Abstract
Background
Irritable bowel syndrome (IBS) is a heterogeneous disorder with abdomen pain as one of the primary symptoms. The etiology of IBS remains unknown. Epidemiological studies found that a subset of these patients have a history of adverse early-life events. We tested the hypothesis that chronic prenatal stress (CPS) epigenetically enhances brain-derived neurotrophic factor (BDNF) in spinal cord to aggravate colon sensitivity to colorectal distension (CRD) differentially in male and female offspring.
Methods
We used heterotypic intermittent chronic stress (HeICS) protocols in pregnant dams from E11 until delivery.
Results
CPS induced significant visceral hypersensitivity (VHS) to CRD in male and female offspring. A second exposure to HeICS in adult offspring exacerbated VHS greater in female offspring that persisted longer than in male offspring. CPS upregulated BDNF expression in the lumbar-sacral dorsal horn that correlated with the exacerbation of VHS in female, but not in male offspring. The upregulation of BDNF was due to a significant increase in RNA Pol II binding, histone H3 acetylation and significant decrease in histone deacetylase 1 association with the core promoter of BDNF in female offspring. Other chronic prenatal and neonatal stress protocols were less effective than HeICS.
Conclusion & Inferences
The development of visceral hypersensitivity, which contributes to the symptom of intermittent abdominal pain, is a two-step process, chronic in utero stress followed by chronic stress in adult-life. This two-step process induces aggravated and persistent colon hypersensitivity in female than in male offspring. Our preclinical model explains several clinical features in IBS patients.
INTRODUCTION
Irritable bowel syndrome (IBS) is a heterogeneous disorder characterized primarily by episodes of abdomen pain and altered bowel habits (diarrhea, constipation or alternating diarrhea and constipation). These symptoms show inter- and intra-individual variations and IBS has significant overlap with other gastrointestinal (functional dyspepsia, nausea and vomiting) and nongastrointestinal (fibromyalgia, lower back pain, neuroticism, anxiety headaches and non-specific fatigue) pain and affective disorders (1–6). Together, these findings suggest that IBS is a complex disease rooted in environmental factors that may concurrently affect multiple cell-types and organs in a variable profile.
Overwhelming clinical, experimental and epidemiological findings have built support for a strong role of early-life stressors in complex diseases, including metabolic syndrome, cardiovascular disease and impaired neural development (7–9). A subset of IBS patients also report a history of childhood adverse events (10–17), suggesting that these events might have aberrantly programmed some genes in gut-related organs to cause dysfunction. Preclinical studies in rodents support this concept; maternal deprivation or inflammation in male rodent neonates induces visceral hypersensitivity (VHS) to colorectal distension (CRD) and impairs smooth muscle function, which respectively contribute to visceral hyperalgesia and motility dysfunction (18–27). The window of susceptibility to develop adult diseases by epigenetic programming in response to environmental stressors is a continuum from in utero to adult-life. Epigenetic programming is highly sensitive to changes in cellular microenvironment during in utero development, it persists to a lesser degree during neonatal development and decreases further in adult-life (10, 28–30). The greater sensitivity during in utero development is partly due to de novo programming of all genes in rapidly dividing cells. The effect of early-life adverse events on organ dysfunction in adult-life depends also on the stress paradigm and its intensity (10, 31–34). The effects of stressful events during in utero development on gut-related organ dysfunctions with respect to IBS-like symptoms remain unknown.
We tested the hypothesis that chronic prenatal stress (CPS) induces visceral hypersensitivity in male and female offspring in adult-life. However, the female offspring show a markedly greater and prolonged increase of visceral sensitivity to CRD than the male offspring, when challenged by chronic stress in adult-life. We also investigated whether adult chronic stress increases BDNF expression in the lumbar-sacral dorsal horn differentially in female and male offspring, which underlies the differential sensory responses between sexes, following chronic stress in adult-life. We found that CPS modifies the chromatin structure of select Bdnf promoters to upregulate BDNF expression.
Materials and Methods
Animal models
Experiments were performed on pregnant Sprague-Dawley rats and their adult offspring. IACUC at UTMB approved all procedures. Pregnant dams were subjected to various psychological stress protocols. A heterotypic intermittent chronic stress (HeICS) protocol consisted of a random sequence of twice-daily (9–11 AM and 3–5 PM) applications of one of three stress sessions, one-hour water avoidance stress (WAS), 45-min cold restraint stress (CRS) or 20-min forced swim stress (FSS) (35, 36), starting on E11 and continuing until delivery. Male and female offspring from the stressed dams were designated chronic prenatally stressed (CPS) rats. Control dams received sham stress and their offspring designated control rats. Adult CPS and control rats (8–12 weeks old) were subjected to the same twice daily HeICS protocol (2× HeICS), as above for nine days. In a separate experiment, pups born to unstressed dams were subjected to maternal deprivation (MD) for 3 hours each morning from postnatal day 2 through day 14 (18). Visceromotor response (VMR) to graded CRD was measured after at least one-week recovery from abdominal electrode implantation (36).
Measurement of viscero-motor response (VMR) to graded CRD
Two electrodes were implanted under general 2% isoflurane anesthesia in the external oblique abdominal muscle and exteriorized in the subscapular region. Rats were allowed one week to recover from surgery, a 5 cm long balloon constructed from a surgical glove finger and attached to tygon tubing was inserted 7 cm into the descending colon and rectum. Rats were placed in small Lucite cubicles (20×8× cm) and allowed to adapt for 30 minutes. CRD was performed by rapidly inflating the balloon to constant pressures: 20, 30, 40, 50, 60 & 80 mm Hg, for 20 seconds followed by 2-minute rest.
Electromyographic (EMG) activity from the external oblique muscle was recorded on Biopac equipment (Biopac Systmes, Inc., Santa Barbara, CA). The EMG signal was amplified, filtered at 300 Hz and digitized. The area under the curve (AUC) for the EMG signal during each 20 seconds of distention was calculated using Acknowledge software (Biopac Systmes, Inc). The net value for each distension period was calculated by subtracting the baseline value derived from the average AUC for 20 seconds before and 20 seconds after the distention period.
Laser capture microscopic (LCM) dissection
A midline abdominal incision was made to expose the descending colon. Cholera Toxin subunit B Alexa 488 conjugate (CTB-488), 0.05 mg/rat in PBS was injected into the wall of the descending colon using a 10 µl Hamilton syringe. Approximately ten, 2 µl injections were made per rat. DRG were collected 6 days later and frozen in Optimal temperature cutting medium on dry ice. Twelve-micron sections, prepared from both S1 DRGs were fixed in 75% ethanol and dehydrated. We identified CTB-488 labeled neuronal profiles and captured them with a Pixel IIe LCM microscope (Applied Biosystems, Foster City, CA). RNA was prepared with a Qiagen microRNA kit. SYBR green RT-PCR was performed with Applied Biosystem’s reagents and Step One Plus real-time PCR apparatus. We used 18S rRNA as a normalizer and compared fold-change to control females subjected to chronic stress by using the DDCt procedure. Primers were designed using Primer Express Software (Applied Biosystems) and validated through control experiments: a single amplimer was observed by melting curve analysis; no amplimer was produced without reverse transcription or template; amplification efficiency was 100%.
Intrathecal treatments
Thirty-two gauge catheters were inserted through the atlanto-occipital membrane and extended to T8 (36). The location of each catheter was confirmed following euthanasia. Rats received either BDNF antagonist trkB-Fc (R&D Systems, Minneapolis, MN), 5 µg in 10 µl sterile saline or vehicle once/day during the 9-day stress period or BDNF siRNA or control siRNA (2 µg of the appropriate siRNA; 1:5 v/v with i-Fect transfection reagent Neuromics, Edina, MN). Each rat received 2ug siRNA/10 µl.) on days 1, 3, 5 and 7 of the 9 day stress period.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed as described previously (37). Antibodies used for immunoprecipitation were Histone H3 acetyl Rabbit pAb (Cat. # 39139), HDAC1 Mouse mAb (cat. # 39531) and RNA pol II mAb (Cat. # 39097), all from Active Motif, Carlsbad, CA. Rabbit IgG was used for mock precipitation. Precipitated DNA, SYBR Green Master Mix (Applied Biosystems, Foster City, CA), and primers specific to the Bdnf promoter IX (−95/−26) were used for real-time polymerase chain reaction (PCR). Fold-differences in precipitated DNA were normalized against input. See table 1 for primers.
Table 1.
Primers for ChIP assays and qRT-PCR
| Application | Primer Name | Primer Sequence (5’ to 3’) |
|---|---|---|
| ChIP Assay | PIX-Core-F | aacaccgtggtccgaagtgct |
| PIX-Core-R | tctgagcaggagagctgggacg | |
| qRT-PCR | Bdnf-III-F | cctttctattttccctccccgagagt |
| Bdnf-IV-F | ctctgcctagatcaaatggagcttc | |
| Bdnf-VI-F | gctggctgtcgcacggtccccatt | |
| Bdnf-VIII-F | gtgtgtgtctctgcgcctcagtgga | |
| Bdnf-IX-F | ccagagctgctaaagtgggaggaag | |
| Bdnf-R | gaagtgtacaagtccgcgtcctta | |
| Hprt-F | gatgatgaaccaggttatgac | |
| Hprt-R | gtccttttcaccagcaagcttg |
Real-time RT-PCR
Total RNA was extracted by using RNeasy Mini Kit (QIAGEN, Valencia, CA). One microgram of total RNA was reverse-transcribed using SuperScript™ III First-Strand Synthesis System for RT-PCR (Invitrogen). Bdnf mRNA levels were measured by TAQMAN RT-PCR using a StepOnePlus thermal cycler with 18S as the normalizer using Applied Biosystems primer/probe set Rn02531967_s1 directed against the translated exon IX. Fold-change relative to control was calculated using the ΔΔCt method (Applied Biosystems). Measurement of untranslated Bdnf exon mRNA was performed with SYBR Green Master Mix (Applied Biosystems, Foster city, CA). Hprt was quantified as internal control for the amount and quality of cDNA. See table 1 for primers.
