Abstract
Background and Aims
Heartwood formation is a unique phenomenon of tree species. Although the accumulation of heartwood substances is a well-known feature of the process, the accumulation mechanism remains unclear. The aim of this study was to determine the accumulation process of ferruginol, a predominant heartwood substance of Cryptomeria japonica, in heartwood-forming xylem.
Methods
The radial accumulation pattern of ferruginol was examined from sapwood and through the intermediate wood to the heartwood by direct mapping using time-of-flight secondary ion mass spectrometry (TOF-SIMS). The data were compared with quantitative results obtained from a novel method of gas chromatography analysis using laser microdissection sampling and with water distribution obtained from cryo-scanning electron microscopy.
Key Results
Ferruginol initially accumulated in the middle of the intermediate wood, in the earlywood near the annual ring boundary. It accumulated throughout the entire earlywood in the inner intermediate wood, and in both the earlywood and the latewood in the heartwood. The process of ferruginol accumulation continued for more than eight annual rings. Ferruginol concentration peaked at the border between the intermediate wood and heartwood, while the concentration was less in the latewood compared wiht the earlywood in each annual ring. Ferruginol tended to accumulate around the ray parenchyma cells. In addition, at the border between the intermediate wood and heartwood, the accumulation was higher in areas without water than in areas with water.
Conclusions
TOF-SIMS clearly revealed ferruginol distribution at the cellular level. Ferruginol accumulation begins in the middle of intermediate wood, initially in the earlywood near the annual ring boundary, then throughout the entire earlywood, and finally across to the whole annual ring in the heartwood. The heterogeneous timing of ferruginol accumulation could be related to the distribution of ray parenchyma cells and/or water in the heartwood-forming xylem.
Keywords: Wood formation, heartwood substances, intermediate wood, earlywood, extractives, ferruginol, Cryptomeria japonica, parenchyma cell, water, time-of-flight secondary ion mass spectrometry, TOF-SIMS, gas chromatography, GC, laser microdissection, LMD, cryo-scanning electron microscopy, cryo-SEM
INTRODUCTION
Heartwood formation is a unique phenomenon of tree species. Heartwood is formed in the centre part of the tree stem, in which such features as the structure and function differ from those of sapwood. The formation of heartwood is important not only for a tree's durability but also for its mechanical strength when using wood materials (reviewed by Bamber and Fukazawa, 1985; Hillis, 1987; Taylor et al., 2002; Kampe and Magel, 2013). Various physiological changes occur during heartwood formation where xylem changes from sapwood to heartwood, including water distribution (Nakada et al., 1999a, b; Nakada, 2006; Merela et al., 2006; Kuroda et al., 2006, 2009), parenchyma cell death (Nobuchi and Harada, 1983; Spicer and Holbrook, 2007; Nakaba et al., 2008, 2012), cell wall structure (Matsumura et al., 1995; Sano and Nakada, 1998), chemical composition (Yoshida et al., 2004, 2006; Bito et al., 2011) and colour (Gierlinger et al., 2004; Lukmandaru et al., 2009; Lukmandaru 2011; Moya et al., 2012). Although these features of heartwood have been widely studied, the mechanism of inducing heartwood formation remains unclear (Kuroda et al., 2009; Nakada and Fukatsu, 2012; Kampe and Magel, 2013).
The change in chemical composition during heartwood formation is one of the most intensively studied features due to the importance of its natural durability and the usage of extractives as pharmaceuticals (Kampe and Magel, 2013). The so-called heartwood substances greatly increase in heartwood. Many researchers have tried to clarify where and how tree species synthesize such substances, and it is now thought that synthesis occurs in the parenchyma cells in intermediate wood and then exudes into adjacent xylem cells (Kuroda and Shimaji, 1983; Nobuchi et al., 1985; Magel, 2000; Nagasaki et al., 2002); alternatively, phenolic precursors of the heartwood substances gradually accumulate in the ageing sapwood tissues and are transformed in the tissues transient between sapwood and heartwood (Burtin et al., 1998; Magel, 2000). Although this is a reasonable theory because living cells exist in sapwood and intermediate wood but not in heartwood (IAWA, 1964), it cannot be proved as direct evidence from these studies.
One reason for the difficulty in studying heartwood formation, particularly heartwood substances, is that heartwood is formed inside the tree stem. Sample trees need to be cut down for investigation, thereby causing a physiological change in condition compared with a standing tree. We applied a sampling procedure whereby the trunk was harvested after the trunk of a standing tree had been frozen by liquid nitrogen (LN2) to reveal the water distribution during heartwood formation (Kuroda et al., 2009). Using this method, we could reduce the artefact of moving water during sample preparations. This method therefore seems useful for chemical analysis, as the distribution of chemicals in the sample is freeze-fixed in the standing tree. Another difficulty arises due to the few methods available to analyse heartwood substances directly. Microscopic analysis in many studies used a dye agent to detect chemicals in the tissue, but the exact site and timing of the start of synthesis could not be proved. Imai et al. (2005) pointed out that these cytological studies did not provide definitive proof that the substances investigated were actually heartwood substances. They therefore attempt to apply time-of-flight secondary ion mass spectrometry (TOF-SIMS) to investigate heartwood substances in the xylem.
