Abstract
Background
Behavioural sensitization has been linked to drug craving in both clinical and preclinical studies of addiction. Increased motor activity is accompanied by enhanced dopamine (DA) release, particularly in the nucleus accumbens (NAcc). The neural bases of sensitization are linked to alterations in synaptic connections that also underlie learning and memory. The present study uses an “interference” peptide, Tat-GluA23Y, that blocks long-term depression (LTD) at glutamatergic synapses by disrupting the endocytosis of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPARs), to explore the role of this form of synaptic plasticity in the induction and maintenance of sensitization.
Methods
Rats were given 5 injections of d-amphetamine (d-AMPH, 1.0 mg/kg, intraperitoneal) every second day. Tat-GluA23Y, was administered by 2 different routes (intravenously and intracerebrally to the ventral tegmental area [VTA] or to the NAcc) before each injection of d-AMPH. After a 14-day drug-free period, expression of behavioural sensitization was evoked by a challenge injection of d-AMPH (0.5 mg/kg, intraperitoneal). Dopamine efflux in the NAcc was measured by high-pressure liquid chromatography with electrochemical detection analyses of brain dialysates on days 1, 9 and 24 of the intravenous peptide experiment.
Results
Systemic administration of Tat-GluA23Y during the induction phase blocked maintenance of behavioural sensitization and attenuated the maintenance of neurochemical sensitization. Intra-VTA infusion of Tat-GluA23Y before each administration of d-AMPH did not affect induction, but inhibited maintenance and subsequent expression of sensitization, whereas intra-NAcc infusion of the peptide did not affect induction or maintenance of sensitization.
Limitations
The relevance of behavioural sensitization in rodents is related to the development of craving and does not provide direct measures of drug reinforcement.
Conclusion
These findings confirm that drug-induced neuroplasticity is labile and may be subject to disruption at a time when long-lasting associations between drug reward and contextual stimuli are formed. Furthermore, the unique ability of Tat-GluA23Y to block maintenance of behavioural sensitization implicates LTD in the consolidation of essential associative memories. Tat-GluA23Y has the unique ability to disrupt functional neuroadaptations triggered by repeated psychostimulant exposure and therefore may protect against the development of craving and drug seeking behaviours.
Introduction
Persistent thoughts and actions related to the procurement and use of illicit drugs, along with vulnerability to relapse following exposure to contextual stimuli associated with drugs of abuse, are hallmark features of drug addiction. Behavioural sensitization, characterized by increased motor activity, is induced by repeated exposure to a variety of illicit drugs, including amphetamines, and may be a key mechanism leading to compulsive drug use in both human and animal models.1–4 Once established, behavioural sensitization is often accompanied by enhanced dopamine (DA) release in the nucleus accumbens (NAcc).5–7 Whereas terminal regions of the mesocorticolimbic DA system are clearly implicated in the long term expression of drug-induced sensitization,8 its induction and initial maintenance depends on modulation of synaptic events in close proximity to DA-containing neurons in the ventral tegmental area (VTA).8–10
The enduring neural adaptations that accompany repeated exposure to drugs of abuse have been linked to specific forms of synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD).9,11–13 This in turn has highlighted possible similarities between neural adaptations related to acquisition and maintenance of addictive behaviours and those that underlie other forms of learning and memory.14,15 Previous work has pointed to a crucial role for the endocytosis of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPARs) in a psychostimulant-induced behavioural sensitization model of drug craving,16,17 extinction of morphine-induced place preference,18 and cue-induced reinstatement of heroin self-administration.17 These studies used a small “interference” peptide, Tat-GluA23Y, to disrupt the regulated endocytosis of AMPARs. This peptide has the unique ability to block LTD by inhibiting facilitated AMPAR endocytosis16,19 via disrupting protein–protein interaction between the GluA2 subunit of AMPAR and Brag2, a clathrin-adaptor protein that activates Arf6 to initiate regulated AMPAR endocytosis.20
In our previous study, acute intravenous administration of Tat-GluA23Y immediately before a drug challenge test, as well as direct intracerebral injection into the NAcc (but not the VTA), blocked the expression of d-amphetamine (d-AMPH)–induced sensitization of motor activity.16 The present study addresses several critical issues of relevance to the potential therapeutic value of Tat-GluA23Y, including the critical question of whether this interference peptide can block the induction and maintenance of behavioural sensitization when given before each of 5 injections of d-AMPH. Given the involvement of the mesocorticolimbic DA system in both the induction and expression of behavioural sensitization8,21–23 as well as the rewarding effects of psychostimulant drugs,24–27 the present study also used brain microdialysis to monitor the efflux of DA in the NAcc during the induction and expression of d-AMPH sensitization. Furthermore, the induction of behavioural sensitization involves neuroplasticity in the VTA,28–30 therefore we also examined whether microinjection of Tat-GluA23Y into the VTA, but not the NAcc, would block the induction and/or maintenance of sensitization.
Methods
Animals
We used male Sprague–Dawley rats (Charles River, Que.) weighing 250–275 g for all experiments. Animals were housed in pairs in transparent cages in a temperature-controlled animal colony and acclimatized to a 12-hour reverse light–dark cycle (lights on at 7 pm) for 7 days before the beginning of the experiments. They were subsequently housed individually once experiments began. Food and water were available ad libitum during all phases of the study. All the experiments were approved by the University of British Columbia Animal Care Committee and conducted in accordance with policies outlined by the Canadian Council on Animal Care.
Drugs
We diluted d-AMPH (USP) in 0.9% sterile saline. Tat-GluA23Y peptide was constituted of 9 amino acids (YKEGYNVYG), and was attached to an HIV-1 Tat peptide (YGRKKRRQRRR) to cross the blood–brain barrier and permeate cells.19 The scram-bled peptide, Tat-GluA23Yscr, comprised the same 9 amino acids placed in random sequence (VYKYGGYNE) and served as a control. Both Tat-GluA23Y and Tat-GluA23Yscr (GL Biochem-Shanghai Ltd) were diluted in 0.9% sterile saline.
