Abstract
Background and Aims
Bromeliaceae is a species-rich neotropical plant family that uses a variety of pollinators, principally vertebrates. Tillandsia is the most diverse genus, and includes more than one-third of all bromeliad species. Within this genus, the majority of species rely on diurnal pollination by hummingbirds; however, the flowers of some Tillandsia species show some characteristics typical for pollination by nocturnal animals, particularly bats and moths. In this study an examination is made of the floral and reproductive biology of the epiphytic bromeliad Tillandsia macropetala in a fragment of humid montane forest in central Veracruz, Mexico.
Methods
The reproductive system of the species, duration of anthesis, production of nectar and floral scent, as well as diurnal and nocturnal floral visitors and their effectiveness in pollination were determined.
Key Results
Tillandsia macropetala is a self-compatible species that achieves a higher fruit production through outcrossing. Nectar production is restricted to the night, and only nocturnal visits result in the development of fruits. The most frequent visitor (75 % of visits) and the only pollinator of this bromeliad (in 96 % of visits) was the nectarivorous bat Anoura geoffroyi (Phyllostomidae: Glossophaginae).
Conclusions
This is the first report of chiropterophily within the genus Tillandsia. The results on the pollination biology of this bromeliad suggest an ongoing evolutionary switch from pollination by birds or moths to bats.
Keywords: Tillandsia macropetala, Bromeliaceae, bat-pollination, Anoura geofroyii, bromeliad, chiropterophily, humid montane forest, floral visitors, Mexico, pollinator effectiveness
INTRODUCTION
The family Bromeliaceae is distributed almost exclusively in the Neotropics (Benzing, 2000), and 56 % of its species are epiphytic (Zotz, 2013). The family comprises about 3160 species in 50 genera, and Tillandsia is the most diverse genus, with more than 670 species, of which 635 are epiphytic (Zotz, 2013). Among bromeliads, pollination by vertebrates predominates over that provided by insects, and most species are pollinated by hummingbirds (Kessler and Krömer, 2000; Canela and Sazima, 2005; Krömer et al., 2006). In some bromeliad genera (e.g. Guzmania, Pitcairnia and Vriesea), however, syndromes of pollination by insects, birds and bats have evolved independently (Benzing, 2000). In particular, species of the subfamily Tillandsioideae (e.g. Tillandsia, Guzmania and Vriesea) have on various occasions modified their floral characteristics to attract a wide range of pollinators (Benzing, 2000).
The epiphytic bromeliad Tillandsia macropetala Wawra, together with T. grandis Schltdl. and T. viridiflora (Beer) Baker, belongs to the Mexican species in the Tillandsia viridiflora complex, among which T. macropetala was recently recognized as a species distinct from T. viridiflora (Krömer et al., 2012). It is distributed in central and southern Mexico, in wet montane or pine–oak forests at elevations of 1100–2500 m. In contrast to the majority of species of the genus, T. macropetala does not present the conspicuously coloured bracts with contrasting flowers and diurnal anthesis typical of pollination by hummingbirds (Endress, 1994; Wilmer, 2011). Instead, this species has a light-green corolla, green bracts and crepuscular anthesis (Krömer et al., 2012).
Tillandsia macropetala was not included (even under its previous name T. viridiflora) in the classic work of Gardner (1986) about the pollination in Tillandsia, but the related species T. heterophylla E. Morren was catalogued as being moth-pollinated. These three species present particular cases within the genus Tillandsia, given their nocturnal anthesis and light-green petals, as seen in other bromeliads of the subgenus Pseudoalcantarea (Benzing, 2000). Whereas Hietz and Hietz-Seifert (1994) suggested that sphingid moths could be pollinators of T. viridiflora, Benzing (2000) argued based on their floral characteristics that the species of Pseudoalcantarea could be pollinated by bats.
The discovery of dilute nectar, rich in hexoses, in T. viridiflora (Krömer et al., 2008), together with other observations also including T. macropetala (T. Krömer and M. C. MacSwiney G., pers. obs.), suggests that bats could be their principal pollinators. In addition, the helicoiform flowers of T. macropetala stay open during the morning following anthesis (Krömer et al., 2012), which could enable pollination by both nocturnal and diurnal visitors (Dar et al., 2006; Ortega-Baes et al., 2011).
In this study, we analysed the floral and reproductive biology of T. macropetala. Specifically, (1) the floral visitors that effectively pollinate the flowers were identified, (2) the floral phenology was described, (3) the production pattern and concentration of nectar were determined, and the floral scent was analysed, and (4) the reproductive system of the bromeliad was assessed. As anthesis in this species begins at dusk and extends into the following day, our primary hypothesis was that T. macropetala receives both nocturnal and diurnal visits, but because nocturnal visitors visit the receptive flower first, they will be more important pollinators, accounting for the majority of fruits or seeds. Finally, as suggested by the dilute and hexose-rich nectar, we hypothesized that bats are the main pollinators of this species.
MATERIALS AND METHODS
Study site
Fieldwork was carried out in March and April of 2011 and 2012, in the municipality of Tlalnelhuayocan, in the central region of Veracruz State, Mexico. The site has an elevation of 1500–1700 m (Mehltreter et al., 2005), with an annual precipitation of 1650 mm and an average temperature of 14 °C (Williams-Linera, 2002). The predominant vegetation is humid montane forest (Williams-Linera, 2007). Monitoring of T. macropetala phenology was conducted in 2011 in a forest fragment (19°31′12·9″N, 96°59′17·9″W; Fig. 1) where the dominant trees were Liquidambar macrophylla Oerst and Quercus spp., and which is surrounded by a mosaic of anthropized vegetation. For logistical reasons, the study in 2012 was conducted in another fragment 1·2 km from the original site (19°31′53·7″N, 96°58′46·8″W; Fig. 1). This second fragment featured similar vegetation, but was surrounded by fruit trees (Citrus sp., Macadamia sp., Musa sp.) and elements of secondary forest.
Fig. 1.
Study area location in the municipality of Tlalnelhuayocan, in central Veracruz, Mexico. Study sites are marked with numbers 1 (2011) and 2 (2012).
