Abstract
Background and Aims
The networks of vessel elements play a vital role in the transport of water from roots to leaves, and the continuous formation of earlywood vessels is crucial for the growth of ring-porous hardwoods. The differentiation of earlywood vessels is controlled by external and internal factors. The present study was designed to identify the limiting factors in the induction of cambial reactivation and the differentiation of earlywood vessels, using localized heating and disbudding of dormant stems of seedlings of a deciduous ring-porous hardwood, Quercus serrata.
Methods
Localized heating was achieved by wrapping an electric heating ribbon around stems. Disbudding involved removal of all buds. Three treatments were initiated on 1 February 2012, namely heating, disbudding and a combination of heating and disbudding, with untreated dormant stems as controls. Cambial reactivation and differentiation of vessel elements were monitored by light and polarized-light microscopy, and the growth of buds was followed.
Key Results
Cambial reactivation and differentiation of vessel elements occurred sooner in heated seedlings than in non-heated seedlings before bud break. The combination of heating and disbudding of seedlings also resulted in earlier cambial reactivation and differentiation of first vessel elements than in non-heated seedlings. A few narrow vessel elements were formed during heating after disbudding, while many large earlywood vessel elements were formed in heated seedlings with buds.
Conclusions
The results suggested that, in seedlings of the deciduous ring-porous hardwood Quercus serrata, elevated temperature was a direct trigger for cambial reactivation and differentiation of first vessel elements. Bud growth was not essential for cambial reactivation and differentiation of first vessel elements, but might be important for the continuous formation of wide vessel elements.
Keywords: Cambial activity, deciduous ring-porous hardwoods, disbudding, formation of earlywood vessel, localized heating, oak, Quercus serrata
INTRODUCTION
Three-dimensional networks of vessel elements play an important role in the movement of water from roots to leaves (Zimmermann, 1982; Kitin et al., 2004). In ring-porous hardwoods, earlywood vessels with wide diameters, which are formed at the beginning of the growth season, function in the transport of water in the current year only (Utsumi et al., 1996, 1999; Umebayashi et al., 2008). Therefore, the continuous formation of earlywood vessels is crucial for the growth of ring-porous hardwoods. It is reasonable to assume that induction of the formation of earlywood vessels is dependent on external and internal factors.
Cambial activity and formation of earlywood vessels are known to be controlled by environmental factors (Denne and Dodd, 1981; Fonti et al., 2007; Dié et al., 2012). Localized heating of stems during the quiescent stage of dormancy revealed that increases in temperature-induced cambial reactivation and xylem differentiation occur directly in evergreen conifers (Savidge and Wareing, 1981; Barnett and Miller, 1994; Oribe and Kubo, 1997; Oribe et al., 2001, 2003; Gricar et al., 2006, Begum et al., 2010a, b, 2012) and in a deciduous diffuse-porous hardwood, Populus (Begum et al., 2007). However, to our knowledge, there are no reports of the effects of localized heating on the induction of cambial reactivation and differentiation of earlywood vessels in deciduous ring-porous hardwoods.
In poplar, cambial reactivation was induced by localized heating without flushing of buds, suggesting that cambial reactivation might be independent of the growth of buds or shoots (Begum et al., 2007). In contrast, xylem differentiation started after bud break (Begum et al., 2007), suggesting that some other factor(s) derived from buds and/or new leaves might be required for xylem differentiation. Indole-3-acetic acid (IAA), which is transported by basipetal polar movement in cambial regions (Uggla et al., 1996, 1998; Tuominen et al., 1997), is one of the most important internal factors in the control of cambial activity and xylem differentiation (Aloni et al., 2000; Sundberg et al., 2000). IAA, which is mainly produced by young developing leaves, appears to be an essential stimulant for the induction of differentiation of vessel elements in hardwoods (Wareing, 1958; Digby and Wareing, 1966; Sachs, 1981; Savidge and Wareing, 1981; Aloni, 1991; Björklund et al., 2007). However, the differentiation of first vessel elements has been observed in deciduous ring-porous hardwoods before leaf expansion (Zasada and Zahner, 1969; Atkinson and Denne, 1988; Aloni and Peterson, 1997; Suzuki et al., 1996; Sass et al., 2011). Aloni (1991) reported that, in stems of a disbudded ring-porous hardwood, approx. 1 month prior to bud break, only a few isolated and very narrow vessels had been formed. The cited studies indicate that bud growth might be only a minor factor in the control of the differentiation of first vessel elements.
The present study was designed to identify the limiting factor(s) in the induction of cambial reactivation and the differentiation of earlywood vessels in seedlings of Quercus serrata. We subjected dormant stems to localized heating to determine whether an increase in temperature could induce cambial reactivation and formation of earlywood vessels in this deciduous ring-porous hardwood. We also subjected stems to disbudding to determine whether the induction of cambial reactivation and formation of earlywood vessels might be independent of bud growth. Our experiment involved three treatments of dormant stems, namely localized heating, disbudding and a combination of localized heating and disbudding, with untreated dormant stems serving as controls. Then we monitored cambial reactivation, differentiation of vessel elements and bud growth for 84 d.
