Abstract
Background
Altered memory processes are thought to be a key mechanism in the etiology of anxiety disorders, but little is known about the neural correlates of fear learning and memory biases in patients with social phobia. The present study therefore examined whether patients with social phobia exhibit different patterns of neural activation when confronted with recently acquired emotional stimuli.
Methods
Patients with social phobia and a group of healthy controls learned to associate pseudonames with pictures of persons displaying either a fearful or a neutral expression. The next day, participants read the pseudonames in the magnetic resonance imaging scanner. Afterwards, 2 memory tests were carried out.
Results
We enrolled 21 patients and 21 controls in our study. There were no group differences for learning performance, and results of the memory tests were mixed. On a neural level, patients showed weaker amygdala activation than controls for the contrast of names previously associated with fearful versus neutral faces. Social phobia severity was negatively related to amygdala activation. Moreover, a detailed psychophysiological interaction analysis revealed an inverse correlation between disorder severity and frontolimbic connectivity for the emotional > neutral pseudonames contrast.
Limitations
Our sample included only women.
Conclusion
Our results support the theory of a disturbed corticolimbic interplay, even for recently learned emotional stimuli. We discuss the findings with regard to the vigilance–avoidance theory and contrast them to results indicating an oversensitive limbic system in patients with social phobia.
Introduction
Social phobia is one of the most prevalent and burdensome anxiety disorders.1 In addition to threat-focused information processing, early experiences and learning mechanisms are assumed to constitute a psychological vulnerability for the onset and maintenance of pathological anxiety.2 Two well-established models of social phobia include memory biases, as relevant for self-perception,3 and/or for preferred processing of negative information before and after a social situation.4 While there is broad evidence for disorder-related attentional biases (see the study by Maidenberg and colleagues5), findings on memory biases in patients with social phobia are still contradictory (see the review by Hirsch and colleagues6). Some studies have failed to find memory alterations for verbal stimuli7 (see the review by Mitte8). However, particularly when using more salient social phobia–related stimuli, such as emotional facial expressions, other authors have reported enhanced memory for critical faces9,10 and a better performance of patients with social phobia when learning names for aversive faces.11
Concerning the neural correlates of threat-processing in patients with social phobia, several functional magnetic resonance imaging (fMRI) studies have suggested a hypersensitivity of emotion-related limbic areas for fearful and angry faces and for disorder-relevant words12,13 (see the reviews by Freitas-Ferrari and colleagues14 and Miskovic and Schmidt15). These results are in accordance with findings of amygdala hyperactivation in patients with other psychiatric disorders, such as specific phobia16 and major depression;17,18 in those with high trait anxiety;19–21 and after childhood maltreatment.22,23 Altered activation patterns in prefrontal executive brain areas (i.e., prefrontal cortex [PFC]) have also been reported in patients with social phobia.24–26 Theories suggest that prefrontal activation may down-regulate anxiety-sensitive subcortical brain areas.27–29 This interplay may be disturbed in patients with pathological anxiety, possibly resulting in increased amygdala responsiveness.15,30 Whereas there is considerable evidence of amygdala hyperactivation in patients with social phobia, a potential prefrontal pathology, particularly the functional interaction between the amygdala and the PFC, remains to be investigated.
Given the ongoing discussion about the neurobiological basis of anxiety disorders and the crucial role of memory biases in current theories on social phobia, a closer look at corticolimbic correlates and connectivity related to learning processes in patients with social phobia seems useful. Therefore, in the present study we applied a statistical learning paradigm with faces as externally valid stimuli in patients with social phobia and healthy controls. Statistical learning is a powerful learning mechanism by which children and adults acquire novel linguistic information.31,32 It involves an implicit computation of distributional frequencies with which specific items (e.g., words, speech sounds) occur relative to others or, as is the case here, with which certain items co-occur. We chose a statistical learning task to allow one-to-one mapping between a specific pseudoname and a person, which resembles natural language acquisition processes.32,33 As such, this method differs from conditioning approaches.34,35 Moreover, statistical learning is assumed to be an implicit learning mechanism and is considered by some authors to be better suited than deterministic learning tasks for functional imaging studies owing to fewer interindividual differences in cognitive strategies across participants.33 From an experimental viewpoint, another advantage is that all items can occur with the same frequency in different experimental conditions, but the consistency of the coupling to other stimuli varies. In our study, participants learned to associate pseudonames with faces with either fearful or neutral expressions and subsequently completed an fMRI measure and several memory tests.
As there is some evidence that patients with social phobia have better memory of negative facial images, we expected that they would learn and remember the pseudonames associated with fearful faces better than controls. On a neural level, we hypothesized a stronger activation of the amygdala for the emotional > neutral pseudonames contrast in the social phobia group, with this activation being positively associated with disorder severity. As there is no evidence so far regarding corticolimbic connectivity for recently acquired emotional stimuli, we merely surmised an influence of disorder severity on corticolimbic coupling in the patient group.
Methods
Participants
We recruited patients with social phobia and healthy controls via local newspaper ads and public notices. All participants were screened by an experienced clinical psychologist (I.L.) using the SCID interview.36 To be included in the study, patients had to fulfil the criteria of current social phobia according to DSM-IV,37 have no comorbid diagnoses of current major depression or generalized anxiety disorder, and have no history of psychotic symptoms or substance abuse. Healthy controls had to have no lifetime history of any psychiatric disorder or treatment. All participants had to be psychotropic medication–free. Further exclusion criteria for both groups were general MRI contraindications, neurologic illnesses or a history of seizure or head trauma, and head movements during scanning of more than 2 mm and/or 2°.