Study species
Tillandsia macropetala is an epiphytic bromeliad that is generally found on the trunks and lower parts of its host trees but can also be lithophytic or terrestrial (Krömer et al., 2012; Fig. 2A). Its leaves form a stemless tank-type rosette of almost 1·2 m in diameter; its inflorescence is 55–83 cm in length and is composed of 3–7 spikes, each with 15–20 flowers. The flowers (Fig. 2B) are distichous and erect, actinomorphic and with a subsessile helicoiform corolla. The petals are light green, strap-shaped, twisted, and 10·7–12·3 cm long and 14–17 mm wide. The light-green to white stamens are subequal, with free filaments 10·4–11·7 cm long; the anthers are green to green-whitish, curved and 7·5–8 mm long. The style is free and has a trilobulate stigma (Krömer et al., 2012).
Fig. 2.
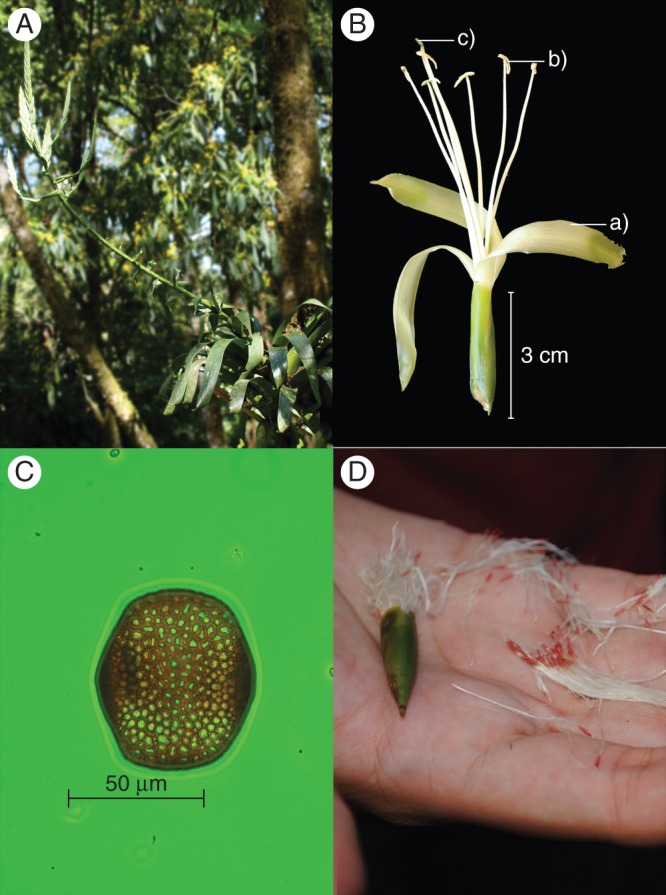
Tillandsia macropetala. (A) Individual with inflorescence in its habitat; (B) flower showing the light green petals (a), as well as the extended stamens (b) and style (c); (C) polar view of a pollen grain (phase contrast technique, 25×, Optovar 2); (D) fruit and seeds, the latter with a plumose appendage. Photographs: P. A. Aguilar-Rodríguez, M. C. MacSwiney G. and A. Knauer.
Phenology and floral anthesis
To follow the phenology of T. macropetala, 17 individuals that were close to flowering were chosen. Each floral bud was marked with a non-toxic indelible pen on the bract to enable monitoring of the development of individual flowers until fruiting. Once flowering began, floral condition was recorded every day for 3 weeks, describing flower growth, initiation of anthesis, opening of the corolla, and withering of the petals and style (Cascante-Marín et al., 2005; Martén-Rodríguez and Fenster, 2008). Receptivity of the stigma was determined by direct observation of the presence of mucilage on its surface (Escobedo, 2007) and to prove their status, the stigmas of six flowers were dipped in hydrogen peroxide 1, 6, 12, 24 or 36 h after anthesis (Martén-Rodríguez and Fenster, 2008).
In the study population, the majority of T. macropetala individuals that were close to flowering were found at heights that impeded manipulation of the flowers (3–5 m). Therefore, nearby plants were translocated and placed at a height of 1·5 m on the trunks of the trees. As some of the individuals of T. macropetala used in this study were found at this lower height, it was considered that the translocation should not affect the incidence of floral visitors (as confirmed by preliminary field observations). Regular trips were made to the study site, from December to February, to determine the onset of flowering.
Reproductive system
To determine the reproductive system of T. macropetala, pollination treatment experiments were carried out on six individuals placed in pots with gravel in a structure covered with mosquito netting that prevented visits to the flowers (hereafter referred to as the ‘planthouse’). The planthouse provided shade and temperatures similar to that found in the trees, and irrigation was carried out sporadically.
In the planthouse, the flowers were subjected to four treatments: (1) emasculation (apomixis) (n = 25 flowers) consisted of the emasculation of the flowers by removal of the anthers, to prevent pollen deposition on the flower; (2) in spontaneous self-pollination (n = 50 flowers), anthers were not removed and no other manipulation was carried out; (3) cross-pollination (xenogamy) (n = 40 flowers), in which the stigma was covered with pollen from the anthers of another plant individual; and (4) self-pollination (autogamy) (n = 29 flowers), in which the stigma was covered with pollen from the anthers of the same individual plant. All treatments were applied to previously covered virgin flowers no more than 2 h after the onset of anthesis. Following treatment, the flowers were kept covered to avoid subsequent visits. Eight weeks after conducting the treatments, the presence of fruits was recorded. Percentage of fructification, or fruit-set, was calculated for each treatment and, once the fruits were ripe, the number of seeds per fruit, or seed-set, was recorded (Fig. 2D).
A chi-square test was used to compare percentage of fructification between treatments. To measure the influence of pollination treatments on seed-set (Schmid et al., 2011a), an ANOVA was performed. As the data were not normally distributed (Kolmogorov–Smirnov test), a non-parametric Kruskal–Wallis test with Tukey-type comparisons was used. Analysis was carried out using the program Statistica (ver. 7, StatSoft Inc., Tulsa, OK, USA) with a level of significance of P ≤ 0·05. To quantify the self-compatibility of T. macropetala, we calculated the self-compatibility index (SC) and the autogamy index (AI) (Wendt et al., 2001; Kamke et al., 2011). The AI was multiplied by 100 to obtain a percentage value of self-compatibility (Martén-Rodríguez and Fenster, 2008).
