Abstract
Due to the emergence of adjuvant and neoadjuvant chemotherapy, the survival rate has been greatly improved in osteosarcoma (OS) patients with localized disease. However, this survival rate has remained unchanged over the past 30 years, and the long-term survival rate for OS patients with metastatic or recurrent disease remains poor. To a certain extent, the reason behind this may be ascribed to the chemoresistance to anti-OS therapy. Chemoresistance in OS appears to be mediated by numerous mechanisms, which include decreased intracellular drug accumulation, drug inactivation, enhanced DNA repair, perturbations in signal transduction pathways, apoptosis- and autophagy-related chemoresistance, microRNA (miRNA) dysregulation and cancer stem cell (CSC)-mediated drug resistance. In addition, methods employed to circumvent these resistance mechanism have been shown to be effective in the treatment of OS. However, almost all the current studies on the mechanisms of chemoresistance in OS are in their infancy. Further studies are required to focus on the following aspects: i) Improving the delivery of efficacy through novel delivery patterns; ii) improving the understanding of the signal transduction pathways that regulate the proliferation and growth of OS cells; iii) elucidating the signaling pathways of autophagy and its association with apoptosis in OS cells; iv) utilizing high-throughput miRNA expression analysis to identify miRNAs associated with chemoresistance in OS; and v) identifying the role that CSCs play in tumor metastasis and in-depth study of the mechanism of chemoresistance in the CSCs of OS.
Keywords: osteosarcoma, chemoresistance
1. Introduction
Osteosarcoma (OS) is the most common malignant primary bone tumor in children and adolescents. OS has a predilection for the metaphyseal portions of the long bone, with the distal femur and proximal tibia accounting for ~50% of all cases (1). OS is highly aggressive and it metastasizes primarily to the lung (2). In ~75% of cases, patients with OS are between 15–25 years old. The median age of an OS patient is 16 years old, with a male predominance. The high incidence of OS during the adolescence growth spurt indicates there may be a link between this disease and bone development. The incidence of OS is also increased in patients with germline mutations in the retinoblastoma and P53 genes, indicating that these genes may be involved in the occurrence of the disease. Histologically, OS is characterized by a proliferation of malignant spindle cells. Although several histological subtypes may exist, including chondroblastic and fibroblastic OS, once the osteoid directly produced by sarcoma cells is found, OS can be diagnosed (3). With regard to the clinical features, pain and swelling of the soft tissue are the most common symptoms of OS patients. Up to 20–25% of recently diagnosed patients present with metastases detectable by radiography, which mainly occur in the lung. Prior to the use of adjuvant and neoadjuvant chemotherapy, the long-term survival rate subsequent to surgical resection alone was <20%. Luckily, multi-agent chemotherapy regimens that were pioneered in the 1970s and early 1980s have dramatically improved the survival rate by up to ~70%, and the necessity for the chemotherapy used for OS patients has been demonstrated by randomized controlled trials (4). The current national and international co-operative trial for patients with recently diagnosed OS mainly builds upon the backbone of cisplatin, doxorubicin and methotrexate (MTX) treatment. Due to the combination of these anti-OS drugs, the 5-year survival rate in patients with localized disease is ~70%. However, the survival rate has plateaued since the mid-1980s, despite advances in anti-OS therapy. In addition, patients with metastatic or recurrent diseases have a <20% chance of long-term survival despite aggressive therapies. These figures have changed little in the past 30 years (5). To a certain extent, the reason behind this may be ascribed to the chemoresistance to anti-OS therapy. The development of chemoresistance in malignant tumors limits the effectiveness of cytotoxic drugs, and this is particularly true in OS, which is characterized by the frequent refractoriness to chemotherapy. Therefore, elucidation of the mechanisms of chemoresistance and implementation of strategies to overcome chemoresistance will definitely play a pivotal role in improving the survival rate of OS patients. This review mainly focuses on the recent studies on the mechanisms of chemoresistance in OS and the methods to overcome chemoresistance.
2. Decreased intracellular drug accumulation
The mechanism behind how the majority of chemotherapy drugs are absorbed by the tumor cells is unclear. Impaired transport of MTX, an effective inhibitor of dihydrofolate reductase, is a common mechanism of resistance in OS (6). As MTX enters cells through the reduced folate carrier (RFC) at the cell membrane, the decreased expression of RFC is proven to be associated with MTX-resistance (Fig. 1) (7). In one study, decreased RFC expression was found in 65% of OS biopsy samples, and decreased RFC expression was more commonly found in samples with a poor histological response to chemotherapy (8). Another study demonstrated that RFC1 expression was moderately decreased in OS samples with a poor histological response to pre-operative treatment, and RFC expression was also lower in initial OS biopsy specimens compared with metastatic specimens (9). A subsequent confirmatory study assessing the RFC protein level found that the protein levels of RFC were lower in primary OS biopsy samples than recurrent tumor specimens, and tumors with poor histological responses to pre-operative chemotherapy exhibited significantly lower RFC levels at diagnosis than those with favorable responses. However, notably, post-chemotherapy progression to recurrence was associated with a significant increase in RFC expression (10). Subsequently, the functionality of the altered RFC proteins has been studied. One of the altered RFC proteins, Leu291Pro, has been demonstrated to confer drug resistance since the carrier is unable to translocate the substrate across the cell membrane, and three alterations, Ser46Asn, Ser4Pro and Gly259Trp, confer a certain degree of resistance to MTX via a decreased rate of transport (11). Furthermore, studies that have focused on the RFC gene have also been reported. Analysis of the RFC gene copy number by dot blot and Southern blot has not identified any variation between the parental cell lines and their MTX-resistant variants, indicating that the reduced RFC expression was not due to gene deletion (12). Sequence alterations in the RFC have been observed, and OS tumor samples with RFC sequence alterations have been shown to possess significantly higher frequencies of inferior histological response (Huvos grade I or II). However, in a study by Yang et al (13), it was not clear if any of these sequence alterations were germline- or tumor-specific, as normal tissue and peripheral blood were not obtained. Although the controversy about RFC remains, trimetrexate, a novel antifolate that does not require the RFC for transport into cells, was enrolled in a phase II study of relapsed or refractory OS patients, and was demonstrated to be effective in 5 out of 38 (13%) patients. In addition, a phase I trial of a combination of trimetrexate and high-dose MTX in patients with recurrent OS is ongoing (14).
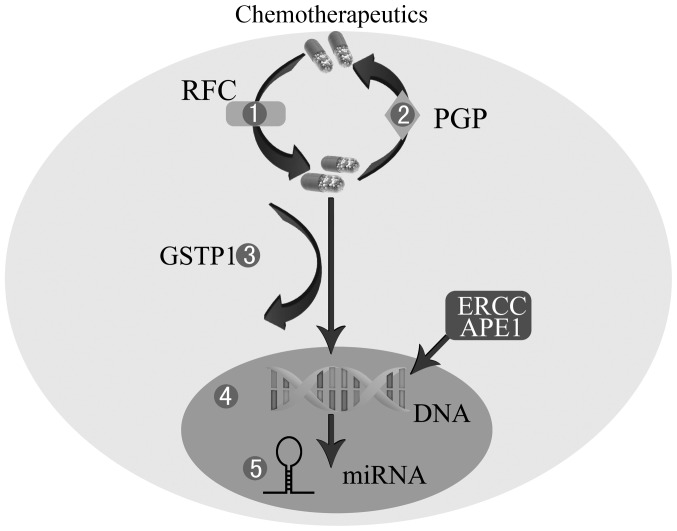
Figure 1.
Mechanisms of chemoresistance in OS. 1, Decreased intracellular drug accumulation mediated by lower RFC. 2, Increased efflux of drugs through P-GP. 3, Drug inactivation by GSTP1. 4, Enhanced DNA repair by APE1 or ERCC. 5, miRNA dysregulation. OS, osteosarcoma; RFC, reduced folate carrier; P-GP, P-glycoprotein; GSTP1, glutathione S-transferase P1; APE1, apurinic endonuclease 1; ERCC, excision repair cross-complementing; miRNA, microRNAs.
Another mechanism leading to decreased intracellular drug accumulation in numerous tumors is the non-specific removal of cytotoxic drugs from tumor cells by the membrane pump P-glycoprotein (P-GP) (15). This membrane-associated protein, a high molecular weight protein of 170 kD coded by the multidrug-resistant (MDR) human gene known as MDR1, belongs to the ATP-binding cassette (ABC) transporters, and is considered to act as an efflux pump extruding drugs from the cell (Fig. 1) (16). A series of studies has found that the high expression of P-GP may be responsible for doxorubicin resistance in human or canine OS cell lines (17–19). Additionally, several retrospective studies have indicated that the overexpression of P-GP appeared to be associated with tumor progression, a higher relapse rate and a trend towards a worse outcome (20,21). By contrast, other studies have found no correlation between P-GP expression and tumor progression or event-free survival (22,23). Similarly, a prospective, multicenter study of 123 non-metastatic OS patients did not reveal any correlation between P-GP mRNA expression and the risk of disease progression or relapse (24). A meta-analysis conclusively showed that P-GP was not associated with the histological responses of OS patients treated with a combination of chemotherapy regimens (25). Subsequently, a comparative clinical pathological study examined histological biopsies from 117 patients and found that P-GP expression could not serve as a predictor of treatment response or survival rate of OS patients (26). Furthermore, in OS cell lines transfected with the MDR gene, an association has been shown between the increased expression of P-GP and a low aggressive phenotype (27). In order to overcome the resistance mechanism caused by P-GP, recent studies have focused on a novel drug delivery system, consisting of a biocompatible and lipid-modified polymeric nanoparticle. The initial results have indicated that this nanoparticle is a promising platform for delivering doxorubicin and small interfering RNAs (siRNAs) to the drug-resistant OS cell lines, which may reverse the decreased intracellular drug accumulation mediated by P-GP (28–30).