All participants completed the German versions of the Beck Depression Inventory (BDI38), the Trait- and State components of the State-Trait Anxiety Inventory (STAI39), the Social Phobia Scale (SPS) and the Social Interaction Anxiety Scale (SIAS40). The latter 2 scales were developed as companion scales40 measuring different aspects of social phobia symptoms. While the SPS measures the fear of being scrutinized during several activities (e.g., drinking, speaking), the SIAS assesses fear of social interactions with others. All procedures were approved by the Ethics Committee of the Medical Faculty of the University of Muenster. The ethical standards of the Declaration of Helsinki were met. All participants provided written informed consent and received financial compensation for their participation. Patients were also offered a complementary psychological consultation.
Stimuli
A total of 72 pictures of 36 different faces (18 men, 18 women, all white) were taken from the Radboud Face Database.42 Each face was shown in a fearful and in a neutral expression on a black background. As pseudonames (i.e., nonexisting names), 36 novel, emotionally neutral word forms were selected. All pseudonames were legal with respect to German phonotactics (see Breitenstein and Knecht33). Importantly, it has been determined previously that the pseudonames are not associated with existing German words and that they are free of emotional valence,33 thus providing a methodological advantage over existing names.
Procedure
All participants completed computer-based training 1 day before fMRI scanning. The training was conducted by means of Presentation Software version 12.1 (Neurobehavioural Systems, Inc.; www.neurobs.com). Participants were trained with face–name associations for about 95 min in 7 learning passes, which were divided by 6 optional breaks. During learning, each pseudoname was presented visually 28 times: 14 times with the identical face (always in the same expression; matches) but only twice with each of 7 other faces (mismatches), balanced for expression across participants. In each of the 7 learning passes, each name was presented twice in the match and twice in the mismatch condition, thus the probability of correctly associating the name with a particular (fearful or neutral) face was at chance level in the first learning pass and successively increased with each learning pass. Note that each matching face for a particular pseudoname served as a mismatch for another pseudoname. To avoid chance associations between specific pseudonames and a particular emotional expression, each name was paired equally often with the neutral and the fearful expression of the same face across participants using a Latin square design. This procedure ensured that each item was neutral before learning and that each item had the same frequency of exposure with regard to negative or neutral pairings. During training, participants were asked to decide intuitively whether the combinations of pseudonames and faces matched. They were first shown a fixation cross (white cross in the centre of a black screen, 400–600 ms), then the pseudoname (700 ms), then another fixation cross (200–300 ms) and finally the face (1000 ms). An exclamation mark (until button press, up to 2500 ms) finished the trial. Participants were instructed to indicate their decision (match or mismatch) via button press while the exclamation mark was visible. No feedback was provided.
One day after training, participants were presented with blocks of the pseudonames previously associated with either fearful or neutral facial expressions in the MRI scanner. They were instructed to read the names attentively. Six blocks, each consisting of 6 different pseudonames, were presented 3 times each in pseudorandomized order. The pseudonames were shown in white at the centre of a black screen for 1500 ms followed by a fixation cross (500 ms) for a total duration of 12 s per block. Each pseudoname block was followed by an 8 second resting phase (fixation cross), to allow participants to take short pauses. In all, the paradigm took 360 s (6 min). The stimuli were projected onto a screen at the rear end of the scanner, using a beamer shielded against radiofrequency interference.
After scanning, the participants performed 2 memory tests. First, they rated the valence of all pseudonames on a self-assessment manikin43 using a 9-point Likert scale ranging from 1 = very unpleasant to 9 = very pleasant. Afterwards, participants completed a multiple-choice test. They were presented with each of the pseudonames with 4 faces from which they had to choose the 1 that matched the name. Each correct face was presented with 3 distractor faces, 1 with the same valence and 2 with the opposite valence (emotional v. neutral). The order of faces was randomized for each question.
Image acquisition
We acquired MRI data using a 3 T scanner (Gyroscan Intera T3.0, Philips Medical Systems) equipped with Quasar Dual gradients (nominal gradient strength in nonenhanced mode 40 mT/m, maximal slew rate 200 mT/m/ms). For spin excitation and resonance signal acquisition, we used a circularly polarized transmit/receive birdcage head coil with a high-frequency reflecting screen at the cranial end. We acquired T2* functional data using a single-shot echo planar sequence (whole brain coverage, echo time 30 ms, repetition time 2.3 s, flip angle 90°, 36 slices, slice thickness 3.6 mm, no gap, matrix 64 × 64 mm, field of view 230 mm, in-plane resolution 3.6 × 3.6 mm). The slices were tilted 25° from the anterior/posterior commissure line to minimize dropout artifacts in the orbitofrontal and mediotemporal regions. For SPM processing, the acquired volumes were resampled to voxels of 2 × 2 × 2 mm.
Statistical analysis
We assessed learning success by testing whether the proportion of correct answers within the last learning pass differed significantly (p < 0.001) from chance (binomial test, as implemented in SPSS version 20 [SPSS Inc.]). Only participants who fulfilled this criterion were included in the subsequent behavioural and fMRI analyses. The proportion of correct button presses (correct “match” or “mismatch” answers) were calculated for each learning pass and separately for pseudonames belonging to emotional or neutral faces. The resulting proportional values were arcsine-transformed44 because the homogeneity assumption was violated given the dependency of variance and mean in binomial distributions.45 We then entered the data into a 7 × 2 × 2 repeated-measures analysis of variance (ANOVA) with learning pass (1–7) and category of pseudoname (emotional v. neutral) as within-subjects factors and group (patients v. controls) as a between-subjects factor. Before analyzing the memory tests, we computed the Cronbach α statistic to check their reliability. Next, the valence ratings and the arcsine- transformed proportions of correct answers in the multiple-choice test were analyzed using 2 × 2 repeated-measures analyses of covariance (ANCOVAs), with emotion as a within-subjects factor and group as a between-subjects factor. We calculated Bonferroni-corrected post hoc tests to explore the nature of effects. For the 2 memory tests, each participant’s arcsine-transformed individual proportion of correct answers in the last learning pass was entered as a covariate of no interest to control for memory effects due to learning differences.
