Abstract
Whereas more than one type of visual opsin is present in the retina of most vertebrates, it was thought that each type of photoreceptor expressed only one opsin. However, evidence has accumulated that some photoreceptors contain more than one opsin, in many cases as a result of a developmental transition from the expression of one opsin to another. The salamander UV-sensitive (UV) cone is particularly notable because it contains three opsins (Makino and Dodd, 1996; J Gen Physiol 108:27–34). Two opsin types are expressed at levels more than a hundred times lower than that of the primary opsin. Here, immunohistochemical experiments identified the primary component as a UV cone opsin and the two minor components as the short wavelength-sensitive (S) and long wavelength-sensitive (L) cone opsins. Based on single cell recordings of 156 photoreceptors, the presence of three components in UV cones of hatchlings and terrestrial adults ruled out a developmental transition. There was no evidence for multiple opsin types within rods or S cones. But immunohistochemistry and partial bleaching in conjunction with single cell recording revealed that both single and double L cones contained low levels of short wavelength-sensitive pigments in addition to the main L visual pigment. These results raise the possibility that co-expression of multiple opsins in other vertebrates was overlooked because a minor component absorbing at short wavelengths was masked by the main visual pigment or because the expression level of a component absorbing at long wavelengths was exceedingly low.
Keywords: electrophysiology, immunocytochemistry, phototransduction, retina, visual pigment
Introduction
Diurnal and most nocturnal vertebrates utilize more than one type of visual pigment in their retinas in order to broaden the wavelength range of their vision and to enable wavelength discrimination (reviewed in Bowmaker, 2008; Jacobs, 2009). The absorption maxima of various vertebrate visual pigments extend from 630 nm down to 350 nm. Visual pigments are composed of an opsin protein and an 11-cis retinal (A1) or a 3,4-dehydro-11-cis retinal (A2) chromophore. For a given opsin, the A2 visual pigment absorbs at longer wavelengths than the corresponding A1 visual pigment. Since only two different chromophores are utilized, the great diversity of pigment absorptions arises from spectral tuning of the chromophore by various interactions with each specific opsin.
In some photoreceptors, a pigment mixture occurs because a fraction of the opsins binds A1 chromophore whereas the remainder binds A2 chromophore. It was long thought that a given photoreceptor expresses only a single type of opsin (reviewed in Mazzoni et al., 2004), but in recent years the number of exceptions has become substantial and continues to grow (reviewed in Lukáts et al., 2005; Isayama and Makino, 2012). Remarkably, the larval salamander UV-sensitive (UV) cone appears to express three different opsins (Makino and Dodd, 1996). In humans, rats and some fish, a developmental change in the type of opsin expressed results in a pigment mixture during the transition period (Wood and Partridge, 1993; Szél et al., 1993, 1994; Archer et al., 1995; Xiao and Hendrickson, 2000; Loew et al., 2002; Cheng et al., 2007; Cheng and Novales Flamarique, 2007). The action spectrum or spectral sensitivity of a photoreceptor’s electrical response to light is determined by the type(s) of visual pigment(s) it expresses. So to discover whether pigment mixtures within larval salamander UV cones reflect one or more changes in the type of opsin expressed as part of a developmental program, we compared the spectral sensitivities of photoreceptors from salamanders a few weeks after hatching, at an advanced larval stage and at the adult, terrestrial stage. Immunohistochemistry with combinations of antibodies specific for the three different salamander cone opsins were used to identify which opsins were present in UV cones.
In addition, other cones and rods were tested for the co-expression of multiple opsins. A red-shifted, secondary visual pigment would give rise to an inflection in the spectral sensitivity at long wavelengths, but because the primary pigment contributes a beta band at short wavelengths to the spectral sensitivity (e.g., Wald, 1968), the presence of a blue-shifted, secondary visual pigment could be masked. Since photons bleach visual pigment, we exposed photoreceptors to judiciously selected wavelengths that preferentially reduced the amount of the primary visual pigment to lower absorption by its main alpha and beta bands. The spectral sensitivity of a photoreceptor expressing a single type of visual pigment would not change following such a treatment. But if another visual pigment were expressed, then partial bleaching of the primary component would increase the relative contribution of the secondary component to the overall spectral sensitivity.
Methods
Animals
Care, use and treatment of animals in this study were in strict agreement with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and institutional guidelines. Large larval tiger salamanders (Ambystoma tigrinum, Charles D. Sullivan, Inc., Nashville, TN) encompassing a wide variation in body morph (Powers, 1907) were maintained in Holtfretter’s solution with a two-fold higher salinity on a 14 h light/10 h dark cycle at 16–20°C for physiological experiments and on a 12 h light/12 h dark cycle at 8–10°C for all other experiments. Early neonates were hatched from eggs (Charles D. Sullivan, Inc.), raised at room temperature and used for recording at 4 to 6 weeks of age. Attempts to record flash responses from rods and cones of individuals younger than 4 weeks were unsuccessful. Terrestrial adults obtained from the same source or converted from larvae from our colony, were kept in terrariums for a minimum of 3 months after complete gill resorption before being used. The terrariums were illuminated by the overhead fluorescent room lights during daytime hours and were darkened at night. The side panels and part of the tops of the terrariums were covered to attenuate the light intensity. Hatchlings were fed live brine shrimp, larger aquatic salamanders were fed live goldfish, and terrestrial animals were fed live crickets dusted with a calcium supplement.
Nomenclature
UV-sensitive cones, blue-sensitive cones and red-sensitive cones will be referred to as UV, S and L cones, respectively. Boll (1877) termed the majority of rods in the frog retina as “red rods” due to the appearance of their outer segments when viewed microscopically during illumination with white light; there were also a fewer number of “green rods”. For consistency in our nomenclature, “red rods” will be referred to as M rods and “green rods” will be called S rods. The primary visual pigments and opsins expressed in rods and cones will be named accordingly.
Single cell recording
Salamanders were dark-adapted overnight, decapitated and retinal tissue prepared for recording under infrared illumination (Makino et al., 1999). A shredded piece of retina was loaded into a recording chamber and perfused continuously with Ringer’s solution at room temperature (20–22°C). Ringer’s consisted of (mM): 108 NaCl, 2.5 KCl, 1.0 MgCl2, 10 HEPES, 1.5 CaCl2, 0.02 EDTA, 10 glucose, 7.5 × 10−4 bovine serum albumin (Fraction V, γ-globulin-free; Sigma); pH 7.6. The inner segment of a single rod or cone was pulled into a suction electrode and the photocurrents recorded with a current-to-voltage converter (Axopatch 200A, Axon Instruments, Foster City, CA). Signals were low-pass filtered (8-pole Bessel, 30 Hz, -3 dB) but no corrections were made for the delay introduced by filtering. Data were digitized at 400 Hz. Cells were stimulated with light from a 75 W xenon arc lamp that passed through a six-cavity interference filter (10 nm bandwidth at half-maximal transmission, Omega Optical, Brattleboro, VT). Stimulus duration was controlled with an electronic shutter. A nominal duration of 22 ms was used for most test flashes. Cells were identified by inspection and by their responses to flashes at 377 nm, 440 nm, 500 nm and 620 nm. The light was calibrated with a photometer (UDT 350, Graseby, Orlando, FL) through a 200 μm diameter pinhole (Melles Griot, Carlsbad, CA) placed at the level of the recording chamber.
The spectral sensitivity of individual salamander photoreceptors was examined by recording flash responses at different wavelengths at two flash strengths in the linear range. For each wavelength, mean response amplitudes were plotted against the corresponding flash strengths, fit with a line, and compared to the relation for a reference wavelength. The relative sensitivity values as a function of wavelength were fit by eye with the sum of two templates designed to weigh contributions of A1 and A2 chromophores (Govardovskii et al., 2000):
| (1) |
where α is the fractional contribution of A1, λ1 is the λmax of A1 visual pigment, and λ2 is the λmax of the A2 pigment. Values used for λ1 and λ2 in nm were (Makino et al., 1999): UV pigment, 361 (A1); S pigment, 428 and 435; M pigment, 501 and 519; L pigment, 562 and 620. Determination of the A1 and A2 composition of the UV pigment was problematic because spectral information on the A1 and A2 UV salamander pigments is limited and their maxima are separated by only a few nanometers (Hárosi, 1994; Ma et al., 2001a), so for simplicity a pure A1 content was assigned throughout the study. The overall λmax of a cell was determined from the fit with eq. 1. The A1-based pigment content of a cell was calculated by adjusting for the difference in absorbance coefficient between A1 and A2 pigments:
| (2) |
where α is given by eq. 1. This component analysis was extended to the spectra of some cells by fitting with the sum of several templates to incorporate the contributions of multiple opsins. To simplify the analyses, the beta bands of the pigment absorption spectra were not considered. For partial bleaching, cells were exposed to: 4–13 × 108 photons μm−2 at 560 to 650 nm for L cones, 3–7 × 108 photons μm−2 at 450 or 465 nm for S rods and cones, 1–3 × 107 photons μm−2 at 360 nm for UV cones, and 4–9 × 108 photons μm−2 at 560 nm for M rods. The extent of pigment bleaching was calculated using the following formula:
| (3) |
where I is the light intensity in photons μm−2 s−1, t is the duration of the exposure, and P is the pigment photosensitivity. Values used for P were 5.4 × 10−9 μm2 for M and L A2 pigments, 7.2 × 10−9 μm2 for A1 pigments, 5.8 × 10−9 μm2 for S A2 pigment (Makino et al., 1991) and 9.3 × 10−9 μm2 for UV pigment (Makino and Dodd, 1996). In estimating the fractional bleaches, P was adjusted for variations in the A1 versus A2 content of individual cells according to the wavelength of the bleaching light. Following the bleach, spectral sensitivities were re-measured after a minimal interlude of 30 min for cones and S rods, and 90 min for the M rods to allow for stabilization of the cell’s condition.
Stimulus-response relations were fit with a modified saturating exponential (Ma et al., 2001b; Igor Pro 3.16, Wavemetrics, Lake Oswego, OR):
| (4) |
where k1, k2 and k3 are constants and i’ is normalized flash strength. Since the fit was sensitive to the starting values, i was normalized by the flash strength that suppressed half of the dark current (i0.5), estimated from a preliminary fit with the Hill equation,
| (5) |
and k1, k2 and k3 were initialized to 2, −1 and 0, respectively. The final i0.5 values for dark-adapted cells found from the fits with eq. 4 (Table 1) were similar to or less than those reported previously (Makino et al., 1999; Ma et al., 2001b).