3. Drug inactivation
Human glutathione S-transferase P1 (GSTP1), one of the cytosolic GSTs that belong to a major group of the phase II detoxification enzyme superfamilies, inactivates a variety of exogenous xenobiotics, including mutagens, anticancer agents and their metabolites (Fig. 1) (31). It is believed that the overexpression of GSTP1 is linked to chemoresistance in numerous cancers (32). A study found that OS-bearing dogs with higher GSTP1 expression had significantly shorter median remission and survival times than dogs with a lower expression of GSTP1 (33). In another study of human OS specimens obtained from 60 OS patient cases, an association was shown between the overexpression of GSTP1 at surgery and a poor histological response to pre-operative chemotherapy (34). Similarly, another study also found that chemotherapy can induce the upregulation of GSTP1 protein expression, and that the high expression of GSTP1 is associated with a poor prognosis (35). In addition, the mRNA expression levels of GSTP1 in human OS xenografts have been assessed, and a significant correlation was shown between a higher GSTP1 expression and a low growth inhibition of OS cells treated with doxorubicin (36). Furthermore, a study by Huang et al (37) indicated that the protective role of GSTP1 in OS cell survival may be mediated in part by promoting the activation of extracellular signal-regulated kinase (ERK)1/2 rather than c-Jun N-terminal kinases (JNK) in HOS OS cells triggered by doxorubicin or cisplatin. Windsor et al (38) investigated the association of 36 candidate genetic polymorphisms in MTX, adriamycin and cisplatin pathway genes with the histological response and survival rate in OS patients and found that a poor histological response was increased in variants of GSTP1, c.313A>G p.lle(105)Val. A study by Zhang et al (39) also showed that individuals with the GSTP1 Val/Val genotype tended to live for less time than those with the IIe/IIe genotype. However, a study by Yang et al (40) found that the GSTP1 Val genotypes exhibited significantly higher rates of response to chemotherapy. These results indicate that GSTP1 polymorphisms may be candidate pharmacogenomic factors to be explored in the future to benefit OS patients with chemotherapy.
To overcome GSTP1-related resistance in OS, the in vitro effectiveness of 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio)hexanol (NBDHEX), a highly efficient inhibitor of GSTP1, was tested. A study found that NBDHEX was extremely active in the resistance to doxorubicin and MTX in the U-2OS or Saos-2 cell lines (41). A further study on NBDHEX confirmed that the in vitro activity of NBDHEX was mostly associated with cytostatic effects, with less evident apoptotic induction and a positive effect against the metastasization of OS cells (42). Subsequently, a proteomic investigation was performed and the result demonstrated that NBDHEX was able to dissociate the GSTP1-tumor necrosis factor receptor-associated factor (TRAF)2 complex, which restores the TRAF2/apoptosis signal-regulating kinase 1 (Arabidopsis) signaling, thereby leading to the simultaneous and prolonged activation of JNK and p38 (43). These findings may support the fact that targeting GSTP1 with NBDHEX may be a promising novel therapeutic possibility for OS patients.
4. Enhanced DNA repair
Chemotherapeutic agents routinely used in the therapy of OS, including cisplatin and cyclophosphamide, work by damaging DNA. Therefore, one of the mechanisms associated with the resistance to these drugs is the enhanced capacity of the cell to carry out repair on damaged DNA (Fig. 1). In general, cells repair DNA damage via four main mechanisms: Direct reversal, base excision repair, nucleotide excision repair and mismatch repair.
Apurinic endonuclease 1 (APE1), one of the main enzymes involved in the base excision repair pathway, has been linked to chemosensitivity and prognosis in a number of cancers, including glioma, melanoma and cervical, prostate and bladder cancer (44–47). High expression levels of APE1 have been demonstrated to significantly correlate with the reduced survival times of OS patients, and decreased APE1 levels in siRNA-treated human OS cells have led to enhanced cell sensitization to the DNA damaging agents (48). Similarly, decreased APE1 levels mediated by siRNA also enhance the sensitivity of human OS cells to endostatin in vivo (49). Subsequent to these findings, a study found that the APE1 gene is amplified in siRNA and APE1 expression, and can serve as an independent predictor of OS patients with local recurrence or metastasis (50). Furthermore, to overcome the increased resilience in cells to chemotherapy caused by APE1, small molecule inhibitors of the APE1 endonuclease, including lucanthone, 7-nitroindole-2-carboxylic acid, resveratrol and arylstibonic acids, have been gradually reported (47,51–53). However, these inhibitors are either fairly weak or non-specific, or the effects in cell culture are difficult to reproduce (54). Therefore, the development of effective small molecule inhibitors of APE1 may benefit OS patients.
The excision repair cross-complementing (ERCC) set of proteins, including ERCC1, 2 and 4, belongs to the nucleotide excision repair system. A study has shown a correlation between ERCC4 and the histological response to chemotherapy in OS patients (55). Similarly, the expression of ERCC4 and ERCC2 mRNA in OS cells has been shown to correlate with the chemotherapeutic effect in OS patients (56). Subsequent to these findings, a study found that a polymorphism in the ERCC2 gene was associated with a positive tumor response and survival rate in cisplatin-treated OS patients (57). Another study of common polymorphisms also found a positive association between ERCC2 polymorphisms and an increased event-free survival rate, and the result indicated that the variant allele of ERCC2, rs1799793, could serve as a marker of OS associated with an improved prognosis following platinum therapy (58). In addition, an association between polymorphisms in ERCC2 and an improved cisplatin response and survival rate in OS patients was also shown in a Chinese population (59).
5. Perturbations in signal transduction pathways
Perturbations in signal transduction pathways are likely to be involved in the chemoresistance of tumors. One pathway that has been intensely studied is the mammalian target of rapamycin (mTOR) pathway (Fig. 2). The serine-threonine kinase, mTOR, plays a major role in the regulation of protein translation, cell growth and metabolism. Alterations of the mTOR signaling pathway are common in various cancers, including OS, and the mTOR signaling pathway is being actively pursued as a therapeutic target (60). In OS cells lines from dogs, mTOR and its downstream product have been shown to be present and active, and pathway inhibition by rapamycin decreased the survival rate of the tumor cells (61). In the human OS cell lines, HOS and KHOS/NP, the mTOR inhibitor, rapamycin, downregulated the activity of mTOR and strongly inhibited cell growth, as apparent by an increase in the G1 phase and a decrease in the S-phase of the cell cycle, linked with the downregulation of cyclin D1 (62). Clinical studies have also been started. A correlation has been shown between the mTOR/p70S6K signal transduction pathway and OS patient prognosis, indicating the prognostic significance of the mTOR/p70S6K signal transduction pathway (63). In an initial testing (phase I) of rapamycin by the pediatric pre-clinical testing program (PPTP), rapamycin was demonstrated to possess broad anti-tumor activity against the PPTP tumor panels in vivo, including that of OS (64). In a murine model of OS, the blockade of the mTOR pathway with rapamycin or its analog, cell cycle inhibitor-779, led to a significant inhibition of experimental lung metastasis in vivo (65). In addition, a recent study has revealed that rapamycin treatment reduces the gene expression of vascular endothelial growth factor (VEGF) and bone morphogenetic protein-2, and that it inhibits the invasion, proliferation and migration of murine K7M2 OS cells in vitro (66). These results indicate that the mTOR pathway may not only decrease the survival of OS tumor cells, but that it also plays a significant role in metastasis.
Figure 2.
Mechanisms of chemoresistance in OS. 1 and 2, Perturbations in mTOR or IGF-IR signal transduction pathways. 3 and 4, Apoptosis and autophagy-related chemoresistance. 3-MA, 3-methyladenine; AKT (PKB), protein kinase B; Bad, basal cell lymphoma 2-associated death protein; Bak, basal cell lymphoma 2 homologous antagonist killer protein; Bax, basal cell lymphoma 2-associated X protein; Bcl-2, basal cell lymphoma 2 protein; Bcl-xl, basal cell lymphoma extra large protein; ERK, extracellular signal-regulated kinase; FIP200, family interacting protein of 200 kDa; HMGB1, high mobility group box 1 protein; IGF-I, insulin-like growth factor I; IGF-IR, IGF-I receptor; MAPK, mitogen activated protein kinase; mAtg13, mammalian autophagy-related gene 13; Mdm2, murine double minute 2; mTOR, mammalian target of rapamycin; OS, osteosarcoma; p70S6K, ribosomal protein S6 kinases, 70 kDa; PI3K, phosphoinositide 3-kinase; PI3KCIII, PI3K class III; ULK1, Unc-51-like kinase 1.
