Abstract
Objectives
Recent positron emission tomography studies of cerebral glucose metabolism have identified the functional neural circuitry associated with mood and cognitive responses to antidepressant treatment in late life depression (LLD). The structural alterations in these networks are not well understood. The present study used magnetic resonance (MR) imaging and voxel-based morphometry (VBM) to evaluate the association between grey matter volumes and changes in mood symptoms and cognitive function with treatment with the antidepressant citalopram.
Design
Open label trial with baseline brain MR scan. Mood and cognitive assessments performed at baseline and during citalopram treatment.
Setting
Outpatient clinics of an academic medical center.
Participants
17 previously unmedicated patients age 55 or older with a major depressive episode and 17 non-depressed comparison subjects.
Intervention
12 week trial of flexibly dosed citalopram.
Measurements
Grey matter volumes, Hamilton Depression Rating Scale, California Verbal Learning Test, Delis–Kaplan Executive Function System™.
Results
In LLD, higher grey matter volumes in the cingulate gyrus, superior and middle frontal gyri, middle temporal gyrus and precuneus was associated with greater mood improvement. Higher grey matter volumes in primarily frontal areas were associated with greater improvement in verbal memory and verbal fluency performance.
Conclusions
Associations with antidepressant induced improvements in mood and cognition were observed in several brain regions previously correlated with normalization of glucose metabolism after citalopram treatment in LLD. Future studies will investigate molecular mechanisms underlying these associations (e.g. beta-amyloid, inflammation, glutamate).
Keywords: late-life depression, magnetic resonance imaging, voxel-based morphometry, citalopram, cognition
Objective
Late-life depression (LLD) has a substantial public health impact given its association with serious disability, completed suicide, and mortality in medically ill elderly.(1, 2) Despite effective antidepressant agents, developed for younger people, more than half of patients with LLD respond only partially or are refractory to medical intervention.(3) Cognitive impairment is a frequent feature of LLD and often persists after improvement in mood symptoms.(4) Cognitive domains consistently affected in LLD include attention, executive function, memory and speed of processing.(5, 6) In LLD patients with remission of symptoms, recent studies demonstrate persisting cognitive deficits that are of sufficient severity to meet criteria for mild cognitive impairment (MCI) or Alzheimer’s dementia (AD).(7) A study of LLD patients evaluated prior to treatment and a year later (when in remission) showed that 45% were cognitively impaired despite remission. The majority of patients (94%) who were impaired at baseline remained impaired, while 23% of cognitively normal patients at baseline developed impairment 1 year later.(4) Furthermore, depression increases risk of conversion from normal aging to MCI, as well as from MCI to AD. (8, 9)
The mechanisms of cognitive impairment in LLD are poorly understood. Ultimately, understanding these mechanisms may allow identification of individuals with LLD at increased risk for subsequent cognitive decline and identification of treatment targets to prevent or delay transition to dementia.(10) MR imaging studies have reported volumetric differences between LLD patients and comparison subjects in some, but not all studies (as recently reviewed in (11, 12)). Consistent findings in LLD include decreased volumes in the hippocampus and orbitofrontal cortex. Decreased volumes in the basal ganglia and anterior cingulate cortex are also reported, though not consistently among all studies. With respect to methodological approaches, hypothesis-driven region of interest (ROI) and data driven voxel-based morphometry (VBM) methods have identified similar patterns of structural changes in LLD (11).
Despite growing knowledge regarding structural brain changes in LLD, the relationship between these volumetric changes and the mood and cognitive responses to antidepressant treatment are not well understood. The logical first step to identify the mechanisms underlying cognitive impairment in LLD is to identify the neural circuits that are associated with changes in cognition with antidepressant treatment so that specific mechanistic hypotheses can be generated.
To evaluate the neural circuitry underlying the mood and cognitive response to antidepressant treatment in LLD, a prior study utilized functional connectivity analysis of positron emission tomography (PET) studies of cerebral glucose metabolism performed before and during antidepressant treatment.(13) This study identified two orthogonal networks associated with the mood and cognitive response to antidepressant treatment. A subcortical-limbic-frontal network was associated with improvement in mood symptoms and a medial temporal-parietal-frontal network was associated with improvement in cognition (verbal memory and verbal fluency). While cerebral glucose metabolism is a final common pathway of neural activity, a remaining question is whether there are structural alterations underlying the metabolic effects observed.
