Abstract
Background and Aims
The number of nodules formed on a legume root system is under the strict genetic control of the autoregulation of nodulation (AON) pathway. Plant hormones are thought to play a role in AON; however, the involvement of two hormones recently described as having a largely positive role in nodulation, strigolactones and brassinosteroids, has not been examined in the AON process.
Methods
A genetic approach was used to examine if strigolactones or brassinosteroids interact with the AON system in pea (Pisum sativum). Double mutants between shoot-acting (Psclv2, Psnark) and root-acting (Psrdn1) mutants of the AON pathway and strigolactone-deficient (Psccd8) or brassinosteroid-deficient (lk) mutants were generated and assessed for various aspects of nodulation. Strigolactone production by AON mutant roots was also investigated.
Key Results
Supernodulation of the roots was observed in both brassinosteroid- and strigolactone-deficient AON double-mutant plants. This is despite the fact that the shoots of these plants displayed classic strigolactone-deficient (increased shoot branching) or brassinosteroid-deficient (extreme dwarf) phenotypes. No consistent effect of disruption of the AON pathway on strigolactone production was found, but root-acting Psrdn1 mutants did produce significantly more strigolactones.
Conclusions
No evidence was found that strigolactones or brassinosteroids act downstream of the AON genes examined. While in pea the AON mutants are epistatic to brassinosteroid and strigolactone synthesis genes, we argue that these hormones are likely to act independently of the AON system, having a role in the promotion of nodule formation.
Keywords: Autoregulation of nodulation, AON, brassinosteroids, CLAVATA2, NARK, Pisum sativum, RDN1, strigolactones
INTRODUCTION
Legume nodulation occurs as a result of a symbiosis with rhizobia soil bacteria, leading to the formation of novel structures called nodules. Hosted inside the specialized nodule, rhizobia fix atmospheric nitrogen into a form that is accessible to the plant and in exchange are provided with plant carbohydrates (Ferguson et al., 2010). Although beneficial, it is essential that the plants balance nitrogen gained with carbon expended, and so nodulation is strictly controlled both locally and systemically through a specialized autoregulation of nodulation (AON) pathway (Reid et al., 2011a).
Although not fully defined, significant progress has been made in our understanding of the AON pathway (Reid et al., 2011a; Okamoto et al., 2013). Following exposure to rhizobia bacteria, genes are induced that encode mobile CLAVATA3/ESR-related (CLE) peptides (Okamoto et al., 2009, 2013; Mortier et al., 2010; Lin et al., 2011; Reid et al., 2011b, 2013; Hayashi et al., 2012). These peptides move up into the shoot where they are perceived by a leucine-rich repeat receptor-like kinase (LRR RLK) (Krusell et al., 2002; Nishimura et al., 2002; Searle et al., 2003; Schnabel et al., 2005). Orthologues of this LRR RLK have been given different names, but we refer to the receptor here as Nodulation Autoregulation Receptor Kinase (NARK), consistent with its name in soybean (Searle et al., 2003). Activation of NARK triggers the production of an as yet unidentified shoot-derived factor that is translocated to the roots to inhibit further nodulation (e.g. Delves et al., 1986; Lin et al., 2010, 2011). Additional receptors, called KLAVIER (KLV) and CLAVATA2 (CLV2), also act in the shoot to control nodule numbers. A model has been proposed in which NARK complexes with CLV2 and/or KLV in the shoot to control nodule numbers in the root (Miyazawa et al., 2010; Krussel et al., 2011). Mutations in the NARK, KLV or CLV2 genes in several legume species lead to a supernodulation phenotype, with a large increase in the number of nodules formed on the roots (Krusell et al., 2002, 2011; Nishimura et al., 2002; Searle et al., 2003; Schnabel et al., 2005; Miyazawa et al., 2010). As stated above, these genes act in the shoot, as grafting studies have shown that nark, klv or clv2 mutant shoots induce supernodulation in wild-type roots (e.g. Delves et al., 1986; Sagan and Duc, 1996; Oka-Kira et al., 2005).
Autoregulation of nodulation mutants acting in the root that may disrupt induction of the root-to-shoot CLE peptides or perception of the shoot-derived factor have also been identified (e.g. Postma et al., 1988; Takahara et al., 2013). These include the nod3 mutant of pea (Postma et al., 1988). NOD3 has recently been identified as the orthologue of the Medicago truncatula gene RDN1 (Schnabel et al., 2011). RDN1 is expressed in the root vasculature and encodes a protein of unknown function that is a member of a highly conserved gene family unique to green plants, including green algae (Schnabel et al., 2011). Grafting studies with both Mtrdn1 and Psnod3 mutants have indicated that the supernodulating phenotype is root controlled and that RDN1 is likely to act very early in AON, in either the generation or transport of the root-to-shoot CLE peptides or alongside this system (Postma et al., 1988; Li et al., 2009; Schnabel et al., 2011; Osipova et al., 2012). Indeed, elegant grafting studies by Novak (2010) indicate that an allele of Psnod3 (RisfixC) lacks the root-to-shoot AON signal, as wild-type adventitious roots that formed on wild-type shoots grafted to large supernodulating Psnod3 roots also displayed a supernodulating phenotype.
Plant hormones have also been implicated in AON signalling (Ferguson and Mathesius, 2003; Ferguson et al., 2010; Saur et al., 2011; Ryu et al., 2012), with good evidence for a role for polar auxin transport (e.g. van Noorden et al., 2006; Jin et al., 2012). Roles for other hormones such as jasmonic acid, cytokinins and brassinosteroids have also been suggested from studies using a combination of hormone application, hormone measurement and AON mutant studies.