Surgical procedures
Surgeries were performed under isoflurane anesthesia (3% isoflurance [Baxter] in oxygen) and maintained with 1.5%–2.5% isoflurane for the duration of the procedure. As an adjunct to gaseous anesthesia, the analgesic, we administered ketoprofen subcutaneously at the time of surgery to help minimize postsurgical distress. All rats were all given a 1-week recovery period before experimental procedures during which their conditions were closely monitored twice daily for possible postoperative complications.
Jugular catheter implantation
Once a surgical plane of anesthesia was achieved, hair was clipped in 2 areas measuring 3.5 × 6 cm on the dorsal surface and 2.5 × 4 cm on the anterior right region of the ventral surface of each rat. We applied an iodophor scrub to the skin surface, alternated twice with 70% ethyl alcohol to disinfect the skin. A single incision measuring 2.5 cm was made using a No. 10 surgical blade on the dorsal surface, and an incision measuring 2 cm was made on the ventral surface above the right jugular vein. A chronic indwelling silastic catheter connected to a guide cannula (Plastics One, 22-gauge) was secured to the dorsal surface of the rat with surgical mesh. The silastic tubing was adhered to the vein by a series of sutures (4–0 silk, nonabsorbable). We closed surgical wounds with polyglactin 910 absorbable sutures.
Microdialysis and intracerebral cannulation
Animals were secured in a stereotaxic frame with the dorsal surface of the cranium oriented in a horizontal plane. For the microdialysis experiments, rats were implanted with bilateral guide cannulae (nitric acid passivated stainless steel, 19-gauge × 15 mm), positioned 1 mm below the dura directly above the NAcc (+ 1.7 mm anteroposterior and ± 1.1 mm mediolateral from the bregma). For the microinjection experiments, rats were implanted with bilateral cannulae (stainless steel, 23-gauge) directed at the VTA (−5.8 mm anteroposterior, ± 0.6 mm mediolateral, −7.0 mm dorsoventral) or NAcc (+1.7 mm anteroposterior, ± 1.1 mm mediolateral, −6.8 mm dorsoventral) at the border of the NAcc core and shell. We secured guide cannulae to the skull via 4 stainless steel screws and dental cement. Sterile obdurators were placed into the guides to maintain patency.
Microdialysis and high-pressure liquid chromatogaphy
Microdialysis probes were constructed from Filtral 12 AN69HF semipermeable hollow fibres (2 mm long, 340 μm outer diameter, 65 kDa molecular weight cut-off; Hospal) and silica inlet-outlet lines (75 of 150 μm inner diameter/outer diameter). Samples were analyzed via high-pressure liquid chromatography with electrochemical detection (HPLC-EC). The HPLC systems were composed of the following: an ESA model 582 pump (Bedford), a pulse damper (Scientific Systems), an inert manual injector (Rheodyne), a Super ODS TSK column (Tosoh Bioscience) and an Intro Electrochemical detector (Antec Leyden). The mobile phase (70 mM sodium acetate buffer, 40 mg/L ethylenediaminetetraacetic acid and 5 mg/L sodium dodecyl sulfate [adjustable]; pH 4.0, 10% methanol) flowed through the system at 0.10 mL/min. We used EZChrome Elite software (Scientific Software) to acquire and analyze chromatographic data.
Behavioural apparatus
To measure horizontal locomotion, we used 8 open-field poly(methyl methacrylate) chambers, measuring 40 × 40 × 40 cm. Top-mounted cameras allowed video capture and tracking of locomotor activity measured and scored by the digital monitoring software Ethovision 3.1 (Noldus). Chambers were also fitted with a liquid-swivel required for in vivo microdialysis. All experiments were run in a darkened room under dim red lighting between 8 am and 6 pm.
Experimental design
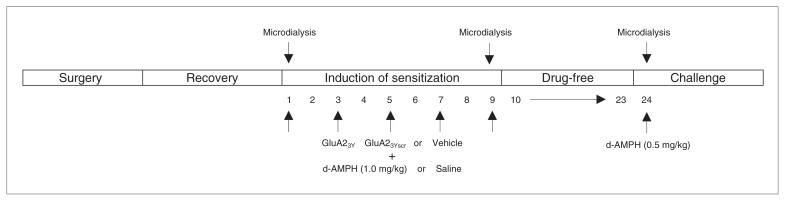
Figure 1 outlines the procedural timeline from the initial day of surgery to the final day of drug challenge to measure induction and expression of d-AMPH–induced behavioural sensitization. Prior to the start of experiments, animals were habituated to the locomotor activity testing chambers for 30 min on 2 separate occasions. During the induction phase, animals received a total of 5 injections of either d-AMPH (5 × 1.0 mg/kg, intraperitoneal) or saline on alternate days, all given in the testing chambers. Following the fifth day of treatment (day 9), all rats remained in the colony room for a 2-week drug-free period. On Day 24, all groups were challenged with a lower dose of d-AMPH (0.5 mg/kg, intraperitoneal) to reduce the possible confounding effect of drug-induced stereotypy.
Fig. 1.
Procedural timeline from the initial day of surgery to the final day of drug challenge to measure induction and expression of d-amphetamine (d-AMPH)–induced behavioural sensitization.
Intravenous administration of Tat-GluA23Y peptide and microdialysis
Dopamine efflux in the NAcc was measured by microdialysis in each animal at 2 of 3 time points: the first or fifth exposure (day 1 or 9) to d-AMPH or saline and during d-AMPH challenge on day 24. The day before microdialysis experiments on days 1, 9 and 24, probes were flushed with artificial cerebrospinal fluid (aCSF; 10.0 mM sodium phosphate buffer with 147.0 mM NaCl, 3.0 mM KCl, 1.0 mM MgCl2 and 1.2 mM CaCl2; pH 7.4) and inserted unilaterally via the guide cannulae into the NAcc (dialysis membrane spanned −4.8 to −6.8 mm, ventrally). Rats remained in the locomotor activity testing chamber overnight (14–16 h) with continuous perfusion of aCSF at 1μL/min. Food and water were available ad libitum. In the morning, we removed the food and water, and we collected 15 μL dialysis samples at 15-minute intervals from the NAcc and immediately assayed them for DA using HPLC-EC. Baseline sampling continued until 4 samples showed less than 10% fluctuation in DA content. Animals were then treated with Tat-GluA23Y (5 × 1.5 nM/g), Tat-GluA23Yscr (5 × 1.5 nM/g), or vehicle control (5 × 0.9% saline) administered intravenously. Microdialysis sampling and behavioural recording continued for 60 minutes, followed by injections of d-AMPH. A fourth group received vehicle control intravenously and saline intraperitoneally during the conditioning period. Microdialysis samples were collected and locomotor activity was measured for a further 2 hours, after which rats were returned to their home cages. Experimental procedures on challenge day 24 were similar except peptide treatment was not given; instead, all animals received d-AMPH injections immediately after baseline sampling.