Previous studies suggest that antidepressant medication may be related to volumetric changes in LLD in components of the subcortical-limbic-frontal network (as reviewed by (14)). For example, Gunning et al. reported smaller dorsal and rostral anterior cingulate grey matter volumes using a ROI method in LLD patients who did not remit after a course of escitalopram treatment compared to those who did remit.(15) Hsieh et al. reported that LLD patients with small right and total hippocampal volumes were less likely to achieve remission after 12 weeks of treatment.(16)
The present study focused on evaluating the relationship between regional variation in grey matter volumes and the antidepressant response to mood and cognitive symptoms in LLD. The goals of the study were: 1) To apply VBM in brain structural magnetic resonance (MR) imaging to evaluate grey matter volumetric differences in LLD patients relative to comparison subjects; and 2) To evaluate the association between changes in mood symptoms and cognitive function after a course of antidepressant treatment with pre-treatment volumes. We hypothesized that: 1) Greater improvement in mood symptoms would be associated with higher brain volumes in the previously identified subcortical-limbic-frontal network including anterior cingulate, superior frontal, middle frontal and insular regions; and 2) Greater improvement in cognition would be associated with higher brain volumes in the previously identified medial temporal-parietal-frontal network including parahippcampal, temporal and parietal regions.
Methods
Subject Screening and Citalopram Treatment
We recruited participants from the outpatient geriatric psychiatry clinics of the Johns Hopkins Bayview Medical Center, as well as through advertisements in the community. After a complete description of the study to potential participants, we obtained written informed consent according to procedures established by the Institutional Review Board and the Radiation Safety Committee of the Johns Hopkins University School of Medicine.
LLD patients and non-depressed comparison subjects underwent psychiatric evaluation by a board-certified geriatric psychiatrist (CMM), structured clinical interview using the SCID (17), and laboratory testing (including complete blood counts, comprehensive metabolic panel, thyroid function tests, vitamin B12 level, folic acid level, and toxicology screen). We utilized the 17-item Hamilton Depression Rating Scale (Ham-D) to assess depressive symptoms.(18)
LLD patients met DSM-IV criteria for a current major depressive episode (non-bipolar, non-psychotic) with a Ham-D score of ≥ 15. Comparison subjects had no history of DSM-IV Axis I psychiatric diagnosis. Exclusion criteria included: neurological diagnosis (including stroke), other past or current Axis I psychiatric diagnosis (including substance abuse), and lack of medical stability (including diabetes mellitus requiring insulin or uncontrolled hypertension). Neither patients nor comparison participants took any psychotropic medications at time of study entry.
After undergoing an MR scan and neuropsychological testing, LLD patients began treatment with flexibly dosed citalopram at a starting dose of 10mg daily under supervision of the study psychiatrist. LLD patients met with the study psychiatrist approximately once every 2 weeks for medication evaluation and administration of the Ham-D. LLD patients continued with citalopram treatment in the study for twelve weeks to allow adequate time to assess treatment response.
MR Imaging Procedures
MR images of the brain were acquired using a Phillips 3.0T Achieva MRI instrument with an 8 channel head coil (Philips Medical Systems, Best, Netherlands). The magnetization-prepared rapid acquisition with gradient echo (MPRAGE) pulse sequence was used for volumetric analyses (TE = 4, TR = 8.9, flip angle = 8 degrees, NSA = 1, n 0.7mm slice thickness).(19)
Neuropsychological Testing
The patients underwent neuropsychological testing prior to initiating citalopram treatment and again at 8-12 weeks after starting treatment (after achieving a stable clinical response for at least 2 weeks based on the Ham-D score). In the present study, the Mini-Mental State Examination (MMSE) (20) is reported as a measure of global cognitive function. The neuropsychological battery included tests of executive function, attention, verbal and visuo-spatial memory and decision making (data not shown). The majority of the tests administered did not show significant differences between patients or comparison subjects or changes with treatment in the patients. The analyses in the present study included two tests which are widely used in LLD research, the California Verbal Learning Test (CVLT) (21) and a measure of verbal (letter) fluency, the letter fluency score of the Delis–Kaplan Executive Function System™ (D-KEFS).(22) Based on prior studies, these measures demonstrate deficits in LLD patients which improve with antidepressant treatment and correlate with cerebral metabolic networks that were altered by citalopram treatment (5, 6) For the CVLT, the parameter used for analysis was the immediate verbal learning/memory scale (sum total of the words recalled without perseverations and intrusions in the first 5 trials of the CVLT). For the letter fluency task, the total number of words recalled with three letters was used.