Brassinosteroids are an especially interesting case. Pea mutants defective in brassinosteroid biosynthesis or receptors display a reduction in nodulation, suggesting a positive role for brassinosteroids in nodule development (Ferguson et al., 2005). However, brassinosteroids appear to be relatively immobile in pea (Symons and Reid, 2004). Thus, restoration of nodulation in brassinosteroid-deficient pea roots by grafting to wild-type shoots indicates that brassinosteroids may influence nodulation via a shoot-derived signal (Ferguson et al., 2005). There is no evidence that this signal is shoot-derived auxin or gibberellins, as grafting did not modify the levels of these hormones (Ferguson et al., 2005). This systemic effect of shoot-derived brassinosteroids on nodulation was also seen in soybean application studies, but, interestingly, in these studies, brassinosteroids appeared to act as an inhibitor of nodulation. Exogenous application of a brassinosteroid synthesis inhibitor actually enhanced nodule formation in wild-type soybean, and brassinosteroid application to the shoots suppressed the nodule number in the soybean nark mutant but not in wild-type plants (Terakado et al., 2005). Clearly, additional studies are required to investigate how brassinosteroids interact with the AON pathway.
The most recent group of plant hormones to be implicated in the control of nodulation is the strigolactones. Strigolactones suppress axillary shoot branching, and roles in other aspects of plant development are currently emerging (Foo and Reid, 2013). Pea mutants disrupted in strigolactone biosynthesis have reduced nodulation, and nodulation can be increased in the mutant by application of the synthetic strigolactone, GR24 (Foo and Davies, 2011). GR24 application can also enhance nodulation in wild-type pea, Medicago sativa and Lotus japonicus (Soto et al., 2010; Foo and Davies, 2011; Liu et al., 2013). Suppression of strigolactone biosynthesis gene expression in transgenic L. japonicus plants also results in reduced nodulation (Liu et al., 2013), indicating a positive role for strigolactones in both determinate (L. japonicus) and indeterminate (pea, M. sativa) nodule development. Although mobile, strigolactones appear to move only in the direction of root to shoot (Foo et al., 2001; Foo and Davies, 2011). Grafting studies indicate that unlike brassinosteroids, strigolactones do not influence nodulation by controlling a shoot-derived factor (Foo and Davies, 2011). Genetic studies indicate that strigolactones may act relatively early in nodule initiation, rather than in nodule organogenesis (Foo et al., 2013a, b), similar to the developmental stage at which the AON pathway is thought to be initiated (Reid et al., 2011a).
In this study, a genetic approach was used to analyse the interaction between brassinosteroids and strigolactones and the AON pathway in pea. Double mutants were generated containing mutations in elements of the AON pathway (Psnark, Psclv2, Psrdn1; Krusell et al., 2002, 2011; Schnabel et al., 2011) and either brassinosteroid biosynthesis (lk; Nomura et al., 2004) or strigolactone biosynthesis (Psccd8; Gomez-Roldan et al., 2008). The nodulation phenotypes of these double mutants were subsequently assessed. We also monitored strigolactone levels in AON mutants to explore potential links between strigolactone production and the AON pathway.
MATERIALS AND METHODS
Plant material
Crosses were performed with Pisum sativum parental lines lk (brassinosteroid-deficient, line 212–, on a line 212+ background; Reid, 1986; Nomura et al., 2004) or Psccd8 (strigolactone-deficient, formerly rms1-2 T, crossed into a ‘Torsdag’ background; Foo and Davies, 2011), with Psnark (P88, formerly Pssym29), Psclv2 (P64, formerly Pssym28) and Psrdn1 (P79, formerly Psnod3) (all supernodulating lines are derived from the parental line ‘Frisson’; Duc and Messager, 1989; Sagan and Duc, 1996). Double mutants were selected in the F2, F3 or F4 generations. As the parental AON mutant lines, Psnark, Psclv2 and Psrdn1 carry the dwarf le-1 mutation, while lk and Psccd8 are on wild-type (LE) backgrounds at this locus, only double mutants that displayed the wild-type LE phenotype were selected. Double mutant phenotypes were confirmed by segregation from heterozygous plants already confirmed as homozygous for the other mutation.
Nodulation experiments
Nodulation experiments were carried out as previously described (Foo and Davies, 2011). Briefly, unless otherwise stated, plants were grown under glasshouse conditions in 2 L pots in a 1:1 mixture of dolerite chips and vermiculite topped with vermiculite. Plants were inoculated 7 d after planting with Rhizobium leguminosarum bv viciae (RLV248) grown in yeast–mannitol broth and received modified Long Ashton solution (Hewitt, 1966) with no nitrogen and 5 mm NaH2PO4 weekly. After 35 or 49 d, shoot height, shoot branching and the total number of nodules on each root system were scored. Nodule spacing data were obtained by placing secondary roots on a grid and selecting nodules that intersected the grid. The distance along the root to the next nodule was recorded and the average space between nodules of six plants per genotypes was calculated (note that roots that contained <3 nodules could not be scored). Root, shoot and nodules were separated and dried at 55 °C to obtain the dry weight. Nodule number is expressed as the number of nodules per g root dry weight for each plant to account for difference in root size, and hence sites for nodule formation, between individual plants.
