Abstract
Background and Aims
The interaction between forest fragmentation and predicted climate change may pose a serious threat to tree populations. In small and spatially isolated forest fragments, increased homozygosity may directly affect individual tree fitness through the expression of deleterious alleles. Climate change-induced drought stress may exacerbate these detrimental genetic consequences of forest fragmentation, as the fitness response to low levels of individual heterozygosity is generally thought to be stronger under environmental stress than under optimal conditions.
Methods
To test this hypothesis, a greenhouse experiment was performed in which various transpiration and growth traits of 6-month-old seedlings of Quercus robur differing in multilocus heterozygosity (MLH) were recorded for 3 months under a well-watered and a drought stress treatment. Heterozygosity–fitness correlations (HFC) were examined by correlating the recorded traits of individual seedlings to their MLH and by studying their response to drought stress.
Key Results
Weak, but significant, effects of MLH on several fitness traits were obtained, which were stronger for transpiration variables than for the recorded growth traits. High atmospheric stress (measured as vapour pressure deficit) influenced the strength of the HFCs of the transpiration variables, whereas only a limited effect of the irrigation treatment on the HFCs was observed.
Conclusions
Under ongoing climate change, increased atmospheric stress in the future may strengthen the negative fitness responses of trees to low MLH. This indicates the necessity to maximize individual multilocus heterozygosity in forest tree breeding programmes.
Keywords: Climate change, drought stress, forest fragmentation, greenhouse experiment, growth traits, heterozygosity–fitness correlations, pedunculate oak, Quercus robur, transpiration
INTRODUCTION
Forests are essential to life on earth as they provide a multitude of ecosystem services, including climate mitigation, water regulation and biomass production (Lindenmayer and Franklin, 2002; Thompson et al., 2009). Over recent decades the functioning and sustainability of forests have been increasingly challenged by various anthropogenic threats (Simberloff, 1999; Millennium Ecosystem Assessment, 2005). One of the most important threats is climate change (Noss, 2001; Lindner et al., 2010). For Europe, climate projections predict increasing temperatures and irregular precipitation patterns during summer, which will increase the number and intensity of drought events (Stocker et al., 2013). During such events, soil water shortage can be expected to induce the closure of stomata, which may directly damage leaf tissue of trees through the inhibition of leaf cooling (Bréda et al., 2006). At more severe levels of drought stress, water transfer within the xylem may be irreversibly disrupted through vessel embolism (cavitation), which may result in losses of roots or twigs. Next to cavitation, tree mortality after long-term drought may also be caused by carbon starvation. This process induces the depletion of carbon resources during drought stress, as stomatal closure will lower carbon assimilation such that it is insufficient to supply the required amounts of carbon (McDowell et al., 2008). Drought-induced physiological disorders will not only affect biomass production in the short term, but may also increase the susceptibility of trees to secondary stresses such as frost and fungal and insect attacks, which may affect tree health and eventually lead to tree death (Bréda et al., 2006; Lindner et al., 2010).
Next to climate change, the loss and fragmentation of forests through land-use changes poses a second major worldwide threat to forest sustainability (Hamrick, 2004; Millennium Ecosystem Assessment, 2005). Forest fragmentation results in small and spatially isolated forest fragments in which increased random genetic drift and inbreeding may erode genetic diversity of the tree populations (Jump and Peñuelas, 2006; Vranckx et al., 2012). Genetic diversity is crucial, however, for the maintenance of vital and productive forests (Jump et al., 2009), since population genetic variation provides the raw material for evolution, allowing adaptation of forest trees to environmental changes (Willi et al., 2006). Moreover, increased homozygosity resulting from inbreeding may directly affect individual tree fitness, through the expression of deleterious alleles that influence morphological, physiological and life-history traits. The relationships between heterozygosity and fitness traits are generally known as heterozygosity–fitness correlations (HFCs), and are more likely to occur in the small, non-random mating populations that typically occur in fragmented habitats (David, 1998; Hansson and Westerberg, 2002; Szulkin et al., 2010). Quantifying HFCs may give insight into the short-term fitness of tree populations (Hedrick and Kalinowski, 2000; Reed and Frankham, 2003) and is also relevant to forest management and forest tree breeding programmes designed to maximize biomass production (Aravanopoulos and Zsuffa, 1998; Alig et al., 2003).
The detrimental genetic consequences of forest fragmentation may be exacerbated by climate change-induced drought stress because the fitness response of tree species to low levels of heterozygosity is generally thought to be more pronounced under environmental stress than under optimal conditions (Armbruster and Reed, 2005). However, the effects of environmental stress on HFCs are not always clear (Keller and Waller, 2002), and beyond a certain level of stress HFCs may become less apparent (Audo and Diehl, 1995; David, 1998). Whereas the relationships between heterozygosity and fitness traits have been examined frequently in conifers (e.g. Mitton et al., 1981; Ledig et al., 1983; Bush et al., 1987; Savolainen and Hedrick, 1995), similar studies are rare for broadleaved species (Aravanopoulos and Zsuffa, 1998). Furthermore, we are not aware of any study that has focused on the potentially detrimental interaction between climate change and habitat fragmentation by studying the effects of drought stress on HFCs.
Here we investigated HFCs and their response to drought stress in the economically important broadleaved tree species pedunculate oak (Quercus robur). We selected different transpiration variables and various growth traits as fitness variables (Van Hees, 1997; David, 1998; Bréda et al., 2006). First, growth is an important fitness component, especially in indeterminate growers such as trees, which are characterized by size-dependent fecundity (David, 1998). Rapid early growth and strong biomass production will increase the competitive ability of seedlings, through which they may outcompete neighbouring seedlings when competition for light and resources is strong (King, 1981; Bush et al., 1987; Scotti-Saintagne et al., 2004). Furthermore, since the crown size of many tree species is strongly correlated with stem diameter (Hemery et al., 2005), larger oak trees may also have greater crown areas for flowering and acorn production (Greenberg, 2000). Second, transpiration variables, such as stomatal conductance, water potential and the water content of seedlings, may give an indication of the water status of a plant and may influence the physiological processes that determine carbon fixation and growth (Bréda et al., 2006). Low soil water content and high atmospheric evaporative demand may decrease the leaf water potential of oak seedlings and induce stomatal closure (Fort et al., 1997; Cavender-Bares and Bazzaz, 2000). This may reduce the rate of stomatal conductance, limiting water fluxes at the cost of reduced photosynthesis and biomass production (Bréda et al., 2006). Furthermore, although transpiration efficiency (ratio of biomass production to transpiration) generally increases during drought stress, Donovan and Ehleringer (1991) and Cavender-Bares and Bazzaz (2000) have suggested that this increase in transpiration efficiency may be lower during seedling establishment, when it is accompanied by increased seedling growth.
HFCs are likely to emerge in Q. robur since forest stands of this tree species are small in many parts of Western Europe due to past deforestation and fragmented forest ownership (Wiersum et al., 2005). Moreover, recent research has revealed variation in individual multilocus heterozygosity within small oak stands in northern Belgium, despite high heterozygosity levels at the population scale (Vranckx et al., 2014). Therefore, it can be hypothesized (1) that this within-stand variation in individual multilocus heterozygosity may be correlated to the variation in transpiration and growth traits of the seedlings; and (2) that these HFCs are stronger under stress conditions. To test these hypotheses, we quantified multilocus heterozygosity based on nine neutral microsatellite loci in 150 seedlings originating from three populations (50 seedlings per population). A greenhouse experiment was performed in which seedlings of Q. robur that differed in multilocus heterozygosity were grown under standardized environmental conditions. Various transpiration and growth traits of 6-month-old seedlings were recorded for 3 months under both a well-watered and a drought stress treatment.
MATERIALS AND METHODS
Study species
Pedunculate oak (Quercus robur) is a keystone tree species of many European forest ecosystems, with a large natural range extending from southern Scandinavia to sub-Mediterranean Europe, and eastwards to the Ural Mountains (Bary-Lenger and Nebout, 1993). This monoecious, wind-pollinated tree species occurs on a wide range of soils, and displays a medium degree of drought tolerance, which may be attributed to its deep rooting system and the maintenance of high rates of stomatal conductance during moderate levels of drought stress (Epron and Dreyer, 1993). Compared with its closest congener, Quercus petraea, Q. robur prefers to grow on neutral soils characterized by good water-holding capacity or soils with a permanent water table within reach of the root system (Bary-Lenger and Nebout, 1993). Flower fertilization is followed by rapid development of acorns, which are dispersed during autumn by gravity, small rodents and birds. Although acorns can germinate and establish under a closed canopy, forest canopy openings are required for further seedling growth and development (Bary-Lenger and Nebout, 1993).
