Abstract
Compensatory mechanisms in dopamine (DA) signaling have long been proposed to delay onset of locomotor symptoms during Parkinson’s disease (PD) progression until ~80% loss of striatal DA occurs. Increased striatal dopamine turnover has been proposed to be a part of this compensatory response, but may occur after locomotor symptoms. Increased tyrosine hydroxylase (TH) activity has also been proposed as a mechanism, but the impact of TH protein loss upon site-specific TH phosphorylation in conjunction with the impact on DA tissue content is not known. The tissue content of DA was determined against TH protein loss in the striatum and substantia nigra (SN) following 6-OHDA lesion in the medial forebrain bundle in young Sprague-Dawley male rats. Although DA predictably decreased in both regions following 6-OHDA, there was a significant difference in DA loss between the striatum (75%) and SN (40%), despite similar TH protein loss. Paradoxically, there was a significant decrease in DA against remaining TH protein in striatum, but a significant increase in DA against remaining TH in SN. In the SN, increased DA per remaining TH protein was matched by increased ser31, but not ser40, TH phosphorylation. In striatum, both ser31 and ser40 phosphorylation decreased, reflecting decreased DA per TH. However, in control nigral and striatal tissue, only ser31 phosphorylation correlated with DA per TH protein. Combined, these results suggest that the phosphorylation of ser31 in the SN may be a mechanism to increase DA biosynthesis against TH protein loss in an in vivo model of PD.
INTRODUCTION
Bradykinesia is a cardinal symptom of both Parkinson’s disease (PD) and age-related Parkinsonism (Bennet et al, 1996; Prettyman, 1998). Despite established side effects, L-DOPA, the product of tyrosine hydroxylase (TH), has been the gold standard to treat locomotor impairment in PD for over 50 years. Still, the major loss of TH is a significant hurdle to overcome to improve locomotor deficits of PD. Prior to the onset of locomotor impairment, the progressive loss of TH protein occurring during PD progression may be countered with compensatory mechanisms that may maintain normal locomotor activity until 70–80% TH loss in striatum (Bernheimer et al, 1973; Hornykiewicz, 1993), or ~50% loss in the substantia nigra (SN) (Bezard et al., 2001). Dopaminergic compensation in PD progression was first proposed ~40 years ago, with evidence that remaining nigrostriatal neurons were hyperactive (Agid et al., 1973). Axonal sprouting in striatum has also been observed in the established 6-OHDA PD model (Stanic et al., 2003), but may also play a role in motor complications from L-DOPA with increasing lesion severity (Lee et al., 2008). Increased DA turnover has also been proposed to be associated with prevention of locomotor symptoms until there is severe TH loss (Pifl and Hornykiewicz, 2006). However, the observations that increased DA turnover occurs only after locomotor symptoms are present (Bezard et al., 2001) or that it may contribute to toxicity (Zigmond et al., 2002) casts some doubt on the efficacy of this mechanism to compensate for loss of TH protein. Other evidence of compensation is seen with increased D2 receptor expression or activity of the subthalamic nuclei, which are argued to maintain basal ganglia function during progressive DA loss (Bezard et al., 2003). Finally, increased activity of remaining TH protein has also been proposed, as increased TH activity has been observed in post-mortem PD tissue (Nagatsu, 1990, Nagatsu and Sawada, 2011; Nakashima et al., 2013a) or recovery of TH expression after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Jackowec et al., 2004). However, the mechanism by which TH activity may be enhanced or how it may affect DA tissue content in either striatum or SN in a progressive PD animal model is unknown.
To better elucidate the role of DA in locomotor function, the differences in DA regulation between the terminal versus somatodendritic compartments must be considered. Altered DA signaling in the SN may affect locomotor activity, independent of any change in striatum (Bergquist et al., 2003; Trevitt et al., 2004; Anderson et al., 2006; Salvatore et al., 2009a; Pruett and Salvatore, 2013). Furthermore, comparatively less TH protein loss in the SN (~40–50%) than in striatum is associated with locomotor deficits in animal models of PD or aging (Emborg et al., 1998; Bezard et al., 2001; Salvatore et al.,2009a). In PD models, basal levels of extracellular DA in SN are unaffected by 6-OHDA lesion, despite loss of TH-positive cells, unless there is amphetamine challenge (Sarre et al., 2004). Together, these observations lend support to the prospect that compensatory changes in DA signaling may occur in the SN, in addition to striatum, and may contribute to locomotor capacity. For example, in aging models, GDNF, which increases locomotor activity, notably increases DA tissue content in the SN, and not striatum (Hoffer et al.,1994; Gerhardt et al., 1999; Grondin et al., 2003). GDNF also increases TH phosphorylation in the SN (Salvatore et al., 2004). Therefore, the regulation of DA biosynthesis in the SN during loss of TH protein could be an important mechanism for maintaining DA signaling associated with locomotor function. Using the 6-OHDA model of PD, the relationship of how site-specific TH phosphorylation and DA tissue content may be affected by TH protein loss was determined in the stratum and SN, therefore examining the nature of dopaminergic neurotransmission at the biosynthesis step in the nigrostriatal pathway using an established PD model.
METHODS
6-OHDA lesion
Male Sprague-Dawley rats were purchased from Harlan and were 4–6 months of age. Rats were anesthetized with 40 mg/kg Nembutal intraperitoneal (i.p.) (pentobarbital Lundbeck Inc, Deerfield, IL) with supplement of 9.0, 0.6, and 0.3 mg/kg ketamine, xylazine, and acepromazine, respectively and then placed into a stereotaxic frame. 6-OHDA was delivered unilaterally to the medial forebrain bundle at coordinates ML +1.5, AP −3.8, DV −8.0 relative to Bregma according to Paxinos and Watson rat brain atlas, 4th ed. A total of 9 or 16 µg of 6-OHDA in a total of 4 µl in 0.02% ascorbic acid (concentrations of 2.25 or 4 mg/ml) was infused unilaterally at a rate of 1 µl/minute. Notwithstanding possible bilateral effects of the 6-OHDA infusion (see section Phosphorylation of TH), the contralateral striatum was left intact as a naïve tissue control. The syringe was left in place for 10 min before removal to allow for maximal diffusion of drug and to avoid further mechanical damage to the tissue. Body temperature was maintained during surgery using a temperature monitor with probe and heating pad (FHC, Bowdoingham, ME). This protocol produces a range of 60–95% TH and dopamine transporter loss within 9–16 days (Chotibut et al., 2012), with mean loss of 77%, 11 days post lesion. Eleven test subjects were analyzed in this study and exhibited loss of TH protein ranging from 22–98% in striatum and 28–79% in substantia nigra. As the intent of this study was to analyze DA tissue content and TH phosphorylation during progressive TH loss, we dissected striatum and substantia nigra no earlier than 9 days and no later than 16 days after 6-OHDA. Most dissections (9 of 11) were done at 9 or 12 (± 1) days following 6-OHDA. Lesion predictability was previously demonstrated in our laboratory, in which case we addressed dopamine transporter function following 6-ODHA (Chotibut et al., 2012).
On day 7 following lesion, amphetamine (2 mg/kg, i.p.) was given in order to test for behavioral confirmation of lesion onset. A lesion of > 50% can be detected by rotational behavior following amphetamine (Hudson et al., 1993). At least 2 days following amphetamine were allowed to pass before dissection to allow for its virtually complete elimination. The protocols used were approved by the Animal Care and Use Committee of LSU Health Sciences Center-Shreveport. ARRIVE guidelines were observed in the conduct of this study.
Tissue analysis for dopamine and tyrosine hydroxylase
The procedure for dissection and neurochemical analyses is fully explicated in Salvatore et al., (2012a), but a brief description is provided as follows. All rats were very briefly rendered unconscious by isoflurane and decapitated for immediate removal of the whole brain. The brain was briefly rinsed in cold water and placed into an ice-cold rodent brain matrix for coronal dissection, each tissue slice being 1 mm. The substantia nigra was isolated from the ventral tegmental area (VTA) using a #11 scalpel blade to dissect it away free-hand from 2 to 3 coronal slices containing midbrain, as illustrated in Salvatore et al., (2012a), and also conducted previously (Salvatore et al., 2004; 2009a; 2009b; Salvatore and Pruett 2012; Pruett and Salvatore, 2010; 2013). Frozen tissues were sonicated immediately after removal from dry ice in perchloric acid/EDTA solution (Salvatore et al., 2012a). The solubilized sample was spun to precipitate inherent protein and the supernatant was processed for analysis of DA and the DA metabolite dihydroxyphenylacetic acid (DOPAC) by HPLC. The solution is aliquoted in quantities to detect DA and DOPAC in linear range and the resulting ng quantities of DA or DOPAC are normalized, at first, to recovered protein and then, following western blot assay to be described, to total TH protein (fully described in methodological detail in Salvatore et al., 2012a). The protein pellet was sonicated in 1% SDS solution (Salvatore et al., 2012a) and processed for analysis of total protein and then further processed for western blot analyses of TH and site-specific TH phosphorylation (Salvatore et al., 2012a; Salvatore and Pruett, 2012). The primary antibody sources were from the following sources; total TH protein (Millipore (Temecula, CA, cat#AB152, diluted 1:1000), ser19 and ser40 phospho-specific primaries were purchased from PhosphoSolutions (Aurora, CO, cat#p1580-19, diluted 1:1000 (ser19) and cat#1580-40, diluted 1:1000 (Ser40)). The phospho-specific antibody to ser31 is an in-house antibody raised and affinity-purified by 21st Century Biochemicals and phosphorylation-state specificity was verified by the vendor and in-house verification against TH standards with verified differences in phosphorylation (as previously reported in Supplemental Information in Salvatore et al, 2009a). Site-specific TH phosphorylation standards used have been described and characterized in previous studies (Salvatore et al., 2009a; Salvatore and Pruett, 2012; Salvatore et al., 2012a). To be clear, every phosphorylation and TH protein assessment was done in independent assays (blots were not stripped and re-probed with primary antibodies). Phosphorylation stoichiometry was determined against a standard curve generated within the linear dynamic range of the antibody in use and values were normalized to total TH protein loaded for each assay. Comparisons were directly made between lesioned and contralateral control tissues for each test subject.
Phosphorylation of TH
We noted that in the assessment of ser19 and ser40 phosphorylation in the 6-OHDA study only that there were lower bands of ~50 kDa present below the 60 kDa band for TH protein and these were more prominent in the lesioned striatal tissue. There is evidence that both ser19 and ser40 phosphorylation may predispose TH to proteosomal degradation (Nakashima et al, 2011). We assumed these bands reflected a non-functional component of total TH protein and therefore determined phosphorylation stoichiometry (ps) by quantifying the 60 kDa bands alone. However, we also quantified the lower bands to determine if a relationship existed between their relative abundance and lesion severity, as indicated by TH loss against TH protein recovered in the contralateral control.