Strigolactone extraction and measurements
Plants were grown in growth cabinets [20 °C/15 °C day/night under cool-white fluorescent tubes (100 μmol m–2 s–1) and an 18 h photoperiod] under semi-sterile conditions to exclude rhizobial bacteria. Pots, seeds and growth trays were sterilized with 70 % ethanol and seeds were planted into 1 L pots containing sterile vermiculite watered with milliQ water. Plants received no nutrients, to induce nitrogen and phosphate starvation, which enhances strigolactone production in pea (Foo et al., 2013a). When plants were 26 d old, roots were severed from the shoot, washed and weighed. Strigolactones were extracted from root tissue using a modified version of the method reported in Foo and Davies (2011). Following tissue homogenization and overnight extraction in ethyl acetate at 4 °C, samples were filtered and labelled strigolactone standards were added (1 ng each of [6′-2H1]orobanchol, [6′-2H1]orobanchyl acetate and [6′-2H1]fabacyl acetate). Samples were then dried, re-suspended in 0·4 % acetic acid and passed through a 200 mg silica column (Waters Pty Ltd, Australia). The column was washed twice with 15:85 ethyl acetate:hexane and strigolactones were eluted with 45:55 ethyl acetate:hexane. Samples were dried, resuspended in 50:50 acetonitrile:water and detected by UPLC-MS as described by Foo and Davies (2011).
Statistical analysis
For each variable, genotypes were compared by performing one-way analyses of variance (ANOVAs) and post-hoc test (least significant difference) at a threshold of at least P < 0·01 using the SPSS software package (IBM).
RESULTS
Brassinosteroid-deficient AON double mutants display supernodulation
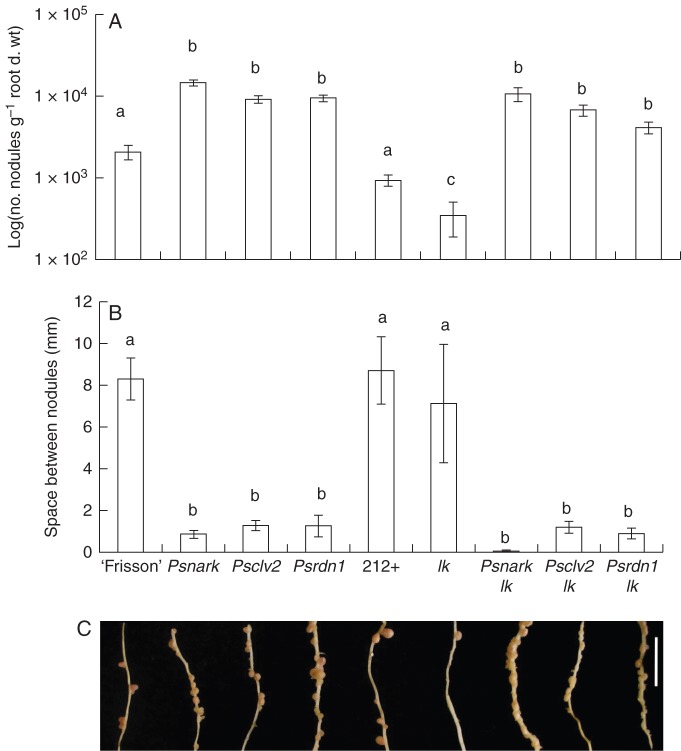
Brassinosteroid-deficient AON double mutants, Psnark lk, Psclv2 lk and Psrdn1 lk, all displayed classic brassinosteroid-deficient shoot phenotypes that were indistinguishable from the lk parent, with smaller, darker leaves, reduced internode lengths and thick stems compared with wild-type or AON parental lines (Fig. 1; Table 1). However, in stark contrast, the roots of Psnark lk, Psclv2 lk and Psrdn1 lk double mutants displayed the classic supernodulating phenotype, indistinguishable from AON parental lines (Fig. 2). Both single-mutant Psnark, Psclv2 and Psrdn1 and double-mutant Psnark lk, Psclv2 lk and Psrdn1 lk plants all had a significantly increased number of nodules for a given amount of root compared with either wild-type line (‘Frisson’, 212+) or lk (Fig. 2A). Indeed, for all double-mutant plants, the nodule number was not significantly different from that of Psnark, Psclv2 or Psrdn1 single mutants at the P < 0·01 level (Fig. 2A).
Fig. 1.
Phenotype of 35-day-old wild type (‘Frisson’, 212+) and various single- and double-mutant combinations of brassinosteroid-deficient lk and AON mutants Psnark, Psclv2 and Psrdn1. ‘Frisson’ wild type, Psnark, Psclv2 and Psrdn1 single-mutant plants carry Mendel's dwarf allele le-1, while all other plants carry the wild-type LE allele at this locus. Scale bar = 10 cm.
Table 1.
Shoot and root phenotypes of 35-day-old wild type (‘Frisson’, 212+ ) and various single- and double-mutant combinations of brassinosteroid-deficient lk and AON mutants Psnark, Psclv2 and Psrdn1 (LE/le genotype indicated in parentheses)
| Genotype | Leaves expanded | L1–6 | Shoot d. wt | Root d. wt | Individual nodule d. wt |
|---|---|---|---|---|---|
| ‘Frisson’ (le) | 8·25 ± 0·31a | 62·3 ± 3·6a | 188·2 ± 8·8a | 52·6 ± 2·9a | 0·18 ± 0·06ab |
| Psnark (le) | 8·58 ± 0·27a | 42·8 ± 2·1b | 67·9 ± 10·4b | 25·0 ± 2·3b | 0·06 ± 0·01a |
| Psclv2 (le) | 8·08 ± 0·15a | 68·0 ± 4·2a | 117·9 ± 5·4c | 32·8 ± 2·8b | 0·04 ± 0·01a |
| Psrdn1 (le) | 7·08 ± 0·33a | 52·7 ± 0·8a | 121·9 ± 10·4c | 38·1 ± 2·8ab | 0·03 ± 0·01a |
| 212+ (LE) | 8·75 ± 0·21a | 184·5 ± 3·8c | 266·9 ± 7·5d | 92·5 ± 5·9c | 0·17 ± 0·03ab |
| lk (LE) | 5·83 ± 0·17b | 38·5 ± 2·4b | 66·8 ± 6·2b | 23·9 ± 3·9b | 0·11 ± 0·03ab |
| Psnark lk (LE) | 7·75 ± 1·2a | 38·2 ± 6·3b | 85·4 ± 9·9bc | 26·68 ± 4·2b | 0·07 ± 0·01a |
| Psclv2 lk (LE) | 7·92 ± 0·93a | 39·7 ± 7·4b | 60·37 ± 8·4bc | 18·9 ± 1·1b | 0·23 ± 0·05ab |
| Psrdn1 lk (LE) | 7·83 ± 0·77a | 39·3 ± 2·2b | 87·8 ± 7·9b | 24·0 ± 2·6b | 0·13 ± 0·02ab |
The number of leaves expanded, length of shoot from node 1 to 6 (L1–6) and shoot, root and individual nodule dry weight (mg) are indicated.