Seed collection and experimental set-up
Acorns of Q. robur were collected in the autumn of 2011 in three small (<4 ha) monospecific pedunculate oak stands in the centre of Flanders (northern Belgium). These forest stands had been studied previously (Vranckx et al., 2014) and showed high heterozygosity values at the population level, which were consistent with what was found in other population genetic studies of Q. robur (Mariette et al., 2002; Hampe et al., 2010). The maximum distance between these stands was less than 15 km, and all were located in a matrix of forest stands composed of other tree species and/or agricultural land (Table 1). Pollen exchange among the three forest stands (Vos, Keffers and Chartreuse) was rather limited, as the minimum geographical distance between stands (∼3400 m) was much greater than the average pollen dispersal distances (δp = 130–210 m) for the three studied stands, based on the neighbourhood model implemented in the program NM+ (Chybicki and Burczyk, 2010). A circular plot containing 35 adult trees was established in the centre of each Q. robur stand, in which five seed traps (1·5 m2 each) were randomly located. At the end of September, ten acorns from each seed trap were collected and stored at 5 °C for 4 weeks. Cold storage increased both the percentage and synchronization of seed germination (Manzanera et al., 1993). After cold storage, 50 Q. robur seeds per forest stand were weighed and sown in the centre of 3 L open-bottom pots filled with commercial soil (20 % organic and 10 % dry matter, pH ∼ 6, electrical conductivity = 750 µS cm−1, 14:16:18 N:P:K 1 kg m−3). The pots were randomly placed in a greenhouse under controlled climatic conditions with a 12/12 h day/night light regime, and kept at field capacity using an automatic drip irrigation system. The irrigation water contained a Peters professional nutrient mixture (20:20:20 N:P:K, including trace elements; Everris International, Geldermalsen, the Netherlands). High germination percentages were obtained (>95 %), with most of the seeds germinating in week 4 after sowing.
Table 1.
Characteristics of the three pedunculate oak (Q. robur) stands where acorns were collected
| Site | Latitude (N) | Longitude (E) | Area (ha) | Population size | Density (per ha) | Plot size (ha) | Isolation (m) |
|---|---|---|---|---|---|---|---|
| Keffers | 50°50′26″ | 4°42′00ì | 3·04 | 328 | 118 | 0·49 | 400 |
| Vos | 50°49′27″ | 4°39′33ì | 3·97 | 682 | 195 | 0·24 | 175 |
| Chartreuse | 50°54′45″ | 4°46′25ì | 0·43 | 32 | 74 | 0·43 | 135 |
Microsatellite analyses
At the end of the growth phase (April 2012) all seedlings were genotyped. A single leaf was taken from each seedling and stored in silica prior to DNA extraction. Dried leaf samples (200 mg) were ground before DNA extraction with a Nucleospin Plant II kit (Macherey-Nagel). We selected ten simple sequence repeat (SSR) loci that had been developed for Q. petraea (QpZAG9, QpZAG108, QpZAG46, QpZAG15, QpZAG110, QpZAG104; Steinkellner et al., 1997), Quercus macrocarpa (MSQ4, MSQ13, MSQ16; Dow et al., 1995; Dow and Ashley, 1996) and Q. robur (QrZAG112; Kampfer et al., 1998). Polymerase chain reaction amplifications were carried out using a Multiplex PCR Master Mix kit (Qiagen) with the thermocycler programme of 15 min at 94 °C followed by 30 cycles of 45 s at 94 °C, 45 s at 50 °C and 45 s at 72 °C and final extension at 72 °C for 10 min. The amplified fragments were analysed with an ABI 3500 genetic analyser (Applied Biosystems, Foster City, CA, USA) and GeneMapper software (version 4·1). The microsatellite data were first analysed using the software Micro-Checker version 2·2·3 (Van Oosterhout et al., 2004) to check for possible genotyping errors, including null alleles, stutter peaks and large allele drop-out. We observed a consistent high null allele frequency at one locus (MSQ16), which was removed from further analyses. Neutrality of the microsatellites was checked with the Ewens–Watterson homozygosity test of neutrality (Manly, 1985) using Popgene version 1·32 (100 000 permutations; Yeh et al., 1999). This test compares observed allele frequencies with those expected under mutation–drift equilibrium, and is therefore useful for detecting deviations from neutrality due to selection or demographic changes. We found no significant departures from neutrality for any loci; however, the observed F (sum of squares of allele frequencies) for locus QpZAG104 was close to the lower limit of the 95 % confidence interval (Supplementary Data Table S1).
Three different metrics were calculated to detect HFCs in the genotyped oak seedlings. First, multilocus heterozygosity (MLH) was measured in each seedling as the percentage of microsatellite loci for which an individual was heterozygous, corrected for non-scored loci (Slate and Pemberton, 2002; Chapman et al., 2009). A second microsatellite-based metric that has been proposed for the study of HFCs is mean d2, which is the squared difference (in repeat units) between the two alleles at a locus, averaged over all the microsatellite loci for which an individual was examined (Coulson et al., 1998; Hedrick et al., 2001; Slate and Pemberton, 2002). Mean d2 was calculated as follows:
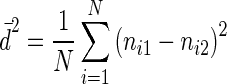 |
where ni1 and ni2 are the lengths in repeat units of the two alleles at the ith locus and N is the number of scored loci examined in an individual. Finally, we also calculated the standardized mean d2 (sMd2), which limits the influence of highly polymorphic loci on the metric. Therefore, d2 was standardized with its locus-specific variance (Pemberton et al., 1999). By studying the above genetic metrics, we actually investigated different ecological processes. MLH has been considered to better detect recent inbreeding events than d2, whereas d2 may also contain information about historical events in an individual's ancestry, such as the influence of population admixture (hybrid vigour, high d2) (Coulson et al., 1998; Pemberton et al., 1999). To assess the relationship between the three tested genetic measures (MLH, d2 and sMd2), Spearman rank correlation coefficients (rs) were calculated. All abbreviations and variables are listed in the Appendix.
Treatment phase
After 6 months at field capacity (growth phase), 100 of the 150 seedlings were exposed to the treatment phase (8 May 2012) and the 50 remaining seedlings were harvested for initial biomass estimation. The 100 seedlings subjected to the treatment phase were selected in such a way that individuals with high and low MLH values originated equally from all three forest stands. Subsequently, these seedlings were assigned to two irrigation treatments such that MLH levels and forest stands were uniformly represented within and between treatments. These drought stress regimes were established based on relative extractable soil water (REW):
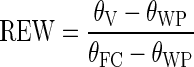 |
and measurements of the actual volumetric moisture content (θV) with a TRIME TDR FM3 sensor (IMKO, Ettlingen, Germany). The volumetric soil water contents at field capacity [θFC, suction pressure (pF) = 2·0] and at wilting point (θWP, pF = 4·2) were measured and calculated for the applied commercial soil (40·8 and 5·8 %, respectively). The normal water treatment consisted of 50 seedlings subjected to a relative extractable soil water above 0·80 (θV = 33·8 %), which greatly exceeds the general water deficit threshold for trees (REW = 0·30–0·40; Granier et al., 2007). To determine the effect of severe drought stress, the remaining seedlings were irrigated up to a relative extractable soil water content of 0·10 (θV = 9·3 %). This value corresponds to the estimates (REW = 0·05–0·2) recorded by Bréda et al. (2006) in European forests during the severe summer drought of 2003. Target weights for watering were calculated for all seedlings based on relative extractable soil water and the relationship between pot weight and soil moisture content. This relationship was regularly checked (weeks 1, 4 and 10) and adjusted for changes in plant weight. Seedlings were watered three times a week up to their target weight and pots were weighed before and after irrigation. To prevent soil evaporation and percolation, the surface and bottom of the pots were covered with aluminium and plastic foil respectively. Five control pots without seedlings were weighed to correct for soil evaporation. During the entire experiment, air temperature (Ta), relative air humidity (RH) and photosynthetically active radiation (PAR) were measured every 5 min. PAR was converted from W m−2 to the more usual μmol m−2 s−1, based on the conversion factors of McCree (1972). Mean daytime values of Ta, PAR and vapour pressure deficit (VPD, calculated using Ta and RH) are given in Table 2. Pots were fully randomized every week to reduce position effects within the greenhouse. Sulphur vaporization and preventive spraying with acaricides (Floramite, Nissorun) were successfully applied for the control of powdery mildew (Microsphaera alphitoides) and red spider mite (Tetranychus urticae).
Table 2.
Mean values and their standard errors (in parentheses) for air temperature (Ta), VPD and PAR during the treatment phase and during the measurements of gs and ψ in Q. robur
| Time | Plant variable | Ta (°C) | VPD (kPa) | PAR (μmol m−2 s−1) |
|---|---|---|---|---|
| Treatment period | ||||
| Entire day | 22·8 (0·1) | 1·30 (0·017) | 359·3 (29·9) | |
| Morning | 20·7 (0·1) | 0·90 (0·019) | 285·2 (39·1) | |
| Midday | 24·7 (0·2) | 1·67 (0·028) | 619·6 (50·6) | |
| Day 35 | ||||
| Morning | gs | 20·7 (1·2) | 0·81 (0·211) | 237·8 (62·1) |
| Midday | gs | 23·0 (0·5) | 1·31 (0·097) | 432·2 (109·5) |
| Day 49, midday | gs | 25·6 (0·4) | 1·88 (0·094) | 511·1 (155·9) |
| Day 65, midday | gs | 22·6 (0·8) | 1·61 (0·108) | 868·9 (159·2) |
| Day 92, midday | ψmd | 21·8 (0·3) | 0·97 (0·047) | 145·8 (12·9) |
| Day 93, predawn | ψpd | 17·9 (0·1) | 0·19 (0·004) | 0 |
Measurements
At the start of the treatment phase, we recorded the following morphological variables: number of leaves (Ln) and branches (Bn), stem length (SL), stem diameter at base (SD,base) and top (SD,top), branch length (BL) and diameter at the base (BD,base) and top of the branches (BD,top). These measurements were repeated every 3 weeks and at the end of the treatment phase. Total woody volume (Vtot, stem + branches) was derived using Smalian's formula (West, 2009):
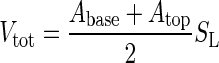 |
where Abase and Atop are the areas at the base and top of the stem or branches, respectively. On days 35, 49 and 65, leaf stomatal conductivity (gs) was measured on two randomly selected top leaves of all seedlings using a steady-state SC-1 leaf porometer (Degacon Devices, Pullman, WA, USA). These measurements were performed in two rounds between 0900–1200 h and 1230–1530 h local time on day 35, whereas on the other days gs was measured only once (between 1230 and 1530 h), since climatic conditions were not homogeneous during the mornings of days 49 and 65. Midday (ψmd) and predawn (ψpd) leaf water potentials were determined on day 92 (1230–1500 h) and 93 (0300–0530 h) respectively, using a Scholander pressure chamber (model 615, PMS instruments, Albany, USA). In total, we examined ψmd and ψpd for 60 seedlings, in each of which two top leaves were randomly selected. These seedlings were characterized by low (≤0·67) and high (≥0·89) levels of MLH. The water potential range (Δψ) per seedling was calculated as ψpd – ψmd. Climatic conditions during measurements of leaf stomatal conductivity and water potential are given in Table 2.