Table 1.
Physiological parameters of salamander rods and cones.
| Cell Type | rmax | i0.5 | tp | Ti | λmax | %A1 |
|---|---|---|---|---|---|---|
| Hatchling | ||||||
| M Rod | 17±9, 2 | 3±1, 2 | 631, 1 | 1459, 1 | 512±4, 2 | 50±25, 2 |
| S Rod | 4, 1 | 11, 1 | 530, 1 | 428, 1 | 94, 1 | |
| UV Cone | 4±1, 7 | 291±119, 8 | 112±27, 2 | 135±53, 2 | 361* | 100* |
| S Cone | 3±0.4, 8 | 59±11, 7 | 674±100, 2 | 429±0, 4 | 82±10, 4 | |
| L Cone Single | 4±1, 4 | 602±160, 4 | 181±34, 2 | 494±46, 2 | 563±0.2, 5 | 92±1, 5 |
| Larval | ||||||
| M Rod | 31±3, 18 | 4±0.5, 17 | 610±19, 9 | 1470±76, 9 | 516±3, 9 | 28±8, 9 |
| S Rod | 11±1, 22 | 51±9, 26 | 310±18, 21 | 686±55, 21 | 431±1, 17 | 43±8, 17 |
| UV Cone | 4±0.5, 11 | 1344±295, 9 | 109±9, 7 | 210±26, 6 | 361* | 100* |
| S Cone | 6±1, 10 | 349±57, 14 | 301±24, 10 | 759±86, 10 | 434±1, 6 | 23±13, 7 |
| L Cone Single | 8±1, 11 | 959±163, 13 | 169±16, 6 | 512±119, 6 | 584±4, 12 | 49±5, 12 |
| L Cone Double | 10±1, 24 | 1098±129, 24 | 188±15, 9 | 534±66, 9 | 586±2, 25 | 51±3, 24 |
| Terrestrial | ||||||
| M Rod | 27±2, 2 | 3±1, 2 | 592, 1 | 505±2, 2 | 82±10, 2 | |
| S Rod | 16±2, 12 | 17±5, 9 | 529±61, 8 | 1405±165, 8 | 429±1, 12 | 73±10, 12 |
| UV Cone | 8±1, 3 | 701±370, 3 | 299±93, 3 | 545±369, 2 | 361* | 100* |
| L Cone Single | 5±1, 4 | 1812±551, 4 | 184±39, 3 | 380±161, 3 | 568±4, 3 | 73±12, 3 |
| L Cone Double | 7±1, 5 | 383±78, 5 | 242, 1 | 481, 1 | 576±3, 5 | 64±3, 3 |
Values given as mean ± SEM, number of cells analyzed. rmax, is the maximal response amplitude. i0.5, is the flash strength at the λmax that elicited a half maximal response. Kinetics of the single photon response were determined from dim flash responses whose mean amplitudes were less than or equal to 0.15rmax. The time-to-peak, tp, was measured from mid-flash to the peak of the response. The integration time, Ti, was calculated as the integral of the dim flash response divided by the response amplitude. Values for λmax were taken from fits of spectral sensitivity with eq. 1 (see Methods).
for UV cones, the sensitivity maximum was set to 361 nm and the %A1 was assigned to be 100% (see Methods).
Immunohistochemistry
The specificities of the anti-opsin antibodies (Table 2): CERN886 (Schalken and DeGrip, 1986), CERN874 (Foster et al., 1993), CERN933 (Sherry et al., 1998), and CERN956 (Vissers and DeGrip, 1996), were characterized by western blot analysis with recombinant salamander opsin proteins. All recombinant opsins were modified with the C-terminal eight amino acid residues of bovine rhodopsin, recognized by the 1D4 antibody (Molday and MacKenzie, 1983), and expressed and purified as described previously (Ma et al., 2001b; Das et al., 2004). SDS-PAGE was run using precast 4–12% Bis-Tris gels in a NuPAGE gel system (Life Technologies, Grand Island, NY) and followed by semi-dry transfer of proteins onto nitrocellulose. Blots were probed with the 1D4 antibody to confirm the loading of each opsin, and repeated with the antibodies listed above. Antibodies were diluted as follows: CERN886, 1:>4000; CERN874, 1:>2000; CERN933, 1:1000; CERN956, 1:1000. Labeling was visualized by chemiluminescence using Pierce’s SuperSignal West Dura (Thermo Fisher Scientific, Rockville, IL).
Table 2.
Antibodies
| Antigen | Source/Citation | Type | |
|---|---|---|---|
| CERN886 | Bovine rod rhodopsin | W. DeGrip, Dept. of Biochemistry, Radboud University Nijmegen Medical Centre; Schalken and DeGrip, 1986 | Rabbit polyclonal |
| CERN956 | N-terminus peptide (AGRHPQDSSEDSTQSSNSTRGPFEGPNY), human/mouse red/green cone (MLWS) rhodopsin | W. DeGrip, Dept. of Biochemistry, Radboud University Nijmegen Medical Centre; Vissers and DeGrip, 1996 | Rabbit polyclonal |
| CERN933 | Peptide (ISSVGPWDGPQYH), human/mouse S cone rhodopsin | W. DeGrip, Dept. of Biochemistry, Radboud University Nijmegen Medical Centre; Sherry et al., 1998 | Rabbit polyclonal |
| CERN874 | Mixture of chicken M and L cone rhodopsins | W. DeGrip, Dept. of Biochemistry, Radboud University Nijmegen Medical Centre; Foster et al., 1993 | Rabbit polyclonal |
| Blue-N | N-terminus peptide (MYKGKQEMMAELSD), S cone and rod (SWS2) opsin | J.-X. Ma, Dept. of Cell Biology, Univ. of Oklahoma Medical Center; Ma et al., 2001b | Rabbit polyclonal |
| UV-N | Peptide (ISKVGPWDGPQY), UV cone (SWS1) opsin | J.-X. Ma, Dept. of Cell Biology, Univ. of Oklahoma Medical Center; Chen et al., 2008 | Rabbit polyclonal |
| TA-2 | Peptide (YASNQRAEDGRL), cone transducin α-subunit (Gαt2) | J.-X. Ma, Dept. of Cell Biology, Univ. of Oklahoma Medical Center; Ryan et al., 2000 | Rabbit polyclonal |
| 1D4 | Bovine rod opsin | R.S. Molday, Dept. of Biochemistry, University of British Columbia; Molday and MacKenzie, 1983 | Mouse monoclonal |
Procedures for immunofluorescence labeling have been described previously (Ma et al., 2001b). In short, lens-attached retinas were dissected from salamanders and transferred to phosphate-buffered saline (PBS); pH 7.4. Retinas were fixed by immersion in 4% paraformaldehyde in PBS for 1 h followed by permeabilization with 50% methanol in PBS for 5 min at −20 °C. Tissues were then washed five times with PBS at room temperature and blocked with 1% horse serum in PBS for 45 min. The antibodies to cone opsins, Blue-N (Ma et al., 2001b), UV-N (Chen et al., 2008), CERN956 (Vissers and DeGrip, 1996) were labeled with the Alexa Fluor antibody labeling kit (Invitrogen) with either Alexa Fluor 350, Alexa Fluor 488 or Alexa Fluor 594. Antibodies were diluted to 1:200 in PBS. Retinas were incubated with primary antibodies for 1 h at room temperature. After three washes with PBS, retinas were mounted on slides with one drop of Prolong anti-fade solution (Molecular Probes, Eugene, OR) and a coverslip.
Double- and triple-labeling experiments with anti-opsin antibodies and anti-cone transducin α-subunit (Gαt) antibody TA2 (Table 2), followed a protocol described earlier (Ryan et al., 2000). All antibodies were from rabbit. Opsin antibodies were conjugated with Alexa Fluor as described above, and the TA2 antibody was biotinylated (ImmunoProbe Biotinylation Kit, Sigma). Tissue was first labeled with biotinylated TA2 antibody for 2 h at room temperature, followed by three PBS washes. Then primary antibodies to opsins (1:100) were added simultaneously with FITC-conjugated mouse-anti-biotin secondary antibody (1:200), and incubated for 2 h. Sections were washed and mounted with coverslips as above. Samples were analyzed using an Axioplan research microscope (Carl Zeiss Inc., Jena, Germany) equipped with a 100 W mercury light source, a 100x plan-neofluar N.A. 1.3 objective and a Dage CCD100 integrating camera (Dage-MTI, Michigan City, IN). Images were recorded with a Flashpoint 128 capture board (Integral Technologies, Indianapolis, IN) and a Dual Pentium Pro 200 Imaging workstation (Dell Computers, Round Rock, TX) and processed using Image Pro Plus software (Media Cybernetics, Baltimore, MD). Panels containing images of Alexa Fluor 350-labeled tissues were pseudo-colored with Adobe Photoshop to enhance visualization of the blue fluorescence.
Results
What types of photoreceptors are present in the salamander retina?