MATERIALS AND METHODS
Plant materials
Fifty seedlings of Quercus serrata (2–3 years old, average height, 102·6 ± 8·7 cm), potted in the field at Tokyo University of Agriculture and Technology in Fuchu, Tokyo (35°40′N, 139°29′E), Japan, were subjected to analysis.
Heating and disbudding treatments
Electric heating ribbon (Nippon Heater Co., Ltd, Tokyo, Japan), 6 m long and 0·5 cm wide, was wrapped around individual stems to produce a 5 cm wide band, at 40 cm above the ground, on each heated stem (Fig. 1) (Begum et al., 2012). The temperature of the interface between the heating ribbon and the outer bark was adjusted to approx. 20 ± 5 °C with a thermometer (TC-1NP; As One Co., Osaka, Japan) and recorded with a data logger (Ondotori Jr. TR-52; T&D Co., Matsumoto, Japan). Localized heating treatment was initiated on 1 February 2012 and continued until 25 April 2012 (84 d).
Fig. 1.
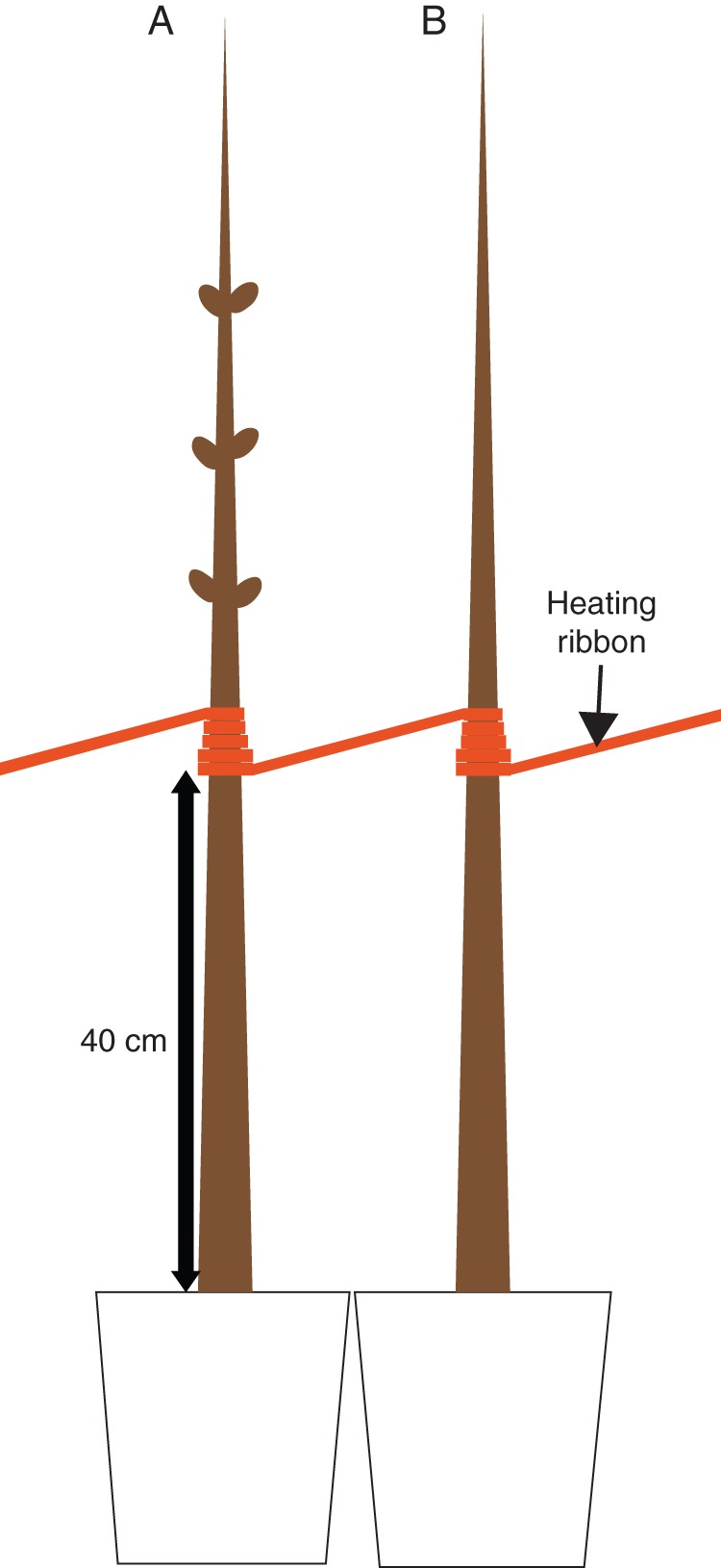
Electric heating ribbon wrapped around the stems of seedlings of Quercus serrata. The heated (A) and the heated plus disbudded (B) seedlings.