The insulin-like growth factor I receptor (IGF-IR), a transmembrane receptor with tyrosine kinase activity, is involved in the initiation and progression of a variety of cancers (67). Activation of the phosphorylation of IGF-IR leads to subsequent activation of at least two pro-survival signaling pathways. Following IGF-1R phosphorylation, stimulation of the phosphoinositol 3-kinase (PI3K)-protein kinase B signaling pathway is the main event, which leads to cell survival. The second pathway consists of Ras, Raf and ERK/mitogen-activated protein kinase (MAPK) activation, which leads to proliferation and tumor growth (Fig. 2) (68). These two key downstream pathways of the IGF-IR have also been demonstrated to be activated in OS cell lines (69). Pre-clinical data has indicated that IGF-IR may constitute a significant therapeutic target in a variety of pediatric solid tumors, including neuroblastoma and musculoskeletal tumors cells (70,71). Similarly, IGF-1R has been found to be abundantly expressed in OS, indicating that the inhibition of IGF-IR may be effective in the therapy of OS (72). A study by Luk et al (73) indicated that IGF-1R inhibition by tyrphostin AG1024 together with doxorubicin achieves an additive anti-OS growth effect, accompanied with increased apoptosis, cytotoxicity and dual cell cycle arrest, which indicates that IGF-1R inhibition can enhance the effect of doxorubicin chemotherapy in OS cell lines. Another study by Wang et al (74) has shown that targeting IGF-1R using lentivirus-mediated short hairpin RNA could lead to growth suppression and the enhanced caspase-3-mediated apoptosis of OS cells not only in vitro, but also in vivo. In addition, a recent study indicated that IGF-1R was involved in the in vitro behavior of metastatic OS cell lines (75). A subsequent study found that the expression of the IGF-1R protein was closely associated with the surgical stage and distant metastasis of OS patients, and that IGF-1R can be used as an independent prognostic marker for OS patients (76).
Although the resistance mechanism of IGF-1R inhibitors remains largely unclear, candidate drugs, including monoclonal antibodies, small molecule tyrosine kinase inhibitors and ligand binding antibodies, are being introduced in phase I and II studies for a wide variety of cancers (77). Ewing sarcoma provides the most clear evidence of clinical activity. The results of a recently published phase II trial found that AMG 479 (a fully human monoclonal antibody to IGF-1R) achieved a clinical benefit rate of 17% in recurrent refractory Ewing’s family tumors (78). In addition, the efficacy of SCH-717454 (robatumumab, a fully human neutralizing anti-IGF-1R antibody) in OS patients is planned to be investigated in a phase II trial, and the result of the study is eagerly awaited (79).
Additionally, other receptor tyrosine kinases, including human epidermal growth factor receptor 2 (HER2/neu) and VEGF have also been recognized as potential targets for the therapy of OS, as studies have shown that HER2/neu and VEGF expression correlate with the malignant phenotype in OS (80,81). Cediranib (AZD-2171), a specific VEGF receptor inhibitor, has been demonstrated to possess a growth inhibitory effect in solid tumor xenograft models, including those of OS (82). However, in a phase II trial of the HER2-targeted agent trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic OS patients, no significant difference was found between the HER2-positive and HER2-negative groups (83). Therefore, the therapeutic benefit of this HER2-targeted agent remains uncertain, and a definitive assessment of the potential role of trastuzumab in treating OS requires further studies of patients with HER2-positive disease.
6. Apoptosis and cell cycle-related gene expression turbulence
Apoptosis is the primary mode of cell death induced by chemotherapy. Conversely, cell cycle arrest allows the host cell to repair its damaged DNA prior to cell division, while cells with excessive DNA damage undergo apoptosis. Therefore, cell cycle and apoptosis-related gene expression may be involved in the modulation of chemotherapeutic cytotoxicity (Fig. 2).
The P53 gene, which plays a pivotal role in cell cycle arrest and in the regulation of apoptosis has been demonstrated to be involved in the modulation of anticancer drug cytotoxicity (84). Wild-type or mutant P53 genes were shown to be associated with the chemoresistance in OS cells (85). However, whether the P53 gene takes part in the elevated or decreased chemoresistance in OS has been confusing. A Study by Wong et al (86) showed that the transfection of a mutated form of P53, p53-R273H, can downregulate the procaspase-3 level and induce resistance to drug toxicity in the p53-null human Saos-2 cell line. However, the various available studies have not yielded consistent results. A study by Fan and Bertino revealed that the induction of p53 conferred resistance to cisplatin when OS cells were cultured in media containing normal serum concentrations, whereas p53 induction led to increased cisplatin sensitivity when cells were grown in low serum media (87). A study by Tsuchiya et al (88) demonstrated that the human OS cell line, Saos2, transfected with wild-type p53, was twice as sensitive to cisplatin as the parental cells. Furthermore, another study revealed that the enhanced expression of murine double minute 2 (Mdm2), a downstream mediator of p53, may inhibit p53-mediated apoptosis and endow cells with resistance to DNA damaging agents (89). A further study found that modified p53, particularly p53 14/19, retains the pro-apoptotic and transcriptional activity of wide-type p53, and augments the effectiveness of chemotherapy even in cells overexpressing Mdm2 (90).
Contradictory results also exist between studies that focus on the expression of P53 in clinical OS patients. OS patients with a p53 gene deletion were found to be more sensitive to pre-operative chemotherapy compared to those without such gene loss (91). Similarly, several studies demonstrated a direct correlation between p53-positive expression and the resistance to therapy or the survival of OS patients, and concluded that p53 expression may be a useful prognostic factor in OS patients (92,93). However, a prospective study found no evidence that P53 mutations can predict the development of metastases, chemotherapy response and clinical outcome in patients with high-grade OS (94). Therefore, additional studies are required to obtain an improved explanation.
B-cell lymphoma 2 (Bcl-2) is the founding member of a family of proteins associated with cell death signaling, and was first isolated as the product of an oncogene (95). The Bcl-2 protein family is comprised of anti-apoptotic proteins, including Bcl-2 and Bcl-xL, and pro-apoptotic proteins, including Bax, Bak and Bad (96). These proteins mainly regulate apoptosis at the mitochondrial outer membrane and control the initiation of mitochondrial outer membrane permeabilization (97). Studies have shown that Bcl-2 and Bax have a role in affecting drug-induced apoptosis and regulating the resistance to chemotherapy in various tumor cells, including hepatocellular carcinoma and bladder, lung and ovarian cancer (98–101).
In vitro studies of anti-apoptotic proteins, the downregulation of Bcl-2 and Bcl-xL by lentivirus-mediated Bcl-2-knockdown or stable transfection with Bcl-xL cDNA could significantly enhance the in vitro chemosensitivity of OS cells to doxorubicin and cisplatin (93,102,103). A synergistic effect, created by co-silencing Bcl-2 and cyclin D1, was also found to enhance the chemosensitivity of OS cells (104). Subsequently, a study revealed that Bcl-xL may exert an anti-apoptotic effect by stimulating oxidative phosphorylation or inhibiting caspase activation (105). Conversely, downregulation of a pro-apoptotic protein, Bax, by lentivirus-mediated knockdown decreases the in vitro chemosensitivity of OS cells (106,107). In addition, upregulated Bax gene expression by runt-related transcription factor 2 (Runx2), which can directly bind to two Runx-specific regulatory elements on the human Bax promoter, could increase the apoptosis and drug sensitivity of OS cells (108). Additionally, another study has demonstrated that although Bax expression is not affected by the knockdown of c-Myc or caspase-2, since caspase-2 is important for cytosolic Bax to integrate into the outer mitochondrial membrane, and as c-Myc is critical for the oligomerization of Bax, that during cytotoxic drug-induced apoptosis, c-Myc and caspase-2 remain involved in activating Bax (109).
In clinical trials, a higher cellular expression level of Bcl-2 has been shown in high-grade OS patients with recurrent pulmonary metastases compared with those with primary tumors, and the expression of Bcl-2 was also shown to be closely associated with the prognosis of OS patients (110,111). Subsequently, a higher mRNA expression level of Bcl-xL was found to significantly correlate with an advanced clinical stage and a poorer survival rate in OS patients (112). However, although Bc1–2 is highly expressed in the specimens of OS patients, no correlation between the expression of Bc1–2 and chemosensitivity and the overall survival of high-grade OS patients was shown in the study by Nedelcu et al (113). Similarly, Bax and Bcl-2 protein expression was observed in OS patients, but proteins were found to be unable to predict the overall or disease-free survival rate. Nevertheless, an increased Bax/Bcl-2 protein expression ratio was associated with a decreased 4-year survival and disease-free survival rate of OS patients (114,115).
7. Autophagy-related chemoresistance
Autophagy is a homeostatic and evolutionarily conserved process that degrades cellular organelles and proteins and maintains cellular biosynthesis (116). This process can be triggered under physiological conditions, including nutrient starvation and growth factor deprivation, or in response to a variety of stress stimuli, including hypoxia and the exposition to cytotoxic compounds (117). Autophagy has been referred to as a double-edged sword. On one hand, it allows tumor cells to survive bioenergetic stress via clearance of damaged organelles and proteins under adverse conditions (116). On the other hand, in certain cellular contexts, sustained or excessive tumor cell autophagy promotes programmed cell death, particularly in apoptosis-defective cells, although certain studies have considered autophagic cell death to be a misnomer (118,119). In recent years, numerous studies have focused on the association between autophagy and the chemoresistance of tumor cells. In leukemia and colon cancer cell lines, the inhibition of autophagy was shown to sensitize resistant cells to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis (120). In addition, the ability of autophagy inhibition to enhance chemosensitivity and tumor regression was confirmed in various animal models. Firstly, the inhibition of autophagy by chloroquine was shown to increase apoptosis and enhance tumor cell death in lymphoma, colon cancer and prostate cancer xenograft mouse models (121–123). Secondly, autophagy inhibition triggered by 3-methyladenine (3-MA) increased apoptotic induction by 5-fluorouracil in association with tumor regression in colon cancer xenografts (124). Thirdly, multiple studies have revealed that the inhibition of autophagy by the knockdown of autophagy-related genes can effectively enhance tumor cell death induced by diverse anti-cancer drugs in pre-clinical models (125,126).
