Abstract
β-phenylethylamine (βPEA) is an endogenous amine that has been shown to increase the synaptic levels of dopamine (DA). A number of in vitro and behavioral studies suggest the dopamine transporter (DAT) plays a role in the effects generated by βPEA, however the mechanism through which βPEA affects DAT has not yet been elucidated. Here, we used Caenorhabditis (C.) elegans DAT (DAT-1) expressing LLC-pk1 cells and neuronal cultures to investigate whether the βPEA-induced increase of extracellular DA required DAT-1. Our data show that βPEA increases extracellular dopamine both in DAT-1 transfected cells and cultures of differentiated neurons. RTI-55, a cocaine homologue and DAT inhibitor, completely blocked the βPEA-induced effect in transfected cells. However in neuronal cultures, RTI-55 only partly inhibited the increase of extracellular DA generated by βPEA. These results suggest that βPEA requires DAT-1 and other, not yet identified proteins, to increase extracellular DA when tested in a native system. Furthermore, our results suggest that βPEA-induced increase of extracellular DA does not require functional monoamine vesicles as genetic ablation of the C. elegans homologue vesicular monoamine transporter, cat-1, did not compromise the ability of βPEA to increase extracellular DA. Finally, our electrophysiology data show that βPEA caused fast-rising and self-inactivating amperometric currents in a subset of wild-type DA neurons but not in neurons isolated from dat-1 knockout animals. Taken together, these data demonstrate that in both DA neurons and heterogeneous cultures of differentiated C. elegans neurons, βPEA releases cytoplasmic DA through DAT-1 to ultimately increase the extracellular concentration of DA.
Keywords: dopamine transporter, β-phenylethylamine; dopamine release, electrophysiology, C. elegans
1. INTRODUCTION
β-phenylethylamine (βPEA) is an endogenous trace amine present in the central nervous system, however, its role in the mammalian physiology is still unknown. Previous studies demonstrated that βPEA is synthetized in neurons that also contain tyrosine hydroxylase and coexists with dopamine (DA) in the nigrostriatal brain regions (Juorio et al. 1991). In striatal tissue, βPEA synthesis occurs with a rate similar to that of DA, but since it is more efficiently metabolized by monoamine oxidase enzymes, striatal βPEA concentrations are about three orders of magnitude lower than DA levels (Paterson et al. 1990). These data suggest that it is crucial the DA neurons keep low concentrations of βPEA to guarantee a proper physiological activity. In fact, changes in urinary βPEA levels have been documented in various human disorders including schizophrenia, attention deficit hyperactive disorder (ADHD) and depression (Baker et al. 1991, O'Reilly and Davis 1994, Sandler et al. 1980). A direct interaction between dopaminergic neurons and βPEA has also been demonstrated in in vivo and in vitro experiments showing that βPEA induces DA release (Bailey et al. 1987, Ishida et al. 2005, Kuroki et al. 1990, Nakamura et al. 1998, Sotnikova et al. 2004, Yamada et al. 1998), and inhibits DA uptake (Liang and Rutledge 1982, Raiteri et al. 1976). Moreover, in vivo studies showed that physiological βPEA concentrations directly and transiently inhibit the firing rate of the DA neurons through the activation of the DA D2 autoreceptors (Ishida et al. 2005, Mercuri et al. 1997, Rodriguez and Barroso 1995). Interestingly, the firing inhibition caused by βPEA as well as βPEA-induced behaviors (Barroso and Rodriguez 1996) were not affected by pretreatment with the vesicular monoamine transporter (VMAT) blocker reserpine. These data suggested that βPEA stimulates the release of DA from a non-vesicular cytoplasmic pool. In this study, we investigated the effect of βPEA on extracellular levels of DA in C. elegans cultured neurons. We found that in isolated DA neurons, βPEA requires DAT to induce transient DA efflux. Furthermore, our data suggest that βPEA-induced DA efflux utilizes cytosolic DA since genetic ablation of VMAT did not affect the increase of extracellular DA induced by βPEA.