Prior to localized heating, some stems were disbudded on 1 February 2012. All buds on the entire stem were removed and petroleum jelly was applied to the sites from where buds had been removed in order to prevent dehydration.
Collection and preparation of samples for microscopy
Four groups of stems, namely controls without any treatment, heated stems, disbudded stems and heated plus disbudded stems were prepared. Two seedlings for each of treatment were harvested at approx. 2-week intervals from 1 February to 25 April 2012. On each sampling date, the conditions of buds and/or shoots were recorded with a digital camera. Samples containing phloem, cambium and some xylem cells were collected from positions 40–45 cm above the ground, where heating ribbon had been applied. Samples were fixed in 4 % glutaraldehyde in 0·1 m phosphate buffer (pH 7·3) at room temperature. Fixed samples were washed in 0·1 m phosphate buffer and trimmed to small blocks. Small blocks were dehydrated in a graded ethanol series and embedded in epoxy resin. Transverse and radial sections were cut at a thickness of 2 μm on a rotary microtome (HM 340E; Carl Zeiss, Germany) and at a thickness of 1 μm with glass knives on an ultramicrotome (Ultracut N; Reichert, Vienna, Austria). Sections were stained with a solution of 0·1 % safranin in water. The sections were examined by light and polarized-light microscopy (Axioskop; Carl Zeiss, Oberk-ochen, Germany) as described by Begum et al. (2012).
RESULTS
No division of fusiform cambial cells and ray cambial cells was observed on 1 February 2012, when we started our experiment (Fig. 2A, B). The cambial zone consisted of 3–5 radial layers of narrow and compactly arranged cells. These observations indicated that the cambium was dormant (Begum et al., 2013). No cell division was observed on 17 February and buds had not burst on 17 February 2012 (Fig. 7A).
Fig. 2.
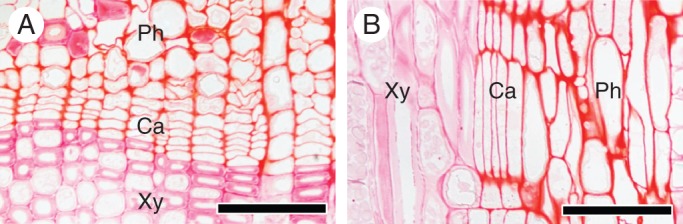
Light micrographs showing the cambial zone on 1 February 2012. The transverse view (A) and radial view (B) show that, in the cambium, there were no new thin cell plates and cells were arranged compactly. Thus, the cambium was dormant. Ph, phloem; Ca, cambium; Xy, xylem. Scale bars = 50 μm.
Fig. 7.

Bud growth on control and heated seedlings. Buds had not yet burst on 1 February (A) and 27 March, when cambial cell divisions were observed in heated and heated plus disbudded seedlings (B). Swelling of buds was observed on 13 April, when natural cambial reactivation and the differentiation of vessel elements were observed (C). Bud burst had occurred by 25 April (D). Scale bars = 0·5 cm.
On 13 March after 41 d, the control and the disbudded seedlings had no obvious cell divisions in the cambial zone (Fig. 3A, B). In contrast, in heated and in heated plus disbudded seedlings, new thin cell plates were observed in the cambial zone and in phloem cells, an indication that cambial reactivation had occurred earlier in heated seedlings than in non-heated seedlings and was independent of disbudding (Fig. 3C, D). The majority of first divisions of cambial cells were observed in the second layer of fusiform cambial cells, counted from the previous year's xylem, but cell division sometimes occurred in the first layer of fusiform cambial cells (Fig. 3C, D). On 13 March, buds on the control and the heated (but not disbudded) seedlings still had not burst.
Fig. 3.
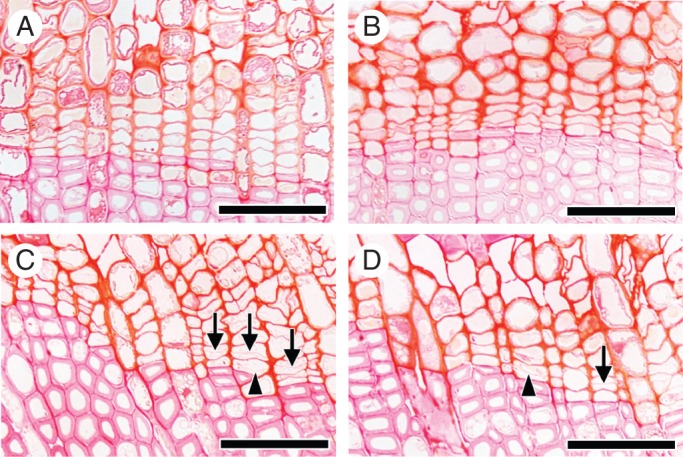
Light micrographs showing transverse views of the cambial zone after 41 d on 13 March 2012. In the control (A) and the disbudded seedlings (B), there were no new thin cell plates in the cambial zone. In contrast, in the heated portion of a heated seedling (C) and of a heated plus disbudded seedling (D), new thin cell plates were visible in the cambial zone. These new thin cell plates were observed in the first layer (arrowheads; C and D) and second or third layer (arrows; C and D) of fusiform cambial cells from the previous year's xylem. Division of cambial cells was observed in the heated portion of stem. Scale bars = 50 μm.
