Abstract
Background. Interferon α (IFN-α) and ribavirin can induce a sustained virologic response (SVR) in some but not all hepatitis C virus (HCV)–infected patients. The mechanism of effective treatment is unclear. One possibility is that IFN-α differentially improves the functional capacity of classic myeloid dendritic cells (mDCs) by altering expression of surface molecules or cytokines. Others have proposed that antigen-presenting cell activation could be paradoxically detrimental during HCV infection because of the production by monocytes of substances inhibitory or toxic to plasmacytoid dendritic cells.
Methods. We examined responses to in vitro IFN-α treatment of peripheral blood leukocyte samples from a retrospective treatment cohort of nearly 200 HCV-seropositive patients who had undergone antiviral therapy with ribavirin and pegylated IFN. We analyzed the variable responses of antigen-presenting cell subsets to drug.
Results. We found that patients achieving SVR were no more likely to have robust mDC activation in response to IFN-α than those who did not achieve SVR. Rather, patients achieving SVR were distinguished by restrained monocyte activation in the presence of IFN-α, a factor that was second in importance only to IL28B genotype in its association with SVR.
Conclusions. These results suggest that interindividual variability in the response of monocytes to IFN-α is an important determinant of treatment success with IFN-α–based regimens.
Keywords: hepatitis C virus, interferon-α, monocytes, activation, dendritic cells, sustained virologic response, treatment, ribavirin, Toll-like receptor 2
Hepatitis C virus (HCV) persists in many people despite induction of immune responses that might be expected to clear the virus, including robust production of interferon α (IFN-α) and other cytokines, antibodies that are frequently neutralizing, and virus-specific T cells. An understanding of the immune mechanisms underlying treatment-mediated clearance might provide opportunities to improve existing and future treatments for HCV.
Individuals who spontaneously clear infection generally have stronger and broader T-cell responses than those who progress to chronicity, who display weaker or functionally impaired responses characterized by low levels of T-cell proliferation, cytokine production, or cytotoxicity in response to HCV antigens [1–4]. There is also some correlative evidence that robust T-cell function contributes to treatment-mediated clearance, although this question is controversial. In particular, patients achieving sustained virologic response (SVR) develop stronger CD4+ T-cell responses than those who do not respond to treatment [5]. However, few studies have examined the longitudinal changes occurring in HCV-specific T cells as a result of treatment with IFN-α and/or ribavirin. Barnes et al followed peripheral blood T-cell responses in a group of 31 patients and reported a decline in the frequency of interleukin 2– and IFN-γ–secreting HCV-specific T cells following the start of treatment [6].
IFN-α could augment T-cell responses and prompt SVR through a mechanism involving activation of antigen-presenting cells (APCs). For example, IFN-α alters the surface phenotype and functional capacity of dendritic cells, enhancing expression of HLA class I molecules, HLA class II molecules, and CD86 on immature myeloid dendritic cells (mDCs) and increasing production of interleukin 12 and tumor necrosis factor α by mature cells [7–10]. Miyatake et al found that DCs differentiated from monocytes in the presence of IFN-α displayed dramatically higher CD86 expression than did untreated DCs and a greater capacity to prime production of IFN-γ and interleukin 2 by CD4+ T cells [11]. In the absence of exogenous IFN-α, DCs from patients chronically infected with HCV are relatively deficient in CD80 and CD86 expression and CD4+ T-cell priming [12]; therefore, IFN-α can act to overcome deficiencies conferred by HCV infection.
In contrast to this potentially beneficial effect of mDC activation, however, it has been proposed that exuberant monocyte activation contributes to HCV persistence [13]. HCV core protein may trigger monocyte activation via effects on Toll-like receptor 2 [14]; this activation leads to production of tumor necrosis factor α and IL-10, which together induce apoptosis in plasmacytoid DCs (pDCs) [13]. The result of this cascade is reduced frequency of circulating pDCs and diminished endogenous IFN-α production.
We have addressed the question of variable APC response to IFN-α in a cohort of 192 patients who underwent treatment for HCV in a large integrated healthcare organization. Expression of activation markers was evaluated on monocytes, mDCs, and pDCs before and after in vitro exposure to IFN-α. We hypothesized that subjects who responded to therapy would demonstrate robust mDC responses to IFN-α. Unexpectedly, we found that subjects with SVR were clearly distinguished by lower monocyte activation responses while having mDC responses equivalent to those of subjects without SVR.
METHODS
Study Population
This retrospective case-control study population was derived from all patients positive for HCV genotype 1 who were treated with combination pegylated IFN-α plus ribavirin between July 2003 and January 2011 in the Northern California Kaiser Permanente Medical Care Program. The cohort consisted of 192 patients chosen to achieve equal representation of the following 4 racial or ethnic groups: non-Hispanic white, Hispanic, Asian, and black. Within each group, approximately equal numbers of SVR and non-SVR patients were included with approximately 40% women, consistent with the sex distribution of HCV in the general population. We recruited all eligible Asians and blacks (consecutive sample) and a random sample of non-Hispanic whites and Hispanics. Patients were considered ineligible if they had either chronic hepatitis B virus or human immunodeficiency virus (HIV) coinfection, had received >1 course of HCV treatment, or had cirrhosis at the time of treatment. Eligible patients were aged 18–65 years at treatment initiation, with documented laboratory evidence in the medical record of treatment outcome, defined as SVR, if they had undetectable HCV RNA 24 weeks after treatment cessation, or as non-SVR, if there was no evidence of early viral response (defined as a <2-log decline in viral load at treatment weeks 10–14 or detectable HCV RNA during weeks 15–24 of treatment). On the basis of patient report and/or medical record review, additional patients were excluded if they developed liver cancer or decompensated cirrhosis or were pregnant. Patients receiving immunosuppressive therapy in the 6-month period before enrollment were also excluded.
