CV, Retief JD, et al. Characterization of the serogroup O11 O-antigen locus of Pseudomonas aeruginosa PA103. J Bacteriol. 1999;181:4275–84. doi: 10.1128/jb.181.14.4275-4284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones C. Revised structures for the capsular polysaccharides from Staphylococcus aureus types 5 and 8, components of novel glycoconjugate vaccines. Carbohydr Res. 2005;340:1097–106. doi: 10.1016/j.carres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Ragle BE, Bubeck Wardenburg J. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun. 2009;77:2712–8. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis. 2012;54:560–7. doi: 10.1093/cid/cir828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaffer AC, Lee JC. Staphylococcal vaccines and immunotherapies. Infect Dis Clin North Am. 2009;23:153–71. doi: 10.1016/j.idc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Lin L, Ibrahim AS, Xu X, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joshi A, Pancari G, Cope L, et al. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum Vaccin Immunother. 2012;8:336–46. doi: 10.4161/hv.18946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narita K, Hu DL, Mori F, Wakabayashi K, Iwakura Y, Nakane A. Role of interleukin-17A in cell-mediated protection against Staphylococcus aureus infection in mice immunized with the fibrinogen-binding domain of clumping factor A. Infect Immun. 2010;78:4234–42. doi: 10.1128/IAI.00447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.