At day 100 of the treatment phase (16 August 2012) all seedlings were harvested and measurements of fresh (WF) and dry weight (WD, 48 h at 85 °C) of the woody parts (stem + branches), leaves, roots and whole plants were performed. We calculated for each seedling above-ground biomass (WAG) and water content (WC) as follows:
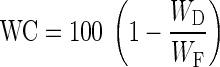 |
Calculated variables
To estimate the initial biomass of the seedlings followed during the treatment phase, we established linear regression models between biomass data (WF, WD and WAG) and morphological input variables [ln(Vtot), ln(SL), ln(Ln) and Bn], which were obtained from the 50 seedlings harvested at the start of the treatment phase. The models with the highest R2adj (0·84–0·97) were selected for initial biomass estimation (Supplementary Data Table S2). A good model fit was also confirmed by Mallow's Cp selection criterion, as the Cp value was equal to the number of regressors in the chosen models (Gagné and Dayton, 2002). The relative growth rates (RGRs) of various morphological and biomass variables were calculated as:
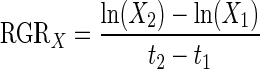 |
where X1 and X2 are the values of the studied variables at times t1 and t2 respectively (Evans, 1972). Daily transpiration rate (TR) was determined by weighing the pots before and after watering and correcting for soil evaporation (blank pots) and leaf loss. We also accounted for differences in seedling size by dividing the transpiration rate by the ln(Vtot) of the seedlings. Transpiration efficiency (TE) was calculated as the ratio between the amount of dry biomass produced during the treatment phase and the total amount of water consumed during this period. We also estimated biomass water productivity (WP), which can be defined as the total dry biomass produced per unit of water transpired, normalized for atmospheric conditions (Steduto, 2003; Steduto et al., 2007). Many studies have shown that WP is approximately constant for a given species, regardless of the growth conditions (irrigation treatment), after the variation in atmospheric conditions is normalized (Steduto, 2003; Steduto et al., 2007). To calculate WP we first normalized cumulative transpiration (CT) for daily mean VPD measured between 0800 and 1800 h on day j (VPDj)
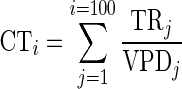 |
Secondly, we constructed a linear regression model that included irrigation treatment, the cumulative transpiration normalized for atmospheric conditions (CTi), MLH and irrigation treatments as input variables, and total dry biomass production as dependent variable. Main effects and interaction terms that were not significant (P > 0·05), were excluded from this model using a backward selection procedure. Finally, biomass water productivity was estimated as the regression coefficient of the final regression model with CTi and irrigation treatment as input variables and dry biomass production as dependent variable (Steduto et al., 2007).
Statistical analyses
We used linear regression models to examine the effects of irrigation treatment (factor), multilocus heterozygosity (covariate) and their interaction term on the response variables measured and calculated during the treatment phase. To test for variation caused by environmental differences between the three seed collection sites, the forest stand in which the acorns were collected was included in the model as a fixed factor. Forest stand was not included as a random effect in our model, since the number of independent clusters within forest stands was too limited (three stands) to properly estimate its standard deviation. We also accounted for the effect of seed size on the measured response variables by including log seed weight in the initial model. Non-significant stand and seed size effects were excluded from the initial models (Supplementary Data Table S3), such that well-fitting final models (highest R2adj) were obtained. For all tested models, R2adj values and their significance levels were calculated as a measure of goodness of fit, and partial R2 coefficients (R2p) were obtained for each fixed effect to compare them between the different final models. To examine the effect of time of measurement, repeated measures ANOVAs with between-subject factors forest stand, irrigation regime and MLH were applied to transpiration rates corrected for seedling size (within-subject effect = irrigation event, 39 levels) and stomatal conductance day 35 (within-subject effect = time of measurement, 2 levels). If the assumption of sphericity was not met (significant Mauchly's test), the Huyn–Feldt statistic was used for within-subject tests. All statistical analyses were performed using SPSS software (SPSS 20·0; SPSS, Chicago, IL).
RESULTS
A total of 146 of the 150 studied seedlings (97·3 %) were successfully genotyped for all nine microsatellite loci. The markers were highly polymorphic, with an average number of 17·7 ± 2·01 (s.e.) alleles per locus. Multilocus heterozygosity ranged between 0·44 and 1, whereas d2 varied between 9·3 and 751·7 and sMd2 between 4·3 × 10−5 and 8·0 × 10−3. The three variables were significantly correlated with each other. Multilocus heterozygosity was more strongly correlated to standardized mean sMd2 (rs = 0·39, P < 0·0001) than to mean d2 (rs = 0·27, P = 0·006). In addition, the statistical power of d2 and sMd2 to detect significant HFCs was much more limited [lower R2p values (<0·1–2·1 %) compared with MLH (3–11 %)] (cf. Coltman and Slate, 2003; Slate and Pemberton, 2002; Chapman et al., 2009). Therefore, we only report the outcome of the HFC tests based on MLH.
Water use and transpiration
Almost all transpiration and water use variables differed strongly (P < 0·05) between the two irrigation treatments (Table 3A), with significantly higher estimates for seedlings subjected to normal water conditions compared with drought-stressed seedlings (Figs 1 and 2). Changes in gs during the day also differed significantly between the two irrigation treatments (significant time × stress effect, P < 0·05). For drought-stressed seedlings, gs decreased by 9·6 %, from 67·7 ± 5·8 (s.e.) mmol m−2 s−1 in the morning of day 35 to 61·3 ± 5·2 mmol m−2 s−1 at midday, whereas gs of well-watered seedlings significantly increased (P < 0·05) by 32·5 %, from 337·6 ± 28·5 to 447·1 ± 44·3 mmol m−2s−1 at midday. More importantly, the results of the linear regression models indicated that gs and water content were significantly correlated (P < 0·05) with the MLH of the seedlings, whereas ψmd and Δψ showed marginally significant relationships (0·05 ≤ P < 0·1) (Table 3A). For all of these variables, estimates increased with increasing MLH (Fig. 2), except for Δψ, for which the opposite relationship was observed in the drought-stressed seedlings (Fig. 2D). Although significant HFCs were found, only a small proportion of the variance (R2p = 4–11 %) could be explained by MLH (Table 3A). Marginally significant interaction terms (0·05 ≤ P < 0·1) between irrigation treatment and MLH were obtained for measures of gs on day 49 and for Δψ (Table 3A). These interactions are visualized in the non-parallel regression lines in Fig. 2A, D, where estimates of gs on day 49 and Δψ were more strongly correlated with MLH under water stress than under normal water conditions. The effects of forest stand and seed weight on the transpiration variables were limited. A few variables showed significant differences between the three seed collection sites (Table 3), but no effects of seed weight were found.
Table 3.