The study was conducted in accordance with international guidelines on good clinical practice and was approved by institutional review boards at all participating institutions [15, 16]. Patients provided written informed consent before collection of a blood specimen for the study and were offered a $25 gift card for participation.
Clinical and Demographic Data
Clinical information regarding medical assessment before treatment and clinical and laboratory parameters during treatment were obtained from the KPNC Viral Hepatitis Registry database, which derives its data from health plan electronic records. Race and ethnicity were based on self-report.
Treatment response status was confirmed using blood samples collected for the study. All patients had congruent results: the selected SVR patients had undetectable HCV RNA (detection limit, 17 IU/mL) and all non-SVR patients had quantifiable serum HCV RNA levels.
Biospecimen Processing
Blood samples were processed at a central laboratory, using standard methods to prepare plasma and peripheral blood mononuclear cells (PBMCs). Specimens were distributed for research labeled only with a study identification number, without clinical information.
IFN-α Stimulation
PBMCs were thawed quickly at 37°C, transferred into 13 mL thaw buffer (phosphate buffered saline with 2% fetal bovine serum by volume and 5 μg of DNase I per mL), centrifuged for 15 minutes, resuspended in 3 mL AIM-V, and rested overnight at 37°C. Cells were counted the following day and adjusted to a final concentration of 10 M/mL in AIM-V. A 96-well, U-bottomed plate was prepared by addition of 10 µL of IFN-α2b stock containing 44 U or 880 U of cytokine. Wells were then seeded with 100 µL cells, giving a final concentration of 400 U/mL or 8000 U/mL IFN-α2b. The dose of IFN and the incubation time were determined by reference to earlier literature [7–10, 17] followed by simple range-finding experiments to confirm stimulation of cell types of interest under the conditions used. Plates were incubated at 37°C for 24 hours. Data shown are differences in expression between cells cultured without IFN and those cultured with IFN. Stimulated and unstimulated cells were cultured for an identical duration.
Flow Cytometry
Cells were stained using standard methods in 2 panels containing anti-CD14-Qdot605, anti-CD20-ECD, anti-CD123-PerCPCy5.5, anti-HLA-DR-PE-Cy7, anti-CD11c-Alexa700, anti-CD16-V450, anti-CD83-PE, anti-CD80-FITC, anti-CD86-APC, anti-PD-L1-PE, anti-HLA class I-APC, and anti-CD69-APC-Cy7. Data were collected on an LSRII flow cytometer and analyzed using FlowJo. A stain reagent for dead cells (Invitrogen Live/Dead Fixable Dead Cell Stain) was included in both panels. An insignificant amount of cell death (<5% of cells) was observed during the brief period of culture, and dead cells were excluded from the analysis.
Statistics
Data were stored in a MySQL database and analyzed with R. Principal components, lasso regression, random forests, and logistic regression used the prcomp function, glmnet package, party package, and glm function, respectively.
RESULTS
STRIDE Retrospective Treatment Cohort
The STRIDE cohort included 192 patients of diverse race and ethnicity treated for HCV infection at a large integrated healthcare organization in northern California. All patients were infected with genotype 1 virus and treated with pegylated IFN-α and ribavirin. The cohort comprised roughly equal numbers of black, Asian, Hispanic, and non-Hispanic white patients (Table 1), of which approximately 51% achieved SVR. Baseline levels of fibrosis were determined for 69% of the cohort by liver biopsy and did not vary significantly between racial or ethnic groups. Genotype 1 subtype did vary between racial and ethnic groups, as would be expected on the basis of the known epidemiology of subtype infections (ie, with genotype 1b most commonly found in Asian subjects) [18]. The frequency of IL28B CC genotype at rs12979860 also varied as expected, with Asians most likely to have this variation and blacks least likely.
Table 1.
Demographic and Clinical Parameters of Study Subjects, Overall and by Race/Ethnicity
| Variable | Total (n = 192) | White (n = 63) | Black (n = 40) | Asian (n = 30) | Hispanic (n = 51) | Native American (n = 8) | P |
|---|---|---|---|---|---|---|---|
| Age at treatment, y, | 50.65 ± 7.50 | 51.79 ± 5.99 | 52.60 ± 6.72 | 48.97 ± 10.31 | 49.00 ± 7.69 | 48.63 ± 5.85 | .02 |
| Sex | |||||||
| Female | 73 (38.0) | 29 (46.0) | 14 (35.0) | 9 (30.0) | 17 (33.3) | 4 (50.0) | .42 |
| Male | 119 (62.0) | 34 (54.0) | 26 (65.0) | 21 (70.0) | 34 (66.7) | 4 (50.0) | |
| HCV genotype | |||||||
| 1a | 85 (44.3) | 31 (49.2) | 18 (45.0) | 8 (26.7) | 23 (45.1) | 5 (62.5) | .02 |
| 1b | 76 (39.6) | 21 (33.3) | 19 (47.5) | 19 (63.3) | 15 (29.4) | 2 (25.0) | |
| Subtype unavailable | 31 (16.1) | 11 (17.5) | 3 (7.5) | 3 (10.0) | 13 (25.5) | 1 (12.5) | |
| Baseline fibrosis stage | |||||||
| 0 | 6 (3.1) | 3 (4.8) | 2 (5.0) | 0 | 1 (2.0) | 0 | .39 |
| 1 | 49 (25.5) | 21 (33.3) | 10 (25.0) | 4 (13.3) | 14 (27.5) | 0 | |
| 2 | 40 (20.8) | 12 (19.0) | 8 (20.0) | 9 (30.0) | 7 (13.7) | 4 (50.0) | |
| 3 | 37 (19.3) | 11 (17.5) | 11 (27.5) | 6 (20.0) | 8 (15.7) | 1 (12.5) | |
| Missing | 60 (31.3) | 16 (25.4) | 9 (22.5) | 11 (36.7) | 21 (41.2) | 3 (37.5) | |
| Log baseline viral load, IU/mL | 5.88 ± 0.56 | 5.96 ± 0.56 | 5.92 ± 0.50 | 5.75 ± 0.68 | 5.80 ± 0.56 | 6.02 ± 0.46 | .52 |
| Diabetes at baseline | 24 (12.5) | 3 (4.8) | 6 (15.0) | 5 (16.7) | 9 (17.6) | 1 (12.5) | .11 |
| SVR | 99 (51.6) | 33 (52.4) | 16 (40.0) | 21 (70.0) | 25 (49.0) | 4 (50.0) | .09 |
| IL28B genotype | |||||||
| CC | 71 (40.0) | 26 (41.3) | 4 (10.0) | 20 (66.7) | 17 (33.3) | 4 (50.0) | <.001 |
| CT or TT (non-CC) | 121 (60.0) | 37 (58.7) | 36 (90.0) | 10 (33.3) | 34 (66.7) | 4 (50.0) | |
Data are mean ± SD or no. (%) of subjects.
