Abstract
Congenital abnormalities of the kidney and urinary tract (CAKUT) account for approximately half of children with chronic kidney disease and they are the most frequent cause of end-stage renal disease in children in the US. However, its genetic etiology remains mostly elusive. VACTERL association is a rare disorder that involves congenital abnormalities in multiple organs including the kidney and urinary tract in up to 60% of the cases. By homozygosity mapping and whole exome resequencing combined with high-throughput mutation analysis by array-based multiplex PCR and next-generation sequencing, we identified recessive mutations in the gene TNF receptor-associated protein 1 (TRAP1) in two families with isolated CAKUT and three families with VACTERL association. TRAP1 is a heat shock protein 90-related mitochondrial chaperone possibly involved in antiapoptotic and endoplasmic reticulum-stress signaling. Trap1 is expressed in renal epithelia of developing mouse kidney E13.5 and in the kidney of adult rats, most prominently in proximal tubules and in thick medullary ascending limbs of Henle’s loop. Thus, we identified mutations in TRAP1 as highly likely causing CAKUT or CAKUT in VACTERL association.
Introduction
Congenital abnormalities of the kidney and urinary tract (CAKUT) occur in 3–6 per 1,000 live births. CAKUT are the most frequent cause for chronic kidney disease in children (~50%)1, 2 in the US. The acronym “CAKUT” comprises heterogeneous malformations involving the kidney (e.g. renal agenesis, hypodysplasia), and the urinary tract (e.g. vesicoureteral reflux, ureteropelvic junction obstruction)3. These congenital anomalies are related because a part of their pathogenesis is an impaired co-development of nephrogenic tissues derived from the metanephric mesenchyme and the ureteric bud4. Twenty monogenic causes of isolated CAKUT in humans have been published to date as reviewed recently by Yosypiv5. However, they only account for ~10% - 20% of all cases indicating a broad genetic heterogeneity of CAKUT. A recent study on copy number variations (CNVs) in a large cohort of individuals with CAKUT and two publications identifying novel monogenic causes of CAKUT bring further evidence that CAKUT is a condition of extensive genetic heterogeneity6–8. CAKUT most frequently occur isolated, but might be associated with extra-renal phenotypes, for instance with VACTERL association (MIM [#192350]). The acronym “VACTERL” describes the combination of at least three of the following congenital anomalies: vertebral defects (V), anorectal malformations (A), cardiac defects (C), tracheoesophageal fistula with or without esophageal atresia (TE), renal malformations (R), and limb defects (L). VACTERL association is a rare disease that occurs mostly sporadic in 1/10,000–40,000 live births9. Its etiology is enigmatic, although animal models suggest an involvement of Sonic hedgehog signaling10. In humans, ZIC3 mutations are the cause of a closely related non-classic VACTERL condition (VACTERL-X, MIM [#314390])11, 12. Additionally, there are six case reports published of individuals with VACTERL association in conjunction with mitochondrial dysfunction as summarized recently by Siebel and Solomon13. In order to identify new recessive genes that cause isolated CAKUT or CAKUT in VACTERL association, we performed homozygosity mapping and whole exome resequencing in 24 affected individuals with CAKUT from 16 families, and in 4 individuals with CAKUT in VACTERL.