Values within a column with different letters indicate significant differences (ANOVA, P < 0·01).
Values are the mean ± s.e. (n = 5–6).
Fig. 2.
Nodulation phenotype of 35-day-old wild type (‘Frisson’, 212 + ) and various single- and double-mutant combinations of brassinosteroid-deficient lk and AON mutants Psnark, Psclv2 and Psrdn1. (A) Log of number of nodules per gram dry root weight, (B) space between nodules and (C) photographs of nodules on secondary roots (tertiary roots have been removed); the scale bar is 1 cm. Different letters above bars indicate significant differences between genotypes (ANOVA, P < 0·01). For A and B, values are the mean ± s.e. (n = 6–7).
The nodules that formed on these double-mutant lines were also significantly more closely spaced than observed in wildtype (‘Frisson’, 212+) or lk roots, and this close spacing was indistinguishable from that of AON single mutants (Fig. 2B, C). Interestingly, in contrast to the small nodules formed on AON single-mutant parents, two of the double-mutant plants (Psclv2 lk and Psrdn1 lk) had somewhat larger individual nodules (P < 0·05; Table 1). Previous studies have found that brassinosteroid-deficient or insensitive pea mutants had fewer but consistently larger nodules than seen on wild-type plants (Ferguson et al., 2005), although that trend was not observed in lk mutants in this study (possibly due to the very small number of nodules that could be weighed, as many individual lk plants formed no nodules at all; Table 1).
The supernodulating phenotype of brassinosteroid-deficient AON double mutants contrasts with the significantly reduced nodulation observed in brassinosteroid-deficient lk single-mutant parents (Fig. 2A; Ferguson et al., 2005). Clearly, a dwarf shoot due to brassinosteroid deficiency does not limit nodulation per se, as supernodulating brassinosteroid-deficient AON double mutants all displayed a similar dwarf shoot. Further, this maintenance of the supernodulating phenotype in a brassinosteroid-deficient background (i.e. recessive epistasis of the AON mutants over alleles at the LK locus) indicates that brassinosteroids do not act downstream of the AON genes examined.
Strigolactone-deficient AON double mutants display supernodulation
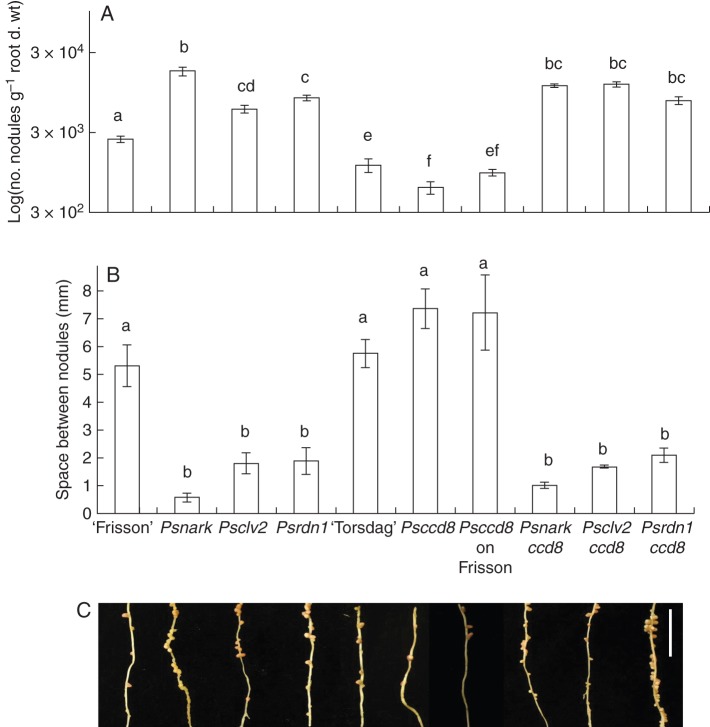
We also used a genetic approach to examine whether disrupting strigolactone production influenced the supernodulating phenotype of AON mutants. Strigolactone-deficient AON double mutants, Psnark ccd8, Psclv2 ccd8 and Psrdn1 ccd8, all displayed increased shoot branching characteristic of strigolactone-deficient Psccd8 parents (Fig. 3; Table 2). The number of branches formed on strigolactone-deficient AON double mutants was not significantly different from that on strigolactone-deficient plants on a ‘Frisson’ background (Psccd8 on ‘Frisson’; Table 2). However, the length of the branches on double mutants was somewhat shorter (Fig. 3), probably due to somewhat reduced root size and increased resource allocation to nodule development in these plants (Table 2).
Fig. 3.