Intracerebral administration of Tat-GluA23Y in the VTA or NAcc
In separate groups of rats, Tat-GluA23Y (15 pmol), Tat-GluA23Yscr (15 pmol), or vehicle control, was microinjected bilaterally into either the VTA or NAcc 40 minutes before each d-AMPH (5 × 1.0 mg/kg, intraperitoneal) or saline injection. Injection needles were inserted via guide cannulae and connected to an infusion pump (Harvard Apparatus). A total volume of 0.3 μL/side was delivered over 1 minute. Injection needles remained in place for 2 minutes to allow for diffusion. Rats were then placed in open field chambers, and locomotor activity was recorded and scored for 90 minutes. At the end of each testing session, animals were returned to their home cages. After the fifth treatment session, rats remained in the animal colony for a 2-week drug-free period. On Day 24, all animals were challenged with a lower dose of d-AMPH (0.5 mg/kg, intraperitoneal) and locomotor activity was recorded.
Histology
Following completion of all testing procedures, animals were given a lethal dose of sodium pentobarbitol and perfused transcardially with 0.9% saline followed by 10% formaldehyde solution. Brains were rapidly removed and cryoprotected in 20% sucrose in 10% formaldehyde for several days. Serial 30 μm coronal sections were cut on a cryostat, sections were mounted on glass slides, dried, stained with cresyl violet and cover slipped. Placements of the microdialysis probe or guide cannulae were verified under a light microscope and located on figures adapted from the Paxinos and Watson atlas of the rat brain,31 presented in the Appendix, Figure S1, available at jpn.ca.
Injection of dansyl-tagged Tat-GluA23Y peptide in the brain
A dansyl-lysine group was added to the Tat-peptide to serve as a fluorescent marker to estimate the diffusion of peptide from the injection sites in the VTA or NAcc. Rats were prepared with bilateral guide cannulae directed at the VTA or NAcc according to the stereotaxic procedure described previously. One week after surgery, the dansyl-Tat-GluA23Y peptide was microinjected with needles inserted through guide cannulae connected to an infusion pump (0.3 μL/side over 1 min). Injection needles were left in place for an additional 2 minutes to allow for diffusion. Sixty minutes after the microinjection, rats were deeply anesthetized and perfused with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer for 30 minutes. Brains were stored overnight in 10% sucrose containing fixative, and fixed brains were sectioned at 50 μm on a cryostat. Sections were then mounted on slides and examined by fluorescent microscopy (Appendix, Fig. S2).
Statistical analysis
All values are expressed as mean ± standard error of the mean (SEM). We assessed statistical significance using 1-way analysis of variance (ANOVA) or 2-way repeated-measures ANOVA with time as the within-subject factor and treatment groups as the between-subjects factor. Where appropriate, we performed post hoc analyses using the Tukey honestly significant difference test to examine simple main effects. We considered results to be significant at p < 0.05.
Results
Intravenous Tat-GluA23Y administration blocks induction of behavioural sensitization and attenuates d-AMPH–induced DA efflux in the NAcc
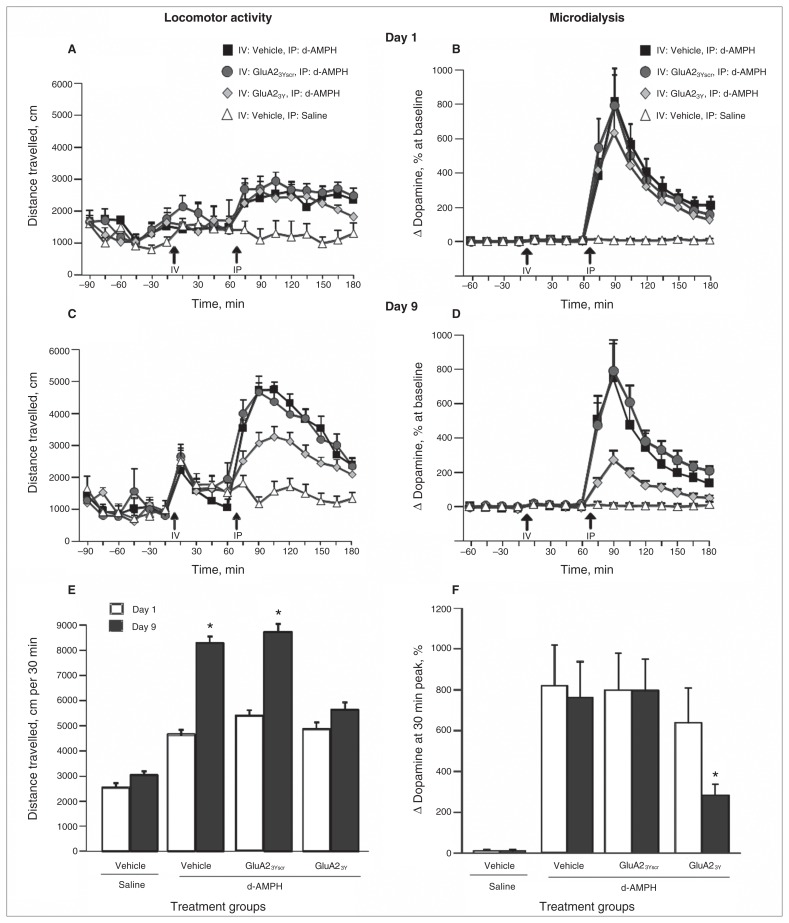
Figure 2 illustrates d-AMPH–induced locomotor activity and DA efflux in the NAcc on day 1 and day 9 of induction of sensitization. Based on the time course of behavioural response to each d-AMPH injection, we observed peak locomotor activity during the initial 30-minute period postinjection. Therefore, we performed statistical analyses on the cumulative data of distance travelled in the 30 minutes following d-AMPH injection. Repeated d-AMPH treatment (5 × 1.0 mg/kg) led to a progressive increase in locomotor activity in rats that received vehicle (n = 12) or Tat-GluA23Yscr (n = 9), whereas the interference peptide Tat-GluA23Y (n = 13) blocked the induction of locomotor sensitization (day × treatment group: F1,3 = 17.364, p = 0.001; post hoc: vehicle + d-AMPH group, day 1 v. day 9, p = 0.010; Tat-GluA23Yscr + d-AMPH group, day 1 v. day 9, p = 0.002; Tat-GluA23Y + d-AMPH group, day 1 v. day 9, p = 0.51).