Nectar analysis
To determine the production pattern and availability of nectar for potential visitors, 21 flowers were randomly chosen from the six individuals kept in the planthouse (comprising at least three flowers from each individual). There was no experimental pollination treatment on any of these flowers, and all measurements of nectar were carried out during periods of no rain. Nectar production was recorded in these flowers every 2 h, using 80- and 10-μL capillary tubes. Extraction continued until no nectar was left. Total nectar volume per flower was calculated as the sum of the hourly values (Tschapka and von Helversen, 2007). The percentage of sugars contained in each nectar sample was measured using a field refractometer (Mod. HRT32, range: 0–32 %, w/w, precision: 0·2 %; A. Krüss Optronic, Hamburg, Germany). A Pearson correlation was conducted to determine the relationship between volume and concentration of sugars in the nectar and the period during which the measurements were taken.
Floral scent analysis
Scent was collected from two single flowers for about 3 h between 0000 and 0330 h. We used the dynamic pull headspace method for the collection of floral volatiles as described by Huber et al. (2005), using Tenax GR (Tenax TR 60/80, Scientific Instrument Services, Old York, RD, USA) as absorbent. We collected scent from an empty oven bag as a control for air contamination.
For chemical analysis of headspace samples, gas chromatography with mass selective detection (GC-MSD) with thermodesorption was used as described by Sun et al. (2014). Compound identification was conducted using a mass-selective detector (Agilent MSD 5975, Agilent Technologies, Palo Alto, CA, USA) and ChemStation Enhanced Data Analysis program (version E.02·02). Compounds were identified by comparison of spectra obtained from the samples with those from a reference library (NIST ‘05 library, http://www.nist.gov/). Only floral scent compounds found with larger amounts in the samples collected from flowers than from the control were considered to be emitted by T. macropetala flowers.
Observations of floral visitors
Any animal that made contact with the corolla of the flower was considered a floral visitor (sensu Schmid et al., 2011b). To be considered potential pollinators, they also had to enter the corolla (Muchhala, 2006) and come into contact with the reproductive organs of the flowers (Schmid et al., 2011b).
Recording of nocturnal visitors was carried out using a video camera (DCR-SR65, Sony Corporation, Tokyo, Japan) in night vision mode, equipped with an infrared light (HVL-HILR, Sony). The camera was placed on a tripod 1·3–1·5 m above the ground and a distance of 1·5 m from the flower. Nocturnal recording took place from 1900 to 2300 h, which included the initiation of anthesis close to dusk, over a period of 13 d (dictated by a combination of equipment capacity and environmental conditions).
Diurnal visitors were observed directly with binoculars and a digital camera from 0600 to 1100 h (morning period) and from 1500 to 1800 h (evening period), at a distance of around 3 m from the flowers. Direct observations were subsequently made for 1 h before dawn and after dusk, over a period of 16 d. Recordings and observations of visitors were carried out from February to April in both years.
During the observations, the species (or morphospecies) of the visitor was recorded, along with the time of the visit, number of flowers visited and the reward sought. The recordings were analysed using the program Final Cut Pro 7 (Apple Inc. 2009) at a speed of 3 f.p.s. (10 % of real time). For the analysis, the duration of each visit was defined as the amount of time that the mouthparts of the pollinator remained within the corolla (Muchhala, 2006). If a visitor moved the whole inflorescence when placing its head inside the flower, it was considered that contact had been made with the reproductive organs of the flower (Slauson, 2000).
To identify the bat species, sampling was carried out with mist-nets (6 × 12 m) for a period of six nights. The nets were placed at least 1 m from a different T. macropetala individual in flower per night, and checked every 30 min. Nets were opened at dusk (1900 h) and closed after 6 h. Species were identified using field guides (Reid, 2009) and following the taxonomy of Simmons (2005) and Velazco and Patterson (2013). Samples of pollen were taken from the bodies of the captured individuals using a moist brush, and placed in vials with 70 % ethanol. Six preparations were made from each sample (18 preparations in total) for subsequent comparison with reference samples of T. macropetala pollen grains (Fig. 2C), using an optical microscope (40×, Carl Zeiss, Oberkochen, Germany).
Effectiveness of floral visitors
The effectiveness of the floral visitors was evaluated using floral visitor exclusion treatments (Montalvo and Ackerman, 1986; Sahley, 2001; Wendt et al., 2001). These treatments were conducted on 17 plants in the field, as follows: (1) diurnal exposure (DE) in which the stigmas of the treated flowers (n = 33) were covered with a plastic tube (of approx. 3 × 0·5 cm) closed with cotton during the night and uncovered for the diurnal visitors; (2) nocturnal exposure (NE) in which the stigmas of the flowers (n = 79) were left exposed during the night and covered during the day; (3) emasculated diurnal exposure (EDE) was carried out as in DE, but in previously emasculated flowers (n = 33); (4) emasculated nocturnal exposure (ENE) was carried out as in NE, but in previously emasculated flowers (n = 62); and (5) control in which flowers were left continuously exposed to visitors (n = 136).
To determine the effectiveness of the visitors, as well as the fruit-set and seed-set produced by the field treatments, we considered the frequency of visits, number of visits in which the visitors pollinated the flower and the behaviour of each visitor during its visit to the flower (e.g. consumption of pollen or petals, form of approach, number of flowers visited and reward sought; Montalvo and Ackerman, 1986).