Measures of DA, TH protein, and site-specific TH phosphorylation from the striatum and SN of the 6-OHDA lesioned side were compared against values obtained from the inherently-matched intact contralateral tissues, respectively. It is known that a unilateral 6-OHDA lesion can produce bilateral effects on neuronal activity in the basal ganglia (Breit et al., 2008), striatal DA release and turnover (Dzahini et al., 2010), and striatal glutamate release (Lindefors and Ungerstedt, 1990). These neuronal events could conceivably affect the dependent measures in the contralateral control tissues. Therefore, a comparison of control values to previously published data on these measures (Salvatore et al., 2009a; Salvatore and Pruett, 2012; Pruett and Salvatore, 2013) was done. In the contralateral control tissues, DA per protein (as ng/mg protein) in striatum was ~225 and in SN ~7.5, and was in range of previously reported values (striatum 190 –300, SN 7.0 – 8.0), TH protein (as ng/µg protein) in striatum was ~0.4 and in SN ~0.07, and was in range of previously reported values (striatum 0.3 –0.5, SN 0.05 – 0.10), ser19 phosphorylation stoichiometry in striatum was ~0.15 and in SN ~0.20, and was above or in range of previously reported values (striatum 0.06 – 0.10, SN 0.15 – 0.25), ser31 phosphorylation stoichiometry in striatum was ~0.4 and in SN ~0.08, and was in range of previously reported values (striatum 0.3 – 0.4, SN 0.05 – 0.10), ser40 phosphorylation stoichiometry in striatum was ~0.06 and in SN ~0.05, and the value for striatum was above previously reported values (striatum 0.02 – 0.04, SN 0.03 – 0.05). Finally, DA turnover (DOPAC: DA ratio) in the contralateral control in striatum was ~0.06 and in SN ~0.14, and for the striatum, this value was higher than previously reported value (0.02), but the value in SN was lower than previously reported value (0.2) (Salvatore and Pruett, 2012). While it is possible that the unilateral 6-OHDA lesion contributed to the differences, primarily seen in striatum, in values as compared to previously reported measures, it stands to reason that an inherent contralateral tissue control still serves as the best possible control, given the variance in measures that occur among rats from bilateral analyses. This design controls for such variance in these measures among rats.
Statistics
With the exception of the correlational analyses, the data are presented as mean and S.E.M. The DA, DOPAC, and TH protein and phosphorylation measures in the lesioned side were compared against the values obtained from the contralateral control tissue, and therefore a two-tailed paired Student’s t-test was used for each region (striatum or SN). In both experiments, outliers were detected with alpha set to 0.05 as dictated by the sample size. A Pearson correlational analysis was done to compare DA turnover (as the ratio of ng DOPAC: ng DA) to the % of 6-OHDA-induced loss of TH against contralateral striatum, to determine if significant relationships of TH phosphorylation for each phosphorylation site existed with inherent recovered DA tissue content (control tissues and lesioned and control together) in the striatum and SN, and to examine the relationship of lower than 60 kDA immunoreactive bands against TH loss. The Grubb’s test was employed to detect outliers in the data sets presented.
RESULTS
TH protein and DA tissue content
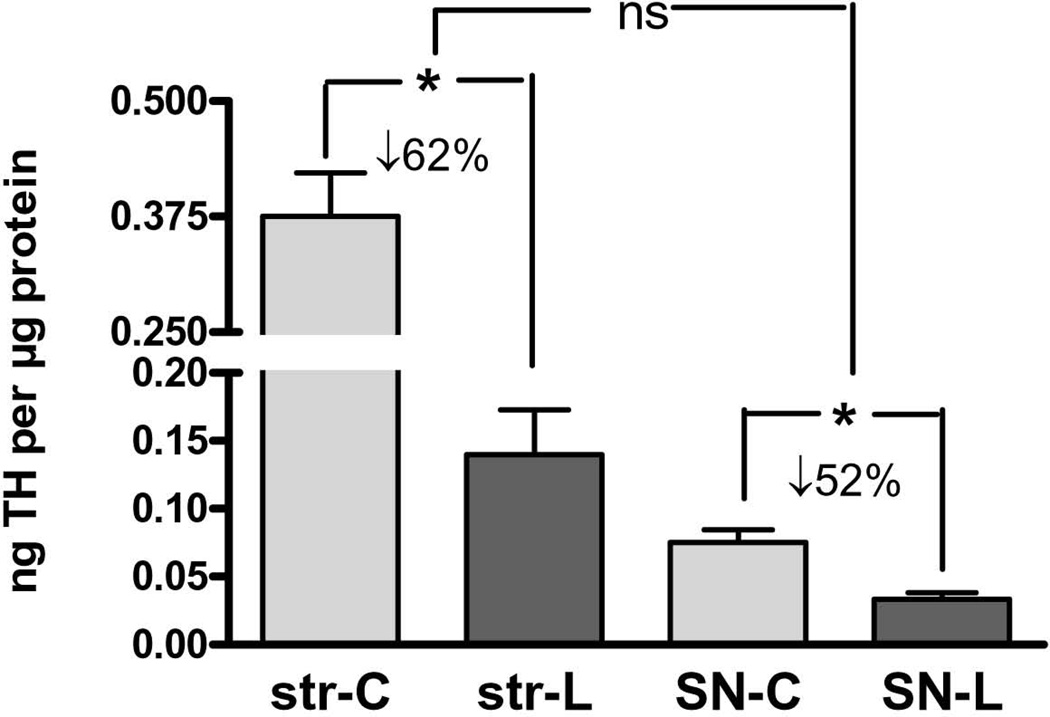
As expected, 6-OHDA lesion to the medial forebrain bundle decreased TH protein in both striatum and SN, with a mean TH loss ranging from 22–98% in striatum and 28–79% in SN, with no significant difference in TH loss between striatum and SN (Fig. 1).
Figure 1. Tyrosine hydroxylase (TH) loss in striatum and substantia nigra (SN) following 6-OHDA.
TH protein decreased on average 63% following 6-OHDA lesion in striatum (n=11, *p=0.0002, t=5.76, df=10) and 52% in SN (n=11, *p=0.0015, t=4.31, df=10). There was no significant difference in TH protein loss between these two regions following medial forebrain bundle lesion with 6-OHDA (n=11, p=0.24, t=1.24, df=10).
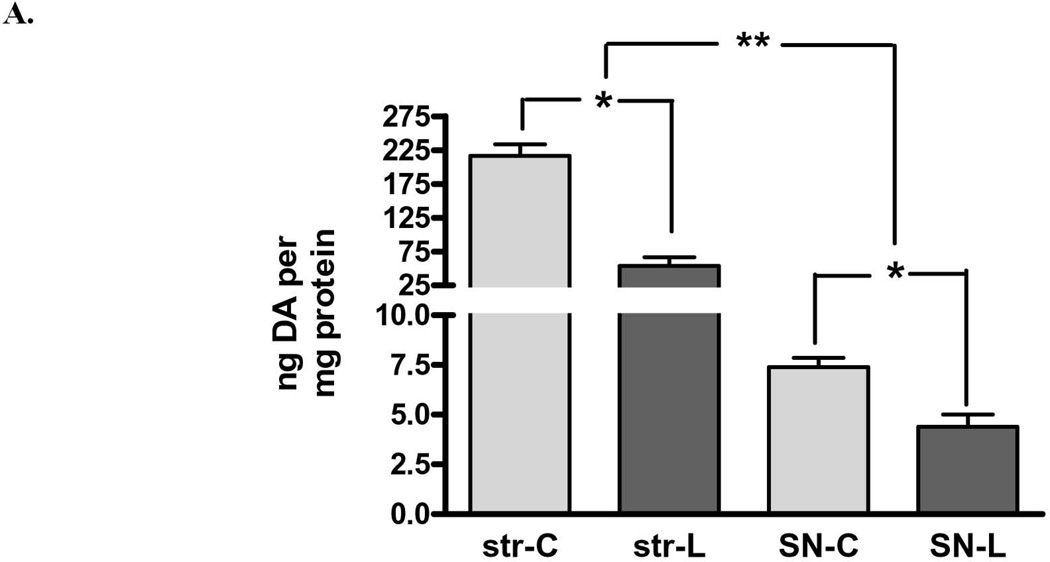
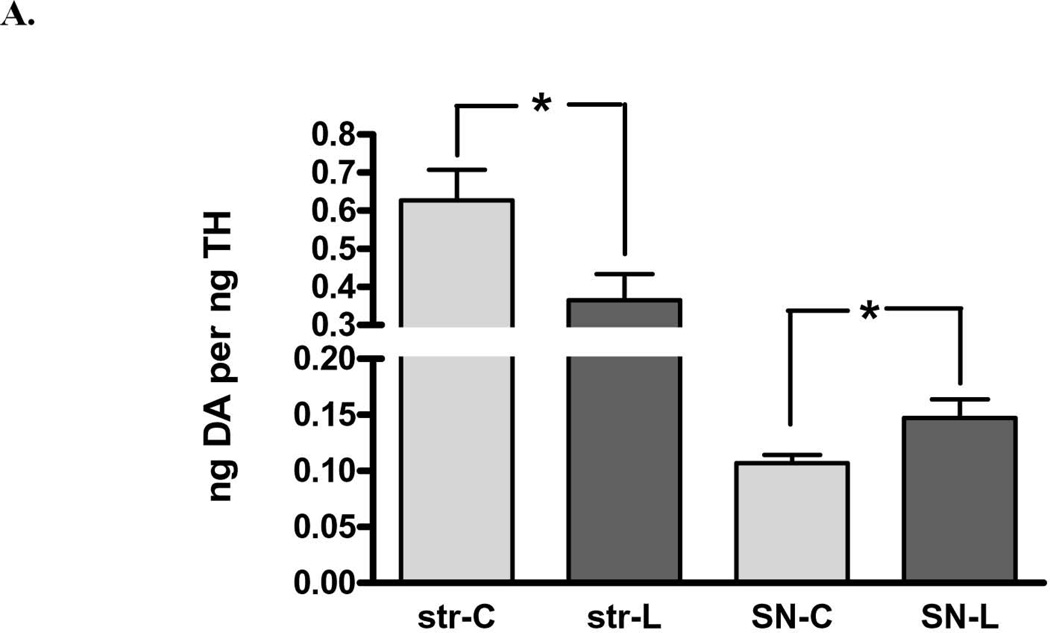
There was also a significant loss of DA in both regions, but there was significantly greater loss in striatum (75%) compared to that in the SN (40%) (Fig. 2A). Examined in another way with regard to remaining DA, there was a significant increase (2.4-fold) in DA in the lesioned SN compared to striatum, despite the similar decrease in TH protein arising from 6-OHDA lesion (Fig. 1). These differences in DA recovery between the striatum and SN were reflected in differences in DA recovered per remaining TH protein, being significantly decreased in striatum, but increased in SN, as compared to this index in the respective contralateral control tissues (Fig. 3 A,B).