Western Blot
Previously described procedures were used (38). BDNF antibody (Santa Cruz Biotechnologies, Santa Cruz, CA) was used at 1/200 dilution; β-actin antibody (Sigma Aldrich, St Louis) was used at 1/5000 dilution. Secondary antibodies used were donkey anti-rabbit alexa fluor 680 (Invitrogen) and goat anti-mouse IRDye 800 (Rockland). Images were acquired and band intensities measured using the Li-Cor Odyssey system (Li-Cor, Lincoln, Nebraska).
Statistics
The area under the colorectal distention-response curve was used for analyzing colon sensitivity. We performed a two-way ANOVA to determine CPS, adult HeICS and sex effects on colonic sensitivity among different groups of rats. If significant main effects were present, the individual means were compared using the Tukey post-hoc test. For intrathecal experiments, two-way repeated measures ANOVA with treatment as the between factor and distention pressure as the repeated factor; Tukey post-hoc test was used to compare individual means.
Results
Chronic prenatal stress exacerbates visceral sensitivity to CRD following adult chronic stress
We measured baseline VMR to graded CRD in four groups of rats: Control female (Ctr. F), CPS female (CPS F), control male (Ctr. M) and CPS male (CPS M). Two-way ANOVA detected a significant main effect of CPS (F1, 49=46.3, p<0.001), but no significant main effect of sex (F1, 49=1.1, p=0.3) or interaction (F1, 49=0.54, p=0.47) (Figure 1A, baseline). Within each sex, Tukey post-hoc test found significant increase in VMR to CRD in CPS male and female rats vs. their respective controls (p<0.001 for each sex), suggesting that CPS enhanced visceral sensitivity similarly in male and female adult offspring.
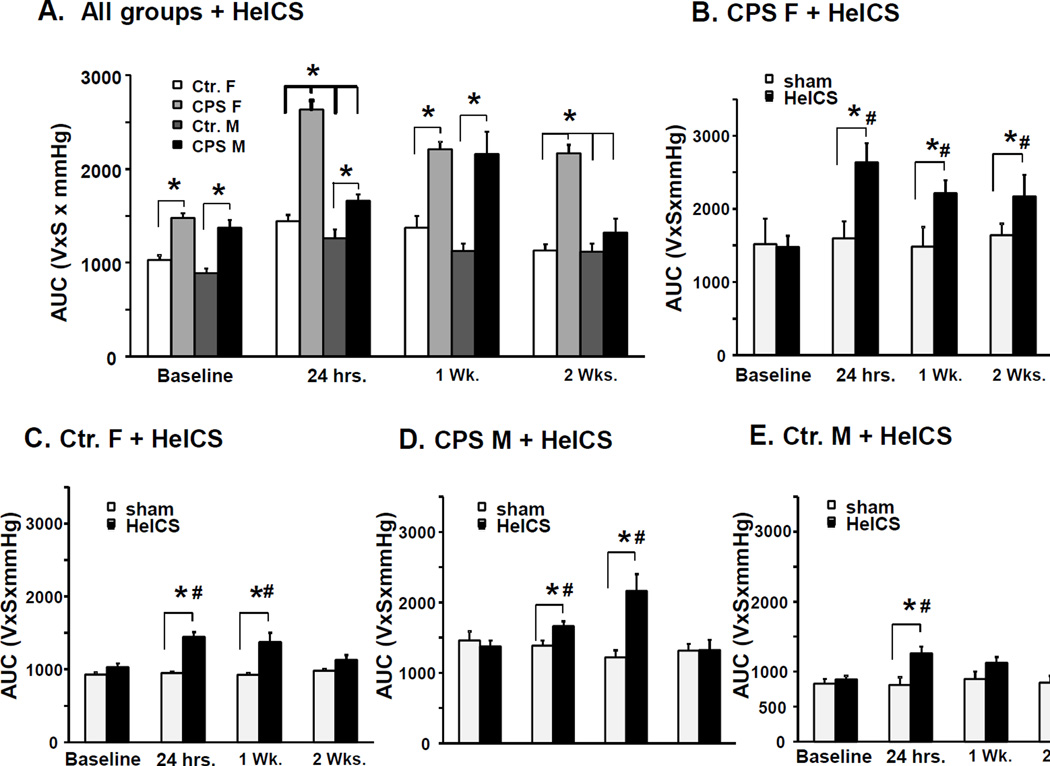
Figure 1.
Effects of CPS followed by adult chronic stress on colon sensitivity to CRD in male and female rats. A. Bar graphs display area under the curve of EMG to graded CRD of male and female CPS and control rats before stress at 24 hours, 1 week and 2 weeks after the end of adult 2× HeICS, *p<0.05 vs. baseline. Baseline measurements were obtained from female Ctr (n=12), female CPS (n=12), male Ctr (n=20) and male CPS (n=10) rats. Ctr. (n=8), female CPS (n=8), male Ctr (n=13) and male CPS (n=6) rats were subjected to the nine day 2× HeICS protocol, the remaining rats were sham stressed. B. Bar graphs display the average response to CRD of female CPS rats before and 24 hours, 1 week and 2 weeks after adult 2× HeICS compared to sham stressed female CPS rats. We observed a significant main effect of the interaction of stress×time (F3, 30=4.4, p=0.01). Sensitivity to CRD in stressed CPS female rats was significantly greater than the pre-stress baseline and the sham stressed CPS female rats at 24 hours (p<0.001 and p=0.01, respectively), 1 week (p=0.001and p=0.02, respectively) and two weeks (p=0.002 and p=0.04, respectively). C. Bar graphs display the average response to CRD of female control rats before (baseline) and 24 hours, 1 week and 2 weeks after adult HeICS compared to sham stressed female control (Ctr.) rats. There was a significant main effect of stress (F3, 30=21.2, p<0.001), but not of time (F3, 30=2.12, p=0.12) or interaction between stress and time (F3, 30=2.42, p=0.085). Sensitivities to CRD were significantly greater in stressed rats compared to pre-stress baseline at 24 hours (p<0.001) and one week (p<0.001) but not at 2 weeks (p=0.24), and to sham stressed females at 24 hours (p=0.014) and 1 week (p<0.001) but not at 2 weeks (p=0.95). D. Bar graphs display the average response to CRD of male CPS rats before (baseline) and 24 hours, 1 week and 2 weeks after adult HeICS compared to sham stressed male CPS rats. There was a significant main effect of the interaction of stress×time (F3, 30=7.88, p<0.001). Sensitivity to CRD was significantly greater than pre-stress baseline at 24 hours (p=0.001) and 1 week (p=0.018), and greater than sham stressed male rats at 24 hours (p<0.001). E. Bar graphs display the average response to CRD of male Ctr. rats before (baseline) and 24 hours, 1 week and 2 weeks after adult 2× HeICS compared to sham stressed male Ctr. rats. *p<0.05 vs. sham; # p< 0.05 vs. baseline.
Next, we investigated whether the male and female CPS rats responded differentially to adult chronic stress. We subjected rats in each group to a nine-day 2× HeICS protocol or sham stress and measured VMR to graded CRD at 24 hours, 1 week and 2 weeks after the last stressor. We detected a significant interaction between CPS and sex (F1, 30=6.53, p=0.016), 24-hours following the end of adult chronic stress (Figure 1A). Post hoc tests showed that CPS female rats were significantly more sensitive than control, CPS male (p<0.001) and female control rats (p<0.001) (Figure 1A). Stressed male CPS rats were not significantly more sensitive than the stressed male control rats (p=0.072) or stressed female control rats (p=0.36). After one week post-2× HeICS, we detected a significant main effect of prenatal 2× HeICS (F1, 30=28.9, p<0.001), but no significant effect of sex (F1, 30=0.01, p=0.95) or interaction (F1, 30=2.86, p=0.1) (Figure 1A). Post-hoc tests showed that CPS female rats were more sensitive than control male and female rats (p<0.015) and that CPS male rats were more sensitive than control female and male rats (p<0.001). After two weeks, we detected a significant interaction (F1, 30=4.3, p=0.046) between sex and CPS (Figure 1A). Post hoc tests showed that CPS female rats were more sensitive than control and CPS male (p=0.01) and control female rats (p<0.001).
Adult chronic stress significantly increased visceral sensitivity in all four groups compared to sham stressed controls and to pre-stress baseline (statistics presented in figure legend). Within CPS females, sensitivity to CRD in stressed CPS female rats was significantly greater than the pre-stress baseline and the sham stressed CPS female rats at 24 hours, 1 week and two weeks (Figure 1B). Within female controls, the sensitivities to CRD were significantly greater in stressed rats compared to pre-stress baseline at 24 hours and one week, but not at 2 weeks, and to sham stressed females at 24 hours and 1 week, but not at 2 weeks (Figure 1C). Within CPS male rats, sensitivity to CRD was significantly greater than pre-stress baseline at 24 hours (p=0.001) and 1 week (p=0.018), and greater than sham stressed male rats at 24 hours (p<0.001) (Figure 1D). Within male control rats, sensitivity to CRD was significantly greater than pre-stress baseline at 24 hours, but not at 2 weeks, and greater than sham stressed male rats at 24 hours and 1 week, but not at 2 weeks (Figure 1E). No significant differences in visceral sensitivity at any time-point were detected in sham stressed rats within each experimental group. These findings demonstrated that the combination of adult chronic stress and prenatal stress produced significantly greater sensitivity in female compared to male CPS rats and between CPS female and control female rats at 24 hours, 1 week and 2 weeks after the last stressor, indicative of sex specific effects of adult chronic stress on colonic visceral sensitivity. The sensitizing effects of adult chronic stress lasted for at least two weeks in CPS female rats, but not in the other groups. The increase of visceral sensitivity following adult chronic stress occurred without any increase of MPO activity in the muscularis externa or the mucosa/submucosa (data not shown).