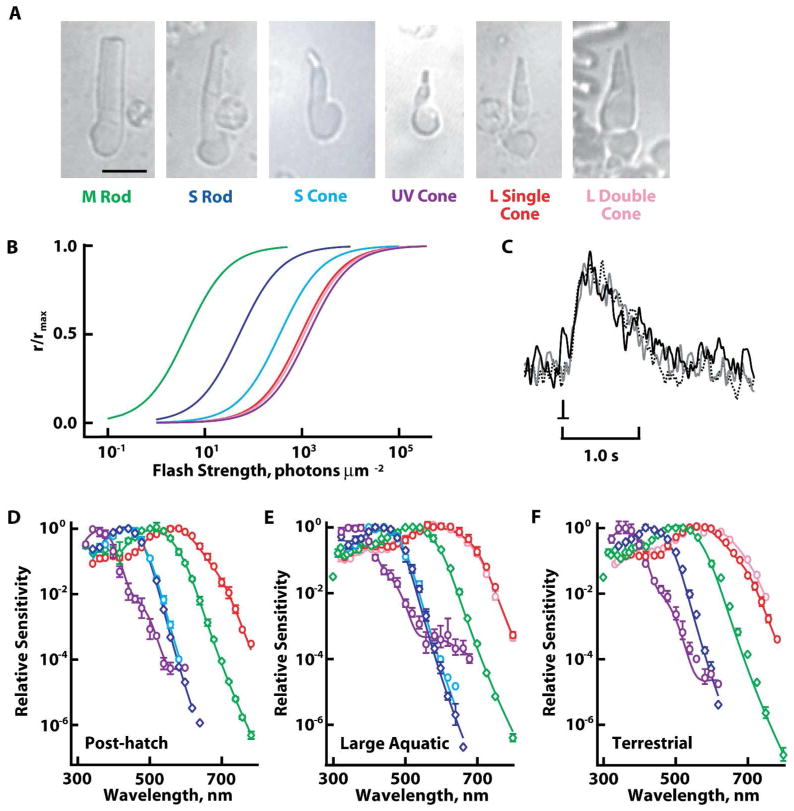
Aquatic salamander retinas are populated mainly by rods. Most are M, although a small fraction are S (Hárosi, 1975; Sherry et al., 1998; Chen et al., 2008; Zhang and Wu, 2009). Compared to M rods, S rods typically have a shorter outer segment (Mariani, 1986; Sherry et al., 1998) that abuts an inner segment whose length is roughly twice that of their outer segment. A rare form of S rod extended a thinner, tapered outer segment attached to a short inner segment. Single cones with the largest outer segments were generally L, however, not all L cones were large (cf. Sherry et al., 1998). Single cones with the thinnest outer segments were usually UV, but some UV cones had medium-sized outer segments and large inner segments. S cone outer segments were intermediate in size; the largest ones were cylindrical in shape, in contrast to the tapered outer segments of L cones. Given the morphological variability, it was not useful to differentiate single cones by size, but single cones were distinguished from double cones. The latter consisted of two closely opposed members (Mariani, 1986), of which the principal member had a thicker outer segment than the accessory member. Some recordings of double cones were made with the inner segments of both members inside the electrode, whereas in others, one member was stripped off. Nevertheless, all recorded double cones were L (n=30), consistent with previous reports (Attwell et al., 1984; Sherry et al., 1998). A few paired cones had very large inner segments capped off with small, tapered outer segments. The inner as well as the outer segments of each member were equal in size, making these cells twin cones (cf. Walls, 1942). Due to their scarcity, twin cones will not be discussed further. Although L double cones were identified with reasonable certainty, we cannot rule out the possibility that a few L single cones were double cones lacking the accessory member. Representative cells are shown in figure 1 along with averaged spectra. Averaged response properties are summarized in Table 1.
Figure 1.
Spectral classes of rods and cones. A. Morphologies of representative photoreceptors of tiger salamanders. Images were captured with an infrared light-sensitive camera. Scale bar = 20 μm. B. Relative sensitivities of salamander rods and cones to flashes at their respective spectral maxima. Colors of the individual curves correspond to the labels for the cells in A. C. Averaged responses of a UV cone from a terrestrial salamander to dim flashes at 620 nm (continuous black trace), 440 nm (gray trace), and 360 nm (dashed black trace) flashes. Responses were scaled to their peak amplitudes. D–F. Evidence for lack of change in opsin content of rods and cones during development. Spectral sensitivities of: S rods, blue diamonds; M rods, green diamonds; L single cones, red circles; L double cones, pink circles; S cones, blue circles; and UV cones, violet circles from hatchling (D), large larval (E), and terrestrial (F) salamanders were fit with sums of visual pigment templates (continuous lines, see Methods). L double cones from hatchlings were not recorded. S cones were not found in terrestrial salamanders. Error bars represent SEM.
The spectral sensitivity of the UV cone was unique among salamander photoreceptors in that it had two inflections on the descending, long wavelength limb (Figs. 1E, 2A). From a component analysis (see Methods), the average waveform was well described by the sum of contributions from a UV pigment along with A1 and A2 forms of S and L cone pigments. One UV cone, for which spectral sensitivity was the most complete, contained 96% A1 P361, 2.8% A1 P428, 1.5% A2 P435, 0.02% A1 P562, and 0.008% A2 P620 (Fig. 2A). The fractional A1 content of the S component was 0.65, while that of the L component was similar at 0.71. Analysis of another cell plus five cells from Makino and Dodd (1996) with more extensive spectral data gave a mean ratio of fractional A1 content for the S component to that of the L component equal to 0.92 ± 0.16 (mean ± SEM), indicating that both components generally contained similar A1 and A2 distributions. One or both of the inflections on the descending limb of the spectral sensitivity diminished or disappeared after partial bleaching with long or middle wavelength light as described previously by Makino and Dodd (1996). The component analysis for the cell in figure 2B, C indicated that approximately 99% of the S component was eliminated by the partial bleach, which matched the estimate from eq. 3 (>99% pigment bleached). Taken together, all of these observations support the contention of Makino and Dodd (1996) that two chromophores combine with three opsins to form five, if not six different visual pigments in UV cones.
Figure 2.
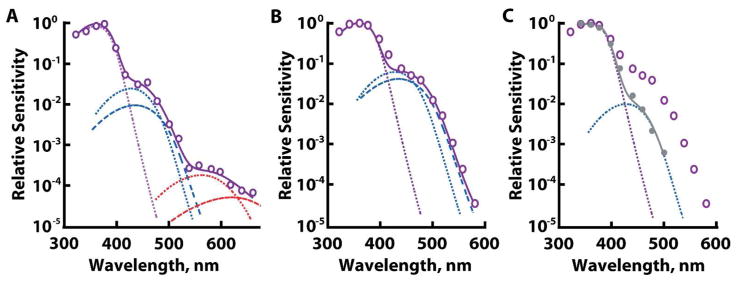
Evidence for three opsin types in UV cones by a component analysis and by partial bleaching. Dotted and dashed lines show A1 and A2 spectra, respectively, of a UV cone pigment in violet, S pigments in blue, and L pigments in red. Summation of the components is shown with a continuous line. A. Dark-adapted UV cone from a large aquatic salamander. B. Dark-adapted UV cone from a terrestrial salamander. L pigment in this cell, if present, fell below our limit of detection. C. Reduction of the S pigment components after exposure of the UV cone from B to bright light, λ >400 nm (filled symbols). The dark-adapted spectrum (open symbols) is redrawn from B.
Does UV cone spectral sensitivity change during development?
All of the cells from the present study described thus far, as well as those of Makino and Dodd (1996), were taken from large, larval salamanders (exceeding eight inches in length), so multiple visual pigments in UV cones could have been linked to changes in opsin expression in preparation for metamorphosis to the terrestrial form. During the transition, two or more opsins expressed at an earlier period might have persisted for some time after their expression ceased. To test this hypothesis, we recorded single photoreceptors from salamanders at two additional developmental stages: 4–6 week old hatchlings and terrestrial adults. Had there been changes in opsin expression, the ratios of visual pigments in cones at progressive stages of development would have varied systematically.
Overall, hatchling photoreceptors tended to be small. In particular, the very large M rods exceeding 13 μm in diameter and large single L cones that were sometimes encountered in retinas of large aquatic salamanders were not present. Hatchling M rods had a high A2 content, but the spectral sensitivities of all other photoreceptors reflected an unexpectedly high proportion of A1 chromophore (Fig. 1D, Table 1). Selective A1 incorporation over A2 by individual photoreceptors within a retina has been documented previously (Reuter et al., 1971; Bowmaker et al., 1988; Saarinen et al., 2012). A few double cones were observed in the hatchling preparations, but none were recorded. A1 chromophore predominated in salamanders after metamorphosis to the terrestrial stage, although the changeover from A2 was seldom complete (Fig. 1F, Table 1). S cones were no longer found, consistent with their disappearance at the terrestrial stage (Chen et al., 2008). Otherwise all morphological and spectral kinds of photoreceptors were present at all three developmental stages (Fig. 1D–F).
Notably, UV cones did not show a systematic change in spectral maximum and two inflections in their spectral sensitivities at longer wavelengths were present from post-hatch to the terrestrial stage (Fig. 1D–F). The L component was somewhat variable, occurring in four out of five UV cones from hatchlings, in five out of six UV cones from large larvae and in two out of three UV cones from adults. An example of a UV cone lacking the L component is shown in figure 2B. However, the frequency of occurrence did not change with developmental stage. Furthermore, the apparent absence of an L component may have resulted from a technical limitation because demonstrating its presence often required the brightest flash available. For hatchling and terrestrial UV cones, sensitivity to blue light was approximately 100 times lower than that to UV light, while sensitivity to red light was more than 10,000 times lower. Levels of the S component in post-hatch and terrestrial UV cones were similar but were approximately two-fold less than those in UV cones of large larval salamanders (Fig. 1D–F). Levels of the L component for post-hatch and terrestrial cells were also similar to each other but tended to be lower than the amount expressed by UV cones of large larval animals.
The spectral positions of the S and L components in UV cones of hatchlings and terrestrial salamanders were slightly blue shifted from those of UV cones of large larval animals. Our component analysis attributed the shift to a higher A1 content, as was the case for other kinds of cones (Table 1). Thus the premise that multiple pigments in the UV cones from large larval salamanders exist during a transitional period following one or more changes in the type of opsins expressed was not supported.
In Nrl−/− mouse “cones” that express both M and S opsins, dim flash responses generated by the two opsins have different recovery kinetics (Nikonov at al., 2005). To find out whether such differences occur in salamander UV cones, a comparison was made of responses to flashes at short, middle and long wavelengths that preferentially excited UV, S and L pigments, respectively. For a given UV cone, the kinetics were independent of wavelength for hatchling and terrestrial (Fig. 1C) as well as for large larval salamanders (cf. figure 1 of Makino and Dodd, 1996). For that matter, flash response kinetics were also invariant with respect to wavelength for all other photoreceptors.
Do other salamander photoreceptors contain more than one type of visual pigment?
Apart from UV cones, the absence of a shoulder(s) on the long wavelength limb of the spectral sensitivities of other salamander photoreceptors (Fig. 1D–F) argued strongly against the co-expression of minor levels of secondary visual pigments absorbing at wavelengths longer than those absorbed by the primary visual pigment. The presence of a secondary visual pigment absorbing at shorter wavelengths was more difficult to assess because it would have been obscured by the beta band (or cis peak) of the primary visual pigment. The chromophore mixture complicated matters further because the beta band of A2 pigment is more pronounced than that of the corresponding A1 pigment (cf. Govardovskii et al., 2000) and the beta bands for salamander L pigments have not been well characterized. Notwithstanding, a marked individual variation in relative sensitivity to short wavelengths for dark-adapted L cones with similar A1 content suggested that cells in our sample pool expressed different levels of secondary pigments. On that assumption, the L double cone with the highest relative sensitivity to short wavelengths was estimated by the component analysis to contain 14% UV and 18% S pigment (Fig. 3A). There was less spectral variation across L single cones and the component analysis suggested upper limits of 9% UV and 12% S pigments (Fig. 3B). Variation in the spectra of rods and S cones fell within experimental error.