RESULTS
Phenology and floral anthesis
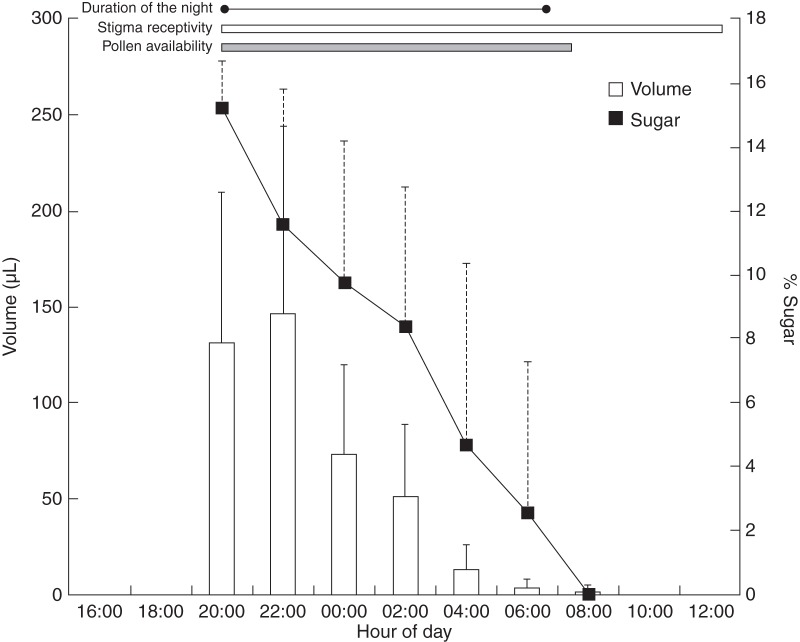
Flowering of T. macropetala began in the third week of December and finished in the second week of April. Opening of the floral bud initiated at approx. 1600 h. The stigma protruded almost 2 h before anthesis and, on reaching its maximum length (12·90 ± 1·51 cm, n = 12 flowers), the petals opened to expose the stamens with the dehiscent anthers. Anthesis began around 1900 h (range: 1820–2140 h, n = 71 flowers), coinciding with the local time of dusk (approx. 1845–1900 h; Fig. 3). Receptivity of the stigma lasted for around 18 h (n = 12 flowers) after opening of the flower.
Fig. 3.
Production (volume) and concentration (% sugar) of nectar in Tillandsia macropetala flowers. Error bars denote s.d. The duration of the receptivity of the stigma and the duration of the night at the study site are indicated, together with the period of dehiscence of the anthers and the time at which pollen grains are found in these.
Senescence began when the floral structures lost colour and turgor between 20 and 24 h after anthesis, completely restricting the entrance of the calyx at around 34 h after the onset of anthesis. In covered flowers without visits, it took about 11 h for the pollen to fall from the anthers (n = 12 flowers) by anther senescence. The inflorescence of an individual plant flowered over a period of 36·36 ± 9·51 d (n = 16 inflorescences), with an average of 0·7 ± 0·3 flowers d−1 (mean ± s.d.; range: 0–5 flowers daily; n = 282 flowers from 14 individuals). The flowering of a spike started at the base and progressed towards the apex over the following days.
Reproductive system
Highest fruit-set was obtained in the self-pollination (34·78 %) and cross-pollination (34·48 %) treatments. There were no fruits formed by the emasculation treatment and fructification was reduced in the spontaneous self-pollination treatment (Table 1). There was a significant difference in the percentage of fructification among treatments (χ2 = 37·169, d.f. = 4, P < 0·05). Excluding the emasculation treatment, which did not produce seeds, there were no significant differences in the number of seeds between the cross-pollination and self-pollination treatments, whereas there was a significant difference between both of these and the spontaneous self-pollination treatment (H = 5·46, d.f. = 2, P < 0·05; n = 98, flowers of six plants; Table 1). According to the SC and AI indices, T. macropetala is a self-compatible species (SC = 0·96, AI = 0·34 or 34 %).
Table 1.
Results of the pollination experiments carried out with Tillandsia macropetala flowers for determination of the reproductive system
| Treatment | No. of flowers manipulated | No. of fruits | Fruit-set | Seed-set (mean ± s.d.) |
|---|---|---|---|---|
| Emasculation | 24 | 0 | 0 | 0 |
| Spontaneous self-pollination | 46 | 6 | 13·04 | 50·6 ± 138·5a |
| Cross-pollination | 29 | 10 | 34·48 | 154·0 ± 225·6b |
| Self-pollination | 23 | 8 | 34·78 | 148·0 ± 215·0b |
Different letters denote significant differences (H = 5·46, d.f. = 2, P < 0·05; the emasculation treatment was excluded).
Nectar analysis
Mean total volume of nectar of the T. macropetala flowers was 434·04 µL (±178·03 s.d., n = 21 flowers in six individuals) per flower per night, and the average concentration of dissolved sugars was 7·43 % (±6·34 % s.d., n = 21 flowers). Nectar was produced for 12 h. The highest quantity of nectar was found at 2200 h (147·02 ± 97·38 µL), subsequently declining to almost zero by 0800 h (1·03 ± 3·90 µL; Fig. 3). The highest concentration of dissolved sugar was recorded at 2000 h, with 15·26 % (±1·45 %) and this decreased with diminishing nectar production (Fig. 3). Pearson correlation showed that a significant negative relationship existed between the time at which nectar was extracted (conducted at intervals of 2 h) and the volume produced (r = –0·72; P < 0·05), as well as between the time and the concentration of dissolved sugars (r = –0·78; P < 0·05).
Floral scent analysis
Nine floral scent compounds were identified in the samples collected from two T. macropetala flowers [amounts are given in per cent of total peak area (%)]: three fatty acid derivatives (nonanal, 19·8 %; heptanal, 10 %; 1-octen-3-ol, 2·5 %) and six terpenoids (limonene, 24·2 %; geraniol, 11·5 %; methyl geranate, 11 %; β-pinene, 8·3 %; 6-methyl-5-hepten-2-one, 7·2 %; (Z)-β-ocimene, 5·6 %).
Observations of floral visitors
A total of 107 h of observation were carried out (29 h at night and 78 h during the day) at 158 T. macropetala flowers (53 at night and 105 during the day) during which 210 visits were recorded (170 at night and 40 during the day), comprising nine different species of floral visitors (three during the night and six during the day; Supplementary Data Table S1).
Thirty-three individual bats of six species were captured near the flowers: Anoura geoffroyi Gray (n = 3 individuals), Artibeus lituratus Olfers (n = 2), Carollia sowelli Baker, Solari & Hoffmann (n = 8), Diphylla ecaudata Spix (n = 2), Myotis volans Allen (n = 3) and Sturnira hondurensis Anthony (n = 15). All individuals of A. geoffroyi had pollen on their snouts, chest, forearms and/or wing membranes, and even on the top of the head (Fig. 4A). All the pollen grains identified in the preparations of pollen taken from the fur of A. geoffroyi (Fig. 4A) belonged to bromeliads of the subfamily Tillandsioideae (Halbritter 1992), probably from T. macropetala, as it was the only flowering bromeliad with crepuscular anthesis at the time of this study. The video recordings showed that A. geoffroyi (identified by its size and reduced uropatagium) was the only bat species visiting T. macropetala.
Fig. 4.