Results of linear regression models performed to examine the effect of irrigation regime, MLH and their interaction on (A) transpiration and (B) growth trait variables for Q. robur
| Parameter | Variable | Correlation model |
Irrigation |
MLH |
Irrigation × MLH |
Forest stand |
Log seed weight |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fmodel | R2adj | F | R2p | F | R2p | F | R2p | F | R2p | F | R2p | ||
| (A) Transpiration | |||||||||||||
| Stomatal conductance | log gs day 35 a.m. | 43·03*** | 0·68 | 10·27** | 0·10 | 4·76** | 0·05 | 0·36 | <0·01 | 4·66** | 0·09 | ||
| log gs day 35 p.m. | 73·79*** | 0·69 | 10·02** | 0·10 | 7·13** | 0·07 | 0·26 | <0·01 | |||||
| log gs day 49 p.m. | 110·81*** | 0·85 | 35·55*** | 0·27 | 9·06** | 0·09 | 3·20(*) | 0·03 | 5·57** | 0·11 | |||
| log gs day 65 p.m. | 121·81*** | 0·86 | 16·43*** | 0·15 | 11·14** | 0·11 | 0·17 | <0·01 | 2·75(*) | 0·06 | |||
| Leaf water potential | ψmd | 40·35*** | 0·68 | 14·01*** | 0·21 | 2·81(*) | 0·05 | 1·81 | 0·03 | ||||
| ψpd | 58·93*** | 0·75 | 5·87** | 0·10 | 0·10 | <0·01 | 0·34 | <0·01 | |||||
| Water potential range | Δψ | 9·52*** | 0·31 | 8·61** | 0·14 | 3·14(*) | 0·06 | 3·93(*) | 0·07 | ||||
| Total transpiration, corrected | TR | 124·84*** | 0·80 | 9·74** | 0·10 | 1·33 | 0·01 | 0·25 | <0·01 | ||||
| Water content | WC | 61·47*** | 0·66 | 6·10** | 0·06 | 4·11** | 0·04 | < 0·01 | <0·01 | ||||
| (B) Growth traits | |||||||||||||
| RGR (Evans, 1972) | RGRdiameter | 69·40*** | 0·74 | 20·06*** | 0·18 | 3·67(*) | 0·04 | 2·17 | 0·02 | 2·75 | 0·03 | ||
| RGRlength | 3·58** | 0·08 | 0·03 | <0·01 | 2·95(*) | 0·03 | 0·45 | <0·01 | |||||
| RGRwoody volume | 46·26*** | 0·66 | 15·21*** | 0·14 | 4·53** | 0·05 | 2·22 | 0·02 | 2·25 | 0·02 | |||
| RGRdry biomass | 64·86*** | 0·67 | 7·55** | 0·08 | 3·23(*) | 0·03 | 0·04 | <0·01 | |||||
| RGRfresh biomass | 102·28*** | 0·76 | 14·10*** | 0·13 | 2·78(*) | 0·03 | 0·28 | <0·01 | |||||
| RGRabove ground | 18·28*** | 0·48 | 8·47** | 0·09 | 2·51 | 0·03 | 1·76 | 0·02 | 5·31** | 0·11 | |||
| RGRroots | 20·80*** | 0·56 | 2·93(*) | 0·03 | 0·12 | < 0·01 | 0·05 | <0·01 | 6·55** | 0·13 | 4·03** | 0·04 | |
| Transpiration efficiency | TE | 2·41(*) | 0·06 | 0·47 | <0·01 | 0·05 | <0·01 | 0·41 | <0·01 | 8·51** | 0·09 | ||
To account for differences between forests and seed sizes, forest stand and log seed weight were included in the analysis as fixed effects. Non-significant stand and seed size effects were excluded from the final models, such that well-fitting models (highest R2adj) were obtained. F-statistics, R2p coefficients and significance levels for main effects and interactions are presented for the final models (after model reduction).
(*)0·05 ≤ P < 0·1; **0·001 ≤ P < 0·05; ***P < 0·001.
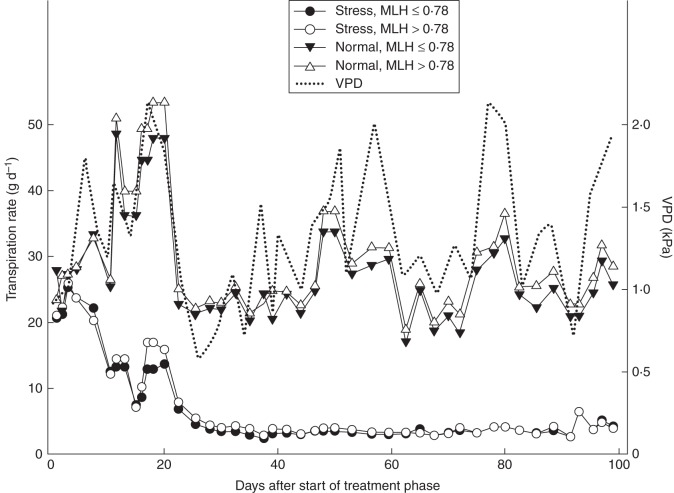
Fig. 1.
Daily transpiration rates of Q. robur corrected for seedling size, under drought stress and normal water conditions (see key). Seedlings were divided into two groups with MLH ≤0·78 and >0·78.
Fig. 2.
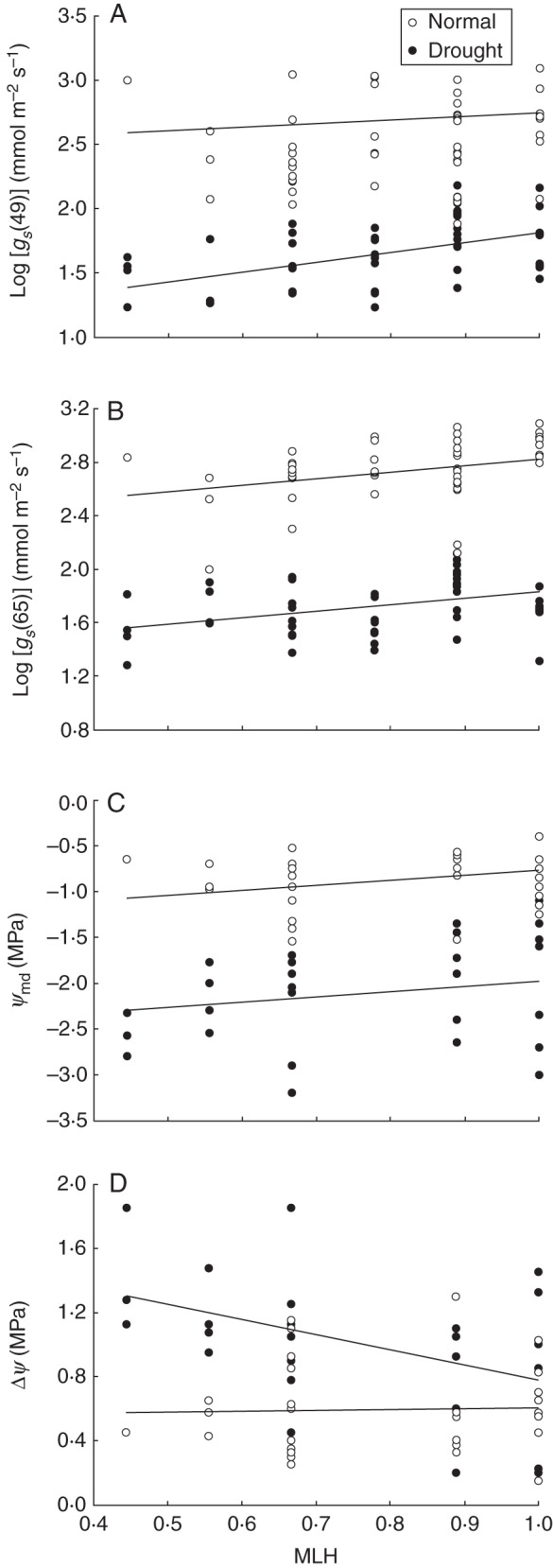
Relationships between MLH of Q. robur and the following transpiration variables: gs at (A) day 49 and (B) day 65, (C) ψm and (D) Δψ. HFCs were compared between seedlings subjected to normal water conditions and drought-stressed seedlings (see key). The plotted regression lines are based on estimates of the significant fixed effects of the linear regression models (Table 3).
The repeated measures ANOVA model used to examine the effect of time on TR indicated a highly significant time and time × irrigation effect (both P < 0·001) (Fig. 1). The mean TR of drought-stressed Q. robur seedlings declined strongly from 25·6 ± 1·5 g (s.e.) on day 5 to 4·4 ± 0·5 g on day 28, after which TR stabilized. Seedlings under normal water conditions showed daily TRs that were highly correlated (rs = 0·45, P < 0·05) to atmospheric demand (VPD), with a slightly but not significantly higher TR when MLH was >0·78 compared with seedlings with MLH ≤0·78 (Fig. 1).
Growth rate and biomass production
Seedling growth was strongly affected by water availability of the soil. First, we observed a highly significant (P < 0·001) positive relationship between dry biomass production and the total amount of water transpired during the treatment phase (Fig. 3). Second, most growth traits were influenced by the irrigation regime, with significantly higher RGRs (P < 0·05) under normal water conditions than under drought stress (Table 3B). Efficiency in producing a certain amount of dry biomass per unit of water transpired (TE) was 23 % higher in drought-stressed seedlings [5·01 ± 0·32 (s.e.)] than in well watered seedlings (4·07 ± 0·23). However, this difference between irrigation treatments was not significant (P > 0·05), and TE was also not associated with the MLH of a seedling (Table 3B). Similar results were obtained for biomass WP, since the regression coefficients of the linear regression model between CTi and dry biomass production were not significantly (P > 0·05) influenced by the other input variables of the model (irrigation treatment and MLH). This was indicated by the non-significant interaction terms (CTi × irrigation treatment and CTi × MLH), which were removed, together with the non-significant main effect MLH, from the linear regression model.
Fig. 3.