Figure 3.
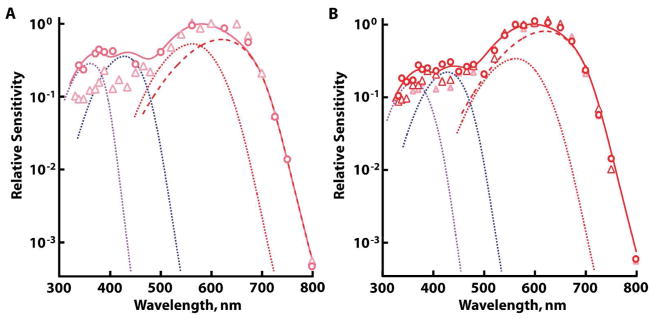
Opsin mixtures indicated by spectral variation at short wavelengths in dark-adapted L cones. Cones with A1 L pigment content of 20–40% were selected for comparison. Component analysis (thick continuous line) of the cone with higher sensitivity to short wavelengths (circles) suggested the presence of UV A1 pigment (violet dotted line), S A1 pigment (blue dotted line), and L A1 (red dotted line) and A2 (red dashed line) pigments. A. Two double cones (circles and triangles). B. Two single cones (red symbols). The spectral sensitivity of the double cone with low sensitivity to short wavelengths is redrawn from A, for reference (pink triangles).
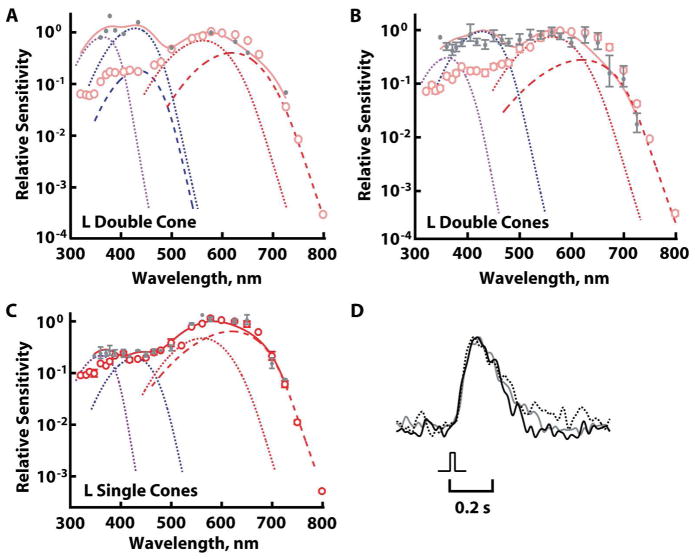
The expression of a secondary visual pigment(s) at short wavelengths was tested in photoreceptors from aquatic salamanders (except where noted otherwise) by partial bleaching, this time to selectively remove the primary visual pigment. Had the cell contained a secondary visual pigment(s), we would have observed a relative increase in spectral sensitivity at shorter wavelengths post-bleach. Exposure to bright light that bleached the primary visual pigment often lowered relative sensitivity to long wavelengths, due to preferential bleaching of the A2 over the A1 primary visual pigments. The effect was most apparent in L cones (e.g., Fig. 4A–C), where the spectral difference between corresponding A1 and A2 pigments was greatest (Makino et al., 1999).
Figure 4.
Opsin mixtures in L cones revealed by partial bleaching with 600–650 nm light. Error bars represent SEM. Dark-adapted cones (open circles) were partially bleached (filled circles) and subjected to the component analysis (continuous line) with contributions of A1 components (dotted traces) and A2 components (dashed traces) for UV, S, and L pigments. Although symbols plot average values in B and C, not every cone was represented at each wavelength, so the component analyses were carried out on collected results. A. L double cone for which partial bleaching of >96% of its primary visual pigment increased relative sensitivity to short wavelengths by more than 10-fold. B. Averaged increase in relative sensitivity to short wavelengths of 5-fold for five L double cones following >90% bleach. C. Increase in relative sensitivity to UV wavelengths in four L single cones following ~60% bleach. D. Indistinguishable kinetics in the responses of a partially bleached L double cone (93% pigment bleached) to dim flashes at 625 (black trace), 450 (gray trace), and 360 (dashed black trace) nm. Responses were scaled to their peak amplitudes.
Double cones from 23 aquatic and four terrestrial salamanders were subjected to partial bleaching. An increased sensitivity to middle and/or short wavelengths was observed nine times, with the greatest sensitization exceeding 10-fold (Fig. 4A). The average sensitization for five experiments after >90% pigment bleach was about 5-fold (Fig. 4B). The change in the shape of the spectral sensitivity was broader than expected for the presence of only one additional visual pigment. The component analysis attributed the elevated short wavelength sensitivity to UV and S pigments. The deduced fractional content for the double cone in figure 4A was 6% UV pigment and 8% S pigment when dark-adapted, with mean values of 7% and 10% for UV and S pigments for the six double cones presented in figure 4A and B. These values estimate upper limits of opsin co-expression, because levels of UV and S components were indeterminate in the majority of partially bleached double cones, including one for which more than 99% of the primary pigment was bleached.
Partial bleaching of approximately 60% of the primary pigment increased relative sensitivity to short wavelengths by 2-fold in four L single cones (Fig. 4C). Component analyses of the spectral sensitivities suggested levels of UV and S pigments as high as 4% and 3%, respectively, upon extrapolating back to the dark-adapted condition.
In two of three S rods and in two S cones, post-bleach sensitivity improved slightly at UV wavelengths (Fig. 5B, C). This change was not attributed to the unmasking of a UV visual pigment because the peak of sensitization was located at wavelengths shorter than the spectral maximum of the UV cone (see discussion). Otherwise, the spectral sensitivities of M and S rods and S cones were largely unchanged by 75–98% bleach (Fig. 5A–C). Unfortunately, more extensive bleaching lowered absolute sensitivity below our ability to measure relative sensitivity to UV light. Thus, UV cone opsin, if co-expressed in M rods and in S rods and cones, was present at a concentration lower than that of the primary visual pigment by at least an order of magnitude.
Figure 5.
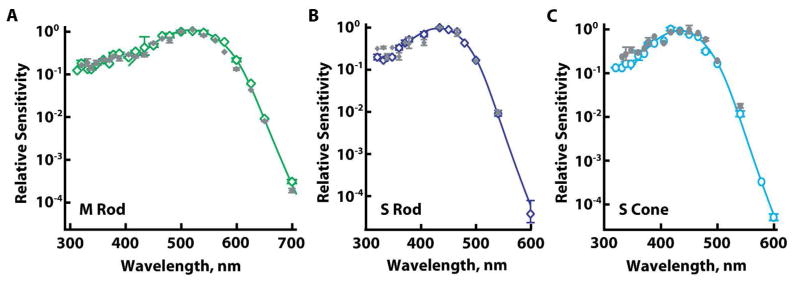
Lack of physiological evidence for opsin mixtures in rods and in S cones. Averaged, dark-adapted, spectral sensitivities (open symbols) were unchanged by partial bleaching (filled symbols). Solid lines are template fits to dark-adapted results. A. M rods (n=5) exposed to 560 nm light that bleached 85 ± 3% pigment. B. S rods (n=3) exposed to 450 nm light to bleach 94 ± 2% pigment. C. S cones (n=2) exposed to 450 or 465 nm to bleach 86 ± 11% pigment. Error bars represent SEM.
Partial bleaching accelerated the flash response kinetics in salamander cones, as part of bleaching adaptation. However, as was the case before partial bleaching, flashes produced responses that were similar in form independent of wavelength in all kinds of cones. Figure 4D shows dim flash responses from an L double cone in which partial bleaching improved relative sensitivity to short wavelengths. Selective stimulation of L, S and UV pigment components yielded responses with similar kinetics, consistent with each visual pigment component being coupled to the same phototransduction pathway within the outer segment.
What types of opsin are co-expressed in UV cones and in L cones?
The preceding analyses indicated co-expression of three different visual opsins in UV cones and L single and double cones. To verify that the minor components in these cells were the same primary opsins of UV, S or L cones, a collection of antibodies was employed to identify the opsins expressed in each kind of photoreceptor (Table 2). Antibody specificity was determined by immunoblotting against recombinant salamander opsins (Fig. 6). Blue-N and UV-N were antibodies raised against the amino termini of the opsins in S rods/cones and UV cones, respectively, segments that show little homology across salamander pigments (Chen et al., 1996; Xu et al., 1998; Ma et al., 2001a). Blue-N does not recognize the opsins of M rods or those of UV and L cones (Ma et al., 2001b). Unfortunately, the supply of UV-N was exhausted before its specificity could be verified. CERN956 was originally raised against the amino terminus of human L/M cone opsins (Vissers and DeGrip, 1996) and as expected, it recognized L cone opsin only (Fig. 6B). Three polyclonal antibodies used in a previous study on salamander by Sherry et al. (1998): CERN874, CERN933 and CERN886, were also characterized. CERN886 was raised against purified bovine rhodopsin (Schalken and DeGrip, 1986). Since all the recombinant salamander opsins contained the 1D4 epitope of bovine rhodopsin, CERN886 (Fig. 6A) as well as the 1D4 antibody (not shown) confirmed protein loading. CERN933 was raised against human S opsin (Sherry et al., 1998). Under our experimental conditions, it recognized salamander S opsin but not any of the other opsins, including the positive controls (Fig. 6C). CERN874 was raised against a mixture of chicken visual pigments that was enriched for L cone pigment (Foster et al., 1993). It bound salamander L opsin > S opsin, but also very weakly bound UV opsin (Fig. 6D).
Figure 6.