On 27 March, after 55 d, no new thin cell plates were found in the cambium and phloem cells in the non-heated control and disbudded seedlings (Fig. 4A, B). In contrast, in heated and in heated plus disbudded seedlings, vessel elements with deposition of secondary walls were observed under polarized light (Fig. 4C, D). The deposition of secondary walls in a few cells that surrounded mature vessel elements was also observed. On 27 March, buds still had not opened (Fig. 7B). Thus, differentiation of vessel elements progressed without buds and without bud burst. Most vessel elements in the heated and the combination-treated seedlings were found in the second layer from the boundary of the previous year's xylem, but a few vessel elements were also found in the first layer.
Fig. 4.
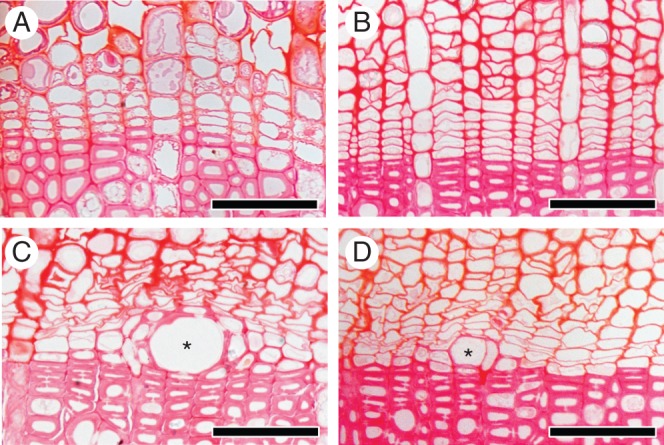
Light micrographs showing transverse views of the cambial zone after 55 d on 27 March 2012. In the control (A) and disbudded seedlings (B), there were no new thin cell plates in the cambial zone. In the heated portion of the heated seedlings (C) and of a heated plus disbudded seedling (D), vessel elements with deposition of secondary walls were visible (asterisks; C and D). Scale bars = 50 μm.
On 13 April, after 72 d, many wide vessel elements, located in the first to third layers from the previous year's xylem boundary, were observed in the non-heated control seedlings (Fig. 5A). In disbudded seedlings, many new cell plates were observed in the cambial zone and in phloem cells, but no differentiating vessel elements were detected (Fig. 5B). Swelling of buds was evident for the first time on control and heated (but not disbudded) seedlings (Fig. 7C).
Fig. 5.
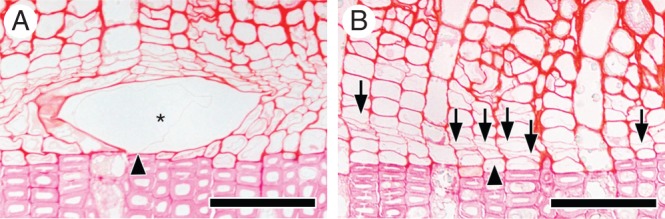
Light micrographs showing transverse views of the cambial zone and differentiating xylem after 72 d on 13 April 2012. In the control seedlings (A), enlarging vessel elements were observed (asterisk; A). Some vessel elements were located in the first layer from the previous year's xylem (arrowhead; A). In the disbudded seedling (B), many new thin cell plates were visible (arrows and arrowhead; B), demonstrating that cambial cell division occurred in the absence of buds. In some radial files, cell divisions in the first layer of fusiform cells from the previous year's xylem were observed (arrowhead; B). Scale bars = 50 μm.