Figure 2. Dopamine (DA) tissue content in striatum and substantia nigra (SN) following 6-OHDA A.
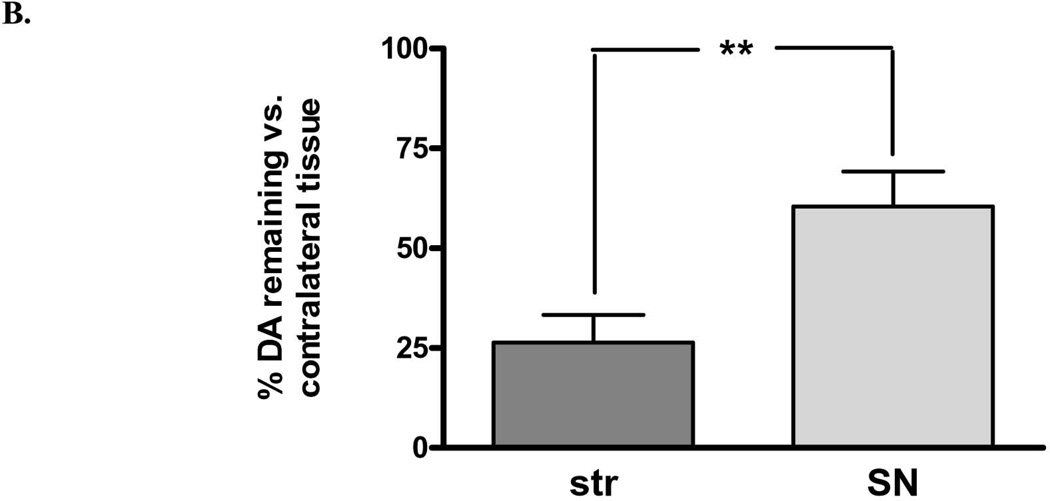
DA tissue content decreased on average 75% following 6-OHDA lesion in striatum (n=11, *p<0.0001, t=8.58, df=10) and 40% in SN (n=11, *p=0.0016, t=4.29, df=10). The degree of tissue DA loss between these two regions following 6-OHDA differed significantly (n=11, **p=0.0044, t=3.67, df=10). B. Remaining DA in the striatum versus the SN differed significantly, being 2.4-fold greater in the SN (60%) compared to striatum (25%) against corresponding contralateral tissues.
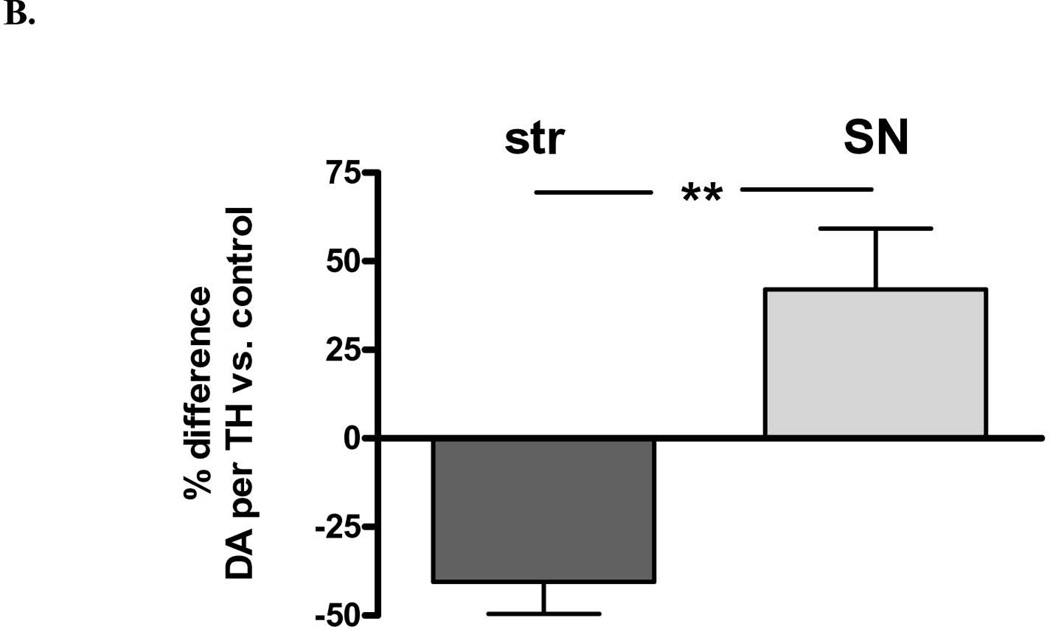
Figure 3. DA tissue content normalized to remaining TH protein following 6-OHDA lesion.
A. DA tissue content against recovered TH protein decreased on average 42% following 6-OHDA lesion in striatum (n=11, *p=0.0025, t=4.01, df=10) but increased 37% in SN (n=11, *p=0.025, t=2.64, df=10). B. DA per TH against contralateral control tissue, striatum vs. SN There was a significant difference in ng DA recovered per remaining TH protein in the striatum (decreased 40 ± 9% (mean ± SEM) against the control value of the contralateral striatum), compared to that in SN (increased 42 ± 17% (n=11, **p=0.005, t=3.54, df=10).
Dopamine turnover
There was no significant increase in DA turnover in either the striatum or substantia nigra (data not shown). However, with increasing TH protein loss in the striatum, there was a significant correlation with the DOPAC:DA ratio difference between lesioned and contralateral control striatum (Fig. 4), indicating that when DA loss exceeded 80%, striatal DA turnover increased.
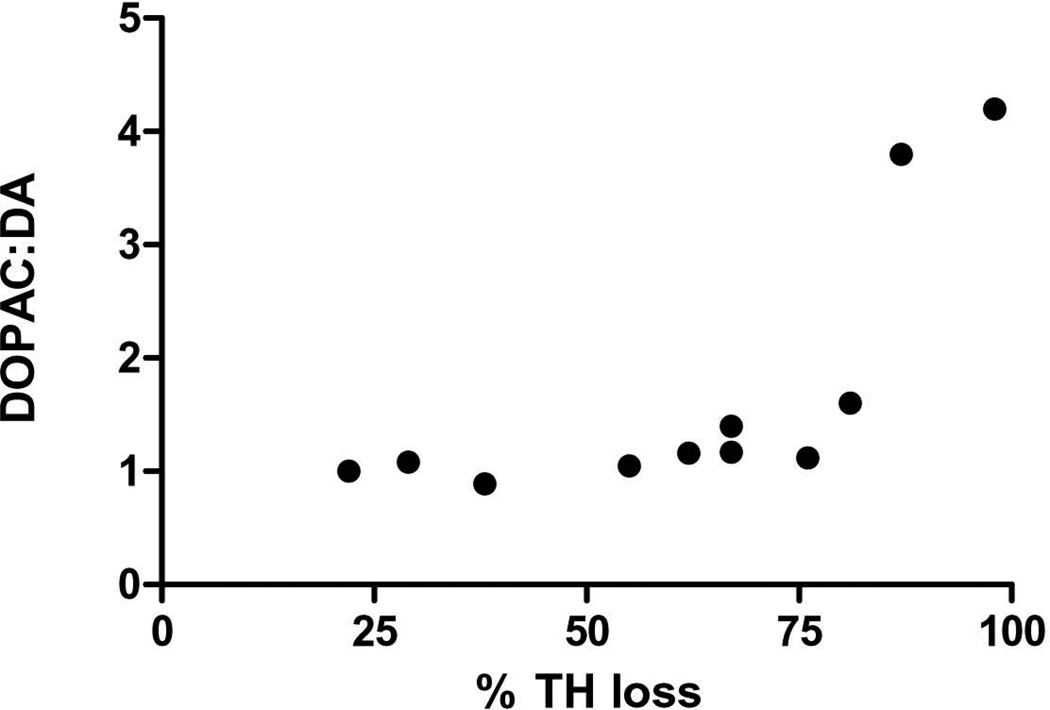
Figure 4. Relationship of TH protein loss to DOPAC:DA ratio.
As TH protein loss increased following 6-OHDA lesion, there was a significant positive correlation with DA turnover (as determined by the DOPAC:DA ratio) in lesioned striatum compared with contralateral control striatum (p=0.014, Pearson r= 0.7119, n=11). Note, number on y-axis reflects the fold difference in the DOPAC:DA ratio value from lesioned side compared to matched contralateral control value.
Relationship of ser 19, ser31, and ser40 TH phosphorylation to DA tissue content
Increased TH phosphorylation at either ser31 or ser40 affects TH activity in vitro or in situ (Salvatore et al., 2001; Dunkley et al., 2004), but recent evidence suggests that ser31 phosphorylation may affect TH regulation in vivo (Salvatore et al., 2009a; Damanhuri et al., 2012; Salvatore and Pruett, 2012; Shi et al., 2012). In control striatal and nigral tissue together (data not shown), there was a significant correlation of DA recovered per TH protein with ser31 (p<0.0001, Pearson r=0.945, n=14), but not ser40 (p=0.16, Pearson r=0.397, n=14), or ser19 (p=0.087, Pearson r= −0.473, n=14). This relationship of site-specific TH phosphorylation with DA per recovered TH protein was then analyzed to include the tissues from the lesioned striatum and SN, which provided a larger range of recovered DA against recovered TH protein. We note these comparisons were made from the same tissue sources, with repeating the phosphorylation assessment for each phosphorylation site as described in the methods. In striatum, there was a significant correlation of ser31 (Fig. 5A) or ser40 (Fig. 5B) TH phosphorylation versus DA recovered to TH protein. However, in the SN, ser31, and not ser40, TH phosphorylation had significant correlation with DA recovered to TH protein (Fig. 5C, D). No significant correlation was observed between ser19 TH phosphorylation and DA recovered to TH protein in the striatum or SN (n=14 for striatum, 13 for SN).