2. MATERIALS AND METHODS
2.1 C. elegans husbandry and transgenic animals
wild-type animals (N2) and knockout animals for DAT-1 (dat-1(ok157)III) and the C. elegans VMAT homologue CAT-1 (cat-1(ok411) were obtained from the Caenorhabditis Genetic Center (University of Minnesota, Minneapolis, MN). All C. elegans strains were grown on bacteria lawns of NA22 and maintained at 22-24°C using standard methods (Brenner 1974). For amperometry recordings, we used the BY250 strain (gift from Dr. Blakely, Vanderbilt University) expressing cytosolic GFP under the control of the dat-1.
2.2 Extracellular [3H]DA in DAT-1 transfected cells and C. elegans embryonic cultures
C. elegans embryonic cultures were prepared as previously described (Carvelli et al. 2004, Strange and Morrison 2006). Embryonic cells were seeded on 22x22 mm glass cover slips coated with peanut lectin (Sigma) and maintained with L15 media (GIBCO) enriched with 5% inactivated FBS and 100 units/ml of penicillin and streptomycin. Cells were assayed 2 days after been seeded. LLC-pk1 (pig epithelial kidney) cells were grown in DMEM medium enriched with 5% FBS, 2mM L-glutamine, 100 units/ml penicillin and 100 mg/ml streptomycin. 100,000 cells were seeded into 12 well plates and after 18-24 hours were transfected with 0.5 μg DAT-1 in the pEGFP vector using X-tremeGENE HP DNA transfection reagent (Roche). Control cells were transfected with pEGFP vector alone. 24-30 hours after transfection, cells were washed 2 times with KRH buffer (120 mM NaCl or MNDG+/4.7 mM KCl/1.2 mM KH2PO4/10 mM Hepes/2.2 CaCl2/10 mM glucose) containing 100 μM of ascorbic acid and tropolone (Sigma) and incubated for 30 minutes at room temperature with 20 nM [3H]DA (PerkinElmer). Cells were then washed 3 times with ascorbic acid/tropolone containing KRH buffer and treated with drugs (βPEA and/or RTI-55) or KRH (control) for 1 minute. Supernatant was collected from each well and counted for radioactivity. We used the same protocol to measure extracellular concentration of [3H]DA in cultured C. elegans neurons except 5 nM of [3H]DA was used and the buffer contained 145 mM NaCl or NMDG+, 5 mM KCl, 1 mM CaCl2, 5 mM MgCl2, 10 mM Hepes, and 20 mM D-glucose (pH 7.2 and 350 osmolarity).
2.3 Amperometric Recordings
4-5 days after C. elegans embryonic cells were seeded in peanut lectin coated glass dishes (MatTech Corporation, Cincinnati, OH), cells were washed twice with bath solution containing 145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 5 mM MgCl2, 10 mM HEPES, and 20 mM d-glucose (pH 7.2; 325 osmolarity adjusted with sucrose). DA fluxes were induced by applying 50 μM βPEA and recorded using a carbon fiber electrodes connected to an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Electrodes were placed in proximity to an isolated dopaminergic neuron expressing cytosolic GFP. Amperometric currents were digitized with an Axon Digidata 1440A, collected with pClamp 10.2 (Molecular Devices), filtered with a low-pass Bessel filter set at 5 Hz, and analyzed using pClampfit 10.2 (Molecular Devices) and Origin 8.5 software (OriginLab Corp, Northampton, MA).