The TOF-SIMS method is a powerful tool to reveal chemical distribution features on the surfaces of solid samples with high spatial resolution (approx. 0·5 µm) without any chemical pre-treatment. Recently, TOF-SIMS was applied to plant samples to analyse either inorganic ions or molecular ions (Imai et al., 2005; Kuroda et al., 2008; Saito et al., 2008, 2012; Tokareva et al., 2010, 2011). The key feature of TOF-SIMS is that it can reveal the existence of a chemical itself by mass spectrometry of the sample. Imai et al. (2005) revealed that two positive ion peaks of the mass-to-charge ratio (m/z) of 285 and 301 generated from the heartwood of Cryptomeria japonica originated from ferruginol, a heartwood substance, and that the ferruginol distribution was almost even in C. japonica heartwood tissue. However, where the ferruginol accumulation starts and how it spreads into heartwood remains to be determined.
The final goal of our study is to clarify the mechanism of the synthesis of heartwood substances. We used C. japonica, which is one of the most useful plantation tree species in Japan, and various features of heartwood were studied (e.g. Nobuchi and Harada, 1983; Nakada et al., 1999). In addition, many types of chemicals were found as heartwood substances (e.g. Nobuchi et al., 1985; Nagahama and Tazaki, 1993), indicating that C. japonica is advantageous as a material to study heartwood formation. In this study, we tried to reveal the accumulation pattern of ferruginol in the heartwood-forming C. japonica xylem at the cellular level by direct mapping using TOF-SIMS and quantitative data using gas chromatography (GC). Based on the results, we discuss the synthesis and migration of heartwood substances in detail together with the mechanism of heartwood formation.
MATERIALS AND METHODS
Plant material
A tree of Cryptomeria japonica D.Don approx. 30 years of age growing on the campus of the Forestry and Forest Products Research Institute (FFPRI; Tsukuba, Ibaraki, Japan) was used in this study. It was 9 m high, with a diameter at breast height of 15 cm, and had blackish-coloured heartwood with a high water content. The sample was collected in the morning in May 2006. A water-tight receptacle was attached to the sample tree at breast height. The receptacle was filled with LN2, the trunk was allowed to freeze for approx. 20 min and then the tree was felled. Several discs 1 cm thick were cut from the frozen trunk, immediately immersed in LN2, and then stored in a deep freeze (at –80 °C) (Kuroda et al., 2009).
In this study, the sapwood, intermediate wood and heartwood were distinguished by colour, where intermediate wood was defined as a zone with an easily distinguishable white colour in the frozen state, existing inside the sapwood (Nakada et al. 1999a). In the analysis area of our sample, the sapwood, intermediate wood and heartwood had seven, eight and 11 annual rings, respectively.
TOF-SIMS analysis
The frozen sample was cut into small blocks in the LN2 pool (final size was approx. 5 × 5 × 5 mm). The transverse surface of the blocks was smoothly cut by using a cryostat at –30 °C (Kuroda et al., 2009). The blocks were freeze-dried with a freeze dryer (FD-1000, Tokyo Rikakikai, Tokyo, Japan) and then subjected to TOF-SIMS.
The TOF-SIMS measurements were performed using a TRIFT III spectrometer (ULVAC-PHI, Kanagawa, Japan). Positive spectra were obtained using a 22 keV Au1+ gold ion at a current of 0·6 pA, with a pulse width of 15 ns. A low-energy pulsed electron gun (28·0 eV) was used for surface charge compensation. All measurements were acquired while maintaining the primary ion dose at <1012 ions/cm2. The measured surface areas were 300 × 300 µm2 or 500 × 500 µm2. The relative ion intensities of the expected mass were obtained as the percentages of the total ion counts.
GC analysis with laser microdissection (LMD) sampling
Serial transverse sections approx. 80 µm thick were prepared from a frozen block sample and freeze-dried. Using an LMD system (LMD6000, Leica Microsystem, Tokyo, Japan), the xylem areas of the latewood and earlywood in each annual ring were cut out and then individually placed into plastic tubes. Samples of >0·1 mg each were collected from several serial sections. These samples were weighed, and then extracted with n-hexane. The solution was subjected to GC analysis. GC was performed using a model GC-2010 equipped with an FID (Shimadzu, Tokyo, Japan). The column used was InertCap 1 (ID × L = 0·25 mm × 30 m, film thickness 0·25 µm, GL Sciences, Tokyo, Japan). The column temperature was programmed from 100 to 280 °C at a heating rate of 5 °C/min. The quantity of compounds was determined by integrating the peak area of spectrograms using a calibration curve prepared with standard ferruginol.
Statistical analysis
Statistical analyses were performed with freeware ‘R’ version 2·1·12 with the base package (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). Welch two-sample t-test was used to compare mean levels of the ferruginol concentration between earlywood and latewood, and of the relative ion intensities between the area with and without water.
Cryo-scanning electron microscopy (cryo-SEM)
The water distribution was observed in several small blocks before TOF-SIMS analysis. A frozen sample with a smooth transverse surface was attached to the specimen holder and transferred to a cryo-SEM system (S4500; Hitachi, Tokyo, Japan). Secondary electron images were obtained at an acceleration voltage of 5 kV after freeze-etching (Kuroda et al., 2009). The observed samples were freeze-dried and then subjected to TOF-SIMS.