Subsequent studies on the dual role of autophagy in OS have been published. A study by Lambert et al (128) found that induction of autophagy was shown in U2OS cells treated with doxorubicin and roscovitine, and it was considered that autophagy may be the cause of increased cytotoxicity. One study by Meschini et al (129) demonstrated that autophagy induced by a natural product, bisindolic alkaloid voacamine, showed a lethal effect, which is effective against drug-resistant OS cell lines either used alone or in association with conventional chemotherapeutics. A study by Kim et al (130) found that in the Saos-2 cell line, the inhibition of autophagy along with 3-MA significantly increased paclitaxel (PCX)-induced apoptotic cell death. It was indicated that a combination of treatment involving autophagy inhibitor therapy and low-dose PCX therapy could be an effective and potent strategy for improving the chemotherapy for OS, although PCX has not been incorporated in the current protocols for OS treatment. By contrast, a study by Zhang et al (131) found that following the downregulation of autophagy in the MG63 cells by the autophagy inhibitor, 3-MA, the chemotherapeutic sensitivity of MG63 cells treated with cisplatin was enhanced, which indicated that autophagy may have a protective effect on OS cells. Similarly, a study by Coupienne et al (132) found that autophagy protected OS cells against photodynamic therapy-induced cell death and thus provided an improved survival rate for the OS cells. The protective role of OS cells mediated by autophagy was also shown in studies from the Central Laboratory of the Second Xiangya Hospital (Changsha, China). The results demonstrated that autophagy induced by the high mobility group box 1 protein (HMGB1), a highly conserved nuclear protein, increased chemoresistance to conventional anti-OS agents, including doxorubicin, cisplatin and MTX. Subsequently, our further studies identified that HMGB1 bound to the autophagy regulator Beclin1, and the interaction between HMGB1 and Beclin1 was dependent upon the autophagic complex, ULK1-mAtg13-FIP200. The formation of the Beclin1-PI3K class 3 complex that facilitates autophagic progression was also regulated by HMGB1 (Fig. 2) (133,134).
8. microRNA (miRNA) dysregulation
miRNAs are a class of small non-coding regulatory RNA molecule that have recently been shown to be involved in a wide array of biological processes (135). The abnormal expression of miRNA has been indicated to be associated with various cancers (136,137). When miR-34a expression was enforced, as shown by the functional analysis of miR-34a in Ewing’s sarcoma cell lines, this indicated that the cells were sensitized to doxorubicin and vincristine (138). A study by Gougelet et al (139) found that OS of rat and human origins showed an miRNA signature that could discriminate promising from unpromising responders for ifosfamide treatment. The study also identified five discriminating miRNAs (miR-92a, miR-99b, miR-132, miR-193a-5p and miR-422a) in tumors of OS patients, which could be used as a potent diagnostic tool to predict tumor sensitivity to ifosfamide. In addition, a study by Song et al (140) found that the expression of miR-140 was involved in the chemoresistance to OS xenografts by reduced cell proliferation via G1- and G2-phase arrest. Their subsequent study indicated that G2 arrest was induced by a decrease in cell proliferation stimulated by miR-215 via the suppression of denticleless protein homolog expression, which resulted in an increase in MTX-chemoresistance in the human OS cell lines, U-2 and MG63 (141). Furthermore, a study by Cai et al (142) found that miR-15a and miR-16-1 downregulated cyclin D1 and induced apoptosis and cell cycle arrest in OS, indicating that miR-15a and miR-16-1 may be used for OS therapy (Fig. 1).
9. Cancer stem cells (CSCs) and drug resistance
The CSC hypothesis, first proposed ~50 years ago, postulates that a small subpopulation of cancer cells with an unlimited proliferative capacity drives tumor self-renewal and differentiation (143). However, no substantial progress was made in the CSC hypothesis until Bonnet and Dick (144) first isolated a subpopulation of human acute myeloid leukemia cells with a CD34++/CD38− phenotype, where CD34++ has a stronger affinity to the antigen. Subsequently, CSCs have been shown to be indicated in the pathogenesis of numerous tumors, including leukemia, brain tumors and cortical glial tumors (144–146). Studies have also found that CSCs may be involved in the mechanisms of chemoresistance (147–149).
Although the specific role that CSCs play in the chemoresistance of OS cells has not been clearly elucidated, several of the aforementioned mechanisms could mediate the intrinsic chemoresistance in CSCs. A study by Di Fiore et al (150) found that a novel CSC cell line, 3AB-OS, irreversibly selected from human OS MG-63 cells by long-term treatment with 3-aminobenzamide (3AB), expressed higher levels of the ABC transporter, ABCG2 (a drug resistance marker), with a high-drug efflux capacity and anti-apoptosis genes, including FADD-like apoptosis regulating protein-L, Bcl-2, X-linked inhibitor of apoptosis protein, inhibitor of apoptosis proteins and survivin. A study by Fujii et al (151) found that the MG63 OS cell line possessed an ability to form clonal expanding colonies (sarcospheres), which show a strong resistance to doxorubicin and cisplatin due to the increased expression of the DNA repair enzyme genes, MutL homolog 1 and MutS protein homolog 2. Additionally, caffeine, a DNA repair inhibitor, enhanced the efficacy of these drugs, indicating that the drug resistance in sarcosphere cells was partly associated with the efficient DNA repair ability. Their subsequent study indicated that CSCs and the sarcosphere cells from the MG63 cell line showed a strong chemoresistance against doxorubicin and cisplatin, which may be attributed to the efficient detoxification by elevated aldehyde dehydrogenase 1 mRNA expression (152). In addition, a study by Martins-Neves et al (153) showed that OS cells contained a CSC population relatively resistant to doxorubicin and MTX, and this resistant phenotype appeared to be associated with the high expression of the drug efflux transporter, P-GP.
10. Conclusions
Although great progress has been made by combination chemotherapy and aggressive surgical resection in the treatment of OS, the survival rate of OS patients with localized disease at diagnosis has plateaued at ~70% since the mid-1980s, and the long-term survival rate of patients with metastatic or recurrent disease remains at <20% (154). Accordingly, an improved understanding of the molecular mechanisms of chemoresistance and the identification of novel strategies to circumvent the resistance mechanisms are desperately required. As mentioned in the present review, chemoresistance in OS has been shown to occur by a variety of mechanisms, including decreased intracellular drug accumulation mediated by RFC or P-GP, drug inactivation by GSTP1, enhanced DNA repair by APE1 or ERCC, perturbations in mTOR or IGF-IR signal transduction pathways, apoptosis and autophagy-related chemoresistance, miRNA dysregulation and CSC-mediated drug resistance. In addition, the interaction between OS cells and their micro-environment has also been shown to be involved in the chemoresistance in OS, and therapies disrupting this interaction have been demonstrated to be efficacious in OS treatment in pre-clinical studies. However, almost all these studies on the mechanisms of chemoresistance in OS are at an early stage, and further studies are eagerly anticipated on the following aspects.
On the basis of the current understanding of the mechanism of resistance mediated by RFC, a novel antifolate, trimetrexate, which does not require the RFC for transport into cells, has been already demonstrated to be effective in OS patients in a phase II study (14). Prior to its use in clinical trials, further clinical studies are required to assess the effect of trimetrexate in OS patients either used alone or in combination with other anti-OS drugs. To circumvent the mechanism of resistance mediated by P-GP and to improve intracellular drug accumulation, novel delivery patterns, including biocompatible nanoparticles and liposomal encapsulation, have emerged and have been shown to improve delivery efficacy in several studies (155). Further studies should be focused on the co-administration of nanoparticles combined with conventional chemotherapy and an efflux pump inhibitor, and the precise mechanism of the interaction between these drugs also deserves further investigation.
In the past two decades, studies about the signal transduction pathways and targets involved in the malignant behavior of OS led to the development of a variety of novel targeted therapeutic agents for OS, including IGF-1R antibodies and mTOR inhibitors (65,70). The following challenge is to identify promising agents in the treatment of OS, which requires more trials for successful design and completion. In addition, an improved understanding of the targeted molecules of signal transduction pathways that regulate cell proliferation and growth and the interaction between these pathways will lead to the development of numerous novel targeted agents.
The association between autophagy and chemoresistance in tumors attracts more and more attention in studies. However, the exact role that autophagy plays in cancer drug-resistance remains controversial, and studies on the autophagy and chemoresistance of OS remain rare (156). Similarly, little is known about the autophagy-related pathways and the association with apoptosis. Therefore, elucidating the signaling pathways of autophagy and the association with apoptosis in OS cells is definitely of great significance, and will bring a novel perspective on the therapy of OS.
Recently, miRNA has become a hot spot in the area of molecular biology. The majority of studies are focusing on elucidating the impact of miRNAs in the chemoresistance of a variety of tumors, including OS. However, almost all these studies are immature (140,141). In the future, utilizing high-throughput miRNA expression analysis to identify miRNAs associated with chemoresistance should be continued. Meanwhile, further studies are required to define chemoresistance-related molecular pathways mediated by miRNA.