Results
Whole exome resequencing identifies a homozygous mutation in TRAP1 in CAKUT and in VACTERL association
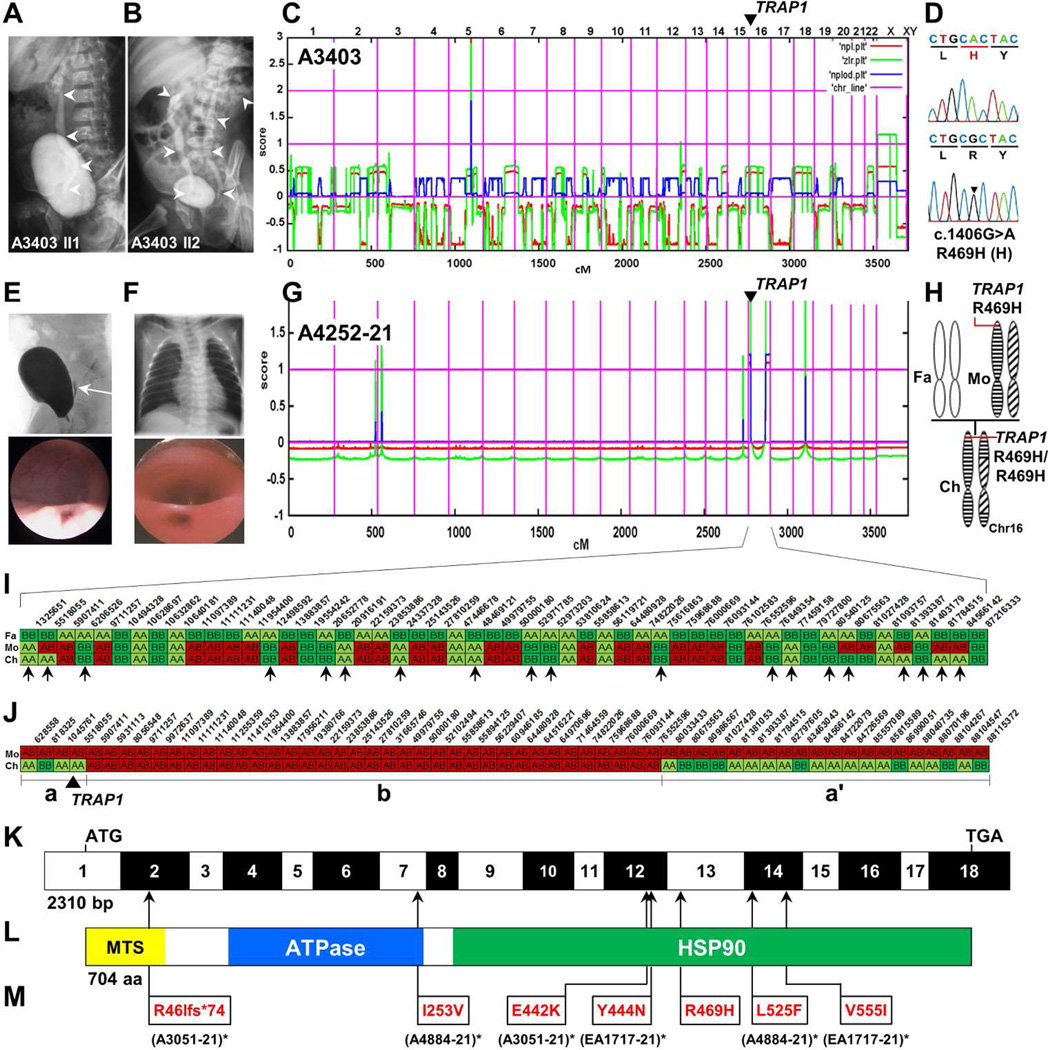
By homozygosity mapping in a family of two sibs (A3403) with unilateral and bilateral vesicoureteral reflux (VUR) III°, respectively (Figure 1A, B and Table 1), we identified a short 5.2 Mb segment of homozygosity on chromosome 5 (Figure 1C), indicating distant consanguinity of the parents. This finding suggested that in this family CAKUT are most likely caused by a homozygous recessive mutation in an unknown CAKUT gene. We performed whole exome resequencing in individual A3403-21 as described previously by the authors14, 15. In order not to miss either a homozygous mutation in a short run of homozygosity or a compound heterozygous mutation (which, as in this case, cannot be excluded a priori in families with remote consanguinity16), we considered variants not only in the homozygosity peak but within regions of genetic linkage for both sibs (coverage ≥ 4; minor variant frequency, MVF ≥ 0.2). Following variant filtering we retained 38 variants in 13 genes for Sanger confirmation and segregation analysis (Supplementary Table S1 online). Only a single homozygous missense mutation (R469H) in the gene TRAP1 on chromosome 16p13.3 survived the variant filtering process and segregation analysis (Figure 1D). This homozygous variant in TRAP1 in A3403-21 and -22 was positioned in a ~1.5 Mb run of apparent homozygosity that was not detected by homozygosity mapping (Figure 1C), because the threshold for detection of “homozygosity peaks” is 2.1 Mb17.
Figure 1. Homozygosity mapping and whole exome resequencing identifies mutations in TRAP1 as causing CAKUT or VACTERL association.
(A, B) Voiding cysturethrograms (VCUG) of CAKUT siblings A3403-21 and -22 showing unilateral vesicoureteral reflux (VUR) grade III and bilateral VUR, respectively (white arrow heads).
(C) Non-parametric LOD (NPL) scores across the human genome in 2 affected sibs. X-axis represents Affymetrix 250k StyI array SNP positions across human chromosomes concatenated from p-terminal (left) to q-terminal (right). Genetic distance is given in cM. A single peak indicates distantly related parents.
(D) Chromatogram of newly identified homozygous missense mutation (arrow head) in the gene encoding TNF receptor-associated protein 1 (TRAP1) over wild type control.
(E) VCUG (upper panel) and cystoscopy (lower panel) demonstrating VUR and a dilated ureteral orifice, respectively.
(F) Chest X-ray (top panel) and esophagoscopy (bottom panel) showing esophageal atresia and esophagotracheal fistula in individual A4252-21 with CAKUT in VACTERL association.
(G) NPL score in an individual A4252-21 with VACTERL association. Two maximum peaks indicate homozygosity at the p-terminus and q-terminus of chromosome 16.
(H) Panel on the right illustrates maternal heterodisomy of chromosome 16 and partial uniparental isodisomy (p-ter and q-ter) of the child (Fa, father; Mo, mother; Ch, child).
(I) Partial haplotypes of selected markers and their physical positions across chromosome 16 in the father (Fa), the mother (Mo), and the affected child (Ch) of CAKUT family A4252. Selected markers (biallelic SNPs; MAF = 0.496 – 0.5) homozygous in the father are shown in green (alleles AA) and light green (alleles BB).