RESULTS
Typical TOF-SIMS spectrum of C. japonica xylem
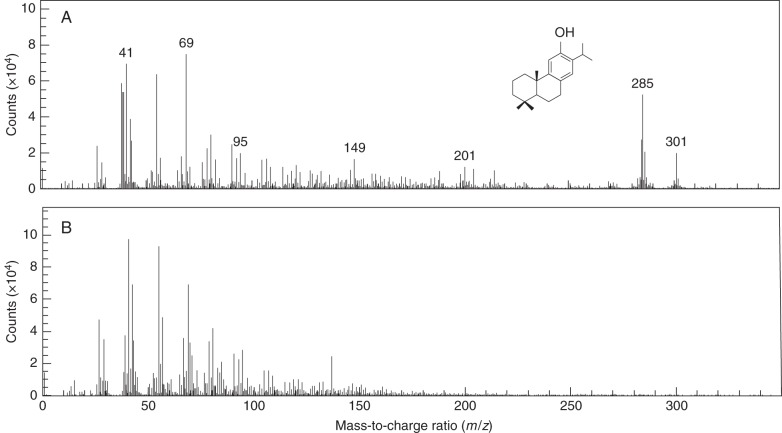
Figure 1 shows the typical positive ion spectra obtained from the earlywood of the heartwood and sapwood of C. japonica xylem. Two clear peaks of m/z 285 and 301 were detected in the heartwood, but not in the sapwood. These two peaks were known to be generated from ferruginol (C20H30O, mol. wt 286) in C. japonica heartwood (Imai et al., 2005; Kuroda et al., 2008). The intensity of m/z 285 always exceeded that of m/z 301. Thus, herein, we used m/z 285 to analyse ferruginol distribution in the heartwood-forming C. japonica xylem.
Fig. 1.
Typical positive TOF-SIMS spectra of C. japonica xylem. The spectra were obtained from the earlywood areas of heartwood (A) and sapwood (B). Two peaks of m/z 285 and 301 generated from ferruginol were detected in the heartwood but not in the sapwood. Note that the chemical structure of ferruginol is indicated in (A).
Mapping of ferruginol
To analyse the distribution pattern of ferruginol in the heartwood-forming C. japonica xylem, mapping data of m/z 285 were obtained from the sapwood to the heartwood. Figure 2 shows typical TOF-SIMS images of C. japonica xylem. Ferruginol is absent or at low concentrations in black areas, present at mid-concentrations in red areas and is at the highest concentrations in yellow/white areas. The image of the total ion, which is the sum of all ion intensities at each pixel, showed the cell structure like a microscopic image. Each image in Fig. 2 included the earlywood and latewood of the previous year with an annual ring boundary. The distribution of m/z 285 differed among the xylem parts. It was hard to produce the image of m/z 285 over the entire area of sapwood and outer intermediate wood due to the lack of ion detection except for background counts (Fig. 2A). In the inner intermediate wood, m/z 285 was clearly detected in the earlywood area but not in the latewood area (Fig 2B). Elsewhere in the inner heartwood area, m/z 285 was detected in both the latewood and earlywood areas, and evenly distributed in the earlywood (Fig. 2C).
Fig. 2.
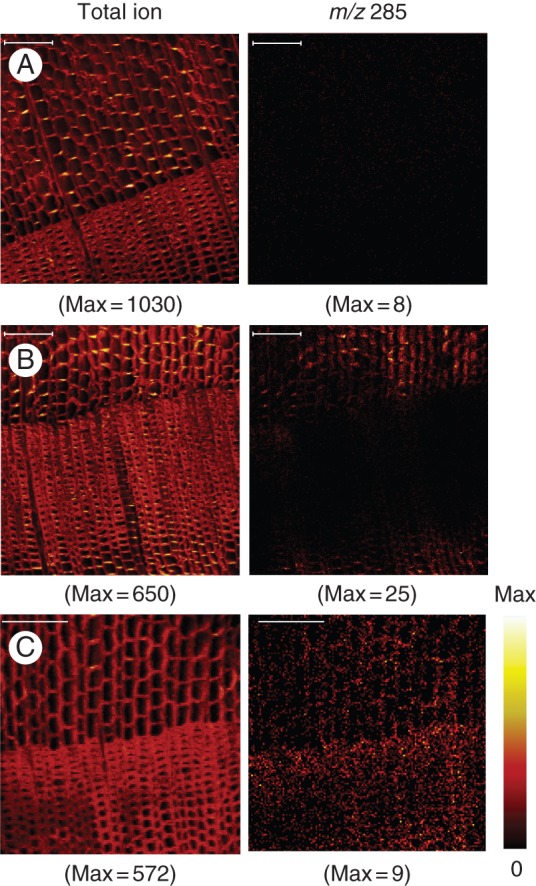
Typical positive TOF-SIMS images of total ion and m/z 285 in C. japonica xylem. Total ion (left) representing the xylem structure and m/z 285 (right) representing ferruginol localization were obtained from sapwood (A), intermediate wood (B) and heartwood (C). Maximum counts are shown in parentheses. Scale bars = 100 µm.