3. RESULTS AND DISCUSSION
3.1. β-phenylethylamine increases extracellular levels of DA in DAT-1 transfected cells and C. elegans cultured embryonic cells
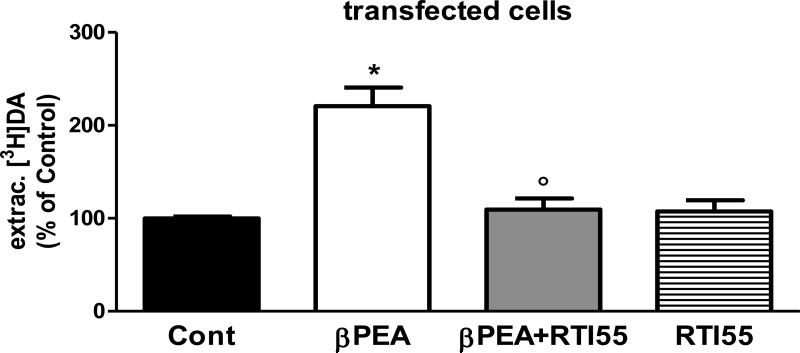
Previous studies showed that βPEA induces DA release and/or inhibits DA uptake. We investigated whether βPEA increased extracellular DA in cultures of LLC-pk1 cells expressing C. elegans DAT (DAT-1). After preloading with 20 nM [3H]DA, cells were treated with 100 μM βPEA for 1 minute. As shown in Figure 1, βPEA caused a statistically significant increase (120 ± 20%) of extracellular [3H]DA with respect to control treated samples (one-way ANOVA test; *p = 0.0001), whereas no change in extracellular [3H]DA was measured in non-transfected cells (data not shown). Previous reports demonstrated that DA uptake through DAT-1 is specifically antagonized by monoamine transporters inhibitors such as mazindol, nisoxetine and cocaine (Jayanthi et al. 1998). Here we tested whether RTI-55, a cocaine homologue, was also capable of blocking the βPEA-induced increase of extracellular [3H]DA in DAT-1 transfected cells. We found that 100 μM RTI-55 completely blocked the effect of βPEA on the extracellular levels of [3H]DA (Figure 1, gray bar). In fact, βPEA/RTI-55 treatment caused a decrease of extracellular [3H]DA comparable to control-treated samples, 109 ± 12% and 100 ± 2%, respectively. The extracellular levels of [3H]DA following βPEA/RTI-55 treatment were statistically different than those measured after βPEA treatment (one-way ANOVA test, ○p = 0.05). To test whether RTI-55 itself had an effect on extracellular [3H]DA, we treated DAT-1 transfected cells with RTI-55 alone (stripe bar, Figure 1) and found that 100 μM RTI-55 caused no effect on extracellular [3H]DA with respect to controls, 108 ± 12 and 100% respectively. Taken together, these data demonstrate that βPEA causes an increase of extracellular DA in DAT-1 transfected cells which is selectively blocked by the DAT-1 inhibitor RTI-55.
Figure 1.
βPEA increases the amount of extracellular DA in DAT-1 transfected cells. When DAT-1 expressing LLC-pk1 cells were treated for 1 minute with 100 μM βPEA, we measured a significant increase of extracellular [3H]DA with respect to control-treated samples (*p = 0.0001). Cells treated with βPEA/RTI-55 showed a significant reduction in extracellular [3H]DA with respect to βPEA-treated cells (○p = 0.05, one-way ANOVA). RTI55 applied alone did not have any effect.
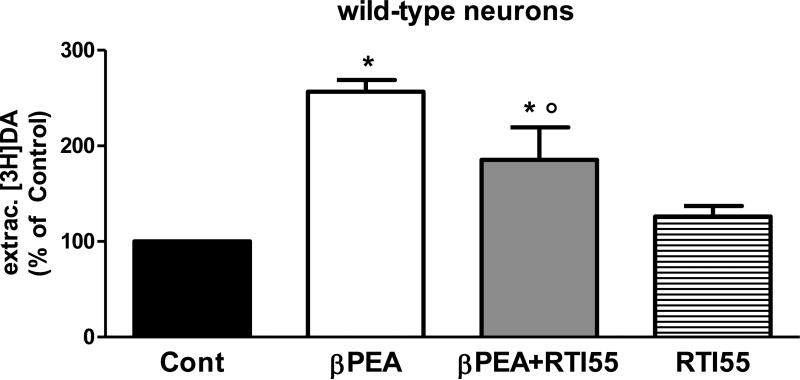
Previously, it was shown that cultured C. elegans embryonic cells are instrumental in studying the activity of proteins in their native cells. C. elegans embryonic cells, which undergo terminal differentiation within a few days after being seeded, have been used to characterize protein function and regulation and to determine specific gene expression patterns (Strange and Morrison 2006, Carvelli et al. 2008). Particularly, we used this preparation to investigate the ability of DAT-1 to accumulate [3H]DA in the dopaminergic neurons and showed that like the mammalian homologue, C. elegans DAT-1 efficiently accumulated DA, whereas genetic and pharmacological ablation of DAT-1 prevented [3H]DA uptake (Carvelli et al. 2004). Importantly, our previous studies demonstrated that functional characterization of DAT-1 in differentiated neurons recapitulates the pharmacological properties observed in mammalian DAT expressed in heterologous systems. In this study, we used differentiated cultures of C. elegans embryonic cells to investigate the effects of βPEA on dopaminergic neurons. Three days after preparation, we preloaded C. elegans embryonic cells with 5 nM [3H]DA and then applied 100 μM βPEA for 1 minute. Similarly to what we saw in DAT-1 transfected cells, βPEA significantly increased the amount of extracellular [3H]DA (one-way ANOVA test, *p = 0.0001). We measured a 157 ± 12% increase in extracellular [3H]DA following βPEA treatment with respect to control treated samples (Figure 2). When βPEA was applied together with 100 μM RTI-55 we measured an increase of 85 ± 34% in extracellular [3H]DA with respect to control-treated samples (Figure 2, gray bar). Interestingly, this value was statistically different to both control- and βPEA-treated sample (*p = 0.0001 vs controls and ○p = 0.001 vs βPEA-treated samples). This result showed that in primary cultures RTI-55 partly blocked the βPEA-induced increase in extracellular DA, and RTI-55 applied alone did not change the extracellular amount of [3H]DA with respect to controls (Figure 2, stripe bar), 126 ± 11 and 100% respectively.