The fact that for 19 of 52 alleles there is paternal non-contribution in the child strongly suggests maternal heterodisomy of chromosome 16. No paternal non-contribution was observed in the child on any other chromosome (data not shown).
(J) Selected markers (biallelic SNPs; MAF = 0.497 – 0.5) heterozygous in the mother (Mo) of family A4252 are shown for alleles coded in red (AB; phase unknown). Note that in the central segment (b), separated by vertical lines, the child’s (Ch) haplotype is identical to the mother’s. In the p-ter (a) and q-ter (a’) segments (a, a’) the child is homozygous, indicating maternal isodisomy in these segments.
(K) Exon structure of human TRAP1 cDNA. Positions of start codon (ATG) and of stop codon (TGA) are indicated.
(L) Domain structure of the TRAP1 protein. HSP, heat shock protein; MTS, mitochondrial targeting sequence.
(M) Translational changes of detected mutations are shown relative to their positions in TRAP1 cDNA (see L) and TRAP1 protein (see M) for affected individuals with CAKUT or CAKUT in VACTERL association with recessive TRAP1 mutations. Family numbers are shown in parenthesis. (* denotes an individual carrying a compound hete)
Table 1.
Mutations of TRAP1 in 5 families with isolated CAKUT or CAKUT in VACTERL association
| Family -Individ. (sex) |
Ethnic origin |
Nucleotide alterationa |
Deduced Protein change |
Continuous amino acid sequence conservation |
MutTb | Poly Phen2c |
SIFTd | MAF in EVSe |
Exon (state; segregation) |
Urinary tract phenotypes |
Other phenotypes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A3403 −21 (F) −22 (F) |
Serbian | c.1406G>A | p.R469H | E. coli (C. elegans has L) | 0.99 | 0.997 | 0.00 | 0.77% | 13 (Hom; Fa, Mo) | −21: VUR-III° R −22: VUR-III° R and L |
None |
| A4252 −21 (F) |
Central Euro-pean | c.1406G>A | p.R469H | E. coli (C. elegans has L) | 0.99 | 0.997 | 0.00 | 0.77% | 13 (Hom; Mo; partial maternal isodisomy) | Double kidney R VUR L | VACTERL association including esophageal atresia IIIb, anal atresia, vestibular fistula |
| A3051 −21 (M) |
Mace-donian | c.127_137dup c.1324G>A |
p.R46Sfs*75 p.E442K |
N/A D. rerio |
N/A 0.99 |
N/A 0.003 |
N/A 0.3 |
absent 0.08% |
2 (het; Mo) 12 (het; Fa) |
MCDK L | None |
| A4884 −21 (F) |
Dutch | c.757A>G c.1573C>T |
p.I253V p.L525F |
E. coli (X. tropicalis has V, S. cerevisiae has L) E. coli |
0.99 0.99 |
0.433 0.942 |
0.00 0.00 |
0.91% absent |
7 (het; Mo) 14 (het; Fa) |
Renal agenesis R | VACTERL association including cervical/thoracic hemivertebrae, 5 dysplastic short ribs R, anal atresia with rectoperineal fistula, ASD type II, esophageal atresia, abnormal position of thumbs |
| EA1717 −21 (F) |
Dutch | c.1330T>A c.1663G>A |
p.Y444N p.V555I |
C. elegans C. intestinalis |
0.99 0.99 |
0.985 0.115 |
0.03 0.39 |
0.91%f absent |
12 (het; Fa) 14 (het; Mo) |
Pyelectasis and VUR L | VACTERL association including anal atresia, esophageal atresia, ASD, VSD, hypoplastic/absent humerus, persistent L vena cava superior, cloaca |
TRAP1 cDNA mutations are numbered according to human cDNA reference sequence NM_016292.2, where +1 corresponds to the A of ATG start translation codon.
MutationTaster score. Range: 0 – 1.0, 1.0 being most deleterious.
PolyPhen2 (HumVar) score. Range: 0 – 1.0, 1.0 being most deleterious.
SIFT score. Range: 0 – 1.0, 0 being most deleterious.
Minor allele frequency in 8,600 alleles of Americans of European descent.
One individual is homozygous for this allele.
The following abbreviations are used: ASD, atrial septum defect; CAKUT, congenital abnormalities of the kidney and urinary tract; F, female; Fa, mutation segregating from the father; L, left; N/A, not applicable; M, male; MCDK, multicystic dysplastic kidney; Mo, mutation segregating from the mother; MutT, MutationTaster; ND, no data; R, right; VSD, ventricular septum defect; VUR-III°, vesicoureteral reflux 3rd degree.