Following a period of silence, CSCs have returned to the study horizons again. An increase in CSC studies has revealed implications for CSCs in the drug resistance and tumor metastasis of OS. However, numerous problems remain. For instance, methods used for the isolation and identification of CSCs require a degree of improvement, and the role that CSCs play in OS metastasis and the in-depth mechanism of CSC-mediated drug resistance in OS require further systemic study (146–153).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (grant no. 81272947).
References
- 1.Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer There. 2006;6:1075–1085. doi: 10.1586/14737140.6.7.1075. [DOI] [PubMed] [Google Scholar]
- 2.Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32:423–436. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma: where do we go from here? Paediatr Drugs. 2008;10:315–327. doi: 10.2165/00148581-200810050-00005. [DOI] [PubMed] [Google Scholar]
- 4.Eilber FR, Rosen G. Adjuvant chemotherapy for osteosarcoma. Semin Oncol. 1989;16:312–322. [PubMed] [Google Scholar]
- 5.Sakamoto A, Iwamoto Y. Current status and perspectives regarding the treatment of osteo-sarcoma: chemotherapy. Rev Recent Clin Trials. 2008;3:228–231. doi: 10.2174/157488708785700267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertino JR. Karnofsky memorial lecture. Ode to methotrexate. J Clin Oncol. 1993;11:5–14. doi: 10.1200/JCO.1993.11.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Hattinger CM, Reverter-Branchat G, Remondini D, et al. Genomic imbalances associated with methotrexate resistance in human osteosarcoma cell lines detected by comparative genomic hybridization-based techniques. Eur J Cell Biol. 2003;82:483–493. doi: 10.1078/0171-9335-00336. [DOI] [PubMed] [Google Scholar]
- 8.Guo W, Healey JH, Meyers PA, Ladanyi M, Huvos AG, Bertino JR, Gorlick R. Mechanisms of methotrexate resistance in osteosarcoma. Clin Cancer Res. 1999;5:621–627. [PubMed] [Google Scholar]
- 9.Patiño-García A, Zalacaín M, Marrodán L, San-Julián M, Sierrasesúmaga L. Methotrexate in pediatric osteosarcoma: response and toxicity in relation to genetic polymorphisms and dihydrofolate reductase and reduced folate carrier 1 expression. J Pediatr. 2009;154:688–693. doi: 10.1016/j.jpeds.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Ifergan I, Meller I, Issakov J, Assaraf YG. Reduced folate carrier protein expression in osteosarcoma: implications for the prediction of tumor chemosensitivity. Cancer. 2003;98:1958–1966. doi: 10.1002/cncr.11741. [DOI] [PubMed] [Google Scholar]
- 11.Flintoff WF, Sadlish H, Gorlick R, Yang R, Williams FM. Functional analysis of altered reduced folate carrier sequence changes identified in osteosarcomas. Biochim Biophys Acta. 2004;1690:110–117. doi: 10.1016/j.bbadis.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Serra M, Reverter-Branchat G, Maurici D, et al. Analysis of dihydrofolate reductase and reduced folate carrier gene status in relation to methotrexate resistance in osteosarcoma cells. Ann Oncol. 2004;15:151–160. doi: 10.1093/annonc/mdh004. [DOI] [PubMed] [Google Scholar]
- 13.Yang R, Sowers R, Mazza BA, et al. Sequence alterations in the reduced folate carrier are observed in osteosarcoma tumor samples. Clin Cancer Res. 2003;9:837–844. [PubMed] [Google Scholar]
- 14.Trippett T, Meyers P, Gorlick R, et al. High dose trimetrexate with leucovorin protection in recurrent childhood malignancies: a phase II trial. J Clin Oncol (ASCO Annual Meeting Abstracts) 1999;9:889. [Google Scholar]
- 15.Weinstein RS, Kuszak JR, Kluskens LF, Coon JS. P-glycoproteins in pathology: the multidrug resistance gene family in humans. Hum Pathol. 1990;21:34–48. doi: 10.1016/0046-8177(90)90073-e. [DOI] [PubMed] [Google Scholar]
- 16.Safa AR, Stern RK, Choi K, et al. Molecular basis of preferential resistance to colchicine in multidrug-resistant human cells conferred by Gly-185→Val-185 substitution in P-glycoprotein. Proc Natl Acad Sci USA. 1990;87:7225–7229. doi: 10.1073/pnas.87.18.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bramwell VH. osteosarcomas and other cancers of bone. Curr Opin Oncol. 2000;12:330–336. doi: 10.1097/00001622-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Park YB, Kim HS, Oh JH, Lee SH. The co-expression of p53 protein and P-glycoprotein is correlated to a poor prognosis in osteosarcoma. Int Orthop. 2001;24:307–310. doi: 10.1007/s002640000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomes CM, van Paassen H, Romeo S, et al. Multidrug resistance mediated by ABC transporters in osteosarcoma cell lines: mRNA analysis and functional radiotracer studies. Nucl Med Biol. 2006;33:831–840. doi: 10.1016/j.nucmedbio.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Serra M, Pasello M, Manara MC, et al. May P-glycoprotein status be used to stratify high-grade osteosarcoma patients? Results from the Italian/Scandinavian Sarcoma Group 1 treatment protocol. Int J Oncol. 2006;29:1459–1468. [PubMed] [Google Scholar]
- 21.Baldini N, Scotlandi K, Serra M, Picci P, Bacci G, Sottili S, Campanacci M. P-glycoprotein expression in osteosarcoma: a basis for risk-adapted adjuvant chemotherapy. J Orthop Res. 1999;17:629–632. doi: 10.1002/jor.1100170502. [DOI] [PubMed] [Google Scholar]
- 22.Kusuzaki K, Hirata M, Takeshita H, Murata H, Hashiguchi S, Ashihara T, Hirasawa Y. Relationship between P-glycoprotein positivity, doxorubicin binding ability and histologic response to chemotherapy in osteosarcomas. Cancer Lett. 1999;138:203–208. doi: 10.1016/s0304-3835(99)00018-x. [DOI] [PubMed] [Google Scholar]
- 23.Trammell RA, Johnson CB, Barker JR, Bell RS, Allan DG. Multidrug resistance-1 gene expression does not increase during tumor progression in the MGH-OGS murine osteosarcoma tumor model. J Orthop Res. 2000;18:449–455. doi: 10.1002/jor.1100180318. [DOI] [PubMed] [Google Scholar]
- 24.Wunder JS, Bull SB, Aneliunas V, et al. MDR1 gene expression and outcome in osteosarcoma: a prospective, multicenter study. J Clin Oncol. 2000;18:2685–2694. doi: 10.1200/JCO.2000.18.14.2685. [DOI] [PubMed] [Google Scholar]
- 25.Pakos EE, Ioannidis JP. The association of P-glycoprotein with response to chemotherapy and clinical outcome in patients with osteosarcoma. A meta-analysis. Cancer. 2003;98:581–589. doi: 10.1002/cncr.11546. [DOI] [PubMed] [Google Scholar]
- 26.Sorensen FB, Jensen K, Vaeth M, et al. Immunohistochemical estimates of angiogenesis, proliferative activity, p53 expression, and multiple drug resistance have no prognostic impact in osteosarcoma: A comparative clinicopathological investigation. Sarcoma. 2008;2008:874075. doi: 10.1155/2008/874075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshita H, Kusuzaki K, Murata H, et al. Osteoblastic differentiation and P-glycoprotein multidrug resistance in a murine osteosarcoma model. Br J Cancer. 2000;82:1327–1331. doi: 10.1054/bjoc.1999.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Susa M, Iyer AK, Ryu K, Choy E, Hornicek FJ, Mankin H, Milane L, Amiji MM, Duan Z. Inhibition of ABCB1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS One. 2010;5:e10764. doi: 10.1371/journal.pone.0010764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Susa M, Iyer AK, Ryu K, Hornicek FJ, Mankin H, Amiji MM, Duan Z. Doxorubicin loaded polymeric nanoparticulate delivery system to overcome drug resistance in osteosarcoma. BMC Cancer. 2009;9:399. doi: 10.1186/1471-2407-9-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi E, Iyer AK, Hornicek FJ, Amiji MM, Duan Z. Lipid-functionalized dextran nanosystems to overcome multidrug resistance in cancer: a pilot study. Clin Orthop Relat Res. 2013;471:915–925. doi: 10.1007/s11999-012-2610-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- 33.Shoieb A, Hahn K. Detection and significance of glutathione-S-transferase pi in osteogenic tumors of dogs. Int J Oncol. 1997;10:635–639. doi: 10.3892/ijo.10.3.635. [DOI] [PubMed] [Google Scholar]
- 34.Uozaki H, Horiuchi H, Ishida T, Iijima T, Imamura T, Machinami R. Overexpression of resistance-related proteins (metallothioneins, glutathione-S-transferase pi, heat shock protein 27, and lung resistance-related protein) in osteosarcoma. Relationship with poor prognosis. Cancer. 1997;79:2336–2344. doi: 10.1002/(sici)1097-0142(19970615)79:12<2336::aid-cncr7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 35.Wei L, Song XR, Wang XW, Li M, Zuo WS. Expression of MDR1 and GST-pi in osteosarcoma and soft tissue sarcoma and their correlation with chemotherapy resistance. Zhonghua Zhong Liu Za Zhi. 2006;28:445–448. (In Chinese) [PubMed] [Google Scholar]