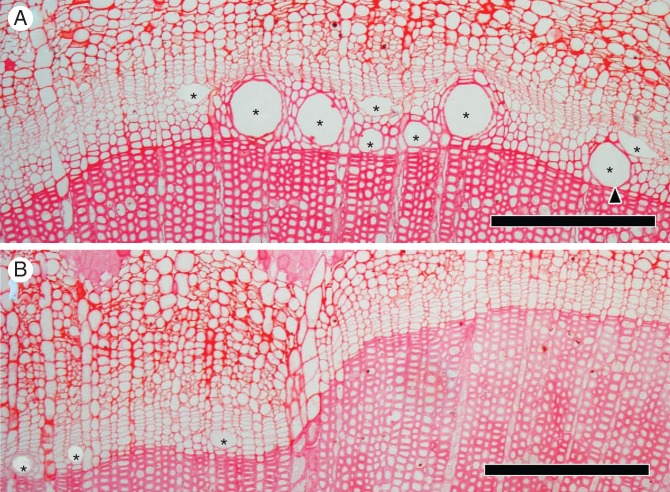
On 25 April, after 84 d, there were many wide vessel elements with secondary walls in heated (but not disbudded) seedlings (Fig. 6A). In contrast, a small number of narrow vessel elements with secondary walls and enlarging vessel elements were observed in the heated plus disbudded seedlings (Fig. 6B). On 25 April, buds had burst on both control and heated (but not disbudded) seedlings (Fig. 7D).
Fig. 6.
Light micrographs showing transverse views of the cambial zone and differentiating xylem after 84 d on 25 April 2012. In the heated seedling (A), there were many wide vessel elements (asterisks; A). Some vessel elements were found in the first layer from the previous year's xylem (arrowhead; A). In the heated portion of heated and disbudded seedlings (B), only a few narrow vessel elements were observed (asterisks; B). Scale bars = 200 μm.
The timing of cambial reactivation, differentiation of first vessel elements and bud development in all of treatments is summarized in Table 1. In heated seedlings, cambial reactivation and differentiation of first vessel elements started earlier than in non-heated seedlings.
Table 1.
Timing of cambial reactivation, differentiation of first vessel elements and bud development in seedlings of Quercus serrata
| Stages of cambial reactivation and differentiation of first vessel elements |
Stage of bud development |
|||||
|---|---|---|---|---|---|---|
| Date | Control | Disbudding | Heating | Heating plus disbudding | Control | Heating |
| 1 February | Dormant | – | – | – | Winter bud | Winter bud |
| 17 February | Dormant | Dormant | Dormant | Dormant | Winter bud | Winter bud |
| 13 March | Dormant | Dormant | Cambial reactivation | Cambial reactivation | Winter bud | Winter bud |
| 27 March | Dormant | Dormant | Deposition of secondary wall in vessel elements | Deposition of secondary wall in vessel elements | Winter bud | Winter bud |
| 13 April | Cambial reactivation and expansion of vessel elements | Cambial reactivation | Swelling | Swelling | ||
| 25 April | Deposition of secondary wall in vessel elements | Deposition of secondary wall in vessel elements | Bud break | Bud break | ||
DISCUSSION
Cambial reactivation and the differentiation of vessel elements were observed earlier in heated seedlings than in non-heated seedlings. The first vessel elements with secondary walls in heated seedlings were observed when the cambium of non-heated seedlings was still dormant. Thus, localized heating of dormant stems induced earlier cambial reactivation and the subsequent differentiation of first vessel elements in seedlings of the deciduous ring-porous hardwood Quercus serrata. It has been reported that localized heating of dormant stems induces earlier cambial reactivation and subsequent formation of secondary xylem than natural conditions in evergreen conifers, such as Pinus contorta (Savidge and Wareing, 1981), Picea sitchensis (Barnett and Miller, 1994), Cryptomeria japonica (Oribe and Kubo, 1997; Begum et al., 2012), Abies sachalinensis (Oribe et al., 2001, 2003), Abies firma (Begum et al., 2012) and Picea abies (Gricar et al., 2006), and a deciduous diffuse-porous hardwood, namely hybrid poplar (Populus sieboldii × P. grandidentata; Begum et al., 2007). In addition, under natural conditions, earlier cambial reactivation and differentiation of secondary xylem cells were observed in poplar when the weather was warmer than normal in late winter and early spring (Begum et al., 2008). Begum et al. (2008) proposed that the timing of cambial reactivation can be predicted from the accumulation of maximum daily temperature in degrees above a threshold value. Our observations indicate that an increase in temperature around the stem during dormancy is the most important limiting factor for cambial reactivation and the differentiation of first vessel elements in seedlings of the ring-porous hardwood Q. serrata. The increase in temperature around the dormant stem might provide a direct trigger for cambial reactivation and xylem differentiation in all types of temperate-zone trees.
Localized heating for 6 weeks in seedlings of Q. serrata studied here and 4 weeks in hybrid poplar (Begum et al., 2007) was required for cambial reactivation. In addition, in a deciduous conifer Larix leptolepis, 2 weeks of localized heating failed to induce cambial reactivation (Oribe and Kubo, 1997). In contrast, in evergreen conifers, such as Abies sachalinensis (Oribe et al., 2001, 2003), C. japonica (Begum et al., 2010a, b, 2012) and seedlings of A. firma (Begum et al., 2012), localized heating for 2–6 d induced cambial reactivation. Longer localized heating of the stems of deciduous trees, as compared with those of evergreen conifers, might be required for the conversion of cambium from a quiescent dormant state to an active state. Therefore, we suggest that, in deciduous trees, the state of dormancy is deeper than in evergreen conifers.