Abbreviations: HCV, hepatitis C virus; SVR, sustained virologic response.
Immune Cells and Activation Markers Tested
PBMCs from all patients were treated for 24 hours in vitro with either 400 U/mL or 8000 U/mL of IFN-α. Treated and untreated cell aliquots were stained with fluorochrome-labeled antibodies and evaluated by flow cytometry for expression of lineage and activation markers, the latter including HLA class I, class II (HLA-DR), CD80, CD83, CD86, CD69, and PD-L1. Monocytes, mDCs, and pDCs were identified as follows: monocytes had a high side-scatter profile and were HLA-DR+CD20−CD14+, mDCs were HLA-DR+CD14−CD16−CD20−CD11c+, and pDCs were HLA-DR+CD14−CD16−CD20−CD123+. Up to 10 combinations of markers were used to assess activation of these subsets in response to in vitro IFN-α stimulation. The change in mean fluorescence intensity (MFI) and the percentage of positive cells was assessed for CD80, CD83, CD86, HLA class I, HLA-DR, CD69, and/or PD-L1. The change in percentage of positive cells was also assessed for 3 two-marker combinations: CD80 and CD86, CD83 and HLA class I molecules, and CD69 and PD-L1.
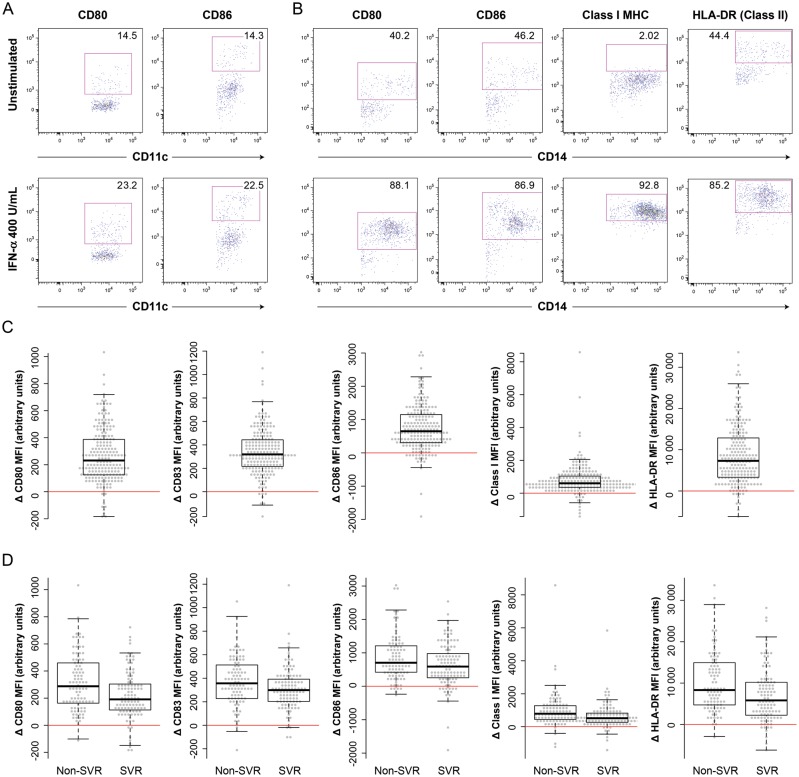
IFN-α stimulation, at both doses, caused significant changes in the MFI of all markers tested on the surface of monocytes and mDCs (P < .05, by the t test, for the absolute change in MFI after stimulation; Figure 1 and Table 2). Changes in MFI for certain markers on pDCs were more variable (Table 2), indicating either a lesser change or greater difficulty detecting change because of a smaller numbers of cells. Changes in the percentage of positive cells were less frequently significant, particularly at the lower dose of IFN-α. Figures 1A and 1B demonstrate upregulation of activation markers on the surface of both mDCs and monocytes. In most instances, the change in MFI appeared to be driven by upregulation of CD80 and CD86 on a subset of cells, rather than by generalized upregulation in the whole population. In the case of monocytes, for which MFI changes were most clearly significant, most but not all patients demonstrated an increase in the density of activation markers (ie, CD80, CD83, CD86, and MHC class I and class II molecules) on the cell surface (Figure 1C).
Figure 1.