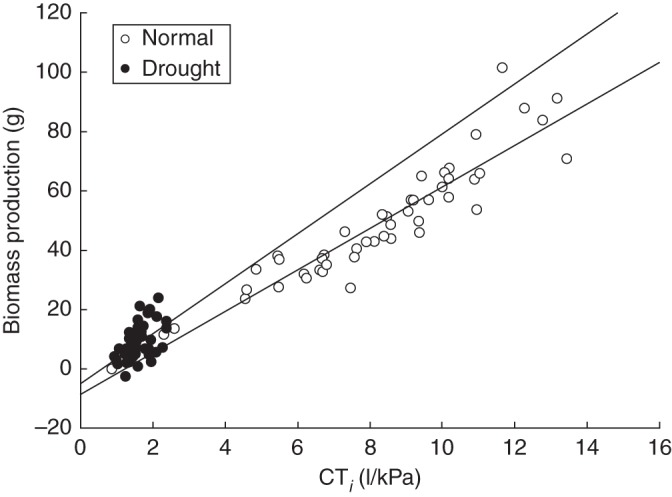
Biomass production of Q. robur as a function of cumulative transpiration standardized for VPD (CTi), compared between seedlings under normal water conditions and drought-stressed seedlings (see key). Regression lines are plotted for each irrigation treatment and are based on the coefficients of the linear regression model.
A significant effect of MLH (P < 0·05) on growth variables was obtained when the RGR of woody volume was tested as trait (Table 3B). Under drought stress, mean values of RGRwoody volume were 7·5 times larger in seedlings of the highest MLH class (MLH = 1) compared with seedlings with the lowest MLH values (MLH = 0·44) (Fig. 4A). The RGR of stem diameter and RGRs of dry and fresh biomass showed a weak (R2p = 3–5 %), marginally significant (0·05 ≤ P < 0·1), positive relationship with MLH (Table 3B, Fig. 4B). In contrast to some of the transpiration variables, no significant interaction terms between MLH and irrigation treatment were found for any growth trait (Table 3B, Fig. 4). Finally, most of the growth traits were not significantly influenced by the forest stand in which the acorns were collected or by the weight of the acorns. Furthermore, the weight of the acorns was not significantly correlated with the MLH of the acorns (rs = 0·12; P = 0·23).
Fig. 4.
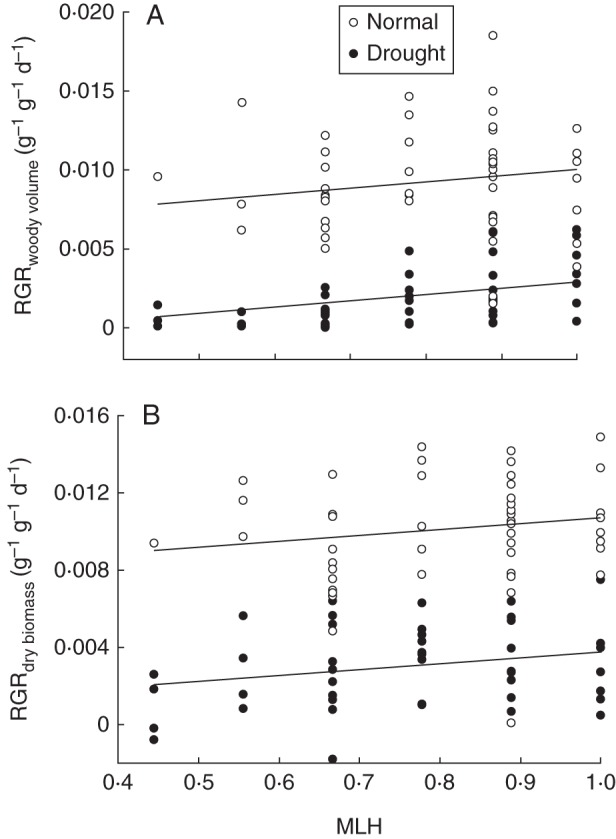
Relationships between MLH of Q. robur and (A) RGR of woody volume and (B) RGR of dry biomass. HFCs were compared between seedlings subjected to normal water conditions and drought-stressed seedlings (see key). The plotted regression lines are based on estimates of the significant fixed effects of the linear regression models (Table 3).
DISCUSSION
Our results showed weak but significant effects of MLH on several transpiration and growth traits in the temperate broadleaved tree species pedunculate oak (Q. robur). Since selectively neutral molecular markers were used to quantify genetic diversity, HFCs cannot readily be attributed to functional overdominance at the scored loci (the so-called direct effect hypothesis) (Queller et al., 1993; Savolainen and Hedrick, 1995), but they would require small non-random mating population structures to emerge. In these populations, processes such as genetic drift, inbreeding and recent bottlenecks are more likely to occur (Young et al., 1996), and consequently HFCs may arise as a result of associative overdominance, either at linked fitness loci (the local effect hypothesis) or at genome wide distributed loci (the general effect hypothesis; i.e. classical inbreeding depression) (Szulkin et al., 2010). Although Q. robur is a wind-pollinated species with a highly outcrossing breeding system (selfing rate 2–5 %; Steinhoff, 1993), it has been shown that, in tree species with similar traits, biparental inbreeding may lower the number of heterozygous individuals in small, fragmented forest stands (Jump and Peñuelas, 2006; Vranckx et al., 2012). Furthermore, recent research conducted in the same forest stands by Vranckx et al. (2014) has demonstrated less diverse pollen pools and increased correlated paternity in small stands. Ultimately, this may lead to stronger effects of biparental inbreeding in subsequent generations. In this study some variation in MLH was already detected for individual seedlings. However, at the population scale we found high heterozygosity levels, which were consistent with what was found in other population genetic studies on Q. robur (Mariette et al., 2002; Hampe et al., 2010).
Strength of HFCs in Q. robur seedlings
The observed proportion of the variance in transpiration and growth traits that was explained by MLH was substantially larger than that reported in earlier HFC studies (0·07–3·3 %; Szulkin et al., 2010). Furthermore, explained variances of ≤1 % for MLH have been reported to be common when microsatellite markers are used to quantify genetic diversity (Coltman and slate, 2003; Chapman et al., 2009). The stronger HFCs that we obtained can be attributed to several factors. First, since most empirical HFC studies have been performed on animal species with separate sexes (Coltman and Slate, 2003; Chapman et al., 2009; Szulkin et al., 2010), significant publication bias is likely. Plant species can be expected to show stronger HFCs than animal species, which may be related to the sessile nature and often self-compatible breeding systems of plants. This makes them more prone to selfing and biparental inbreeding, especially when habitat fragmentation occurs (González-Varo et al., 2012). Second, the choice of genetic metric may also influence the strength of the HFC (Slate and Pemberton, 2002; Chapman et al., 2009). Our study indicated that MLH had a higher power to detect HFCs than the variables d2 and sMd2. This is consistent with the results of previous meta-analyses (Coltman and Slate, 2003; Chapman et al., 2009) and may indicate the occurrence of recent inbreeding events rather than population admixture (Coulson et al., 1998; Pemberton et al., 1999). As already mentioned, d2 may show stronger HFCs than MLH when population admixture strongly increases the number of highly heterozygous individuals and when fast-evolving molecular markers (mutation rate >0·001) are used (Goudet and Keller, 2002). Third, contrary to previous studies in conifers (Ledig et al., 1983; Savolainen and Hedrick, 1995), we examined Q. robur seedlings under controlled common garden conditions, which increased the probability of detecting HFCs (David and Jarne, 1997; Keller and Waller, 2002). Heterozygosity–fitness correlations are expected to be stronger in seedlings compared with adult trees, as growth and survival are most strongly affected during the earliest life stages (David, 1998). Adult trees may cope better with stressful conditions such as drought stress as their roots may access deeper water sources, whereby higher rates of transpiration and photosynthesis are maintained (Bond, 2000; Cavender-Bares and Bazzaz, 2000). Furthermore, less fit homozygotes, still present in the seedling cohort, may be absent from older generations (Honnay et al., 2008). Because of this gradual decrease in the number of homozygous individuals from seedlings to adult trees and the reduced environmental stress perceived by the adults, one would expect that under natural conditions HFCs would have been absent or undetectable in adult cohorts. However, Ziehe and Hattmer (2002) still found positive associations between heterozygosity level and diameter growth of adult trees in natural populations of the temperate broadleaved tree species Fagus sylvatica.
Perhaps the most important factor influencing the magnitude of HFCs is the fitness trait under study. In our study, transpiration and growth traits showed (marginally) significant relationships with MLH. HFCs were, however, stronger for transpiration variables (R2p = 4–11 %) than for growth traits (R2p = 3–5 %). When Q. robur seedlings are facing drought stress, stomatal conductance is directly reduced as a result of a highly efficient stomatal control mechanism. This allows the leaf water potential to remain above the critical threshold value at which cavitation damage occurs (Vivin et al., 1993; Cochard et al., 1996). Contrary to the growth traits, which were recorded over a longer period of time, the transpiration variables were measured at midday (1230–1530 h). Consequently, transpiration was strongly affected, as the seedlings were exposed to the highest possible values of VPD and high levels of PAR (Table 2). Similar midday depression of gas exchange has been reported frequently in oaks and many other tree species (Weber and Gates, 1990; Epron and Dreyer, 1993) and was confirmed in this study by the decreased midday stomatal conductance (−9·6 %) of the drought-stressed seedlings on day 35.