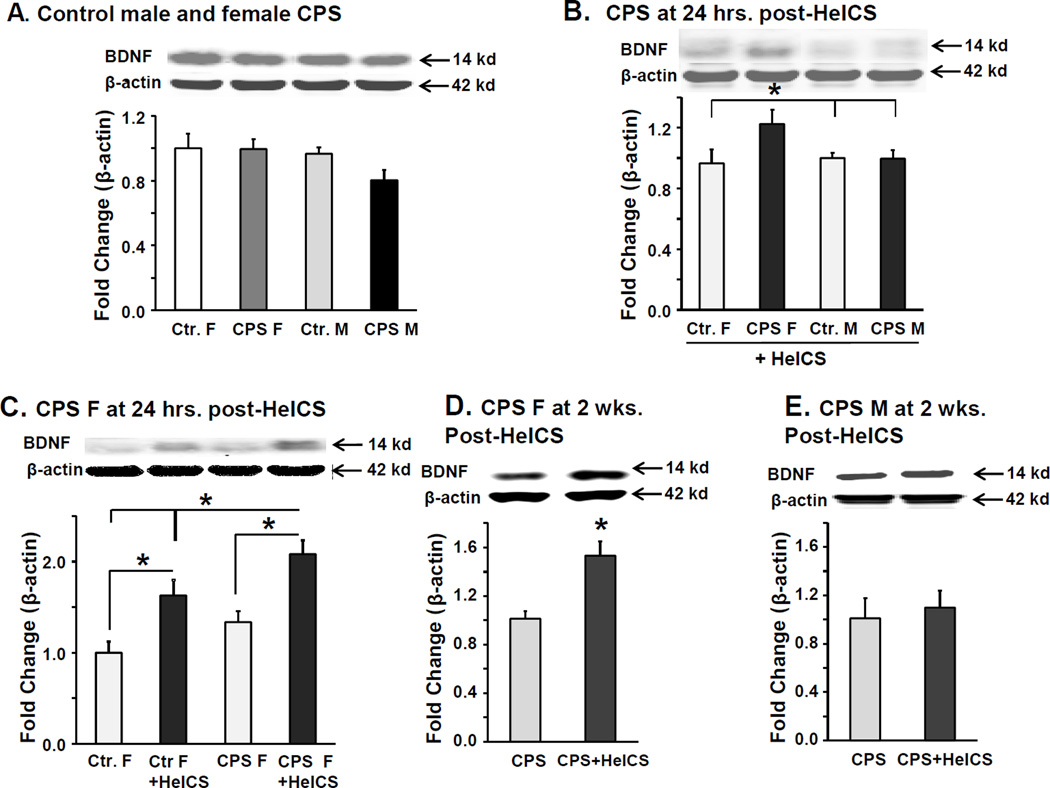
Upregulation of BDNF in LS spinal cord of female CPS rats following 2× HeICS correlated with the magnitude of visceral hypersensitivity
Given the pro-nociceptive role of BDNF in the spinal cord, we tested whether BDNF protein levels in in spinal cord LS dorsal horn correlated with the magnitude of visceral hypersensitivity in female CPS rats following HeICS. No significant differences in BDNF protein levels were detected between adult control female, CPS female, control male, and CPS male rats (Figure 2A). Twenty-four hours following 2× HeICS, we found a significant increase in BDNF protein expression in CPS female rats vs. the other three groups, which were not significantly different from each other (Figure 2B). Within females, 2× HeICS significantly increased BDNF protein in both control and CPS rats compared to sham stressed rats (Figure 2C). This increase was significantly greater in female CPS+HeICS rats vs. Ctr.+HeICS rats (p=0.014), consistent with the findings in Figure 2B. BDNF protein levels remained significantly elevated in CPS female rats 2 weeks after HeICS vs. the unstressed CPS rats (Figure 2D), whereas, in similarly treated male CPS rats, no difference was observed at 2-weeks (Figure 2E). These findings show a correlation between LS spinal cord BDNF and the magnitude of the VMR to CRD in female CPS+HeICS rats.
Figure 2.
BDNF is preferentially upregulated in LS spinal cord of female CPS+HeICS rats. A. Western blots show BDNF expression in LS spinal cord dorsal horns from female and male Ctr. and CPS rats at baseline, n=6. B. Western blots show BDNF expression in LS spinal cord dorsal horns from female and male control (Ctr.) and CPS rats, 24 hours after adult HeICS (*p<0.05). (2 way ANOVA: prenatal, F1,24=9.27, p=0.006, sex, , F1,24=0.61, p=0.44, interaction, F1,24=3.36, p=0.079, CPS F vs. Ctr. F p=0.002, vs. Ctr. M p=0.006, vs. CPS M p=0.047. n=6) C. Western blots show BDNF expression in LS spinal cord dorsal horns from female sham stressed and stressed Ctr. and CPS rats 24 hours after adult HeICS (*p<0.05). (2 way ANOVA: prenatal, F1,24=10.7, p=0.003, adult stress, F1,24=32.7, p<0.001, interaction, F1,24=.25, p=0.62, within HeICS, CPS F vs. Ctr. F p=0.014, within CPS F, stress vs. sham, p<0.001, within Ctr. F, stress vs. sham, p=0.001, n=6). D. Western blots show BDNF expression in LS spinal cord dorsal horns from female sham stressed and 2× HeICS stressed CPS rats 2 weeks after adult HeICS (*p<0.05, n=6). E. Western blots show BDNF expression in LS spinal cord dorsal horns from male sham stressed and 2× HeICS stressed CPS rats 2 weeks after adult HeICS, n=6.
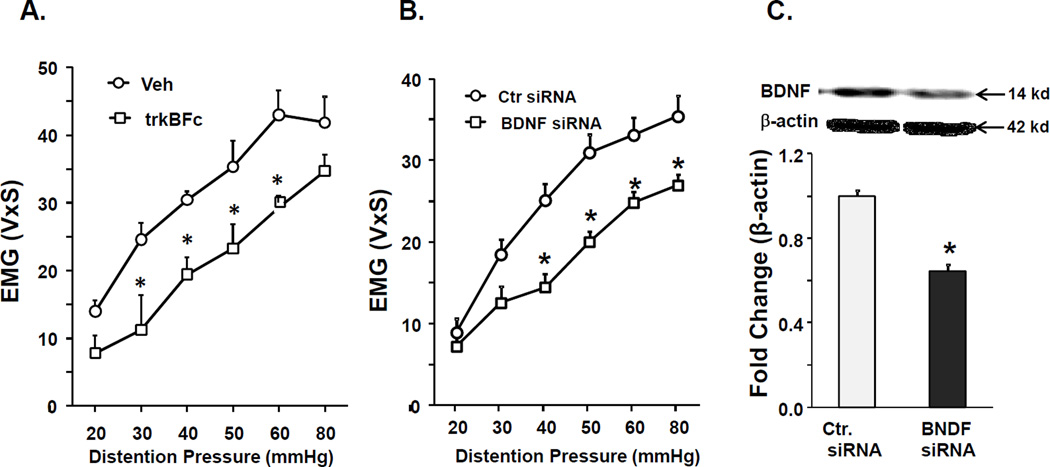
We performed interventional experiments to investigate the functional relevance of BDNF upregulation to visceral hypersensitivity in female CPS+HeICS rats using intrathecal infusion of either BDNF sequestration agent trkBFc or BDNF siRNA, followed by measurement of VMR at 24 hours after the last stressor. Rats received trkBFc once per day for the last five days of the 2× HeICS protocol. This treatment produced a significant decrease in VMR to CRD 24 hours after 2× HeICS compared to vehicle treated CPS+HeICS rats (Figure 3A). siRNA was administered on days 4, 6 and 8 of the stress protocol. This treatment produced a significant decrease in VMR to CRD in CPS+HeICS female rats 24 hours after HeICS compared to CPS+HeICS rats treated with control siRNA (Figure 3B). BDNF siRNA treatment significantly reduced BDNF protein expression in the LS dorsal horn (Figure 3C). These data supported a role of BNDF increase in the LS spinal cord in enhancing visceral sensitivity to CRD in female CPS+HeICS rats.
Figure 3.
Intrathecal treatment with BDNF antagonists reduced VMR to CRD in female CPS+HeICS rats. A. Graph shows that intrathecal administration of BDNF antagonist trkBFc in female CPS rats significantly decreased VMR to CRD, 24 hours following adult 2× HeICS (2 way repeated measures ANOVA found a significant main effect of treatment, F1,53=10.4, p=0.015; post hoc tests found significant differences at 30 mmHg, 40 mmHg, 50 mmHg and 60 mmHg, n=4). B. Graph shows that intrathecal administration of BDNF siRNA in female CPS rats significantly decreased VMR to CRD, 24 hours following adult 2× HeICS (2 way repeated measures ANOVA: treatment×pressure interaction, F1,77=3.49, p=0.008, tukey post hoc tests found significance at 30 mmHg, p=0.013 and at 40, 50 50, 80 mmHg, p<0.001, n=7 Ctr., n=6 BDNF siRNA). C. Western blot shows a significant decrease in spinal cord BDNF protein expression in rats treated with BDNF siRNA (*p<0.05).