Figure 2.
βPEA increases the amount of extracellular DA in C. elegans differentiated neuronal cultures. 100 μM βPEA applied for 1 minute in C. elegans cells caused a significant increase of extracellular [3H]DA with respect to control-treated cells (*p = 0.0001, one-way ANOVA). RTI-55 partly inhibited the βPEA-induced effect (*p=0.0001 vs. controls and ○p = 0.001 vs. βPEA-treated samples, one-way ANOVA), but caused no effect when applied alone.
In conclusion, these results demonstrate that βPEA caused an increase of extracellular DA in DAT-1 transfected cells and differentiated C. elegans neurons. Moreover, the ability of βPEA to increase extracellular DA was similar in DAT-1 transfected cells (157%) and neuronal cultures (120%), suggesting that DAT-1 accounts for the increase of extracellular DA measured after βPEA treatment. Interestingly though, in neuronal cultures the DAT-1 blocker RTI-55 only partly inhibited the βPEA-induced increase of extracellular DA. This result suggests that in native neurons DAT-1 and other unknown proteins are required by βPEA to elevate extracellular DA. Perhaps the DA D2 receptors as well as low affinity neurotransmitter transporters might be involved (Ishida et al. 2005, Mercuri et al. 1997, Rodriguez and Barroso 1995, Horton et al. 2013), however, we can not exclude that a dose-related dependency of βPEA and/or RTI-55 in transfected cells versus neuronal culture underlies the different effect of RTI55 seen in these two preparations.
3.2. β-phenylethylamine-induced increase of extracellular dopamine does not involve vesicle-dependent dopamine release
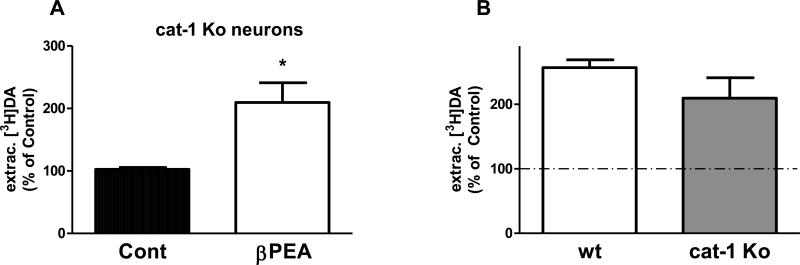
Neurotransmitters like DA and serotonin are released in the synaptic cleft through 2 different mechanisms: a vesicle-dependent mechanism or a Ca2+-independent and DAT mediated mechanism. In C. elegans, all major proteins involved in the dopaminergic system have been identified (Chase and Koelle 2007), including the vesicular monoamine transporter (VMAT) homologue CAT-1. Moreover, animals made knockout for cat-1 have been previously created and characterized (Duerr et al. 1999). We investigated whether the effects of βPEA seen in Figure 2 required intact DA vesicles. Thus, we measured the increase of extracellular [3H]DA induced by βPEA in cultures of differentiated cells obtained from cat-1 knockout animals. We found that βPEA significantly (*p = 0.01; t-test) augmented the extracellular amount of [3H]DA of about 110 ± 32% with respect to control treated cells (Figure 3A). The comparison of βPEA effects between cat-1 knockout and wild type neurons did not show a statistically significant difference, 110 ± 32 and 137 ± 12 respectively (Figure 3B). These data demonstrated that genetic ablation of cat-1 did not compromise the ability of βPEA to increase extracellular DA in native neuronal cultures, suggesting that CAT-1 (VMAT) does not play a significant role in the βPEA-induced increase of extracellular DA. Therefore, these data suggest that the elevated levels of extracellular DA measured during βPEA treatment are mediated by DAT-1.