Upregulation of activation markers on myeloid dendritic cells (mDCs) and monocytes after in vitro stimulation with interferon α (IFN-α). A, The expression of CD80 and CD86 on mDCs is shown before (top panels) and after (bottom panels) in vitro stimulation with 400 U/mL IFN-α. Gates shown in this panel and those for CD80 and CD86 in panel B were set on the basis of fluorescence minus one controls [19]. B, The expression of various activation markers on CD14+ monocytes is shown before (top panels) and after (bottom panels) in vitro stimulation with 400 U/mL IFN-α. Gates for major histocompatibility (MHC) class I and class II (HLA-DR) molecules were set so as to maximize differences between the unstimulated and stimulated samples. C, The mean change in expression of activation markers on monocytes after in vitro stimulation with IFN-α. All changes were statistically significant (Table 2). D, The change in expression of activation markers on monocytes, by sustained virologic response (SVR) status. The uncorrected between-group differences shown here were all significant (from left to right, P = .003, .013, .049, .0007, and .002, respectively, by the rank sum test). Abbreviation: MFI, mean fluorescence intensity.
Table 2.
Statistical Significance of Changed Expression of Activation Markers on Treatment With Interferon α (IFN-α)
| Marker |
P, IFN-α 400 U/mL |
P, IFN-α 8000 U/mL |
||||
|---|---|---|---|---|---|---|
| Monocytes | mDCs | pDCs | Monocytes | mDCs | pDCs | |
| MFI | ||||||
| CD80 | <.0001 | .0145 | .036 | <.0001 | <.0001 | .3174 |
| CD83 | <.0001 | .0037 | .1283 | <.0001 | <.0001 | <.0001 |
| CD86 | <.0001 | <.0001 | .0319 | <.0001 | <.0001 | .2606 |
| HLA class I | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| HLA-DR | <.0001 | .0138 | .0068 | <.0001 | <.0001 | .2385 |
| CD69 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| PD-L1 | <.0001 | <.0001 | <.0001 | <.0001 | .0004 | .001 |
| Positive cells, % | ||||||
| CD80 | <.0001 | .3843 | .1815 | <.0001 | <.0001 | .2142 |
| CD83 | <.0001 | .2046 | .1465 | <.0001 | <.0001 | <.0001 |
| CD86 | <.0001 | .1604 | .974 | <.0001 | <.0001 | .4819 |
| HLA class I | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| CD69 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| PD-L1 | <.0001 | .0469 | .0878 | <.0001 | .0005 | <.0001 |
| CD80, CD86 | <.0001 | <.0001 | .0851 | <.0001 | <.0001 | .9742 |
| CD83, HLA class I | <.0001 | .0098 | .0167 | Not done | Not done | Not done |
| CD69, PD-L1 | <.0001 | .0029 | .0017 | <.0001 | <.0001 | <.0001 |
Nonstatistically significant data are denoted in bold.
Abbreviations: mDC, myeloid dendritic cell; MFI, mean fluorescence intensity; pDC, plasmacytoid dendritic cell.
Association of Monocyte Activation With Failure to Achieve SVR
We hypothesized that robust mDC responsiveness to IFN-α stimulation might be key to effective treatment and that, contrariwise, monocyte activation would be linked to pDC destruction and reduced viral clearance. Assuming that expression of many activation markers would be changed coordinately in different cell types, presenting an aggregate profile that is typical of each, we used principal component analysis of changes occurring with IFN-α stimulation to identify profiles and to test their association with SVR.
For each cell type, principal component analysis was performed using standardized values from all markers for which we obtained expression data from at least 180 individuals. The first principal components for each cell type, accounting for 30%, 23%, and 38% of variance in responsiveness of monocytes, mDCs, and pDCs, respectively, are presented in Table 3. In all cases, the greatest variability between patients was contributed by differences in CD80, CD83, and/or CD86 expression, as might have been expected on the basis of published literature [7, 17, 20].
Table 3.
Variable Loadings in First Principal Component and Statistical Significance
| Marker | IFN-α Dose | Loadings in First Component |
||
|---|---|---|---|---|
| Monocytes | mDCs | pDCs | ||
| MFI | ||||
| CD80 | 400 | 0.338339252 | 0.079236453 | 0.22082063 |
| CD83 | 400 | 0.266021681 | 0.359287011 | 0.34824769 |
| CD86 | 400 | 0.341448336 | 0.386120736 | 0.34861322 |
| HLA class I | 400 | 0.117921892 | 0.107787781 | −0.08790404 |
| HLA-DR | 400 | 0.26539493 | 0.377048872 | 0.17278328 |
| HLA-DR | 8000 | 0.26265885 | Not included | Not included |
| PD-L1 | 8000 | 0.275359612 | −0.017719164 | Not included |
| Positive cells, % | ||||
| CD80 | 400 | 0.076624761 | 0.167950116 | 0.38387577 |
| CD83 | 400 | 0.332959174 | 0.315446584 | 0.32378585 |
| CD86 | 400 | 0.241068561 | 0.437919506 | 0.42894487 |
| HLA class I | 400 | 0.179220616 | 0.095267179 | −0.1327744 |
| HLA class I | 8000 | −0.125031012 | 0.007902132 | Not included |
| CD80, CD86 | 400 | 0.197644914 | 0.448410621 | 0.46586351 |
| P | .00167 | .435 | .038343 | |
Data denote loading (contribution) of indicated markers to the first principal component, unless otherwise indicated. The greatest loading in each column is indicated in bold.
Abbreviations: mDC, myeloid dendritic cell; MFI, mean fluorescence intensity; pDC, plasmacytoid dendritic cell.