HeICS epigenetically upregulates spinal cord BDNF in CPS rats
To distinguish between the relative contributions of primary afferents and spinal cord neurons in upregulating BDNF in female CPS+HeICS rats, we measured BDNF mRNA in colon projecting primary afferents in S1 DRG isolated by laser capture microdissection and in LS dorsal horns. In LS dorsal horns, BDNF mRNA was significantly greater in CPS+HeICS female rats vs. CPS control and Ctr.+HeICS rats (Figure 4A). By contrast, in colon projecting neurons, there was no significant difference in BDNF mRNA levels between CPS+HeICS and Ctr.+HeICS rats (Figure 4B). These findings suggested that the source of enhanced spinal cord dorsal horn BNDF expression in CPS+HeICS female rats was the cells within the spinal cord.
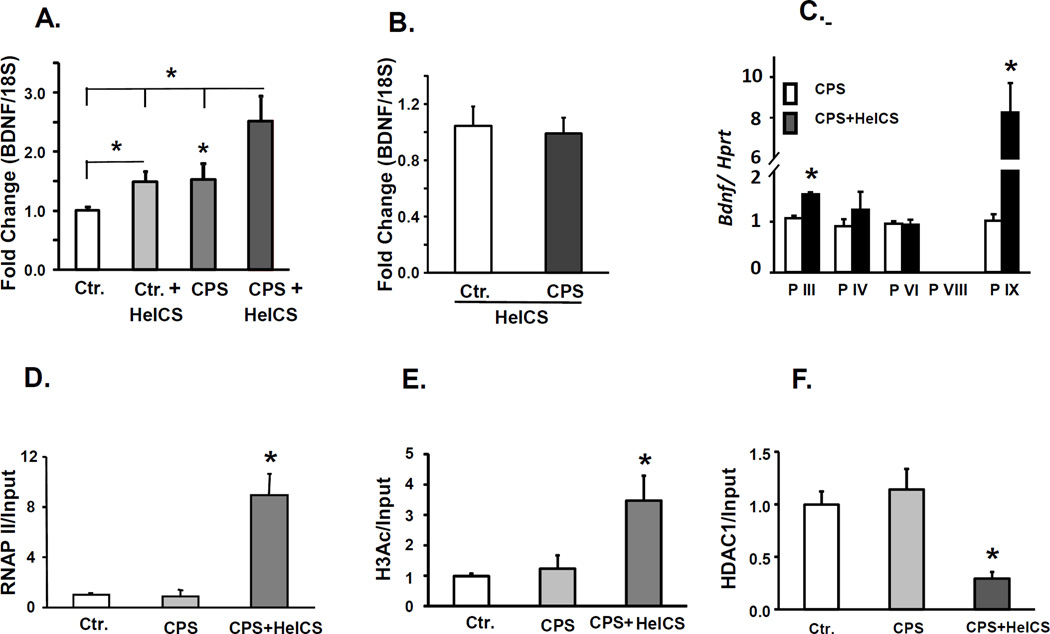
Figure 4.
Epigenetic changes associated with enhanced BDNF mRNA expression in LS dorsal horns in female CPS+HeICS rats. A. Bar graph showing BDNF mRNA levels by quantitative RT-PCR relative to β-III-tubulin mRNA in female Ctr. and CPS stressed rats measured in S1 DRG colon projecting neurons isolated by laser capture microdissection 24 hours after last stressor, n=6. B. Bar graph showing BDNF mRNA levels measured by quantitative RT-PCR relative to 18S RNA in female Ctr. and CPS sham and stressed rats 24 hours after last stressor. Two way repeated measures ANOVA found significant main effects of prenatal treatment F1,28=36.2, p<0.001 and of adult stress F1,28 =25.7, p<0.001. post hoc tests found significant effects of stress within Ctr., p=0.015, stress within CPS, P<0.001, CPS within sham, p=0.004 and CPS within stress, p<0.001, n=6–8. C. Expression levels of Bdnf transcripts from promoters III, IV, VI, VIII and IX in spinal cord dorsal horn of female CPS and CPS+HeICS rats, 24 hours after the last stressor. mRNA levels were quantified by real-time RT-PCR. Hprt served as internal control. N=5–6. *p < 0.05. ChIP assays by using RNA polymerase II (RNAP II), acetylated histone H3 (H3Ac) and histone deacetylase 1 (HDAC1) antibodies showed that HeICS increases RNAP II binding (D) and histone H3 acetylation (E), and suppresses HDAC1 interaction (F) at the core promoter region (−95/−26) of Bdnf promoter IX in spinal cord dorsal horn of female CPS+HeICS rats. Immunoprecipitated DNA was quantified by qPCR and normalized to input. Rabbit IgG was used for mock immunoprecipitation that did not pull down detectable chromatin. N=3 independent experiments each. * p < 0.05 vs. Ctr.
We used ChIP assay to investigate whether the upregulation of spinal cord BDNF after application of adult 2× HeICS to female CPS rats occurred by epigenetic mechanisms. Since BDNF has multiple promoters (39), each followed by a unique untranslated exon, we performed RT-PCR for five of the untranslated exons (III. IV, VI, VIII and IX) to investigate whether adult chronic stress altered the activity of one or more promoters to upregulate BDNF transcription in female CPS rats. We found significant increase in BDNF mRNA containing exons III and IX in female CPS+HeICS rats vs. unstressed female CPS rats (Figure 4C). Given the greater magnitude of increase in exon IX, we focused ChIP assays on this promoter. We found a significant increase in RNA Pol II binding (Figure 4D), histone H3 acetylation (Figure 4E) and significant decreases in HDAC1 association (Figure 4F) with the core promoter region of this promoter in CPS+HeICS female rats vs. unstressed CPS and Ctr. rats. These findings demonstrated that increased histone acetylation at BDNF promoter IX was associated with increased mRNA levels and RNA pol II binding with promoter IX.
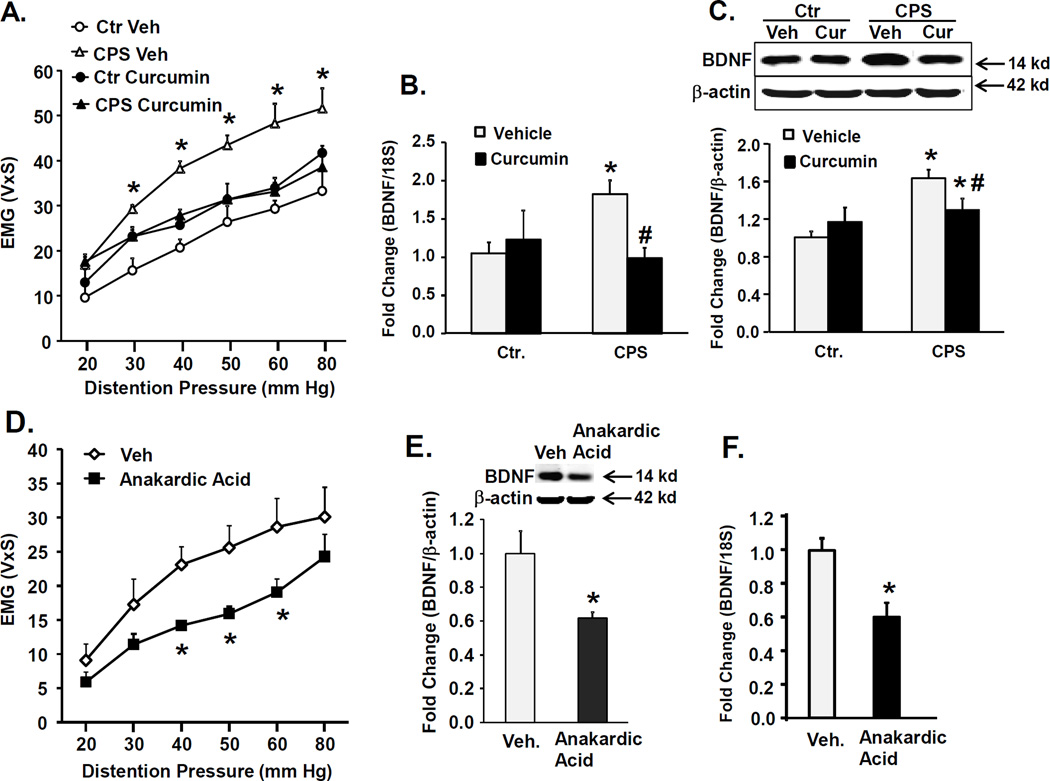
To investigate the physiological significance of increased histone acetylation, we measured the effects of treating female CPS and control rats with a non-specific histone acetyltransferase (HAT) inhibitor curcumin (200 mg/kg, p.o., twice daily during the HeICS protocol) on VMR to CRD and spinal cord BDNF expression following 2× HeICS. Curcumin treatment in stressed female CPS rats significantly decreased the VMR to CRD (Figure 5A), suppressed BDNF mRNA (Figure 5B) and protein expression (Figure 5C) in the LS dorsal horn, but it was without significant effect on any of these parameters in stressed control female rats (Figures 5A, B and C).
Figure 5.