Figure 3.
VMAT is not required by βPEA to increase the extracellular levels of DA. A, In cat-1 (VMAT) knockout neurons, 100 μM βPEA caused a significant increase of extracellular [3H]DA with respect to control-treated cells (*p = 0.01, t test). B, The increase of extracellular DA measured in cat-1 knockout neurons was comparable to that measured in wild-type neurons.
3.3. βPEA generates amperometric currents in a subset of C. elegans DA neurons
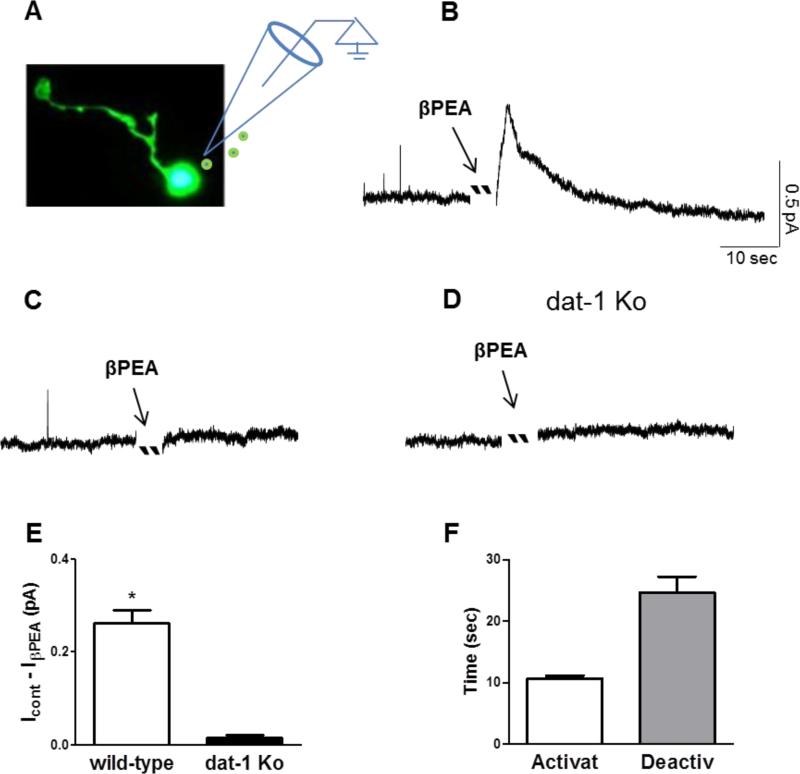
Previously, we demonstrated that amphetamine promoted DA release through DAT-1 (efflux) in C. elegans dopaminergic neurons (Carvelli et al. 2010). Since our data (Figure 3) demonstrated that βPEA-induced increase of [3H]DA does not occur through vesicle release, we investigated whether βPEA induces DA efflux by measuring amperometric currents. Microamperometry is a technique which allows detecting DA by measuring the electrons released during its oxidizing reaction. When DA touches a carbon fiber electrode held at a voltage of +700 mV, each DA molecule releases 2 electrons that are detected by the same carbon fiber electrode. Thus, DA efflux stimulated by βPEA can be detected by a carbon fiber electrode as a measure of DA oxidation (Carvelli 2010, Khoshbouei et al. 2003, Schroeder et al. 1994). When C. elegans DA neurons engineered to express cytosolic GFP (Figure 4A) were perfused with 50 μM βPEA, we observed fast-rising and outward amperometric currents (0.26 ± 0.02 pA) in 44% of DA neurons tested (Figure 4B and E). This result demonstrated that βPEA transiently increases the extracellular levels of DA in proximity of a single dopaminergic neuron. However, 33 dopaminergic neurons of 59 total neurons tested exhibited no effect following βPEA application (Figure 4C). Interestingly, the amperometric currents, which reached their maximal levels within 11 ± 0.5 seconds after βPEA perfusion, spontaneously returned to basal levels after 24 ± 3 seconds even though βPEA was still present in the recording chamber (Figure 4B and F). To test whether DAT-1 was required in βPEA-induced currents, we performed recordings in DA neurons lacking expression of DAT-1. βPEA did not cause significant amperometric currents (0.016 ± 0.004 pA) in any of the dat-1 knockout neurons (N=20) we tested (Figure 3D and E). This result demonstrated that the increase of extracellular DA measured during βPEA treatment required a functional DAT-1 and suggested that βPEA needs DAT-1 to increase extracellular DA.