Fig. 2.
d-Amphetamine (d-AMPH)–induced locomotor activity and dopamine efflux in the nucleus accumbens (NAcc) on day 1 and day 9 of induction of sensitization after intravenous (IV) administration of Tat-GluA23Y or controls. (A) Time course of locomotor activity on day 1 of induction. (B) Dopamine efflux in the NAcc on day 1 of induction. (C) Time course of locomotor activity on day 9 of induction. (D) Dopamine efflux in the NAcc on day 9 of induction. (E) Cumulative locomotor activity over 30 min on days 1 and 9 of induction. (F) Dopamine efflux in the NAcc at the 30 min peak on days 1 and 9 of induction. IP = intraperitoneal. *p < 0.01.
Consistent with previous microdialysis experiments, d-AMPH treatment on both day 1 and day 9 was accompanied by a significant increase in DA efflux in the NAcc in rats treated with vehicle (n = 9) or Tat-GluA23Yscr (n = 10; repeated-measures ANOVA, F8,24 = 30.75, p = 0.005; Fig. 2B; repeated-measures ANOVA, F8,24 = 37.12, p = 0.005; Fig. 2D). Tat-GluA23Y peptide pretreatment (n = 8) did not significantly affect levels of d-AMPH–induced DA efflux on day 1. However, repeated treatment with Tat-GluA23Y significantly attenuated the magnitude of d-AMPH–induced DA efflux in the NAcc by the fifth d-AMPH injection (pairwise comparison, 633% ± 170% on day 1 v. 278% ± 53% on day 9, p = 0.001).
Repeated intravenous Tat-GluA23Y administration during induction phase blocks maintenance and expression of behavioural and neurochemical sensitization to d-AMPH
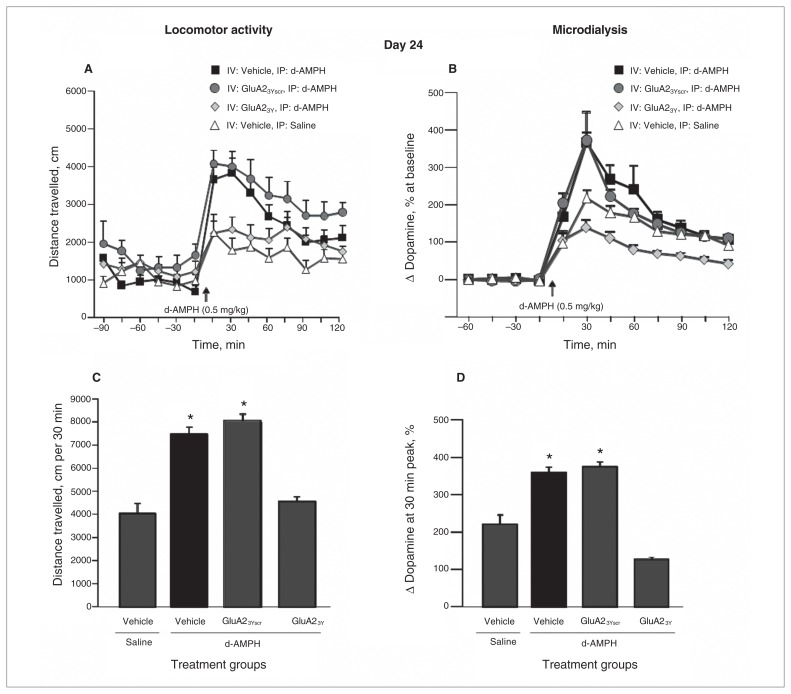
Following a 14-day drug-free period, rats previously given Tat-GluA23Y before each d-AMPH injection showed a significantly reduced locomotor response to the d-AMPH challenge compared with groups that received vehicle or Tat-GluA23Yscr before d-AMPH in the induction phase. Furthermore, distance travelled for rats given repeated Tat-GluA23Y and d-AMPH did not differ significantly from that in rats receiving the challenge dose of d-AMPH for the first time (treatment group: F3,40 = 5.89, p = 0.008; post hoc: Tat-GluA23Y + d-AMPH v. vehicle + d-AMPH, p = 0.012; Tat-GluA23Y + d-AMPH v. Tat-GluA23Yscr + d-AMPH, p = 0.020; Tat-GluA23Y + d-AMPH v. vehicle + saline, p = 0.10; Fig. 3). These data indicate that Tat-GluA23Y peptide treatment blocked the induction of behavioural sensitization.
Fig. 3.
d-Amphetamine (d-AMPH)–induced locomotor activity and dopamine efflux in the nucleus accumbens (NAcc) on day 24 after a 14-day drug-free period in animals that received intravenous (IV) administration of Tat-GluA23Y or controls during induction. (A) Time course of locomotor activity on challenge day 24. (B) Dopamine efflux in the NAcc on challenge day 24. (C) Cumulative locomotor activity over 30 min following drug challenge. (D) Dopamine efflux in the NAcc at the 30 min peak following drug challenge. IP = intraperitoneal. *p < 0.05.