Functional MRI data were motion-corrected, spatially normalized to standard Montreal Neurological Institute (MNI) space, and smoothed with a 6 mm full-width at half-maximum Gaussian kernel using Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm). The onsets and durations of the 2 conditions were modelled with a canonical hemodynamic response function within the context of the general linear model. The movement parameters from the realignment step were further entered as nuisance regressors. Fixed-effects analyses were performed at an individual level, including the generation of individual contrast maps for the emotional > neutral pseudoname contrast. The resulting contrast images were then entered in second-level (group) random-effects analyses. Again, each participant’s arcsine transformed proportion of correct answers within the last learning pass was entered as a covariate of no interest in the group analysis to control for individual learning differences.
With respect to our hypotheses, we used a 2-sample t test to compare activations for emotional > neutral pseudoname contrasts. We chose a region of interest (ROI) approach for the left and right amygdala (defined according to the automated anatomic labelling (AAL) atlas46 and dilated by 1 mm using the WFU pickatlas47). In addition, we conducted a whole-brain analysis to ensure that we did not miss relevant task-related activations outside the ROI. We explored the association between amygdala activation and disorder severity by conducting a voxel-wise regression analysis, correlating each patient’s amygdala activity for emotional > neutral pseudoname contrasts with his/her individual SPS and SIAS scores. Furthermore, a psychophysiological interaction (PPI) analysis48 was conducted to explore task-dependent connectivity patterns based on the emotional > neutral pseudoname contrasts. Volumes of interest (VOI) included the left or right amygdala, which were separately defined as seed regions according to the AAL atlas.46 We used a 2-sample t test based on the resulting contrast images of the PPI analysis for group comparison. Again, the regression analysis included correlating individual SPS and SIAS scores with the connectivity analysis for each patient separately. We used a mask of the whole frontal lobe (defined according to the WFU pickatlas47), including all frontal areas, for the PPI analysis as well as the regression analysis between disorder severity and the PPI.
The group results were calculated using a combined height and extend significance threshold based on Monte Carlo simulations, as implemented in the AlphaSim procedure,49 to control for multiple statistical testing. Corrected false-positive detection rates were maintained, with a cluster extent (k) empirically determined by computing 5000 simulations. For the amygdala ROI, this analysis was based on an uncorrected threshold of p < 0.05 (k = 29 for each amygdala as the empirically determined cluster extent). Given the undirected hypothesis, for the (much larger) frontal-lobe ROI and the additional whole-brain analysis, we selected a threshold of p < 0.005 (k = 260 for the frontal lobe ROI and k = 389 for the whole brain).
The results of the regression analyses were checked in a second step by extracting the significant mean contrast values for each participant and entering them in a subsequent SPSS multiple regression analysis, predicting each cluster’s activation by the variables of interest (SPS/SIAS) as well as BDI, STAI-T, STAI-S, age, years of education, and verbal intelligence.
Results
Participants
Twenty-one patients with social phobia and 21 healthy controls participated in our study. Table 1 summarizes the demographic and clinical characteristics of the 2 groups. All participants were native German speakers and had normal or corrected-to-normal vision. Five participants in the patient group and 4 in the control group were left-handed. Comorbid diagnoses in the patient sample were specific phobia (n = 2), currently remitted anorexia nervosa (n = 1), panic disorder (n = 1) and currently remitted depressive disorder (n = 6). Some patients received current (n = 3) or former (n = 7) psychotherapeutic treatment (post hoc analyses revealed no differences within the patient group regarding behavioural data or brain responses as a function of treatment). Intercorrelations between the clinical measures can be found in the Appendix, Table S1, available at jpn.ca.
Table 1.
Demographic and clinical characteristics of patients with social phobia and healthy controls
| Group; mean ± SD | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Social phobia | Control | t value | p value* |
| Age, yr | 29.19 ± 7.98 | 29.29 ± 9.14 | 0.04 | 0.97 |
| Education, yr | 15.14 ± 1.93 | 15.52 ± 1.91 | 0.64 | 0.52 |
| Verbal intelligence† | 111.67 ± 14.09 | 114.14 ± 12.39 | 0.61 | 0.55 |
| BDI | 14.09 ± 10.12 | 1.48 ± 1.54 | −5.65 | < 0.001 |
| STAI-T | 53.86 ± 11.54 | 31.52 ± 3.75 | −8.43 | < 0.001 |
| STAI-S | 41.38 ± 9.55 | 30.61 ± 3.51 | −4.85 | < 0.001 |
| SPS | 37.67 ± 14.41 | 3.62 ± 3.75 | −10.48 | < 0.001 |
| SIAS | 46.14 ± 14.45 | 8.38 ± 5.88 | −11.09 | < 0.001 |
BDI = Beck Depression Inventory; SD = standard deviation; SIAS = Social Interaction Anxiety Scale; SPS = Social Phobia Scale; STAI-S = State–Trait Anxiety Inventory, state version; STAI-T = State–Trait Anxiety Inventory, trait version.
2-tailed.
Assessed with the Mehrfachwahl–Wortschatz–Intelligenztest.41
Behavioural data
Pseudoname training
In the course of training, the numer of correct responses (correct “match” or “mismatch” answers) increased significantly from chance level (51.86%) to about 82% in the last learning pass (Tables 2 and 3). Learning increased in a linear fashion (F1,40 = 223.35, p < 0.001). Overall, neutral pseudonames were learned better than emotional pseudonames (t = 4.86, p < 0.001). The emotion × learning pass interaction was due to significantly better hit rates for neutral than for emotional pseudonames in learning passes 1 and 4–7 (all t > 2.50, p < 0.05), but not in learning passes 2 and 3 (all t < 1.82, p > 0.05).
Table 2.