Data and Image Analysis
We performed a VBM analysis using Statistical Parametric Mapping version 8 (SPM8; Institute of Neurology, London) and MATLAB 7.10 (MathWorks, Natick, Massachusetts) using the following steps: 1) MPRAGE images were manually aligned to the anterior commissure. 2) Images were segmented into grey matter, white matter, and cerebrospinal fluid using the unified segmentation model.(23) 3) Nonlinear deformations to a grey matter template generated through iterative registration of individual grey matter images to a group average were estimated using the “Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra” (DARTEL) algorithm.(24) 4) A nonlinear transformation was estimated for the grey matter template generated in the previous step to normalize to Montreal Neurological Institute (MNI) space. 5) Individual grey matter images were normalized to MNI space using deformations estimated in steps 4 and 5, modulated to preserve tissue volumes, and smoothed with an isotropic Gaussian kernel (8mm full width at half maximum for all directions).
Statistical analyses were performed in SPM8 using the smoothed, modulated (tissue volume), and normalized grey matter images. To correct for individual differences in total brain volume, grey matter images were normalized (proportional scaling) using total intracranial volume computed for each subject in SPM8. First, a two-sample t-test was performed to evaluate between-group (LLD patients/comparison subjects) differences in regional grey matter volumes. Second, in LLD patients, one-sample t-tests were performed to evaluate for regional grey volumes whether the covariate of mood and cognitive variables (change with pre and post treatment scores for Ham-D, CVLT sum of trials 1 – 5, and D-KEFS letter fluency) contributed significant variance to the grey matter volume measures when considering other covariates (total intracranial volume and the baseline score for the respective measure). The comparisons were considered significant at a t threshold greater than 3.51 (z > 2.98, p < 0.003; uncorrected for multiple independent comparisons) and a cluster size greater than 50 voxels.
Results
Seventeen LLD patients and 17 comparison subjects enrolled in the study. Table 1 shows participant characteristics. Groups did not differ significantly in age, level of education, MMSE score, total CVLT score (baseline or follow-up), or D-KEFS letter fluency score (baseline or follow-up), although, as expected, the pre-treatment HAM-D score was significantly greater in the patients than comparison subjects (t = -25.282, df = 32, p < 0.0005). Furthermore, we observed a significant change in Ham-D score between pre- and post-treatment in LLD patients (t = -22.490, df = 16, p < 0.0005). Eight of the 17 patients had no prior treatment with antidepressant medication. Of the 9 previously treated patients, no patient received treatment within 6 months of the study. All subjects had a negative urine toxicology screen for psychotropic drugs or supplements. All participants successfully completed the study including follow-up testing. The mean Ham-D score in LLD patients decreased from 17.2 at study entry to 3.8 at study completion. All 17 patients met criteria for treatment response (defined by a 50% reduction from baseline Ham-D and final Ham-D score ≤ 10). Mean citalopram dose was 21.8mg/day (range 10-40mg/day).
Table 1.