Clear evidence of a sensitized neurochemical effect was provided by a comparison of the magnitude of d-AMPH–induced DA efflux in the NAcc on challenge tests on day 24 in rats treated repeatedly with d-AMPH versus those receiving the challenge dose of d-AMPH for the first time (treatment group: F3,32 = 36.285, p = 0.001; post hoc: vehicle + d-AMPH v. vehicle + saline, p = 0.036; Tat-GluA23Yscr + d-AMPH v. vehicle + saline, p = 0.009; Fig. 3D). Most importantly, repeated treatment with Tat-GluA23Y during the induction phase blocked the expression of neurochemical sensitization to a challenge injection of d-AMPH in the absence of peptide (pairwise comparison, Tat-GluA23Yscr + d-AMPH v. Tat-GluA23Y + d-AMPH, p = 0.025).
Microinjection of Tat-GluA23Y into the VTA or NAcc has no effect on induction of d-AMPH sensitization
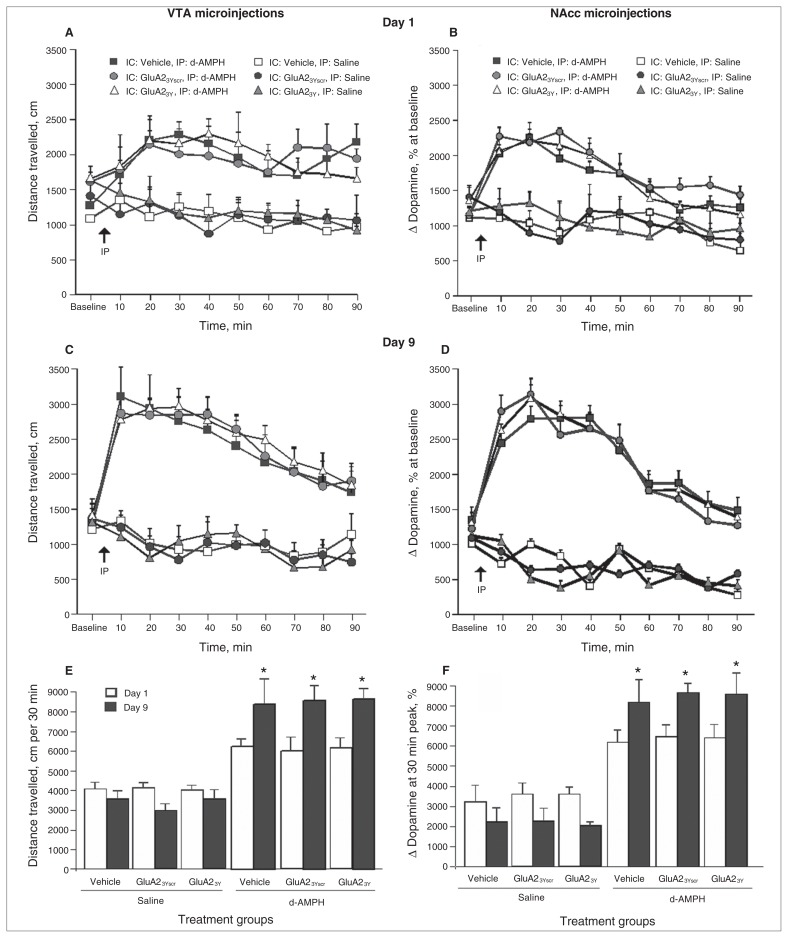
Infusion of Tat-GluA23Y into the VTA before a systemic d-AMPH injection did not significantly affect d-AMPH–induced locomotion on the first day of exposure (repeated-measures ANOVA, F9,38 = 8.34, p = 0.040; post hoc: Tat-GluA23Y + d-AMPH v. Tat-GluA23Yscr + d-AMPH, p > 0.99; Tat-GluA23Y + d-AMPH v. vehicle + d-AMPH, p > 0.99; Fig. 4A). Furthermore, repeated d-AMPH injections led to significant increases in activity and total distance travelled on day 9 of the induction phase in all groups, including those pretreated with Tat-GluA23Y (day × treatment group: F1,5 = 5.47, p = 0.001; post hoc: vehicle + d-AMPH group, day 1 v. day 9, p = 0.008; Tat-GluA23Yscr + d-AMPH group, day 1 v. day 9, p = 0.001; Tat-GluA23Y + d-AMPH group, day 1 v. day 9, p =0.002; Fig. 4C and 4E).
Fig. 4.
d-Amphetamine (d-AMPH)–induced locomotor activity on day 1 and day 9 of induction of sensitization following microinjection of Tat-GluA23Y or controls into the ventral tegmental area (VTA) or nucleus accumbens (NAcc). (A) Time course of locomotor activity on day 1 of induction following VTA microinjection of peptide or controls. (B) Time course of locomotor activity on day 1 of induction following NAcc microinjection of peptide or controls. (C) Time course of locomotor activity on day 9 of induction following VTA microinjection of peptide or controls. (D) Time course of locomotor activity on day 9 of induction following NAcc microinjection of peptide or controls. (E) Cumulative locomotor activity over 30 min on days 1 and 9 of induction following VTA microinjections. (F) Cumulative locomotor activity over 30 min on days 1 and 9 of induction following NAcc microinjections. IC = intracerebral; IP = intraperitoneal. *p < 0.01.
Similarly, infusions of Tat-GluA23Y into the NAcc before each systemic d-AMPH injection did not affect d-AMPH–induced locomotion on the first or ninth day of the induction phase (Fig. 4B and 4D). Repeated d-AMPH injections led to a significant increase in peak activity and total distance travelled from day 1 to day 9 in all groups, including the one pretreated with Tat-GluA23Y (day × treatment group: F1,5 = 19.85, p = 0.005; post hoc: vehicle + d-AMPH group, day 1 v. day 9, p = 0.021; Tat-GluA23Yscr + d-AMPH group, day 1 v. day 9, p = 0.024; Tat-GluA23Y + d-AMPH group, day 1 v. day 9, p = 0.011; Fig. 4F).