Figure 4.
βPEA generates amperometric currents in a subset of dopaminergic neurons. A, C. elegans dopaminergic neuron visualized with cytosolic GFP. B, Representative recording of fast-rising and self-inactivated amperometric currents generated by 50 μM βPEA. C, Representative recording from a subset of dopaminergic neurons that did not respond to βPEA. D, Representative recording from a DAT-1 knockout neuron treated with 50 μM βPEA. E, Average of amperometric currents measured in wild-type (N=26) and dat-1 knockout (N=20) neurons (*p = 0.0001, t test). F, Average of time required to activate and inactivate the βPEA-induced amperometric currents.
Taken together, these data demonstrate that in cultures of C. elegans DA neurons βPEA generates amperometric currents. Moreover, they show that about 40% of dopaminergic neurons did not respond to βPEA treatment suggesting, as previously shown for mammalian DA neurons, that physiological differences might exist among different subtypes of C. elegans DA neurons (Grenhoff et al. 1988, Murata et al. 2009, Li et al. 2012, Weihe et al. 2006). Importantly, our data support that the amperometric currents induced by βPEA are mediated by DAT-1. In fact, in DA neurons lacking expression of dat-1, βPEA did not generate amperometric currents.
4. CONCLUSION
In the brain, the amount of extracellular DA is controlled by a tight balance between DA release and uptake events. In vitro and in vivo studies have showed that βPEA enhances the extracellular levels of DA by inducing DA release and/or preventing DA uptake through mammalian DAT. Here we found that in C. elegans neuronal cultures, the βPEA-induced increase of extracellular DA was partly inhibited by the DAT blocker RTI-55, whereas RTI-55 did not have any effect when applied alone (Figure 2). Moreover, our data demonstrate that βPEA-induced release of DA requires DAT. This conclusion was supported by two major observations: I) the increase of extracellular DA induced by βPEA in cat-1 (VMAT) knockout neurons was comparable to that measured in wild-type neurons (Figure 3B), and II) transient amperometric DA currents generated by βPEA were lost in neurons lacking expression of DAT-1 (Figure 4). Interestingly, our data show that in DAT-1 transfected cells, RTI55 completely blocked βPEA effect on extracellular levels of [3H]DA whereas, in neuronal culture this effect was only partial. These results could simply reflect a different dose-related dependency of βPEA and/or RTI55 in transfected cells versus neuronal culture or suggest that βPEA involved proteins other than DAT-1, e.g. low affinity neurotransmitter transporter and/or DA D2 receptors (Horton et al. 2013, Duan and Wang 2010; Ishida et al. 2005, Mercuri et al. 1997, Rodriguez and Barroso 1995). Finally, our data demonstrate that C. elegans represents a powerful genetic model to perform in vitro studies in differentiated dopaminergic neurons.
HIGHLIGHTS.
βPEA increases the extracellular concentrations of DA in differentiated C. elegans neurons.
The dopamine transporter (DAT) blocker RTI-55 partly blocks the βPEA-induced increase of extracellular DA.
The vesicular monoamine transporter is not required to generate the βPEA-induced increase of extracellular DA.
βPEA generates DA efflux in a subset of dopaminergic neurons
βPEA does not generate DA efflux in DAT knockout neurons.