Characteristics of late-life depression (LLD) patients and comparison subjects (mean ± standard deviations [range])
| LLD Patients (n = 17) | Comparison Subjects (n = 17) | |
|---|---|---|
| Age | 66.9 ± 6.4 | 66.0 ± 7.9 |
| Sex (M/F) | 7 / 10 | 9 / 8 |
| Years of Education | 15.1 ± 2.4 | 15.4 ± 2.8 |
| MMSE | 28.8 ± 0.7 | 28.2 ± 1.9 |
| Final Citalopram Dose (mg) | 21.8 ± 8.8 (10 to 40) | |
| Ham-D (Baseline) 1 | 17.2 ± 2.0 | 1.2 ± 1.7 |
| Ham-D (Follow-Up) 2 | 3.8 ± 2.2 | |
| Ham-D Change | −13.4 ± 2.4 (−17 to −8) | |
| Total CVLT Score (Baseline) | 56.7 ± 9.3 | 54.8 ± 9.3 |
| Total CVLT Score (Follow-Up) | 56.4 ± 10.7 | 59.3 ± 11.9 |
| Total CVLT Score Change | −0.4 ± 6.9 (−13 to 10) | 3.9 ± 7.4 (−10 to 14) |
| D-KEFS Letter Fluency (Baseline) | 41.0 ± 11.5 | 42.8 ± 12.2 |
| D-KEFS Letter Fluency (Follow-Up) | 45.2 ± 11.0 | 45.3 ± 11.6 |
| D-KEFS Letter Fluency Change | 4.2 ± 8.2 (−11 to 19) | 0.2 ± 6.9 (−9 to 11) |
p < 0.0005 between untreated LLD patients and comparison subjects at baseline
p < 0.0005 between pre- (untreated) and post-treatment in LLD patients
Ham-D = Hamilton Depression Rating Scale
CVLT= California Verbal Learning Test
D-KEFS = Delis–Kaplan Executive Function System™
The results of the VBM analyses of MR volumetric data and the association between the volumetric data with mood and cognitive outcomes appear in Tables 2-4 The comparison of the LLD patients to comparison subjects did not reveal any significant volumetric differences between groups.
Table 2.
Relationship between grey matter volumes and Hamilton Depression Rating Scale (Ham-D) in late-life depression patients treated with citalopram
| LEFT | Brain Region | RIGHT | ||||||
|---|---|---|---|---|---|---|---|---|
| X (mm) | Y (mm) |
Z (mm) | Z-Score | X (mm) |
Y (mm) |
Z (mm) |
Z-Score | |
| Decrease in Ham-D score with treatment associated with smaller grey matter volumes (p < 0.01) | ||||||||
| Caudate | 9 | 11 | 14 | 2.15 | ||||
| Decrease in Ham-D score with treatment associated with larger grey matter volumes (p < 0.01) | ||||||||
| −2 | −28 | 41 | 3.05 | Cingulate Gyrus (BA 31) | 6 | −30 | 37 | 3.04 |
| −32 | 42 | 31 | 3.24 | Superior Frontal Gyrus (BA 9/8) | 15 | 42 | 44 | 3.62 |
| Middle Frontal Gyrus (BA 6) | 38 | −3 | 47 | 3.13 | ||||
| Middle Frontal Gyrus (BA 46) | 43 | 36 | 17 | 3.27 | ||||
| −29 | 43 | 19 | 3 | Middle Frontal Gyrus (BA 10) | ||||
| −56 | −17 | −4 | 3.04 | Middle Temporal Gyrus (BA 21) | 55 | −26 | −12 | 3.23 |
| Precuneus (BA 7) | 1 | −37 | 44 | 3.7 | ||||
Table 4.
Relationship between grey matter volumes and Delis–Kaplan Executive Function System™ (D-KEFS) Letter Fluency score in late-life depression patients treated with citalopram
| LEFT | Brain Region | RIGHT | ||||||
|---|---|---|---|---|---|---|---|---|
| X (mm) | Y (mm) | Z (mm) | Z-Score | X (mm) | Y (mm) | Z (mm) | Z-Score | |
| Improvement in D-KEFS Letter Fluency score with treatment associated with smaller grey matter volumes (p < 0.01) | ||||||||
| −10 | −52 | 40 | 4.69 | Precuneus (BA 7) | 6 | −48 | 56 | 3.45 |
| Improvement in D-KEFS Letter Fluency score with treatment is associated with larger grey matter volumes (p < 0.01) | ||||||||
| Superior Frontal Gyrus (BA 8) | 17 | 46 | 39 | 3.27 | ||||
| Middle Frontal Gyrus (BA 10) | 27 | 51 | 7 | 3.11 | ||||
| −20 | −62 | −6 | 3.07 | Fusiform Gyrus (BA 19) | ||||
| −6 | −63 | −5 | 3.42 | Cerebellum (Culmen) | ||||
Relationship between change in Ham-D score and grey matter volumes in LLD patients (Table 2)
Greater decreases in Ham-D score with treatment was associated with smaller grey matter volumes in the right caudate. Greater decreases in Ham-D score with treatment was associated with larger grey matter volumes in the bilateral cingulate gyrus (BA 31), bilateral superior frontal gyrus (BA 9/8), right middle frontal gyrus (BA 6, BA 46), left middle frontal gyrus (BA10), bilateral middle temporal gyrus (BA21), and right precuneus (BA7). These results, superimposed on a three-dimensional MR rendered brain template are shown graphically in figure 1.