It has been reported that, under natural conditions, cambial reactivation and the differentiation of earlywood vessel begin prior to bud break in deciduous ring-porous hardwoods, such as Quercus rubur (Zasada and Zahner, 1969; Aloni and Peterson, 1997; Sass et al., 2011), Fraxinus excelsior (Atkinson and Denne, 1988; Sass et al., 2011), and Q. serrata, Q. acutissima, Castanea crenata, Zelkova serrata and Hovenia dulcis (Suzuki et al., 1996). In the cited studies, there was no obvious correlation between the initiation of differentiation of first vessel elements and bud growth. In the present study, earlier cambial reactivation and differentiation of first vessel elements were induced by localized heating in the absence of buds. Aloni (1991) reported similarly that only a few very narrow vessel elements were evident in stems of the deciduous ring-porous hardwood Melia azedarach that had been disbudded approx. 1 month prior to bud break. In addition, our data showed that there was no difference in terms of the timing of cambial reactivation and differentiation of first vessel elements between heated stems and heated plus disbudded stems. Apparently, the timing of cambial reactivation and differentiation of first vessel elements was unaffected by disbudding. Therefore, in the seedlings of the deciduous ring-porous hardwood Q. serrata, bud growth is not required for cambial reactivation and the differentiation of first vessel elements.
The auxin IAA, which is mainly produced by young and developing leaves, is essential for the induction of differentiation of vessel elements in deciduous diffuse-porous hardwood poplar (Wareing, 1958; Digby and Wareing, 1966; Björklund et al., 2007), in sycamore (Acer pseudoplatanus) and in deciduous ring-porous ash (F. excelsior; Wareing, 1958). However, our present results indicate that bud growth, which is associated with new supplies of auxin and an increase in the amount of endogenous IAA in cambium, is not essential for cambial reactivation and the differentiation of first vessel elements in Q. serrata. In winter dormancy, endogenous IAA was found in the cambial region of the deciduous ring-porous hardwood Q. rubur (Savidge and Wareing, 1982) and the deciduous conifer L. kaempferi (Funada et al., 2002). The total amount of endogenous IAA in L. kaempferi was constant during cambial reactivation (Funada et al., 2002). Thus, increases in total amounts of endogenous IAA that accompany the growth of the current year's buds do not act as the trigger for cambial reactivation and the differentiation of first vessel elements. The level of endogenous IAA in dormant quiescent cambium might be adequate for the maintenance of cambial cells, cambial reactivation and the differentiation of first vessel elements.
Previous reports have suggested that overwintering cells in the first or second layer of cambial cells, counted from the previous year's xylem boundary, might differentiate directly into vessel elements without cell division at the beginning of the growth season in ring-porous hardwoods such as F. excelsior (Doley and Leyton, 1968; Frankenstein et al., 2005), Q. rubur (Zasada and Zahner, 1969) and Fraxinus mandshurica var. japonica, Kalopanax pictus, Ulmus davidiana, Phellodendron amurense, Robinia pseudoacacia and Quercus mongolica var. grosseserrata (Imagawa and Ishida, 1972a, b). In the present study, we observed cell division from the first to the third layer in cambial cells from the previous year's xylem boundary. The first vessel elements were also found in the first to the third layers of cambial cells from the previous year's xylem boundary. Therefore, it was unclear whether overwintering cells had differentiated into vessel elements without cambial cell division. We observed the cell division of cambial cells before the expansion of vessel elements in all groups of seedlings examined. If overwintering cells differentiate directly into first vessel elements, sensitivity to increases in temperature might differ between cambial cells that will undergo cell division and overwintering cells that will expand.
After 84 d, on 25 April, when buds had opened in control stems, a few narrow vessel elements were observed in the heated plus disbudded seedlings, while many large earlywood vessel elements were found in heated seedlings. In deciduous ring-porous Melia azedarach, disbudding approx. 1 month prior to bud break resulted in differentiation of a few isolated and very narrow vessels (Aloni, 1991). This observation indicates that buds or bud growth, which might provide a continuous supply of IAA to cambium, is needed for the continuous formation of wide vessel elements.
In conclusion, in the present study, we found that, in seedlings of a deciduous ring-porous hardwood oak, localized heating of dormant stems induced cambial reactivation and the differentiation of first vessel elements in the absence of buds. Our results suggest that an increase in temperature around the stem might be one of the most important limiting factors for the start of cambial reactivation and the differentiation of first vessel elements. The initiation of cambial reactivation and differentiation of first vessel elements did not require buds or bud growth. However, buds and/or bud growth, which might increase the supply of IAA, appear to be essential for the continuous formation of wide vessel elements.