In family A4252 with CAKUT in VACTERL we performed whole exome resequencing in an affected individual (A4252-21). This girl was born with a right double kidney and duplex ureter, left VUR, esophageal atresia type IIIb, and anal atresia with a vestibular fistula (Figure 1E, F and Table 1). Although there was no consanguinity reported in this family, homozygosity mapping showed unusually broad homozygosity peaks on chromosome 16 on the p-terminus and q-terminus (5.5 and 9.6 Mb, respectively) (Figure 1G). In this case, we hypothesized that CAKUT in VACTERL is caused by a homozygous mutation within these homozygous regions. When evaluating whole exome resequencing data in this individual, the 512,733 variants initially detected (MVF ≥ .55; coverage ≥ 2) were reduced to only 11 variants within the “homozygosity peaks” on chromosome 16 and 18 (Supplementary Table S2 online). The only variant that was confirmed by Sanger sequencing and that altered a conserved amino acid residue was TRAP1 R469H, the same allele as in family A3403. By comparison of SNPs in the affected girl and her parents, we demonstrated that partial maternal isodisomy of chromosome 16 with two recombinants (one located on the p-arm and one located on the q-arm) was the underlying cause of homozygosity for TRAP1 R469H (Figure 1G-J).
The TRAP1 allele c.1406G>A, p.R469H alters an evolutionary highly conserved amino acid residue and it is predicted to be deleterious for protein function by publically available software programs (Table 1 and Supplementary Figure S1 online). In the Exome Variant Server (EVS) database, R469H has a minor allele frequency (MAF) of 0.9% in Americans of European descent. In our cohort of 675 individuals with CAKUT, most of them European, the MAF is 1.9%. The three affected individuals from two unrelated families with homozygous TRAP1 R469H, as well as 6 additional heterozygous carriers share haplotypes at the TRAP1 locus (Figure S2 online) which speaks for TRAP1 R469H being a European founder mutation.
Mutation analysis reveals three additional families with TRAP1 mutations
We subsequently analyzed the coding sequence of TRAP1 in a cohort of 675 individuals with isolated CAKUT (Supplementary Table S3 online) and 300 individuals with classic VACTERL association (i.e. VACTERL-X and other related disorders have been excluded) using a barcoded multiplex PCR approach and consecutive next generation sequencing as described previously by the authors18. As a control group, we included 800 individuals with the distinct renal phenotype of nephronophthisis.
We detected six additional recessive mutations in TRAP1 in a compound heterozygous state in three additional unrelated families with CAKUT or CAKUT in VACTERL (Table 1, Figure 1K, L, M, Supplementary Figure S1, and S3 online). In individual A3051-21 with a left-sided multicystic dysplatic kidney (MCDK), we found a maternally inherited protein-truncating frame-shift mutation (c.127_137dup, p.R46fs*75). This mutation abrogates the N-terminal mitochondrial targeting sequence of TRAP1, which makes this a null allele. The second allele was a missense mutation (c.1324G>A, p.E442K) which segregated from the father.
In individual A4884-21 with CAKUT in VACTERL, including right renal agenesis, vertebral malformations, anal atresia with a rectoperineal fistula, atrial septum defect type II, esophageal atresia, and abnormal position of the thumbs (Table 1 and Supplementary Figure S4 online), we detected compound heterozygous missense mutations in TRAP1 located in the ATPase-domain (c.757A>G, p.I253V) and in the HSP90-domain (c.1573C>T, p.L525F) (Figure 1L).
In individual EA1717 with CAKUT in VACTERL, including pyelectasis, left VUR, a complex anorectal malformation including anal atresia and persistent cloaca, esophageal atresia, cardiac defects, limb defects and, persistent left vena cava superior (Table 1), we detected compound heterozygous missense mutations which are both located in the HSP90-domain of TRAP1 (c.1330T>A, p.Y444N and c.1663G>A, p.V555I).
In order to exclude the presence of recessive mutations in controls, we sequenced the TRAP1 coding sequence in 800 individuals with the distinct renal phenotype of nephronophthisis (NPHP). We detected the TRAP1 allele I253V seven times (MAF 0.87%), T444N twice (MAF 0.25%), and R469H twice (MAF 0.025%), all of them as single heterozygous alleles. TRAP1 R46Sfs*75, E442K, L525F, and V555I were absent from our control cohort. Furthermore, no other possibly deleterious variants were present in a homozygous or compound heterozygous state in 800 individuals with NPHP.