Another possible explanation for the stronger correlations between transpiration variables and MLH is that these HFCs could be the result of an association with a microsatellite near the coding region of the studied trait (the local effect hypothesis). Although theoretical papers often suggest that transpiration and growth traits are both typically controlled by numerous loci (David, 1998; Szulkin et al., 2010), recent research has detected a relatively limited number of quantitative trait loci (QTLs) for growth rate and several transpiration traits in Q. robur (Scotti-Saintagne et al., 2004; Brendel et al., 2008; Gailing et al., 2008). For example, in the study of Scotti-Saintagne et al. (2004) three QTLs for height growth were found which explained between 9·5 and 18·7 % of the mean variance. One of the problems of QTLs is that they may be specific to a given environment, growth stage or genetic background, because of which QTL consistency across studies is often low (Teulat et al., 2001). Only in the study of Gailing et al. (2008) were some of the microsatellites used in our study located within QTLs for height growth and stomatal density. For height increment, two microsatellites (QpZag 104 and QpZag 46) were positioned within one QTL region (LG2M), explaining 3·4 % of the variance in height growth, whereas for stomatal density three microsatellites (QpZag 104, QpZag 46 and QpZag 9) occurred within two QTLs (LG2F and LG7F), explaining a higher percentage (3·7 and 7·2 %, respectively) of the phenotypic variance (Gailing et al., 2008). It has been shown that increased stomatal density may improve drought resistance, as stomata present at high density are often smaller and have small guard cells, which contributes to better control of transpiration (Roussel et al., 2009).
Interaction between drought stress and heterozygosity
It has been claimed that HFCs are more pronounced under elevated environmental stress levels than under optimal conditions (Armbruster and Reed, 2005; Lesbarrères et al., 2005), possibly exacerbating climate change effects in small, inbred tree populations. In our study, little evidence was found to support this hypothesis, as most of the examined fitness traits showed no significant interaction between irrigation treatment and MLH. However, for the water potential range, we found stronger HFCs in drought-stressed seedlings compared with well watered seedlings. Plants can recover from water deficits overnight through internal hydraulic redistribution, which removes water potential gradients among leaves and roots (Domec et al., 2004; Bauerle et al., 2008). This will alleviate plant water stress, as root function, cell turgor for growth and leaf water content are maintained (Nardini and Pitt, 1999). The variation in recovery rate of the water potential that was observed in the drought-stressed seedlings was influenced by the level of midday leaf water potential, which, in turn was affected by the individual MLH.
Next to the limited effect of the irrigation treatment, the strength of the HFCs may also have been influenced by the atmospheric stress level. Stomatal conductance was more correlated with MLH on days 49 and 65, which were both characterized by high VPD and PAR levels (Table 2), whereas on day 35 there were lower atmospheric stress levels and weaker HFCs (Table 3). Previous studies on tree transpiration have indicated not only that the physiological response of trees to drought stress depends on the water status of the soil, but also that the VPD and PAR level may also play a major role in transpiration and growth (Van Hees, 1997; Oren and Pataki, 2001; Zweifel et al., 2005). The significant interaction between irrigation treatment and MLH that was observed on day 49 might be attributed to the combined effect of low soil moisture and high atmospheric stress (e.g. high VPD and PAR), which may have imposed stronger overall drought stress conditions on seedlings (Van Hees, 1997). This is in contrast with the findings of Mitton (1993) and Audo and Diehl (1995), who found stronger HFCs at moderate stress levels, whereas we demonstrated that higher stress levels (especially atmospheric stress) exacerbated the effects of low genetic diversity on tree transpiration.
Implications for future forest management under climate change
The existence of HFCs in natural populations of pedunculate oak indicate that increased homozygosity could ultimately limit biomass production below the potential yield. We found that relative growth rates of biomass production and woody volume declined with increasing number of homozygous loci under both irrigation treatments. Considering ongoing climate change, the projected increase in temperature of 2·0–3·1 °C for Central Europe by 2100 (CMIP5 model, scenario RCP4·5, 25th–75th percentile), will raise the VPD of the air by 3–6 % °C−1 (Kirschbaum, 2000; Stocker et al., 2013), leading to greater exposure of trees to atmospheric drought stress. Since we have shown stronger relationships between transpiration rates and MLH under higher VPD levels and since biomass production was strongly correlated with total transpiration, one can expect lower biomass production in homozygous individuals in the future. Moreover, more frequent and severe drought events may also limit the water availability in the soil, which may worsen the effect of high levels of atmospheric stress (Kirschbaum, 2000; Zweifel et al., 2005). In our study, however, the effect of limited soil water availability on the strength of the HFCs was only observed for measurements of stomatal conductance on day 49 (highest VPD), indicating a large tolerance of pedunculate oak seedlings to soil water stress (Van Hees, 1997; Bréda et al., 2006). Furthermore, since the roots of adult trees may tap into deeper water sources, they will be even less susceptible to soil water stress than seedlings (Bond, 2000; Cavender-Bares and Bazzaz, 2000).
To narrow the gap between average and potential yields under current and future environmental conditions, individual MLH should be maximized in forest tree breeding programmes. In naturally regenerating forest stands, intense natural selection at the seedling stage may preserve high levels of MLH in older age classes (Bush and Smouse, 1991). However, natural regeneration of oak is often problematic due to factors such as low seed quantity and quality, strong predation and the lack of appropriate site conditions (Lorimer, 1992; Abrams, 2003). Moreover, in small fragmented forest stands, which are common in many parts of Western Europe, inbreeding may reduce the number of seedlings with high MLH. Extensive gene flow between stands may mitigate loss of genetic diversity. However, we previously showed that even in wind-pollinated species, such as oak, a reduction in tree density or population size may decrease local pollen diversity and increase correlated paternity (Vranckx et al., 2014), which may lead to stronger effects of biparental inbreeding in subsequent generations. So, even under high pollen immigration rates from outside the stand, an important fraction of mating events will occur at short distances between neighbouring trees (Sork et al., 2002; Breed et al., 2013). Processes such as biparental inbreeding and reduced gene flow may be avoided by the maintenance of large continuous forest stands (Jump and Peñuelas, 2006; Vranckx et al., 2012). Not only may this increase MLH, but it will also retain and enlarge the gene pool for adaptation, which is probably the best strategy to counter current and future environmental changes (Hamrick, 2004; Jump et al., 2009).
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are grateful to the following institutions and persons for their contribution to this study: the Institute for Nature and Forest Research (INBO) of the Flemish Government for assistance with conducting the genetic analyses; the Agency for Nature and Forests (ANB) for providing research permits; and Kasper Van Acker and Poi Verwilt for assistance with the measurements.
APPENDIX
List of abbreviations and variables
- Abase
area at base of stem or branches
- Atop
area at top of stem or branches
- BD,base
branch diameter at base
- BD,top
branch diameter at top
- BL
branch length
- Bn
number of branches
- CT
cumulative transpiration
- CTi
cumulative transpiration standardized for vapour pressure deficit
- gs
leaf stomatal conductivity
- HFC
heterozygosity–fitness correlation
- Ln
number of leaves
- MLH
multilocus heterozygosity
- PAR
photosynthetically active radiation
- pF
suction pressure
- REW
relative extractable soil water
- RGR
relative growth rate
- RH
relative humidity
- R2p
partial R2 coefficient
- SD,base
stem diameter at base
- SD,top
stem diameter at top
- SL
stem length
- Ta
air temperature
- TE
transpiration efficiency
- TR
transpiration rate
- VPD
vapour pressure deficit
- VPDj
VPD on day j
- Vtot
total woody volume (stem + branches)
- WAG
above-ground biomass
- WC
water content
- WD
dry weight
- WF
fresh weight
- WP
water productivity
- Δψ
water potential range
- θFC
volumetric soil water content at field capacity
- θWP
volumetric soil water content at wilting point
- θV
volumetric moisture content
- ψmd
midday leaf water potential
- ψpd
predawn leaf water potential
LITERATURE CITED
- Abrams MD. Where has all the white oak gone? Bioscience. 2003;53:927–939. [Google Scholar]