Pseudoname training and memory test results in patients with social phobia and healthy controls
| Group; mean ± SD | ||
|---|---|---|
|
|
||
| Result | Social phobia | Control |
| Pseudoname training* | ||
| Emotional | 80.18 ± 10.83 | 79.41 ± 11.49 |
| Neutral | 85.05 ± 11.04 | 82.06 ± 10.21 |
| Multiple choice test† | ||
| Emotional | 68.76 ± 20.06 | 69.81 ± 20.73 |
| Neutral | 73.62 ± 16.99 | 72.12 ± 17.80 |
| Valence rating‡ | ||
| Emotional | 4.85 ± 0.73 | 5.14 ± 0.45 |
| Neutral | 5.33 ± 0.71 | 5.18 ± 0.52 |
SD = standard deviation.
Data refer to the proportion of correct responses (%) in the last learning pass.
Proportions of correct responses are reported as percentages.
Higher scores indicate more positive pseudoname ratings.
Table 3.
Repeated-measures ANOVA and ANCOVA for the pseudoname training and the memory tests in patients with social phobia and healthy controls
| Response; comparison | F | p value | Partial η2 |
|---|---|---|---|
| Pseudoname training* | |||
| Learning pass, F2,240 | 151.51 | < 0.001 | 0.791 |
| Learning pass × group, F2,240 | 0.24 | 0.79 | 0.006 |
| Emotion, F1,40 | 21.74 | < 0.001 | 0.352 |
| Emotion × group, F1,40 | 1.06 | 0.31 | 0.026 |
| Learning pass × emotion, F6,240 | 2.24 | 0.040 | 0.053 |
| Learning pass × emotion × group, F6,240 | 1.01 | 0.42 | 0.025 |
| Group, F1,40 | 0.29 | 0.59 | 0.007 |
| Multiple choice test,* F1,39 | |||
| Emotion | 2.01 | 0.17 | 0.049 |
| Emotion × group | 0.96 | 0.33 | 0.024 |
| Group | 0.51 | 0.48 | 0.013 |
| Valence rating, F1,39 | |||
| Emotion | 0.00 | 0.95 | 0.000 |
| Emotion × group | 4.55 | 0.039 | 0.104 |
| Group | 0.23 | 0.64 | 0.006 |
ANCOVA = analysis of covariance; ANOVA = analysis of variance.
Analysis based on the arcsine-transformed proportions of correct answers.
Memory tests
Both memory tests showed a sufficient internal consistency (Cronbach α = 0.78 for the valence rating and α = 0.83 for the multiple-choice test). Patients with social phobia rated the valence of pseudonames more negatively for emotional than for neutral pseudonames (t = 3.34, p = 0.002), whereas the control group rated the valence of pseudomyms similarly (t = 0.30, p = 0.77), resulting in a significant emotion × group interaction (Table 3). There were no other main effects or interactions in the valence rating, and there were no significant effects at all in the multiple-choice test (Tables 2 and 3).
Functional imaging data
A whole-brain analysis of general task-related effects revealed robust activations of areas related to perception (e.g., temporal and occipital cortex), emotion regulation (e.g., the whole frontal gyrus) and limbic areas, such as the amygdala, the cingulate gyrus and the hippocampus, underlining the appropriateness of the task to elicit responses in crucial emotion-related brain regions.
ROI analysis comparing amygdala activation to emotional > neutral pseudonames
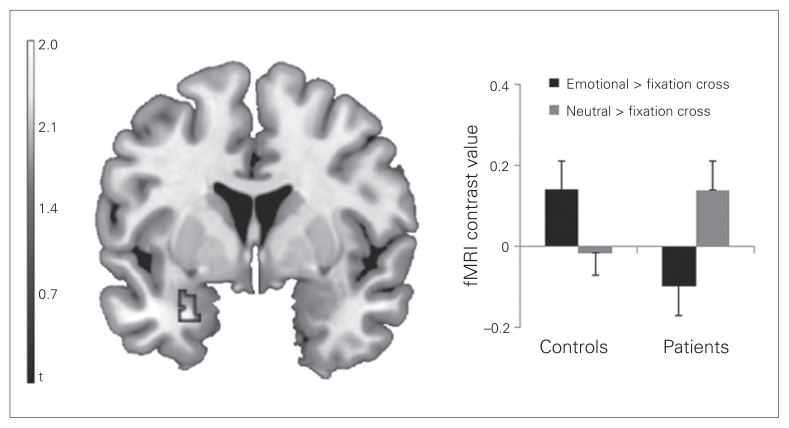
The 2-sample t test revealed that controls showed a stronger activation of the left amygdala than patients with social phobia for the emotional > neutral pseudoname contrast (MNI coordinates x, y, z = −30, 2, −26, t39 = 2.82, p = 0.029, corrected, k = 54 voxels; Fig. 1). The additional whole-brain analysis for the same contrast revealed no other significant differences between the groups.
Fig. 1.
Coronal view (Montreal Neurological Institute [MNI] coordinate y = 2) depicting the significant cluster of the comparison between healthy controls and patients with social phobia based on the emotional > neutral pseudonames contrast. The bar graphs depict the mean contrast values for emotional pseudonames > fixation cross and neutral pseudonames > fixation cross contrasts extracted from MNI coordinates x, y, z = −30, 2, −26. Error bars represent standard errors of the mean. fMRI = functional magnetic resonance imaging.
Psychophysiological interaction analysis
The PPI analysis based on the emotional > neutral pseudo-name contrast did not reveal any significant clusters within frontal regions that differed between patients and controls.