Figure 1.
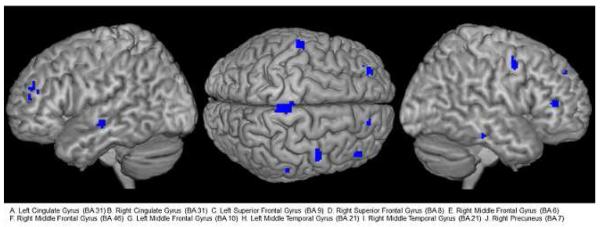
Brain regions where greater decreases in Ham-D score with treatment was associated with larger grey matter volumes in late-life depression patients (Results superimposed on a three dimensional MR rendered brain template)
Relationship between change in CVLT score and grey matter volumes in LLD patients (Table 3)
Table 3.
Relationship between grey matter volumes and total California Verbal Learning Test (CVLT) score (trials 1 – 5) in late-life depression patients treated with citalopram
| LEFT | Brain Region | RIGHT | ||||||
|---|---|---|---|---|---|---|---|---|
| X (mm) | Y (mm) | Z (mm) | Z-Score | X (mm) | Y (mm) | Z (mm) | Z-Score | |
| Improvement in total CVLT score with treatment associated with smaller grey matter volumes (p < 0.01) | ||||||||
| −12 | 42 | 45 | 3.63 | Superior Frontal Gyrus (BA 8) | 7 | 20 | 52 | 3.01 |
| Improvement in total CVLT score with treatment associated with larger grey matter volumes (p < 0.01) | ||||||||
| −42 | 11 | 32 | 4.36 | Middle Frontal Gyrus (BA 9) | ||||
| −40 | 38 | 1 | 3.14 | Inferior Frontal Gyrus (BA 46) | ||||
| Superior Temporal Gyrus (BA 38) | 52 | −24 | 5 | 3.38 | ||||
| Uncus (BA 20) | 33 | −16 | −29 | 3.01 | ||||
| −42 | −70 | −14 | 3.14 | Fusiform Gyrus (BA 37) | 48 | −59 | −11 | 3.59 |
| Angular Gyrus (BA 39) | 48 | −61 | 34 | 3.47 | ||||
| Lingual Gyrus (BA 18) | 4 | −85 | −8 | 4.03 | ||||
Greater improvement in CVLT score with treatment was associated with smaller grey matter volumes in the bilateral superior frontal gyrus (BA 8). Greater improvement in CVLT score with treatment was associated with larger grey matter volumes in the left middle frontal gyrus (BA 9), left inferior frontal gyrus (BA 46), right superior temporal gyrus (BA 38), right uncus (BA 20), bilateral fusiform gyrus (BA 39), right angular gyrus (BA 39), and right lingual gyrus (BA 18).
Relationship between change in D-KEFS letter fluency score and grey matter volumes in LLD patients (Table 4)
Greater improvement in D-KEFS letter fluency score with treatment was associated with smaller grey matter volumes in the bilateral precuneus (BA 7). Greater improvement in D-KEFS letter fluency score with treatment was associated with larger grey matter volumes in the right superior frontal gyrus (BA 8), right middle frontal gyrus (BA 10), left fusiform gyrus (BA 19), and left cerebellum (culmen).
Conclusions
The primary findings of the study are that difference in grey matter volumes are not observed in this sample of patients relative to comparison subjects. Greater improvement in depressive symptoms and cognitive function (episodic verbal memory and verbal fluency) were associated with larger grey matter volumes. While the LLD patients did not differ significantly as a group in pre- and post-treatment cognitive function, associations between improvement in cognition and larger grey matter volumes was observed. The specific frontal, temporal and parietal cortical regions implicated in the structural analyses are a subset of regions shown by cerebral glucose metabolism studies to be associated with improvement of depressive symptoms and cognitive function. The unique aspects of this preliminary study are that associations between changes in both mood and cognitive function after a course of antidepressant treatment and regional differences in brain grey matter volume are reported. The study also includes a non-depressed comparison group.