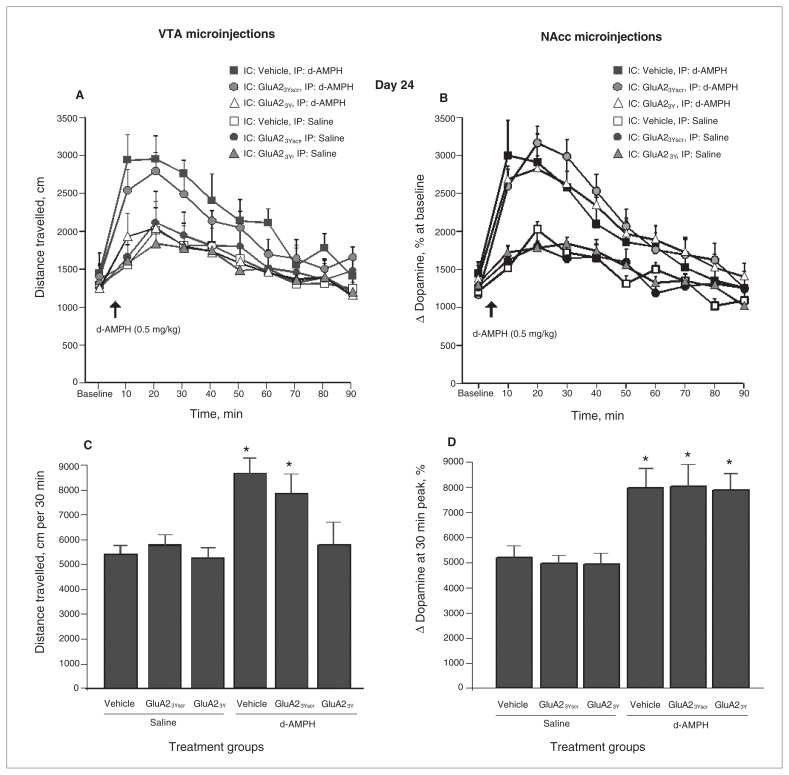
Microinjection of Tat-GluA23Y into the VTA, but not the NAcc, inhibits maintenance and expression of d-AMPH sensitization
When challenged with d-AMPH (0.5mg/kg) after a 14-day drug-free period, rats treated with microinjections of Tat-GluA23Y into the VTA during the induction phase failed to express behavioural sensitization on day 24 (treatment group: F5,38 = 3.25, p = 0.001; post hoc: Tat-GluA23Y + d-AMPH v. vehicle + d-AMPH, p = 0.003; Tat-GluA23Y + d-AMPH v. Tat-GluA23Yscr + d-AMPH, p = 0.025; Fig. 5A and 5C). In contrast, all sensitized animals, including those pretreated with microinjections of Tat-GluA23Y into the NAcc, displayed an enhanced response to the d-AMPH challenge relative to control rats that received d-AMPH for the first time (d-AMPH main effect: F5,36 = 6.54, p = 0.010; Fig. 5B and 5D).
Fig. 5.
d-Amphetamine (d-AMPH)–induced locomotor activity on day 24 after a 14-day drug-free period in animals that received microinjections of Tat-GluA23Y or controls during induction. (A) Time course of locomotor activity on challenge day in animals previously given ventral tegmental area (VTA) microinjections of Tat-GluA23Y or controls during induction. (B) Time course of locomotor activity on challenge day in animals previously given nucleus accumbens (NAcc) microinjections of Tat-GluA23Y or controls during induction. (C) Cumulative locomotor activity over 30 min following drug challenge in animals previously given VTA microinjections of Tat-GluA23Y or controls during induction. (D) Cumulative locomotor activity over 30 min following drug challenge in animals previously given NAcc microinjections of Tat-GluA23Y or controls during induction. IC = intracerebral; IP = intraperitoneal. *p < 0.05.
Discussion
Consistent with the behavioural sensitization literature, locomotor activity increased significantly from day 1 to day 9 as a result of repeated amphetamine exposure.32,33 Importantly, this effect was maintained in all control groups after a 14-day drug-free period, as evidenced by a significantly enhanced locomotor response relative to drug-naive controls following the challenge dose of d-AMPH (0.5 mg/kg) on day 24. Intravenous administration of Tat-GluA23Y did not affect the initial acute locomotor response on day 1; however, repeated coadministration of Tat-GluA23Y with each amphetamine injection prevented the induction of behavioural sensitization normally observed after 5 intermittent injections of d-AMPH. A challenge injection of d-AMPH after a 14-day drug-free period confirmed that coadministration of Tat-GluA23Y peptide during the induction phase blocked the induction and maintenance of d-AMPH–induced behavioural sensitization observed in the Tat-GluA23Yscr and vehicle-treated groups.
The microdialysis experiment confirmed that acute d-AMPH induced a significant increase in DA efflux in the NAcc in the control groups on the first and fifth day of drug exposure. It is important to note that the induction of sensitization is not always accompanied by an increase in DA release in the NAcc, especially after a short incubation period. Paulson and Robinson34 measured motor behaviour and DA neurotransmission at different times after discontinuation of repeated d-AMPH treatment, and they failed to observe a sensitized neurochemical response to a challenge dose of d-AMPH after either 3 or 7 days of withdrawal. In contrast, animals that received the same sensitization protocol displayed enhanced DA release when tested after a 28-day drug-free period. These results indicate that the development of neurochemical sensitization, expressed as an increase of DA release in the NAcc, may develop more slowly than enhanced motor activity. In the present study, when tested after a 14-day incubation period, both Tat-GluA23Yscr and vehicle-treated groups displayed significantly higher levels of evoked DA than the d-AMPH–naive group, thereby confirming the occurrence of neurochemical sensitization.33
Here we also identify a distinct role for regulated endocytosis of GluA2–containing AMPARs in modulating the efflux of DA within the NAcc after repeated but not acute exposure to d-AMPH. A transient (1–5 d) synaptic potentiation of AMPAR currents in DA neurons in the VTA35 is attributed to a change from GluA2-containing to GluA2-lacking AMPARs, induced by the initial exposure to a psychostimulant drug.36,37 In the present study, the significant attenuation of DA efflux observed after the fifth injection of d-AMPH, when GluA2-containing AMPAR endocytosis is blocked by coadministration of Tat-GluA23Y, clearly implicates the development of LTD in the maintenance of a robust neurochemical response to repeated psychostimulant exposure. The behavioural pharmacological response to d-AMPH, whether in the form of a conditioned response associated with repeated drug exposure or as a drug-induced behavioural sensitization, is highly influenced by associative processes,33,38 which in turn are encoded as enduring memories of drug-related environmental stimuli. We hypothesize that formation of such memories is dependent on an LTD-like process that may well involve the replacement of GluA2-containing by GluA2-lacking AMPARs. When AMPAR endocytosis is disrupted by the interference peptide Tat-GluA23Y, the inhibition of LTD results in the absence of both neurochemical and behavioural sensitization in rats subsequently exposed to a challenge dose of d-AMPH 14 days later.