Effects of treatment with HAT inhibitors on VMR to CRD and BDNF expression in female Ctr. and CPS rats. (A) Graph shows a significant reduction in VMR to graded CRD in female CPS+HeICS rats treated with curcumin (200 mg/kg p.o.) twice daily during the 9-day 2× HeICS protocol compared to vehicle treated rats. Two-way ANOVA found a significant prenatal treatment×drug interaction F1,21=20.9, p<0.001; post-hoc tests found that within CPS, drug vs. vehicle, p<0.001; within prenatal treatment CPS vs. Ctr., p<0.001, n=6 for drug, n=5 for vehicle). B. Bar graph shows effects of curcumin treatment on BDNF mRNA in LS spinal cord dorsal horns measured by quantitative RT-PCR relative to 18S rRNA. Curcumin treatment produced a significant decrease in BNDF mRNA in CPS+HeICS, but not in control females. Two way ANOVA: prenatal treatment×drug interaction F1,27=6.8, p=0.015; post-hoc tests found within CPS drug vs. vehicle, p=0.007; within prenatal treatment CPS vs Ctr, p=0.01, n=6). C. Western blots show that curcumin treatment significantly reduced BDNF protein expression in female CPS+HeICS rats but was without significant effects in control rats. Two way ANOVA: prenatal treatment×drug interaction F1,28=8.51, p=0.007; post-hoc tests found that within CPS, drug vs. vehicle, p=0.015; within prenatal treatment CPS vs. Ctr, p<0.00, n=6). D. Graph showing a significant reduction in VMR to graded CRD in female CPS rats after intrathecal administration of HAT inhibitor anakardic acid (1 µg/hr. via osmotic pump) for 14 days compared to vehicle infusion. Two way repeated measures ANOVA found a significant interaction between treatment and pressure, F5,45=3.27, p=0.013. Post hoc tests revealed significant differences at 40 mmHg, p=0.012, 50 mmHg, p=0.008, and 60 mmHg, p=0.008. n=7–8). E and F. This treatment produced a significant reduction in BDNF mRNA and protein expression detected in LS spinal cord dorsal horns by western blot and qRT-PCR, normalized to 18S and β-actin respectively (*p<0.05, n=7–8).
Next, we treated unstressed female CPS and control rats with the HAT inhibitor anakardic acid (1 µg/hour) or vehicle delivered via an intrathecal catheter connected to an osmotic pump for 14 days. The drug treatment significantly reduced the VMR to CRD (Figure 5D), BDNF mRNA (Figure 5E) and protein expression (Figure 5F) in LS spinal cord dorsal horns compared to vehicle control, suggesting that the increase of BDNF in the spinal cord contributed to the increase of VMR to CRD.
Relative effects of intensity, timing and type of perinatal stressor on visceral sensitivity in adult-life
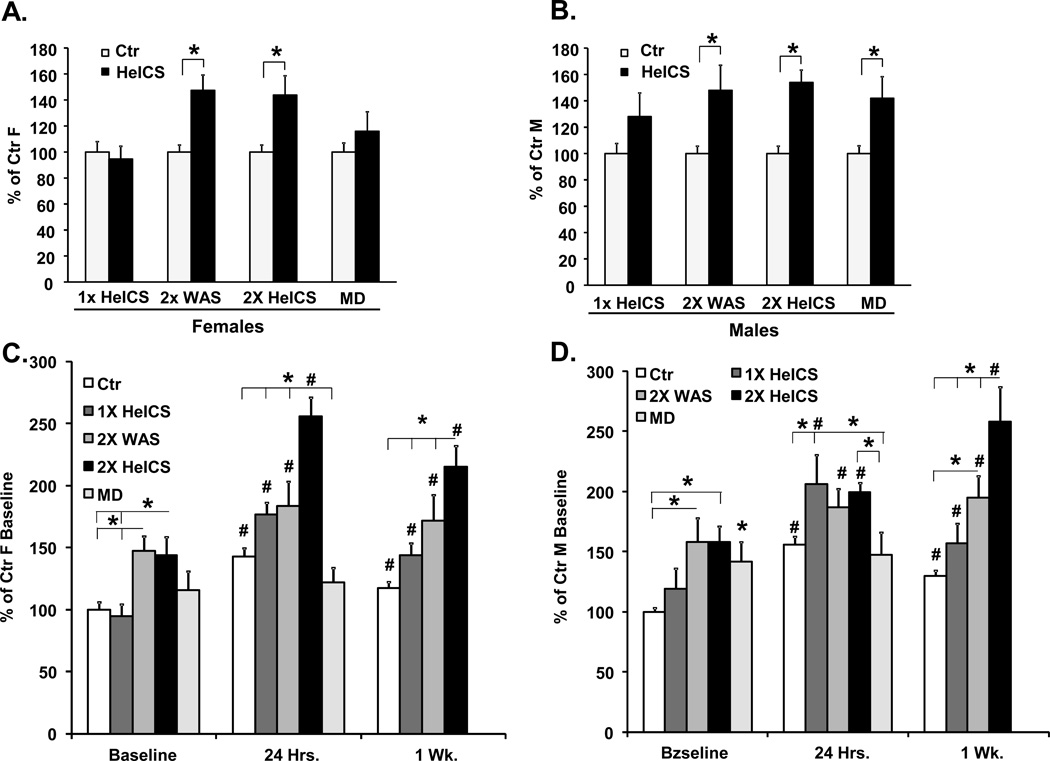
As stated in the introduction section, the effect of perinatal stressors on organ dysfunction in adult-life depends on the type of stressor, its intensity and timing. We investigated whether these variables have differential effects in inducing VHS to CRD in male and female offspring. We used three additional stress protocols. First, we reduced the intensity of stress by applying only one heterotypic stressor in the morning (1× HeICS), instead of two as in the regular protocol. Second, we used homotypic, instead of heterotypic, stress by applying only WAS twice a day (2× WAS). Third, we used maternal deprivation (MD) stress in neonates, instead of prenatal stress. Each group of rats received double HeICS when 6 to 8 weeks old. Prenatal 2× HeICS and 2× WAS significantly increased colon sensitivity in female offspring, while neonatal MD and prenatal 1× HeICS had no significant effect (Fig. 6A). In male offspring, prenatal 2× HeICS, 2× WAS and neonatal MD increased colon sensitivity, but 1× HeICS had no effect (Figure 6B).
Figure 6.
Comparison of the effects of various pre-natal stress protocols and neonatal maternal deprivation on colonic sensitivity in male and female adult rats before and after adult chronic stress. Pregnant dams were subjected to either once a day heterotypic stress (1× HeICS) or twice a day homotypic stress, water avoidance stress (2× WAS). A third set of pups were subjected to maternal separation for 3 hours per day from PND2 to PND 14. A, B. Bar graphs display the average VMR to CRD of female, A and male, B adult offspring of dams subjected to one of three prenatal HeICS protocols or pups subjected to maternal deprivation (MD) expressed as a percentage of their respective age matched control rats, *p<0.05 compared to controls. C, D. Bar graphs display the effects of adult chronic stress on the average VMR to CRD of female © and male (D) adult offspring of dams subjected to one of three prenatal HeICS protocols or pups subjected to maternal deprivation (MD) compared to pre-stress baseline and to controls. Data are expressed as a percentage of their respective average baseline control values. #p<0.05 compared to corresponding baseline; *p<0.05 compared to other prenatal or neonatal treatments. No data were collected for MD at one week. MD, n=8 all groups. 1× HeICS: Ctr. (n=18), female HeICS (n=18), male Ctr. (n=12) and male HeICS (n=12) rats. Of these, Ctr. (n=12), female CPS (n=11), male Ctr. (n=8) and male CPS (n=6) rats were stressed as adults. 2× WAS: N=7 all groups. Within females, two way ANOVA with repeated measures revealed a significant interaction between the factors type of early-life adverse event and adult stress, F6,137=6.12, p<0.001. Post-hoc comparisons of the effect of adult stress on the VMR to CRD at 24 hr. and 1 week compared to baseline within each group revealed the following significant differences: Ctr.: 24 hr. vs. Baseline p<0.001,1 week vs. Baseline p=0.004, 24 hr. vs. 1 week p=0.024; Within 1× CPS: 24 hr. vs. Baseline p<0.001, 1 week vs. Baseline p=0.041 24 hr. vs. 1 week p<0.001; Within 2× CPS: 24 hr. vs. baseline p<0.001, 1 week vs. Baseline p<0.001, 24 hr. vs. 1 week p=0.009; Within 2× WAS: 24 hr. vs. Baseline p<0.001,1 week vs. Baseline p=0.037 24 hr. vs. 1 week p=0.42; Within MD: 24 hr. vs. Baseline p>0.05.
We then investigated whether the intensity, type and timing of early-life stress affects the duration of VHS following chronic stress in adult-life. The colon sensitivity in response to adult chronic stress was significantly elevated in female offspring subjected to prenatal 2× HeICS, 2× WAS and 1× HeICS at 24 hours and one week after the last stressor (Figure 6C). The increase of VHS at both time points was greater in females subjected to 2× HeICS than in those subjected to either 2× WAS or 1× HeICS (Figure 6C). We detected no significant increase in colonic sensitivity of female MD rats at 24 hours. Colonic sensitivity in all three prenatal stress groups was significantly greater than their respective pre-stress baselines at both 24 hours and 1 week.
In males, we detected no significant differences in colonic sensitivity amongst the three prenatally stress groups 24 hours after the last stressor, although sensitivities were significantly greater than their respective baselines in each group (Figure 6D). Adult stress did not significantly increase sensitivity in male MD rats. At one week, colonic sensitivity in all three prenatally stressed groups and controls remained significantly greater than their respective baselines. The offspring subjected to 2× HeICS were significantly more sensitive than the other groups and 2× WAS offspring were more sensitive than controls. Taken together, these findings indicated that female offspring subjected to 2× HeICS show a significantly greater and more prolonged response to adult chronic stress compared to the other groups; this effect was not observed in male offspring.
Discussion
Our findings in a preclinical model show that chronic stress during in utero development sets up molecular conditions by epigenetic programming so that exposure to chronic stress in adult offspring exacerbates visceral sensitivity to colorectal distension. Visceral hypersensitivity to colorectal distension correlates with the symptom of abdominal pain in IBS patients (40–43). Clinical studies in IBS patients also show that chronic stress correlates with the severity of symptoms, especially abdominal pain (44–48). The slope of the regression line relating stress intensity to bowel symptoms is significantly greater in IBS patients than in non-IBS patients or healthy controls (44), suggesting greater stress reactivity in IBS patients as the underlying cause of the greater intensity of symptoms. The clinical and preclinical findings together suggest that chronic stress is a prominent underlying environmental factor that determines the severity of abdominal pain in IBS patients.
We found that chronic prenatal stress by itself modestly, but significantly, increased visceral sensitivity to CRD in adult male and female offspring. However, the reactivity to chronic stress in prenatally stressed rats was significantly greater than in control rats. The exacerbation of visceral sensitivity persisted longer in prenatally stressed than in prenatally unstressed controls. In addition, the intensity of hypersensitivity and its duration were greater in female than in male prenatally stressed rats. These findings are consistent with the findings of multiple retroactive and prospective studies in IBS patients that major stressful events have contemporaneous and delayed effects on symptoms (44–48).
IBS is a heterogeneous disorder: 1. Symptom intensity varies among patients and with time in individuals (49), 2. The ratio of female to male IBS is about 2:1 among patients seen in medical clinics and tertiary centers (50–54), 3. Female IBS patients report greater intensity (41), frequency (52) and longer episodes of abdominal pain (55). Stressful events in childhood and adolescence have been identified as risk factors for the development of IBS (10–14). Work-, family-, illness- and environment-related stressors are common in daily life and their intensities vary. Female IBS patients report higher levels of stress and greater reactivity to stress than non-IBS controls and normal subjects (44, 56). Our preclinical findings suggest that the intensity, susceptibility and sex effects on IBS symptoms may depend on the intensity, timing, type and duration of stress during early develoopment as well as in adult-life. Together, these observations explain the greater intensity of symptoms (57, 58) and morbidity, at least from abdominal pain (41, 52, 55) and greater prevalence of IBS in women than in men. In addition, variations in the intensity of stressors with time may explain the variations in the intensities of symptoms.
Chronic homotypic stress induced by twice-daily water avoidance stress yielded results similar to those of twice-daily heterotypic stress. However, the intensity of increase in visceral sensitivity depended on the intensity of psychological stress; twice-daily heterotypic threatening stressors were more effective in inducing visceral hypersensitivity and prolonging its post-stress persistence than a single daily stressor. The timing of application of stress in the perinatal period affected the intensity of visceral hypersensitivity and sex-effect in adult-life. Psychological stress by maternal deprivation in neonates was less effective in inducing visceral hypersensitivity. In addition, it induced visceral hypersensitivity in male, but not in female rats and it did not set up molecular conditions to exacerbate visceral sensitivity following adult chronic stress. Another recent study found a sexually dimorphic effect of unpredictable exposure to odor-shock in neonate rats (59). Most investigators found sexual dimorphism among IBS patients; lower discomfort thresholds in female than in male IBS patients (42, 60–63), while some did not (40, 64). Together, our findings suggest that the intensity of pathogenic effects and sex effects of adverse early life experiences (AELEs) on visceral sensitivity depend upon the type of stressor, its severity and timing of adverse experiences in perinatal development; these variables should be considered in retrospective studies to establish a correlation between AELEs and development of IBS. One retrospective study found no effect of war-time conditions, specifically food restriction, during in utero development on the prevalence of IBS in adulthood (65); data on stress level, type of stress and its timing during pregnancy were not included or were not available in this study.
Blanchard and colleagues (46) found a bidirectional relationship between the intensity of chronic stress and IBS symptoms. This implies that while initially chronic stress might precipitate IBS, with the passage of time the symptoms of IBS may sustain or enhance chronic stress. These investigators argued that stress management might not be effective in reducing IBS symptoms because of lack of causal relationship between chronic stress and IBS symptoms. Our preclinical study demonstrated a causal relationship between chronic stress and exacerbation of visceral sensitivity that relates to the severity of abdominal pain (40–43). An improvement in IBS symptoms does not occur until all stressors are eliminated for a period of time (45). This time lag might be because the upregulation of nociceptive genes by chronic stress persists long after the stressors are eliminated (see later).
The prenatal development profiles of the neuroendocrine, immune and other systems in rodents differ from those in humans (66–68). Therefore, the prenatal period during which the human fetus is susceptible to chronic psychological maternal stress in developing visceral hypersensitivity in adult-life is likely to differ from that in the rat species.
Previous studies indicate that the visceromotor response fluctuates during estrous cycle, with rats in proestrus being more sensitive than those in metestrus/diestrus (69), we did not control for the estrous cycle in female CPS rats. However, since all treatment groups contained a mix of females at different stages of the cycle, effects of the cycle on parameters measured would be expected to average out between groups. The finding of enhanced VHS in the CPS+chronic stress females was reproducible across several experiments and was not observed in stressed control females, demonstrating that our finding was not due to a chance clustering of CPS females in the proestrus (more sensitive part) of the cycle. Note that most clinical studies in female IBS patients to show differential pain sensitivity between female and male patients were performed at random times during estrus cycle.
BDNF is a critical pronociceptive neurotrophin in spinal cord dorsal horn where increase of its expression enhances neurotransmission (70–72). We found that chronic prenatal stress upregulates the expression of BDNF in LS dorsal horn, but not in the LS DRG, in female offspring. The elevation of BDNF in LS dorsal horn was critical in inducing visceral hypersensitivity in prenatally stressed female offspring, but not in male offspring. Chronic stress in adult female offspring further increased BDNF expression, which correlated with the exacerbation of visceral sensitivity for at least two weeks after the end of stress. The exaggeration of symptoms in female IBS patients also persists long after stressful events (45). We project that the modulation of BDNF expression in spinal cord may underlie the greater morbidity of abdominal pain in female IBS patients. BDNF expression did not correlate with VHS in male prenatally stressed offspring; we did not investigate the cellular mechanisms of VHS in male offspring in this study.
This study identified only one of the neurotrophins in LS spinal cord, BDNF, which related to increase in reactivity to chronic stress in adult CPS female offspring. Although a previous study demonstrated primary afferent input into the thoraco-lumbar spinal cord (73), we chose to focus our initial studies in LS spinal cord segments, where pelvic nerve afferents terminate in rats; sensory input from the colon that activates pseudo-affective reflexes travels primarily through the LS DRG (74). We do not rule out that chronic prenatal stress also targets BDNF in other parts of the spinal cord as well as additional nociceptive genes, including other neurotrophins and their receptors and ion channels, to enhance reactivity to chronic stress in female offspring. Human tissue is seldom available to investigate the alterations in BDNF expression in spinal cord. However, one study found elevated BDNF in the colon biopsies of IBS patients vs. healthy controls (75), suggesting that BDNF upregulation might also occur in non-spinal cord tissue in IBS patients. The contribution of the elevated BDNF in the mucosa to visceral hypersensitivity remains unknown.
Bdnf gene has a complex structure that contains at least eight 5 and one 3 untranslated exons, each with its own promoter (39). Transcription can be initiated by one or more of these promoters in a developmental-, tissue-, and activity-dependent manner (39, 72). However, whichever promoter is used, the first transcribed exon is spliced to form a bipartite mRNA with exon IX, which contains the complete Bdnf coding sequence. Therefore, the resulting BDNF protein is the same. The functional significance of the above transcriptional organization is to provide differential mRNA stability and translatability or differential subcellular localization of the protein (72, 76). Therefore, the degree and duration of transcription may vary with the use of each promoter. Of the five promoters we investigated, prenatal stress upregulated the activities of promoters III and IX in female prenatally stressed rats.
Acetylation of lysine residue is one of the best-understood epigenetic modifications of chromatin to modulate transcription (77, 78); the acetylation of lysine residues relaxes the chromatin to allow increased interaction between DNA and transcription regulating complexes, while deacetylation condenses the chromatin to suppress transcription. Histone acetyltransferases (HATs) and histone deacetylases (HDACs), recruited to target genes via their direct association with transcriptional activators and repressors, regulate acetyl group turnovers. Our findings show that CPS reduced the association of HDAC1 with the core promoter region of Bdnf promoter IX, which increased the acetylation of histone 3 allowing greater interaction of RNA pol II with the core prompter to enhance Bdnf transcription. Intrathecal administration of anakardic acid to CPS unstressed female offspring or oral gavage with curcumin during adult chronic stress suppressed the VMR to CRD by reducing the transcription of BDNF mRNA and protein expression. Together, these findings show that epigenetic modification of Bdnf by CPS exacerbates VHS in adult female offspring in the resting state and following chronic stress. HAT inhibitors may serve as potential modulators of visceral pain in female IBS patients. Epigenetic modulations by adverse early life experiences can have transgenerational (79); one study showed that susceptibility to stress induced visceral hypersensitivity in maternally separated rats transferred to the next generation.
Previous maternal stress studies found that elevated fetal corticosterone levels were responsible for many of the lasting deleterious metabolic, neurobiological and stress axis changes observed in adult offspring (80–86). One study utilizing maternal stress protocols found significant effects of prenatal stress on maternal behavior toward pups, in particular, arched-back nursing and nesting/grouping of pups, were reduced in pre-natal stressed dams over post-natal days 1–10 (87). Maternal separation-induced susceptibility to stress-triggered visceral hypersensitivity is transferred across generations and this transfer depends on maternal care (88). Our data suggest a primary role of direct effects of pre-natal stress on the fetus. When we subjected maternal deprivation rats to adult chronic stress, we did not find significant post-stress enhancement of VHS in MD male or female rats. These findings suggest that the effects on nociceptive circuits producing enhanced female VHS in CPS rats following chronic stress occurred during fetal development. This hypothesis is further supported by lack of differences in LS spinal cord expression of BDNF between male and female stressed MD rats. However, we cannot rule out the possibility that CPS also adversely affects dam behavior during the suckling period.
Acetylation of lysine residue is one of the best-understood epigenetic modifications of chromatin to modulate transcription (77, 78); the acetylation of lysine residues relaxes the chromatin to allow increased interaction between DNA and transcription regulating complexes, while deacetylation condenses the chromatin to suppress transcription. Histone acetyltransferases (HATs) and histone deacetylases (HDACs), recruited to target genes via their direct association with transcriptional activators and repressors, regulate acetyl group turnovers. Our findings show that CPS reduced the association of HDAC1 with the core promoter region of Bdnf promoter IX, which increased the acetylation of histone 3 allowing greater interaction of RNA pol II with the core prompter to enhance Bdnf transcription. Oral gavage with curcumin during adult chronic stress suppressed the VMR to CRD by reducing the transcription of BDNF mRNA and protein expression. Curcumin is a naturally occurring p300 histone acyl transferase inhibitor that has anti-nociceptive effects in a number of rodent pain models (89–93), in some reports due to down-regulation of specific pro-nociceptive genes. These findings are consistent with our finding that curcumin down-regulates spinal cord BDNF expression and reduces colonic hypersensitivity in stressed female CPS rats. However, curcumin might act at multiple genes to suppress affective responses or pain. With these limitations in mind, our findings show that epigenetic modification of Bdnf by CPS exacerbates VHS in adult female offspring in the resting state and following chronic stress. HAT inhibitors may serve as potential modulators of visceral pain in female IBS patients. It is noteworthy that epigenetic modulations by adverse early life experiences can have transgenerational (79); one study showed that susceptibility to stress induced visceral hypersensitivity in maternally separated rats transferred to the next generation.
In conclusion, the development of IBS-like visceral hypersensitivity is a two-step process. The first step is exposure to chronic stress in early-life that epigenetically modifies the chromatin structure of nociceptive genes to modestly, but significantly, increase visceral sensitivity in male and female offspring. In the second step, chronic stress in adult offspring exacerbates visceral sensitivity, more in female than in male rats, again by epigenetic mechanisms. The intensity of these effects and sex specificity depend on the type of psychological stress and its intensity and timing in the perinatal period; prenatal stress appears to be more potent than the neonatal stress. In female offspring, prenatal stress epigenetically modified the chromatin structure of the Bdnf gene in spinal cord LS dorsal horn, which played a major role in the exacerbation of visceral hypersensitivity following adult chronic stress. HAT inhibitors can reverse the epigenetic modulation and its pathogenic effects on visceral sensitivity. Together, these findings show that the intensity of visceral hypersensitivity in prenatally stressed rats depends on the intensity of chronic stress in early- as well as in adult-life. Our findings in a preclinical model suggest that several features of IBS, such as greater prevalence and morbidity in women than in men and the variability of symptom intensity with time might relate to the varying intensity of daily-life stressors. The inclusion of a metric of co-existing stress (mild, moderate and severe) in the widely used Rome (94) and Manning (95) criteria might be beneficial in stratifying IBS patients in obtaining consistent findings and charting a course for the management of symptoms. It is important to understand that early-life stressors are one of the potential etiologies of IBS; severe inflammatory stress in adults in the presence of psychological morbidity can also induce IBS symptoms (96–98).
Acknowledgments
Supported in part by NIDDK Grant 5R01DK088796
Footnotes
- Designed research study SKS
- Performed research JHW, QL
- Contributed essential reagents or tools SKS, JHW and QL
- Wrote the paper SKS and JHW
Each author has read the final manuscript and approved it.
No author has any conflict of interest.
References
- 1.Veale D, Kavanagh G, Fielding JF, Fitzgerald O. Primary fibromyalgia and the irritable bowel syndrome: different expressions of a common pathogenetic process. British journal of rheumatology. 1991;30:220–222. doi: 10.1093/rheumatology/30.3.220. [DOI] [PubMed] [Google Scholar]
- 2.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–1156. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- 3.Hazlett-Stevens H, Craske MG, Mayer EA, Chang L, Naliboff BD. Prevalence of irritable bowel syndrome among university students: the roles of worry, neuroticism, anxiety sensitivity and visceral anxiety. J Psychosom Res. 2003;55:501–505. doi: 10.1016/s0022-3999(03)00019-9. [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ, Boyce P, Jones M. Identification of distinct upper and lower gastrointestinal symptom groupings in an urban population. Gut. 1998;42:690–695. doi: 10.1136/gut.42.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitehead WE, Winget C, Fedoravicius AS, Wooley S, Blackwell B. Learned illness behavior in patients with irritable bowel syndrome and peptic ulcer. Dig Dis Sci. 1982;27:202–208. doi: 10.1007/BF01296915. [DOI] [PubMed] [Google Scholar]
- 6.Whorwell PJ, Prior A, Colgan SM. Hypnotherapy in severe irritable bowel syndrome: further experience. Gut. 1987;28:423–425. doi: 10.1136/gut.28.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 8.Schuurmans C, Kurrasch DM. Neurodevelopmental consequences of maternal distress: what do we really know? Clinical genetics. 2013;83:108–117. doi: 10.1111/cge.12049. [DOI] [PubMed] [Google Scholar]
- 9.Rinaudo P, Wang E. Fetal programming and metabolic syndrome. Annu Rev Physiol. 2012;74:107–130. doi: 10.1146/annurev-physiol-020911-153245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hislop IG. Childhood deprivation: an antecedent of the irritable bowel syndrome. Med J Aust. 1979;1:372–374. doi: 10.5694/j.1326-5377.1979.tb126963.x. [DOI] [PubMed] [Google Scholar]
- 11.Bradford K, Shih W, Videlock EJ, et al. Association between early adverse life events and irritable bowel syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012;10:385–390. e381–e383. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Annals of internal medicine. 1990;113:828–833. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 14.Drossman DA. Abuse, trauma, and GI illness: is there a link? Am J Gastroenterol. 2011;106:14–25. doi: 10.1038/ajg.2010.453. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Fett SL, Zinsmeister AR. Self-reported abuse and gastrointestinal disease in outpatients: association with irritable bowel-type symptoms. Am J Gastroenterol. 1995;90:366–371. [PubMed] [Google Scholar]
- 16.Salmon P, Skaife K, Rhodes J. Abuse, dissociation, and somatization in irritable bowel syndrome: towards an explanatory model. Journal of behavioral medicine. 2003;26:1–18. doi: 10.1023/a:1021718304633. [DOI] [PubMed] [Google Scholar]
- 17.Ross CA. Childhood sexual abuse and psychosomatic symptoms in irritable bowel syndrome. Journal of child sexual abuse. 2005;14:27–38. doi: 10.1300/J070v14n01_02. [DOI] [PubMed] [Google Scholar]
- 18.Coutinho SV, Plotsky PM, Sablad M, et al. Neonatal maternal separation alters stressinduced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 19.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 20.Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004;127:524–534. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Christianson JA, Bielefeldt K, Malin SA, Davis BM. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151:540–549. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwetz I, McRoberts JA, Coutinho SV, et al. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am J Physiol Gastrointest Liver Physiol. 2005;289:G704–G712. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- 23.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Schwetz I, Bradesi S, McRoberts JA, et al. Delayed stress-induced colonic hypersensitivity in male Wistar rats: role of neurokinin-1 and corticotropin-releasing factor-1 receptors. Am J Physiol Gastrointest Liver Physiol. 2004;286:G683–G691. doi: 10.1152/ajpgi.00358.2003. [DOI] [PubMed] [Google Scholar]
- 25.Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 26.Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–2048. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 27.Winston JH, Sarna SK. Developmental origins of functional dyspepsia-like gastric hypersensitivity in rats. Gastroenterology. 2013;144:570–579. doi: 10.1053/j.gastro.2012.11.001. e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 29.Holladay SD, Smialowicz RJ. Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environmental health perspectives. 2000;108(Suppl 3):463–473. doi: 10.1289/ehp.00108s3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faulk C, Dolinoy DC. Timing is everything: the when and how of environmentally induced changes in the epigenome of animals. Epigenetics : official journal of the DNA Methylation Society. 2011;6:791–797. doi: 10.4161/epi.6.7.16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- 32.Bale TL, Baram TZ, Brown AS, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunton PJ. Resetting the dynamic range of hypothalamic-pituitary-adrenal axis stress responses through pregnancy. J Neuroendocrinol. 2010;22:1198–1213. doi: 10.1111/j.1365-2826.2010.02067.x. [DOI] [PubMed] [Google Scholar]
- 34.Waffarn F, Davis EP. Effects of antenatal corticosteroids on the hypothalamic-pituitary-adrenocortical axis of the fetus and newborn: experimental findings and clinical considerations. American journal of obstetrics and gynecology. 2012 doi: 10.1016/j.ajog.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choudhury BK, Shi XZ, Sarna SK. Norepinephrine mediates the transcriptional effects of heterotypic chronic stress on colonic motor function. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.90712.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology. 2010;138:294–304. doi: 10.1053/j.gastro.2009.09.054. e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Sarna SK. Nuclear myosin II regulates the assembly of preinitiation complex for ICAM-1 gene transcription. Gastroenterology. 2009;137:1051–1060. doi: 10.1053/j.gastro.2009.03.040. 1060 e1051-e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi XZ, Winston JH, Sarna SK. Differential immune and genetic responses in rat models of Crohn's colitis and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G41–G51. doi: 10.1152/ajpgi.00358.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 41.Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simren M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology. 2007;133:1113–1123. doi: 10.1053/j.gastro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Bouin M, Plourde V, Boivin M, et al. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 43.Kuiken SD, Klooker TK, Tytgat GN, Lei A, Boeckxstaens GE. Possible role of nitric oxide in visceral hypersensitivity in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:115–122. doi: 10.1111/j.1365-2982.2005.00731.x. [DOI] [PubMed] [Google Scholar]