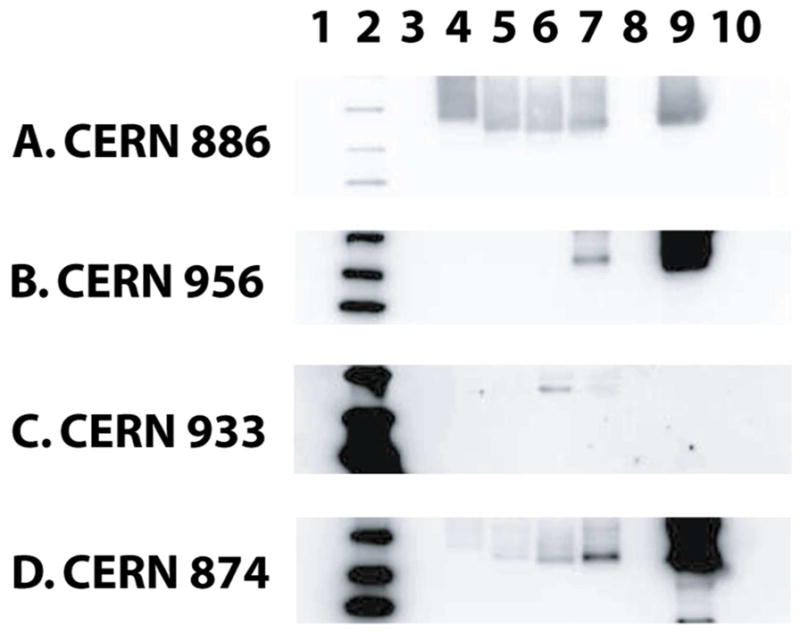
A. Characterization of antibodies against recombinant salamander opsins. Gel lanes were loaded with: (1) protein elution buffer and sample buffer (i.e., blank), (2) MW markers (kDa): 40, 30, 20, (3) blank, (4) salamander M rod opsin, (5) salamander UV opsin, (6) salamander S opsin, (7) salamander L opsin, (8) blank, (9) positive control (specified for individual antibodies, below), (10) blank except where stated otherwise. A. CERN886. Lane 9 was loaded with bovine rhodopsin, while lane 10 was a blank. B. CERN956. Lane 9 was loaded with recombinant human M cone opsin. C. CERN933. Lane 9 was loaded with recombinant human S opsin. Lane 10 was loaded with mouse UV opsin as a positive control. D. CERN874. Lane 9 was loaded with a retinal extract from chicken. Approximately equal amounts of opsins were loaded onto the gels based on 1D4 immunoreactivity (not shown). Blots in B–D are shown overexposed to check for weak cross-reactivity to more than one opsin.
In whole mounted retinas, outer segments of some cones and rods were labeled by Blue-N. Most of the Blue-N-labeled cone outer segments were co-labeled by UV-N (Fig. 7A–C); cones labeled exclusively by Blue-N were scarce in aquatic animals and were never encountered in terrestrial animals, consistent with distributions reported previously (Chen et al., 2008). Taken together with the results of the physiological recordings, cells labeled exclusively by Blue-N were the S rods and cones, whereas the doubly labeled cones were L and UV cones. Some cones appeared to contain much more S opsin than others in the same field (Fig. 7A, arrows), while double labeling indicated that outer segments that were labeled strongly with Blue-N were weakly labeled with UV-N (Fig. 7B, arrows). These outer segments belonged to L cones. Cone outer segments that were stained very weakly by Blue-N (Fig. 7A, arrowhead), but were labeled quite well by UV-N (Fig. 7B, arrowhead), were UV cones.
Figure 7.
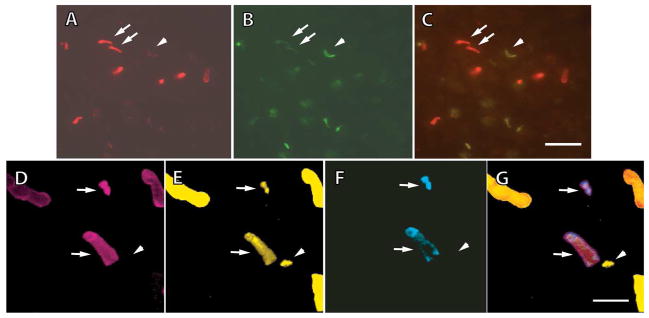
Immunohistochemical evidence for the coexistence of multiple visual pigments within UV cones and L cones of larval salamanders. A–C. Staining with Blue-N, an antibody to S opsin (A), and UV-N, an antibody to UV opsin (B); Blue-N was conjugated with Alexa Fluor 594, and UV-N with Alexa Fluor 488. Blue-N labeled some outer segments brightly (arrows) and others dimly (arrowhead), while UV-N labeled the same outer segments in a reverse pattern, indicating that some cones contained a mixture of S and UV visual pigments. The composite image is shown in (C). D–G. Retina triply labeled with CERN956 for L opsin (D), TA2 for cone transducin α-subunit (E), and UV-N (F); all four images are of the same field with the composite shown in G. CERN956 and UV-N were conjugated with Alexa Fluor 594 and Alexa Fluor 350, respectively; TA2 was biotinylated and visualized with a FITC-conjugated secondary. Some outer segments were positive for L and UV opsins (arrows). One cone outer segment showing TA2 immunoreactivity belonged to an S cone (arrowhead). Scale bar = 16 μm in A–C, and 8 μm in D–G.
Labeling with CERN956 and cell counts supported these assignments. Quite a large fraction of cones were labeled by UV-N. In samples from three aquatic salamander retinas, 122 cones were UV-N positive compared to 464 1D4 positive M rods. In separate samples of 143 UV-N positive cones, 103 co-labeled with Blue-N. Since the relative frequency of UV-N positive cones far exceeded estimates for the relative occurrence of UV cones (Sherry et al., 1998; Chen et al., 2008), the labeling must have included L cones, which are the most prevalent kind of cone. Indeed there were cone outer segments that labeled with both CERN956 and UV-N (Fig. 7D, F, arrows). These cones were L, because the miniscule levels of L pigment in UV cones would have precluded detection with CERN956 (Figs. 2, 3, and Discussion). Many other cone outer segments were labeled by CERN956 alone, consistent with very low levels of UV pigment in some L cones (Fig. 7D, F). Upon triple labeling with both cone opsin antibodies and TA2, the few cells containing cone transducin but not L or UV pigment were S cones (Fig. 7E, arrowhead). Unexpectedly, a few UV cones failed to show labeling for cone transducin (results not shown).
Discussion
Multiple visual pigments in salamander cones
Nearly all aquatic and terrestrial salamander photoreceptors contained pigment mixtures due to the combination of one type of opsin with A1 and A2 chromophores. The oddly shaped spectral sensitivity of UV cones, with three spectral maxima distributed over a range exceeding 200 nm, provided an important clue that they also contained multiple opsins. The types were identified electrophysiologically and immunohistochemically as a UV opsin, with lesser amounts of the S and L opsins (Figs. 1, 2, 6, 7). The expression levels for the various minor opsins were estimated on the following grounds. All of the salamander visual pigments have comparable photosensitivities (Makino et al. 1991; Jones et al., 1993), dim flashes produce responses in a salamander rod or cone that vary in amplitude but not kinetics as a function of wavelength (Figs. 1C, 4D; see also Makino and Dodd, 1996), and various cone pigments are capable of producing responses of similar magnitude when expressed in the same photoreceptor (Kefalov et al., 2003; Shi et al., 2007; Fu et al., 2008). Therefore, based on spectral sensitivity, a small UV cone from a terrestrial animal with an outer segment diameter of ~1 μm, a length of 10 μm, and a combined pigment concentration of 3 mM contains 1.4 million visual pigment molecules in total; a few tens of thousands are S pigment, and only a few hundred are L pigment.
Although the descending limb of the spectral sensitivity at long wavelengths was not diagnostic for mixed opsin populations in other salamander photoreceptors, inspection of the spectral sensitivity to short wavelengths suggested that UV and S pigments were present in at least some L single and double cones. Partial bleaching with red light increased relative sensitivity to short wavelengths in many L cones. The increase could not be attributed to photoexcitation of metarhodopsin photointermediates formed after bleaching because those of cone pigments decay within minutes (Ala-Laurila et al., 2006) and our spectral measurements were made long after their disappearance. The presence of small amounts of UV and S opsins in L cones was corroborated by immunohistochemical experiments in which a subset of L cones were doubly labeled by antibodies for L and UV opsins (Fig. 7D–G). In L double cones, UV and S pigments could each comprise as much as ~10% of the total amount of visual pigment, whereas in L single cones, levels of these secondary pigments were only as high as a few percent each. Even lower levels of pigment co-expression in some L single and double cones could explain why not all co-labeled with UV pigment antibody.
Our results on double cones confirm those of Zhang and Wu (2009) who used the same S pigment antibody to label the accessory member. Since the principal member and L single cones were not labeled, we can estimate the threshold for detection of S opsin as a few percent under their conditions. In our physiological experiments, very low or undetectable levels of S pigment in some double cones may have resulted from the accessory member being stripped off or compromised, leaving the principal member as the lone functional cell in the electrode. While our results also compare favorably to immunohistochemical studies by Chen et al. (2008) and Sherry et al. (1998), we now offer a re-interpretation of their findings. It is likely that some single cones labeled for S and UV pigments in Chen et al. (2008) were L cones, rather than UV cones. Sherry et al. (1998) were not able to conclude that opsin mixtures occurred within UV and L cones because the specificities of their antibodies were not known at the time. That information, now provided in figure 6, along with the knowledge that S rods and S cones express the same visual opsin (Ma et al., 2001b), suggests that the designations of cones as “UV” and “S” should be reversed in Sherry et al. (1998). A further argument for reversal is that the “UV” cones were labeled by 1D4, an antibody recognizing an epitope on bovine rhodopsin that is particularly sensitive to one of the C-terminal alanines (Hodges et al., 1988). The alanine is replaced by a serine in salamander rod opsin and in UV and L opsin sequences, but is conserved in the salamander S opsin sequence.
Partial bleaching also improved slightly, the relative sensitivity to UV wavelengths in S rods and cones. However, this spectral change was hypsochromically shifted from the UV cone spectral sensitivity. It is unlikely that a novel opsin was present because none were found after previous searches for additional visual opsins in the retina using low stringency screens and degenerative RT-PCR (Ma et al., 2001b). Instead, we suspect that vitamin A (λmax near 330 nm), formed from all-trans retinal that was released from opsin upon bleaching, served as an accessory chromophore that absorbed UV light and isomerized the remaining S pigment by radiationless transfer of the photic energy (Miyazono et al., 2011). Despite washing with albumin, vitamin A lingers for tens of minutes in isolated bleached cells (Ala-Laurila et al., 2006). Postbleach UV sensitization was negligible in M rods because there was a longer delay after bleaching (see Methods) before UV sensitivity was determined that allowed more complete removal of vitamin A. Although opsin mixtures in S rods and cones could not be dismissed with certainty, our results place upper limits on the expression level of a secondary opsin. The shape of the long wavelength limb of the spectral sensitivity argues for less than one A1 L pigment per 10,000 S pigment molecules and less than one A2 L pigment per 1,000,000. Partial bleaching experiments exclude the presence of UV pigment at levels more than one per 10. It would have been desirable to carry out more extensive partial bleaches, but the accompanying loss in circulating current and in overall sensitivity due to bleaching adaptation was prohibitive.