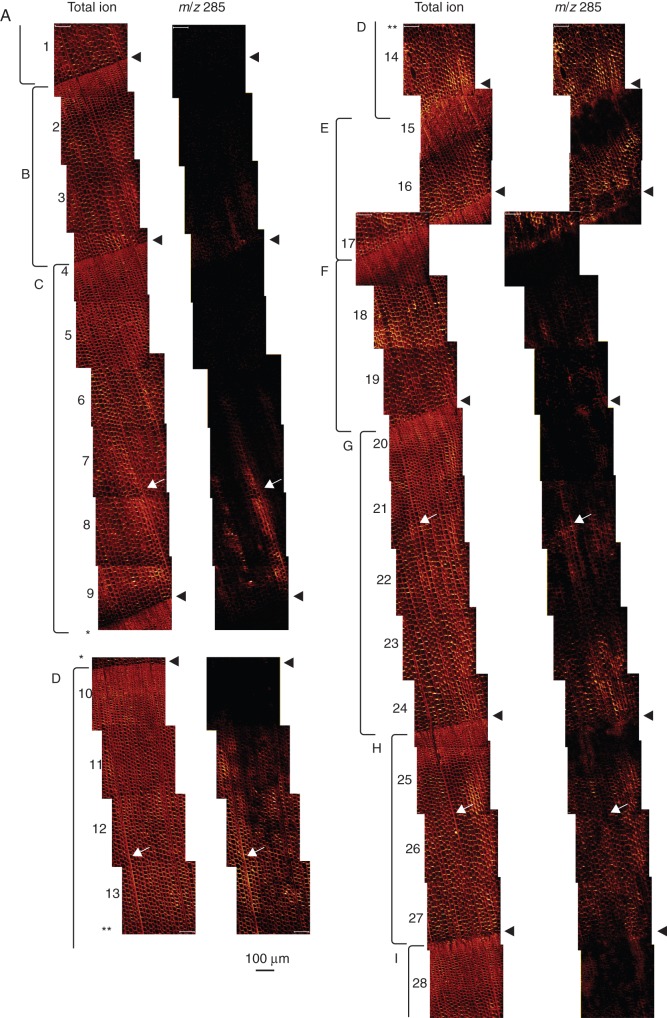
Figure 3 shows the ferruginol distribution from intermediate wood to outer heartwood. Twenty-eight images were obtained and arranged in a contiguous picture. Among them, the two parts marked with asterisks in Fig. 3 were separated due to the use of two block samples or charging-up during analysis. However, we could obtain mapping data at almost the same radial position, and made a figure almost contiguously. The images of total ion showed a clear structure of nine (Fig. 3A–I) annual rings with eight annual ring boundaries. Note that m/z 285 was not detected in either the latewood or earlywood in the outer intermediate wood (Fig. 3A). In the intermediate wood, m/z 285 was detected in the earlywood near the annual ring boundary with the previous year (Fig. 3B), in the inner part of the earlywood (Fig. 3C, D) or in the entire earlywood (Fig. 3E, F). Moreover, m/z 285 was detected in both the earlywood and latewood areas in the heartwood (Fig. 3G–I).
Fig. 3.
TOF-SIMS images from intermediate wood to heartwood of C. japonica xylem. Numbers 1–28 represent the order of the images. The annual rings are labelled (A–I), intermediate wood (A–F) and heartwood (G–I). The arrowheads represent annual ring boundaries. The arrows represent the ray parenchyma cells. The two parts marked with asterisks (*, **) were obtained at almost the same radial position. Scale bars = 100 µm.
Relative ion intensity of ferruginol
To reveal the radial change in ferruginol distribution, the relative ion intensity of m/z 285 to the total ion counts was calculated from the intermediate wood to the outer heartwood (Fig. 4). The relative ion intensity of m/z 285 increased from middle intermediate wood to inner intermediate wood, while the relative ion intensity peaked at the border of intermediate wood and heartwood. In each annual ring, the intensity of m/z 285 was high in the earlywood area but low in the latewood area, both in the intermediate wood and in the outer heartwood.
Fig. 4.
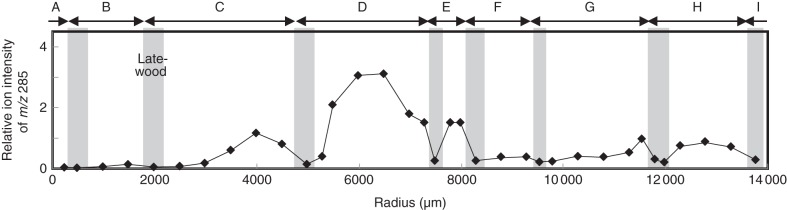
Radial change of ferruginol distribution from intermediate wood to heartwood of C. japonica xylem. The relative ion intensities of m/z 285 were obtained as the percentage of the total ion counts. The annual rings are labelled (A–I), which correspond to those in Fig. 3. Arrows indicate an annual ring. Grey shading indicates the latewood area in each annual ring.