Several studies using ROI analysis comparing depressed patients to non-depressed comparison subjects report decreased regional brain volumes in areas including the orbitofrontal cortex, as well as the anterior cingulate gyrus, caudate and hippocampus.(25-28) However, of note, other ROI studies observe no regional volume differences between LLD patients and comparison subjects, consistent with the present study (e.g.(29, 30)). VBM studies are more limited. Bell-McGinty et al. observed decreased right hippocampal and bilateral middle frontal volumes in LLD patients compared to non-depressed subjects.(31) Egger et al. found decreased volumes in the right rostral hippocampus, right amygdala, and right medial orbitofrontal cortex.(32)
Yuan et al. reported decreased volumes in the right superior frontal cortex, left postcentral cortex and right middle temporal gyrus in remitted LLD patients versus comparison subjects with increased left cingulate volumes in the depressed patients.(33) Hwang et al. reported decreased insula and posterior cingulate volumes in hospitalized men with late-onset depression.(34) Interestingly in the present study, despite the lack of grey matter volume difference between the LLD and comparison subjects, larger frontal volumes including orbitofrontal cortex are associated with mood and cognitive response to citalopram treatment. Thus, the LLD patients with more “normal” brains may be the most likely to benefit from treatment.
While the limitations of older versions of the VBM method have been reviewed (e.g.(35)), the use of the DARTEL method in the present study increases the accuracy of inter-subject alignment, which is one of the major issues with the use of VBM. Similar to the present results, Colloby et al., using VBM-DARTEL, reported that grey matter volumes were indistinguishable when comparing 38 older subjects with a lifetime history of major depression with 30 healthy comparison subjects, though significant age effects were observed independent of diagnosis. Unlike the present study, a current major depressive episode was not an enrollment requirement for this study.(36) The absence of regional volume differences between LLD patients and comparison subjects in the present study may be due to the relative “health” of the enrolled LLD patients who did not meet criteria for MCI or AD and responded to selective serotonin reuptake inhibitor treatment.
Our findings are particularly interesting in light of functional neuroimaging studies evaluating cerebral glucose metabolism in LLD. Smith et al reported elevated cerebral glucose metabolism in untreated LLD patients in frontal regions (right and left superior frontal gyrus) relative to non-depressed comparison subjects.(37) Diaconescu et al reported decreased cerebral glucose metabolism during citalopram treatment for LLD in the anterior cingulate gyrus (BA 32), middle temporal gyrus, precuneus, amygdala, and parahippocampal gyrus, as well as increased metabolism in the putamen, occipital cortex, and cerebellum.(13) Using a functional connectivity analysis, the authors reported 2 distinct networks associated with treatment response, a subcortical-limbic-frontal network associated with improvement in mood symptoms and a medial temporal-parietal-frontal network associated with improvement in cognitive (verbal memory and verbal fluency) symptoms. Many of the regions in which an association between mood symptom improvement and higher grey matter volumes was observed in the present study overlap with the regions of the proposed subcortical-limbic-frontal mood network. Furthermore, many of the regions in which an association between improvement in verbal memory and verbal fluency (via CVLT and D-KEFS Letter Fluency respectively) and higher grey matter volumes was observed overlap with regions of the proposed medial temporal-parietal-frontal cognitive network. Improvement in CVLT score was more strongly associated with higher grey matter volumes in temporal regions than the D-KEFS Letter Fluency score. Improvement in D-KEFS Letter Fluency score was more strongly associated with higher grey matter volumes in frontal and parietal regions. Thus, both functional and structural imaging methods have shown consistent abnormalities in regions that comprise cortico-cortico and cortico-limbic pathways. Of note, these differences were observed despite the lack of volumetric and cognitive differences between the LLD patients and comparison subjects.