A long held belief is that retinal photoreceptors follow a general rule: barring a few exceptions, a given cell expresses only a single type of opsin. Yet, the expression of more than one opsin in a vertebrate rod or cone can no longer be considered to be a rare occurrence (reviewed in Lukáts et al., 2005; Isayama and Makino, 2012) and several considerations argue that more cases await discovery. First, the expression level of a secondary opsin can fall two to four orders of magnitude below that of the primary opsin (Makino and Dodd, 1996; Nikonov et al., 2005, 2006; Leung et al., 2007; present study), severely limiting the methods available for detection. Second, a secondary opsin for absorbing at short wavelengths could lie spectrally hidden. Unless its expression level approaches that of the primary opsin for absorbing longer wavelength, its functional presence would only be revealed after partial bleaching of the primary pigment. Third, opsin co-expression often involves a UV pigment but tests of UV wavelengths are not always included in physiological, spectroscopic and behavioral studies. Fourth, opsin mixtures may only be present transiently and/or restricted to a small subpopulation of photoreceptors (Wood and Partridge, 1993; Archer et al., 1995; Xiao and Hendrickson, 2000; Cheng and Novales Flamarique, 2004; Cornish et al., 2004). Fifth, “nonvisual” opsins are present in rods and cones of some retinas (Taniguchi et al., 2001; Bailey and Cassone, 2005; Chaurasia et al., 2005; Bellingham et al., 2006; Dkhissi-Benyahya et al., 2006), however, it is not generally known whether these opsins get expressed in the cell to the exclusion of all others.
Basis for expression of multiple opsins
The expression of multiple types of opsins within a photoreceptor could have a trivial explanation. With ancient gene duplications, separate promoters, locus control regions and repressors evolve for specifying the expression of each gene variant (Wang et al., 1992; Smallwood et al., 2002; Takechi et al., 2008) to a single receptor type. But with recent gene duplications, polymorphisms, or interbreeding of closely related taxons, mechanisms specifying opsin expression may not differentiate between the variants. The astonishing occurrence of 11 or more opsins in guppy including six L cone opsins (Hoffmann et al., 2007; Weadick and Chang, 2007; Laver and Taylor, 2011) may underlie the visual pigment mixtures in their cones (Archer and Lythgoe, 1990). Such a mechanism is not at play in salamander because recent gene duplications or polymorphisms should produce closely related genes. In salamander, the amino acid sequence of UV opsin bears only 45% and 51% homology to the S and L opsins, respectively, and the S and L opsins have only 42% homology (Xu et al., 1998; Ma et al., 2001a). The lack of primary sequence conservation places the three opsins into separate classes on a phylogenetic tree: UV opsin in the SWS1 class, S opsin in the SWS2 class, L opsin in the LWS class. These opsin classes are ancient, tracing back to the earliest vertebrates. Furthermore, salamander photoreceptors did demonstrate some specificity in opsin expression. Even though SWS2 opsin alone was expressed in S rods and cones, it was one of the opsins co-expressed in UV cones and L cones.
In another scheme, different opsins could be expressed sequentially in a given photoreceptor during development, with long-lasting retention of the earlier pigment type. In rats, gerbils and humans, immature cones initially express S opsin (Szél et al., 1993, 1994; Xiao and Hendrickson, 2000). Later, some or all of these cones up-regulate the expression of genes for MWS or LWS opsin and down-regulate SWS opsin gene expression. The change in opsin expression during development in some fish can be restricted to cones, to certain kinds of cone (Cheng and Novales Flamarique, 2004; Cheng et al., 2007; Cheng and Novales Flamarique, 2007; Temple et al., 2008) or to rods (Wood and Partridge, 1993; Archer et al., 1995). Rods and cones constantly turn over their complement of opsin. Newly synthesized opsins are added to disc membranes of the proximal outer segment, and over time discs are displaced distally and are eventually shed from the tip of the outer segment (Young, 1967; Hogan et al., 1974; Anderson and Fisher, 1975). Even with a discrete switch in opsin expression, there will be a transition period during which both opsin types coexist within the cell. The transition period lasts for one to several weeks, depending upon the turnover period characteristic of that species. Thyroid hormone affects the type of chromophore used, eliciting the conversion from A1 to A2 visual pigments in salmonid fishes (Beatty, 1969; Temple et al., 2008; Suliman and Novales Flamarique, 2014), and drives changes in opsin expression (Roberts et al., 2006; Applebury et al., 2007; Cheng et al., 2009; Gan and Novales Flamarique, 2010). In a variation on this scheme, cues for opsin specification may vary spatially across the retina rather than temporally (Roberts et al., 2006; Applebury et al., 2007; Temple et al., 2010; Alfano et al., 2011). Cells at the boundaries may then be subjected to mixed messages. For example, cones in the dorsal retina of mouse, hamster and guinea pig express predominantly M opsin and those in the ventral retina express S opsin. Cones in the transition zone near the midline co-express both pigments (Röhlich et al., 1994; Applebury et al., 2000; Glösmann and Ahnelt, 2002; Parry and Bowmaker, 2002).
None of these schemes applies to the salamander. Metamorphosis in salamander from aquatic to terrestrial stages is controlled by thyroid hormone (reviewed in Rosenkilde and Ussing, 1996). Although terrestrial salamanders lose S cones, increase their complement of S rods (Chen et al., 2008) and rely more heavily on A1 than A2 chromophore (e.g., Hárosi, 1975), which is the opposite switch in chromophore that occurs in salmon, there did not appear to be any change in opsin co-expression associated with developmental stage. In particular, all three types of opsin were present in the UV cone and they remained in roughly the same proportions from early after hatching until well after metamorphosis to the terrestrial form (Fig. 1). UV and S pigments were also present in L single cones of large aquatic and terrestrial salamanders. Therefore, opsin co-expression in salamander resulted from a genetic program with purposeful expression of multiple opsin types in certain kinds of cones.
Functional significance of expressing multiple opsins
Photoreceptors of the parietal eye contain both a S pinopsin and a M parietopsin (Su et al., 2006) that mediate hyperpolarizing responses to short wavelengths and depolarizing responses to long wavelengths (Solessio and Engbretson, 1993). The two pigments generate opposing responses by coupling to different G proteins (Su et al., 2006). In contrast, all visual pigments are thought to couple to the same transducin G protein in salamander cones (Ma et al., 2001b), and no such response differences were detected within individual salamander cones that expressed multiple pigments. Instead, response waveform was the same for all wavelengths, in accordance with the principle of univariance (Naka and Rushton, 1966).
Color vision relies upon the relative excitation of receptors with overlapping, but spectrally different sensitivities. Pigment mixtures within a receptor may broaden the spectral sensitivity, but wavelength discrimination is not necessarily precluded. Co-expression of two pigments could provide a means for fine tuning spectral sensitivity. Yet in salamander UV and L cones, the levels of co-expressed opsins may differ by one or more orders of magnitude. Furthermore, in L cones, the small complements of S and UV pigments are redundant because L pigment already absorbs the same wavelengths. Thus, under dark-adapted conditions, a role in color vision is unlikely. With brighter lighting, the signal from L cones would change, dependent upon the relative fractional bleach of each pigment type they contain.
Color vision was demonstrated for Salamandra salamandra (Przyrembel et al., 1995), but it has not yet been shown in tiger salamanders. So, perhaps L and UV cones serve achromatic functions in the latter. Double cones support color vision in some fish (Pignatelli et al., 2010), but they may operate as luminosity detectors in other fish and in birds (reviewed in Osorio and Vorobyev, 2005). Co-expression of UV pigment with other visual pigments may support UV vision for the purposes of feeding, mate selection, and communication (reviewed in Losey et al., 1999; Hunt et al., 2001; Hart and Hunt, 2007). For example, some fish rely on UV cones for prey-catching behavior (Novales Flamarique, 2012); UV pigment in UV and in L cones may be used for a similar purpose in salamanders. UV light is damaging to biological tissue so behavioral avoidance may be a mechanism for coping, particularly in selection of sites for oviposition (reviewed in Blaustein and Belden, 2003; Dahms and Lee, 2010). Salamanders reproduce as both “larvae” and “adults,” therefore expression of UV pigment in L cones may be important for recognizing brightly lit areas with probable exposure to harmful levels of UV wavelengths over a wide range of lighting conditions at all developmental stages. For such achromatic functions, the expression level of the accessory pigments may not need to be tightly regulated.
Acknowledgments
We thank Ahmet Altiner for participating in some experiments, Karen Schroeder for assistance with data analysis and David Sherry for discussions.
Support Information: This work was supported by the National Eye Institute: EY011358, EY012231, EY014104, EY019515, the Howe Laboratory Endowment of the Massachusetts Eye and Ear Infirmary, and Research to Prevent Blindness, Inc.: unrestricted grants to the Massachusetts Eye and Ear Infirmary and Medical University of South Carolina and a Career Development Award. Rosalie K. Crouch is an RPB Senior Scientific Investigator. The authors are responsible for the contents, which do not necessarily reflect the official views of the National Eye Institute.
Footnotes
Conflict of Interest Statement
T. Isayama, none; Y. Chen, none; M. Kono, none; E. Fabre, none; M. Slavsky, none; W.J. DeGrip, none; Ma, J.-X., none; Crouch, R.K., none; Makino, C.L., none.
Role of Authors
Tomoki Isayama: carried out electrophysiological recordings, analyzed data, wrote the manuscript.
Ying Chen: carried out immunohistochemistry.
Masahiro Kono: expressed and purified recombinant opsins, and characterized antibody specificities.
Eduard Fabre: carried out electrophysiological recordings and analyzed data.
Michael Slavsky: carried out electrophysiological recordings and analyzed data.
Willem J. DeGrip: provided antibodies and contributed to the discussion of the results.
Jian-Xing Ma: helped to design the study, generated antibodies, carried out immunohistochemistry.
Rosalie K. Crouch: helped to design the study.
Clint L. Makino: conceived and designed the study, carried out electrophysiological recordings, analyzed data, wrote the manuscript.