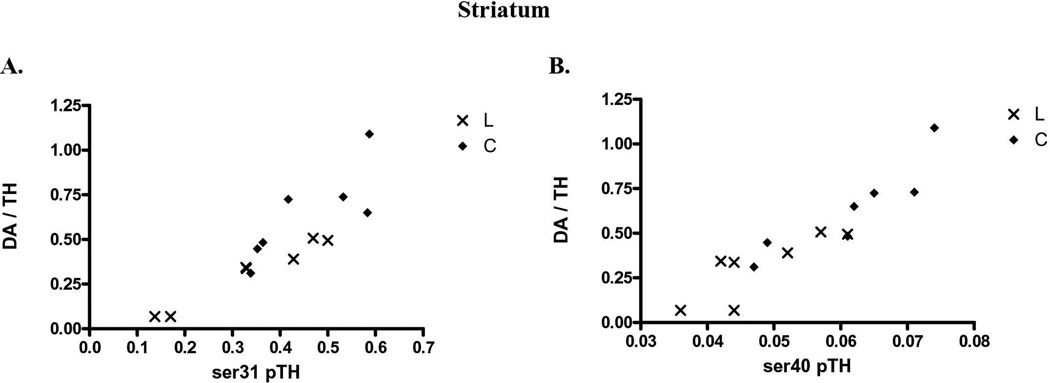
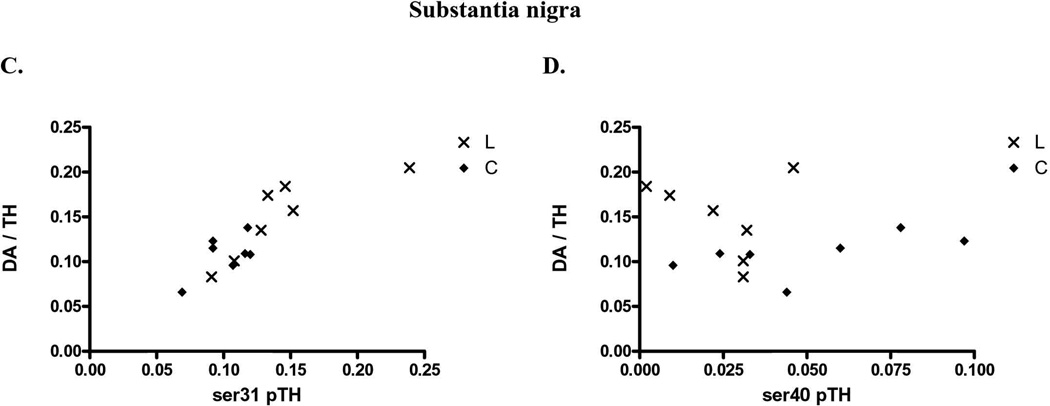
Figure 5. Relationship of ser31 and ser40 TH phosphorylation in vivo.
Striatal tissue from control (◆) and 6-OHDA lesioned mfb (X) sides was used to compare DA tissue content (normalized to inherent TH protein to eliminate the influence of TH protein to inherent DA tissue content) with associated TH phosphorylation stoichiometries. A. ser31 to striatal DA tissue content. There was a significant correlation of TH-normalized DA tissue content with ser31 (n=14, ***p<0.0001, Pearson r=0.878). B. ser40 to striatal DA tissue content. There was a significant correlation of TH-normalized DA tissue content with ser40 (n=14, ***p<0.0001, Pearson r=0.917) C. ser31 phosphorylation to nigral DA tissue content. There was a significant correlation of TH-normalized DA tissue content with ser31 (n=14, ***p=0.0001, Pearson r=0.853). ser40 to nigral DA tissue content. No significant correlation of TH-normalized DA tissue content existed in the SN with ser40 (n=14, p=0.617, Pearson r= −0.147). As noted in the text, no relationship of striatal or nigral DA tissue content existed with ser19 phosphorylation stoichiometries determined in the lesioned and control tissues.
Impact of 6-OHDA on TH phosphorylation
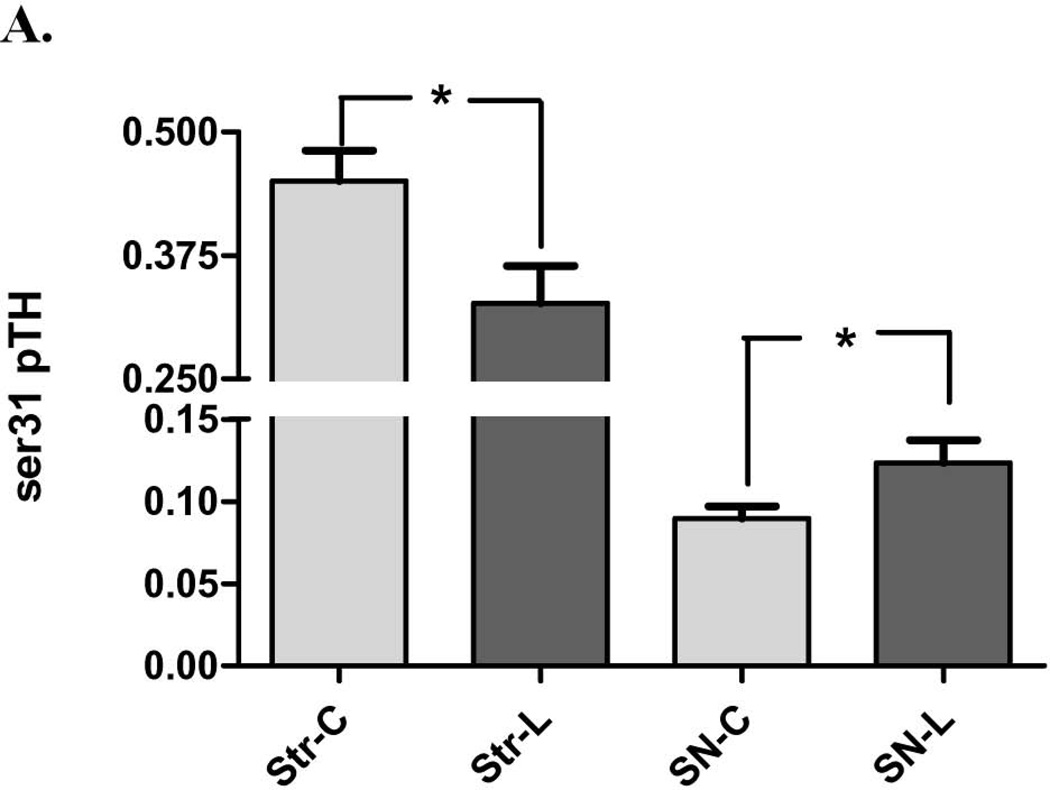
As with DA recovered to remaining TH protein, the 6-OHDA lesion also produced significant differences in ser31 TH phosphorylation compared to control tissues between the striatum and SN. Whereas ser31 TH phosphorylation significantly decreased in the striatum following 6-OHDA, there was a significant increase in the SN (Fig. 6A). This pattern of phosphorylation differences between lesioned and contralateral intact tissue matched the differences in DA recovered per TH protein (Fig. 3A) between the striatum and SN, suggesting ser31 phosphorylation, as affected by 6-OHDA, plays a significant role in affecting DA tissue content in the nigrostriatal pathway.
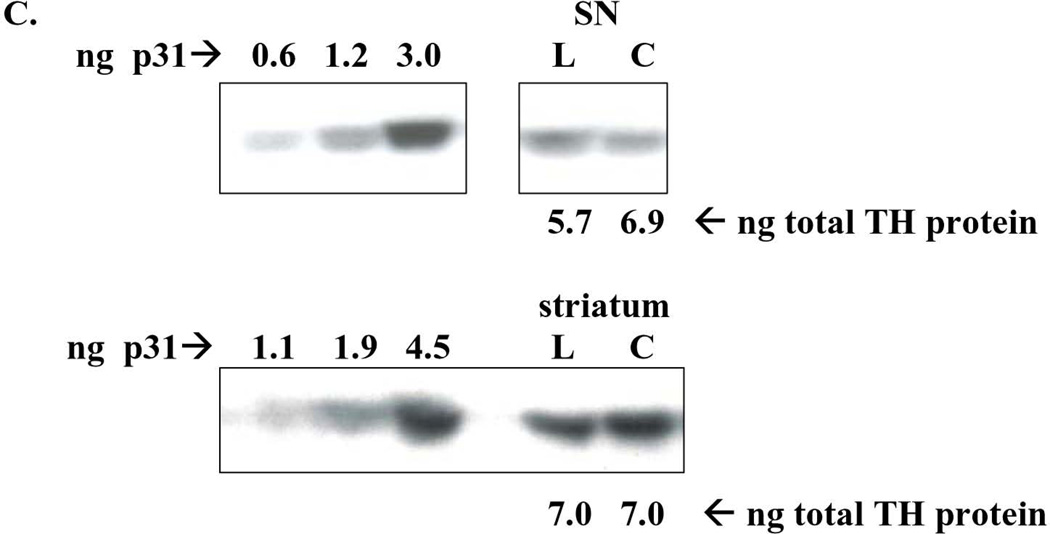
Figure 6. Impact of 6-OHDA lesion on TH phosphorylation stoichiometry. A. ser31 TH phosphorylation in striatum (str) vs. substantia nigra (SN).