Regression analysis with SPS and SIAS scores
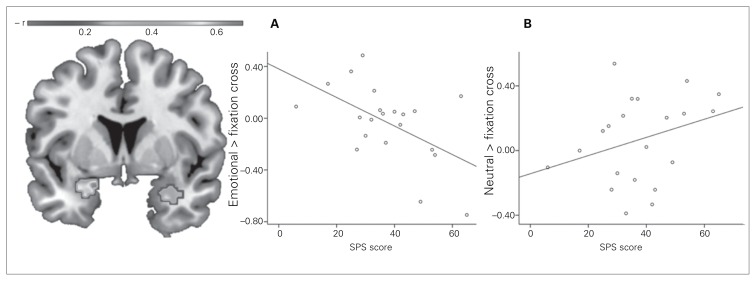
The regression analyses within the patient group revealed a negative correlation between the activation of both the left and right amygdala to the emotional > neutral pseudoname contrast and the SPS score (left amygdala: MNI coordinates x, y, z = −18, 4, −20, t18 = 4.06, p < 0.001, r = −0.69, k = 223 voxels; right amygdala: MNI coordinates x, y, z = 30, 6, −28, t18 = 3.72, p = 0.017, r = −0.66, k = 70 voxels; Fig. 2). Similarly, there was a negative correlation between the SIAS score and the activation of the left amygdala when comparing emotional and neutral pseudonames (MNI coordinates x, y, z = −18, 2, −16, t18 = 3.89, p = 0.020, r = −0.68, k = 67 voxels). In the subsequent multiple regression analysis predicting the activation of the significant clusters by SPS, SIAS, BDI, STAI-T, STAI-S, age, years of education and verbal intelligence, the strong influence of the SPS and SIAS, respectively, remained virtually unchanged (β = −0.51 for the correlation with the SPS and β = −0.58 for the correlation with the SIAS, all p < 0.05).
Fig. 2.
Association of the patients’ Social Phobia Scale (SPS) scores with left and right amygdala responsiveness to emotional > neutral pseudoname contrasts (coronal view, Montreal Neurological Institute [MNI] coordinate y = 0). The scatter plots depict the correlations between the SPS score and the (A) emotional pseudonames > fixation cross contrast and (B) the neutral pseudonames > fixation cross contrast at the maximum of the left panel (MNI coordinates x, y, z = −18, 4, −20).
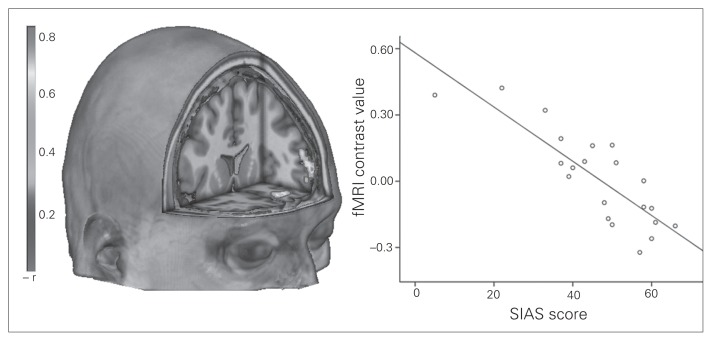
Figure 3 and Table 4 show the results of the regression analysis between SPS and SIAS score and the outcome of the PPI analysis. Most importantly, disorder severity, as indicated by the SIAS, was inversely related to the coupling between the right and left amygdala and several frontal regions for the comparison of emotional and neutral pseudonames. There were no significant correlations between the PPI analysis and the SPS. Again, all significant correlations remained stable when including the SPS, BDI, STAI-T, STAI-S, age, years of education and verbal intelligence into the analyses (all β > −0.54, all p < 0.05).
Fig. 3.
Negative association of the patients’ Social Interaction Anxiety Scale (SIAS) scores and the connectivity between the left amygdala and frontal regions rendered on an anatomic template in Montreal Neurological Insititute space. The scatter plot depicts the negative correlation of the mean cluster activation values and the SIAS scores. fMRI = functional magnetic resonance imaging.
Table 4.
Results of the multiple regression analysis between psychophysiological interactions (emotional > neutral pseudonames) and the Social Interaction Anxiety Scale in patients with social phobia*
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Anatomic region | BA | Cluster size | x | y | z | r value | p value |
| Left amygdala seed | |||||||
| SFG, SFG (orbital), | 10, 11 | 386 | −34 | 64 | 6 | −0.84 | 0.011 |
| MFG, Medial frontal gyrus (orbital) | |||||||
| Right amygdala seed | |||||||
| SFG (medial), SFG, | 6, 8, 32 | 325 | 12 | 32 | 60 | −0.74 | 0.023 |
| SMA, MCG | |||||||
BA = Brodmann area; MCG = middle cingulate gyrus; MFG = middle frontal gyrus; MNI = Montreal Neurological Institute; SFG = superior frontal gyrus; SMA = supplementary motor area.
p < 0.005, uncorrected (corrected at p < 0.05 on the cluster level using the AlphaSim procedure, which resulted in an empirically determined cluster-extent threshold of k = 260 voxels).
Discussion
Our findings provide further evidence for aberrant neural connectivity patterns and alterations in amygdala activation in patients with social phobia, although the latter occurred in the opposite direction than expected. First, for the contrast of pseudonames associated with fearful faces versus those associated with neutral faces, contrary to our hypothesis, patients with social phobia showed decreased rather than increased amygdala activation compared with controls. A regression analysis in the patient group revealed that amygdala activation to emotional > neutral pseudoname contrasts even decreased as a function of social phobia severity. Correlating task-dependent connectivity (PPI) with social phobia severity revealed that with increasing disorder severity (SIAS score), the connectivity between the amygdala and several frontal regions (Brodmann areas [BA] 6, 8, 10, 11 and 32) decreased. The behavioural data showed no group differences with respect to the performance in pseudoname learning or memory tests except for the more negative valence ratings for emotional than for neutral pseudo-names in the patient group but not in the control group.