Acknowledgments
The authors thank the support from NIH grant R21 DA024797 and the NIH funded COBRE P20 GM103329.
Abbreviations
- DA
dopamine
- DAT
DA transporter
- βPEA
β-phenylethylamine
- VMAT
vesicular monoamine transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- Bailey BA, Philips SR, Boulton AA. In vivo release of endogenous dopamine, 5-hydroxytryptamine and some of their metabolites from rat caudate nucleus by phenylethylamine. Neurochem Res. 1987;12(2):173–178. doi: 10.1007/BF00979534. [DOI] [PubMed] [Google Scholar]
- Baker GB, Bornstein RA,, Rouget AC,, Ashton SE,, van Muyden JC, Coutts RT. Phenylethylaminergic mechanisms in attention-deficit disorder. Biol Psychiatry. 1991;29(1):15–22. doi: 10.1016/0006-3223(91)90207-3. [DOI] [PubMed] [Google Scholar]
- Barroso N, Rodriguez M. Action of beta-phenylethylamine and related amines on nigrostriatal dopamine neurotransmission. Eur J Pharmacol. 1996;297:195–203. doi: 10.1016/0014-2999(95)00757-1. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Blakely RD,, DeFelice LJ. Dopamine transporter/syntaxin 1A interactions regulate transporter channel activity and dopaminergic synaptic transmission. PNAS. 2008;105(37):14192–14197. doi: 10.1073/pnas.0802214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Matthies DS, Galli A. Molecular mechanisms of amphetamine actions in Caenorhabditis elegans. Mol. Pharmacol. 2010;78(1):151–156. doi: 10.1124/mol.109.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, McDonald PW, Blakely RD, DeFelice LJ. Dopamine transporters depolarize neurons by a channel mechanism. Proc. Natl. Acad. Sci USA. 2004;101(45):16046–16051. doi: 10.1073/pnas.0403299101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase DL, Koelle MR. Biogenic amine neurotransmitters in C. elegans. WormBook. 2007:1–15. doi: 10.1895/wormbook.1.132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335(3):743–53. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerr JS, Frisby DL, Gaskin J, Duke A, Asermely K, Huddleston D, Eiden LE, Rand JB. The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J.Neurosci. 1999;19(1):72–84. doi: 10.1523/JNEUROSCI.19-01-00072.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff J, Ugedo L, Svensson TH. Firing patterns of midbrain dopamine neurons: differences between A9 and A10 cells. Acta Physiol Scand. 1988;(134):1. doi: 10.1111/j.1748-1716.1988.tb08468.x. [DOI] [PubMed] [Google Scholar]
- Ishida K, Murata M, Katagiri N, Ishikawa M, Abe K, Kato M, Utsunomiya I, Taguchi K. Effects of beta-phenylethylamine on dopaminergic neurons of the ventral tegmental area in the rat: a combined electrophysiological and microdialysis study. J Pharmacol Exp Ther. 2005;314(2):916–922. doi: 10.1124/jpet.105.084764. [DOI] [PubMed] [Google Scholar]
- Horton RE, Apple DM, Owens WA, Baganz NL, Cano S, Mitchell NC, Vitela M, Gould GG, Koek W, Daws LC. Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: uncovering novel targets to treat depression. J Neurosci. 2013;33(25):10534–43. doi: 10.1523/JNEUROSCI.5687-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi LD, Apparsundaram S, Malone MD, Ward E, Miller DM, Eppler M, Blakely RD. The Caenorhabditis elegans gene T23G5.5 encodes an antidepressant- and cocaine-sensitive dopamine transporter. Mol.Pharmacol. 1998;54(4):601–609. [PubMed] [Google Scholar]
- Juorio AV, Paterson IA, Zhu MY, Matte G. Electrical stimulation of the substantia nigra and changes of 2-phenylethylamine synthesis in the rat striatum. J Neurochem. 1991;56(1):213–220. doi: 10.1111/j.1471-4159.1991.tb02583.x. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, Wang H, Lechleiter DJ, Javitch JA, Galli A. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem. 2003;278(14):12070–12077. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Tsutsumi T, Hirano M, Matsumoto T, Tatebayashi Y, Nishiyama K, Uchimura H, Shiraishi A, Nakahara T, Nakamura K. Behavioral sensitization to beta-phenylethylamine (PEA): enduring modifications of specific dopaminergic neuron systems in the rat. Psychopharmacology (Berl) 1990;102(5):10. doi: 10.1007/BF02245736. [DOI] [PubMed] [Google Scholar]