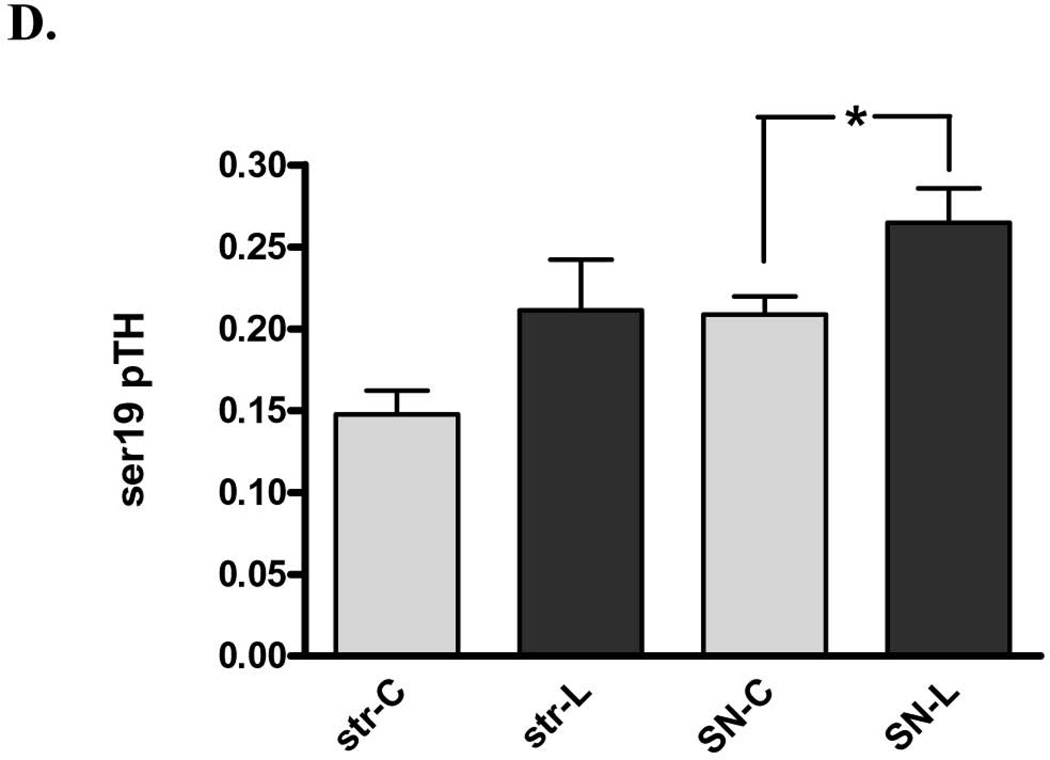
Ser31 decreased on average 28% following 6-OHDA lesion in striatum (n=11, *p=0.0195, t=2.78, df=10) but increased 37% in SN (n=11, *p=0.0125, t=3.04, df=10). B. ser40 TH phosphorylation in striatum (str) vs. substantia nigra (SN) Ser40 decreased on average 22% following 6-OHDA lesion in striatum (n=7, *p=0.011, t=3.65, df=6) but did not produce a consistent increase or decrease in the SN (n=5, p=0.27, t=1.32, df=3). C. representative western blot results from ser31 phosphorylation in the SN (top panel) and striatum (bottom panel). ng of phospho-ser31 loaded for standard curve is indicated and selected image from a lesioned (L) and contralateral control tissue (C) from the same rat in the same assay to show, with a comparable total TH protein load (~6 – 7 ng) that the phosphorylation at ser31 is increased in lesioned SN (represented pair not adjacent to standard curve), but decreased in lesioned striatum, compared to corresponding contralateral control tissue (represented pair adjacent to standard curve). D. ser19 TH phosphorylation in striatum (str) vs. substantia nigra (SN). Following 6-OHDA (SN-L), Ser19 phosphorylation (60 kDa band only) increased 27% in SN relative to control (SN-C) (n=7, *p=0.006, t=4.22, df=6). There was a trend (p=0.061) toward an increase in ser19 TH phosphorylation in the lesioned striatum. Note: results reflect 60 kDa immunoreactive band only for all measures.
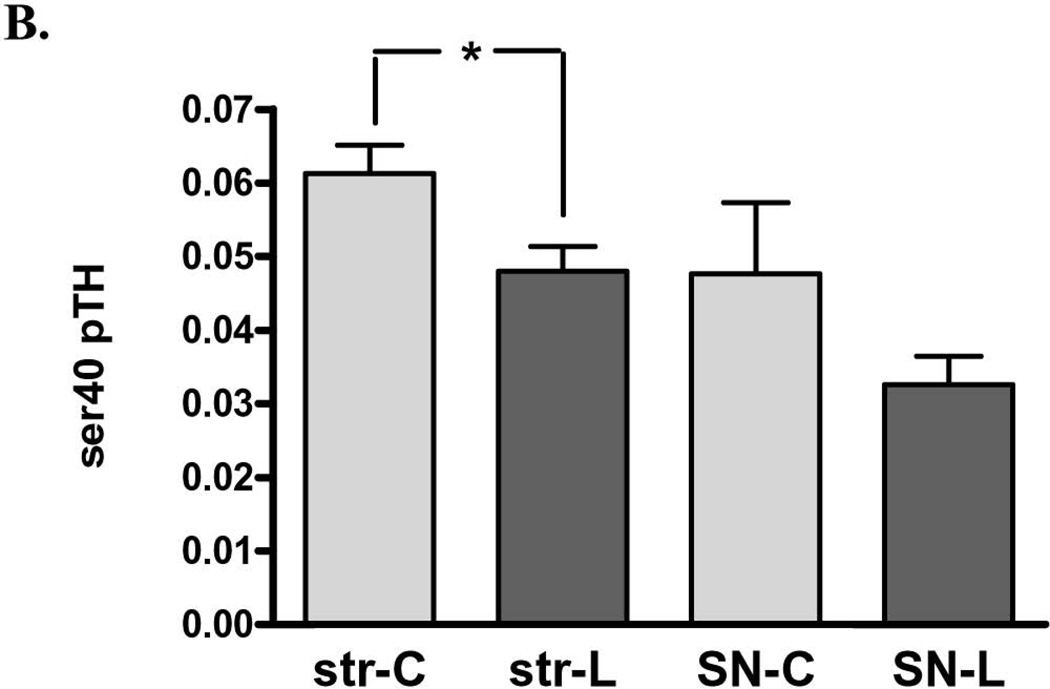
There was a significant decrease in ser40 TH phosphorylation (~28%) in striatum, but there was no significant difference observed in the SN (Fig. 6B).
Ser19 TH phosphorylation does not directly affect TH activity. However, the impact of 6-OHDA on ser19 may reveal several changes in signaling or pathology within and outside of the nigrostriatal neurons, given its Ca2+-dependence (Salvatore et al, 2001), influence by glutamate uptake dynamics (Salvatore et al., 2012b; Chotibut et al., 2013), and possible association with TH proteolysis (Nakashima et al., 2011). In the SN, there was a significant increase in ser19 TH phosphorylation (60 kDa band only) in the lesioned side (Fig. 6D). A trend toward an increase (p<0.06) was observed in striatum (Fig. 6D).
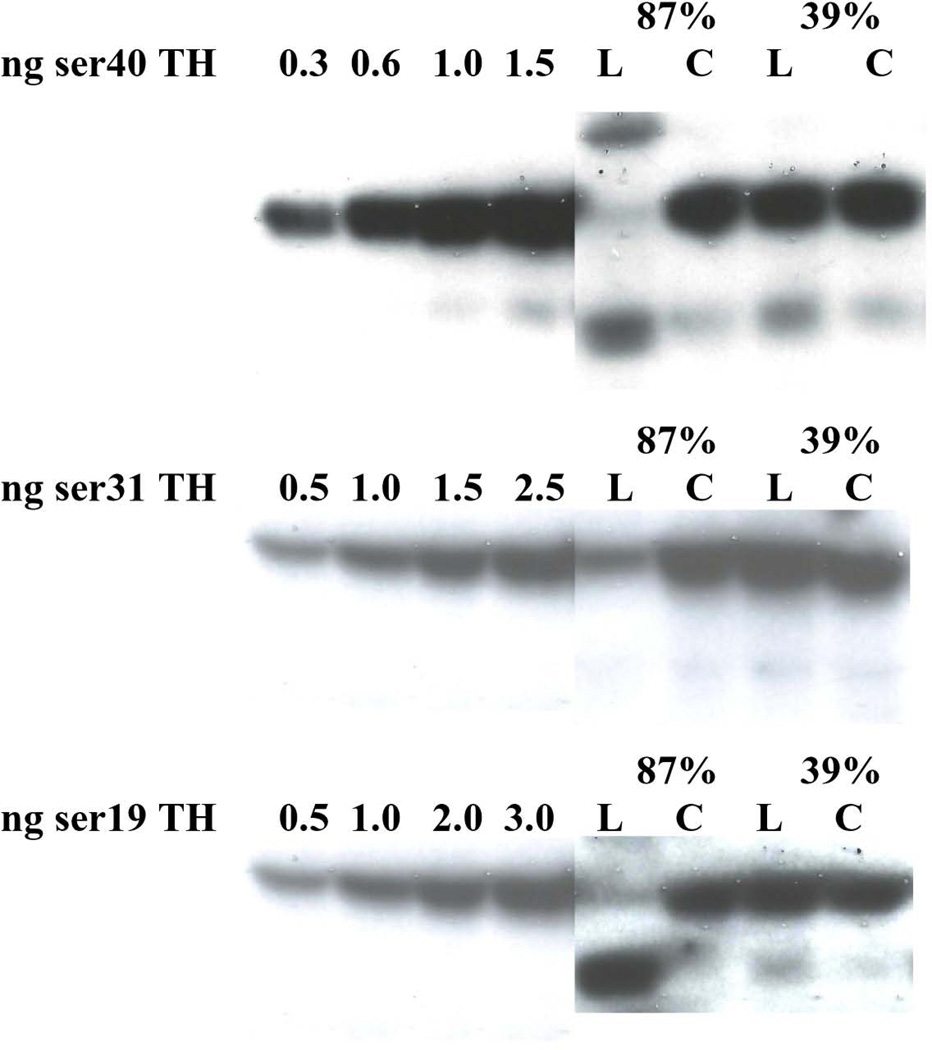
In the 6-OHDA-lesioned striatal tissue, there was an increase in the presence of ser40-reactive bands, other than the ~60 kDa TH protein band (Fig. 7, top panel). These bands were also present in the lesioned SN and contralateral SN. Their proportion in the lesioned SN trended toward an increase with >50% TH protein loss, (a low n made statistical conclusion inconclusive (data not shown)). When accounting for the lower band, in addition to the ~60 kDa TH band, there was no difference in ser40 immunoreactivity following 6-OHDA in striatum and the result in SN remained insignificant. However, the overall ser40 stoichiometry in the SN, when accounting for both the 60 kDa and lower band, was ~0.2, (data not shown), a stoichiometry that has yet to be reported in any previous in vivo study. With regard to ser31 phosphorylation, these lower MW bands were not observed in either region under equivalent assay parameters (Fig. 7, middle panel).
Figure 7. Western blot depicting impact of 6-OHDA lesion on ser40-reactive (top panel), ser31-reactive (middle panel), and ser19 TH protein in striatum.
The presence of an upper and lower MW bands relative to ser40 TH immunoreactivity at ~60 kDa was evident with TH protein loss following 6-OHDA lesion (L) as compared to contralateral control striatum (C). A predominant lower MW band was also detected at ~50 kDa in the phospho-ser19 assay of striatum. These non ~60 kDa bands were not present in detection of phosho-ser31. Standard curves of phosphorylated TH standards are presented and the relative loss of TH protein is described for two test subjects above the pairs of striatal tissue analyzed. Note that the exposures were enhanced for this particular figure to demonstrate that a 60 kDa band is still detected in the lesioned striatum.