Trap1 is expressed in developing and adult kidney
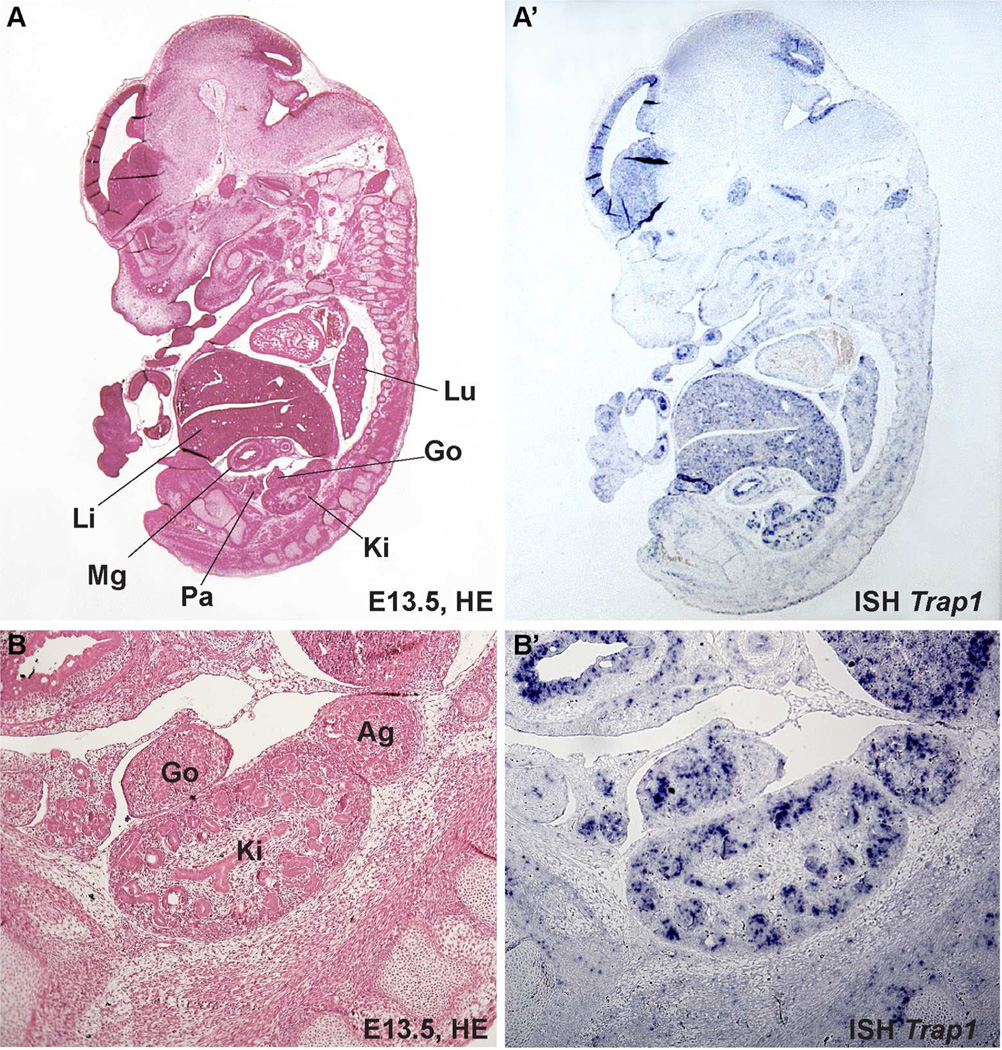
In order to determine whether TRAP1 has a function during kidney development, we analyzed Trap1 expression in developing kidney in mouse embryos 13.5 dpc. Trap1 expression seemed to be expressed at this stage in renal vesicles according to Trap1 transcription assays publically available through the Gudmap project. By in-situ hybridization in E13.5 mouse embryos, we found Trap1 to be strongly expressed in kidney, adrenal gland, and gonad. Trap1 expression specifically localized to renal epithelia (Figure 2).
Figure 2. Trap1 is highly expressed in renal epithelia of E13.5 mouse embryos.
The upper panel shows an HE-stained sagittal section (A) and a Trap1-ISH (A’) in consecutive sections of a mouse embryo E13.5. Note the prominent Trap1 expression in the developing kidney (marked “Ki” in the left panel). The lower panel shows higher magnifications of E13.5 mouse kidney. (B) HE staining, (B’) Trap1-ISH. The Trap1-ISH staining pattern in consistent with Trap1 being expressed specifically in renal epithelia (B’).
Ag, adrenal gland; Go, gonad; HE, hematoxylin-eosin; ISH, in-situ hybridization; Ki, kidney (i.e. metanephros); Li, liver; Lu, lung; Mg, midgut; Pa, pancreatic primordium.
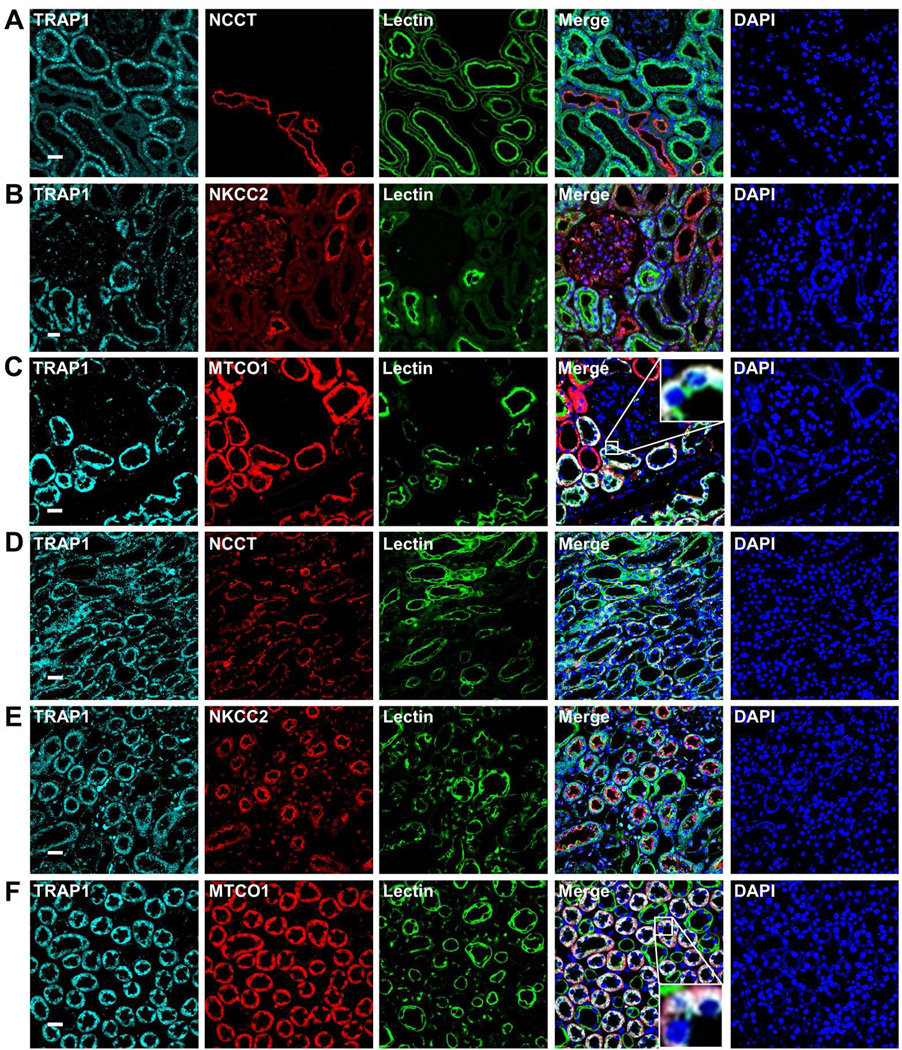
In order to characterize TRAP1 localization in adult kidney, we performed immunofluorescence stainings in rat using a monoclonal TRAP1 antibody in conjunction with established renal markers (Figure 3). TRAP1 is present most prominently in peanut-lectin-marked proximal tubules in the renal cortex (Figure 3A-B). In renal medulla, we detected TRAP1 in peanut-lectin-negative tubular segments and in NKCC-marked (Na+K+2Cl− co-transporter) thick ascending limbs of Henle’s loop (Figure 3C-D). TRAP1 co-localizes with mitochondrial marker MTCO1 in renal cortex and medulla.
Figure 3. Trap1 is highly expressed in renal epithelia of E13.5 mouse embryos.
The upper panel shows an HE-stained sagittal section (A) and a Trap1-ISH (A’) in consecutive sections of a mouse embryo E13.5. Note the prominent Trap1 expression in the developing kidney (marked “Ki” in the left panel). The lower panel shows higher magnifications of E13.5 mouse kidney. (B) HE staining, (B’) Trap1-ISH. The Trap1-ISH staining pattern in consistent with Trap1 being expressed specifically in renal epithelia (B’).
Ag, adrenal gland; Go, gonad; HE, hematoxylin-eosin; ISH, in-situ hybridization; Ki, kidney (i.e. metanephros); Li, liver; Lu, lung; Mg, midgut; Pa, pancreatic primordium.
Discussion