Our results on amygdala activation and its negative correlation with disorder severity seem to be in contrast with those of several previous reports on limbic hyperactivity in social phobia.14,15,30,50 However, previous studies applied very salient stimuli, such as emotional faces or well-known existing words, which all have the potential to provoke immediate and strong emotional reactions. In contrast, the pseudonames used in our study did not carry any emotional salience by themselves. They were associated with emotional stimuli only 1 day before the fMRI measurement and might thus have a less established and less accessible emotional salience. One prominent model of information processing in anxiety disorders is the vigilance-avoidance theory.51 This approach suggests that anxiety leads to a preferred processing of threatening information in initial stages of stimulus processing but to avoidance in later stages. Given that accessing the meaning of very recently acquired emotional stimuli might require a deeper and more conscious processing than the rather automatic processing of faces or long-known words, it is possible that patients with social phobia do not activate aversive information about the emotional quality of the pseudonames. This idea is especially highlighted by the negative correlation between disorder severity and amygdala activation to emotional pseudonames that we observed (Fig. 2A). In contrast, controls might not feel threatened and have no need to avoid the pseudonames’ emotional connotation, resulting in the expected pattern of amygdala activation (see the study by LaBar and Cabeza52 for an overview on the association between amygdala activation and memory). This pattern of results contradicts the theories presented in the introduction that consider amygdala hyperactivation in combination with PFC-related emotion regulation alterations to be a crucial factor in pathological anxiety (see the review by Miskovic and Schmidt15). On the other hand, amygdala hypoactivation for less salient and less accessible anxiety-related stimuli may help to explain why stimuli that strongly induce anxiety constitute such an excessive demand to the limbic–prefrontal system, resulting in the reported aberrant amygdala and PFC response patterns.
Considering that the interplay between prefrontal and limbic regions might be disturbed in patients with pathological anxiety,15 we expected an influence of social phobia severity on corticolimbic coupling. Although the association we observed between disorder severity and connectivity between the amygdala and the premotor cortex/frontal eye field (BA 6 and 8) is difficult to interpret, the negative correlations with the anterior and orbitofrontal cortex (BA 10 and 11) and the middle cingulate gyrus (BA 32) are of considerable interest. Both regions are considered to be part of a central executive network, which, among other functions, is assumed to implement control when subcortical limbic areas are downregulated.53,54 Social phobia has been associated with several frontal pathologies, such as decreased activation in the orbitofrontal cortex (OFC)55 and dorsolateral PFC (DLPFC)56 and reduced connectivity within the PFC57 and between the amygdala and the medial OFC,24 and with abnormal white-matter tracts connecting the amygdala and the PFC.58 More specifically, it has been suggested that less inferior areas, such as BA 10, exert an inhibiting influence on the amygdala, possibly mediated by the OFC.29 Interestingly, Demenescu and colleagues26 recently reported a positive correlation of anxiety severity with left dorsal, but not ventro-medial amygdala–PFC coupling during fearful face viewing, in a combined group of patients with social phobia or panic dis-order; they interpreted the finding as a possible correlate of anxiety-driven hypervigilance. Complementing these findings, our data from a pure social phobia sample using nonestablished emotional stimuli revealed a negative association between disorder severity and the connectivity to the amygdala for more dorsally located areas as well as the OFC. This weaker connectivity might represent less deep emotional processing, such as less vigilance and less use of emotion regulation strategies, possibly owing to a more general avoidance of anxiety-related stimuli that is especially present in severely affected patients and when less salient emotional stimuli are used. Our finding has 2 implications. First, our results support the view of disturbed interplay between cortical and subcortical regions, even for recently learned emotional stimuli. Second, given that the corticolimbic connectivity with areas related to emotion regulation was weaker in more severely affected patients, it is not likely that the decreased amygdala activation in these patients for the emotional > neutral pseudoname contrast was due to inhibiting influences by BA 10, 11 or 32.
However, and aside from prefrontal activation, other authors stressed the relevance of sensory areas in early stages of emotion processing.59 It is thus tempting to speculate that in patients with social phobia, a disruption of emotional processing might have occurred before relevant frontal areas were affected. This coping mechanism might no longer work for stimuli that induce strong anxiety, leading to the frequently reported limbic hyperactivation in response to threatening information, which may represent the vigilance part of the vigilance–avoidance theory (see the study by Larson and colleagues60 for comparison).
On a behavioural level, we expected to find better learning and memory results for emotional pseudonames in the patient group than in controls. This assumption was not supported by our results. In both groups, neutral pseudonames were learned better than emotional pseudonames. This result is in accordance with a previous finding,7 but contradicts the view that emotional information has a processing advantage in memory.52 Thus, further research is needed to explore the circumstances under which memory biases for emotional material occur. Except for the valence rating, where in contrast to controls patients with social phobia rated emotional pseudo-names more negatively than neutral ones, we found no group differences in the pseudoname training or the multiple-choice test; this finding is in accordance with those of some previous studies7,8 but in contrast to those of other studies.9–11 However, avoidance mechanisms in the patient group could also account for the absence of group differences in explicit memory tests, such as the multiple-choice task. More implicit memory biases might thus only become obvious in a memory test in which pseudonames are not consciously recalled but rated (see the study by Mitte8 for contradicting results).