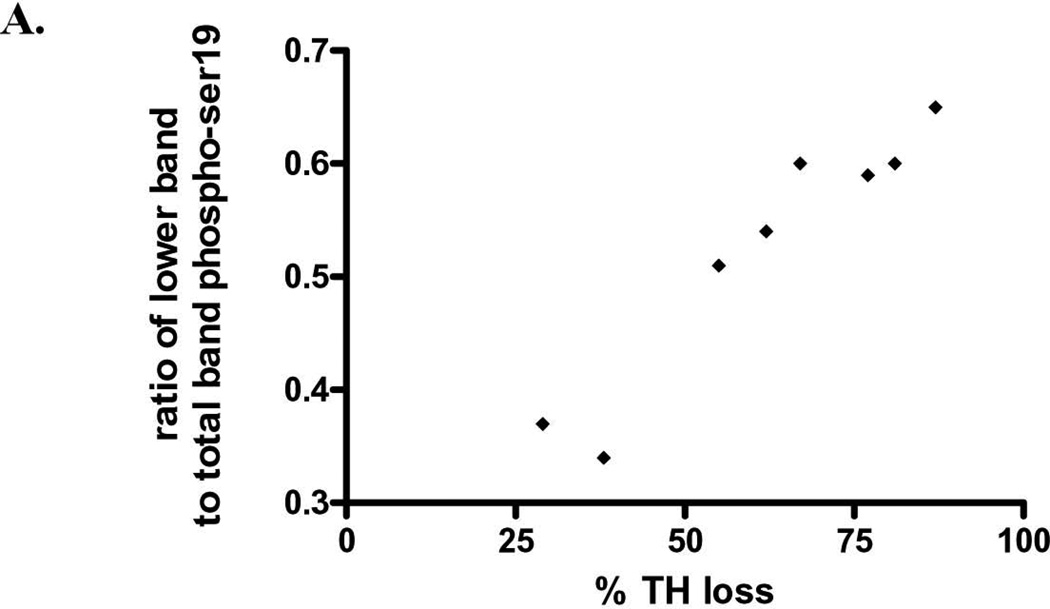
In striatum, but not SN, a lower molecular weight band reactive to the phosphospecific ser19 antibody (Fig. 7, lower panel), was correlated with TH protein loss following 6-OHDA (Fig. 8). This relationship was not observed in the case of ser40 in striatum, but there was a two-fold increase in percentage of lower band immunoreactivity observed in verified lesion of >75% (data not shown).
Figure 8. ser19 phosphorylation following 6-OHDA Relationship of striatal TH loss from 6-OHDA versus presence of lower MW phospho-ser19-reactive band (as shown in Fig. 7, lower panel).
The y-axis depicts the percentage of the lower 50 kDa band detected against the sum of this band and the 60 kDa immunoreactive band. This value is compared against the inherent loss of TH protein (60 kDa band only) detected for each 6-OHDA lesioned rat. As TH protein loss increased, the proportion of this lower MW band significantly increased (n=8, p=0.0002, Pearson r=0.956).
DISCUSSION
Presynaptic plasticity, or dopaminergic compensation, has been long proposed to be one possible mechanism by which locomotor impairment does not manifest until loss of TH protein exceeds 70% (Agid et al, 1973; Bezard et al., 2001; Zigmond et al., 2002). Here, the results reveal that the impact of 6-OHDA on TH regulation in the nigrostriatal pathway has distinct and dichotomous effects on ser31 TH phosphorylation between the striatum compared with the SN. Notably, on our assumption that DA tissue content can at least partially reflect TH activity, there is an overall decrease in DA per remaining TH protein in the striatum, whereas this index of DA biosynthesis is increased in the SN, following 6-OHDA. In so far as other mechanisms of DA regulation in the 6-OHDA model, increased reuptake of DA per remaining dopamine transporter in striatum has been recently reported (Chotibut et al., 2012). Accelerated DA turnover is also reported in lesioned striatum, (Bezard et al., 2001; Pifl and Horykiewicz, 2006). Our data indicate such increases occur only when TH loss exceeds 75% (Fig. 4). Increased DA release occurs in striatal slices from 6-OHDA-lesioned rats (Snyder et al., 1990). Therefore, these results add additional insight in how DA is regulated with TH protein loss.
The loss of TH protein following 6-OHDA was comparable between the striatum and the SN at the time of analysis, averaging ~60%, but the inclusion of tissues wherein there was less and greater than 60% TH loss allowed for determining if phosphorylation changes were consistent during TH protein loss. Overall, the results indicate that ser31 phosphorylation is differentially regulated between the striatum and SN during TH protein loss, as it is under basal conditions (Salvatore et al., 2004; 2009a; 2009b; Salvatore and Pruett, 2012). Determining TH phosphorylation in response to 6-OHDA may indicate whether increased DA biosynthesis occurs to offset TH loss. The results indicate that such a compensatory mechanism does not exist in striatum in this PD model, given a decrease in either ser31 or ser40, and DA recovered per remaining TH protein. However, increased ser31 phosphorylation and DA recovered per remaining TH protein in the SN suggests increased DA biosynthesis may occur in response to 6-OHDA-induced TH loss in the SN.
Consequences of TH phosphorylation in response to 6-OHDA
Multiple protein kinase signaling cascades regulate site-specific TH phosphorylation (Haycock, 1993; Dunkley et al., 2004). Thus, differences in TH phosphorylation following 6-OHDA may reveal potential signaling mechanisms associated with loss of TH protein. Recent studies support that ser31 may regulate DA biosynthesis in vivo (Salvatore et al, 2009a; Damanhuri et al., 2012; Salvatore and Pruett, 2012; Shi et al., 2012; Ong et al., 2013) and in the current study (Fig. 5). The increase in nigral ser31 may be related to ERK activation (Haycock et al., 1992; Salvatore et al., 2001; Lindgren et al., 2002; Salvatore et al., 2004). ERK activation can be accomplished by growth factors (Haycock, 1993), including GDNF (Salvatore et al.,2004; 2009b). Studies indicate 6-OHDA can increase GDNF-signaling (Smith et al., 2003), and ERK activation (Kulich and Chu, 2001; Lin et al., 2008). Therefore, ERK activation in SN may maintain DA signaling during nigrostriatal neuron loss. Our data support this possibility, given that ERK phosphorylates ser31, and the dichotomous changes between striatum and SN at ser31 that are congruent with DA recovered per remaining TH protein.
Recent evidence also suggests that TH phosphorylation at ser19 or ser40 could contribute to proteolytic loss of TH or DA neuron function (Nakashima et al., 2011; Nakashima et al., 2013b; Shi et al., 2012). As PD lesion progresses, increased TH proteolysis in striatum may be followed by TH loss in the SN by this mechanism, given that DA terminal fields may be more vulnerable (Chu et al., 2012). Increased TH phosphorylation at ser19, as TH protein loss increases in the striatum (Fig. 8), may be a key regulatory component for increased proteosomal digestion via the ubiquitin-proteasome pathway (Nakashima et al., 2011; 2013a; 2013b). Hyperactive nigrostriatal neurons have been thought to hasten progressive loss of DA neurons (Agid et al., 1973; Zigmond et al., 2002; Nakashima et al., 2013). Increased ser40 TH phosphorylation is also seen in post-mortem tissue of Restless Legs Syndrome patients (Connor et al., 2009) and is observed in models of iron dysregulation, wherein mild TH protein loss occurs (Salvatore et al., 2005). One could presume with our results that phosphorylation, at least at ser19, with the lower MW band suggesting TH proteolysis, may contribute to at least some level of proteolytic TH loss in the striatum (Figs.7,8). The presence of a band slightly above the 60 kDa band with the ser19 phosphorylation state specific antibody has only been observed in one other in vivo study, which also employed the use of 6-OHDA (Chotibut et al., 2013). The etiology of this band therefore appears to be associated with molecular events associated with 6-OHDA alone or in combination with TH protein loss. It is also important to note that in our study and of Nakashima (2011) that ser31 phosphorylation assessment did not detect proteolytic TH bands to an extent near that observed for ser19 or ser40 (Fig. 7). Nonetheless, a 60 kDa band was detected in assessment of all three phosphorylation sites and for total TH protein, indicating that a functional component of TH still remains following 6-OHDA.
Both ser31 and ser40 TH phosphorylation were significantly correlated with DA per recovered TH protein only in the striatum. However, only ser31 phosphorylation was significantly correlated with DA per TH protein in the control striatal and nigral tissue, an observation that has been previously reported (Salvatore et al., 2009a; Salvatore and Pruett, 2012). A summary is presented in table 1.
TABLE 1.
| 6-OHDA lesion impact vs. contralateral control tissue |
striatum | Substantia nigra |
|---|---|---|
| DA (per protein) | ↓ | ↓ |
| DA (per TH) | ↓ | ↑ |
| TH | ↓ | ↓ |
| Ser19 | ns | ↑ |
| Ser31 | ↓ | ↑ |
| Ser40 | ↓ | ns |
Relevance of Ser 19 & 31 TH phosphorylation to upstream signaling
The increase in ser19 and ser31 TH phosphorylation in SN following 6-OHDA may signify increased DA neuron excitability. However, under depolarizing conditions, a Ca2+-dependent increase in TH phosphorylation also occurs at ser40, in addition to ser19 and ser31 (Haycock and Haycock, 1991; Salvatore et al., 2001). Under basal conditions in vivo, there is a significant correlation of ser19 TH phosphorylation with ser31, but not ser40, TH phosphorylation in the midbrain DA centers (Salvatore and Pruett, 2012). However, in the striatum, ser19 TH phosphorylation correlates with ser40, but not ser31 phosphorylation (Salvatore and Pruett, 2012). However, the data herein indicate decreased ser40 phosphorylation in the striatum. GDNF also increases TH phosphorylation at all three sites (Salvatore et al., 2004; 2009b), which may be related to increased DA neuron excitability (Yang et al., 2001). Yet, the results herein indicate incongruent relationships; in the SN, increased ser19 and ser31 occur with 6-OHDA without increase in ser40. These phosphorylation changes represent end-points in signal transduction that are affected in the nigrostriatal neuron compartments with TH loss. The extracellular signal driving these changes may be related to differences in growth factor expression and the innervation by glutamatergic inputs from the subthalamic nucleus (Reese et al., 2007; Spieles-Engemann et al., 2010; 2011). The impact of such signaling on human TH may differ compared with PD models, given differential expression of TH isoforms (Gordon et al, 2009).
Significance of the differences in striatum v. substantia nigra
It is not particularly surprising that there was a dichotomous response to 6-OHDA in terms of DA regulation with TH protein loss, given that compartmental differences exist between striatum and SN in the intact nigrostriatal pathway in TH phosphorylation (Salvatore and Pruett, 2012), DA uptake (Cragg et al., 1997; Chen and Rice, 2001), and DA biosynthesis (Nissbrandt et al., 1989; Salvatore and Pruett, 2012). Disparities in TH expression between SN and striatum are reported in aging and PD models. In aging, there is direct and indirect evidence of decreased TH expression in SN (Fearnley and Lees, 1991; Emborg et al., 1998; Rudow et al., 2008; Salvatore et al., 2009a) but no loss of striatal TH in rats (Salvatore et al., 2009a; Salvatore and Pruett, 2012) or in the human lifespan (Haycock et al., 2003). In PD models, TH loss may be more severe in striatum, followed by loss of a lesser magnitude in SN at the locomotor symptom stage (Bezard et al., 2001; Sarre et al., 2004; Chu et al., 2012). Thus, less striatal DA recovery per TH, compared with opposite impact in SN, in our study indicates increased vulnerability of nigrostriatal terminals from the additional perspective of decreased DA biosynthesis (inferred by decreased ser31 TH phosphorylation) during nigrostriatal degeneration.