Phenotype of 49-day-old wild type (‘Frisson’, ‘Torsdag’) and various single- and double-mutant combinations of strigolactone-deficient Psccd8 and AON mutants Psnark, Psclv2 and Psrdn1. Arrows indicate shoot branches. ‘Frisson’ wild type, Psnark, Psclv2 and Psrdn1 single-mutant plants carry Mendel's dwarf allele le-1, while all other plants carry the wild-type LE allele at this locus. Scale bar = 10 cm.
Table 2.
Shoot and root phenotypes of 49-day-old wild type (‘Frisson’, ‘Torsdag’) and various single- and double-mutant combinations of strigolactone-deficient Psccd8 and AON mutants Psnark, Psclv2 and Psrdn1 (LE/le genotype indicated in parentheses)
| Genotype | No. of branches | Leaves expanded | L1–6 | Shoot d. wt | Root d. wt | Individual nodule d. wt |
|---|---|---|---|---|---|---|
| ‘Frisson’ (le) | 0a | 13 ± 0·3a | 74 ± 3a | 580 ± 62a | 86 ± 9a | 0·13 ± 0·02a |
| Psnark (le) | 0a | 10·7 ± 0·3b | 54 ± 4a | 200 ± 44a | 36 ± 6a | 0·05 ± 0·01a |
| Psclv2 (le) | 0a | 12·7 ± 0·5a | 65 ± 2a | 349 ± 4a | 58 ± 4a | 0·11 ± 0·02a |
| Psrdn1 (le) | 0·3 ± 0·3a | 12·8 ± 0·2a | 57 ± 2a | 375 ± 20a | 49 ± 3a | 0·07 ± 0·01a |
| ‘Torsdag’ (LE) | 0a | 14 ± 0a | 194 ± 16b | 1012 ± 79b | 176 ± 21bc | 0·36 ± 0·05b |
| Psccd8 (LE) | 9·5 ± 0·2b | 15 ± 0c | 205 ± 7bc | 1178 ± 58b | 229 ± 41c | 0·27 ± 0·02b |
| Psccd8 on ‘Frisson’ (LE) | 4·2 ± 0·3bc | 13 ± 0a | 206 ± 5bc | 1242 ± 53b | 120 ± 13ab | 0·29 ± 0·02b |
| Psnark ccd8 (LE) | 5·3 ± 1·2c | 13·6 ± 0·9a | 241 ± 7c | 1102 ± 131b | 60 ± 6a | 0·08 ± 0·01a |
| Psclv2 ccd8 (LE) | 3·2 ± 0·3bc | 15 ± 0·3ac | 190 ± 18b | 680 ± 21ab | 68 ± 9a | 0·03 ± 0·01a |
| Psrdn1 ccd8 (LE) | 4·5 ± 0·6bc | 13·5 ± 0·2a | 234 ± 9c | 845 ± 79b | 46 ± 5a | 0·11 ± 0·01a |
The number of leaves expanded, number of shoot branches (>3 mm) and shoot, root and individual nodule dry weight (mg) are given.
Values within a column with different letters indicate significant differences (ANOVA, P < 0·01).
Values are the mean ± s.e. (n = 5–6).
However, unlike strigolactone-deficient single mutant plants that had significantly fewer nodules than the respective wild types (Fig. 4B; Foo and Davies, 2011), strigolactone-deficient AON double mutants displayed a clear supernodulating phenotype (Fig. 4). Both single-mutant Psnark, Psclv2 and Psrdn1 and double-mutant Psnark ccd8, Psclv2 ccd8 and Psrdn1 ccd8 plants all had a significantly increased number of nodules for a given amount of root compared with either wild-type line (‘Frisson’, ‘Torsdag’) or Psccd8 lines (Fig. 4A). Although there were some small differences in the total number of nodules formed on the double mutants compared with the AON single-mutant parents (Fig. 4A), there was no consistent reduction in nodule numbers across the double mutants. Indeed, the characteristic disruption in nodule spacing observed in AON mutants (e.g. Sagan and Gresshoff, 1996; Fig. 4B, C) was observed in all the double-mutant plants investigated here, with a significant decrease in nodule spacing compared with wild-type (‘Frisson’, ‘Torsdag’) or Psccd8 roots (Fig. 4B). This phenotype was almost indistinguishable from the AON single-mutant parents (Fig. 4B, C). This is in contrast to the small, but not significant, increase in nodule spacing observed in Psccd8 mutants compared with the respective wild-type parents (Fig. 4B).
Fig. 4.
Nodulation phenotype of 49-day-old wild type (‘Frisson’, ‘Torsdag’) and various single- and double-mutant combinations of strigolactone-deficient Psccd8 and AON mutants Psnark, Psclv2 and Psrdn1. (A) Log of number of nodules per gram dry root weight, (B) space between nodules and (C) photographs of nodules on secondary roots (tertiary roots have been removed); the scale bar is 1 cm. Different letters above bars indicate significant differences between genotypes (ANOVA, P < 0·01). For A and B, values are the mean ± s.e. (n = 5–6).
Strigolactone levels in AON mutants
The double-mutant studies outlined above indicate that strigolactones are not essential for the supernodulating phenotype. However, given the strong regulation of strigolactone production by nitrogen and phosphate deficiency (e.g. Foo et al., 2013a; Yoneyama et al., 2012), and the proposed roles for elements of the AON pathway in the control of nodulation under nitrogen and phosphorus limitation (Carrol et al., 1985; Sagan and Duc, 1996; Reid et al. 2011a, b; Foo et al., 2013b), we investigated strigolactone production in the AON mutants. Plants were grown in the absence of rhizobia and no plants formed nodules (data not shown). This was important in case nodules themselves produce strigolactones, which would influence the results.