- 36.Bruheim S, Bruland OS, Breistol K, Maelandsmo GM, Fodstad O. Human osteosarcoma xenografts and their sensitivity to chemotherapy. Pathol Oncol Res. 2004;10:133–141. doi: 10.1007/BF03033741. [DOI] [PubMed] [Google Scholar]
- 37.Huang G, Mills L, Worth LL. Expression of human glutathione S-transferase P1 mediates the chemosensitivity of osteosarcoma cells. Mol Cancer Ther. 2007;6:1610–1619. doi: 10.1158/1535-7163.MCT-06-0580. [DOI] [PubMed] [Google Scholar]
- 38.Windsor RE, Strauss SJ, Kallis C, Wood NE, Whelan JS. Germline genetic polymorphisms may influence chemotherapy response and disease outcome in osteosarcoma: a pilot study. Cancer. 2012;118:1856–1867. doi: 10.1002/cncr.26472. [DOI] [PubMed] [Google Scholar]
- 39.Zhang SL, Mao NF, Sun JY, Shi ZC, Wang B, Sun YJ. Predictive potential of glutathione S-transferase polymorphisms for prognosis of osteosarcoma patients on chemotherapy. Asian Pac J Cancer Prev. 2012;13:2705–2709. doi: 10.7314/apjcp.2012.13.6.2705. [DOI] [PubMed] [Google Scholar]
- 40.Yang LM, Li XH, Bao CF. Glutathione S-transferase P1 and DNA polymorphisms influence response to chemotherapy and prognosis of bone tumors. Asian Pac J Cancer Prev. 2012;13:5883–5886. doi: 10.7314/apjcp.2012.13.11.5883. [DOI] [PubMed] [Google Scholar]
- 41.Pasello M, Michelacci F, Scionti I, Hattinger CM, Zuntini M, Caccuri AM, Scotlandi K, Picci P, Serra M. Overcoming glutathione S-transferase P1-related cisplatin resistance in osteosarcoma. Cancer Res. 2008;68:6661–6668. doi: 10.1158/0008-5472.CAN-07-5840. [DOI] [PubMed] [Google Scholar]
- 42.Pasello M, Manara MC, Michelacci F, et al. Targeting glutathione-S transferase enzymes in musculoskeletal sarcomas: a promising therapeutic strategy. Anal Cell Pathol (Amst) 2011;34:131–145. doi: 10.3233/ACP-2011-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sau A, Filomeni G, Pezzola S, et al. Targeting GSTP1-1 induces JNK activation and leads to apoptosis in cisplatin-sensitive and -resistant human osteosarcoma cell lines. Mol Biosyst. 2012;8:994–1006. doi: 10.1039/c1mb05295k. [DOI] [PubMed] [Google Scholar]
- 44.Sak SC, Harnden P, Johnston CF, Paul AB, Kiltie AE. APE1 and XRCC1 protein expression levels predict cancer-specific survival following radical radiotherapy in bladder cancer. Clin Cancer Res. 2005;11:6205–6211. doi: 10.1158/1078-0432.CCR-05-0045. [DOI] [PubMed] [Google Scholar]
- 45.Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 46.Silber JR, Bobola MS, Blank A, Schoeler KD, Haroldson PD, Huynh MB, Kolstoe DD. The apurinic/apyrimidinic endonuclease activity of Ape1/Ref-1 contributes to human glioma cell resistance to alkylating agents and is elevated by oxidative stress. Clin Cancer Res. 2002;8:3008–3018. [PubMed] [Google Scholar]
- 47.Yang S, Irani K, Heffron SE, Jurnak F, Meyskens FL., Jr Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (Ape/Ref-1) in human melanoma and identification of the therapeutic potential of resveratrol as an Ape1/Ref-1 inhibitor. Mol Cancer Ther. 2005;4:1923–1935. doi: 10.1158/1535-7163.MCT-05-0229. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Luo M, Kelley MR. Human apurinic endonuclease 1 (APE1) expression and prognostic significance in osteosarcoma: enhanced sensitivity of osteosarcoma to DNA damaging agents using silencing RNA APE1 expression inhibition. Mol Cancer Ther. 2004;3:679–686. [PubMed] [Google Scholar]
- 49.Wang D, Zhong ZY, Li MX, Xiang DB, Li ZP. Vector-based Ape1 small interfering RNA enhances the sensitivity of human osteosarcoma cells to endostatin in vivo. Cancer Sci. 2007;98:1993–2001. doi: 10.1111/j.1349-7006.2007.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang JL, Yang D, Cogdell D, et al. APEX1 gene amplification and its protein overexpression in osteosarcoma: correlation with recurrence, metastasis, and survival. Technol Cancer Res Treat. 2010;9:161–169. doi: 10.1177/153303461000900205. [DOI] [PubMed] [Google Scholar]
- 51.Luo M, Kelley MR. Inhibition of the human apurinic/apyrimidinic endonuclease (APE1) repair activity and sensitization of breast cancer cells to DNA alkylating agents with lucanthone. Anticancer Res. 2004;24:2127–2134. [PubMed] [Google Scholar]
- 52.Madhusudan S, Smart F, Shrimpton P, et al. Isolation of a small molecule inhibitor of base excision repair. Nucleic Acids Res. 2005;33:4711–4724. doi: 10.1093/nar/gki781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seiple LA, Cardellina JH, II, Akee R, Stivers JT. Potent inhibition of human apurinic/apyrimidinic endonuclease 1 by arylstibonic acids. Mol Pharmacol. 2008;73:669–677. doi: 10.1124/mol.107.042622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fishel ML, Kelley MR. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol Aspects Med. 2007;28:375–395. doi: 10.1016/j.mam.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Nathrath M, Kremer M, Letzel H, Remberger K, Höfler H, Ulle T. Expression of genes of potential importance in the response to chemotherapy in osteosarcoma patients. Klin Padiatr. 2002;214:230–235. doi: 10.1055/s-2002-33189. (In German) [DOI] [PubMed] [Google Scholar]
- 56.Li X, Guo W, Shen DH, Yang RL, Liu J, Zhao H. Expressions of ERCC2 and ERCC4 genes in osteosarcoma and peripheral blood lymphocytes and their clinical significance. Beijing Da Xue Xue Bao. 2007;39:467–471. (In Chinese) [PubMed] [Google Scholar]
- 57.Caronia D, Patiño-García A, Milne RL, Zalacain-Díez M, Pita G, Alonso MR, Moreno LT, et al. Common variations in ERCC2 are associated with response to cisplatin chemotherapy and clinical outcome in osteosarcoma patients. Pharmacogenomics J. 2009;9:347–353. doi: 10.1038/tpj.2009.19. [DOI] [PubMed] [Google Scholar]
- 58.Biason P, Hattinger CM, Innocenti F, Talamini R, Alberghini M, Scotlandi K, Zanusso C, Serra M, Toffoli G. Nucleotide excision repair gene variants and association with survival in osteosarcoma patients treated with neoadjuvant chemotherapy. Pharmacogenomics J. 2012;12:476–483. doi: 10.1038/tpj.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hao T, Feng W, Zhang J, Sun YJ, Wang G. Association of four ERCC1 and ERCC2 SNPs with survival of bone tumour patients. Asian Pac J Cancer Prev. 2012;13:3821–3824. doi: 10.7314/apjcp.2012.13.8.3821. [DOI] [PubMed] [Google Scholar]
- 60.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–2287. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon IK, Ye F, Kent MS. Evaluation of the mammalian target of rapamycin pathway and the effect of rapamycin on target expression and cellular proliferation in osteosarcoma cells from dogs. Am J Vet Res. 2008;69:1079–1084. doi: 10.2460/ajvr.69.8.1079. [DOI] [PubMed] [Google Scholar]
- 62.Gazitt Y, Kolapathi V, Moncada K, Thomas C, Freeman J. Targeted therapy of human osteosarcoma with 17AAG or rapamycin: characterization of induced apoptosis and inhibition of mTOR and Akt/MAPK/Wnt pathways. Int J Oncol. 2009;34:551–561. [PubMed] [Google Scholar]
- 63.Zhou Q, Deng Z, Zhu Y, Long H, Zhang S, Zhao J. mTOR/p70S6K signal transduction pathway contributes to osteosarcoma progression and patients’ prognosis. Med Oncol. 2010;27:1239–1245. doi: 10.1007/s12032-009-9365-y. [DOI] [PubMed] [Google Scholar]
- 64.Houghton PJ, Morton CL, Kolb EA, et al. Initial testing (stage 1) of the mTOR inhibitor rapamycin by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:799–805. doi: 10.1002/pbc.21296. [DOI] [PubMed] [Google Scholar]
- 65.Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005;65:2406–2411. doi: 10.1158/0008-5472.CAN-04-3135. [DOI] [PubMed] [Google Scholar]
- 66.Mu X, Isaac C, Schott T, Huard J, Weiss K. Rapamycin inhibits ALDH activity, resistance to oxidative stress, and metastatic potential in murine osteosarcoma cells. Sarcoma. 2013;2013:480713. doi: 10.1155/2013/480713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 68.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 69.Chou AJ, Merola PR, Sowers R, et al. Analysis of aberrant signal transduction pathways in osteosarcoma cell lines. Proc Amer Assoc Cancer Res. 2005;46:4551. [Google Scholar]