Quantitative analysis of ferruginol
Gas chromatography was used to analyse the ferruginol distribution in intermediate wood and heartwood using LMD sampling in order to reveal the ferruginol distribution quantitatively (Fig. 5). In the outer intermediate wood, no ferruginol was detected in either the latewood or earlywood. In the innermost intermediate wood and heartwood, the amount of ferruginol in the earlywood exceeded that in the latewood in each annual ring. In the earlywood, the concentration of ferruginol was higher in the outermost heartwood than that in the inner part or intermediate wood.
Fig. 5.
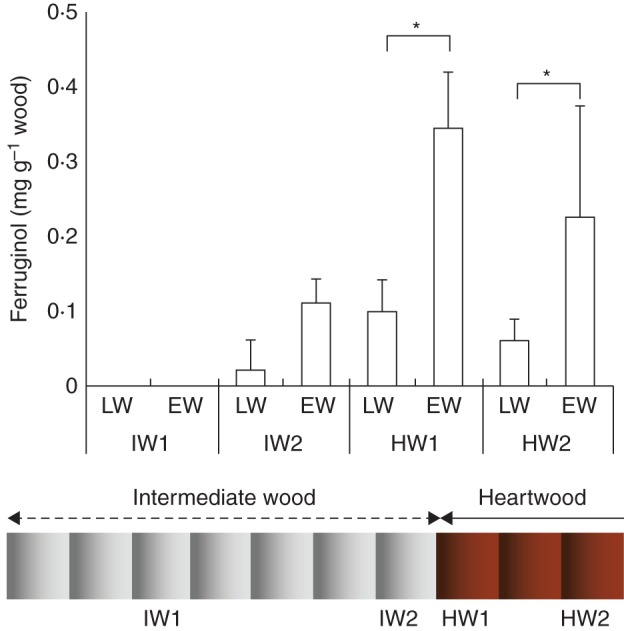
Concentration of ferruginol in the intermediate wood and heartwood in C. japonica xylem as measured by GC analysis using laser microdissection (LMD) sampling. LW, latewood; EW, earlywood; IW1 and IW2, intermediate wood; HW1 and HW2, heartwood. The data are mean values ± s.d. of the results from at least three replicates. Asterisks indicate significantly different means between LW and EW (P < 0·05).
Region of interest (ROI) data of ferruginol accumulation as compared with water distribution
During heartwood formation, a change in water distribution is one of the typical features. Accordingly, we tried to compare ferruginol and water distribution at the border between the intermediate wood and heartwood. Areas with or without water in the tracheids were selected, whereupon the relative ion intensities of m/z 285 were calculated separately. The relative ion intensity of m/z 285 in areas with and without water in the tracheids was 2·43 ± 0·43 and 3·42 ± 0·76, respectively (Fig. 6).
Fig. 6.
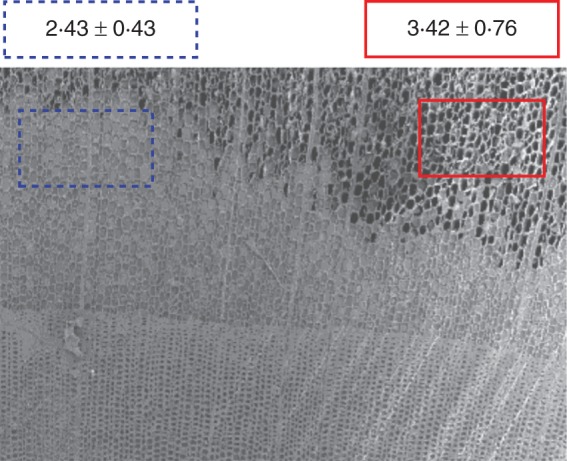
Relationship between ferruginol and water distribution at the border of intermediate wood and heartwood of C. japonica xylem. The figure shows an example of an ROI on a cryo-SEM micrograph. The relative ion intensities of m/z 285 were calculated from the ROI of areas with (blue) and without (red) water in the lumina of tracheids. The data are mean values ± s.d. of the results from six areas (P < 0·05).
DISCUSSION
In this study, we attempted to reveal the accumulation pattern of ferruginol in heartwood-forming C. japonica xylem in order to understand the mechanism of heartwood formation. TOF-SIMS analysis clearly revealed ferruginol distribution in the xylem at the cellular level. Ferruginol was not detected in the sapwood and outer intermediate wood (Figs. 1B, 2A and 3A). Ferruginol accumulation was first observed in the middle of the intermediate wood, where it only accumulated in the earlywood near the annual ring boundary with the previous year (Fig. 3B). From the middle to the inner part of the intermediate wood, ferruginol accumulated in the outer part of the earlywood (Fig. 3C–F). In the heartwood, ferruginol accumulated in the latewood area (Fig. 3G–I). In the inner heartwood, ferruginol accumulated almost evenly (Fig. 2C), and thus showed no discrepancy from the result obtained by Imai et al. (2005). Accordingly, ferruginol might accumulate evenly at the final accumulation stage in heartwood formation. We found the same tendency for the accumulation pattern of ferruginol in three other C. japonica trees (data not shown).