The relationships between SVR and scores on these first principal components were then tested in a logistic regression model for achievement of SVR that also included IL28B genotype, the presence of diabetes, age at treatment start, sex, and race. After we controlled for other variables, the model showed that monocyte activation was a highly significant negative predictor of SVR (P = .002; Table 3), whereas mDC and pDC activation were not. Those variables that were most heavily loaded in the first principal component for monocyte activation were also significant negative predictors of SVR; for example, all variables shown in the first column of Table 3 with loadings of >0.3 were significant individual negative predictors of SVR (Figure 1D).
Association of CD80 and MHC Class I Molecules on Monocytes With Non-SVR
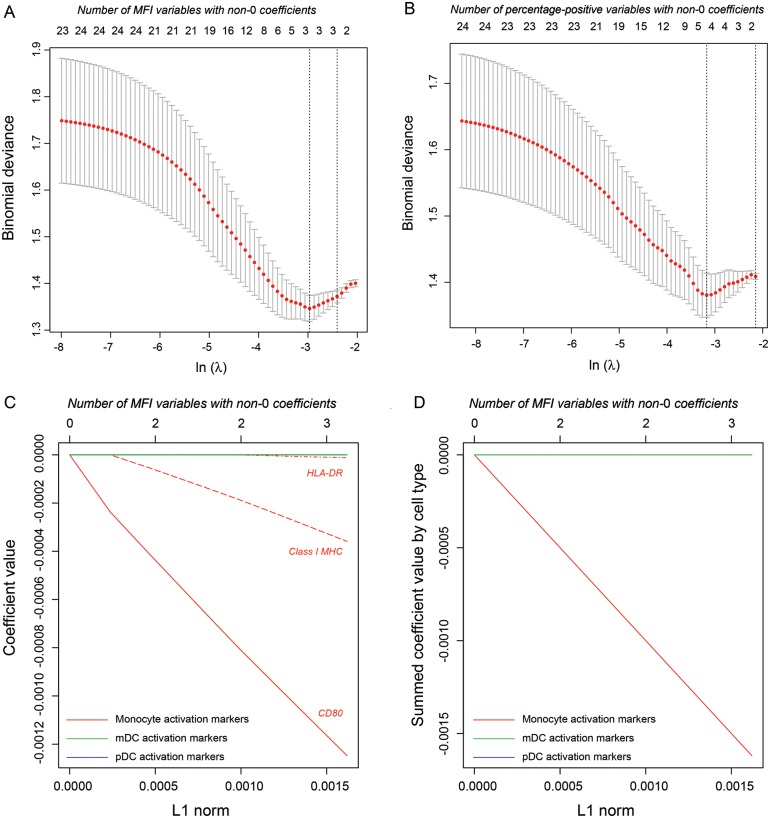
Although we initially hypothesized that robust mDC responsiveness to IFN-α stimulation would be key to effective treatment response, the first principal component of this response showed no association with SVR. Nonetheless, it was possible that analysis of only the first principal component of responsiveness had obscured other significant associations (eg, a significant positive association between some measure of mDC activation and SVR). We therefore used lasso logistic regression analysis, a technique for performing variable shrinkage and selection via least absolute deviation (L1) coefficient penalization [21], to search for other variables in the data set that might be negatively or positively associated with SVR.
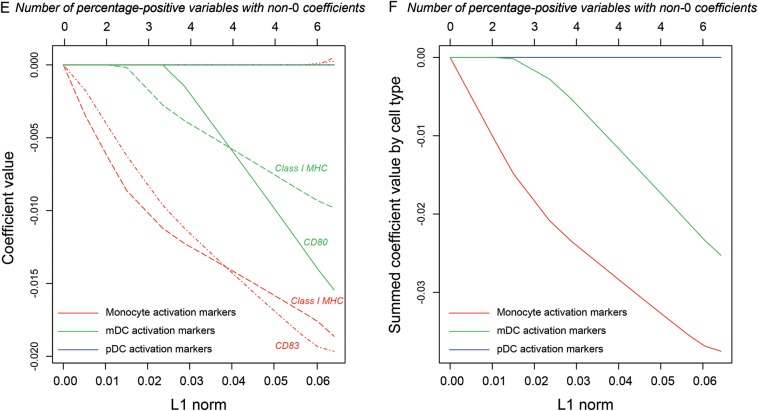
Lasso analysis was performed separately with MFI and percentage-positive variables. Cross-validation of binomial deviance for the logistic regression models showed a minimum after inclusion of 3 or 4 variables (Figure 2A and 2B, respectively). We applied lasso logistic regression with the corresponding penalty, as well as slightly relaxed values that corresponded to inclusion of 6 variables (Figures 2C and 2E, respectively). Coefficients for regression on MFI variables again demonstrated a dominant relationship between monocyte activation in response to IFN-α and failure to achieve SVR, with expression of CD80 and MHC class I molecules on monocytes most highly associated (Figure 2C). We also plotted summed coefficients to demonstrate the joint tendency of monocyte activation markers after IFN-α stimulation to be associated with failure to achieve SVR (Figure 2D). Regression on percentage-positive variables revealed a negative association between SVR and activation markers on monocytes and possibly also on pDCs (Figures 2E and 2F).
Figure 2.
Lasso regression of activation markers as predictors of achievement of sustained virologic response. A, Cross-validation of the regression model for mean fluorescence intensity (MFI) variables as the penalty parameter lambda is decreased (abscissa, bottom label) and the number of included variables is correspondingly increased (abscissa, top label). The cross-validated deviance reaches a minimum after inclusion of 3 variables. B, Cross-validation of the regression model for percentage-positive variables. C, Coefficient values in the regression model for MFI variables as the L1 norm (ie, the sum of the absolute values of coefficients) increases (abscissa) and the number of variables with non-0 coefficients correspondingly increases. All MFI variables chosen by the lasso algorithm for inclusion in the model are measures of monocyte activation. This panel shows individual coefficient values. D, Coefficients in the regression model for MFI variables summed by cell type. E, Individual coefficient values in the regression model for percentage-positive variables. F, Coefficients in the regression model for percent positive variables summed by cell type. Abbreviations: mDC, myeloid dendritic cell; MHC, major histocompatibility complex; pDC, plasmacytoid dendritic cell.