- 70.Scotlandi K, Manara MC, Nicoletti G, et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 2005;65:3868–3876. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 71.Tanno B, Mancini C, Vitali R, et al. Down-regulation of insulin-like growth factor I receptor activity by NVP-AEW541 has an antitumor effect on neuroblastoma cells in vitro and in vivo. Clin Cancer Res. 2006;12:6772–6780. doi: 10.1158/1078-0432.CCR-06-1479. [DOI] [PubMed] [Google Scholar]
- 72.Hassan SE, Bekarev M, Kim MY, Lin J, Piperdi S, Gorlick R, Geller DS. Cell surface receptor expression patterns in osteosarcoma. Cancer. 2012;118:740–749. doi: 10.1002/cncr.26339. [DOI] [PubMed] [Google Scholar]
- 73.Luk F, Yu Y, Walsh WR, Yang JL. IGF1R-targeted therapy and its enhancement of doxorubicin chemosensitivity in human osteosarcoma cell lines. Cancer Invest. 2011;29:521–532. doi: 10.3109/07357907.2011.606252. [DOI] [PubMed] [Google Scholar]
- 74.Wang YH, Xiong J, Wang SF, Yu Y, Wang B, Chen YX, Shi HF, Qiu Y. Lentivirus-mediated shRNA targeting insulin-like growth factor-1 receptor (IGF-1R) enhances chemosensitivity of osteosarcoma cells in vitro and in vivo. Mol Cell Biochem. 2010;341:225–233. doi: 10.1007/s11010-010-0453-2. [DOI] [PubMed] [Google Scholar]
- 75.Rettew AN, Young ED, Lev DC, Kleinerman ES, Abdul-Karim FW, Getty PJ, Greenfield EM. Multiple receptor tyrosine kinases promote the in vitro phenotype of metastatic human osteosarcoma cell lines. Oncogenesis. 2012;1:e34. doi: 10.1038/oncsis.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang YH, Han XD, Qiu Y, et al. Increased expression of insulin-like growth factor-1 receptor is correlated with tumor metastasis and prognosis in patients with osteosarcoma. J Surg Oncol. 2012;105:235–243. doi: 10.1002/jso.22077. [DOI] [PubMed] [Google Scholar]
- 77.Gombos A, Metzger-Filho O, Dal Lago L, Awada-Hussein A. Clinical development of insulin-like growth factor receptor-1 (IGF-1R) inhibitors: at the crossroad? Invest New Drugs. 2012;30:2433–2442. doi: 10.1007/s10637-012-9811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tap WD, Demetri GD, Barnette P, et al. AMG 479 in relapsed or refractory Ewing’s family tumors (EFT) or desmoplastic small round cell tumors (DSRCT): Phase II results. J Clin Oncol. 2010;28(15 Suppl):10001. [Google Scholar]
- 79.Natinoal Institutes of Health. [Accessed April 6, 2011];A Study to Determine the Activity of SCH 717454 in Subjects with Relapsed Osteosarcoma or Ewing’s Sarcoma (Study P04720AM3) http://clinicaltrials.gov/ct2/show/NCT00617890?term=sch-717454&rank=2.
- 80.Akatsuka T, Wada T, Kokai Y, et al. ErbB2 expression is correlated with increased survival of patients with osteosarcoma. Cancer. 2002;94:1397–1404. doi: 10.1002/cncr.10360. [DOI] [PubMed] [Google Scholar]
- 81.Zhou Q, Zhu Y, Deng Z, Long H, Zhang S, Chen X. VEGF and EMMPRIN expression correlates with survival of patients with osteosarcoma. Surg Oncol. 2011;20:13–19. doi: 10.1016/j.suronc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Maris JM, Courtright J, Houghton PJ, et al. Initial testing of the VEGFR inhibitor AZD2171 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:581–587. doi: 10.1002/pbc.21232. [DOI] [PubMed] [Google Scholar]
- 83.Ebb D, Meyers P, Grier H, et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: a report from the children’s oncology group. J Clin Oncol. 2012;30:2545–2551. doi: 10.1200/JCO.2011.37.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liebermann DA, Hoffman B, Steinman RA. Molecular controls of growth arrest and apoptosis: p53-dependent and independent pathways. Oncogene. 1995;11:199–210. [PubMed] [Google Scholar]
- 85.Asada N, Tsuchiya H, Tomita K. De novo deletions of p53 gene and wild-type p53 correlate with acquired cisplatin-resistance in human osteosarcoma OST cell line. Anticancer Res. 1999;19:5131–5137. [PubMed] [Google Scholar]
- 86.Wong RP, Tsang WP, Chau PY, Co NN, Tsang TY, Kwok TT. p53-R273H gains new function in induction of drug resistance through down-regulation of procaspase-3. Mol Cancer Ther. 2007;6:1054–1061. doi: 10.1158/1535-7163.MCT-06-0336. [DOI] [PubMed] [Google Scholar]
- 87.Fan J, Bertino JR. Modulation of cisplatinum cytotoxicity by p53: effect of p53-mediated apoptosis and DNA repair. Mol Pharmacol. 1999;56:966–972. doi: 10.1124/mol.56.5.966. [DOI] [PubMed] [Google Scholar]
- 88.Tsuchiya H, Mori Y, Ueda Y, Okada G, Tomita K. Sensitization and caffeine potentiation of cisplatin cytotoxicity resulting from introduction of wild-type p53 gene in human osteosarcoma. Anticancer Res. 2000;20:235–242. [PubMed] [Google Scholar]
- 89.Sato N, Mizumoto K, Maehara N, Kusumoto M, Nishio S, Urashima T, Ogawa T, Tanaka M. Enhancement of drug-induced apoptosis by antisense oligodeoxynucleotides targeted against Mdm2 and p21WAF1/CIP1. Anticancer Res. 2000;20:837–842. [PubMed] [Google Scholar]
- 90.Tang HJ, Qian D, Sondak VK, Stachura S, Lin J. A modified p53 enhances apoptosis in sarcoma cell lines mediated by doxorubicin. Br J Cancer. 2004;90:1285–1292. doi: 10.1038/sj.bjc.6601653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goto A, Kanda H, Ishikawa Y, et al. Association of loss of heterozygosity at the p53 locus with chemoresistance in osteosarcomas. Jpn J Cancer Res. 1998;89:539–547. doi: 10.1111/j.1349-7006.1998.tb03295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pápai Z, Féja CN, Hanna EN, Sztán M, Oláh E, Szendrôi M. P53 overexpression as an indicator of overall survival and response to treatment in osteosarcomas. Pathol Oncol Res. 1997;3:15–19. doi: 10.1007/BF02893346. [DOI] [PubMed] [Google Scholar]
- 93.Ozger H, Eralp L, Atalar AC, et al. The effect of resistance-related proteins on the prognosis and survival of patients with osteosarcoma: an immunohistochemical analysis. Acta Orthop Traumatol Turc. 2009;43:28–34. doi: 10.3944/AOTT.2009.028. (In Turkish) [DOI] [PubMed] [Google Scholar]
- 94.Wunder JS, Gokgoz N, Parkes R, et al. TP53 mutations and outcome in osteosarcoma: a prospective, multicenter study. J Clin Oncol. 2005;23:1483–1490. doi: 10.1200/JCO.2005.04.074. [DOI] [PubMed] [Google Scholar]
- 95.Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Ann Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 96.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 97.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59(7 Suppl):1693s–1700s. [PubMed] [Google Scholar]
- 98.Ye D, Li H, Qian S, Sun Y, Zheng J, Ma Y. bcl-2/bax expression and p53 gene status in human bladder cancer: relationship to early recurrence with intravesical chemotherapy after resection. J Urol. 1998;160:2025–2029. [PubMed] [Google Scholar]
- 99.Han JY, Chung YJ, Park SW, Kim JS, Rhyu MG, Kim HK, Lee KS. The relationship between cisplatin-induced apoptosis and p53, bcl-2 and bax expression in human lung cancer cells. Korean J Intern Med. 1999;14:42–52. doi: 10.3904/kjim.1999.14.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luo D, Cheng SC, Xie H, Xie Y. Chemosensitivity of human hepatocellular carcinoma cell line QGY-7703 is related to bcl-2 protein levels. Tumour Biol. 1999;20:331–340. doi: 10.1159/000030097. [DOI] [PubMed] [Google Scholar]
- 101.Murata T, Haisa M, Uetsuka H, et al. Molecular mechanism of chemoresistance to cisplatin in ovarian cancer cell lines. Int J Mol Med. 2004;13:865–868. [PubMed] [Google Scholar]
- 102.Perego P, Righetti SC, Supino R, et al. Role of apoptosis and apoptosis-related proteins in the cisplatin-resistant phenotype of human tumor cell lines. Apoptosis. 1997;2:540–548. doi: 10.1023/a:1026442716000. [DOI] [PubMed] [Google Scholar]
- 103.Zhao Y, Zhang CL, Zeng BF, Wu XS, Gao TT, Oda Y. Enhanced chemosensitivity of drug-resistant osteosarcoma cells by lentivirus-mediated Bcl-2 silencing. Biochem Biophys Res Commun. 2009;390:642–647. doi: 10.1016/j.bbrc.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 104.Zhang C, Zhao Y, Zeng B. Enhanced chemosensitivity by simultaneously inhibiting cell cycle progression and promoting apoptosis of drug-resistant osteosarcoma MG63/DXR cells by targeting Cyclin D1 and Bcl-2. Cancer Biomark. 2012;12:155–167. doi: 10.3233/CBM-130305. [DOI] [PubMed] [Google Scholar]
- 105.Dey R, Moraes CT. Lack of oxidative phosphorylation and low mitochondrial membrane potential decrease susceptibility to apoptosis and do not modulate the protective effect of Bcl-x(L) in osteosarcoma cells. J Biol Chem. 2000;275:7087–7094. doi: 10.1074/jbc.275.10.7087. [DOI] [PubMed] [Google Scholar]