The level of the three major strigolactones found in pea root tissue, orobanchol, orobanchyl acetate and fabacyl acetate, was similar in wild-type (‘Frisson’), Psnark and Psclv2 roots (Fig. 5). However, there was a significant, almost 2-fold increase in all three strigolactones in the root tissue of Psrdn1 mutant plants compared with wild-type roots.
Fig. 5.
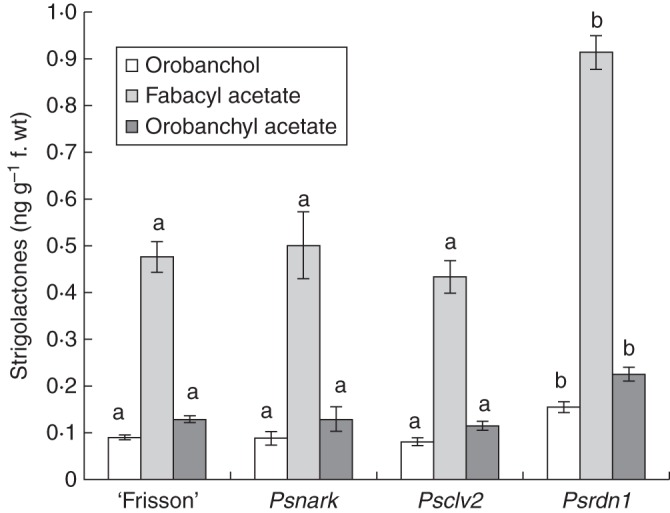
Strigolactone levels in the root tissue of 21-day-old wild type (‘Frisson’) and AON mutant lines, Psnark, Psclv2 and Psrdn1. Different letters above bars indicate significant differences between genotypes (separate ANOVAs were carried out on each strigolactone, P < 0·01). Values are the mean ± s.e. (n = 4).
DISCUSSION
In pea, both strigolactones and brassinosteroids have been shown to have a positive effect on nodule initiation (Ferguson et al., 2005; Foo and Davies, 2011), although the exact nature of their influence on nodulation is still emerging. This study indicates that neither brassinosteroids nor strigolactones are likely to play a major role in the AON pathway, since double mutants deficient in either of these hormones and a shoot- and/or root-acting element of AON exhibit a supernodulation phenotype.
In most cases, the roots of the double-mutant plants generated were indistinguishable from those of their single-mutant AON parents, on the basis of elevated nodule number and reduced nodule spacing (Figs 2 and 4). This epistasis of AON mutations to either brassinosteroid or strigolactone deficiency can be interpreted in one of two ways. A classical genetic interpretation would be that strigolactones and brassinosteroids act earlier in the nodulation pathway than the AON system, and hence disruption of the synthesis of either hormone results in no phenotype in an AON mutant. However, given that disruption of the AON system is known to result in a qualitative shift in nodulation (supernodulation; in this study up to a 7-fold increase in nodule number), while strigolactone or brassinosteroid deficiency results in only a relatively small quantitative change in nodulation (in this study an approx. 50 % decrease in nodule number), there is a second way to interpret this data. If strigolactones and brassinosteroids influence nodulation independently of the AON pathway, the qualitative shift to a supernodulaing phenotype in an AON mutant may mask any small, quantitative effects of strigolactone or brassinosteroid deficiency in a double-mutant plant. Regardless of which interpretation is correct, the data certainly show that the strigolactones and brassinosteroids do not act further down the AON pathway than the PsNARK, PsCLV2 and PsRDN1 genes.
An independent role for strigolactones and brassinosteroids in nodulation is consistent with previous studies examining the role of gibberellins in the AON pathway (Ferguson et al., 2011). As observed for double mutants in this study, gibberellin-deficient AON double mutants exhibited a supernodulation phenotype similar to their supernodulating AON parents. However, it is interesting to note that the large number of nodules that formed on gibberellin-deficient AON double mutants were aberrant in structure and appeared to be arrested in development, indicating an important role for gibberellins in nodule organogenesis (Ferguson et al., 2005, 2011). This is in contrast to the fully formed nodules observed on strigolactone- and brassinosteroid-deficient single- or double-mutant plants (Figs 2C and 4C), consistent with previous studies suggesting that these hormones are likely to be involved in nodule initiation but appear less critical for nodule organogenesis (Ferguson et al., 2005; Foo and Davies, 2011; Foo et al., 2013a).
The observation that strigolactone levels are elevated in the roots of Psrdn1 single mutants but not shoot-acting elements of the AON pathway (Fig. 5) is intriguing. The action of the RDN1 protein is still unknown, but studies indicate that it acts in the vasculature of the root to induce early AON responses either downstream of or at the level of root-derived CLE peptides (Li et al., 2009; Schnabel et al., 2011; Osipova et al., 2013). It is possible that RDN1, but not the AON pathway more generally, normally suppresses strigolactone production in the root. The elevated strigolactones do not appear to contribute to elevated nodulation in Psrdn1, as Psrdn1 ccd8 double mutants had a similar number of nodules to the Psrdn1 parents (Fig. 4). However, given that strigolactones appear to play a small role in some aspects of root development (e.g. Ruyter-Spira et al., 2011), it would be interesting to explore whether the altered root development observed in Psrdn1 mutant plants (Schnabel et al., 2011) may be in part due to altered strigolactone levels.
ACKNOWLEDGEMENTS
We wish to thank A/Professor Noel Davies for assistance with strigolactone measurements, Anthony Cummings, Tiernan O'Rourke, Shelley Urquart, Nicole Bezemer, Helen Paul, Ian Cummings, Michelle Lang and Tracy Winterbottom for technical support, and Professor Koichi Yoneyama (Utsunomiya University) and Dr Kohki Akiyama (Osaka Prefecture University) for their kind gifts of deuterated strigolactone standards. This work was supported by funding from the Australian Research Council.