The microinjection studies targeting the NAcc and VTA revealed important differences in the neuroplasticity involved in the induction of sensitization as distinct from those processes related to the maintenance of behavioural sensitization. Notably, microinjection of Tat-GluA23Y into either the VTA or the NAcc had no effect on the initial increase in drug-induced locomotion from the first to the fifth injection. In contrast, disruption of GluA2 endocytosis in the VTA during the induction phase did have a marked effect on d-AMPH–induced locomotion in response to the final challenge dose of this drug on day 24. A direct comparison of the VTA microinjection data to results obtained with systemic administration of Tat-GluA23Y suggests that the blockade of sensitization observed in the latter condition involves actions of Tat-GluA23Y at sites beyond the VTA, which remain to be identified. Accordingly, there is a distinction between processes responsible for the immediate increase in motor activity during repeated injections of a psychostimulant drug and those neuroadaptations related to the encoding and augmentation of behavioural sensitization over a drug-free period before the expression of sensitization.38 Importantly, our findings are consistent with the literature linking neural activity within the VTA to neuroadaptations that establish and maintain sensitization,39–42 as distinct from other forms of adaptation in the NAcc responsible for the expression of behavioural sensitization.40,43 The imposition of a prolonged drug-free period after repeated noncontingent treatments with cocaine is related to enhanced AMPAR function in medium spiny neurons (MSNs) in the NAcc shell, which is linked to increased surface expression of GluA1 and GluA2 subunits.44,45 This state may be permissive for the expression of behavioural sensitization, as both enhanced AMPAR currents and surface expression receptor subunits are reversed immediately following a challenge dose of cocaine.13,44,46
A recent discussion of synaptic plasticity induced by drugs of abuse and its contributions to the neuropathology of addiction highlights the many and varied processes involved.47 Initial findings emphasize the induction of LTP by acute exposure to drugs of abuse that was subsequently linked to an increase in AMPAR trafficking.48 Of particular importance to the present discussion is the finding that the prominence of GluA2-containing AMPARs in a drug-naive state is replaced by increased expression of the GluA2-lacking variant of AMPARs following exposure to a psychostimulant drug.36,37 Together, these findings suggest that increased Ca2+ permeability and conductance of GluA2-lacking AMPA receptors are essential factors in the sensitized response to a psychostimulant drug challenge. If this change in AMPAR subtype is a critical step in the modification of glutamatergic synapses on DA neurons in the VTA required for the encoding of associations between contextual stimuli and drug reward, it follows that interference with regulated endocytosis of GluA2-containing AMPARs by Tat-GluA23Y is an ideal tool to prevent the ascendency of drug-related memories.
Limitations
Behavioural sensitization in rodents, as a model of addiction, does not provide direct measures of drug reinforcement. One may also question the relevance of experimenter-administered drug treatment to an understanding of processes related to human addiction. Critics of noncontingent drug administration methods favour the use of drug self-administration protocols; however, it is also important to use procedures that lead to behavioural, psychological or neuro-biological outcomes that parallel those seen in clinical addiction.49 Animals that develop behavioural sensitization acquire self-administration more readily, indicating increased motivation for drug reward.50 They also exhibit impairments in tests of cognition51 and display prolonged neurochemical alterations, such as enhanced glutamate release52 or drug-induced DA efflux,53 as observed in the present study. The intermittent treatment and relatively high dose of drug per injection may contribute to the utility of sensitization in mimicking the initial period of irregular drug use and experimentation, while the maintenance of a heightened response seen after a prolonged drug-free period may effectively model drug craving. Although we have not observed adverse side effects in rats treated repeatedly with the Tat-GluA23Y peptide, formal toxicology is required before it may be considered as a candidate for use in human clinical trials.
Conclusion
The interplay between glutamatergic synapses and dopaminergic projections onto MSNs within the NAcc core has long been recognized as a critical factor in the expression of behavioural sensitization as well as different forms of relapse to drug-seeking behaviour. In a similar manner, AMPARs in the VTA have also been implicated in the induction of behavioural sensitization.12 The present study provides conclusive evidence linking AMPAR endocytosis within the VTA to the induction and maintenance of behavioural sensitization. In this context, inhibition of the endocytosis of GluA2-containing AMPARs by the interference peptide Tat-GluA23Y may serve to maintain or restore the balance of GluA2-containing to GluA2-lacking AMPARs. Furthermore, these effects may reflect modulation of DA transmission within the NAcc. Our findings complement our previous observation that disruption of GluA2 AMPAR endocytosis by direct microinjection of Tat-GluA23Y peptide into the NAcc can prevent the expression of behavioural sensitization to d-AMPH.16
By preventing the induction of LTD, the interference peptide Tat-GluA23Y may retain the mesocorticolimbic DA system and related glutamatergic modulation in a drug-naive state. An immediate consequence of this mode of action would be to block the encoding of critical drug–contextual stimuli associations essential for the maintenance and expression of behavioural sensitization. Given the arguments that support a close relationship between behavioural sensitization and drug craving54,55 and its relevance to clinical addiction in humans,3 one may contemplate the use of the interference peptide Tat-GluA23Y to protect against the development of craving and drug-seeking behaviours.
Acknowledgements
This project was supported by operating grants from the Canadian Institutes of Health Research (CIHR) to A.G. Phillips (MOP 38069) and to Y.T. Wang (MOP 38090). F.Y. Choi was supported by a doctoral fellowship from the CIHR-funded Integrated Mentor Program in Addictions Research Training program and subsequently by a research assistantship from CIHR (MOP 38069). We thank Ms. Kitty So for her invaluable technical assistance with the microdialysis studies. Portions of these data were included in the Eli Lilly Canada Heinz Lehmann Award Lecture presented by A.G. Phillips during the Joint Meeting of the CCNP/SSNP in Copenhagen, Denmark, in April 2009.