ACKNOWLEDGEMENTS
This work was supported, in part, by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (nos 20120009, 20·5659, 21380107, 22·00104, 23380105, 24380090 and 25850121). We are very grateful to the referees and editor for their constructive comments and suggestions.
LITERATURE CITED
- Aloni R. In: Wood formation in deciduous hardwood trees. Raghavendra AS, editor. NewYork: Wiley and Sons; 1991. pp. 175–197. Physiology of trees. [Google Scholar]
- Aloni R, Peterson CA. Auxin promotes dormancy callose removal from the phloem of Magnolia kobus and callose accumulation and earlywood vessel differentiation in Quercus robur. Journal of Plant Research. 1997;110:37–44. doi: 10.1007/BF02506841. [DOI] [PubMed] [Google Scholar]
- Aloni R, Feigenbaum P, Kalev N, Rozovsky S. In: Hormonal control of vascular differentiation in plants: the physiological basis of cambium ontogeny and xylem evolution. Savidge RA, Barnnet JR, Napier R, editors. Oxford: BIOS Scientific Publishers; 2000. pp. 223–236. Cell and molecular biology of wood formation. [Google Scholar]
- Atkinson CJ, Denne MP. Reactivation of vessel production in ash (Fraxinus excelsior L.) trees. Annals of Botany. 1988;61:679–688. [Google Scholar]
- Barnett JR, Miller H. The effect of applied heat on graft union formation in dormant Picea sitchensis (Bong.) Carr. Journal of Experimental Botany. 1994;45:135–143. [Google Scholar]
- Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii × P. grandidentata) Annals of Botany. 2007;100:439–447. doi: 10.1093/aob/mcm130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Bayrmzadeh V, Oribe Y, Kubo T, Funada R. Response to ambient temperature of cambial reactivation and xylem differentiation in hybrid poplar (Populus sieboldii × P. grandidentata) under natural conditions. Tree Physiology. 2008;28:1813–1819. doi: 10.1093/treephys/28.12.1813. [DOI] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Cambial sensitivity to rising temperature from late winter to early spring in the evergreen conifer Cryptomeria japonica. Trees. 2010a;24:43–52. [Google Scholar]
- Begum S, Nakaba S, Oribe Y, Kubo T, Funada R. Changes in the localization and levels of starch and lipids in cambium and phloem during cambial reactivation by artificial heating of main stems of Cryptomeria japonica trees. Annals of Botany. 2010b;106:885–895. doi: 10.1093/aob/mcq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Yamagishi Y, et al. A rapid decrease in temperature induces latewood formation in artificially reactivated cambium of conifer stems. Annals of Botany. 2012;110:875–885. doi: 10.1093/aob/mcs149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum S, Nakaba S, Yamagishi Y, Oribe Y, Funada R. Regulation of cambial activity in relation to environmental conditions: understanding the role of temperature in wood formation of trees. Physiologia Plantarum. 2013;147:46–54. doi: 10.1111/j.1399-3054.2012.01663.x. [DOI] [PubMed] [Google Scholar]
- Björklund S, Antti H, Uddestrand I, Moritz T, Sundberg B. Cross-talk between gibberellin and auxin in development of Populus wood: gibberellin stimulates polar auxin transport and has a common transcriptome with auxin. The Plant Journal. 2007;52:499–511. doi: 10.1111/j.1365-313X.2007.03250.x. [DOI] [PubMed] [Google Scholar]
- Denne MP, Dodd RS. In: The environmental control of xylem differentiation. Barnett JR, editor. Tunbridge Wells, UK: Castle House; 1981. pp. 236–255. Xylem cell development. [Google Scholar]
- Dié A, Kitin P, N'Guessan Kouamé F, Van den Blucke J, Van Acker J, Beeckman H. Fluctuations of cambial activity in relation to precipitation result in annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis) in Ivory Coast. Annals of Botany. 2012;110:861–873. doi: 10.1093/aob/mcs145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby J, Wareing PF. The effect of applied growth hormones on cambial division and the differentiation of the cambial derivatives. Annals of Botany. 1966;30:539–548. [Google Scholar]
- Doley D, Leyton L. Effects of growth regulating substances and water potential on the development of secondary xylem in Fraxinus. New Phytologist. 1968;67:579–594. [Google Scholar]
- Fonti P, Solomonoff N, Garcia-Gonzalez I. Earlywood vessels of Castanea sativa record temperature before their formation. New Phytologist. 2007;173:562–570. doi: 10.1111/j.1469-8137.2006.01945.x. [DOI] [PubMed] [Google Scholar]
- Funada R, Kubo T, Sugiyama T, Fushitani M. Changes in levels of endogenous plant hormones in cambial regions of stem of Larix kaempferi at the onset of cambial activity in springtime. Journal of Wood Science. 2002;48:75–80. [Google Scholar]