In the present study, we identified by whole exome resequencing and high-throughput mutation analysis five unrelated families with CAKUT or CAKUT in VACTERL association with recessive mutations in TRAP1. Two sibs with CAKUT had a homozygous missense mutation (R469H), which segregated from a common ancestor of their parents. A girl with VACTERL association had the identical homozygous mutation due to maternal isodisomy of chromosome 16 p-ter and q-ter. In a cohort of 675 individuals with CAKUT and 300 individuals with classic VACTERL association we identified 3 additional individuals carrying compound heterozygous mutations in TRAP1. Homozygous or compound heterozygous deleterious variants were absent from 800 control individuals. By ISH and IF, we showed that Trap1 is expressed in early mouse renal epithelia whereas the Trap1 protein is present only in defined segments of developed nephrons in rat.
In 6,500 individuals recorded in the EVS server there are several nonsynonymous variants present in TRAP1, including heterozygous truncating variants in 11 individuals. However, deleterious alleles in recessive disease-genes, unlike in dominant disease-genes, do not underlie direct negative selection through evolution. Consequently the presence of rare deleterious variants in recessive disease genes in a large cohort is an expected finding.
The allele TRAP1 Y444N, detected as compound heterozygous mutation in an individual with CAKUT in VACTERL, is present homozygously in a single individual of the ESP cohort of 6,500 healthy Americans. However, in the context of CAKUT, this does not necessarily mean the variant is non-pathogenic. CAKUT frequently remain completely asymptomatic. For instance, a double-kidney or unilateral renal agenesis typically are an “accidental finding” in renal ultrasound.
The fact that the homozygous mutation TRAP1 R469H was found in an individual with CAKUT and an individual with VACTERL association is surprising. However, in CAKUT and in VACTERL association intra-familial phenotypic variability is very common19–21. Even in a single individual different CAKUT phenotypes may be present, for instance left renal agenesis and right VUR.
The frequencies of individuals with recessive TRAP1 mutations in our cohorts (0.15% in CAKUT, 0.6% in CAKUT with VACTERL) suggest that mutations in TRAP1 are a rare cause of these conditions. Similarly, mutations in two recently identified CAKUT-causing genes, WNT4 and DSTYK, are rare causes of CAKUT7, 8. These findings in humans, along with numerous CAKUT-mouse models, indicate that CAKUT are a common clinical phenotype arising from a multitude of different single-gene causes.