In the annual ring shown in Fig. 3I, ferruginol accumulated heterogeneously, suggesting that this annual ring had not reached the final accumulation stage. Therefore, there were more than eight annual rings from the initial to the final accumulation stage, indicating that the accumulation of ferruginol takes several years. Due to the extended ferruginol accumulation period, we hypothesize two possibilities: (1) ferruginol synthesis occurs over several years in intermediate wood and heartwood; or (2) ferruginol once synthesized moves slowly to the outer part of the earlywood. In response to hypothesis (1), it is difficult to believe that ferruginol synthesis occurs in heartwood, since heartwood lacks living cells (IAWA, 1964). However, it is possible that ferruginol synthesis occurs over several years in intermediate wood, because the area of intermediate wood of C. japonica includes several annual rings, meaning that the duration of synthesis could be several years. Thus, such a duration may result in the extended ferruginol accumulation period. Regarding hypothesis (2), chemicals dissolved in water can move as rapidly as water itself. Ferruginol, however, is hydrophobic and insoluble in water. Although no information exists on the form of chemicals in living trees, ferruginol might exist independently of water, suggesting that ferruginol moves slowly. Thus, ferruginol accumulation could continue for a long period, even if the synthesis occurs over a short period. Currently, these hypotheses are all plausible.
Parenchyma cells in the intermediate wood are believed to be the site of synthesis of heartwood substances in C. japonica (Nobuchi and Harada, 1983). Nagasaki et al. (2002) showed direct evidence via immunohistochemistry that agatharesinol, a heartwood substance of C. japonica, was synthesized in the ray parenchyma cells in intermediate wood and then migrated to the neighbouring tracheids. By carefully observing our TOF-SIMS data, it could be seen that ferruginol tended to accumulate in the ray parenchyma cells and their neighbouring tracheids (arrows in Fig. 3). This result supports the fact that heartwood substances are biosynthesized in ray parenchyma cells and infused into neighbouring tracheids, indicating that the presence of ray parenchyma cells affects the heterogeneous accumulation of ferruginol.
Moreover, our results from TOF-SIMS indicated that less ferruginol was present in the latewood as compared with the earlywood in the same annual ring (Figs 3 and 4). The quantitative results confirmed the results from the TOF-SIMS data. The method of GC analysis using LMD sampling is effective for quantitative chemical analysis at the cellular or tissue level. These results suggest that the timing of ferruginol accumulation differed between the earlywood and latewood. Our previous finding indicated different water distribution patterns in intermediate wood between the earlywood and latewood (Kuroda et al., 2009). The tracheids in the earlywood lost water from their lumina, while tracheids in the latewood retained water in the intermediate wood. In this study, we compared ferruginol accumulation with water distribution, and revealed that ferruginol accumulation increased in areas without water as compared with areas with water. These data suggest that less accumulation of ferruginol in the latewood is related to water in the latewood area. Ohashi et al. (1990, 1991) revealed that a decrease in moisture content induced ferruginol accumulation in sapwood during the withering process, supporting our inference about the relationship of water to ferruginol accumulation. However, the earlywood tracheids in the inner heartwood of this sample had water in their lumina, where ferruginol accumulation was even in an annual ring. The effect of water on ferruginol accumulation might differ between heartwood-forming xylem and the heartwood. Further studies are needed to reveal whether water is involved in ferruginol accumulation.
In this study, we found an interesting accumulation pattern of ferruginol in intermediate wood and heartwood. The accumulation pattern of certain heartwood substances may show the same tendency as that of ferruginol, but that of others may differ, e.g. agatharesinol in C. japonica not being distributed throughout the entire area of an annual ring but only in the ray parenchyma cells and neighbouring tracheids (Nagasaki et al., 2002). Also, the type of intermediate wood differs among tree species, such as the narrow width of intermediate wood in Larix kaempferi at less than one annual ring (Nakada and Fukatsu, 2012). To understand the accumulation pattern of heartwood substances, the exact site and timing of synthesis, and how these substances migrate should be carefully investigated in each chemical compound in each tree species.
Conclusions
In this study, we investigated the accumulation pattern of ferruginol in heartwood-forming C. japonica xylem in order to understand the accumulation process of heartwood substances. TOF-SIMS clearly revealed ferruginol distribution at the cellular level. Ferruginol accumulation begins in the middle of the intermediate wood, in the earlywood near the annual ring boundary. Further into the intermediate wood, ferruginol accumulates throughout the earlywood, and finally to the whole annual ring, including the latewood, in heartwood. This accumulation process takes several years due to factors whereby (1) ferruginol synthesis may occur for several years in intermediate wood and (2) synthesized ferruginol moves slowly. Also, the timing of ferruginol accumulation could be related to the distribution of ray parenchyma cells and water in the heartwood-forming xylem.
ACKNOWLEDGEMENTS
We thank Dr Satoshi Kubo of FFPRI for supporting the GC analysis, Dr Atsushi Kato of FFPRI for providing the ferruginol standard, Dr Ryogo Nakada of the Forest Tree Breeding Centre for the use of cryo-SEM, and Dr Kenichi Yazaki for the statistical analysis. We also thank Toshiyuki Kato and Ruka Takama of the Technical Center at Nagoya University for their technical support with the TOF-SIMS measurements. LMD was supported by the NIMS Molecule & Material Synthesis Platform in ‘Nanotechnology Platform Project’ operated by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. This work was supported by JSPS KAKENHI grant nos 21228004, 22580188, 23380105, 25292110 and 25114508.