Limitations
Some limitations must be acknowledged. First, all participants performed the pseudoname training on their own without the assistance of an investigator. However, we included only those participants whose learning success fulfilled a strong criterion (see the Methods section). This ensured that all included participants carefully performed the training. Second, as pointed out by other authors,61,62 there is evidence for genetic influence on amygdala activation and connectivity in patients with social phobia. This should be addressed by future studies. As in many previous studies (see the review by Miskovic and Schmidt15), we used faces with fearful expressions as emotional stimuli in the learning task. However, it is yet unknown which negative facial expression is most suitable for the pathology of social phobia, for statistical learning or any other paradigm. This is of special relevance, as there are also some results indicating altered neural responses to neutral faces in patients with social phobia.63 With respect to the lack of increased amygdala activation in response to emotional versus neutral pseudo-names in patients in the present study, it might be questioned whether the patients perceived the fearful faces used in the learning task as threatening. However, given the current literature and the differences in valence ratings between emotional and neutral pseudonames in the patient group that were in the expected direction, we consider it unlikely that the emotional quality of fearful faces was insufficient. Future studies, however, should include subjective ratings of the facial stimuli. An important difference between our study and others is that most studies used facial expressions as stimuli in the scanner, but we did not. Furthermore, we included only female participants in our study, limiting the generalizability of our results. On the other hand, the generally greater prevalence of social phobia in women1 is reflected, and additional variance owing to existing differences in brain activation between men and women64 has been avoided. Another limitation is that we did not control for menstrual cycle, which has been shown to influence brain activation.65 Finally, according to the current state of the literature, we chose a hypotheses-driven approach mainly focusing on selected brain regions (i.e., the amygdala and the frontal cortex) to allow the comparison of our results with brain activation in response to facial images or to existing words. However, to our knowledge, our study is the first that uses statistical learning in combination with fMRI in patients with social phobia. Future studies might also investigate activation of the hippocampus given its importance in fear learning66 as well as temporal and occipital brain areas being relevant for language but also emotion processing.
Conclusion
Our work provides supporting evidence for distorted corticolimbic connectivity in patients with social phobia. In our data, this finding is particularly highlighted by alterations in connectivity between the amygdala and frontal areas related to disorder severity. Contrary to our expectation, using a statistical learning paradigm we found decreased amygdala activation in patients with social phobia compared with controls for recently learned emotional stimuli. Future research should thus consider different approaches to investigate the functional role of the amygdala and the relevance of corticolimbic interactions in pathological anxiety.
Acknowledgements
This work was supported by grants of Inter-disziplinäres Zentrum für klinische Forschung (IZKF) of the Medical Faculty of Muenster University (grant number Do3/021/10 to CD and PZ) and the SFB TRR 58 subproject C1 to PZ. The named funding sources were not involved in study design, collection, analysis or interpretation of data. We acknowledge support by the Deutsche Forschungsgemeinschaft and the Open Access Publication Fund of the University of Muenster.
Footnotes
Competing interests: None declared.
Contributors: I. Laeger, K. Keuper, H. Kugel, C. Dobel, A. Eden, V. Arolt, P. Zwitserlood, U. Dannlowski and P. Zwanger designed the study. I. Laeger, C. Heitmann and P. Zwanger acquired the data, which I. Laeger, and U. Dannlowski analyzed. I. Laeger, K. Keuper and U. Dannlowski wrote the article, which all authors reviewed and approved for publication.
References
- 1.Kessler RC. The impairments caused by social phobia in the general population: implications for intervention. Acta Psychiatr Scand Suppl. 2003;108:19–27. doi: 10.1034/j.1600-0447.108.s417.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Barlow D. Anxiety and its disorders: the nature and treatment of anxiety and panic. 2nd ed. New York (NY): Guilford Press; 2002. [Google Scholar]
- 3.Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther. 1997;35:741–56. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 4.Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg RG, Liebowitz MR, Hope DA, et al., editors. Social phobia: diagnosis, assessment, and treatment. New York (NY): Guilford Press; 1995. pp. 69–93. [Google Scholar]
- 5.Maidenberg E, Chen E, Craske M, et al. Specificity of attentional bias in panic disorder and social phobia. J Anxiety Disord. 1996;10:529–41. [Google Scholar]
- 6.Hirsch CR, Clark DM. Information-processing bias in social phobia. Clin Psychol Rev. 2004;24:799–825. doi: 10.1016/j.cpr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Rapee RM, Mccallum SL, Melville LF, et al. Memory bias in social phobia. Behav Res Ther. 1994;32:89–99. doi: 10.1016/0005-7967(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 8.Mitte K. Memory bias for threatening information in anxiety and anxiety disorders: a meta-analytic review. Psychol Bull. 2008;134:886–911. doi: 10.1037/a0013343. [DOI] [PubMed] [Google Scholar]
- 9.Lundh LG, Öst LG. Recognition bias for critical faces in social phobics. Behav Res Ther. 1996;34:787–94. doi: 10.1016/0005-7967(96)00035-6. [DOI] [PubMed] [Google Scholar]
- 10.Coles ME, Heimberg RG. Recognition bias for critical faces in social phobia: a replication and extension. Behav Res Ther. 2005;43:109–20. doi: 10.1016/j.brat.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Foa EB, Gilboa-Schechtman E, Amir N, et al. Memory bias in generalized social phobia: remembering negative emotional expressions. J Anxiety Disord. 2000;14:501–19. doi: 10.1016/s0887-6185(00)00036-0. [DOI] [PubMed] [Google Scholar]