- Alig RJ, Plantinga AJ, Ahn S, Kline JD. Land use changes involving forestry in the United States: 1952 to 1997, with projections to 2050. Portland: US Department of Agriculture, Forest Service, Pacific Northwest Research Station; 2003. [Google Scholar]
- Audo MC, Diehl WJ. Effect of quantity and quality of environmental stress on multilocus heterozygosity-growth relationships in Eisenia fetida (Annelida: Oligochaeta) Heredity. 1995;75:98–105. [Google Scholar]
- Aravanopoulos FA, Zsuffa L. Heterozygosity and biomass production in Salix eriocephala. Heredity. 1998;81:396–403. [Google Scholar]
- Armbruster P, Reed DH. Inbreeding depression in benign and stressful environments. Heredity. 2005;95:235–242. doi: 10.1038/sj.hdy.6800721. [DOI] [PubMed] [Google Scholar]
- Bary-Lenger A, Nebout JP. Le chêne. Alleur-Liège, Belgium: Éditions du Perron; 1993. [Google Scholar]
- Bauerle TL, Richards JH, Smart DR, Eissenstat DM. Importance of internal hydraulic redistribution for prolonging lifespan of roots in dry soil. Plant Cell & Environment. 2008;31:177–186. doi: 10.1111/j.1365-3040.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- Bond BJ. Age-related changes in photosynthesis of woody plants. Trends in Plant Science. 2000;5:349–353. doi: 10.1016/s1360-1385(00)01691-5. [DOI] [PubMed] [Google Scholar]
- Bréda N, Huc R, Granier A, Dreyer E. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Annals of Forest Science. 2006;63:625–644. [Google Scholar]
- Breed MF, Ottewell KM, Gardner MG, Marklund MHK, Dormontt EE, Lowe AJ. Mating patterns and pollinator mobility are critical traits in forest fragmentation genetics. Heredity. 2013. doi:10.1038/hdy.2013.48. [DOI] [PMC free article] [PubMed]
- Brendel O, Le Thiec D, Scotti-Saintagne C, Bodénès C, Kremer A, Guehl JM. Quantitative trait loci controlling water use efficiency and related traits in Quercus robur L. Tree Genetics & Genomes. 2008;4:263–278. [Google Scholar]
- Bush RM, Smouse PE. The impact of electrophoretic genotype on life history traits in Pinus taeda. Evolution. 1991;45:48l–498. doi: 10.1111/j.1558-5646.1991.tb04325.x. [DOI] [PubMed] [Google Scholar]
- Bush RM, Smouse PE, Ledig FT. The fitness consequences of multiple-locus heterozygosity: the relationship between heterozygosity and growth rate in pitch pine (Pinus rigida Mill.) Evolution. 1987;41:787–798. doi: 10.1111/j.1558-5646.1987.tb05853.x. [DOI] [PubMed] [Google Scholar]
- Cavender-Bares J, Bazzaz FA. Changes in drought response strategies with ontogeny in Quercus rubra: implications for scaling from seedlings to mature trees. Oecologia. 2000;124:8–18. doi: 10.1007/PL00008865. [DOI] [PubMed] [Google Scholar]
- Chapman JR, Nakagawa S, Coltman DW, Slate J, Sheldon BC. A quantitative review of heterozygosity–fitness correlations in animal populations. Molecular Ecology. 2009;18:2746–2765. doi: 10.1111/j.1365-294X.2009.04247.x. [DOI] [PubMed] [Google Scholar]
- Chybicki IJ, Burczyk J. Realized gene flow within mixed stands of Quercus robur L. and Q. petraea (Matt.) L. revealed at the stage of naturally established seedling. Molecular Ecology. 2010;19:2137–2151. doi: 10.1111/j.1365-294X.2010.04632.x. [DOI] [PubMed] [Google Scholar]
- Cochard H, Bréda N, Granier A. Whole tree hydraulic conductance and water loss regulation in Quercus during drought: evidence for stomatal control of embolism? Annales des Sciences Forestières. 1996;53:197–206. [Google Scholar]
- Coltman DW, Slate J. Microsatellite measures of inbreeding: a meta-analysis. Evolution. 2003;57:971–983. doi: 10.1111/j.0014-3820.2003.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Coulson TN, Pemberton JM, Albon SD, et al. Microsatellites reveal heterosis in red deer. Proceedings of the Royal Society of London B: Biological Sciences. 1998;265:489–495. doi: 10.1098/rspb.1998.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David P. Heterozygosity–fitness correlations: new perspectives on old problems. Heredity. 1998;80:531–537. doi: 10.1046/j.1365-2540.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- David P, Jarne P. Context-dependent survival differences among electrophoretic genotypes in natural populations of the marine bivalve Spisula ovalis. Genetics. 1997;146:335–344. doi: 10.1093/genetics/146.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domec JC, Warren JM, Meinzer FC, Brooks JR, Coulombe R. Native root xylem embolism and stomatal closure in stands of Douglas-fir and ponderosa pine: mitigation by hydraulic redistribution. Oecologia. 2004;141:7–16. doi: 10.1007/s00442-004-1621-4. [DOI] [PubMed] [Google Scholar]
- Donovan L, Ehleringer JR. Ecophysiological differences among juvenile and reproductive plants of several woody species. Oecologia. 1991;86:594–597. doi: 10.1007/BF00318327. [DOI] [PubMed] [Google Scholar]
- Dow BD, Ashley MV. Microsatellite analysis of seed dispersal and parentage of saplings in bur oak, Quercus macrocarpa. Molecular Ecology. 1996;5:615–627. [Google Scholar]
- Dow BD, Ashley MV, Howe HF. Characterization of highly variable (GA/CT) microsatellites in the bur oak, Quercus macrocarpa. Theoretical and Applied Genetics. 1995;91:137–141. doi: 10.1007/BF00220870. [DOI] [PubMed] [Google Scholar]
- Epron D, Dreyer E. Photosynthesis of oak leaves under water stress: maintenance of high photochemical efficiency of photosystem II and occurrence of non-uniform CO2 assimilation. Tree Physiology. 1993;13:107–117. doi: 10.1093/treephys/13.2.107. [DOI] [PubMed] [Google Scholar]
- Evans GC. The quantitative analysis of plant growth. Berkeley: University of California Press; 1972. [Google Scholar]
- Fort C, Fauveau ML, Muller F, Label P, Granier A, Dreyer E. Stomatal conductance, growth and root signaling in young oak seedlings subjected to partial soil drying. Tree Physiology. 1997;17:281–289. doi: 10.1093/treephys/17.5.281. [DOI] [PubMed] [Google Scholar]
- Gagné P, Dayton CM. Best regression model using information criteria. Journal of Modern Applied Statistical Methods. 2002;1:57. [Google Scholar]
- Gailing O, Langenfeld-Heyser R, Polle A, Finkeldey R. Quantitative trait loci affecting stomatal density and growth in a Quercus robur progeny: implications for the adaptation to changing environments. Global Change Biology. 2008;14:1934–1946. [Google Scholar]
- González-Varo JP, Aparicio A, Lavergne S, Arroyo J, Albaladejo RG. Contrasting heterozygosity-fitness correlations between populations of a self-compatible shrub in a fragmented landscape. Genetica. 2012;140:31–38. doi: 10.1007/s10709-012-9655-8. [DOI] [PubMed] [Google Scholar]
- Goudet J, Keller L. The correlation between inbreeding and fitness: does allele size matter? Trends in Ecology & Evolution. 2002;17:201–202. [Google Scholar]
- Granier A, Reichstein M, Bréda N, et al. Evidence for soil water control on carbon and water dynamics in European forests during the extremely dry year 2003. Agricultural and Forest Meteorology. 2007;143:123–145. [Google Scholar]
- Greenberg CH. Individual variation in acorn production by five species of southern Appalachian oaks. Forest Ecology and Management. 2000;132:199–210. [Google Scholar]
- Hampe A, El Masri L, Petit RJ. Origin of spatial genetic structure in an expanding oak population. Molecular Ecology. 2010;19:459–471. doi: 10.1111/j.1365-294X.2009.04492.x. [DOI] [PubMed] [Google Scholar]
- Hamrick JL. Response of forest trees to global environmental changes. Forest Ecology and Management. 2004;197:323–335. [Google Scholar]
- Hansson B, Westerberg L. On the correlation between heterozygosity and fitness in natural populations. Molecular Ecology. 2002;11:2467–2474. doi: 10.1046/j.1365-294x.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- Hedrick PW, Kalinowski ST. Inbreeding depression in conservation biology. Annual Review of Ecology and Systematics. 2000;31:139–162. [Google Scholar]
- Hedrick PW, Fredrickson R, Ellegren H. Evaluation of d2, a microsatellite measure of inbreeding and outbreeding, in wolves with a known pedigree. Evolution. 2001;55:1256–1260. doi: 10.1111/j.0014-3820.2001.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Van Hees AFM. Growth and morphology of pedunculate oak (Quercus robur L) and beech (Fagus sylvatica L) seedlings in relation to shading and drought. Annales des Sciences Forestières. 1997;54:9–18. [Google Scholar]
- Hemery GE, Savill PS, Pryor SN. Applications of the crown diameter–stem diameter relationship for different species of broadleaved trees. Forest Ecology and Management. 2005;215:285–294. [Google Scholar]
- Honnay O, Bossuyt B, Jacquemyn H, Shimono A, Uchiyama K. Can a seed bank maintain the genetic variation in the above ground plant population? Oikos. 2008;117:1–5. [Google Scholar]
- Jump AS, Peñuelas J. Genetic effects of chronic habitat fragmentation in a wind-pollinated tree. Proceedings of the National Academy of Sciences of the USA. 2006;103:8096–8100. doi: 10.1073/pnas.0510127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump AS, Marchant R, Peñuelas J. Environmental change and the option value of genetic diversity. Trends in Plant Science. 2009;14:51–58. doi: 10.1016/j.tplants.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Kampfer S, Lexer C, Glössl J, Steinkellner H. Characterization of (GA) microsatellite loci from Quercus robur. Hereditas. 1998;129:183–186. [Google Scholar]
- Keller LF, Waller DM. Inbreeding effects in wild populations. Trends in Ecology & Evolution. 2002;17:230–241. [Google Scholar]