Literature Cited
- Ala-Laurila P, Kolesnikov AV, Crouch RK, Tsina E, Shukolyukov SA, Govardovskii VI, Koutalos Y, Wiggert B, Estevez ME, Cornwall MC. Visual cycle: Dependence of retinol production and removal on photoproduct decay and cell morphology. J Gen Physiol. 2006;128:153–169. doi: 10.1085/jgp.200609557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano G, Conte I, Caramico T, Avellino R, Arnò B, Pizzo MT, Tanimoto N, Beck SC, Huber G, Dollé P, Seeliger MW, Banfi S. Vax2 regulates retinoic acid distribution and cone opsin expression in the vertebrate eye. Development. 2011;138:261–271. doi: 10.1242/dev.051037. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Fisher SK. Disc shedding in rodlike and conelike photoreceptors of tree squirrels. Science. 1975;187:953–955. doi: 10.1126/science.1145180. [DOI] [PubMed] [Google Scholar]
- Applebury ML, Farhangfar F, Glösmann M, Hashimoto K, Kage K, Robbins JT, Shibusawa N, Wondisford FE, Zhang H. Transient expression of thyroid hormone nuclear receptor TRβ2 sets S opsin patterning during cone photoreceptor genesis. Dev Dyn. 2007;236:1203–1212. doi: 10.1002/dvdy.21155. [DOI] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LLY, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Archer S, Hope A, Partridge JC. The molecular basis for the green-blue sensitivity shift in the rod visual pigments of the European eel. Proc R Soc Lond B. 1995;262:289–295. doi: 10.1098/rspb.1995.0208. [DOI] [PubMed] [Google Scholar]
- Archer SN, Lythgoe JN. The visual pigment basis for cone polymorphism in the guppy, Poecilia reticulata. Vision Res. 1990;30:225–233. doi: 10.1016/0042-6989(90)90038-m. [DOI] [PubMed] [Google Scholar]
- Attwell D, Wilson M, Wu SM. A quantitative analysis of interactions between photoreceptors in the salamander (Ambystoma) retina. J Physiol (Lond) 1984;352:703–737. doi: 10.1113/jphysiol.1984.sp015318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MJ, Cassone VM. Melanopsin expression in the chick retina and pineal gland. Mol Brain Res. 2005;134:345–348. doi: 10.1016/j.molbrainres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Beatty DD. Visual pigment changes in juvenile kokanee salmon in response to thyroid hormones. Vision Res. 1969;9:855–864. doi: 10.1016/0042-6989(69)90093-5. [DOI] [PubMed] [Google Scholar]
- Bellingham J, Chaurasia SS, Melyan Z, Liu C, Cameron MA, Tarttelin EE, Iuvone PM, Hankins MW, Tosini G, Lucas RJ. Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates. PLoS Biol. 2006;4:e254. doi: 10.1371/journal.pbio.0040254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein AR, Belden LK. Amphibian defenses against ultraviolet-B radiation. Evol Dev. 2003;5:89–97. doi: 10.1046/j.1525-142x.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- Boll F. Zur anatomie und physiologie der retina. Archiv fur Anatomie und Physiologie 4–36. Translated by R Hubbard. 1977. Vision Res. 1877;17:1249–1265. [Google Scholar]
- Bowmaker JK. Evolution of vertebrate visual pigments. Vision Res. 2008;48:2022–2041. doi: 10.1016/j.visres.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Bowmaker JK, Dartnall HJA, Herring PJ. Longwave-sensitive visual pigments in some deep-sea fishes: segregation of ‘paired’ rhodopsins and porphyropsins. J Comp Physiol A. 1988;163:685–698. [Google Scholar]
- Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, Natesan A, Zatz M, Tosini G, Liu C, Korf HW, Iuvone PM, Provencio I. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J Neurochem. 2005;92:158–170. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- Chen N, Ma J-X, Corson DW, Hazard ES, Crouch RK. Molecular cloning of a rhodopsin gene from salamander rods. Invest Ophthalmol Vis Sci. 1996;37:1907–1913. [PubMed] [Google Scholar]
- Chen Y, Znoiko S, DeGrip WJ, Crouch RK, Ma J-X. Salamander blue-sensitive cones lost during metamorphosis. Photochem Photobiol. 2008;84:855–862. doi: 10.1111/j.1751-1097.2008.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Novales Flamarique I. New mechanism for modulating colour vision: Single cones start making a different opsin as young salmon move to deeper waters. Nature. 2004;428:279. doi: 10.1038/428279a. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Novales Flamarique I. Chromatic organization of cone photoreceptors in the retina of rainbow trout: single cones irreversibly switch from UV (SWS1) to blue (SWS2) light sensitive opsin during natural development. J Exp Biol. 2007;210:4123–4135. doi: 10.1242/jeb.009217. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Gan KJ, Novales Flamarique I. The ultraviolet opsin is the first opsin expressed during retinal development of salmonid fishes. Invest Ophthalmol Vis Sci. 2007;48:866–873. doi: 10.1167/iovs.06-0442. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Gan KJ, Novales Flamarique I. Thyroid hormone induces a time-dependent opsin switch in the retina of salmonid fishes. Invest Ophthalmol Vis Sci. 2009;50:3024–3032. doi: 10.1167/iovs.08-2713. [DOI] [PubMed] [Google Scholar]
- Cornish EE, Xiao M, Yang Z, Provis JM, Hendrickson AE. The role of opsin expression and apoptosis in determination of cone types in human retina. Exp Eye Res. 2004;78:1143–1154. doi: 10.1016/j.exer.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Dahms H-U, Lee J-S. UV radiation in marine ectotherms: molecular effects and responses. Aquat Toxicol. 2010;97:3–14. doi: 10.1016/j.aquatox.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Das J, Crouch RK, Ma J-X, Oprian DD, Kono M. Role of the 9-methyl group of retinal in cone visual pigments. Biochemistry. 2004;43:5532–5538. doi: 10.1021/bi036097u. [DOI] [PubMed] [Google Scholar]
- Dkhissi-Benyahya O, Rieux C, Hut RA, Cooper HM. Immunohistochemical evidence of a melanopsin cone in human retina. Invest Ophthalmol Vis Sci. 2006;47:1636–1641. doi: 10.1167/iovs.05-1459. [DOI] [PubMed] [Google Scholar]
- Foster RG, Garcia-Fernandez JM, Provencio I, DeGrip WJ. Opsin localization and chromophore retinoids identified within the basal brain of the lizard Anolis carolinensis. J Comp Physiol A. 1993;172:33–45. [Google Scholar]
- Fu Y, Kefalov V, Luo D-G, Xue T, Yau K-W. Quantal noise from human red cone pigment. Nature Neurosci. 2008;11:565–571. doi: 10.1038/nn.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan KJ, Novales Flamarique I. Thyroid hormone accelerates opsin expression during early photoreceptor differentiation and induces opsin switching in differentiated TRα-expressing cones of the salmonid retina. Dev Dyn. 2010;239:2700–2713. doi: 10.1002/dvdy.22392. [DOI] [PubMed] [Google Scholar]
- Glösmann M, Ahnelt PK. A mouse-like retinal cone phenotype in the Syrian hamster: S opsin coexpressed with M opsin in a common cone photoreceptor. Brain Res. 2002;929:139–146. doi: 10.1016/s0006-8993(02)02267-9. [DOI] [PubMed] [Google Scholar]
- Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K. In search of the visual pigment template. Vis Neurosci. 2000;17:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- Hárosi FI. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975;66:357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi FI. An analysis of two spectral properties of vertebrate visual pigments. Vision Res. 1994;34:1359–1367. doi: 10.1016/0042-6989(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Hart NS, Hunt DM. Avian visual pigments: Characteristics, spectral tuning, and evolution. Am Nat. 2007;169(Suppl1):S7–S26. doi: 10.1086/510141. [DOI] [PubMed] [Google Scholar]
- Hodges RS, Heaton RJ, Parker JMR, Molday L, Molday RS. Antigen-antibody interaction. Synthetic peptides define linear antigenic determinants recognized by monoclonal antibodies directed to the cytoplasmic carboxyl terminus of rhodopsin. J Biol Chem. 1988;263:11768–11775. [PubMed] [Google Scholar]
- Hoffmann M, Tripathi N, Henz SR, Lindholm AK, Weigel D, Breden F, Dreyer C. Opsin gene duplication and diversification in the guppy, a model for sexual selection. Proc R Soc Lond B. 2007;274:33–42. doi: 10.1098/rspb.2006.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MJ, Wood I, Steinberg RH. Phagocytosis by pigment epithelium of human retinal cones. Nature. 1974;252:305–307. doi: 10.1038/252305a0. [DOI] [PubMed] [Google Scholar]
- Hunt DM, Wilkie SE, Bowmaker JK, Poopalasundaram S. Vision in the ultraviolet. Cell Mol Life Sci. 2001;58:1583–1598. doi: 10.1007/PL00000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isayama T, Makino CL. Pigment mixtures and other determinants of spectral sensitivity of vertebrate retinal photoreceptors. In: Akutagawa E, Ozaki K, editors. Photoreceptors: Physiology, Types, and Abnormalities. Nova Science Publishers, Inc; Hauppauge, NY: 2012. pp. 1–32. [Google Scholar]
- Jacobs GH. Evolution of colour vision in mammals. Phil Trans R Soc Lond B. 2009;364:2957–2967. doi: 10.1098/rstb.2009.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GJ, Fein A, MacNichol EF, Jr, Cornwall MC. Visual pigment bleaching in isolated salamander retinal cones. J Gen Physiol. 1993;102:483–502. doi: 10.1085/jgp.102.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalov V, Fu Y, Marsh-Armstrong N, Yau K-W. Role of visual pigment properties in rod and cone phototransduction. Nature. 2003;425:526–531. doi: 10.1038/nature01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver CRJ, Taylor JS. RT-qPCR reveals opsin gene upregulation associated with age and sex in guppies (Poecilia reticulata)-a species with color-based sexual selection and 11 visual-opsin genes. BMC Evol Biol. 2011;11:81. doi: 10.1186/1471-2148-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung YT, Fain GL, Matthews HR. Simultaneous measurement of current and calcium in the ultraviolet-sensitive cones of zebrafish. J Physiol (Lond) 2007;579:15–27. doi: 10.1113/jphysiol.2006.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loew ER, McFarland WN, Margulies D. Developmental changes in the visual pigments of the yellowfin tuna, Thunnus albacares. Mar Fresh Behav Physiol. 2002;35:235–246. [Google Scholar]