LITERATURE CITED
- Carroll BJ, McNeil DL, Gresshoff PM. Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proceedings of the National Academy of Sciences; USA. 1985. pp. 4162–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves AC, Mathews A, Day DA, Carter AS, Carroll BJ, Gresshoff PM. Regulation of the soybean–Rhizobium nodule symbiosis by shoot and root factors. Plant Physiology. 1986;82:588–59. doi: 10.1104/pp.82.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc G, Messager A. Mutagenesis of pea (Pisum sativum L.) and the isolation of mutants for nodulation and nitrogen fixation. Plant Science. 1989;60:207–213. [Google Scholar]
- Ferguson BJ, Mathesius U. Signaling interactions during nodule development. Journal of Plant Growth Regulation. 2003;22:47–72. [Google Scholar]
- Ferguson BJ, Ross JJ, Reid JB. Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiology. 2005;138:2396–2405. doi: 10.1104/pp.105.062414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ, Indrasummunar A, Hayashi S, et al. Molecular analysis of legume nodule development and autoregulation. Journal of Intergrative Plant Biology. 2010;52:61–72. doi: 10.1111/j.1744-7909.2010.00899.x. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Foo E, Ross JJ, Reid JB. Relationship between gibberellin, ethylene and nodulation in Pisum sativum. New Phytologist. 2011;189:829–842. doi: 10.1111/j.1469-8137.2010.03542.x. [DOI] [PubMed] [Google Scholar]
- Foo E, Davies NW. Strigolactones promote nodulation in pea. Planta. 2011;234:1073–1081. doi: 10.1007/s00425-011-1516-7. [DOI] [PubMed] [Google Scholar]
- Foo E, Reid JB. Strigolactones: new physiological roles for an ancient signal. Journal of Plant Growth Regulation. 2013;32:429–442. [Google Scholar]
- Foo E, Turnbull CG, Beveridge CA. Long-distance signaling and the control of branching in the rms1 mutant of pea. Plant Physiology. 2001;126:203–209. doi: 10.1104/pp.126.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Yoneyama K, Hugill CJ, Quittenden LJ, Reid JB. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Molecular Plant. 2013a;6:76–87. doi: 10.1093/mp/sss115. [DOI] [PubMed] [Google Scholar]
- Foo E, Yoneyama K, Hugill C, Quittenden LJ, Reid JB. Strigolactones: internal and external signals in plant symbioses? Plant Signaling and Behavior. 2013b;8:e23168. doi: 10.4161/psb.23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Reid DE, Lorenc M, et al. Transient Nod-factor dependent gene expression in the nodulation competent zone of soybean (Glycine max L. Merr.) roots. Plant Biotechnology Journal. 2012;10:995–1010. doi: 10.1111/j.1467-7652.2012.00729.x. [DOI] [PubMed] [Google Scholar]
- Hewitt EJ. Sand and water culture: methods used in the study of plant nutrition, 2nd edn. Farnham Royal, UK: Commonwealth Agricultural Bureau; 1966. [Google Scholar]
- Jin J, Watt M, Mathesius U. The autoregulation gene SUNN mediates changes in root organ formation in response to nitrogen through alteration of shoot-to-root auxin transport. Plant Physiology. 2012;159:489–500. doi: 10.1104/pp.112.194993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krussel L, Madsen LH, Sato S, et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature. 2002;420:422–425. doi: 10.1038/nature01207. [DOI] [PubMed] [Google Scholar]
- Krusell L, Sato N, Fukuhara I, et al. The Clavata2 genes of pea and Lotus japonicus affect autoregulation of nodulation. The Plant Journal. 2011;65:861–871. doi: 10.1111/j.1365-313X.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- Li DX, Kinkema M, Gresshoff PM. Autoregulation of nodulation (AON) in Pisum sativum (pea) involves signaling events associated with both nodule primordia development and nitrogen fixation. Journal of Plant Physiology. 2009;166:955–967. doi: 10.1016/j.jplph.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Lin YH, Ferguson BJ, Kereszt A, Gresshoff PM. Suppression of hypernodulation in soybean by a leaf extracted, NARK- and Nod factor-dependent, low molecular mass fraction. New Phytologist. 2010;185:1074–1086. doi: 10.1111/j.1469-8137.2009.03163.x. [DOI] [PubMed] [Google Scholar]
- Lin YH, Lin MH, Gresshoff PM, Ferguson BJ. An efficient petiole-feeding bioassay for introducing aqueous solutions into dicotyledonous plants. Nature Protocols. 2011;6:36–45. doi: 10.1038/nprot.2010.171. [DOI] [PubMed] [Google Scholar]
- Liu J, Lovisolo C, Schubert A, Cardinale F. Signaling role of strigolactones at the interface between plants, (micro) organisms, and a changing environment. Journal of Plant Interactions. 2013;8:17–33. [Google Scholar]
- Miyazawa H, Oka-Kira E, Sato N, et al. The receptor-like kinase KLAVIER mediates systemic regulation of nodulation and non-symbiotic shoot development in Lotus japonicus. Development. 2010;137:4317–4325. doi: 10.1242/dev.058891. [DOI] [PubMed] [Google Scholar]