LITERATURE CITED
- Bamber RK, Fukazawa K. Sapwood and heartwood: a review. Forestry Abstracts. 1985;46:567–580. [Google Scholar]
- Bito N, Nakada R, Fukatsu E, Matsushita Y, Fukushima K, Imai T. Clonal variation in heartwood norlignans of Cryptomeria japonica: evidence for independent control of agatharesinol and sequirin C biosynthesis. Annals of Forest Science. 2011;68:1049–1056. [Google Scholar]
- Burtin P, Jay-Allemand C, Charpentier JP, Janin G. Natural wood colouring process in Juglans sp. (J. nigra, J. regia and hybrid J. nigra 23×J. regia) depends on native phenolic compounds accumulated in the transition zone between sapwood and heartwood. Trees. 1998;12:258–264. [Google Scholar]
- Gierlinger N, Jacques D, Grabner M, et al. Colour of larch heartwood and relationships to extractives and brown-rot decay resistance. Trees. 2004;18:102–108. [Google Scholar]
- Hillis WE. Heartwood and tree exudates. Berlin: Springer; 1987. [Google Scholar]
- IAWA (Committee on Nomenclature International Association of Wood Anatomists) Multilingual glossary of terms used in wood anatomy. Switzerland: Verlagsanstalt Buchdrucherei Konkordia Winterthur; 1964. [Google Scholar]
- Imai T, Tanabe K, Kato T, Fukushima K. Localization of ferruginol, a diterpene phenol, in Cryptomeria japonica heartwood by time-of-flight secondary ion mass spectrometry. Planta. 2005;221:549–556. doi: 10.1007/s00425-004-1476-2. [DOI] [PubMed] [Google Scholar]
- Kampe A, Magel E. New insights into heartwood and heartwood formation. In: Fromm J, editor. Cellular aspects of wood formation. Plant cell monographs 20. Berlin: Springer-Verlag; 2013. pp. 71–95. [Google Scholar]
- Kuroda H, Shimaji K. Distribution of coloring substances in sugi heartwood. Holzforschung. 1983;37:225–230. [Google Scholar]
- Kuroda K, Yamashita K, Fujiwara T, Hirakawa Y. Water distribution in the xylem of Cryptomeria japonica associated with the distribution in heartwood moisture content. In: Kurjatko S, Kúdela, Lagaňa R, editors. Wood structure and properties '06. Zvolen: Arbora Publishers; 2006. pp. 77–81. [Google Scholar]
- Kuroda K, Imai T, Saito K, Kato T, Fukushima K. Application of ToF-SIMS to the study on heartwood formation in Cryptomeria japonica trees. Applied Surface Science. 2008;255:1143–1147. [Google Scholar]
- Kuroda K, Yamashita K, Fujiwara T. Cellular level observation of water loss and the refilling of tracheids in the xylem of Cryptomeria japonica during heartwood formation. Trees. 2009;23:1163–1172. [Google Scholar]
- Lukmandaru G. Variability in the natural termite resistance of plantation teak wood and its relations with wood extractive content and color properties. Journal of Forestry Research. 2011;8:17–31. [Google Scholar]
- Lukmandaru G, Ashitani T, Takahashi K. Color and chemical characterization of partially black-streaked heartwood in teak (Tectona grandis) Journal of Forestry Research. 2009;20:377–380. [Google Scholar]
- Magel E. Biochemistry and physiology of heartwood formation. In: Savidge RA, Barnett JR, Napier R, editors. Cell and molecular biology of wood formation. Experimental biology reviews. Oxford: BIOS Scientific Publishers; 2000. pp. 363–376. [Google Scholar]
- Matsumura J, Tsutsumi J, Oda K. Relationships of bordered pit aspiration occurring during to longitudinal gas permeability in Karamatsu (Larix leptolepis) woods natural- and freeze-dried. Mokuzai Gakkaishi. 1995;41:33–439. [Google Scholar]
- Merela M, Serša I, Oven P. Research of anatomy and moisture distribution in beech and oak wood by 3D MR imaging technique. In: Kurjatko S, Kúdela, Lagaňa R, editors. Wood structure and properties ‘06. Zvolen: Arbora Publishers; 2006. pp. 105–109. [Google Scholar]
- Moya R, Fallas RS, Bonilla PJ, Tenorio C. Relationship between wood color parameters measured by the CIELab system and extractive and phenol content in Acacia mangium and Vochysia guatemalensis from fast-growth plantations. Molecules. 2012;17:3639–3652. doi: 10.3390/molecules17043639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama S, Tazaki M. Terpenoids of wood oil of sugi (Cryptomeria japonica). Peculiarities of obisugi variety (in Japanese, with English summary, figures and tables) Mokuzai Gakkaishi. 1993;39:1077–1083. [Google Scholar]
- Nagasaki T, Yasuda S, Imai T. Immunohistochemical localization of agatharesinol, a heartwood norlignan, in Cryptomeria japonica. Phyto-chemistry. 2002;60:461–466. doi: 10.1016/s0031-9422(02)00141-3. [DOI] [PubMed] [Google Scholar]