- Losey GS, Cronin TW, Goldsmith TH, Hyde D, Marshall NJ, McFarland WN. The UV visual world of fishes: a review. J Fish Biol. 1999;54:921–943. [Google Scholar]
- Lukáts Á, Szabo A, Röhlich P, Vígh B, Szél Á. Photopigment coexpression in mammals: comparative and developmental aspects. Histol Histopathol. 2005;20:551–574. doi: 10.14670/HH-20.551. [DOI] [PubMed] [Google Scholar]
- Ma J-X, Kono M, Xu L, Das J, Ryan JC, Hazard ES, 3rd, Oprian DD, Crouch RK. Salamander UV cone pigment: Sequence, expression, and spectral properties. Vis Neurosci. 2001a;18:393–399. doi: 10.1017/s0952523801183057. [DOI] [PubMed] [Google Scholar]
- Ma J-X, Znoiko S, Othersen KL, Ryan JC, Das J, Isayama T, Kono M, Oprian DD, Corson DW, Cornwall MC, Cameron DA, Hárosi FI, Makino CL, Crouch RK. A visual pigment expressed in both rod and cone photoreceptors. Neuron. 2001b;32:451–461. doi: 10.1016/s0896-6273(01)00482-2. [DOI] [PubMed] [Google Scholar]
- Makino CL, Dodd RL. Multiple visual pigments in a photoreceptor of the salamander retina. J Gen Physiol. 1996;108:27–34. doi: 10.1085/jgp.108.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino CL, Taylor WR, Baylor DA. Rapid charge movements and photosensitivity of visual pigments in salamander rods and cones. J Physiol (Lond) 1991;442:761–780. doi: 10.1113/jphysiol.1991.sp018818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino CL, Groesbeek M, Lugtenburg J, Baylor DA. Spectral tuning in salamander visual pigments studied with dihydroretinal chromophores. Biophys J. 1999;77:1024–1035. doi: 10.1016/S0006-3495(99)76953-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani AP. Photoreceptors of the larval tiger salamander retina. Proc R Soc Lond B. 1986;227:483–492. doi: 10.1098/rspb.1986.0035. [DOI] [PubMed] [Google Scholar]
- Mazzoni EO, Desplan C, Celik A. ‘One receptor’ rules in sensory neurons. Dev Neurosci. 2004;26:388–395. doi: 10.1159/000082281. [DOI] [PubMed] [Google Scholar]
- Miyazono S, Isayama T, Delori FC, Makino CL. Vitamin A activates rhodopsin and sensitizes it to ultraviolet light. Vis Neurosci. 2011;28:485–497. doi: 10.1017/S0952523811000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, MacKenzie D. Monoclonal antibodies to rhodopsin: Characterization, cross-reactivity, and application as structural probes. Biochemistry. 1983;22:653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- Naka KI, Rushton WAH. An attempt to analyse colour reception by electrophysiology. J Physiol (Lond) 1966;185:556–586. doi: 10.1113/jphysiol.1966.sp008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Daniele LL, Zhu X, Craft CM, Swaroop A, Pugh EN., Jr Photoreceptors of Nrl−/− mice coexpress functional S- and M-cone opsins having distinct inactivation mechanisms. J Gen Physiol. 2005;125:287–304. doi: 10.1085/jgp.200409208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novales Flamarique I. Opsin switch reveals function of the ultraviolet cone in fish foraging. Proc R Soc B. 2012;280:20122490. doi: 10.1098/rspb.2012.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc R Soc Lond B. 2005;272:1745–1752. doi: 10.1098/rspb.2005.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry JWL, Bowmaker JK. Visual pigment coexpression in Guinea pig cones: a microspectrophotometric study. Invest Ophthalmol Vis Sci. 2002;43:1662–1665. [PubMed] [Google Scholar]
- Pignatelli V, Champ C, Marshall J, Vorobyev M. Double cones are used for colour discrimination in the reef fish, Rhinecanthus aculeatus. Biol Lett. 2010;6:537–539. doi: 10.1098/rsbl.2009.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers JH. Morphological variation and its causes in Amblystoma tigrinum. University Studies (University of Nebraska) 1907;7:197–274. [Google Scholar]
- Przyrembel C, Keller B, Neumeyer C. Trichromatic color vision in the salamander (Salamandra salamandra) J Comp Physiol A. 1995;176:575–586. [Google Scholar]
- Reuter TE, White RH, Wald G. Rhodopsin and porphyropsin fields in the adult bullfrog retina. J Gen Physiol. 1971;58:351–371. doi: 10.1085/jgp.58.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: Thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci USA. 2006;103:6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhlich P, van Veen T, Szél Á. Two different visual pigments in one retinal cone cell. Neuron. 1994;13:1159–1166. doi: 10.1016/0896-6273(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Rosenkilde P, Ussing AP. What mechanisms control neoteny and regulate induced metamorphosis in urodeles? Int J Dev Biol. 1996;40:665–673. [PubMed] [Google Scholar]
- Ryan JC, Znoiko S, Xu L, Crouch RK, Ma J-X. Salamander rods and cones contain distinct transducin alpha subunits. Vis Neurosci. 2000;17:847–854. doi: 10.1017/s0952523800176047. [DOI] [PubMed] [Google Scholar]
- Saarinen P, Pahlberg J, Herczeg G, Viljanen M, Karjalainen M, Shikano T, Merila J, Donner K. Spectral tuning by selective chromophore uptake in rods and cones of eight populations of nine-spined stickleback (Pungitius pungitius) J Exp Biol. 2012;215:2760–2773. doi: 10.1242/jeb.068122. [DOI] [PubMed] [Google Scholar]
- Schalken JJ, DeGrip WJ. Enzyme-linked immunosorbent assay for quantitative determination of the visual pigment rhodopsin in total-eye extract. Exp Eye Res. 1986;43:431–439. doi: 10.1016/s0014-4835(86)80078-1. [DOI] [PubMed] [Google Scholar]
- Sherry DM, Bui DD, DeGrip WJ. Identification and distribution of photoreceptor subtypes in the neotenic tiger salamander retina. Vis Neurosci. 1998;15:1175–1187. doi: 10.1017/s0952523898156201. [DOI] [PubMed] [Google Scholar]
- Shi G, Yau K-W, Chen J, Kefalov VJ. Signaling properties of a short-wave cone visual pigment and its role in phototransduction. J Neurosci. 2007;27:10084–10093. doi: 10.1523/JNEUROSCI.2211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood PM, Wang Y, Nathans J. Role of a locus control region in the mutually exclusive expression of human red and green cone pigment genes. Proc Natl Acad Sci USA. 2002;99:1008–1011. doi: 10.1073/pnas.022629799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solessio E, Engbretson GA. Antagonistic chromatic mechanisms in photoreceptors of the parietal eye of lizards. Nature. 1993;364:442–445. doi: 10.1038/364442a0. [DOI] [PubMed] [Google Scholar]
- Su C-Y, Luo D-G, Terakita A, Shichida Y, Liao H-W, Kazmi MA, Sakmar TP, Yau K-W. Parietal-eye phototransduction components and their potential evolutionary implications. Science. 2006;311:1617–1621. doi: 10.1126/science.1123802. [DOI] [PubMed] [Google Scholar]
- Suliman T, Novales Flamarique I. Visual pigments and opsin expression in the juveniles of three species of fish (rainbow trout, zebrafish, and killifish) following prolonged exposure to thyroid hormone or retinoic acid. J Comp Neurol. 2014;522:98–117. doi: 10.1002/cne.23391. [DOI] [PubMed] [Google Scholar]
- Szél Á, Röhlich P, Mieziewska K, Aguirre G, van Veen T. Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: A developmental study. J Comp Neurol. 1993;331:564–577. doi: 10.1002/cne.903310411. [DOI] [PubMed] [Google Scholar]
- Szél Á, van Veen T, Röhlich P. Retinal cone differentiation. Nature. 1994;370:336. doi: 10.1038/370336a0. [DOI] [PubMed] [Google Scholar]
- Takechi M, Seno S, Kawamura S. Identification of cis-acting elements repressing blue opsin expression in zebrafish UV cones and pineal cells. J Biol Chem. 2008;283:31625–31632. doi: 10.1074/jbc.M806226200. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Hisatomi O, Yoshida M, Tokunaga F. Pinopsin expressed in the retinal photoreceptors of a diurnal gecko. FEBS Lett. 2001;496:69–74. doi: 10.1016/s0014-5793(01)02395-x. [DOI] [PubMed] [Google Scholar]
- Temple SE, Veldhoen KM, Phelan JT, Veldhoen NJ, Hawryshyn CW. Ontogenetic changes in photoreceptor opsin gene expression in coho salmon (Oncorhynchus kisutch, Walbaum) J Exp Biol. 2008;211:3879–3888. doi: 10.1242/jeb.020289. [DOI] [PubMed] [Google Scholar]
- Temple S, Hart NS, Marshall NJ, Collin SP. A spitting image: specializations in archerfish eyes for vision at the interface between air and water. Proc R Soc B. 2010;277:2607–2615. doi: 10.1098/rspb.2010.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers PMAM, DeGrip WJ. Functional expression of human cone pigments using recombinant baculovirus: compatibility with histidine tagging and evidence for N-glycosylation. FEBS Lett. 1996;396:26–30. doi: 10.1016/0014-5793(96)01064-2. [DOI] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968;219:800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- Walls GL. The Vertebrate Eye and Its Adaptive Radiation. New York: Hafner; 1942. [Google Scholar]
- Wang Y, Macke JP, Merbs SL, Zack DJ, Klaunberg B, Bennett J, Gearhart J, Nathans J. A locus control region adjacent to the human red and green visual pigment genes. Neuron. 1992;9:429–440. doi: 10.1016/0896-6273(92)90181-c. [DOI] [PubMed] [Google Scholar]
- Weadick CJ, Chang BSW. Long-wavelength sensitive visual pigments of the guppy (Poecilia reticulata): six opsins expressed in a single individual. BMC Evol Biol. 2007;7(Suppl 1):S11. doi: 10.1186/1471-2148-7-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P, Partridge JC. Opsin substitution induced in retinal rods of the eel (Anguilla anguilla (L.)): a model for G-protein-linked receptors. Proc R Soc Lond B. 1993;254:227–232. [Google Scholar]
- Xiao M, Hendrickson A. Spatial and temporal expression of short, long/medium, or both opsins in human fetal cones. J Comp Neurol. 2000;425:545–559. [PubMed] [Google Scholar]
- Xu L, Hazard ES, 3rd, Lockman K, Crouch RK, Ma J-X. Molecular cloning of the salamander red and blue cone visual pigments. Mol Vision. 1998;4:10–15. [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu SM. Immunocytochemical analysis of photoreceptors in the tiger salamander retina. Vision Res. 2009;49:64–73. doi: 10.1016/j.visres.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]