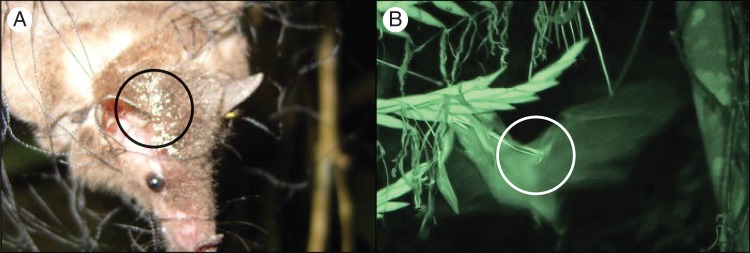
Images of: (A) Tillandsioideae pollen grains, presumably of Tillandsia macropetala, in the fur of an Anoura geoffroyi individual captured in a mist-net (circled in black); (B) nectarivorous bat making contact with the anthers of T. macropetala during its visit (circled in white). Images: P. A. Aguilar-Rodríguez.
Nocturnal video recordings totaling 29 h were obtained, in which 170 visits by three species were recorded: the bat A. geoffroyi, at least two mice Peromyscus sp. and a nocturnal moth (Noctuidae; Supplementary Data Table S1). Anoura geoffroyi made 158 visits of 0·17 ± 0·08 s duration (n = 10 visits). Visits began at dusk and continued throughout the 3-h recording period, peaking between 1930 and 2130 h. In all visits, the bats placed their heads within the flowers and on 152 occasions (96·20 %) made contact with the stigma and the stamens, causing the whole spike to move as a result of the force of contact (Fig. 4B). The Peromyscus sp. mice were recorded making ten visits in the same night to at least six different flowers in one single bromeliad inflorescence (Aguilar-Rodríguez et al., 2013). The moth was recorded on the T. macropetala corolla on two occasions, but was never observed taking the floral rewards.
Forty diurnal visits were recorded. The most frequent diurnal visitor was the hummingbird Lampornis amethystinus Swainson, which made 32 visits to the flowers of T. macropetala. Of these visits, 28 occurred between 0630 and 0915 h and four between 1800 and 1900 h. As a result of its way of approaching the flowers (not pressing the head against the flower, unlike the bat), in combination with the open corolla, L. amethystinus hardly made contact with the reproductive organs of T. macropetala.
Effectiveness of floral visitors
Only the flowers exposed to nocturnal visitors (treatments NE and ENE) developed fruits, with both nocturnal exposure treatments achieving 40 % fructification (Table 2), whereas the flowers exposed to diurnal visitors (treatments DE and EDE) did not produce fruits. Seed-set in treatments NE and ENE did not differ significantly from those of the control treatment (H = 0·17, d.f. = 2, P > 0·05; n = 255 flowers of 17 plants, excluding those of treatments DE and EDE; Table 2).
Table 2.
Results of the exclusion experiments with Tillandsia macropetala flowers for determination of the effectiveness of the pollinators
| Treatment | No. of flowers | No. of fruits | Fruit-set | Seed-set (mean ± s.d.) |
|---|---|---|---|---|
| Diurnal exposure | 33 | 0 | 0 | 0 |
| Nocturnal exposure | 72 | 25 | 34·72 | 184·5 ± 268·8a |
| Emasculated diurnal exposure | 31 | 0 | 0 | 0 |
| Emasculated nocturnal exposure | 57 | 22 | 38·59 | 199·3 ± 284·8a |
| Control | 126 | 49 | 38·88 | 180·5 ± 249·9a |
Different letters denote significant differences at P ≤ 0·05 (diurnal exposure and emasculated diurnal exposure were excluded).
DISCUSSION
Tillandsia macropetala is a self-compatible species and, although anthesis covered both nocturnal and diurnal periods, displays a pollination system that is specialized towards nocturnal visitors, of which the bat A. geoffroyi is the only pollinator. Thus, we report for the first time bat-pollination of a species in the genus Tillandsia. In bromeliads, chiropterophily has been previously reported for species of the genera Encholirium, Guzmania, Pitcairnia, Puya, Vriesea and Werauhia (Sazima et al., 1989; Krömer et al., 2007; Tschapka and von Helversen, 2007; Fleming et al., 2009; Christianini et al., 2013).
Tillandsia macropetala can be pollinated either by its own pollen or by that of another individual, a common characteristic in bromeliads (Matallana et al., 2010). However, differences in fructification between the spontaneous self-pollination (13 %) and those of the cross-pollination and self-pollination (approx. 35 %) treatments indicate that, while it has the potential for self-pollination, more fruits are developed when an external agent delivers pollen from another plant individual. This may be due to the floral morphology of the species, which makes self-pollination difficult.
Contrary to our hypothesis, even if T. macropetala receives nocturnal and diurnal visits, the visitor exclusion treatments conducted in the field showed that only visits made by nocturnal animals produced fruits, thus making the diurnal visitors nectar thieves. Both the duration of stigma receptivity (approx. 18 h, of which 9 h are nocturnal) and the period during which pollen is available on the anthers (approx. 11 h, of which 9 h are nocturnal) make nocturnal pollination more feasible. Nectar production patterns, in terms of volume and concentration, are higher in the initial hours of the night and decrease to a minimum in the initial hours of the morning. In this way, the highest potential reward is available during the night, a trait that has been previously observed in bromeliads visited by bats (Tschapka and von Helversen, 2007).
In accordance with our second hypothesis, the only effective pollinator of T. macropetala was the bat Anoura geoffroyi, and the captured individuals of this species were found to carry bromeliad pollen of presumably this species. Although Anoura bats visit different flowering species, as revealed by different pollen morphotypes (Muchhala and Jarrín-V., 2002), we only found pollen of the same morphotype, which may indicate that night-flowering Tillandsioideae bromeliads were the only source for nectar in the vicinity at the time of this study. It is known that A. geoffroyi visits at least two other bromeliad species, namely Vriesea longiscapa Ule in the coastal tropical lowland forest of south-eastern Brazil (5–90 m; Sazima et al., 1999) and Vriesea platynema Gaudichaud at a montane humid forest of Brazil (1100 m; Kaehler et al., 2005). Early morning field observations on the flowers recorded in the previous night showed that in flowers visited by bats, pollen was frequently found on the stigma and the anthers were found without remaining pollen. Due to this lack of pollen available in the morning, diurnal visits probably had no effect on the production of seeds. The predominance of nocturnal visitors to T. macropetala is clear from the low frequency of diurnal visitors (19 % of the total visits), which act only as nectar thieves (sensu Irwin et al., 2010), as no diurnal visitor was observed to make contact with the anthers and/or stigma while taking nectar.
The morphology of the flowers of T. macropetala enables many animals (e.g. hummingbirds and various insects) to access the floral reward while making no contact with the reproductive organs. The free extended filaments and style imply that arthropods, because of their small body size, cannot make contact with both anthers and stigma during one visit. In the case of the hummingbird, however, it is the form of approach and visit to the flower that impedes pollination (see Muchhala, 2006).
Considering the characteristics of chiropterophilous plants, the floral morphology of T. macropetala does not match the typical traits of large, zygomorphic and bell-shaped flowers with strong scent (Tschapka and Dressler, 2002), such as presented by bromeliads of the genera Vriesea and Werauhia (Vogel, 1969; Sazima et al., 1995; Cascante-Marín et al., 2005; Krömer et al., 2007). Also, sulphur-containing compounds are absent from the scent of T. macropetala, unlike in other bat-pollinated plants (Bestmann et al., 1997; von Helversen et al., 2000). It has also been reported that the nectar of some chiropterophilous bromeliads has a strong and disagreeable odour (e.g. Sazima et al., 1989), whereas the nectar of T. macropetala has a faintly sweet odor in the early hours of the night when the volume of nectar is highest (P. A. Aguilar-Rodríguez, pers. obs.).
However, the nocturnal anthesis of T. macropetala, its temporal pattern and characteristics of nectar production, extended stamens and stylus, petal colour and phenology, and floral display (annual flowering, with about one flower per night for several nights) match those expected for a bat-pollinated species (Sazima et al., 1999; Tschapka and von Helversen, 2007; Wilmer, 2011).
Nectar sugar concentration of T. macropetala is low for a bat-pollinated flower, being about 10 % below the average (Tschapka and Dressler, 2002). Comparing nectar characteristics of chiropterophilous bromeliads, the nectar sugar concentration of T. macropetala is lower than those of Guzmania, Vriesea and most Werauhia species (Krömer et al., 2008), but the nectar volume is higher (434·04 ± 178·03 µL) than those recorded for Vriesea longiscapa and V. bituminosa Wawra (116 – 235 µL; Sazima et al., 1999) and T. heterophylla (82·21 ± 48·13 µL; P. A. Aguilar-Rodríguez, pers. obs.).
The most studied chiropterophilous bromeliad, Werauhia gladioliflora (H. Wendland) J.R. Grant, produces more than twice the fruit-set (87 vs. 38 %) and ten times as many seeds as T. macropetala (1871–2018 vs. 180 seeds; Cascante-Marín et al., 2005; Tschapka and von Helversen, 2007). These differences may be due to the fact that W. gladioliflora has a high potential for self-pollination, and may be pollinated by at least four species of bats in the tropical lowland rain forest of Costa Rica (Tschapka and von Helversen, 2007), whereas T. macropetala has only limited capacity for spontaneous self-pollination and was pollinated only by one bat species at our study site. The higher number of bat pollinators may explain the differences in the volume and sugar concentration in nectar of W. gladioliflora compared with T. macropetala (1129 µL and 17 % vs. 434·04 µL and 7·43 %; Tschapka and von Helversen, 2007).
Unlike the floral scent of W. gladioliflora, which included dimethyl disulphide, an unusual component of floral scents that attracts even naïve flower-visiting glossophagine bats (von Helversen et al., 2000), T. macropetala scent lacks this compound. However, not all bat-pollinated species have dimethyl disulphide in their scent (Knudsen and Tollsten, 1995), and six out of nine detected compounds in the T. macropetala scent have been detected in other bat-pollinated plants (Knudsen and Tollsten, 1995; Bestmann et al., 1997). Also, it is known that plants with an extended floral anthesis change the pattern and components of their floral scent (e.g. Silene otites, Caryophyllaceae; Dötterl et al., 2012). For practical reasons, we could only evaluate the floral scent compounds from 0000 to 0330 h from two different flowers, so perhaps monitoring the scent emission through the entire anthesis of T. macropetala – including the period with maximum number of visits – would reveal additional volatile compounds.
Our results on the pollination biology of this bromeliad are in accordance with the isolated phylogenetic position of T. viridiflora (the type species of Pseudalcantarea) as sister to the rest of Tillandsia/Racinaea/Viridantha (Barfuss et al., 2005). This might indicate an ongoing evolution from bird or moth pollination, which are common in Tillandsia (Benzing, 2000; Kessler and Krömer, 2000), to bat-pollination, in the same way as suggested for the origin of chiropterophily (von Helversen and Winter, 2003). Likewise, the present study supports Krömer et al.'s (2008) suggestion, based on the characteristics of the nectar, that this bromeliad may be considered a chiropterophilous species.
However, note that at our study site T. macropetala is at the northern limits of its distribution, and in the more tropical areas of Mexico and in Central America there are more species of nectar-feeding phyllostomid bats (Espinoza et al., 1998; Estrada and Coates-Estrada, 2001; Laval and Rodríguez-H., 2002), and also other animals that could possibly serve as pollinators (e.g. hummingbirds and moths). Thus, more detailed studies of nectar characteristics, pollinators and reproductive biology of the relevant species, especially realized in more diverse tropical zones, are necessary to understand the evolution of pollination syndromes in the genus Tillandsia in general and the subgenus Pseudoalcantarea in particular.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are grateful to Luz María Ordiales, Aníbal Silva Ayala, Jonathan Ott, Madian Rivera, Jorge Gómez, Juan Manuel Pech, Grecia Benítez and Zuemy Vallado for their help in the field. Emmanuel Solís, Lilia Ruíz and Samaria Armenta helped with editing photographs, videos and the map. Andrew P. Vovides and Sonia Galicia Castellanos kindly allowed use of the Laboratorio de Biología Evolutiva de Cycadales, of INECOL A. C. to process the pollen samples. We also thank Florian Schiestl for providing the gas chromatograph and to Edward Connor for his help with scent analysis. This work was supported by the Consejo Nacional de Ciencia y Tecnología (grant number 59406 awarded to P.A.A.-R.), the Claraz Schenkung and The Bromeliad Society International. The collection permit (SGPA/DGVS/02294/11) was issued by the Secretaría de Medio Ambiente y Recursos Naturales.