The finding of an association between lower brain volumes in several regions and better treatment response is admittedly counterintuitive. Lower caudate volumes were associated with greater improvement of depressive symptoms, while lower volumes of superior frontal gyrus (bilaterally) and precuneus (bilaterally) were associated with greater improvement in verbal memory and verbal fluency performance, respectively. While the neurobiological mechanisms underlying these changes in brain structure cannot be inferred from the MR imaging data, it is possible that the finding may represent a compensatory change in response to preserved or increased volumes in other components of these neural networks, as had been suggested in AD.(38)
The study limitations include the open label treatment aspect of the study design and the small sample size that did not allow us to evaluate the role of genetic polymorphisms. Genetic polymorphisms of the APOE4 allele or the brain-derived neurotrophic factor (val66met polymorphism), for example, could contribute to differences in brain structure or cognition. (39, 40) The results should be regarded as preliminary due to the relatively small sample size of the study.
In conclusion, brain morphological differences that were associated with mood and cognitive outcomes after citalopram treatment were observed in several similar regions that have been shown in a separate study to comprise the functional neural circuits associated with these outcomes after citalopram treatment.(13) The metabolic networks were in a more extensive group of brain regions than the structural changes. Future studies will incorporate multi-modality imaging methods with direct measures of Alzheimer pathology (such as brain beta-amyloid), vascular pathology (such as MR white matter hyperintensities and arterial spin labeling measures of cerebral blood flow), and potential molecular mechanisms (such as inflammation and glutamate) to help understand the mechanisms linking LLD and cognitive impairment and to develop treatments to prevent or delay transition to dementia.
Acknowledgments
The authors gratefully acknowledge Terri Brawner, Ivana Kusevic, and Kathy Kahl for their contribution to the acquisition of the MR data. The authors also acknowledge the constructive input of the peer reviewers.
Supported in part by National Institute of Health/ National Institute of Mental Health: MH 086881, MH 01621, MH 64823, MH 095971 and UL1 TR 000424.
Footnotes
Conflicts of Interest and Source of Funding: Dr. Smith has received research funding from Pfizer. For the remaining authors, none were declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexopoulos GS, Vrontou C, Kakuma T, et al. Disability in geriatric depression. Am J Psychiatry. 1996;153:877–85. doi: 10.1176/ajp.153.7.877. [DOI] [PubMed] [Google Scholar]
- 2.Conwell Y, Duberstein PR, Cox C, et al. Relationships of age and axis I diagnoses in victims of completed suicide: a psychological autopsy study. Am J Psychiatry. 1996;153:1001–8. doi: 10.1176/ajp.153.8.1001. [DOI] [PubMed] [Google Scholar]
- 3.Dew MA, Reynolds CF, Houck PR, et al. Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry. 1997;54:1016–1024. doi: 10.1001/archpsyc.1997.01830230050007. [DOI] [PubMed] [Google Scholar]
- 4.Bhalla RK, Butters MA, Mulsant BH, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14:419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 5.Kramer-Ginsberg E, Greenwald BS, Krishnan KR, et al. Neuropsychological functioning and MRI signal hyperintensities in geriatric depression. Am J Psychiatry. 1999;156:438–444. doi: 10.1176/ajp.156.3.438. [DOI] [PubMed] [Google Scholar]
- 6.Lockwood KA, Alexopoulos GS, Kakuma T, et al. Subtypes of cognitive impairment in depressed older adults. Am J Geriatr Psychiatry. 2000;8:201–208. [PubMed] [Google Scholar]
- 7.Bhalla RK, Butters MA, Becker JT, et al. Patterns of mild cognitive impairment after treatment of depression in the elderly. Am J Geriatr Psychiatry. 2009;17:308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 9.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63:435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 10.Smith GS, Gunning-Dixon FM, Lotrich FE, et al. Translational research in late-life mood disorders: implications for future intervention and prevention research. Neuropsychopharmacology. 2007;32:1857–1875. doi: 10.1038/sj.npp.1301333. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin S, Steffens DC. Structural neuroimaging of geriatric depression. Psychiatr Clin North Am. 2011;34:423–35. ix. doi: 10.1016/j.psc.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sexton CE, Mackay CE, Ebmeier KP. A Systematic Review and Meta-Analysis of Magnetic Resonance Imaging Studies in Late-Life Depression. Am J Geriatr Psychiatry. 