From another perspective, it has also been observed that despite more than 90% nigral TH loss following 6-OHDA, extracellular levels of DA in SN remain normal; only amphetamine challenge reveals decreased DA release capabilities (Sarre et al., 2004). Thus, increased ser31 in the SN may be a mechanism by which DA signaling can be maintained. However, PD is a disease of aging, wherein in the SN there is decreased ser31 TH phosphorylation and DA (Cruz-Miros et al., 2007; Salvatore et al., 2009a), which are associated with aging-related bradykinesia (Salvatore et al., 2009a). Notably, reversing such losses is associated with increased locomotor activity in aged rats (Pruett and Salvatore, 2013). Thus, if increased ser31 following 6-OHDA in the SN can offset TH protein loss, aging-related decreases in TH protein and phosphorylation, on top of PD pathology, could diminish any impact of increased ser31 TH phosphorylation to maintain normal DA signaling. Other work also suggests aging exacerbates aspects of PD pathology (Collier et al., 2007).
Possible relationship to locomotor function
Studies indicate that DA regulation in the SN alone may affect open-field locomotor activity (Bergquist et al., 2003; Trevitt et al., 2004; Andersson et al., 2006; Salvatore et al., 2009a; Pruett and Salvatore, 2013). We have reported evidence that DA regulation and TH expression in the SN alone have a temporal association with locomotor capabilities in aged rats (Salvatore et al., 2009a; Pruett and Salvatore, 2013). The significance of an increase in nigral ser31 TH phosphorylation following 6-OHDA, being associated with increased DA per remaining TH protein, suggests the possibility that this mechanism could sustain normal locomotor activity until a critical threshold of TH protein loss occurs. Indeed, locomotor improvement following GDNF or neurturin is associated with increased levels of nigral DA, without effect upon striatal DA tissue content (Hoffer et al., 1994; Gash et al., 1996; Gerhardt et al., 1999; Grondin et al., 2003; Cass and Peters, 2010). GDNF also increases TH expression in PD models (Grondin et al, 2002) and TH phosphorylation at ser31 in the SN (Salvatore et al., 2004; 2009b). The GDNF receptor, GFRα-1, may also increase nigral TH expression, DA tissue content, and locomotor function (Pruett and Salvatore, 2010; 2013). Therefore, the CNS response in the SN may reflect an avenue of pharmacological treatment (increasing GDNF-signaling) that has been previously shown to restore DA signaling and locomotor function.
Conclusions
These studies indicate signaling pathways that target ser31 TH phosphorylation in vivo may be involved in regulating DA with progressive TH protein loss in the nigrostriatal pathway. In the striatum, there is decreased ser31 TH phosphorylation in association with less DA recovery per remaining TH protein, whereas in the SN, there is increased ser31 phosphorylation in association with greater DA recovery per remaining TH protein. From the perspective of whether such changes are important for locomotor function, future work should delineate whether increasing ser31 TH phosphorylation in the striatum during TH loss would increase DA signaling and increase locomotor activity in a PD model, given its decrease shown here. Likewise, a role for increased nigral ser31 phosphorylation in locomotor function could be revealed if the inhibition of this increase would hasten the appearance of locomotor impairment during TH protein loss.
Acknowledgements
This work was funded in part by the Edward P. Stiles Trust Fund, the Biomedical Research Foundation of Northwest Louisiana, and NIH grant AG04026. Mr. Sandy Spann and Charles Dempsey provided outstanding technical support.
Abbreviations used
- DA
dopamine
- TH
tyrosine hydroxylase
- PD
Parkinson’s disease
- SN
substantia nigra
- 6-OHDA
6-hydroxydopamine
- GDNF
glial cell line-derived neurotrophic factor
- ERK
extracellular signal-regulated protein kinase
Footnotes
Conflict of Interest Statement: The author has no conflict of interest to declare.
REFERENCES
- Agid Y, Javoy F, Glowinski J. Hyperactivity of remaining dopaminergic neurones after partial destruction of the nigro-striatal dopaminergic system in the rat. Nat. New Biol. 1973;245:150–151. doi: 10.1038/newbio245150a0. [DOI] [PubMed] [Google Scholar]
- Andersson DR, Nissbrandt H, Bergquist F. Partial depletion of dopamine in substantia nigra impairs motor performance without altering striatal dopamine neurotransmission. Eur. J. Neurosci. 2006;24:617–624. doi: 10.1111/j.1460-9568.2006.04953.x. [DOI] [PubMed] [Google Scholar]
- Bennet DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, Evans DA. Prevalence of Parkinsonian signs and associated mortality in a community population of older people. New Eng. J. Med. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- Bergquist F, Shahabi HN, Nissbrandt H. Somatodendritic dopamine release in rat substantia nigra influences motor performance on the accelerating rod. Brain Res. 2003;973:81–89. doi: 10.1016/s0006-8993(03)02555-1. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurological Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, Crossman AR, Bioulac B, Brotchie JM, Gross CE. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned Macaque model of Parkinson's disease. J. Neurosci. 2001;21:6853–6861. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends Neurosci. 2003;26:215–221. doi: 10.1016/S0166-2236(03)00038-9. [DOI] [PubMed] [Google Scholar]
- Breit S, Martin A, Lessman L, Cerkez D, Gasser T, Schulz JB. Bilateral changes in neuronal activity of the basal ganglia in the unilateral 6-hydroxydopamine rat model. J. Neurosci. Res. 2008;86:1388–1396. doi: 10.1002/jnr.21588. [DOI] [PubMed] [Google Scholar]
- Cass WA, Peters LE. Neurturin protects against 6-hydroxydopamine-induced reductions in evoked dopamine overflow in rat striatum. Neurochem. Int. 2010;57:540–546. doi: 10.1016/j.neuint.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J. Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotibut T, Apple DM, Jefferis R, Salvatore MF. Dopamine transporter loss in 6-OHDA Parkinson’s model is unmet by parallel reduction in dopamine uptake. PLoS ONE. 2012;7:e52322. doi: 10.1371/journal.pone.0052322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotibut T, Davis RW, Arnold JC, Frenchek Z, Gurwara S, Bondada V, Geddes JW, Salvatore MF. Ceftriaxone increases glutamate uptake and reduces striatal tyrosine hydroxylase loss in 6-OHDA Parkinson's model. Mol. Neurobiol. 2013 doi: 10.1007/s12035-013-8598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Morfini GA, Langhamer LB, He Y, Brady ST, Kordower JH. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson's disease. Brain. 2012;135:2058–2073. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, Lipton J, Daley BF, Palfi S, Chu Y, Sortwell C, Bakay RA, Sladek JR, Kordower JH. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: Diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol. Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JR, Wang X-S, Allen RP, Beard JL, Weisinger JA, Felt BT, Earley CJ. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132:2403–2412. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg S, Rice ME, Greenfield SA. Heterogeneity of electrically evoked dopamine release and reuptake in substantia nigra, ventral tegmental area, and striatum. J Neurophysiol. 1997;77:863–873. doi: 10.1152/jn.1997.77.2.863. [DOI] [PubMed] [Google Scholar]
- Cruz-Muros I, Afonso-Oramas D, Abreu P, Barroso-Chinea P, Rodríguez M. Aging of the rat mesostriatal system: Differences between the nigrostriatal and the mesolimbic compartments. Exp. Neurol. 2007;204:147–161. doi: 10.1016/j.expneurol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Damanhuri HA, Burke PGR, Ong LK, Bobrovskaya L, Dickson PW, Dunkley PR, Goodchild AK. Tyrosine Hydroxylase Phosphorylation in Catecholaminergic Brain Regions: A Marker of Activation following Acute Hypotension and Glucoprivation. PLoS ONE. 2012;7(11):e50535. doi: 10.1371/journal.pone.0050535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J. Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- Dzahini K, Dentresangle C, Cavorsin ML, Bertrand A, Detraz I, Savasta M, Leviel V. Pre-synaptic glutamate-induced activation of DA release in the striatum after partial nigral lesion. J. Neurochem. 2010;113:1459–1470. doi: 10.1111/j.1471-4159.2010.06682.x. [DOI] [PubMed] [Google Scholar]
- Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, Holden JE, Kordower JH. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J. Comp. Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: Substantia nigrregional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, et al. Functional recovery in Parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Huettl P, Brock S, Zhang Z, Gash DM. GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain Res. 1999;817:163–171. doi: 10.1016/s0006-8993(98)01244-x. [DOI] [PubMed] [Google Scholar]
- Gordon SL, Bobrovskaya L, Dunkley PR, Dickson PW. Differential regulation of human tyrosine hydroxylase isoforms 1 and 2 in situ: Isoform 2 is not phosphorylated at Ser35. Biochimica Biophysica Acta. 2009;1793:1860–1867. doi: 10.1016/j.bbamcr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, Elsberry DD, Klein MC, Gerhardt GA, Gash DM. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–2201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA. Glial Cell Line-Derived Neurotrophic Factor Increases Stimulus-Evoked Dopamine Release and Motor Speed in Aged Rhesus Monkeys. J. Neurosci. 2003;23:1974–1980. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, Haycock DA. Tyrosine hydroxylase in rat brain dopaminergic nerve terminals: Multiple-site phosphorylation in vivo and in synaptosomes. J. Biol. Chem. 1991;266:5650–5657. [PubMed] [Google Scholar]