- 106.Zangemeister-Wittke U. Antisense to apoptosis inhibitors facilitates chemotherapy and TRAIL-induced death signaling. Ann NY Acad Sci. 2003;1002:90–94. doi: 10.1196/annals.1281.019. [DOI] [PubMed] [Google Scholar]
- 107.Zhang L, Yu J, Park BH, et al. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 108.Eliseev RA, Dong YF, Sampson E, et al. Runx2-mediated activation of the Bax gene increases osteosarcoma cell sensitivity to apoptosis. Oncogene. 2008;27:3605–3614. doi: 10.1038/sj.onc.1211020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cao X, Bennett RL, May WS. c-Myc and caspase-2 are involved in activating Bax during cytotoxic drug-induced apoptosis. J Biol Chem. 2008;283:14490–14496. doi: 10.1074/jbc.M801107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferrari S, Bertoni F, Zanella L, et al. Evaluation of P-glycoprotein, HER-2/ErbB-2, p53, and Bcl-2 in primary tumor and metachronous lung metastases in patients with high-grade osteosarcoma. Cancer. 2004;100:1936–1942. doi: 10.1002/cncr.20151. [DOI] [PubMed] [Google Scholar]
- 111.Wu X, Cai ZD, Lou LM, Zhu YB. Expressions of p53, c-MYC, BCL-2 and apoptotic index in human osteosarcoma and their correlations with prognosis of patients. Cancer Epidemiol. 2012;36:212–216. doi: 10.1016/j.canep.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 112.Wang ZX, Yang JS, Pan X, Wang JR, Li J, Yin YM, De W. Functional and biological analysis of Bcl-xL expression in human osteosarcoma. Bone. 2010;47:445–454. doi: 10.1016/j.bone.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 113.Nedelcu T, Kubista B, Koller A, et al. Livin and Bcl-2 expression in high-grade osteosarcoma. J Cancer Res Clin Oncol. 2008;134:237–244. doi: 10.1007/s00432-007-0276-z. [DOI] [PubMed] [Google Scholar]
- 114.Kaseta MK, Khaldi L, Gomatos IP, et al. Prognostic value of bax, bcl-2, and p53 staining in primary osteosarcoma. J Surg Oncol. 2008;97:259–266. doi: 10.1002/jso.20913. [DOI] [PubMed] [Google Scholar]
- 115.Kaseta MK, Gomatos IP, Khaldi L, et al. Prognostic value of bax, cytochrome C, and caspase-8 protein expression in primary osteosarcoma. Hybridoma (Larchmt) 2007;26:355–362. doi: 10.1089/hyb.2007.0519. [DOI] [PubMed] [Google Scholar]
- 116.Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 119.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Han J, Hou W, Goldstein LA, et al. Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J Biol Chem. 2008;283:19665–19677. doi: 10.1074/jbc.M710169200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amaravadi RK, Yu D, Lum JJ, et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carew JS, Medina EC, Esquivel JA, II, et al. Autophagy inhibition enhances vorinostat-induced apoptosis via ubiquitinated protein accumulation. J Cell Mol Med. 2010;14:2448–2459. doi: 10.1111/j.1582-4934.2009.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu Z, Chang PC, Yang JC, et al. Autophagy blockade sensitizes prostate cancer cells towards Src family kinase inhibitors. Genes Cancer. 2010;1:40–49. doi: 10.1177/1947601909358324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li J, Hou N, Faried A, Tsutsumi S, Kuwano H. Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer. 2010;46:1900–1909. doi: 10.1016/j.ejca.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 125.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Katayama M, Kawaguchi T, Berger MS, Pieper RO. DNA damaging agent-induced autophagy produces a cytoprotective adenosine triphosphate surge in malignant glioma cells. Cell Death Differ. 2007;14:548–558. doi: 10.1038/sj.cdd.4402030. [DOI] [PubMed] [Google Scholar]
- 127.Carew JS, Nawrocki ST, Kahue CN, et al. Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance. Blood. 2007;110:313–322. doi: 10.1182/blood-2006-10-050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lambert LA, Qiao N, Hunt KK, Lambert DH, Mills GB, Meijer L, Keyomarsi K. Autophagy: a novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model. Cancer Res. 2008;68:7966–7974. doi: 10.1158/0008-5472.CAN-08-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meschini S, Condello M, Calcabrini A, et al. The plant alkaloid voacamine induces apoptosis-independent autophagic cell death on both sensitive and multidrug resistant human osteosarcoma cells. Autophagy. 2008;4:1020–1033. doi: 10.4161/auto.6952. [DOI] [PubMed] [Google Scholar]
- 130.Kim HJ, Lee SG, Kim YJ, Park JE, Lee KY, Yoo YH, Kim JM. Cytoprotective role of autophagy during paclitaxel-induced apoptosis in Saos-2 osteosarcoma cells. Int J Oncol. 2013;42:1985–1992. doi: 10.3892/ijo.2013.1884. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Z, Shao Z, Xiong L, Che B, Deng C, Xu W. Expression of Beclin1 in osteosarcoma and the effects of down-regulation of autophagy on the chemotherapeutic sensitivity. J Huazhong Univ Sci Technolog Med Sci. 2009;29:737–740. doi: 10.1007/s11596-009-0613-3. [DOI] [PubMed] [Google Scholar]
- 132.Coupienne I, Fettweis G, Piette J. RIP3 expression induces a death profile change in U2OS osteosarcoma cells after 5-ALA-PDT. Lasers Surg Med. 2011;43:557–564. doi: 10.1002/lsm.21088. [DOI] [PubMed] [Google Scholar]
- 133.Huang J, Ni J, Liu K, et al. HMGB1 promotes drug resistance in osteosarcoma. Cancer Res. 2012;72:230–238. doi: 10.1158/0008-5472.CAN-11-2001. [DOI] [PubMed] [Google Scholar]
- 134.Huang J, Liu K, Yu Y, et al. Targeting HMGB1-mediated autophagy as a novel therapeutic strategy for osteosarcoma. Autophagy. 2012;8:275–277. doi: 10.4161/auto.8.2.18940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pillai RS, Bhattacharyya SN, Fillipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 136.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 137.Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nakatani F, Ferracin M, Manara MC, et al. miR-34a predicts survival of Ewing’s sarcoma patients and directly influences cell chemo-sensitivity and malignancy. J Pathol. 2012;226:796–805. doi: 10.1002/path.3007. [DOI] [PubMed] [Google Scholar]
- 139.Gougelet A, Pissaloux D, Besse A, et al. Micro-RNA profiles in osteosarcoma as a predictive tool for ifosfamide response. Int J Cancer. 2011;129:680–690. doi: 10.1002/ijc.25715. [DOI] [PubMed] [Google Scholar]
- 140.Song B, Wang Y, Xi Y, et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28:4065–4074. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song B, Wang Y, Titmus MA, Botchkina G, Formentini A, Kornmann M, Ju J. Molecular mechanism of chemoresistance by miR-215 in osteosarcoma and colon cancer cells. Mol Cancer. 2010;9:96. doi: 10.1186/1476-4598-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cai CK, Zhao GY, Tian LY, et al. miR-15a and miR-16-1 downregulate CCND1 and induce apoptosis and cell cycle arrest in osteosarcoma. Oncol Rep. 2012;28:1764–1770. doi: 10.3892/or.2012.1995. [DOI] [PubMed] [Google Scholar]
- 143.Makino S. The role of tumor stem-cells in regrowth of the tumor following drastic applications. Acta Unio Int Contra Cancrum. 1959;15(Suppl 1):196–198. [PubMed] [Google Scholar]
- 144.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 145.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 146.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 147.Liu B, Ma W, Jha RK, Gurung K. Cancer stem cells in osteosarcoma: recent progress and perspective. Acta Oncol. 2011;50:1142–1150. doi: 10.3109/0284186X.2011.584553. [DOI] [PubMed] [Google Scholar]
- 148.Woodward WA, Sulman EP. Cancer stem cells: markers or biomarkers? Cancer Metastasis Rev. 2008;27:459–470. doi: 10.1007/s10555-008-9130-2. [DOI] [PubMed] [Google Scholar]
- 149.Vangipuram SD, Wang ZJ, Lyman WD. Resistance of stem-like cells from neuroblastoma cell lines to commonly used chemotherapeutic agents. Pediatr Blood Cancer. 2010;54:361–368. doi: 10.1002/pbc.22351. [DOI] [PubMed] [Google Scholar]
- 150.Di Fiore R, Santulli A, Ferrante RD, et al. Identification and expansion of human osteosarcoma-cancer-stem cells by long-term 3-aminobenzamide treatment. J Cell Physiol. 2009;219:301–313. doi: 10.1002/jcp.21667. [DOI] [PubMed] [Google Scholar]
- 151.Fujii H, Honoki K, Tsujiuchi T, Kido A, Yoshitani K, Takakura Y. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol. 2009;34:1381–1386. [PubMed] [Google Scholar]
- 152.Honoki K, Fujii H, Kubo A, Kido A, Mori T, Tanaka Y, Tsujiuchi T. