Abstract
Protozoan parasites have a significant impact upon global health, infecting millions of people around the world. With limited therapeutic options and no vaccines available, research efforts are focused upon unraveling cellular mechanisms essential for parasite survival. During its life cycle, Trypanosoma cruzi, the causal agent of Chagas disease, is exposed to multiple external conditions and different hosts. Environmental cues are linked to the differentiation process allowing the parasite to complete its life cycle. Successful transmission depends on the ability of the cells to trigger adaptive responses and cope with stressors while regulating proliferation and transition to different life stages. This review focuses upon different aspects of the stress response in T. cruzi, proposing new hypotheses regarding cross-talk and cross-tolerance with respect to environmental changes and discussing open questions and future directions.
Keywords: Stress response, Adaptation, Survival, Trypanosomatids
1. Introduction
All cells and organisms develop in a changing environment. The nature, magnitude and duration of such changes determine the type of responses that cells must develop in order to cope with these challenges. The fate of the organism, whether uni- or multicellular, will result from the balance between potentially harmful stressors and compensation mechanisms. When environmental forces induce damage in macromolecules, a cellular stress response is set in motion. This includes change in gene expression, DNA repair mechanisms, modifications in protein turnover and restoration of the redox balance. Failure to repair damage or prolonged exposure to stress conditions could end in cell death, either by necrosis or apoptosis [1]. When changes in the environment are transient, stimuli-specific or do not result in permanent damage to macromolecules, cells respond with a different set of adaptive responses oriented toward restoring homeostasis, compensating for variations in conditions with what is called a cellular homeostatic response [1].
Both types of responses are important in Trypanosoma cruzi, the protozoan parasite that causes Chagas disease. T. cruzi has a complex life cycle that includes transmission by an insect vector and invasion of a vertebrate host, where the parasite is able to establish chronic infections. Throughout its developmental cycle, the parasite encounters diverse environmental conditions to which it has successfully adapted in order to maintain its transmission and infectivity. This review summarizes current knowledge, new insights and future questions regarding adaptation mechanisms in response to environmental changes in T. cruzi and compares them with other pathogenic protozoans, including Trypanosoma brucei and Leishmania spp.
2. Life cycle and environmental changes
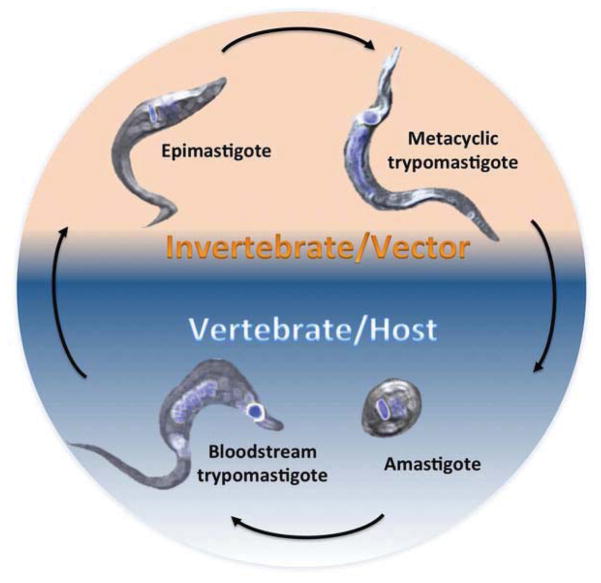
Protozoan parasites from the Trypanosomatidae family are important human pathogens responsible for millions of infections around the world. The most common mechanism for T. cruzi infection in endemic regions is through the bite of insect vectors. Blood transfusion, organ transplant, transplacental and oral routes are also possible and have epidemiological relevance in non-endemic areas [2]. T. cruzi has four main developmental stages, with differences in shape, metabolism and replicative capacity (Fig. 1). Transmission occurs when invertebrates from the Reduvidae family feed from an infected vertebrate, taking a blood meal that contains circulating non-replicative trypomastigotes. In the insect stomach, most trypomastigotes are killed [3], but the surviving parasites differentiate into epimastigotes. They migrate through the midgut, where they actively divide and attach to the perimicrovillar membranes prior to differentiation into non-replicative infective metacyclic trypomastigotes. At the hindgut and rectum, free metacyclics are mixed with urine and feces, and are released by defecation during the next feeding cycle. The human infection commonly occurs by penetration of mucosal and epithelial lesions after an insect bite. Metacyclics invade macrophages, fibroblasts and epithelial cells at the site of the inoculation and are initially contained in a transient endocytic parasitophorous vacuole. After differentiation into replicative amastigote forms, the parasite has several rounds of replication in the cytosol, followed by transformation into trypomastigotes that escape to the bloodstream and reach multiple target tissues, invading and establishing persistent infection. In these locations, trypomastigotes undergo a second round of differentiation to amastigotes, replicating at a low rate and maintaining active infection for over 20 years [4].
Fig. 1. T. cruzi life cycle.
Epimastigotes and metacyclic trypomastigotes develop in the gut of the insect vectors. In the vertebrate host, the developmental stages are circulating bloodstream trypomastigotes and amastigotes. In blue are stained the nucleus and the kinetoplast (the single mitochondrion of T. cruzi). The shape and relative position of both organelles changes through the development of the parasite and is one of the hallmarks of each developmental stage. Background colors indicate the stages found on each host, orange for parasite forms developed in the insect vector and blue for those found in mammalian hosts.
Adding to the complexity of the described cycle, T. cruzi is able to infect over 1,000 different vertebrate species and is transmitted in domestic and sylvatic cycles by more than 40 invertebrates, underscoring the amazing adaptive capacity of this parasite [5].
During its developmental cycle, the parasite encounters changing environmental conditions and faces immunological reactions from both mammalian hosts and insect vectors. The adaptive response to such challenges results in the successful transmission of the parasite and is intertwined with its own differentiation process, since some of the stressors, like acidic pH and starvation, trigger the transition from one developmental stage to another. This is one of the reasons why teasing apart stress responses and homeostatic adaptation is not an easy task in protozoan biology. Our knowledge of the molecules involved in detection of environment changes, transduction of external signals and mechanisms of response in protozoan parasites is limited and particularly scarce in T. cruzi. At the same time, it is a promising field of research in which mechanisms of adaptation and differentiation could emerge as new therapeutic targets.
3. Nature of the stressors and cellular responses
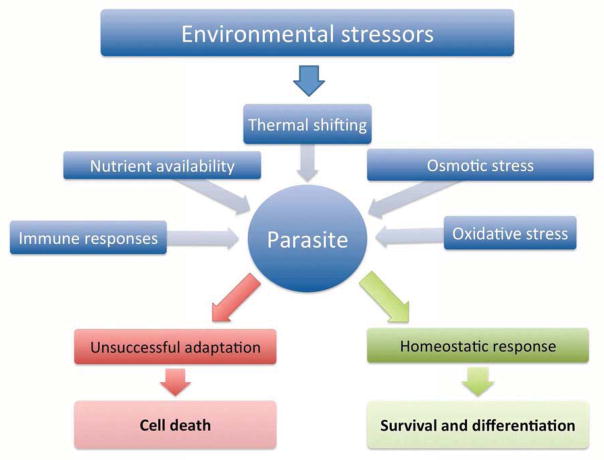
The multiplicity of stressors faced by T. cruzi are derived from the changing environments in which the life cycle takes place, as well as from the interaction with the diverse hosts’ immune systems. Given the extent of the topic, aspects related to immune responses and parasite persistence mechanisms will not be discussed here, but they are extensively reviewed elsewhere [6, 4]. The major environmental variables that T. cruzi faces during its development are summarized in Fig. 2 and include, among others, variations in temperature, fluctuation of the type and availability of nutrients and changes in pH, osmolarity, ion composition and oxidative species of the surrounding environment. In the sections which follow, details of the parasite’s responses to these variables will be discussed.
Fig. 2. Potential stress sources for T. cruzi.
Successful transmission of the parasite is dependent on its ability to adapt to changing hosts and environmental conditions (in blue). Adequate responses (in green) allow the parasite to cope with stressors and differentiate, ensuring continuation of the life cycle. Failure in triggering adaptation mechanisms (in red) will result in cell death.
3.1. Thermal shifting
In the insect, T. cruzi metacyclic forms are exposed to an average temperature of 26°C, with a daily fluctuation of more that 10°C depending on the habitat of the vector. Upon invasion of a vertebrate, a shift to higher temperature occurs and the parasite develops a stress response characterized by an increase in heat shock proteins (HSPs)[7]. A similar increase occurs during differentiation from promastigote to amastigote in Leishmania donovani [8] and in T. brucei bloodstream forms, while there is no apparent change in the total amount of HSP70 mRNA in procyclic forms [9].
HSPs are key components of the thermal stress response, preventing the misfolding and aggregation of proteins and regulating localization and secretion. Some members of these chaperone families are inducible upon stress, while others are constitutively expressed. In trypanosomes, HSPs have been studied mainly by genomic analysis (reviewed in [10]) but the dynamics of the induction and role of specific chaperones during heat shock are far from being understood. This is not surprising given that T. cruzi has more than 60 genes encoding for HSP40 homologues, and at least 14 genes for HSP70 [10].
Upon exposure at 37°C, epimastigotes show an increase in mRNA and protein levels for HSP60, HSP70 and HSP40 (also known as DnaJ), while the general level of transcription is decreased. In parasites incubated at higher temperature (40–129 42°C), HSP70 mRNA decreases, while the level of proteins increases [11], except for the mitochondrial mtHSP70 that is generally reduced by heath shock. Under severe heat shock (42°C) both splicing and polyadenylation are inhibited and HSP70 pre-mRNA accumulates in polysomes [12]. Translocation of the mature mRNA to the nucleus and nucleolus [13] and redistribution of the protein to the periphery of the cells [14] have been observed.
A family of HSP40 homologs was found in T. cruzi, but only one member, TcJ2, shows an increase in mRNA after heat shock [15]. HSP104 mRNA and protein levels [16] as well as transcript abundance for the small chaperonins HSP10 and HSP16, are also increased by thermal stress [17, 18]. Two-dimensional gel electrophoresis and proteomic analysis of 24 differential spots obtained from epimastigotes incubated at 42°C showed an increase in a relatively small number of stress-related proteins, including HSP70 and HSP40, but no large changes in protein expression were observed under this conditions [7].
The mechanism by which HSP70 and possibly other HSPs are upregulated depends on the stabilization of mRNA. In Trypanosomatids, stability and degradation of mRNA is controlled by cis-acting elements in the 3′-UTR [19]. It has been shown that the half-life of HSP70 mRNA increases from 60 min at 29°C to 120 min at 37°C. This effect is mediated by U-rich elements in the 3′ UTR and trans-splicing acceptor sites in the 5′ UTR [20]. Recently, a zinc finger protein inducible by heat shock (ZC3H11) was described in T. brucei [21]. It specifically binds to AU rich 3′-UTRs regions and stabilizes the mRNA for several HSPs and other stress-related proteins. It is reasonable to speculate that similar proteins could be present in T. cruzi, but they have not yet been commonly identified.
Transmission of bloodstream trypomastigotes from vertebrates to invertebrates exposes the parasite to a fluctuating lower temperature. How the parasite responds and whether a “cold stress” response plays a role in T. cruzi differentiation has not been studied, but in T. brucei, transformation from stumpy bloodstream to procyclic forms is triggered by cis-aconitate, and expression of the transporter responsible for its uptake is strongly upregulated at 20°C, suggesting that the temperature shift from the mammalian host to the tsetse fly does play a role in differentiation [22, 23].
An understudied aspect of thermal shifting is the effect on the dynamics and composition of lipids in the membranes and how such modifications affect cellular responses. In order to maintain constant fluidity and preserve protein functions, cells respond to changes in temperature by altering their membrane composition. This is called homeoviscous adaptation and is a key step in the cellular response upon thermal stress in all kind of organisms [24–27]. T. cruzi epimastigotes and metacyclics shifted from 28°C to 37°C, increase the sterol/phospholipid ratio and have a higher proportion of saturated fatty acids in phospholipids, after 24 and 48 h of heat shock. Unlike other cell types, they increase unsaturated fatty acids associated with neutral sterols and triglycerides, suggesting that a switch in fatty acids could be occurring [28]. The functional consequences of homeoviscous adaptation have been studied in prokaryotes and eukaryotes [29], but its role in protozoan thermal adaptation is still to be determined. In bacteria, mammalian cells [30, 31] and plants [32], a rise in temperature results in transient fluidization of the plasma membrane and activation of calcium entry. The channels that play a role in thermosensing-related calcium influx are mechano-sensitive, transient receptor potential (TRP) and cyclic nucleotide gated channels (CNG) [33, 34]. Elevation in cytosolic calcium has multiple consequences, including activation of heat shock factors (Hsf) to regulate heat shock protein expression and triggering of several signaling cascades like PLC/IP3-, CAMKII-, PKC- and PLA-regulated pathways. As a result, in several type of cells analyzed, a global response is induced leading to modification of gene expression, adaptation of metabolic pathways and remodeling of membranes [34]. Successful adaptation will result in cell survival, while failure to adapt or lasting intense stress will conduce to cell death (Fig. 2). Two elements are keys in the integration of the responses: calcium as a pleiotropic second messenger and Hsf for controlling gene expression. In Trypanosomatids, there is no evidence, at the genetic or protein level, of the presence of Hsf homologues and this is not surprising, since the control of heat shock protein expression seems to be mainly post-translational. The remaining question, then, is how parasites detect changes in temperature and trigger compensatory responses? One possibility is that RNA molecules are themselves the sensors due to their ability to undergo conformational changes induced by temperature. A few examples have been described in bacteria [35, 36] and mammalian cells [37, 38] in which translation efficiency for stress and virulence-related genes can be controlled by RNA thermosensors. It would be interesting to explore the presence of this type of mechanism in Trypanosomatids, where post-transcriptional regulation is key to cellular homeostasis.
The second, but not exclusive, possibility is that changes in lipid-protein interactions and lipid membrane dynamic are transducers of thermal stress signals. Temperature shifting induces reorganization of lipid structures and homeoviscous adaptation in membranes. In mammalian cells, a rise in temperature promotes rapid disappearance of cholesterol-rich lipid rafts and replacement for ceramide-rich rafts, forming a more rigid membrane domain that increases the density of receptors and signaling molecules necessary for intracellular communication of external stimuli [34]. Lipid rafts are abundant in the flagellar membranes of T. cruzi [39] and T. brucei [40] promoting and driving the localization of signaling molecules, including cAMP-related molecules and calcium channels [41, 42]. The flagellum has been postulated as a sensing structure in Trypanosomatids; thus, lipid reorganization in response to temperature variations could be a way of triggering directional signals from the flagellum to the cell body in order to elicit adaptive responses in the parasite.
Finally, modification of lipid organization has a profound effect upon the activity and distribution of membrane proteins, including calcium channels and receptors. Calcium is a key player in differentiation [43] and invasion [44, 45] in T. cruzi. Based on the evidence from other cell types, it could also play a fundamental role in stress sensing and adaptation. The channels responsible for temperature-induced calcium influx in trypanosomes await identification, but the presence of mechanosensitive and TRP channels in their genomes encourage our future efforts toward their functional characterization.
3.2. Nutritional availability
When T. cruzi is maintained in axenic culture, general starvation or nutrient-specific restriction of amino acids and serum has important consequences, limiting the growth of epimastigotes [46], modifying the metabolism and inducing differentiation into metacyclics [47]. Metacyclogenesis can be induced by placing epimastigotes in a poor media with low pH that resembles the composition of triatomine urine, followed by incubation in the same media supplemented with glucose and amino acids (TAU3AGG) [48]. Addition of serum prevents the transition to metacyclic forms, suggesting that nutrient availability is an important cue for differentiation.
Nutritional stress induces an overall decrease in translation, mediated by phosphorylation and inhibition of eIF2. Recently, Tonelli et al. showed that in epimastigotes, eIF2α phosphorylation is induced by incubation in metacyclogenesis media, causing a significant reduction in polysomes and decreasing protein synthesis. Interestingly, parasites in which eIF2α phosphorylation is impaired failed to differentiate into metacyclics, indicating that eIF2α signaling pathways could be regulating the progression of the T. cruzi life cycle [49].
The formation of stress granules is a conserved mechanism of translational regulation in which mRNA is mobilized from polysomes and stored in cytoplasmic foci, from where it could be retrieved for translation once the stress insult has passed. It is particularly relevant in Trypanosomatids, since they lack transcriptional regulation and their gene expression is mostly controlled by post-transcriptional mechanisms. Both T. brucei procyclic forms and T. cruzi epimastigotes [50] respond to starvation by increasing the number and size of stress granules. Moreover, this type of granule can be observed in developmental forms of T. brucei collected from tsetse flies [51] and in T. cruzi epimastigotes obtained from the insect gut [50], but they are absent in metacyclics, suggesting that they could have a differential role in replicative forms of the parasite by regulating the availability of mRNA accessible for translation.
Starvation is also a potent inducer of autophagy and organelle recycling in a diversity of cells, and could be a mechanism of cellular remodeling contributing to differentiation [52]. In T. cruzi epimastigotes, starvation induces autophagosome formation and promotes transformation into metacyclic forms [53]. Similarly, in L. major, autophagy is essential for differentiation from promastigotes to metacyclic forms [54, 55]. In pleomorphic strains of T. brucei, transition from long-slender to short-stumpy bloodstream forms, as well as from short-stumpy to procyclic forms, is accompanied by autophagy and organelle remodeling [56].
The effect of specific nutrient withdrawal and the metabolic responses elicited in T. cruzi have been studied and are summarized in Table 1.
Table 1.
Effect of nutrient restriction in T. cruzi
| Nutrient restriction | Effect | Reference |
|---|---|---|
| Starvation | Apoptosis-like cell death | [109] |
| Autophagy and differentiation | [53] | |
| eIF2-α phosphorylation, translation repression, differentiation | [49] | |
| Metacyclogenesis | [48, 47] | |
| Decrease in ATP, reduced infectivity | [110] | |
| Delipidated serum | Cholesterol mobilization from reservosomes | [46] |
| Polyamines | Growth inhibition, increased uptake, translocation to membrane | [111] |
| Inorganic phosphate | Growth inhibition | [112] |
| Amino acids | ||
| Aspartate* | Increased uptake | [113] |
| Arginine** | Increased uptake | [114] |
| Cysteine | Increased uptake | [115] |
| Lysine | Increased uptake | [116] |
Necessary for in vitro metacyclogenesis
High-affinity transporter
An evident question arises: Is starvation a physiological situation during the T. cruzi life cycle? In the mammalian hosts, nutrients are plentiful, with a high concentration of glucose and amino acids in blood, but the situation is different in the vector. In their natural environment, triatomines spend weeks between feedings [57] and the complete digestion of a blood meal could take up to 10 days. During this period, the availability of nutrients and the chemical conditions in the insect gut change drastically, affecting development of the parasite. In Triatoma infestans parasitized with T. cruzi, long periods of starvation (more than 60 days) decrease the total number of trypomastigotes in the rectum, but the infective forms are predominant under these conditions, while non-infective epimastigotes are more abundant after feeding [57]. These results underscore the physiological role of stress responses and their implications during parasite differentiation into infective forms.
Although nutrient restriction is a known inducer of differentiation in vitro, it is still under debate as to whether the absence of one or more specific compounds drives the first steps in differentiation or if a molecule produced by the parasites under nutritional stress regulates transition to the next developmental stage. Quorum sensing mechanisms as a cue for differentiation are present in T. brucei [58], where a soluble factor called SIF (stumpy induction factor) governs transition from slender to stumpy trypomastigotes [59]. No similar factor has been identified in T. cruzi, but in epimastigotes, transition from exponential to stationary phase of growth occurs at a constant cell density characteristic for each strain, independently of the nutrient availability, pointing to the presence of a quorum-sensing-related mechanism that may be triggered by nutrient restriction but not strictly caused by it.
Differentiation into metacyclic forms is dependent on attachment, both in the vector [60] and in vitro [61, 47]. Surface attachment is mediated in part by glycosylated proteins. GP82 and GP90, two glycosylated proteins belonging to the transialidase family are upregulated in metacyclics upon starvation-induced differentiation [62] and both proteins influence the infectivity of the parasite [63]. It is plausible that starvation induces changes in the expression profile of surface molecules that, in turn, will mediate adhesion and regulate progression of the differentiation process.
3.3. Osmotic stress
During the transition of trypomastigotes from the bloodstream to the cytosol of the mammalian host cell or transit along the vector’s gut, T. cruzi encounters variation in osmolarity and ionic composition of the surrounding environment. The concentration of physiological cations like K+ can vary orders of magnitude depending on the feeding cycles of the insect, and between the extra- and intracellular environment. Osmolarity also increases, reaching up to 1,000 mosm/Kg in the rectal content of the vector [64], and about 1,400 mosm/kg when circulating trypomastigotes pass through the renal medulla of a host. Even in human tissues, interstitial osmolality can fluctuate 20–30 mosm/kg under physiological conditions [65].
Almost all cell types have developed compensatory responses to changes in osmolarity, allowing them to re-establish homeostasis. Osmotic stress, if not compensated, severely impairs cell growth, induces DNA and protein damage, alters metabolism and ultimately causes cell death [66].
The mechanisms of osmoregulation in T. cruzi have been extensively studied in the last 20 years, and it is now known that acidocalcisomes and the contractile vacuole complex play a key role in volume regulation. Acidocalcisomes are membrane-bound organelles, rich in calcium and phosphate, present in the cytosol of a wide range of cells [67]. The contractile vacuole complex is a bi-partite structure formed by a bladder or vacuole, surrounded by a network of tubules called spongiomes (Fig. 3A). It is present in T. cruzi [68] and Leishmania spp. [69, 70], but absent from T. brucei. Besides its role in osmoregulation, proteomic analysis and functional studies are suggesting that the contractile vacuole complex could also be involved in calcium homeostasis and protein trafficking (reviewed in [71]).
Fig. 3. Model for regulatory volume decrease in T. cruzi.
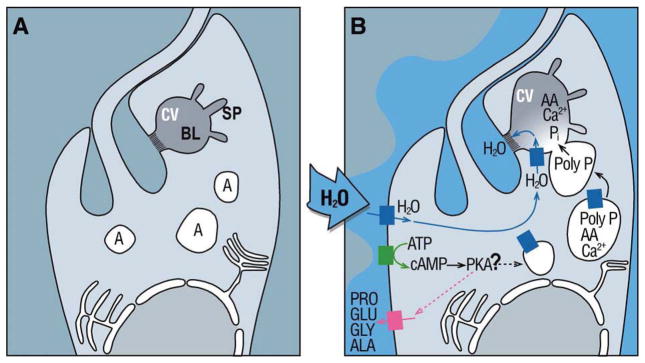
Panel A. Appearance and localization under isosmotic conditions of cellular structures involved in osmotic responses: (A) Acidocalcisomes, (CV) contractile vacuole, (BL)ladder, (SP) spongiome. Panel B. Proposed osmoregulatory response elicited by hyposmotic stress in T. cruzi epimastigotes. Upon exposure to hypo-osmotic stress, a spike of intracellular cAMP is followed by amino acid release and fusion of acidocalcisomes with the contractile vacuole. Osmolytes are transferred from acidocalcisomes to the contractile vacuole, attracting water into the bladder. Accumulated molecules are later expelled into the extracellular space. Question mark in the figure indicates that the molecular targets for cAMP are still under debate. This image has been modified from [108] and reproduced with kind permission from Elsevier.
Under hypoosmotic stress, the three major forms of the parasite initially swell, but they exhibit a rapid regulatory volume decrease (RVD) response, allowing the cells to regain their initial volume in about 5 min (Fig. 3B) [72]. An intense efflux of neutral and anionic amino acids accounts for 50% of the response, but unlike other cell types where taurine plays a central role, alanine and proline are the main amino acids released by T. cruzi [72]. Surprisingly, Na+, phosphate and inositol are not released, and K+ plays only a marginal role. The current model for RVD in T. cruzi proposes that, after hyposmotic stress, cAMP increases by activation of a still unknown adenylyl cyclase, promoting the mobilization and fusion of acidocalcisomes with the contractile vacuole complex. Fusion of acidocalcisomes and activation of an exopolyphosphatase leads to the transfer of proteins (aquaporin-1), amino acids, phosphate and other osmolytes to the lumen of the contractile vacuole complex, resulting in water accumulation followed by the release to the extracellular media through transient fusion of the bladder of the contractile vacuole with the plasma membrane. Once the cells have recovered their normal volume, the response is ended by a phosphodiesterase that hydrolyzes cAMP and shuts down the signaling pathway.
A similar mechanism of RVD has been described in L. donovani amastigotes [70]. L. major possesses five genes encoding for putative aquaporins, but only one of them, LmAQP1, has been characterized [69]. Similarly to T. cruzi AQP1, LmAQP1 is important for RVD and is at least partially localized in the contractile vacuole. Interestingly, LmAQP1 seems to be regulated by MAP kinases. Phosphorylation by LmjMPK2 extends the half-life of LmAQP1 and induces its relocation from the flagellum to the surface of the parasite. Cells overexpressing a phosphorylation-deficient mutant had a severe defect in the RVD, suggesting that this post-translational modification is necessary for its function.
In T. brucei, RVD responses are as efficient as in T. cruzi and Leishmania spp., but the absence of a contractile vacuole complex suggests that the mechanisms involved might be different. Three AQPs have been characterized, localizing AQP1 in the flagellum of bloodstream forms, AQP2 in intracellular organelles and AQP3 in the plasma membrane [73]. Knockdown of AQP1 and AQP3 decreases the swelling of the parasites under hyposmotic stress, supporting their role in water influx, while reduced expression of AQP2 impairs the RVD response and affects growth of the parasite [73].
Responses triggered by hyperosmotic stress are just starting to become elucidated. In epimastigotes, exposure to hyperosmotic stress by sorbitol induces rapid shrinkage [74] but, in contrast to other cell types, the parasites do not have a regulatory volume increase response and do not recover their initial volume, at least for 2 h. The shrinkage is impaired by knockdown or inhibition of APQ1 [74] and by pharmacological blockage of a cation channel (TcCat) [75]. An early phase is characterized by enlarged contractile vacuoles, increased levels of long-chain polyP and free amino acid, followed by a global decrease in protein levels, suggesting that protein catabolism is activated in order to generate compatible osmolytes [74].
The parasites adapt fairly well to sustained hyperosmolarity, undergoing an extensive change in gene expression. At 3 h post-stress, parasites show an increase in mRNA for ribosomal and RNA binding proteins, amino acid transporters, trans-sialidases and flagellar proteins [74]. This phenomenon could be part of a pre-conditioning leading to differentiation into metacyclics observed in the rectum of the insect.
In mammalian cells, hyperosmotic stress increases the expression of HSPs, but in T. cruzi, except for Hsp10, no other significant changes were detected. A decrease in mRNA for glycolytic enzymes, translation factors and enzymes involved in amino acids and fatty acids synthesis was observed, indicating extensive metabolic re-shaping [74].
These results bring up the hypothesis that parasites exposed to increasing osmolarity in the gut and rectum of the insect could have put into place a mechanism of osmoadaptation similar to what has been described for mammalian cells [76]. The variations observed in the level of transcripts are probably the result of selective mRNA stabilization and/or degradation in order to elicit an adaptive response. Recently, Li et al. showed the presence of GC-rich motifs in the 3′UTR of genes upregulated under hyperosmotic stress, as well as an AU-rich element in the 3′UTR of genes downregulated under the same conditions [74]. These could represent signature marks for RNA binding proteins involved in transcript stabilization, similar to what has been described during thermal stress in T. cruzi [20] and T. brucei [77, 21, 78], where 3′ and 5′-UTR elements are required for mRNA stabilization during heat shock.
While our understanding of the osmoregulatory mechanisms has grown, some important questions remain. How do cells sense changes in osmolarity? What is the nature of the sensor? Is it a membrane protein or are the biophysical changes in the lipid bilayer involved?
In yeast [79, 80] and mammalian cells [81], hyperosmotic stress activates MAPK by rearrangement of lipid domains that facilitate molecular crowding and self-activation of membrane receptors, reinforcing the idea that the membrane itself could be a sensor for environmental changes. Alternatively, in many cell types, the primary sensing mechanism for osmotic stress are mechano-sensitive channels located in the plasma membrane. These are mostly cation channels activated directly by changes in the tension of the lipid bilayer or indirectly by traction through their interaction with the cytoskeleton. In bacteria, osmosensing is mediated by MscS, MscK and MscL channels [82]. In mammalian cells, several families of proteins act as osmosensors and transducers of the adaptive responses (reviewed in [83]), including transient potential receptors, two-pore K+ channels, ENaC channels and the recently described Piezo channels [84]. In silico analysis showed that T. cruzi and Leishmania spp. have genes encoding for homologues of bacterial MscS/MscK and channels similar to eukaryotic TRP and Piezo [85, 86]. Piezo channels seem to be absent in T. brucei, underscoring the differences in osmosensing-osmoregulatory mechanisms between the two protozoa. These differences could be the consequence of differential adaptation to the dissimilar environments that both parasites encounter throughout their life cycles.
The presence of osmosensors similar to both mammalian cells and bacteria suggests a strong and probably redundant system of mechano-sensation in protozoan parasites, and has interesting evolutive and therapeutic implications. Homology analysis indicates that Trypanosomatids have two highly divergent groups of Piezo channels, with higher differences between them than between Piezo 1 and 2 in humans, suggesting an independent evolutionary origin for these proteins in protozoans and humans [86]. The overall structural and sequence homology of Piezo channels is very low, with only 5 amino acids conserved among channels in different organisms. These residues are located in a structural context of transmembrane domains compatible with a pore-loop domain. Whether this could be a selectivity filter or a gating-related sequence remains to be seen. Although experimental evidence demonstrating the role of mechanosensitive channels in T. cruzi is necessary to elucidate their function, this type of protein could be implicated in sensing, osmoregulation, adaptation to high potassium intracellular environments, etc. playing a multiplicity of roles in the parasite’s adaptive stress response. The diversity of functions together with the low level of conservation compared with human channels make mechano-sensitive channels a potential therapeutic target that warrants further exploration. Stay tuned.
3.4. Oxidative stress
The generation of reactive oxygen species (ROS) and nitrogen-reactive species (NRS) as byproducts of oxidative metabolism is a general feature in eukaryotic cells. In the same way, all cells possess mechanisms to eliminate oxidative species and restore the redox balance [1]. Intracellular pathogens like T. cruzi encounter an additional source of oxidative stress when invading macrophages, where the oxidative burst is part of the innate immune response directed toward eliminating the parasite [87].
Changes in the redox state and an excess of free radicals are significant stressors; they affect DNA and protein integrity, induce membranes damage, regulate the function of enzymes and alter energetic metabolism [1].
Trypanosomatids have significant differences in their antioxidant defense systems compared with mammalian cells, and these pathways have been under intense study, as they could represent important drug targets. The main scavenger of ROS and NRS is trypanothion, a small molecule that contains two glutathiones covalently bound to spermidine. T. cruzi lacks catalase and glutathione peroxidase, but possesses at least two glutathione-dependent peroxidases, two peroxiredoxins and four iron-dependent superoxide dismutases, as well as a complete biosynthetic pathway for ascorbate (reviewed in [88]). This network protects the parasite against oxidative insult in a very effective way.
Some evidence pointed to H2O2 as being responsible for killing the parasites inside macrophages [87], while other groups showed that the oxidative burst induced by T. cruzi invasion was significantly lower than that expected for an intracellular pathogen and might not be eliminating the parasites [89]. Recently, Paiva et al. [90] demonstrated that oxidative stress in macrophages promotes T. cruzi infection by increasing the availability of iron, an essential nutrient for parasite proliferation. The mechanism by which the parasite prevents full activation of macrophages is still unknown. New evidence indicates that cruzain, a cysteine protease exposed at the surface of intracellular amastigotes, is able to block NF-κB activation, preventing IL-12 production and facilitating intracellular replication of the parasites in the early phases of the infection [91].
Nevertheless, accumulation of ROS and NRS does kill the parasite by either necrosis or apoptosis, probably as a result of accumulated DNA damage [92]. Free radicals induce DNA breaks and formation of 8-oxoguanine adducts that are usually detected and corrected by the base excision repair (BER) system. The mismatch repair pathway is a second line of defense that maintains the integrity of the genome when the BER system is not able to repair oxidative lesions. Parasites exposed to ROS/RNS have a significant increase in 8-oxoguanine in nuclear and kinetoplastid DNA. They also show a decrease in viability that is more pronounced in the presence of inhibitors of the BER pathway [92] and in parasites where MSH2, a key component in the mismatch repair pathway, has been knocked down [93], implying that both pathways are involved in repairing nuclear and mitochondrial DNA from oxidative-stress-induced lesions.
Significant differences in the antioxidant capacity have been observed between developmental stages and strains of the parasite, linking the ability to resist oxidative insult with virulence [94]. Infective forms are more tolerant to oxidative stress [87], although the thiol concentration is higher in epimastigotes [95]. Antioxidant enzymes are expressed at higher levels in metacyclics and it has been suggested that this is a pre-conditioning mechanism for infective forms to cope with the oxidative burst after invading macrophages [96]. Higher expression of these enzymes has been also associated with T. cruzi strains resistant to benznidazol, one of the drugs used for Chagas disease treatment, but the clinical relevance of these findings is not clear [97].
Cross-talk between oxidative stress and responses triggered by other type of stressors is widely spread as a mechanism for adaptation and cross-tolerance to harmful conditions [1, 98, 99]. In Leishmania chagasi promastigotes, a thermal shift at 37°C induces higher tolerance to oxidative stress by H2O2 exposure [100]. In Arabidopsis [101], HsfA1 is activated by either heat shock or low pH, but is also regulated by NADP/NADPH levels, increasing its activity under oxidative stress. In T. cruzi, metacyclogenesis is triggered by the concurrence of low pH, a shift in temperature and nutrient restriction. Therefore, it is possible that the redox state in metacyclics is a metabolic integrator in parasites under multiple stress conditions, triggering or regulating the necessary steps for initiating the differentiation process and for dealing with adverse environmental conditions.
4. Future challenges and conclusions
New and exciting studies are showing that, over the course of the infection, T. cruzi is adapting to changes in external conditions and is also responding to cues from the intracellular environment, reshaping its metabolism in order to optimize the use of host cell pathways that can promote parasite replication. Parasitized cells also undergo significant modifications in their gene expression profiles [102, 103]. These mutual adaptations contribute to the persistence of the parasite in the host tissues, evading clearance by the immune system. Understanding how host and parasite mutually adapt their gene expression profiles, signaling and metabolic pathways could provide valuable information regarding potential drug targets.
There are some important limitations when studying adaptive responses in these parasites. Unlike T. brucei, T. cruzi is a non-amenable organism for genetic manipulation due to high genetic diversity and gene copy number variation [104]. The absence of RNAi machinery rules out the use of high throughput screening methods to identify genes involved in stress responses. At the same time, efficient systems for inducible expression of membrane proteins and endogenous tagging in T. cruzi are long overdue, limiting the available approaches for tackling our questions.
Although detailed proteomic information is available for the major developmental stages in T. cruzi, T. brucei and Leishmania spp., no large-scale proteomics studies have evaluated global or stimulus-specific stress responses in Trypanosomatids. Available data indicate the presence of RNA stabilization and translation regulation mechanisms as part of the stress response, but they also show a weak correlation between mRNA abundance and the amount of proteins. Extensive proteomic and metabolomics studies could contribute significantly to drawing a clearer picture of the players involved in adaptation to environmental challenges in T. cruzi and other protozoan parasites.
One of the main remaining challenges is to find the sensors and transducers that are detecting the environmental variables and communicating them to the intracellular effectors. An emerging line of research is postulating that the flagellum could be a sensory organelle, similar to cilia and flagella in other organisms. Detailed proteomic studies showed enrichment of signaling molecules in the flagella of T. brucei and T. cruzi [41, 42], but our understanding of the membrane molecules involved in the sensing process and articulation with the intracellular signaling pathways linked to adaptive responses is still partial. Part of the problem resides in the technical limitations when studying membrane proteins and the fact that many mechanisms for sensing are mediated by receptors and ion channels. These are, in general, multimeric complexes the function of which is linked to their lipid context and biophysical properties of the membrane. Studying these proteins in motile cells by direct electrophysiological approaches is challenging and heterologous expression does not always reflect the functionality of the native protein.
A high percentage of T. cruzi proteins are still annotated in the databases as hypotheticals. This is particularly significant for ion channels, where the overall conservation of sequences between organisms is very low, making identification rely on laborious functional studies. Nevertheless, exciting times lie ahead. The study of the elements involved in sensory networks and their effect upon parasite biology and population dynamics is fertile ground for research. Growing evidence supports the idea that one or multiple integrators of stress signals and ion channels involved in multimodal sensing are strong candidates for that role. cAMP-dependent pathways are keys to multiple processes leading to adaptation and differentiation, but some elements are still missing, like the adenylyl cyclases activated by stress or the targets downstream of cAMP production. Finally, the role of the plasma membrane itself through remodeling of the lipid domains and modification of the surface proteins is another important paradigm to be addressed. Trypanosomes actively release membrane vesicles of diverse nature containing nucleotide-binding protein, surface glycoproteins, proteases, ion channels, heat shock proteins, etc. [105, 106]. This may represent a rapid way of remodeling the surface upon environmental changes without activating protein degradation pathways [75]. It could also represent a mechanism for signaling and regulation of the interaction between parasites and with the host cells [107, 106].
The developmental complexities of the life cycle as well as the peculiarities of the cellular processes have to be kept in mind when analyzing stress responses in T. cruzi. In trypanosomes, the immediate adaptive response seems to be achieved by modifying the stability of mRNA and regulating the translation rate and half-life of proteins. Identification of RNA binding proteins and translation regulators will help to complete the picture. The fact that differentiation is cued by several environmental variables operating simultaneously makes it difficult to separate specific responses, but reinforces the idea that cross-tolerance to different stressors and cross-talk between adaptive pathways are important for survival and progression of the parasite throughout its life cycle.
Acknowledgments
This work was supported by NIH/NIAID grant R00AI101167.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kultz D. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 2.Milei J, Guerri-Guttenberg RA, Grana DR, Storino R. Prognostic impact of Chagas disease in the United States. Am Heart J. 2009;157:22–29. doi: 10.1016/j.ahj.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Garcia ES, Genta FA, de Azambuja P, Schaub GA. Interactions between intestinal compounds of triatomines and Trypanosoma cruzi. Trends Parasitol. 2010;26:499–505. doi: 10.1016/j.pt.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Nagajyothi F, Machado FS, Burleigh BA, Jelicks LA, Scherer PE, Mukherjee S, Lisanti MP, Weiss LM, et al. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Microbiol. 2012;14:634–643. doi: 10.1111/j.1462-5822.2012.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teixeira AR, Gomes C, Lozzi SP, Hecht MM, Rosa AeC, Monteiro PS, Bussacos AC, Nitz N, et al. Environment, interactions between Trypanosoma cruzi and its host, and health. Cad Saude Publica. 2009;25(Suppl 1):S32–44. doi: 10.1590/s0102-311x2009001300004. [DOI] [PubMed] [Google Scholar]
- 6.Tarleton RL. Immune system recognition of Trypanosoma cruzi. Curr Opin Immunol. 2007;19:430–434. doi: 10.1016/j.coi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Morales D, Lanz-Mendoza H, Hurtado G, Martinez-Espinosa R, Espinoza B. Proteomic analysis of Trypanosoma cruzi epimastigotes subjected to heat shock. J Biomed Biotechnol. 2012(2012):902803. doi: 10.1155/2012/902803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saar Y, Ransford A, Waldman E, Mazareb S, Amin-Spector S, Plumblee J, Turco SJ, Zilberstein D. Characterization of developmentally-regulated activities in axenic amastigotes of Leishmania donovani. Mol Biochem Parasitol. 1998;95:9–20. doi: 10.1016/s0166-6851(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 9.Hausler T, Clayton C. Post-transcriptional control of hsp70 mRNA in Trypanosoma brucei. Mol Biochem Parasitol. 1996;76:57–71. doi: 10.1016/0166-6851(95)02538-3. [DOI] [PubMed] [Google Scholar]
- 10.Folgueira C, Requena JM. A postgenomic view of the heat shock proteins in kinetoplastids. FEMS Microbiol Rev. 2007;31:359–377. doi: 10.1111/j.1574-6976.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- 11.de Carvalho EF, de Castro FT, Rondinelli E, Soares CM, Carvalho JF. HSP 70 gene expression in Trypanosoma cruzi is regulated at different levels. J Cell Physiol. 1990;143:439–444. doi: 10.1002/jcp.1041430306. [DOI] [PubMed] [Google Scholar]
- 12.Engman DM, Henkle-Duhrsen K, Kirchhoff LV, Donelson JE. Trypanosoma cruzi: accumulation of polycistronic hsp70 RNAs during severe heat shock. Exp Parasitol. 1995;80:575–577. doi: 10.1006/expr.1995.1072. [DOI] [PubMed] [Google Scholar]
- 13.Nazer E, Verdun RE, Sanchez DO. Severe heat shock induces nucleolar accumulation of mRNAs in Trypanosoma cruzi. PLoS One. 2012;7:e43715. doi: 10.1371/journal.pone.0043715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giambiagi-deMarval M, Souto-Padron T, Rondinelli E. Characterization and cellular distribution of heat-shock proteins HSP70 and HSP60 in Trypanosoma cruzi. Exp Parasitol. 1996;83:335–345. doi: 10.1006/expr.1996.0081. [DOI] [PubMed] [Google Scholar]
- 15.Tibbetts RS, Jensen JL, Olson CL, Wang FD, Engman DM. The DnaJ family of protein chaperones in Trypanosoma cruzi. Mol Biochem Parasitol. 1998;91:319–326. doi: 10.1016/s0166-6851(97)00214-4. [DOI] [PubMed] [Google Scholar]
- 16.Campos RA, da Silva ML, da Costa GV, Bisch PM, Peralta JM, Silva R, Rondinelli E, Urmenyi TP. Gene expression and molecular modeling of the HSP104 chaperone of Trypanosoma cruzi. Genet Mol Res. 2012;11:2122–2129. doi: 10.4238/2012.August.6.15. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes M, Silva R, Rossle SC, Bisch PM, Rondinelli E, Urmenyi TP. Gene characterization and predicted protein structure of the mitochondrial chaperonin HSP10 of Trypanosoma cruzi. Gene. 2005;349:135–142. doi: 10.1016/j.gene.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Morales D, Ostoa-Saloma P, Espinoza B. Trypanosoma cruzi SHSP16: Characterization of an alpha-crystallin small heat shock protein. Exp Parasitol. 2009;123:182–189. doi: 10.1016/j.exppara.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Clayton C, Shapira M. Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Mol Biochem Parasitol. 2007;156:93–101. doi: 10.1016/j.molbiopara.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues DC, Silva R, Rondinelli E, Urmenyi TP. Trypanosoma cruzi: modulation of HSP70 mRNA stability by untranslated regions during heat shock. Exp Parasitol. 2010;126:245–253. doi: 10.1016/j.exppara.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Droll D, Minia I, Fadda A, Singh A, Stewart M, Queiroz R, Clayton C. Post-transcriptional regulation of the trypanosome heat shock response by a zinc finger protein. PLoS Pathog. 2013;9:e1003286. doi: 10.1371/journal.ppat.1003286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engstler M, Boshart M. Cold shock and regulation of surface protein trafficking convey sensitization to inducers of stage differentiation in Trypanosoma brucei. Genes Dev. 2004;18:2798–2811. doi: 10.1101/gad.323404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean S, Marchetti R, Kirk K, Matthews KR. A surface transporter family conveys the trypanosome differentiation signal. Nature. 2009;459:213–217. doi: 10.1038/nature07997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974;71:522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sojcic Z, Toplak H, Zuehlke R, Honegger UE, Buhlmann R, Wiesmann UN. Cultured human skin fibroblasts modify their plasma membrane lipid composition and fluidity according to growth temperature suggesting homeoviscous adaptation at hypothermic (30 degrees C) but not at hyperthermic (40 degrees C) temperatures. Biochim Biophys Acta. 1992;1104:31–37. doi: 10.1016/0005-2736(92)90128-9. [DOI] [PubMed] [Google Scholar]
- 26.Aloia RC, Raison JK. Membrane function in mammalian hibernation. Biochim Biophys Acta. 1989;988:123–146. doi: 10.1016/0304-4157(89)90007-5. [DOI] [PubMed] [Google Scholar]
- 27.Buda C, Dey I, Balogh N, Horvath LI, Maderspach K, Juhasz M, Yeo YK, Farkas T. Structural order of membranes and composition of phospholipids in fish brain cells during thermal acclimatization. Proc Natl Acad Sci U S A. 1994;91:8234–8238. doi: 10.1073/pnas.91.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florin-Christensen M, Florin-Christensen J, de Isola ED, Lammel E, Meinardi E, Brenner RR, Rasmussen L. Temperature acclimation of Trypanosoma cruzi epimastigote and metacyclic trypomastigote lipids. Mol Biochem Parasitol. 1997;88:25–33. doi: 10.1016/s0166-6851(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 29.Vigh L, Escriba PV, Sonnleitner A, Sonnleitner M, Piotto S, Maresca B, Horvath I, Harwood JL. The significance of lipid composition for membrane activity: new concepts and ways of assessing function. Prog Lipid Res. 2005;44:303–344. doi: 10.1016/j.plipres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Nagy E, Balogi Z, Gombos I, Akerfelt M, Bjorkbom A, Balogh G, Torok Z, Maslyanko A, et al. Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc Natl Acad Sci U S A. 2007;104:7945–7950. doi: 10.1073/pnas.0702557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shigapova N, Torok Z, Balogh G, Goloubinoff P, Vigh L, Horvath I. Membrane fluidization triggers membrane remodeling which affects the thermotolerance in Escherichia coli. Biochem Biophys Res Commun. 2005;328:1216–1223. doi: 10.1016/j.bbrc.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 32.Mittler R, Finka A, Goloubinoff P. How do plants feel the heat? Trends in biochemical sciences. 2012;37:118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Sengupta P, Garrity P. Sensing temperature. Current biology : CB. 2013;23:R304–307. doi: 10.1016/j.cub.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balogh G, Peter M, Glatz A, Gombos I, Torok Z, Horvath I, Harwood JL, Vigh L. Key role of lipids in heat stress management. FEBS letters. 2013;587:1970–1980. doi: 10.1016/j.febslet.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 35.Johansson J, Mandin P, Renzoni A, Chiaruttini C, Springer M, Cossart P. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell. 2002;110:551–561. doi: 10.1016/s0092-8674(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro RS, Cowen LE. Thermal control of microbial development and virulence: molecular mechanisms of microbial temperature sensing. mBio. 2012;3 doi: 10.1128/mBio.00238-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shamovsky I, Ivannikov M, Kandel ES, Gershon D, Nudler E. RNA-mediated response to heat shock in mammalian cells. Nature. 2006;440:556–560. doi: 10.1038/nature04518. [DOI] [PubMed] [Google Scholar]
- 38.Place RF, Noonan EJ. Non-coding RNAs turn up the heat: An emerging layer of novel regulators in the mammalian heat shock response. Cell stress & chaperones. 2013 doi: 10.1007/s12192-013-0456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maric D, McGwire BS, Buchanan KT, Olson CL, Emmer BT, Epting CL, Engman DM. Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein. J Biol Chem. 2011;286:33109–33117. doi: 10.1074/jbc.M111.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyler KM, Fridberg A, Toriello KM, Olson CL, Cieslak JA, Hazlett TL, Engman DM. Flagellar membrane localization via association with lipid rafts. J Cell Sci. 2009;122:859–866. doi: 10.1242/jcs.037721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maric D, Epting CL, Engman DM. Composition and sensory function of the trypanosome flagellar membrane. Curr Opin Microbiol. 2010;13:466–472. doi: 10.1016/j.mib.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberholzer M, Langousis G, Nguyen HT, Saada EA, Shimogawa MM, Jonsson ZO, Nguyen SM, Wohlschlegel JA, et al. Independent analysis of the flagellum surface and matrix proteomes provides insight into flagellum signaling in mammalian-infectious Trypanosoma brucei. Mol Cell Proteomics. 2011;10:M111.010538. doi: 10.1074/mcp.M111.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lammel EM, Barbieri MA, Wilkowsky SE, Bertini F, Isola EL. Trypanosoma cruzi: involvement of intracellular calcium in multiplication and differentiation. Exp Parasitol. 1996;83:240–249. doi: 10.1006/expr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 44.Caler EV, Morty RE, Burleigh BA, Andrews NW. Dual role of signaling pathways leading to Ca(2+) and cyclic AMP elevation in host cell invasion by Trypanosoma cruzi. Infect Immun. 2000;68:6602–6610. doi: 10.1128/iai.68.12.6602-6610.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Epting CL, Coates BM, Engman DM. Molecular mechanisms of host cell invasion by Trypanosoma cruzi. Exp Parasitol. 2010;126:283–291. doi: 10.1016/j.exppara.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pereira MG, Nakayasu ES, Sant’Anna C, De Cicco NN, Atella GC, de Souza W, Almeida IC, Cunha-e-Silva N. Trypanosoma cruzi epimastigotes are able to store and mobilize high amounts of cholesterol in reservosome lipid inclusions. PLoS One. 2011;6:e22359. doi: 10.1371/journal.pone.0022359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Figueiredo RC, Rosa DS, Soares MJ. Differentiation of Trypanosoma cruzi epimastigotes: metacyclogenesis and adhesion to substrate are triggered by nutritional stress. J Parasitol. 2000;86:1213–1218. doi: 10.1645/0022-3395(2000)086[1213:DOTCEM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Contreras VT, Salles JM, Thomas N, Morel CM, Goldenberg S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol Biochem Parasitol. 1985;16:315–327. doi: 10.1016/0166-6851(85)90073-8. [DOI] [PubMed] [Google Scholar]
- 49.Tonelli RR, Augusto LaS, Castilho BA, Schenkman S. Protein synthesis attenuation by phosphorylation of eIF2α is required for the differentiation of Trypanosoma cruzi into infective forms. PLoS One. 2011;6:e27904. doi: 10.1371/journal.pone.0027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cassola A, De Gaudenzi JG, Frasch AC. Recruitment of mRNAs to cytoplasmic ribonucleoprotein granules in trypanosomes. Mol Microbiol. 2007;65:655–670. doi: 10.1111/j.1365-2958.2007.05833.x. [DOI] [PubMed] [Google Scholar]
- 51.Subota I, Rotureau B, Blisnick T, Ngwabyt S, Durand-Dubief M, Engstler M, Bastin P. ALBA proteins are stage regulated during trypanosome development in the tsetse fly and participate in differentiation. Mol Biol Cell. 2011;22:4205–4219. doi: 10.1091/mbc.E11-06-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 (Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarez VE, Kosec G, Sant’Anna C, Turk V, Cazzulo JJ, Turk B. Autophagy is involved in nutritional stress response and differentiation in Trypanosoma cruzi. J Biol Chem. 2008;283:3454–3464. doi: 10.1074/jbc.M708474200. [DOI] [PubMed] [Google Scholar]
- 54.Besteiro S, Williams RA, Morrison LS, Coombs GH, Mottram JC. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J Biol Chem. 2006;281:11384–11396. doi: 10.1074/jbc.M512307200. [DOI] [PubMed] [Google Scholar]
- 55.Williams RA, Mottram JC, Coombs GH. Distinct roles in autophagy and importance in infectivity of the two ATG4 cysteine peptidases of Leishmania major. J Biol Chem. 2013;288:3678–3690. doi: 10.1074/jbc.M112.415372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herman M, Perez-Morga D, Schtickzelle N, Michels PA. Turnover of glycosomes during life-cycle differentiation of Trypanosoma brucei. Autophagy. 2008;4:294–308. doi: 10.4161/auto.5443. [DOI] [PubMed] [Google Scholar]
- 57.Kollien AH, Schaub GA. Development of Trypanosoma cruzi after starvation and feeding of the vector - a review. Tokai J Exp Clin Med. 1998;23:335–340. [PubMed] [Google Scholar]
- 58.Mony BM, Macgregor P, Ivens A, Rojas F, Cowton A, Young J, Horn D, Matthews K. Genome-wide dissection of the quorum sensing signalling pathway in Trypanosoma brucei. Nature. 2013 doi: 10.1038/nature12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vassella E, Reuner B, Yutzy B, Boshart M. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci. 1997;110(Pt 21):2661–2671. doi: 10.1242/jcs.110.21.2661. [DOI] [PubMed] [Google Scholar]
- 60.Kollien AH, Schmidt J, Schaub GA. Modes of association of Trypanosoma cruzi with the intestinal tract of the vector Triatoma infestans. Acta Trop. 1998;70:127–141. doi: 10.1016/s0001-706x(97)00117-4. [DOI] [PubMed] [Google Scholar]
- 61.Bonaldo MC, Souto-Padron T, de Souza W, Goldenberg S. Cell-substrate adhesion during Trypanosoma cruzi differentiation. J Cell Biol. 1988;106:1349–1358. doi: 10.1083/jcb.106.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bayer-Santos E, Cunha-e-Silva NL, Yoshida N, Franco da Silveira J. Expression and cellular trafficking of GP82 and GP90 glycoproteins during Trypanosoma cruzi metacyclogenesis. Parasites & vectors. 2013;6:127. doi: 10.1186/1756-3305-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruiz RC, Favoreto S, Jr, Dorta ML, Oshiro ME, Ferreira AT, Manque PM, Yoshida N. Infectivity of Trypanosoma cruzi strains is associated with differential expression of surface glycoproteins with differential Ca2+ signalling activity. Biochem J. 1998;330(Pt 1):505–511. doi: 10.1042/bj3300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kollien AH, Grospietsch T, Kleffmann T, Zerbst-Boroffka I, Schaub GA. Ionic composition of the rectal contents and excreta of the reduviid bug Triatoma infestans. J Insect Physiol. 2001;47:739–747. doi: 10.1016/s0022-1910(00)00170-0. [DOI] [PubMed] [Google Scholar]
- 65.Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci U S A. 2004;101:10673–10678. doi: 10.1073/pnas.0403139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26:613S–623S. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- 67.Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN. Acidocalcisomes - conserved from bacteria to man. Nat Rev Microbiol. 2005;3:251–261. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 68.Rohloff P, Montalvetti A, Docampo R. Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi. J Biol Chem. 2004;279:52270–52281. doi: 10.1074/jbc.M410372200. [DOI] [PubMed] [Google Scholar]
- 69.Figarella K, Uzcategui NL, Zhou Y, LeFurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol. 2007;65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- 70.Lefurgey A, Gannon M, Blum J, Ingram P. Leishmania donovani amastigotes mobilize organic and inorganic osmolytes during regulatory volume decrease. J Eukaryot Microbiol. 2005;52:277–289. doi: 10.1111/j.1550-7408.2005.00030.x. [DOI] [PubMed] [Google Scholar]
- 71.Docampo R, Jimenez V, Lander N, Li ZH, Niyogi S. New insights into roles of acidocalcisomes and contractile vacuole complex in osmoregulation in protists. Int Rev Cell Mol Biol. 2013;305:69–113. doi: 10.1016/B978-0-12-407695-2.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rohloff P, Rodrigues CO, Docampo R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol Biochem Parasitol. 2003;126:219–230. doi: 10.1016/s0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- 73.Bassarak B, Uzcategui NL, Schonfeld C, Duszenko M. Functional characterization of three aquaglyceroporins from Trypanosoma brucei in osmoregulation and glycerol transport. Cell Physiol Biochem. 2011;27:411–420. doi: 10.1159/000327968. [DOI] [PubMed] [Google Scholar]
- 74.Li ZH, Alvarez VE, De Gaudenzi JG, Sant’Anna C, Frasch AC, Cazzulo JJ, Docampo R. Hyperosmotic stress induces aquaporin-dependent cell shrinkage, polyphosphate synthesis, amino acid accumulation, and global gene expression changes in Trypanosoma cruzi. J Biol Chem. 2011;286:43959–43971. doi: 10.1074/jbc.M111.311530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jimenez V, Docampo R. Molecular and electrophysiological characterization of a novel cation channel of Trypanosoma cruzi. PLoS Pathog. 2012;8:e1002750. doi: 10.1371/journal.ppat.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christoph K, Beck FX, Neuhofer W. Osmoadaptation of Mammalian cells - an orchestrated network of protective genes. Curr Genomics. 2007;8:209–218. doi: 10.2174/138920207781386979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee MG. The 3′ untranslated region of the hsp 70 genes maintains the level of steady state mRNA in Trypanosoma brucei upon heat shock. Nucleic Acids Res. 1998;26:4025–4033. doi: 10.1093/nar/26.17.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kramer S, Queiroz R, Ellis L, Webb H, Hoheisel JD, Clayton C, Carrington M. Heat shock causes a decrease in polysomes and the appearance of stress granules in trypanosomes independently of eIF2(alpha) phosphorylation at Thr169. J Cell Sci. 2008;121:3002–3014. doi: 10.1242/jcs.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reiser V, Raitt DC, Saito H. Yeast osmosensor Sln1 and plant cytokinin receptor Cre1 respond to changes in turgor pressure. J Cell Biol. 2003;161:1035–1040. doi: 10.1083/jcb.200301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alfieri RR, Petronini PG. Hyperosmotic stress response: comparison with other cellular stresses. Pflugers Arch. 2007;454:173–185. doi: 10.1007/s00424-006-0195-x. [DOI] [PubMed] [Google Scholar]
- 82.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–329. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 83.Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 84.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prole DL, Taylor CW. Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS One. 2011;6:e26218. doi: 10.1371/journal.pone.0026218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prole DL, Taylor CW. Identification and analysis of putative homologues of mechanosensitive channels in pathogenic protozoa. PLoS One. 2013;8:e66068. doi: 10.1371/journal.pone.0066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanaka Y, Tanowitz H, Bloom BR. Growth of Trypanosoma cruzi in a cloned macrophage cell line and in a variant defective in oxygen metabolism. Infect Immun. 1983;41:1322–1331. doi: 10.1128/iai.41.3.1322-1331.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krauth-Siegel RL, Comini MA. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim Biophys Acta. 2008;1780:1236–1248. doi: 10.1016/j.bbagen.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 89.McCabe RE, Mullins BT. Failure of Trypanosoma cruzi to trigger the respiratory burst of activated macrophages. Mechanism for immune evasion and importance of oxygen-independent killing. J Immunol. 1990;144:2384–2388. [PubMed] [Google Scholar]
- 90.Paiva CN, Feijo DF, Dutra FF, Carneiro VC, Freitas GB, Alves LS, Mesquita J, Fortes GB, et al. Oxidative stress fuels Trypanosoma cruzi infection in mice. J Clin Invest. 2012;122:2531–2542. doi: 10.1172/JCI58525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Doyle PS, Zhou YM, Hsieh I, Greenbaum DC, McKerrow JH, Engel JC. The Trypanosoma cruzi protease cruzain mediates immune evasion. PLoS Pathog. 2011;7:e1002139. doi: 10.1371/journal.ppat.1002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cabrera G, Barria C, Fernandez C, Sepulveda S, Valenzuela L, Kemmerling U, Galanti N. DNA repair BER pathway inhibition increases cell death caused by oxidative DNA damage in Trypanosoma cruzi. J Cell Biochem. 2011;112:2189–2199. doi: 10.1002/jcb.23138. [DOI] [PubMed] [Google Scholar]
- 93.Campos PC, Silva VG, Furtado C, Machado-Silva A, Darocha WD, Peloso EF, Gadelha FR, Medeiros MH, et al. Trypanosoma cruzi MSH2: Functional analyses on different parasite strains provide evidences for a role on the oxidative stress response. Mol Biochem Parasitol. 2011;176:8–16. doi: 10.1016/j.molbiopara.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Osorio L, Rios I, Gutierrez B, Gonzalez J. Virulence factors of Trypanosoma cruzi: who is who? Microbes Infect. 2012;14:1390–1402. doi: 10.1016/j.micinf.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 95.Maya JD, Repetto Y, Agosin M, Ojeda JM, Tellez R, Gaule C, Morello A. Effects of nifurtimox and benznidazole upon glutathione and trypanothione content in epimastigote, trypomastigote and amastigote forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1997;86:101–106. [PubMed] [Google Scholar]
- 96.Atwood JA, Weatherly DB, Minning TA, Bundy B, Cavola C, Opperdoes FR, Orlando R, Tarleton RL. The Trypanosoma cruzi proteome. Science. 2005;309:473–476. doi: 10.1126/science.1110289. [DOI] [PubMed] [Google Scholar]
- 97.Nogueira FB, Ruiz JC, Robello C, Romanha AJ, Murta SM. Molecular characterization of cytosolic and mitochondrial tryparedoxin peroxidase in Trypanosoma cruzi populations susceptible and resistant to benznidazole. Parasitol Res. 2009;104:835–844. doi: 10.1007/s00436-008-1264-1. [DOI] [PubMed] [Google Scholar]
- 98.Swindell WR, Huebner M, Weber AP. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics. 2007;8:125. doi: 10.1186/1471-2164-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morano KA, Grant CM, Moye-Rowley WS. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics. 2012;190:1157–1195. doi: 10.1534/genetics.111.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miller MA, McGowan SE, Gantt KR, Champion M, Novick SL, Andersen KA, Bacchi CJ, Yarlett N, et al. Inducible resistance to oxidant stress in the protozoan Leishmania chagasi. J Biol Chem. 2000;275:33883–33889. doi: 10.1074/jbc.M003671200. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y, Zhang C, Chen J, Guo L, Li X, Li W, Yu Z, Deng J, et al. Arabidopsis heat shock factor HsfA1a directly senses heat stress, pH changes, and hydrogen peroxide via the engagement of redox state. Plant physiology and biochemistry : PPB / Societe francaise de physiologie vegetale. 2013;64:92–98. doi: 10.1016/j.plaphy.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 102.Manque PA, Probst CM, Probst C, Pereira MC, Rampazzo RC, Ozaki LS, Pavoni DP, Silva Neto DT, et al. Trypanosoma cruzi infection induces a global host cell response in cardiomyocytes. Infect Immun. 2011;79:1855–1862. doi: 10.1128/IAI.00643-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Caradonna KL, Engel JC, Jacobi D, Lee CH, Burleigh BA. Host metabolism regulates intracellular growth of Trypanosoma cruzi. Cell Host Microbe. 2013;13:108–117. doi: 10.1016/j.chom.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Minning TA, Weatherly DB, Flibotte S, Tarleton RL. Widespread, focal copy number variations (CNV) and whole chromosome aneuploidies in Trypanosoma cruzi strains revealed by array comparative genomic hybridization. BMC Genomics. 2011;12:139. doi: 10.1186/1471-2164-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Geiger A, Hirtz C, Becue T, Bellard E, Centeno D, Gargani D, Rossignol M, Cuny G, et al. Exocytosis and protein secretion in Trypanosoma. BMC microbiology. 2010;10:20. doi: 10.1186/1471-2180-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bayer-Santos E, Aguilar-Bonavides C, Rodrigues SP, Cordero EM, Marques AF, Varela-Ramirez A, Choi H, Yoshida N, et al. Proteomic analysis of Trypanosoma cruzi secretome: characterization of two populations of extracellular vesicles and soluble proteins. Journal of proteome research. 2013;12:883–897. doi: 10.1021/pr300947g. [DOI] [PubMed] [Google Scholar]
- 107.Garzon E, Holzmuller P, Bras-Goncalves R, Vincendeau P, Cuny G, Lemesre JL, Geiger A. The Trypanosoma brucei gambiense secretome impairs lipopolysaccharide-induced maturation, cytokine production, and allostimulatory capacity of dendritic cells. Infect Immun. 2013;81:3300–3308. doi: 10.1128/IAI.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rohloff P, Docampo R. A contractile vacuole complex is involved in osmoregulation in Trypanosoma cruzi. Exp Parasitol. 2008;118:17–24. doi: 10.1016/j.exppara.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jimenez V, Paredes R, Sosa MA, Galanti N. Natural programmed cell death in T. cruzi epimastigotes maintained in axenic cultures. J Cell Biochem. 2008;105:688–698. doi: 10.1002/jcb.21864. [DOI] [PubMed] [Google Scholar]
- 110.Martins RM, Covarrubias C, Rojas RG, Silber AM, Yoshida N. Use of L-proline and ATP production by Trypanosoma cruzi metacyclic forms as requirements for host cell invasion. Infect Immun. 2009;77:3023–3032. doi: 10.1128/IAI.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hasne MP, Coppens I, Soysa R, Ullman B. A high-affinity putrescine-cadaverine transporter from Trypanosoma cruzi. Mol Microbiol. 2010;76:78–91. doi: 10.1111/j.1365-2958.2010.07081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dick CF, Dos-Santos AL, Majerowicz D, Paes LS, Giarola NL, Gondim KC, Vieyra A, Meyer-Fernandes JR. Inorganic phosphate uptake in Trypanosoma cruzi is coupled to K(+) cycling and to active Na(+) extrusion. Biochim Biophys Acta. 2013;1830:4265–4273. doi: 10.1016/j.bbagen.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 113.Canepa GE, Bouvier LA, Urias U, Miranda MR, Colli W, Alves MJ, Pereira CA. Aspartate transport and metabolism in the protozoan parasite Trypanosoma cruzi. FEMS Microbiol Lett. 2005;247:65–71. doi: 10.1016/j.femsle.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 114.Pereira CA, Alonso GD, Paveto MC, Flawia MM, Torres HN. L-arginine uptake and L-phosphoarginine synthesis in Trypanosoma cruzi. J Eukaryot Microbiol. 1999;46:566–570. doi: 10.1111/j.1550-7408.1999.tb05132.x. [DOI] [PubMed] [Google Scholar]
- 115.Canepa GE, Bouvier LA, Miranda MR, Uttaro AD, Pereira CA. Characterization of Trypanosoma cruzi L-cysteine transport mechanisms and their adaptive regulation. FEMS Microbiol Lett. 2009;292:27–32. doi: 10.1111/j.1574-6968.2008.01467.x. [DOI] [PubMed] [Google Scholar]
- 116.Inbar E, Canepa GE, Carrillo C, Glaser F, Suter Grotemeyer M, Rentsch D, Zilberstein D, Pereira CA. Lysine transporters in human trypanosomatid pathogens. Amino Acids. 2012;42:347–360. doi: 10.1007/s00726-010-0812-z. [DOI] [PubMed] [Google Scholar]