As noted above, there was a strong and significant relationship between monocyte activation and failure to achieve SVR, even after controlling for the effects of other important predictors such as IL28B genotype, the presence of diabetes, age, sex, and race. It was difficult, however, to test the significance of the association between pDC expression of CD80 and/or MHC class I and SVR, because calculations of statistical significance for the strongly biased estimates that arise from penalized regression methods such as the lasso are currently experimental [22]. Nevertheless, we note that only one of these pDC predictors (MHC class I upregulation) and not the other (CD80 upregulation) was significantly associated with SVR in the full logistic regression model, which included IL28B genotype, the presence of diabetes, age, sex, and race as covariates.
Importance of Monocyte Activation Predictors of SVR
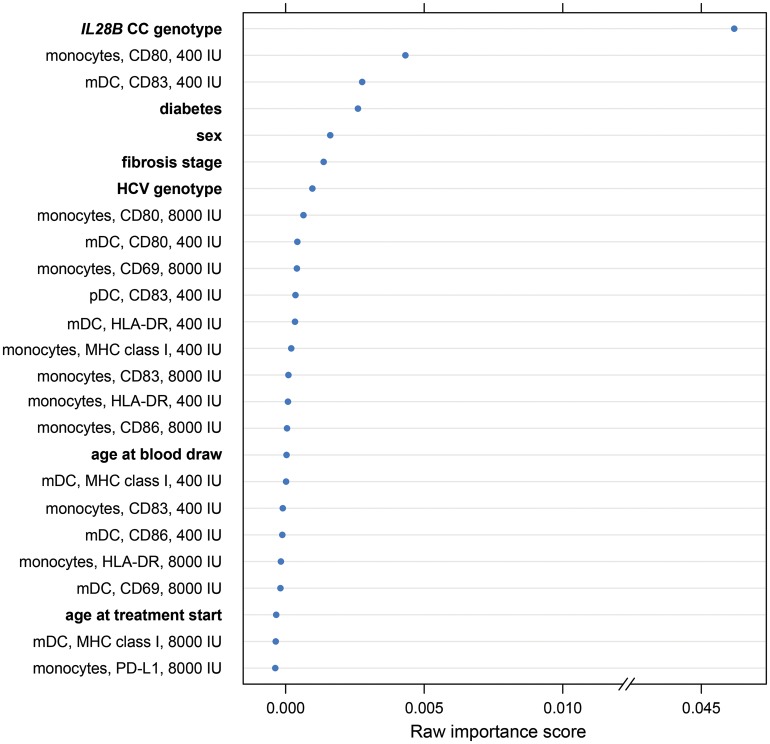
We wished to understand whether variable monocyte activation might have a dominant or at least an important influence on SVR, relative to that of well-known predictors such as IL28B genotype. To complement the lasso approach above, we used a random forest approach that, in addition to achieving good predictive performance, provides measures of variable importance. Thus, random forests consist of a large ensemble of classification trees (here, 1000 trees) that use both bootstrap resampling and random subsets of the independent variables to improve the classification accuracy (here, in predicting SVR status) of individual trees by averaging over the ensemble, thereby reducing prediction variance [23, 24]. The importance of a given variable is reflected by a decrease in successful prediction of SVR status when its values are permuted within the data set; that is, the method tests for a decrease in successful prediction of SVR when the observed values of a given variable are randomly assigned to study subjects. We used a conditional method of testing variable importance so that correlated variables were not given undue weight [25].
Fifty-three different variables, including 42 variables reflecting APC responsiveness to in vitro IFN-α stimulation based on MFI changes, were used in the analysis. The 25 most important variables rated by the forest are shown in Figure 3. As would have been predicted by the existing literature [26,27], interleukin 28B status, the presence of diabetes, and sex were all rated as important variables. Note that the relatively low importance of race is expected because study subjects were chosen so that people of different race/ethnicity would be evenly distributed in SVR and non-SVR groups. The second most important variable identified by the algorithm, however, was upregulation of CD80 on monocytes after in vitro stimulation with IFN-α, while upregulation of MHC class I and of CD83 was rated just after the presence of diabetes. These observations suggest that testing in vitro responsiveness of monocytes to IFN-α stimulation may provide predictive power intermediate between that of diabetes and IL28B genotype.
Figure 3.
Variable importance by random forest methodology. This chart lists the top 25 most important variables for classification of subjects into groups of those with and those without sustained virologic response (SVR), using classification trees. Monocyte activation in the presence of interferon α (IFN-α), as assessed by CD80 mean fluorescence intensity, is second only to IL28B genotype. Abbreviations: HCV, hepatitis C virus; mDC, myeloid dendritic cell; MHC, major histocompatibility complex; pDC, plasmacytoid dendritic cell.
Influence of Continued HCV Infection on Monocyte Activation
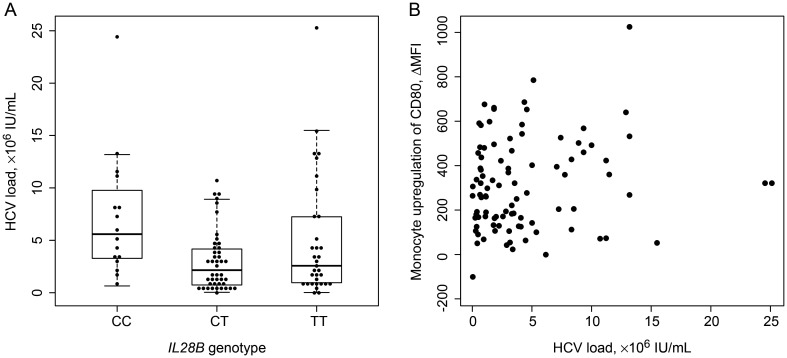
It remained possible that monocyte activation after IFN-α stimulation appeared to be associated with failure to achieve SVR because HCV infection itself alters the capacity of monocytes to respond in the ex vivo assay. Should this be the case, we reasoned that the effect might be greater in those with a higher viral load. To test this possibility, we determined whether there was a consistent relationship (in the non-SVR group) between viral load and the activation parameters identified above as being important. In so doing, we noted that presence of the IL28B CC genotype was associated with higher viral load, indicating that such an association does not necessarily indicate an effect of HCV on the predictor (Figure 4A [26]). In any case, viral load was not correlated with the important predictor identified by a random forest: upregulation of CD80 on monocytes after in vitro stimulation with IFN-α (Figure 4B). In fact, viral load was not significantly associated with changes in the MFI of any of the markers examined. Nonetheless, it remains possible that the presence of HCV affects the conditioning of cells.
Figure 4.
Association of important predictors of sustain virologic response (SVR) with viral load. A, The IL28B CC genotype is associated with higher viral load among subjects not achieving SVR, as previously reported [26]. Note that this association is in contrast to that which might be expected from a genotype associated with clearance. B, Hepatitis C virus (HCV) load is not predictive of in vitro monocyte activation (P = .16, by linear regression). Abbreviation: MFI, mean fluorescence intensity.
DISCUSSION
In this study, we documented a clear and robust association between SVR and lower levels of monocyte activation in response to in vitro IFN-α stimulation. This association was observed when evaluating a broad, overall measure of monocyte activation (the first principal component of monocyte responses to IFN-α) and also in the case of many individual monocyte activation markers. The association was statistically significant after we controlled for other known factors influencing SVR and was important in distinguishing patients who achieved SVR from those who did not. In contrast, and contrary to our initial hypothesis, neither mDC nor pDC activation in response to IFN-α was reliably associated with achievement of SVR.
Because of the cross-sectional nature of this study, we do not know whether monocyte responsiveness is an important cause of treatment failure or, rather, a correlate of ongoing HCV infection. This question is currently being addressed in a prospective clinical study; however, we believe that a direct effect of viremia on in vitro activation by IFN-α is unlikely. First, there was no association between viral load and in vitro monocyte activation, despite the fact that many individuals not achieving SVR had extremely low viral loads. Second, alanine aminotransferase levels at time of blood collection for subjects with ongoing viremia were not correlated with monocyte activation, suggesting that monocyte responses were not linked to inflammation. Third, the assay used in this study was performed ex vivo and presumably in the presence of little or no virus, regardless of infection status. Finally, only ex vivo IFN-α–stimulated, rather than basal, monocyte activation parameters correlated consistently with failure to achieve SVR (data not shown), suggesting that there are intrinsic differences in IFN-α responsiveness rather than baseline effects due to viremia. Nevertheless, priming effects on monocytes due to recent in vivo viral exposure cannot be excluded.
It will be interesting to determine whether low levels of monocyte responses to IFN-α are predictive of responses only to those regimens containing IFN-α or will also predict response to IFN-sparing regimens. It is possible that, as proposed by others [13], monocyte responsiveness and activation are important drivers of HCV immunopathology. Individuals with highly responsive monocytes may produce larger amounts of tumor necrosis factor α and IL-10, resulting in destruction of pDCs and making the disease more difficult to treat, irrespective of the regimen used.
Our observations are reminiscent of previous findings of a paradoxical association between IFN-α responsiveness and treatment failure. Honda et al showed that expression of hepatic IFN-stimulated genes (ISGs) in the liver before treatment is associated with treatment failure and a detrimental IL28B haplotype [28]. This finding is surprising because it suggests that responsiveness to IFN-α can be detrimental even though IFN-α can clearly serve as an antiviral agent. In the present study, monocyte responsiveness to IFN-α was associated with treatment failure but not with IL28B haplotype. A more recent study confirmed that basal intrahepatic expression of ISGs is linked to nonresponse and suggested that Kupffer cells are an important source of the IFN-α that drives ISG expression and may also induce innate immune tolerance [29]. Given the close relationship between blood monocytes and Kupffer cells, it seems possible that our in vitro assay and the differences in Kupffer cell activity proposed by Lau et al are linked.
Finally, these results may have implications for understanding individual outcomes seen in other chronic viral infections. For example, although IFN-α can inhibit HIV replication in vitro, it has a number of regulatory effects that might contribute to immune activation and associated HIV immunopathology in vivo, including upregulation of MHC class I [30], the generation of naive CD8low T-cells that are functionally anergic [31, 32], and the upregulation of CCR5 on intrathymic T-cell progenitors [33]. Variable monocyte responsiveness to HIV infection may therefore play an important role in the balance between productive immunity and immunopathology, just as in the case of HCV infection as described here.
Supplementary Material
Notes
Acknowledgments. We thank the many Northern California Kaiser Permanente HCV treatment providers who allowed recruitment of their patients; the patients who generously participated in the STRIDE retrospective treatment cohort; Roche Molecular Systems, for HCV RNA testing reagents; Laurie Grinnell, Rosemary Murphy, and Sharon Kidd, for extraordinary patient enrollment efforts; and Rosemary McQuaid, for assistance with regulatory compliance.