- Frankenstein C, Eckstein D, Schmitt U. The onset of cambium activity – a matter of agreement? Dendrochronologia. 2005;23:57–62. [Google Scholar]
- Gricar J, Zupancic M, Cufar K, Koch G, Schmitt U, Oven P. Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies) Annals of Botany. 2006;97:943–951. doi: 10.1093/aob/mcl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa K, Ishida S. Study on the wood formation in trees report II. Development of the vessel in earlywood of hari-giri, Kalopanax pictus. Research Bulletin of the College Experimental Forests, College of Agriculture, Hokkaido University. 1972a;29:55–72. [Google Scholar]
- Imagawa K, Ishida S. Study on the wood formation in trees report III. Occurrence of the overwintering cells in cambial zone in several ring-porous trees. Research Bulletin of the College Experimental Forests, College of Agriculture, Hokkaido University. 1972b;29:207–221. [Google Scholar]
- Kitin P, Fujii T, Abe H, Funada R. Anatomy of the vessel network within and between tree rings of Fraxinus lanuginosa (Oleaceae) American Journal of Botany. 2004;91:779–788. doi: 10.3732/ajb.91.6.779. [DOI] [PubMed] [Google Scholar]
- Oribe Y, Kubo T. Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiology. 1997;17:81–87. doi: 10.1093/treephys/17.2.81. [DOI] [PubMed] [Google Scholar]
- Oribe Y, Funada R, Shibagaki M, Kubo T. Cambial reactivation in locally heated stems of evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta. 2001;212:684–691. doi: 10.1007/s004250000430. [DOI] [PubMed] [Google Scholar]
- Oribe Y, Funada R, Kubo T. Relationship between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees. 2003;17:185–192. [Google Scholar]
- Sachs T. The control of the patterned differentiation of vascular tissues. Advances in Botanical Research. 1981;9:152–255. [Google Scholar]
- Sass-Klaassen U, Sabajo CR, Ouden JD. Vessel formation in relation to leaf phenology in pedunculate oak and European ash. Dendrochronologia. 2011;29:171–175. [Google Scholar]
- Savidge RA, Wareing PF. In: Plant growth regulators and the differentiation of vascular elements. Barnett JR, editor. Tunbridge Wells, UK: Castle House; 1981. pp. 192–235. Xylem cell development. [Google Scholar]
- Savidge RA, Wareing PF. Apparent auxin production and transport during winter in the nongrowing pine tree. Canadian Journal of Botany. 1982;60:681–691. [Google Scholar]
- Sundberg B, Uggla C, Tuominen H. In: Cambial growth and auxin gradients. Savidge RA, Barnnet JR, Napier R, editors. Oxford: BIOS Scientific Publishers; 2000. pp. 169–188. Cell and molecular biology of wood formation. [Google Scholar]
- Suzuki M, Yoda K, Suzuki H. Phenological comparison of onset of vessel formation between ring-porous and diffuse-porous deciduous trees in a Japanese temperate forest. IAWA Journal. 1996;17:431–444. [Google Scholar]
- Tuominen H, Puech L, Fink S, Sundberg B. A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiology. 1997;115:577–585. doi: 10.1104/pp.115.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B. Auxin as a positional signal in pattern formation in plants. Proceedings of the National Academy of Sciences, USA. 1996;93:9282–9286. doi: 10.1073/pnas.93.17.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Mellerowicz EJ, Sundberg B. Indole-3-acetic-acid controls cambial growth in Scots pine by positional signaling. Plant Physiology. 1998;117:113–121. doi: 10.1104/pp.117.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umebayashi T, Utsumi Y, Koga S, et al. Conducting pathways in north temperate deciduous broadleaved trees. IAWA Journal. 2008;29:247–263. [Google Scholar]
- Utsumi Y, Sano Y, Ohtani J, Fujikawa S. Seasonal changes in the distribution of water in the outer growth rings of Fraxinus mandshurica var. japonica: a study by cryo-scanning electron microscopy. IAWA Journal. 1996;17:113–124. [Google Scholar]
- Utsumi Y, Sano Y, Funada R, Fujikawa S, Ohtani J. The progression of cavitation in earlywood vessels of Fraxinus mandshurica var. japonica during freezing and thawing. Plant Physiology. 1999;121:897–904. doi: 10.1104/pp.121.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareing PF. Interaction between indole-acetic acid and gibberellic acid in cambial activity. Nature. 1958;181:1744–1745. doi: 10.1038/1811744a0. [DOI] [PubMed] [Google Scholar]
- Zasada JC, Zahner R. Vessel element development in earlywood of red oak (Quercus rubra) Canadian Journal of Botany. 1969;47:1965–1971. [Google Scholar]
- Zimmermann MH. In: Functional xylem anatomy of angiosperm trees. Bass P, editor. The Hague: Martinus Nijhoff Publishers; 1982. pp. 59–70. New perspectives in wood anatomy. [Google Scholar]