- 44.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–830. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256–261. doi: 10.1136/gut.43.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanchard EB, Lackner JM, Jaccard J, et al. The role of stress in symptom exacerbation among IBS patients. J Psychosom Res. 2008;64:119–128. doi: 10.1016/j.jpsychores.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 47.Dancey CP, Taghavi M, Fox RJ. The relationship between daily stress and symptoms of irritable bowel: a time-series approach. J Psychosom Res. 1998;44:537–545. doi: 10.1016/s0022-3999(97)00255-9. [DOI] [PubMed] [Google Scholar]
- 48.Levy RL, Cain KC, Jarrett M, Heitkemper MM. The relationship between daily life stress and gastrointestinal symptoms in women with irritable bowel syndrome. Journal of behavioral medicine. 1997;20:177–193. doi: 10.1023/a:1025582728271. [DOI] [PubMed] [Google Scholar]
- 49.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 50.Hungin AP, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21:1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 51.Muller-Lissner SA, Bollani S, Brummer RJ, et al. Epidemiological aspects of irritable bowel syndrome in Europe and North America. Digestion. 2001;64:200–204. doi: 10.1159/000048862. [DOI] [PubMed] [Google Scholar]
- 52.Taub E, Cuevas JL, Cook EW, 3rd, Crowell M, Whitehead WE. Irritable bowel syndrome defined by factor analysis. Gender and race comparisons. Dig Dis Sci. 1995;40:2647–2655. doi: 10.1007/BF02220455. [DOI] [PubMed] [Google Scholar]
- 53.Saito YA, Schoenfeld P, Locke GR., 3rd The epidemiology of irritable bowel syndrome in North America: a systematic review. Am J Gastroenterol. 2002;97:1910–1915. doi: 10.1111/j.1572-0241.2002.05913.x. [DOI] [PubMed] [Google Scholar]
- 54.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 55.Corney RH, Stanton R. Physical symptom severity, psychological and social dysfunction in a series of outpatients with irritable bowel syndrome. J Psychosom Res. 1990;34:483–491. doi: 10.1016/0022-3999(90)90022-v. [DOI] [PubMed] [Google Scholar]
- 56.Hertig VL, Cain KC, Jarrett ME, Burr RL, Heitkemper MM. Daily stress and gastrointestinal symptoms in women with irritable bowel syndrome. Nursing research. 2007;56:399–406. doi: 10.1097/01.NNR.0000299855.60053.88. [DOI] [PubMed] [Google Scholar]
- 57.Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol. 2011;106:1749–1759. doi: 10.1038/ajg.2011.201. quiz 1760. [DOI] [PubMed] [Google Scholar]
- 58.Lembo A, Ameen VZ, Drossman DA. Irritable bowel syndrome: toward an understanding of severity. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2005;3:717–725. doi: 10.1016/s1542-3565(05)00157-6. [DOI] [PubMed] [Google Scholar]
- 59.Chaloner A, Greenwood-Van Meerveld B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. The journal of pain : official journal of the American Pain Society. 2013;14:270–280. doi: 10.1016/j.jpain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 60.Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98:1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 61.Chang L, Mayer EA, Labus JS, et al. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol Regul Integr Comp Physiol. 2006;291:R277–R284. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- 62.Munakata J, Naliboff B, Harraf F, et al. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55–63. doi: 10.1016/s0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- 63.Naliboff BD, Munakata J, Fullerton S, et al. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997;41:505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HS, Rhee PL, Park J, et al. Gender-related differences in visceral perception in health and irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21:468–473. doi: 10.1111/j.1440-1746.2005.04060.x. [DOI] [PubMed] [Google Scholar]
- 65.Klooker TK, Braak B, Painter RC, et al. Exposure to severe wartime conditions in early life is associated with an increased risk of irritable bowel syndrome: a population-based cohort study. Am J Gastroenterol. 2009;104:2250–2256. doi: 10.1038/ajg.2009.282. [DOI] [PubMed] [Google Scholar]
- 66.Levine S. The ontogeny of the hypothalamic-pituitary-adrenal axis. The influence of maternal factors. Ann N Y Acad Sci. 1994;746:275–288. doi: 10.1111/j.1749-6632.1994.tb39245.x. discussion 289–293. [DOI] [PubMed] [Google Scholar]
- 67.Landreth KS. Critical windows in development of the rodent immune system. Human & experimental toxicology. 2002;21:493–498. doi: 10.1191/0960327102ht287oa. [DOI] [PubMed] [Google Scholar]
- 68.West LJ. Defining critical windows in the development of the human immune system. Human & experimental toxicology. 2002;21:499–505. doi: 10.1191/0960327102ht288oa. [DOI] [PubMed] [Google Scholar]
- 69.Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merighi A, Salio C, Ghirri A, et al. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 71.Thompson SW, Bennett DL, Kerr BJ, Bradbury EJ, McMahon SB. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. Proc Natl Acad Sci U S A. 1999;96:7714–7718. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmed-Choudhury J, Russell CL, Randhawa S, Young LS, Adams DH, Afford SC. Differential induction of nuclear factor-kappaB and activator protein-1 activity after CD40 ligation is associated with primary human hepatocyte apoptosis or intrahepatic endothelial cell proliferation. Mol Biol Cell. 2003;14:1334–1345. doi: 10.1091/mbc.E02-07-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christianson JA, Traub RJ, Davis BM. Differences in spinal distribution and neurochemical phenotype of colonic afferents in mouse and rat. The Journal of comparative neurology. 2006;494:246–259. doi: 10.1002/cne.20816. [DOI] [PubMed] [Google Scholar]
- 74.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 75.Yu YB, Zuo XL, Zhao QJ, et al. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012;61:685–694. doi: 10.1136/gutjnl-2011-300265. [DOI] [PubMed] [Google Scholar]
- 76.Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol. 2007;14:1008–1016. doi: 10.1038/nsmb1337. [DOI] [PubMed] [Google Scholar]
- 78.Bennett EJ, Piesse C, Palmer K, Badcock CA, Tennant CC, Kellow JE. Functional gastrointestinal disorders: psychological, social, and somatic features. Gut. 1998;42:414–420. doi: 10.1136/gut.42.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 80.Bingham BC, Sheela Rani CS, Frazer A, Strong R, Morilak DA. Exogenous prenatal corticosterone exposure mimics the effects of prenatal stress on adult brain stress response systems and fear extinction behavior. Psychoneuroendocrinology. 2013;38:2746–2757. doi: 10.1016/j.psyneuen.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 81.Green MK, Rani CS, Joshi A, et al. Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience. 2011;192:438–451. doi: 10.1016/j.neuroscience.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 82.Fournier S, Steele S, Julien C, et al. Gestational stress promotes pathological apneas and sex-specific disruption of respiratory control development in newborn rat. J Neurosci. 2013;33:563–573. doi: 10.1523/JNEUROSCI.1214-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cottrell EC, Holmes MC, Livingstone DE, Kenyon CJ, Seckl JR. Reconciling the nutritional and glucocorticoid hypotheses of fetal programming. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:1866–1874. doi: 10.1096/fj.12-203489. [DOI] [PubMed] [Google Scholar]
- 84.Franko KL, Forhead AJ, Fowden AL. Differential effects of prenatal stress and glucocorticoid administration on postnatal growth and glucose metabolism in rats. The Journal of endocrinology. 2010;204:319–329. doi: 10.1677/JOE-09-0390. [DOI] [PubMed] [Google Scholar]
- 85.Igosheva N, Taylor PD, Poston L, Glover V. Prenatal stress in the rat results in increased blood pressure responsiveness to stress and enhanced arterial reactivity to neuropeptide Y in adulthood. J Physiol. 2007;582:665–674. doi: 10.1113/jphysiol.2007.130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behavioural brain research. 2006;175:323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 87.Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW. Gestational stress induces postpartum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology. 2004;29:227–244. doi: 10.1016/s0306-4530(03)00025-8. [DOI] [PubMed] [Google Scholar]
- 88.van den Wijngaard RM, Stanisor OI, van Diest SA, et al. Susceptibility to stress induced visceral hypersensitivity in maternally separated rats is transferred across generations. Neurogastroenterol Motil. 2013;25:e780–e790. doi: 10.1111/nmo.12202. [DOI] [PubMed] [Google Scholar]
- 89.Zhao X, Xu Y, Zhao Q, Chen CR, Liu AM, Huang ZL. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: descending monoamine system and opioid receptors are differentially involved. Neuropharmacology. 2012;62:843–854. doi: 10.1016/j.neuropharm.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 90.Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006;536:256–261. doi: 10.1016/j.ejphar.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 91.Li Y, Zhang Y, Liu DB, Liu HY, Hou WG, Dong YS. Curcumin attenuates diabetic neuropathic pain by downregulating TNF-alpha in a rat model. International journal of medical sciences. 2013;10:377–381. doi: 10.7150/ijms.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ji FT, Liang JJ, Liu L, Cao MH, Li F. Curcumin exerts antinociceptive effects by inhibiting the activation of astrocytes in spinal dorsal horn and the intracellular extracellular signal-regulated kinase signaling pathway in rat model of chronic constriction injury. Chinese medical journal. 2013;126:1125–1131. [PubMed] [Google Scholar]
- 93.Zhi L, Dong L, Kong D, et al. Curcumin acts via transient receptor potential vanilloid-1 receptors to inhibit gut nociception and reverses visceral hyperalgesia. Neurogastroenterol Motil. 2013;25:e429–e440. doi: 10.1111/nmo.12145. [DOI] [PubMed] [Google Scholar]
- 94.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manning AP, Thompson WG, Heaton KW, Morris AF. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;2:653–654. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McKendrick MW, Read NW. Irritable bowel syndrome--post salmonella infection. J Infect. 1994;29:1–3. doi: 10.1016/s0163-4453(94)94871-2. [DOI] [PubMed] [Google Scholar]
- 97.Gwee KA, Graham JC, McKendrick MW, et al. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet. 1996;347:150–153. doi: 10.1016/s0140-6736(96)90341-4. [DOI] [PubMed] [Google Scholar]
- 98.Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. Bmj. 1997;314:779–782. doi: 10.1136/bmj.314.7083.779. [DOI] [PMC free article] [PubMed] [Google Scholar]