- Mortier V, Den Herder G, Whitford R, et al. CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiology. 2010;153:222–237. doi: 10.1104/pp.110.153718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu GJ, et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- Nomura T, Jager CE, Kitasaka Y, et al. Brassinosteroid deficiency due to truncated steroid 5α-reductase causes dwarfism in the lk mutant of pea. Plant Physiology. 2004;135:2220–2229. doi: 10.1104/pp.104.043786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Noorden GE, Ross JJ, Reid JB, Rolfe BG, Mathesius U. Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiology, 2006;140:1494–1506. doi: 10.1104/pp.105.075879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák K. Early action of pea symbiotic gene NOD3 is confirmed by adventitious root phenotype. Plant Science. 2010;179:472–478. doi: 10.1016/j.plantsci.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Oka-Kira E, Tateno K, Miura KI, et al. klavier (klv), a novel hypernodulation mutant of Lotus japonicus affected in vascular tissue organization and floral induction. The Plant Journal. 2005;44:505–515. doi: 10.1111/j.1365-313X.2005.02543.x. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Ohnishi E, Sato S, et al. Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant and Cell Physiology. 2009;50:67–77.. doi: 10.1093/pcp/pcn194. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Satoru H, Mori T, Matsubayashi Y, Kawaguchi M. Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nature Communications. 2013;4:2191. doi: 10.1038/ncomms3191. [DOI] [PubMed] [Google Scholar]
- Osipova M, Mortier V, Demchenko KN, et al. Wuschel-related homeobox5 gene expression and interaction of CLE peptides with components of the systemic control add two pieces to the puzzle of autoregulation of nodulation. Plant Physiology. 2012;158:1329–1341. doi: 10.1104/pp.111.188078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JG, Jacobsen E, Feenstra WJ. Three pea mutants with an altered nodulation studied by genetic analysis and grafting. Journal of Plant Physiology. 1988;132:424–430. [Google Scholar]
- Reid JB. Internode length in Pisum. Three further loci, lh, ls and lk. Annals of Botany. 1986;57:577–592. [Google Scholar]
- Reid DE, Ferguson BJ, Hayashi S, Lin Y-H, Gresshoff PM. Molecular mechanisms controlling legume autoregulation of nodulation. Annals of Botany. 2011a;108:789–95. doi: 10.1093/aob/mcr205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DE, Ferguson BJ, Gresshoff PM. Inoculation- and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation. Molecular Plant-Microbe Interactions. 2011b;24:606–618. doi: 10.1094/MPMI-09-10-0207. [DOI] [PubMed] [Google Scholar]
- Reid DE, Li D, Ferguson BJ, Gresshoff PM. Structure–function analysis of the GmRIC1 signal peptide and CLE domain required for nodulation control in soybean. Journal of Experimental Botany. 2013;64:1575–1585. doi: 10.1093/jxb/ert008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyter-Spira C, Kohlen W, Charnikhova T, et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiology. 2011;155:721–734. doi: 10.1104/pp.110.166645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Cho H, Choi D, Hwang I. Plant hormonal regulation of nitrogen-fixing nodule organogenesis. Molecules and Cells. 2012;34:117–126. doi: 10.1007/s10059-012-0131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagan M, Duc G. Sym28 and Sym29, two new genes involved in regulation of nodulation in pea (Pisum sativum L.) Symbiosis. 1996;20:229–245. [Google Scholar]
- Sagan M, Gresshoff PM. Developmental mapping of nodulation events in pea (Pisum sativum L.) using supernodulating plant genotypes and bacterial variability reveals both plant and Rhizobium control of nodulation regulation. Plant Science. 1996;117:167–179. [Google Scholar]
- Saur IML, Oakes M, Djordjevic MA, Imim N. Crosstalk between the nodulation signaling pathway and the autoregulation of nodulation in Medicago truncatula. New Phytologist. 2011;190:865–874. doi: 10.1111/j.1469-8137.2011.03738.x. [DOI] [PubMed] [Google Scholar]
- Schnabel E, Journet EP, De Carvalho-Niebel F, Duc G, Frugoli J. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Molecular Biology. 2005;58:809–822. doi: 10.1007/s11103-005-8102-y. [DOI] [PubMed] [Google Scholar]
- Schnabel EL, Kassaw TK, Smith LS, et al. The ROOT DETERMINED NODULATION1 gene regulates nodule number in roots of Medicago truncatula and defines a highly conserved, uncharacterized plant gene family. Plant Physiology. 2011;157:328–340. doi: 10.1104/pp.111.178756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle IR, Men AE, Laniya TS, et al. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science. 2003;299:109–112. doi: 10.1126/science.1077937. [DOI] [PubMed] [Google Scholar]
- Soto MJ, Fernandez-Aparicio M, Castellanos-Morales V, Garcia-Garrido JA, Delgado MJ, Vierheilig H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa) Soil Biology and Biochemistry. 2010;42:383–385. [Google Scholar]
- Symons GM, Reid JB. Brassinosteroids do not undergo long-distance transport in pea. Implications for the regulation of endogenous brassinosteroid levels . Plant Physiology. 2004;135:2196–2206. doi: 10.1104/pp.104.043034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara M, Magori S, Soyano T, et al. TOO MUCH LOVE, a novel Kelch repeat-containing F-box protein, functions in the long-distance regulation of the legume–rhizobium symbiosis. Plant and Cell Physiology. 2013;54:433–447. doi: 10.1093/pcp/pct022. [DOI] [PubMed] [Google Scholar]
- Terakado J, Fujihara S, Goto S, et al. Systemic effect of a brassinosteroid on root nodule formation in soybean as revealed by the application of brassinolide and brassinazole. Soil Science and Plant Nutrition. 2005;51:389–395. [Google Scholar]
- Yoneyama K, Xie X, Kim HI, et al. How do nitrogen and phosphorus deficiencies affect strigolactone production and exudation? Planta. 2012;235:1197–1207. doi: 10.1007/s00425-011-1568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]