In conclusion, we propose that recessive mutations in TRAP1 are a novel rare cause of isolated CAKUT and the first recessive cause of the VACTERL association.
Subjects and Methods
Human subjects
We obtained blood samples and pedigrees following informed consent from individuals with CAKUT and from individuals with VACTERL association. Approval for human subjects research was obtained from the University of Michigan Institutional Review Board and other institutions involved. The diagnosis of CAKUT and VACTERL association was based on published clinical criteria9.
Homozygosity mapping
We performed homozygosity mapping as described previously17.
Whole exome resequencing (WER)
Exome library preparation and massively parallel resequencing was conducted using the SeqCap EZ Exome v2 (Nimblegen) and Genome Analyzer II (Illumina). Subsequent variant detection, filtering and analysis have been described previously by the authors14, 15. All detected variants were confirmed by Sanger sequencing.
Immunofluorescence microscopy (IF)
IF was performed as previously described by the authors14 using a Leica SP5X system with an upright DM6000 compound microscope and images were processed with the Leica AF software suite. Antibodies used: TRAP1 (Abcam, [TRAP1-6], Cat# ab2721), MTCO1 (Abcam Cat# ab45918), NKCC2 (LSBio Cat# LS-C150446), NCCT (Millipore Cat# AB3553). Specificity of the anti-TRAP1 antibody for rat TRAP1 was confirmed in immunoblot (Figure S5 online).
In-situ hybridization (ISH)
ISH was conducted on sections of wildtype mouse embryos with an NMRI background at embryonic day 13.5. Mouse embryos were dissected into ice cold phosphate buffered saline (PBS), fixed overnight in 4% paraformaldehyde/PBS, and then processed into paraffin wax. ISH was performed on paraffin sections (5]m) using antisense probes generated by PCR from an E11.0 total embryo cDNA library, and specific staining was verified using a sense probe. PCR products contained 3’ T7 and 5’ T3 RNA polymerase binding sites for in vitro transcription and probes were purified using G-50 sephadex columns (GE Healthcare). The 779bp probe for Trap1 spans exons 13–17 (Accession: NM_026508.2).
ISH was performed according to the protocol from (Chotteau-Lelievre et al., 2006) with minor modifications, and detection of AP activity was visualized using BM Purple (Roche Diagnostics). Following staining, slides were quickly dehydrated in 80% and then 100% ethanol, cleared twice for 1 min in xylene (Roth) and coverslips were mounted with Entellan mounting medium (Merck). Photographs were obtained using AxioVision software (Zeiss) with a Zeiss AxioCam and SteREO Discovery.V12 microscope. Three sections from at least 2 different embryos were analyzed.
Bioinformatics
NGS reads alignment and variant detection was done with Genomics Workbench software (CLC Biotech). Mapping parameter: Global alignment, length fraction = 0.9, and similarity fraction = 0.9. Genetic location is according to the assembly of the Genome Reference Consortium GRCh37.
Supplementary Material
Acknowledgements
The authors thank the physicians and families for participating in this study, the German self-help organization for people with anorectal malformations (SoMA e.V.), and all participating physicians, nurses, research assistants, laboratory analysts, and project members of AGORA (Aetiologic research into Genetic and Occupational/Environmental Risk Factors for Anomalies in Children) for their support in building this biobank.
FH is an Investigator of the Howard Hughes Medical Institute, a Doris Duke Distinguished Clinical Scientist, and a Frederick GL Huetwell Professor. ACH, GCD, EB, ES, DS, SG-D, SM, SH, SH-C, MMN, ML, HR, MD are members of the “Network for the Systematic Investigation of the Molecular Causes, Clinical Implications and Psychosocial Outcome of Congenital Uro-Rectal Malformations” (CURE-Net).
This research was supported by grants from the National Institutes of Health (to FH; R01-DK045345 and R01-DK088767), by the March of Dimes Foundation (6FY11-241), by the Division of Intramural Research, by the National Human Genome Research Institute (NHGRI), by the National Institutes of Health and Human Services, by the Bundesministerium fur Bildung und Forschung, (grant 01GM08107), by the BONFOR program of the University of Bonn (to EB; grant O-149.0099, and to GCD; grant O-149.0096), by Sophia Scientific Research Foundation (to EB; grant SSWO S13/9), by the associazione Italiana per la Ricerca sul Cancro (AIRC) (to FE; grant IG13128), and by the Italian Ministry of Health (to FE; grant GR-2010-2310057).
Footnotes
Disclosure
The authors report no conflicts of interest.