Disclaimer. The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by the National Institutes of Health (NIH; grants U19 AI088790 [to the Bay Area Hepatitis C Cooperative Research Center], P30 DK026743 [to the UCSF Liver Center], and 5K23AI081540 [to D. J. H.-O.]); the Intramural Research Program, Center for Cancer Research, National Cancer Institute, NIH (support to M. P. M. and M. C.); and the American Cancer Society (professorship to L. L. L.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–52. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 2.Gruener NH, Lechner F, Jung MC, et al. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol. 2001;75:5550–8. doi: 10.1128/JVI.75.12.5550-5558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mengshol JA, Golden-Mason L, Rosen HR. Mechanisms of disease: HCV-induced liver injury. Nat Clin Pract Gastroenterol Hepatol. 2007;4:622–34. doi: 10.1038/ncpgasthep0961. [DOI] [PubMed] [Google Scholar]
- 4.Wedemeyer H, He XS, Nascimbeni M, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–58. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 5.Rosen HR, Weston SJ, Im K, et al. Selective decrease in hepatitis C virus-specific immunity among African Americans and outcome of antiviral therapy. Hepatology. 2007;46:350–8. doi: 10.1002/hep.21714. [DOI] [PubMed] [Google Scholar]
- 6.Barnes E, Gelderblom HC, Humphreys I, et al. Cellular immune responses during high-dose interferon-alpha induction therapy for hepatitis C virus infection. J Infect Dis. 2009;199:819–28. doi: 10.1086/597072. [DOI] [PubMed] [Google Scholar]
- 7.Barnes E, Salio M, Cerundolo V, et al. Impact of alpha interferon and ribavirin on the function of maturing dendritic cells. Antimicrob Agents Chemother. 2004;48:3382–9. doi: 10.1128/AAC.48.9.3382-3389.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jinushi M, Takehara T, Kanto T, et al. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003;170:1249–56. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 9.Luft T, Luetjens P, Hochrein H, et al. IFN-alpha enhances CD40 ligand-mediated activation of immature monocyte-derived dendritic cells. Int Immunol. 2002;14:367–80. doi: 10.1093/intimm/14.4.367. [DOI] [PubMed] [Google Scholar]
- 10.Padovan E, Spagnoli GC, Ferrantini M, Heberer M. IFN-alpha2a induces IP-10/CXCL10 and MIG/CXCL9 production in monocyte-derived dendritic cells and enhances their capacity to attract and stimulate CD8+ effector T cells. J Leukoc Biol. 2002;71:669–76. [PubMed] [Google Scholar]
- 11.Miyatake H, Kanto T, Inoue M, et al. Impaired ability of interferon-alpha-primed dendritic cells to stimulate Th1-type CD4 T-cell response in chronic hepatitis C virus infection. J Viral Hepat. 2007;14:404–12. doi: 10.1111/j.1365-2893.2006.00814.x. [DOI] [PubMed] [Google Scholar]
- 12.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–91. [PubMed] [Google Scholar]
- 13.Dolganiuc A, Chang S, Kodys K, et al. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758–68. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 14.Dolganiuc A, Oak S, Kodys K, et al. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513–24. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 15.ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice. J Postgrad Med. 2001;47:199–203. [PubMed] [Google Scholar]
- 16.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000;284:3043–5. [PubMed] [Google Scholar]
- 17.Luft T, Pang KC, Thomas E, et al. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–53. [PubMed] [Google Scholar]
- 18.Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84:1744–50. doi: 10.1002/jmv.23399. [DOI] [PubMed] [Google Scholar]
- 19.Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Decalf J, Fernandes S, Longman R, et al. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med. 2007;204:2423–37. doi: 10.1084/jem.20070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tibshirani R. Regression shrinkage and selection via the lasso. J R Statist Soc B. 1996;58:267–88. [Google Scholar]
- 22.Lockhart R, Taylor J, Tibshirani R, Tibshirani R, Johnstone I. A significance test for adaptive linear regression. Annals of Statistics. 2013 [Google Scholar]
- 23.Breiman L. Random forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 24.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning. New York: Springer; 2009. [Google Scholar]
- 25.Strobl C, Boulesteix A-L, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinformatics. 2008;9:307. doi: 10.1186/1471-2105-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 27.Conjeevaram HS, Fried MW, Jeffers LJ, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470–7. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Honda M, Sakai A, Yamashita T, et al. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology. 2010;139:499–509. doi: 10.1053/j.gastro.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 29.Lau DT-Y, Negash A, Chen J, et al. Innate immune tolerance and the role of Kupffer cells in differential responses to interferon therapy among patients with HCV genotype 1 infection. Gastroenterology. 2012;144:402–13. doi: 10.1053/j.gastro.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keir ME, Stoddart CA, Linquist-Stepps V, Moreno ME, McCune JM. IFN-alpha secretion by type 2 predendritic cells up-regulates MHC class I in the HIV-1-infected thymus. J Immunol. 2002;168:325–31. doi: 10.4049/jimmunol.168.1.325. [DOI] [PubMed] [Google Scholar]
- 31.Favre D, Stoddart CA, Emu B, et al. HIV disease progression correlates with the generation of dysfunctional naive CD8(low) T cells. Blood. 2011;117:2189–99. doi: 10.1182/blood-2010-06-288035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keir ME, Rosenberg MG, Sandberg JK, et al. Generation of CD3+CD8low thymocytes in the HIV type 1-infected thymus. J Immunol. 2002;169:2788–96. doi: 10.4049/jimmunol.169.5.2788. [DOI] [PubMed] [Google Scholar]
- 33.Stoddart CA, Keir ME, McCune JM. IFN-alpha-induced upregulation of CCR5 leads to expanded HIV tropism in vivo. PLoS Pathog. 2010;6:e1000766. doi: 10.1371/journal.ppat.1000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.