- 12.Straube T, Kolassa I-T, Glauer M, et al. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:921–30. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt S, Mohr A, Miltner WHR, et al. Task-dependent neural correlates of the processing of verbal threat-related stimuli in social phobia. Biol Psychol. 2010;84:304–12. doi: 10.1016/j.biopsycho.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Freitas-Ferrari MC, Hallak JEC, Trzesniak C, et al. Neuroimaging in social anxiety disorder: a systematic review of the literature. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:565–80. doi: 10.1016/j.pnpbp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Miskovic V, Schmidt LA. Social fearfulness in the human brain. Neurosci Biobehav Rev. 2012;36:459–78. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Straube T, Mentzel HJ, Miltner WHR. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry. 2006;59:162–70. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Suslow T, Konrad C, Kugel H, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. 2010;67:155–60. doi: 10.1016/j.biopsych.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1:10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Etkin A, Klemenhagen KC, Dudman JT, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–55. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Laeger I, Dobel C, Dannlowski U, et al. Amygdala responsiveness to emotional words is modulated by subclinical anxiety and depression. Behav Brain Res. 2012;233:508–16. doi: 10.1016/j.bbr.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Sehlmeyer C, Dannlowski U, Schöning S, et al. Neural correlates of trait anxiety in fear extinction. Psychol Med. 2011;41:789–98. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- 22.Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Dannlowski U, Kugel H, Huber F, et al. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp. 2013;34:2899–909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56:881–9. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 25.Koric L, Volle E, Seassau M, et al. How cognitive performance-induced stress can influence right VLPFC activation: an fMRI study in healthy subjects and in patients with social phobia. Hum Brain Mapp. 2012;33:1973–86. doi: 10.1002/hbm.21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demenescu LR, Kortekaas R, Cremers HR, et al. Amygdala activation and its functional connectivity during perception of emotional faces in social phobia and panic disorder. J Psychiatr Res. 2013;47:1024–31. doi: 10.1016/j.jpsychires.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Wager TD, Davidson ML, Hughes BL, et al. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hariri AR, Mattay VS, Tessitore A, et al. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 29.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saffran JR, Aslin RN, Newport EL. Statistical learning by 8-month-old infants. Science. 1996;274:1926–8. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- 32.Dobel C, Junghöfer M, Breitenstein C, et al. New names for known things: on the association of novel word forms with existing semantic information. J Cogn Neurosci. 2010;22:1251–61. doi: 10.1162/jocn.2009.21297. [DOI] [PubMed] [Google Scholar]
- 33.Breitenstein C, Knecht S. Development and validation of a language learning model for behavioral and functional-imaging studies. J Neurosci Methods. 2002;114:173–9. doi: 10.1016/s0165-0270(01)00525-8. [DOI] [PubMed] [Google Scholar]
- 34.Lissek S, Levenson J, Biggs AL, et al. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am J Psychiatry. 2008;165:124–32. doi: 10.1176/appi.ajp.2007.06091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fritsch N, Kuchinke L. Acquired affective associations induce emotion effects in word recognition: an ERP study. Brain Lang. 2013;124:75–83. doi: 10.1016/j.bandl.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Wittchen H, Wunderlich U, Gruschwitz S, et al. SKID-I, Strukturiertes Klinisches Interview für DSM-IV. [SCID-I, Structured Clinical Interview for DSM-IV]. Göttingen (DE): Hogrefe; 1997. (Ger). [Google Scholar]
- 37.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994. [Google Scholar]
- 38.Beck AT, Steer RA. Beck Depression Inventory: manual. San Antonio (TX): Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 39.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto (CA): Consulting Psychologists Press; 1983. [Google Scholar]
- 40.Mattick RP, Clarke JC. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behav Res Ther. 1998;36:455–70. doi: 10.1016/s0005-7967(97)10031-6. [DOI] [PubMed] [Google Scholar]
- 41.Lehrl S. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. Göttingen (DE): Hogrefe; 1995. [Google Scholar]
- 42.Langner O, Dotsch R, Bijlstra G, et al. Presentation and validation of the Radboud Faces Database. Cogn Emot. 2010;24:1377–88. [Google Scholar]
- 43.Bradley MM, Lang PJ. Measuring emotion: the self-assessment semantic differential manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 44.Winer B. Statistical Principles in Experimental Design. 2nd ed. New York (NY): McGraw-Hill; 1971. [Google Scholar]
- 45.Sokal R, Rohlf F. Biometry. New York (NY): W.H. Freeman and Company; 2012. [Google Scholar]
- 46.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 47.Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 48.Friston KJ, Buechel C, Fink GR, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 49.Forman SD, Cohen JD, Fitzgerald M, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 50.Brühl AB, Rufer M, Delsignore A, et al. Neural correlates of altered general emotion processing in social anxiety disorder. Brain Res. 2011;1378:72–83. doi: 10.1016/j.brainres.2010.12.084. [DOI] [PubMed] [Google Scholar]
- 51.Amir N, Foa EB, Coles ME. Automatic activation and strategic avoidance of threat-relevant information in social phobia. J Abnorm Psychol. 1998;107:285–90. doi: 10.1037//0021-843x.107.2.285. [DOI] [PubMed] [Google Scholar]
- 52.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 53.Davidson RJ. Dysfunction in the neural circuitry of emotion regulation — a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 54.Dannlowski U, Ohrmann P, Konrad C, et al. Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol. 2009;12:11–22. doi: 10.1017/S1461145708008973. [DOI] [PubMed] [Google Scholar]
- 55.Tillfors M, Furmark T, Marteinsdottir I, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry. 2001;158:1220–6. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 56.Lorberbaum JP, Kose S, Johnson MR, et al. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–5. [PubMed] [Google Scholar]
- 57.Ding J, Chen H, Qiu C, et al. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magn Reson Imaging. 2011;29:701–11. doi: 10.1016/j.mri.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 58.Phan KL, Orlichenko A, Boyd E, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66:691–4. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9:585–94. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 60.Larson CL, Schaefer HS, Siegle GJ, et al. Fear is fast in phobic individuals: amygdala activation in response to fear-relevant stimuli. Biol Psychiatry. 2006;60:410–7. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 61.Furmark T, Tillfors M, Garpenstrand H, et al. Serotonin trans-porter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neurosci Lett. 2004;362:189–92. doi: 10.1016/j.neulet.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 62.Domschke K, Dannlowski U. Imaging genetics of anxiety disorders. Neuroimage. 2010;53:822–31. doi: 10.1016/j.neuroimage.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 63.Cooney RE, Atlas LY, Joormann J, et al. Amygdala activation in the processing of neutral faces in social anxiety disorder: Is neutral really neutral? Psychiatry Res. 2006;148:55–9. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Hofer A, Siedentopf CM, Ischebeck A, et al. Sex differences in brain activation patterns during processing of positively and negatively valenced emotional words. Psychol Med. 2007;37:109–19. doi: 10.1017/S0033291706008919. [DOI] [PubMed] [Google Scholar]
- 65.Goldstein JM, Jerram M, Poldrack R, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–16. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lissek S, Powers AS, McClure EB, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005;43:1391–424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]