Footnotes
Competing interests: None declared by F.Y. Choi and S. Ahn. Y.T. Wang and A.G. Phillips declare a pending patent (PCT/CA2004/001813) for an IV formulation of the interference peptide Tat-GluA23Y. A.G. Phillips also declares a grant, unrelated to the present study, from Panora Pharmaceuticals. He served until July 2013 on the board of directors of Allon Therapeutics.
Contributors: All authors designed the study and reviewed and approved it for publication. F.Y. Choi and S. Ahn acquired and analyzed the data, which A.G. Phillips also analyzed. F.Y. Choi, S. Ahn and A.G. Phillips wrote the article.
References
- 1.Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW, Pierce RC, Cornish J, et al. A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol. 1998;12:49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- 3.Boileau I, Dagher A, Leyton M, et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–95. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- 4.Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1601–13. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19:456–63. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson TE, Becker JB. Behavioral sensitization is accompanied by an enhancement in amphetamine-stimulated dopamine release from striatal tissue in vitro. Eur J Pharmacol. 1982;85:253–4. doi: 10.1016/0014-2999(82)90478-2. [DOI] [PubMed] [Google Scholar]
- 7.Imperato A, Obinu MC, Carta G, et al. Reduction of dopamine release and synthesis by repeated amphetamine treatment: role in behavioral sensitization. Eur J Pharmacol. 1996;317:231–7. doi: 10.1016/s0014-2999(96)00742-x. [DOI] [PubMed] [Google Scholar]
- 8.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 9.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–28. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 10.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 11.Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22:545–51. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–63. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas MJ, Beurrier C, Bonci A, et al. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–23. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 14.Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–32. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 15.Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–22. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 16.Brebner K, Wong TP, Liu L, et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310:1340–3. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 17.Van den Oever MC, Goriounova NA, Li KW, et al. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci. 2008;11:1053–8. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- 18.Dias C, Wang YT, Phillips AG. Facilitated extinction of morphine conditioned place preference with Tat-GluA2(3Y) interference peptide. Behav Brain Res. 2012;233:389–97. doi: 10.1016/j.bbr.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadian G, Ju W, Liu L, et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 2004;23:1040–50. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholz R, Berberich S, Rathgeber L, et al. AMPA receptor signaling through BRAG2 and Arf6 critical for long-term synaptic depression. Neuron. 2010;66:768–80. doi: 10.1016/j.neuron.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Gulley JM, Stanis JJ. Adaptations in medial prefrontal cortex function associated with amphetamine-induced behavioral sensitization. Neuroscience. 2010;166:615–24. doi: 10.1016/j.neuroscience.2009.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28:7876–82. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 24.Koob GF, Le MM. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 25.Schultz W. Multiple reward signals in the brain. Nat Rev Neurosci. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- 26.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 27.Fibiger HC, Phillips AG. Mesocorticolimbic dopamine systems and reward. Ann. N.Y. Acad. Sci. 1988;537:206–15. doi: 10.1111/j.1749-6632.1988.tb42107.x. [DOI] [PubMed] [Google Scholar]
- 28.Churchill L, Swanson CJ, Urbina M, et al. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem. 1999;72:2397–403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- 29.Perugini M, Vezina P. Amphetamine administered to the ventral tegmental area sensitizes rats to the locomotor effects of nucleus accumbens amphetamine. J Pharmacol Exp Ther. 1994;270:690–6. [PubMed] [Google Scholar]
- 30.Tolliver BK, Ho LB, Fox LM, et al. Necessary role for ventral tegmental area adenylate cyclase and protein kinase A in induction of behavioral sensitization to intraventral tegmental area amphetamine. J Pharmacol Exp Ther. 1999;289:38–47. [PubMed] [Google Scholar]
- 31.Paxinos G, Watson CR, Emson PC. AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods. 1980;3:129–49. doi: 10.1016/0165-0270(80)90021-7. [DOI] [PubMed] [Google Scholar]
- 32.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 33.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 34.Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electro-physiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–90. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argilli E, Sibley DR, Malenka RC, et al. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown MT, Bellone C, Mameli M, et al. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS ONE. 2010;5:e15870. doi: 10.1371/journal.pone.0015870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- 39.Ungless MA, Whistler JL, Malenka RC, et al. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–7. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 40.Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–75. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vezina P, Stewart J. Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine: lack of conditioned effects. Brain Res. 1990;516:99–106. doi: 10.1016/0006-8993(90)90902-n. [DOI] [PubMed] [Google Scholar]
- 42.Wolf ME, Xue CJ. Amphetamine-induced glutamate efflux in the rat ventral tegmental area is prevented by MK-801, SCH 23390, and ibotenic acid lesions of the prefrontal cortex. J Neurochem. 1999;73:1529–38. doi: 10.1046/j.1471-4159.1999.0731529.x. [DOI] [PubMed] [Google Scholar]
- 43.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–7. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boudreau AC, Reimers JM, Milovanovic M, et al. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–35. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boudreau AC, Ferrario CR, Glucksman MJ, et al. Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. J Neurochem. 2009;110:363–77. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kourrich S, Rothwell PE, Klug JR, et al. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–8. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van den Oever MC, Spijker S, Smit AB. The synaptic pathology of drug addiction. Adv Exp Med Biol. 2012;970:469–91. doi: 10.1007/978-3-7091-0932-8_21. [DOI] [PubMed] [Google Scholar]
- 48.Wolf ME, Sun X, Mangiavacchi S, et al. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47(Suppl 1):61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–39. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–62. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierce RC, Bell K, Duffy P, et al. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–60. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vezina P, Pierre PJ, Lorrain DS. The effect of previous exposure to amphetamine on drug-induced locomotion and self-administration of a low dose of the drug. Psychopharmacology (Berl) 1999;147:125–34. doi: 10.1007/s002130051152. [DOI] [PubMed] [Google Scholar]
- 55.Lorrain DS, Arnold GM, Vezina P. Previous exposure to amphetamine increases incentive to obtain the drug: long-lasting effects revealed by the progressive ratio schedule. Behav Brain Res. 2000;107:9–19. doi: 10.1016/s0166-4328(99)00109-6. [DOI] [PubMed] [Google Scholar]