Web Resources
1000 Genomes Browser, http://browser.1000genomes.org;
Ensembl Genome Browser, http://www.ensembl.org;
Exome Variant Server, http://evs.gs.washington.edu/EVS;
Mutation Taster, http://www.mutationtaster.org;
Gudmap (GenitoUrinary Molecular Anatomy Project), http://www.gudmap.org;
Online Mendelian Inheritance in Man (OMIM), http://www.omim.org;
Polyphen2, http://genetics.bwh.harvard.edu/pph2;
Sorting Intolerant From Tolerant (SIFT), http://sift.bii.a-star.edu.sg;
The Human Protein Atlas, http://www.proteinatlas.org;
UCSC Genome Browser, http://genome.ucsc.edu/cgi-bin/hgGateway.
References
- 1.NAPRTCS: 2011 Annual Dialysis Report. 2011 https://web.emmes.com/study/ped/annlrept/annualrept2011.pdf, [Google Scholar]
- 2.Smith JM, Stablein DM, Munoz R, et al. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Pediatric Transplantation. 2007;11:366–373. doi: 10.1111/j.1399-3046.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 3.Pope JCt, Brock JW, 3rd, Adams MC, et al. How they begin and how they end: classic and new theories for the development and deterioration of congenital anomalies of the kidney and urinary tract, CAKUT. J Am Soc Nephrol. 1999;10:2018–2028. doi: 10.1681/ASN.V1092018. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa I, Kuwayama F, Pope JCt, et al. Paradigm shift from classic anatomic theories to contemporary cell biological views of CAKUT. Kidney Int. 2002;61:889–898. doi: 10.1046/j.1523-1755.2002.00188.x. [DOI] [PubMed] [Google Scholar]
- 5.Yosypiv IV. Congenital anomalies of the kidney and urinary tract: a genetic disorder? Int J Nephrol. 2012;2012:909083. doi: 10.1155/2012/909083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanna-Cherchi S, Kiryluk K, Burgess KE, et al. Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet. 2012;91:987–997. doi: 10.1016/j.ajhg.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivante A, Mark-Danieli M, Davidovits M, et al. Renal hypodysplasia associates with a WNT4 variant that causes aberrant canonical WNT signaling. J Am Soc Nephrol. 2013;24:550–558. doi: 10.1681/ASN.2012010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanna-Cherchi S, Sampogna RV, Papeta N, et al. Mutations in DSTYK and Dominant Urinary Tract Malformations. N Engl J Med. 2013 doi: 10.1056/NEJMoa1214479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon BD. VACTERL/VATER Association. Orphanet J Rare Dis. 2011;6:56. doi: 10.1186/1750-1172-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaze D, Mahalik S, Rao KL. Novel association of VACTERL, neural tube defect and crossed renal ectopia: sonic hedgehog signaling: a point of coherence? Congenit Anom (Kyoto) 2012;52:211–215. doi: 10.1111/j.1741-4520.2011.00354.x. [DOI] [PubMed] [Google Scholar]
- 11.Wessels MW, Kuchinka B, Heydanus R, et al. Polyalanine expansion in the ZIC3 gene leading to X-linked heterotaxy with VACTERL association: a new polyalanine disorder? J Med Genet. 2010;47:351–355. doi: 10.1136/jmg.2008.060913. [DOI] [PubMed] [Google Scholar]
- 12.Chung B, Shaffer LG, Keating S, et al. From VACTERL-H to heterotaxy: variable expressivity of ZIC3-related disorders. American journal of medical genetics Part A. 2011;155A:1123–1128. doi: 10.1002/ajmg.a.33859. [DOI] [PubMed] [Google Scholar]
- 13.Siebel S, Solomon BD. Mitochondrial Factors and VACTERL Association- Related Congenital Malformations. Molecular Syndromology. 2013;4:63–73. doi: 10.1159/000346301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaki M, Airik R, Ghosh AK, et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 2012;150:533–548. doi: 10.1016/j.cell.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Otto EA, Cluckey A, et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat Genet. 2012;44:910–915. doi: 10.1038/ng.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ten Kate LP, Scheffer H, Cornel MC, et al. Consanguinity sans reproche. Human genetics. 1991;86:295–296. doi: 10.1007/BF00202413. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrandt F, Heeringa SF, Ruschendorf F, et al. A Systematic Approach to Mapping Recessive Disease Genes in Individuals from Outbred Populations. PLoS Genet. 2009;5:e1000353. doi: 10.1371/journal.pgen.1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halbritter J, Diaz K, Chaki M, et al. High-throughput mutation analysis in patients with a nephronophthisis-associated ciliopathy applying multiplexed barcoded array-based PCR amplification and next-generation sequencing. J Med Genet. 2012;49:756–767. doi: 10.1136/jmedgenet-2012-100973. [DOI] [PubMed] [Google Scholar]
- 19.Solomon BD, Pineda-Alvarez DE, Raam MS, et al. Evidence for inheritance in patients with VACTERL association. Hum Genet. 2010;127:731–733. doi: 10.1007/s00439-010-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon BD, Pineda-Alvarez DE, Raam MS, et al. Evidence for inheritance in patients with VACTERL association. Human genetics. 2010;127:731–733. doi: 10.1007/s00439-010-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilger A, Schramm C, Draaken M, et al. Familial occurrence of the VATER/VACTERL association. Pediatr Surg Int. 2012;28:725–729. doi: 10.1007/s00383-012-3073-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.