2012 doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Diaconescu AO, Kramer E, Hermann C, et al. Distinct functional networks associated with improvement of affective symptoms and cognitive function during citalopram treatment in geriatric depression. Hum Brain Mapp. 2011;32:1677–1691. doi: 10.1002/hbm.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naismith SL, Norrie LM, Mowszowski L, et al. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Prog Neurobiol. 2012;98:99–143. doi: 10.1016/j.pneurobio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Gunning FM, Cheng J, Murphy CF, et al. Anterior cingulate cortical volumes and treatment remission of geriatric depression. Int J Geriatr Psychiatry. 2009;24:829–836. doi: 10.1002/gps.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh MH, McQuoid DR, Levy RM, et al. Hippocampal volume and antidepressant response in geriatric depression. Int J Geriatr Psychiatry. 2002;17:519–525. doi: 10.1002/gps.611. [DOI] [PubMed] [Google Scholar]
- 17.First M, Spitzer R, Williams J, et al. Structured Clinical Interview for DSM-IV-Non-Patient Edition. SCID-NP, Version 1.0 American Psychiatric Press; Washington, D.C.: 1995. [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mugler JP, 3rd, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE) Magn Reson Med. 1990;15:152–157. doi: 10.1002/mrm.1910150117. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Delis DC, Freeland J, Kramer JH, et al. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. 1988;56:123–130. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- 22.Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System: Examiner’s Manual. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- 23.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Alexopoulos GS, Gunning-Dixon FM, Latoussakis V, et al. Anterior cingulate dysfunction in geriatric depression. Int J Geriatr Psychiatry. 2008;23:347–355. doi: 10.1002/gps.1939. [DOI] [PubMed] [Google Scholar]
- 26.Lai T, Payne ME, Byrum CE, et al. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan KR, McDonald WM, Escalona PR, et al. Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Arch Gen Psychiatry. 1992;49:553–557. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- 28.Steffens DC, Byrum CE, McQuoid DR, et al. Hippocampal volume in geriatric depression. Biol Psychiatry. 2000;48:301–309. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 29.Pantel J, Schroder J, Essig M, et al. Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord. 1997;42:69–83. doi: 10.1016/s0165-0327(96)00105-x. [DOI] [PubMed] [Google Scholar]
- 30.Ashtari M, Greenwald BS, Kramer-Ginsberg E, et al. Hippocampal/amygdala volumes in geriatric depression. Psychol Med. 1999;29:629–638. doi: 10.1017/s0033291799008405. [DOI] [PubMed] [Google Scholar]
- 31.Bell-McGinty S, Butters MA, Meltzer CC, et al. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159:1424–1427. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 32.Egger K, Schocke M, Weiss E, et al. Pattern of brain atrophy in elderly patients with depression revealed by voxel-based morphometry. Psychiatry Res. 2008;164:237–244. doi: 10.1016/j.pscychresns.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Y, Zhu W, Zhang Z, et al. Regional gray matter changes are associated with cognitive deficits in remitted geriatric depression: an optimized voxel-based morphometry study. Biol Psychiatry. 2008;64:541–544. doi: 10.1016/j.biopsych.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 34.Hwang JP, Lee TW, Tsai SJ, et al. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol. 2010;23:171–184. doi: 10.1177/0891988710363713. [DOI] [PubMed] [Google Scholar]
- 35.Hoptman MJ, Gunning-Dixon FM, Murphy CF, et al. Structural neuroimaging research methods in geriatric depression. Am J Geriatr Psychiatry. 2006;14:812–822. doi: 10.1097/01.JGP.0000238588.34205.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colloby SJ, Firbank MJ, Vasudev A, et al. Cortical thickness and VBM-DARTEL in late-life depression. J Affect Disord. 2011;133:158–164. doi: 10.1016/j.jad.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Smith GS, Kramer E, Ma Y, et al. The functional neuroanatomy of geriatric depression. Int J Geriatr Psychiatry. 2009;24:798–808. doi: 10.1002/gps.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seeley WW, Crawford RK, Zhou J, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Y, Zhang Z, Bai F, et al. Genetic variation in apolipoprotein E alters regional gray matter volumes in remitted late-onset depression. J Affect Disord. 2010;121:273–277. doi: 10.1016/j.jad.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Kanellopoulos D, Gunning FM, Morimoto SS, et al. Hippocampal volumes and the brain-derived neurotrophic factor val66met polymorphism in geriatric major depression. Am J Geriatr Psychiatry. 2011;19:13–22. doi: 10.1097/jgp.0b013e3181f61d62. [DOI] [PMC free article] [PubMed] [Google Scholar]