- Li X, Qi J, Yamaguchi T, Wang HL, Morales M. Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct. 2012;218(5):1159–1176. doi: 10.1007/s00429-012-0452-z. [DOI] [PubMed] [Google Scholar]
- Liang NY, Rutledge CO. Evidence for carrier-mediated efflux of dopamine from corpus striatum. Biochem Pharmacol. 1982;31(15):2479–2484. doi: 10.1016/0006-2952(82)90057-0. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Saiardi A, Bonci A, Picetti R, Calabresi P, Bernardi G, Borrelli E. Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience. 1997;79(2):323–327. doi: 10.1016/s0306-4522(97)00135-8. [DOI] [PubMed] [Google Scholar]
- Murata M, Katagiri N, Ishida K, Abe K, Ishikawa M, Utsunomiya I, Hoshi K, Miyamoto K, Taguchi K. Effect of beta-phenylethylamine on extracellular concentrations of dopamine in the nucleus accumbens and prefrontal cortex. Brain Res. 2009;1269(40-6) doi: 10.1016/j.brainres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ishii A, Nakahara D. Characterization of beta-phenylethylamine-induced monoamine release in rat nucleus accumbens: a microdialysis study. Eur J Pharmacol. 1998;349(2-3):163–169. doi: 10.1016/s0014-2999(98)00191-5. [DOI] [PubMed] [Google Scholar]
- O'Reilly RL, Davis BA. Phenylethylamine and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18(1):63–75. doi: 10.1016/0278-5846(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Paterson IA, Juorio AV, Boulton AA. 2-Phenylethylamine: a modulator of catecholamine transmission in the mammalian central nervous system? J Neurochem. 1990;55(6):1827–1837. doi: 10.1111/j.1471-4159.1990.tb05764.x. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Bertollini A, Del Carmine R, Levi G. Effects of phenethylamine derivatives on the release of biogenic amines from synaptosomes. Biochem Soc Trans. 1976;4(1):121–124. doi: 10.1042/bst0040121. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Barroso N. beta-Phenylethylamine regulation of dopaminergic nigrostriatal cell activity. Brain Res. 1995;703(1-2):201–204. doi: 10.1016/0006-8993(95)01098-x. [DOI] [PubMed] [Google Scholar]
- Sandler M, Ruthven CR, Goodwin BL, Reynolds GP, Rao VA, Coppen A. Trace amine deficit in depressive illness: the phenylalanine connexion. Acta Psychiatr Scand Suppl. 1980;280(29-39) [PubMed] [Google Scholar]
- Schroeder TJ, Jankowski A, Senyshyn J, Holz RW, Wightman RM. Zones of exocytotic release on bovine adrenal medullary cells in culture. J Biol Chem. 1994;269(25):17215–17220. [PubMed] [Google Scholar]
- Sotnikova TD, Budygin EA, Jones SR, Dykstra LA, Caron MG, Gainetdinov RR. Dopamine transporter-dependent and -independent actions of trace amine beta-phenylethylamine. J Neurochem. 2004;91(2):362–373. doi: 10.1111/j.1471-4159.2004.02721.x. [DOI] [PubMed] [Google Scholar]
- Strange K, Morrison R. In vitro culture of C. elegans somatic cells. Methods Mol Biol. 2006;351(265-73) doi: 10.1385/1-59745-151-7:265. [DOI] [PubMed] [Google Scholar]
- Weihe E, Depboylu C, Schütz B, Schäfer MK, Eiden LE. Three types of tyrosine hydroxylase-positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co-expression. Cell Mol Neurobiol. 2006;26(4-6):659–678. doi: 10.1007/s10571-006-9053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Harano M, Tanaka M. Antagonistic effects of beta-phenylethylamine on quinpirole-and (−)-sulpiride-induced changes in evoked dopamine release from rat striatal slices. Eur J Pharmacol. 1998;343(2-3):145–150. doi: 10.1016/s0014-2999(97)01529-x. [DOI] [PubMed] [Google Scholar]