LITERATURE CITED
- Aguilar-Rodríguez PA, MacSwiney G MC, Krömer T, García-Franco JG. Pollen consumption by free-living mice. Acta Theriologica. 2013 doi:10.1007/s13364-013-0164-7. [Google Scholar]
- Barfuss MHJ, Samuel R, Till W, Stuessy TF. Phylogenetic relationships in subfamily Tillandsioideae (Bromeliaceae) based on DNA sequence data from seven plastid regions. American Journal of Botany. 2005;92:337–351. doi: 10.3732/ajb.92.2.337. [DOI] [PubMed] [Google Scholar]
- Benzing DH. Bromeliaceae. Profile of an adaptive radiation. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Bestmann H J, Winkler L, von Helversen O. Headspace analysis of volatile flower scent constituents of bat-pollinated plants. Phytochemistry. 1997;46:1169–1172. [PubMed] [Google Scholar]
- Canela MBF, Sazima M. The pollination of Bromelia antiacantha (Bromeliaceae) in Southeastern Brazil: ornithophilous versus melittophilous features. Plant Biology. 2005;7:411–416. doi: 10.1055/s-2005-865619. [DOI] [PubMed] [Google Scholar]
- Cascante-Marín A, Oostermeijer JGB, Wolf JHD, Dennijs JCM. Reproductive biology of the epiphytic bromeliad Werauhia gladioliflora in a premontane tropical forest. Plant Biology. 2005;7:203–209. doi: 10.1055/s-2005-837584. [DOI] [PubMed] [Google Scholar]
- Christianini AV, Forzza RC, Buzato S. Divergence on floral traits and vertebrate pollinators of two endemic Encholirium bromeliads. Plant Biology. 2013;15:360–368. doi: 10.1111/j.1438-8677.2012.00649.x. [DOI] [PubMed] [Google Scholar]
- Dar S, Arizmendi MS, Valiente-Banuet A. Diurnal and nocturnal pollination of Marginatocereus marginatus (Pachycereeae: Cactaceae) in Central Mexico. Annals of Botany. 2006;97:423–427. doi: 10.1093/aob/mcj045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dötterl S, Jahreiß K, Jhumur UM, Jürgens A. Temporal variation of flower scent in Silene otitis (Caryophyllaceae): a species with a mixed pollination system. Botanical Journal of the Linnean Society. 2012;169:447–460. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Escobedo SGJ. México: CIIDIR, Instituto Politécnico Nacional, Santa Cruz Xoxocotlán, Oaxaca; 2007. Biología de la reproducción de Tillandsia prodigiosa (Lem.) Baker Bromeliaceae. Master Thesis. [Google Scholar]
- Espinoza ME, Anzures DA, Cruz AE. Mamíferos de la reserva de la biosfera El Triunfo, Chiapas. Revista Mexicana de Mastozoología. 1998;3:79–94. [Google Scholar]
- Estrada A, Coates-Estrada R. Species composition and reproductive phenology of bats in a tropical landscape at Los Tuxtlas, Mexico. Journal of Tropical Ecology. 2001;17:627–646. [Google Scholar]
- Fleming TH, Geiselman C, Kress WJ. The evolution of bat pollination: a phylogenetic perspective. Annals of Botany. 2009;104:1017–1043. doi: 10.1093/aob/mcp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CS. Inferences about pollination in Tillandsia (Bromeliaceae) Selbyana. 1986;9:76–87. [Google Scholar]
- Hietz P, Hietz-Seifert U. Epífitas de Veracruz. Guía ilustrada para las regiones de Xalapa y Los Tuxtlas, Veracruz. Xalapa: Instituto de Ecología, A.C; 1994. [Google Scholar]
- Huber FK, Kaiser R, Sauter W, Schiestl FP. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae) Oecologia. 2005;142:564–575. doi: 10.1007/s00442-004-1750-9. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Bronstein JL, Manson JS, Richardson L. Nectar robbing: ecological and evolutionary perspectives. Annual Review of Ecology, Evolution, and Systematics. 2010;41:271–292. [Google Scholar]
- Kaehler M, Varassin IG, Goldenberg R. Polinização em uma comunidade de bromélias em floresta atlântica alto-montana no estado do Paraná, Brasil. Brazilian Journal of Botany. 2005;28:219–228. [Google Scholar]
- Kamke R, Schmid S, Zillikens A, Cortés-Lopes B, Steiner J. The importance of bees as pollinators in the short corolla bromeliad Aechmea caudata in Southern Brazil. Flora. 2011;206:749–756. [Google Scholar]
- Kessler M, Krömer T. Patterns and ecological correlates of pollination modes among bromeliad communities of Andean forests in Bolivia. Plant Biology. 2000;2:659–669. [Google Scholar]
- Knudsen JT, Tollsten L. Floral scent in bat-pollinated plants: a case of convergent evolution. Botanical Journal of the Linnean Society. 1995;119:45–57. [Google Scholar]
- Krömer T, Kessler M, Herzog SK. Distribution and flowering ecology of bromeliads along two climatically contrasting elevational transects in the Bolivian Andes. Biotropica. 2006;38:183–195. [Google Scholar]
- Krömer T, Espejo A, López-Ferrari AR, Acebey A. Werauhia noctiflorens (Bromeliaceae), una nueva especie del sureste de México y Belice. Novon. 2007;17:336–340. [Google Scholar]
- Krömer T, Kessler M, Lohaus G, Schmidt-Lebuhn AN. Nectar sugar composition and concentration in relation to pollination syndromes in Bromeliaceae. Plant Biology. 2008;10:502–511. doi: 10.1111/j.1438-8677.2008.00058.x. [DOI] [PubMed] [Google Scholar]
- Krömer T, Espejo-Serna A, López-Ferrari A, Ehlers R, Lautner J. Taxonomic and nomenclatural status of the Mexican species in the Tillandsia viridiflora complex (Bromeliaceae) Acta Botánica Mexicana. 2012;99:1–20. [Google Scholar]
- Laval R, Rodríguez-H. B . Murciélagos de Costa Rica. 1st edn. Santo Domingo de Heredia: Instituto Nacional de Biodiversidad; 2002. [Google Scholar]
- Martén-Rodríguez S, Fenster CB. Pollination ecology and breeding systems of five Gesneria species from Puerto Rico. Annals of Botany. 2008;102:23–30. doi: 10.1093/aob/mcn056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matallana G, Godinho MAS, Guilherme FAG, Belisario M, Coser TS, Wendt T. Breeding systems of Bromeliaceae species: evolution of selfing in the context of sympatric occurrence. Plant Systematics and Evolution. 2010;289:57–65. [Google Scholar]
- Mehltreter K, Flores-Palacios A, García-Franco JG. Host preferences of low-trunk vascular epiphytes in a cloud forest of Veracruz, Mexico. Journal of Tropical Ecology. 2005;21:651–660. [Google Scholar]
- Montalvo AM, Ackerman JD. Relative pollinator effectiveness and evolution of floral traits in Spathiphyllum friedrichsthalii (Araceae) American Journal of Botany. 1986;73:1665–1676. [Google Scholar]
- Muchhala N. The pollination biology of Burmeistera (Campanulaceae): specialization and syndromes. American Journal of Botany. 2006;93:1081–1089. doi: 10.3732/ajb.93.8.1081. [DOI] [PubMed] [Google Scholar]
- Muchhala N, Jarrín-V. P Flower visitation by bats in cloud forests of Western Ecuador. Biotropica. 2002;34:387–395. [Google Scholar]
- Ortega-Baes P, Saravia M, Sühring S, Godínez-Álvarez H, Zamar M. Reproductive biology of Echinopsis terscheckii (Cactaceae): the role of nocturnal and diurnal pollinators. Plant Biology. 2011;13(Suppl. 1):33–40. doi: 10.1111/j.1438-8677.2010.00332.x. [DOI] [PubMed] [Google Scholar]
- Reid F. A field guide to the mammals of Central America and Southeast Mexico. Oxford: Oxford University Press; 2009. [Google Scholar]
- Sahley CT. Vertebrate pollination, fruit production, and pollen dispersal of Stenocereus thurberi (Cactaceae) The Southwestern Naturalist. 2001;46:261–271. [Google Scholar]
- Sazima I, Vogel S, Sazima M. Bat pollination of Encholirium glaziovii, a terrestrial bromeliad. Plant Systematics and Evolution. 1989;168:167–179. [Google Scholar]
- Sazima M, Buzato S, Sazima I. Bat pollination of Vriesea in southeastern Brazil. Bromelia. 1995;2:29–37. [Google Scholar]
- Sazima M, Buzato S, Sazima I. Bat-pollinated flower assemblage and bat visitors at two Atlantic forest sites in Brazil. Annals of Botany. 1999;83:705–712. [Google Scholar]
- Schmid S, Schmid VS, Zillikens A, Harter-Marques B, Steiner J. Bimodal pollination system of the bromeliad Aechmea nudicaulis involving hummingbirds and bees. Plant Biology. 2011a;13:41–50. doi: 10.1111/j.1438-8677.2010.00348.x. [DOI] [PubMed] [Google Scholar]
- Schmid S, Schmid VS, Zillikens A, Steiner J. Diversity of flower visitors and their role for pollination in the ornithophilous bromeliad Vriesea friburgensis in two different habitats in southern Brazil. Ecotropica. 2011b;17:91–102. [Google Scholar]
- Simmons NB. Order Chiroptera. In: Wilson DE, Reeder DM, editors. Mammal species of the world: a taxonomic and geographic reference. Baltimore, MD: Johns Hopkins University Press; 2005. pp. 312–529. [Google Scholar]
- Slauson LA. Pollination biology of two chiropterophilous agaves in Arizona. American Journal of Botany. 2000;87:825–836. [PubMed] [Google Scholar]
- Sun M, Gross K, Schiestl FP. Floral adaptation to local pollinator guilds in a terrestrial orchid. Annals of Botany. 2014;113:289–300. doi: 10.1093/aob/mct219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschapka M, Dressler S. Chiropterophily: on bat-flowers and flower bats. Curtis's Botanical Magazine. 2002;19:114–125. [Google Scholar]
- Tschapka M, von Helversen O. Phenology, nectar production and visitation behaviour of bats on the flowers of the bromeliad Werauhia gladioliflora in Costa Rican lowland rain forest. Journal of Tropical Ecology. 2007;23:385–395. [Google Scholar]
- Velazco PM, Patterson BD. Diversification of the yellow-shouldered bats, genus Sturnira (Chiroptera, Phyllostomidae), in the New World tropics. Molecular Phylogenetics and Evolution. 2013;65:683–698. doi: 10.1016/j.ympev.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Vogel S. Chiropterophilie in der neotropischen Flora-Neue Mitteilungen III. Flora. 1969;158:289–323. [Google Scholar]
- von Helversen O, Winter Y. Glossophagine bats and their flowers: costs and benefits for plants and pollinators. In: Kunz TH, Fenton M B, editors. Bat ecology. Chicago: The University of Chicago Press; 2003. pp. 346–397. [Google Scholar]
- von Helversen O, Winkler L, Bestmann HJ. Sulphur-containing ‘perfumes’ attract flower-visiting bats. Journal of Comparative Physiology A. 2000;186:143–153. doi: 10.1007/s003590050014. [DOI] [PubMed] [Google Scholar]
- Wendt T, Ferreira CMB, Gelli de Faria AP, Iglesias-Rios R. Reproductive biology and natural hybridization between two endemic species of Pitcairnia (Bromeliaceae) American Journal of Botany. 2001;88:1760–1767. [PubMed] [Google Scholar]
- Williams-Linera G. Tree species richness complementarity, disturbance and fragmentation in a Mexican tropical montane cloud forest. Biodiversity and Conservation. 2002;11:1825–1843. [Google Scholar]
- Williams-Linera G. El bosque de niebla del centro de Veracruz: ecología, historia y destino en tiempos de fragmentación y cambio climático. Xalapa: Instituto de Ecología, A. C., CONABIO; 2007. [Google Scholar]
- Wilmer P. Pollination and floral ecology. Princeton, NJ: Princeton University Press; 2011. [Google Scholar]
- Zotz G. The systematic distribution of vascular epiphytes – a critical update. Botanical Journal of the Linnean Society. 2013;177:453–481. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.