- Nakaba S, Kubo T, Funada R. Differences in patterns of cell death between ray parenchyma cells and ray tracheids in the conifers Pinus densiflora and Pinus rigida. Trees. 2008;22:623–630. [Google Scholar]
- Nakaba S, Begum S, Yamagishi Y, Jin HO, Kubo T, Funada R. Differences in the timing of cell death, differentiation and function among three different types of ray parenchyma cells in the hardwood Populus sieboldii×P. grandidentata. Trees. 2012;26:743–750. [Google Scholar]
- Nakada R. Within-stem water distribution in living trees of some conifers. IAWA Journal. 2006;27:313–327. [Google Scholar]
- Nakada R, Fukatsu E. Seasonal variation of heartwood formation in Larix kaempferi. Tree Physiology. 2012;32:1497–1508. doi: 10.1093/treephys/tps108. [DOI] [PubMed] [Google Scholar]
- Nakada R, Fujisawa Y, Hirakawa Y. Soft X-ray observation of water distribution in the stem of Cryptomeria japonica D. Don. I: general description of water distribution. Journal of Wood Science. 1999a;45:188–193. [Google Scholar]
- Nakada R, Fujisawa Y, Hirakawa Y. Soft X-ray observation of water distribution in the stem of Cryptomeria japonica D. Don. II: types found in wet-area distribution patterns in transverse sections of the stem. Journal of Wood Science. 1999b;45:194–199. [Google Scholar]
- Nobuchi T, Harada H. Physiological features of the ‘white zone’ of sugi (Cryptomeria japonica D. Don) – cytological structure and moisture content. Mokuzai Gakkaishi. 1983;29:824–832. [Google Scholar]
- Nobuchi T, Takai K, Harada H. Radial distribution of heartwood phenols and the related cytological structure in the fresh wood of sugi (Cryptomeria japonica D. Don) Mokuzai Gakkaishi. 1985;31:711–718. [Google Scholar]
- Ohashi H, Imai T, Yoshida K, Yasue M. Characterization of physiological functions of sapwood; fluctuation of extractives in the withering process of Japanese cedar sapwood. Holzforschung. 1990;44:79–86. [Google Scholar]
- Ohashi H, Imai T, Yoshida K, Yasue M. Characterization of physiological functions of sapwood; fluctuation of heartwood extractives in the withering process of Japanese cedar sapwood fed an inhibitor of phenylalanine ammonia-lyase. Holzforschung. 1991;45:245–252. [Google Scholar]
- Saito K, Mitsutani T, Imai T, Matsushita Y, Fukushima K. Discriminating the indistinguishable sapwood from heartwood in discolored ancient wood by direct molecular mapping of specific extractives using time-of-flight secondary ion mass spectrometry. Analytical Chemistry. 2008;80:1552–1557. doi: 10.1021/ac7021162. [DOI] [PubMed] [Google Scholar]
- Saito K, Watanabe Y, Shirakawa M, et al. Direct mapping of morphological distribution of syringyl and guaiacyl lignin in the xylem of maple by time-of-flight secondary ion mass spectrometry. The Plant Journal. 2012;69:542–552. doi: 10.1111/j.1365-313X.2011.04811.x. [DOI] [PubMed] [Google Scholar]
- Sano Y, Nakada R. Time course of the secondary deposition of incrusting materials on bordered pit membranes in Cryptomeria japonica. IAWA Journal. 1998;19:285–299. [Google Scholar]
- Spicer R, Holbrook NM. Parenchyma cell respiration and survival in secondary xylem: does metabolic activity decline with cell age? Plant, Cell and Environment. 2007;30:934–943. doi: 10.1111/j.1365-3040.2007.01677.x. [DOI] [PubMed] [Google Scholar]
- Taylor A, Gartner BL, Morrell JJ. Heartwood formation and natural durability – a review. Wood and Fiber Science. 2002;34:587–611. [Google Scholar]
- Tokareva EN, Pranovich AV, Holmbom BR. Determination of anionic groups in wood by time-of-flight secondary ion mass spectrometry and laser ablation-inductively coupled plasma-mass spectrometry. Holzfor-schung. 2010;64:35–43. [Google Scholar]
- Tokareva EN, Pranovich AV, Holmbom BR. Characteristic fragment ions from lignin and polysaccharides in ToF-SIMS. Wood Science and Technology. 2011;45:767–785. [Google Scholar]
- Yoshida K, Hiraide M, Nishiguchi M, Hishiyama S, Kato A. A heartwood norlignan, agatharesinol, was generated in sapwood during withering of a Sugi (Cryptomeria japonica D. Don) log. Bulletin of FFPRI. 2004;3:25–28. [Google Scholar]
- Yoshida K, Nishiguchi M, Hishiyama S, Kato A, Takahashi K. Generation and alteration of norlignans in a transition zone during the drying of a Cryptomeria japonica log. Journal of Wood Science. 2006;52:372–375. [Google Scholar]