- King D. Tree dimensions: maximizing the rate of height growth in dense stands. Oecologia. 1981;51:351–356. doi: 10.1007/BF00540905. [DOI] [PubMed] [Google Scholar]
- Kirschbaum MUF. Forest growth and species distribution in a changing climate. Tree Physiology. 2000;20:309–322. doi: 10.1093/treephys/20.5-6.309. [DOI] [PubMed] [Google Scholar]
- Ledig FT, Guries RP, Bonefeld BA. The relation of growth to heterozygosity in pitch pine. Evolution. 1983;37:1227–1238. doi: 10.1111/j.1558-5646.1983.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Lesbarrères D, Primmer SR, Laurila A, Merilä J. Environmental and population dependency of genetic variability-fitness correlations in Rana temporaria. Molecular Ecology. 2005;14:311–323. doi: 10.1111/j.1365-294X.2004.02394.x. [DOI] [PubMed] [Google Scholar]
- Lindenmayer DB, Franklin JF. Conserving forest biodiversity: a comprehensive multi-scaled approach. Washington: Island Press; 2002. [Google Scholar]
- Lindner M, Maroschek M, Netherer S, et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. Forest Ecology and Management. 2010;259:698–709. [Google Scholar]
- Lorimer CG. Causes of the oak regeneration problem. In: Loftis DL, McGee CE, editors. Oak regeneration: serious problems, practical recommendations. General Technical Report SE-84. Washington: US Forest Service; 1992. pp. 14–39. [Google Scholar]
- Manly BFJ. The statistics of natural selection. London: Chapman and Hall; 1985. [Google Scholar]
- Manzanera JA, Astorga R, Bueno MA. Somatic embryo induction and germination in Quercus suber L. Silvae Genetica. 1993;42:90–93. [Google Scholar]
- Mariette S, Cottrell J, Csaikl UM, et al. Comparison of levels of genetic diversity detected with AFLP and microsatellite markers within and among mixed Q. petraea (Matt.) Liebl. and Q. robur L. stands. Silvae Genetica. 2002;51:72–79. [Google Scholar]
- McCree KJ. Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agricultural Meteorology. 1972;10:443–453. [Google Scholar]
- McDowell N, Pockman WT, Allen CD, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- Millennium Ecosystem Assessment. Ecosystems and human wellbeing: current state and trends. Washington: Island Press; 2005. [Google Scholar]
- Mitton JB. Enzyme heterozygosity, metabolism, and developmental stability. Genetica. 1993;89:47–65. [Google Scholar]
- Mitton JB, Knowles P, Sturgeon KB, Linhart YB, Davis M. Associations between heterozygosity and growth rate variables in three western forest trees. In: Conkle MT, editor. Proceedings of the Symposium on Isozymes of North American Forest Trees and Forest Insects. Washington: US Forest Service; 1981. pp. 27–34. [Google Scholar]
- Nardini A, Pitt F. Drought resistance of Quercus pubescens as a function of root hydraulic conductance, xylem embolism and hydraulic architecture. New Phytologist. 1999;143:485–493. doi: 10.1046/j.1469-8137.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- Noss RF. Beyond Kyoto: forest management in a time of rapid climate change. Conservation Biology. 2001;15:578–590. [Google Scholar]
- Oren R, Pataki DE. Transpiration in response to variation in microclimate and soil moisture in southeastern deciduous forests. Oecologia. 2001;127:549–559. doi: 10.1007/s004420000622. [DOI] [PubMed] [Google Scholar]
- Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley PF. Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes. 2004;4:535–538. [Google Scholar]
- Pemberton JM, Coltman DW, Coulson TN, Slate J. Using microsatellites to measure the fitness consequences of inbreeding and outbreeding. In: Goldstein DB, Schlötterer C, editors. Microsatellites: Evolution and Applications. Oxford: Oxford University Press; 1999. pp. 151–164. [Google Scholar]
- Queller DC, Strassmann JE, Hughes CR. Microsatellites and kinship. Trends in Ecology & Evolution. 1993;8:285–288. doi: 10.1016/0169-5347(93)90256-O. [DOI] [PubMed] [Google Scholar]
- Reed DH, Frankham R. Correlation between fitness and genetic diversity. Conservation Biology. 2003;17:230–237. [Google Scholar]
- Roussel M, Dreyer E, Montpied P, Le-Provost G, Guehl JM, Brendel O. The diversity of 13C isotope discrimination in a Quercus robur full-sib family is associated with differences in intrinsic water use efficiency, transpiration efficiency, and stomatal conductance. Journal of Experimental Botany. 2009;60:2419–2431. doi: 10.1093/jxb/erp100. [DOI] [PubMed] [Google Scholar]
- Savolainen O, Hedrick P. Heterozygosity and fitness: no association in Scots pine. Genetics. 1995;140:755–766. doi: 10.1093/genetics/140.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti-Saintagne C, Bodénès C, Barreneche T, Bertocchi E, Plomion C, Kremer A. Detection of quantitative trait loci controlling bud burst and height growth in Quercus robur L. Theoretical and Applied Genetics. 2004;109:1648–1659. doi: 10.1007/s00122-004-1789-3. [DOI] [PubMed] [Google Scholar]
- Slate J, Pemberton JM. Comparing molecular measures for detecting inbreeding depression. Journal of Evolutionary Biology. 2002;15:20–31. [Google Scholar]
- Sork VL, Davis FW, Smouse PE, et al. Pollen movement in declining populations of California valley oak, Quercus lobata: where have all the fathers gone? Molecular Ecology. 2002;11:1657–1668. doi: 10.1046/j.1365-294x.2002.01574.x. [DOI] [PubMed] [Google Scholar]
- Steduto P. FAO expert meeting on crop water productivity under deficient water supply. Rome: FAO; 2003. Biomass water-productivity. Comparing the growth-engines of crop models. [Google Scholar]
- Steduto P, Hsiao TC, Fereres E. On the conservative behavior of biomass water productivity. Irrigation Science. 2007;25:189–207. [Google Scholar]
- Steinhoff S. Results of species hybridization with Quercus robur L. and Quercus petraea (Matt.) Liebl. Annals of Forest Science. 1993;53:173–180. [Google Scholar]
- Steinkellner H, Fluch S, Turetschek E, et al. Identification and characterization of (GA/CT)n-microsatellite loci from Quercus petraea. Plant Molecular Biology. 1997;33:1093–1096. doi: 10.1023/a:1005736722794. [DOI] [PubMed] [Google Scholar]
- Stocker TF, Qin D, Platner GK, et al. Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2013. [Google Scholar]
- Simberloff D. The role of science in the preservation of forest biodiversity. Forest Ecology and Management. 1999;115:101–111. [Google Scholar]
- Szulkin M, Bierne N, David P. Heterozygosity-fitness correlations: a time for reappraisal. Evolution. 2010;64:1202–1217. doi: 10.1111/j.1558-5646.2010.00966.x. [DOI] [PubMed] [Google Scholar]
- Teulat B, Borries C, This D. New QTLs identified for plant water status, water-soluble carbohydrate and osmotic adjustment in a barley population grown in a growth-chamber under two water regimes. Theoretical and Applied Genetics. 2001;103:161–170. [Google Scholar]
- Thompson I, Mackey B, McNulty S, Mosseler S. Forest resilience, biodiversity, and climate change. A synthesis of the biodiversity/resilience/stability relationship in forest ecosystems. Montreal: Secretariat of the Convention on Biological Diversity; 2009. [Google Scholar]
- Vivin P, Aussenac G, Levy G. Differences in drought resistance among three deciduous oak species grown in large boxes. Annales des Sciences Forestières. 1993;50:221–233. [Google Scholar]
- Vranckx G, Jacquemyn H, Muys B, Honnay O. Meta-analysis of susceptibility of woody plants to loss of genetic diversity through habitat fragmentation. Conservation Biology. 2012;26:228–237. doi: 10.1111/j.1523-1739.2011.01778.x. [DOI] [PubMed] [Google Scholar]
- Vranckx G, Jacquemyn H, Mergeay J, et al. Transmission of genetic variation from the adult generation to naturally established seedling cohorts in small forest stands of pedunculate oak (Quercus robur L.) Forest Ecology and Management. 2014;312:19–27. [Google Scholar]
- Weber JA, Gates DM. Gas exchange in Quercus rubra (northern red oak) during a drought: analysis of relations among photosynthesis, transpiration and leaf conductance. Tree Physiology. 1990;7:215–225. doi: 10.1093/treephys/7.1-2-3-4.215. [DOI] [PubMed] [Google Scholar]
- West P. Tree and forest measurement. Heidelberg: Springer; 2009. [Google Scholar]
- Wiersum KF, Elands BHM, Hoogsta MA. Small-scale forest ownership across Europe: characteristics and future potential. Small-scale Forest Economics, Management And Policy. 2005;4:1–9. [Google Scholar]
- Willi Y, Van Buskirk J, Hoffmann AA. Limits to the adaptive potential of small populations. Annual Review of Ecology, Evolution, and Systematics. 2006;37:433–458. [Google Scholar]
- Yeh FC, Yang R-C, Boyle T. POPGENE, version 1·32, the user friendly software for population genetic analysis. Edmonton: Molecular Biology and Biotechnology Centre, University of Alberta; 1999. [Google Scholar]
- Young AG, Boyle T, Brown AHD. The population genetic consequences of habitat fragmentation for plants. Trends in Ecology & Evolution. 1996;11:413–418. doi: 10.1016/0169-5347(96)10045-8. [DOI] [PubMed] [Google Scholar]
- Ziehe M, Hattemer HH. Target-diameter felling and consequences for genetic structures in a beech stand (Fagus sylvatica L.) In: von Gadow K, Nagel J, Saborowski J, editors. Continuous cover forestry: assessment, analysis, scenarios. Dordrecht: Kluwer Academic Publishers; 2002. pp. 91–105. [Google Scholar]
- Zweifel R, Zimmermann L, Newbery DM. Modeling tree water deficit from microclimate: an approach to quantifying drought stress. Tree Physiology. 2005;25:147–156. doi: 10.1093/treephys/25.2.147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.