- Haycock JW, Ahn NG, Cobb MH, Krebs EG. ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc. Natl. Acad. Sci. 1992;89:2365–2369. doi: 10.1073/pnas.89.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW. Multiple signaling pathways in bovine chromaffin cells regulate tyrosine hydroxylase phosphorylation at Ser19, Ser31, and Ser40. Neurochem. Res. 1993;18:15–26. doi: 10.1007/BF00966919. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J. Neurochem. 2003;87:574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Hoffer BJ, Hoffman AF, Bowenkamp KE, Huettl P, Hudson J, Martin D, Lin LF, Gerhardt GA. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci. Lett. 1994;192:107–111. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. In: Parkinson's disease and the adaptive capacity of the nigrostriatal system: Possible neurochemical mechanisms, Advances in Neurology. Narabayashi H, Nagatsu T, Yanagisawa N, Mizuno Y, editors. New York: Raven Press; 1993. pp. 140–147. [PubMed] [Google Scholar]
- Hudson JL, Van Horne CG, Stromberg I, Brock S, Clayton J, Masserano JM, Hoffer BJ, Gerhardt GA. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 1993;626:167–174. doi: 10.1016/0006-8993(93)90576-9. [DOI] [PubMed] [Google Scholar]
- Jakowec MW, Nixon K, Hogg E, McNeill T, Petzinger GM. Tyrosine hydroxylase and dopamine transporter expression following 1-methyl-4phenyl-1,2,3,6-tetrahydropiridine-induced neurodegeneration of the mouse nigrostriatal pathway. J. Neurosci. Res. 2004;76:539–550. doi: 10.1002/jnr.20114. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson's disease. J. Neurochem. 2001;77:1058–1066. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Zhu WM, Stanic D, Finkelstein DI, Horne MH, Henderson J, Lawrence AJ, O’Connor L, Tomas D, Drago J, Horne MK. Sprouting of dopamine terminals and altered dopamine release and uptake in Parkinsonian dyskinesia. Brain. 2008;131:1574–1587. doi: 10.1093/brain/awn085. [DOI] [PubMed] [Google Scholar]
- Lin E, Cavanaugh JE, Leak RK, Perez RG, Zigmond MJ. Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. J. Neurosci. Res. 2008;86:108–117. doi: 10.1002/jnr.21478. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Ungerstedt U. Bilateral regulation of glutamate tissue and extracellular levels in caudate-putamen by midbrain dopamine neurons. Neurosci. Lett. 1990;115:248–252. doi: 10.1016/0304-3940(90)90463-j. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Goiny M, Herrera-Marschitz M, Haycock JW, Hokfelt T, Fisone G. Activation of extracellular signal-regulated kinases 1 and 2 by depolarization stimulates tyrosine hydroxylase phosphorylation and dopamine synthesis in rat brain. Eur. J. Neurosci. 2002;15:769–773. doi: 10.1046/j.1460-9568.2002.01901.x. [DOI] [PubMed] [Google Scholar]
- Nagatsu T. Change of tyrosine hydroxylase in the Parkinsonian brain an in the the of MPTP-treated mice as revealed by homospecific activity. Neurochem Res. 1990;15:425–429. doi: 10.1007/BF00969928. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Biochemistry of postmortem brains in Parkinson's disease: historical overview and future prospects. J. Neural Transm. 2011;72:113–120. doi: 10.1007/978-3-211-73574-9_14. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Mori K, Kaneko YS, Hayashi N, Nagatsu T, Ota A. Phosphorylation of the N-terminal portion of tyrosine hydroxylase triggers proteasomal digestion of the enzyme. Biohem.Biophys.Res.Comm. 2011;407:343–347. doi: 10.1016/j.bbrc.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Ota A, Kaneko YS, Mori K, Nagasaki H, Nagatsu T. A possible pathophysiological role of tyrosine hydroxylase in Parkinson's disease suggested by postmortem brain biochemistry: a contribution for the special 70th birthday symposium in honor of Prof. Peter Riederer. J. Neural Trans. 2013a;120:49–54. doi: 10.1007/s00702-012-0828-5. [DOI] [PubMed] [Google Scholar]
- Nakashima A, Kaneko YS, Kodani Y, Mori K, Nagasaki H, Nagatsu T, Ota A. Intracellular stability of tyrosine hydroxylase: phosphorylation and proteosomal digestion of the enzyme. Adv. Pharmacol. 2013b;68:3–11. doi: 10.1016/B978-0-12-411512-5.00001-4. [DOI] [PubMed] [Google Scholar]
- Nissbrandt H, Sundstrom E, Jonsson G, Hjorth S, Carlsson A. Synthesis and release of dopamine in rat brain: comparison between substantia nigra pars compacts, pars reticulata, and striatum. J. Neurochem. 1989;52:1170–1182. doi: 10.1111/j.1471-4159.1989.tb01863.x. [DOI] [PubMed] [Google Scholar]
- Ong LK, Guan L, Damanhuri H, Goodchild AK, Bobrovskaya L, Dickson PW, Dunkley PR. Neurobiological consequences of acute footshock stress; effects on tyrosine hydroxylase phosphorylation and activation in the rat brain and adrenal medulla. J. Neurochem. 2013 doi: 10.1111/jnc.12482. [DOI] [PubMed] [Google Scholar]
- Pifl C, Hornykiewicz O. Dopamine turnover is upregulated in the caudate/putamen of asymptomatic MPTP-treated rhesus monkeys. Neurochem. Int. 2006;49:519–524. doi: 10.1016/j.neuint.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Prettyman R. Extrapyramidal signs in cognitively intact elderly people. Age and Ageing. 1998;27:557–560. doi: 10.1093/ageing/27.5.557. [DOI] [PubMed] [Google Scholar]
- Pruett BS, Salvatore MF. GFR a-1 receptor expression in the aging nigrostriatal and mesoaccumbens pathways. J. Neurochem. 2010;115:707–715. doi: 10.1111/j.1471-4159.2010.06963.x. [DOI] [PubMed] [Google Scholar]
- Pruett BS, Salvatore MF. Nigral GFR a-1 infusion in aged rats increases locomotor activity, nigral tyrosine hydroxylase, and dopamine content in synchronicity. Mol. Neurobiol. 2013 doi: 10.1007/s12035-013-8397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R, Winter C, Nadjar A, Harnack D, Morgenstern R, Kupsch A, Bezard E, Meissner W. Subthalamic stimulation increases striatal tyrosine hydroxylase phosphorylation. NeuroReport. 2007;19:179–182. doi: 10.1097/WNR.0b013e3282f417b4. [DOI] [PubMed] [Google Scholar]
- Rudow G, O’Brien R, et al. Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol. 2008;115:461–470. doi: 10.1007/s00401-008-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Waymire JC, Haycock JW. Depolarization-stimulated catecholamine biosynthesis: involvement of protein kinases and tyrosine hydroxylase phosphorylation sites in situ. J. Neurochem. 2001;79:349–360. doi: 10.1046/j.1471-4159.2001.00593.x. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Zhang JL, Large DM, Wilson PE, Gash CR, Thomas TC, Haycock JW, Bing G, Stanford JA, Gash D, Gerhardt GA. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J. Neurochem. 2004;90:245–254. doi: 10.1111/j.1471-4159.2004.02496.x. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Fisher B, Surgener SP, Gerhardt GA, Rouault TA. Neurochemical investigations of dopamine neuronal systems in iron-regulatory protein 2 (IRP-2) knockout mice. Mol. Brain Res. 2005;139:341–347. doi: 10.1016/j.molbrainres.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS, Spann SL, Dempsey C. Aging reveals a role for nigral tyrosine hydroxylase ser31 phosphorylation in locomotor activity generation. PLoS ONE. 2009a;4:e8466. doi: 10.1371/journal.pone.0008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Gerhardt GA, Dayton RD, Klein RL, Stanford JA. Bilateral effects of unilateral GDNF administration on dopamine- and GABA-regulating proteins in the rat nigrostriatal system. Exp. Neurol. 2009b;219:197–207. doi: 10.1016/j.expneurol.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS. Dichotomy of Tyrosine Hydroxylase and Dopamine Regulation between Somatodendritic and Terminal Field Areas of Nigrostriatal and Mesoaccumbens Pathways. PLoS ONE. 2012;7:e29867. doi: 10.1371/journal.pone.0029867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Pruett BS, Dempsey C, Fields V. Comprehensive Profiling of Dopamine Regulation in Substantia Nigra and Ventral Tegmental Area. J. Vis. Exp. 2012a;66:e4171. doi: 10.3791/4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Davis RW, Arnold JC, Chotibut T. Transient striatal GLT-1 blockade increases EAAC1 expression, glutamate reuptake, and decreases tyrosine hydroxylase phosphorylation at ser19. Exp. Neurol. 2012b;234:428–436. doi: 10.1016/j.expneurol.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Sarre S, Yuan H, Jonkers N, Van Hemelrijck A, Ebinger G, Michotte Y. In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. J. Neurochem. 2004;90:29–39. doi: 10.1111/j.1471-4159.2004.02471.x. [DOI] [PubMed] [Google Scholar]
- Shi X, Woodward WR, Habecker BA. Ciliary neurotrophic factor stimulates tyrosine hydroxylase activity. J. Neurochem. 2012;121:700–704. doi: 10.1111/j.1471-4159.2012.07712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Antion M, Zigmond MJ, Austin MC. Effect of 6-hydroxydopamine on striatal GDNF and nigral GFR[alpha]1 and RET mRNAs in the adult rat. Mol. Brain Res. 2003;117:129–138. doi: 10.1016/s0169-328x(03)00289-4. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Keller RW, Zigmond MJ. Dopamine efflux from striatal slices after intracerebral 6-hydroxydopamine: evidence for compensatory hyperactivity of residual terminals. J. Pharmacol. Exp. Ther. 1990;253:867–876. [PubMed] [Google Scholar]
- Spieles-Engemann AL, Behbehani MM, Collier TJ, Wohlgenant SL, Steece-Collier K, Paumier K, Daley BF, Gombash S, Madhavan L, Mandybur GT, Lipton JW, Terpstra BT, Sortwell CE. Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiol. Dis. 2010;39:105–115. doi: 10.1016/j.nbd.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieles-Engemann AL, Steece-Collier K, Behbehani MM, Collier TJ, Wohlgenant SL, Kemp CJ, Cole-Strauss A, Levine ND, Gombash S, Thompson VB, Lipton JW, Sortwell CE. Subthalamic Nucleus Stimulation Increases Brain Derived Neurotrophic Factor in the Nigrostriatal System and Primary Motor Cortex. J. Parkinsons Dis. 2011;1:123–136. [PMC free article] [PubMed] [Google Scholar]
- Stanic D, Finkelstein DI, Bourke DW, Drago J, Horne MK. Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. Eur. J. Neurosci. 2003;18:1175–1188. doi: 10.1046/j.1460-9568.2003.02800.x. [DOI] [PubMed] [Google Scholar]
- Trevitt JT, Carlson BB, Nowend K, Salamone JD. Substantia nigra pars reticulata is a highly potent site of action for the behavioral effects of the D1 antagonist SCH 23390 in the rat. Psychopharmacology (Berl) 2004;156:32–41. doi: 10.1007/s002130100708. [DOI] [PubMed] [Google Scholar]
- Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, Wu C, Lu B. GDNF acutely modulates excitability and A-type K+ channels in midbrain dopaminergic neurons. Nat. Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Hastings TG, Perez RG. Increased dopamine turnover after partial loss of dopaminergic neurons: compensation or toxicity? Parkinsonism & Related Dis. 2002;8:389–393. doi: 10.1016/s1353-8020(02)00019-6. [DOI] [PubMed] [Google Scholar]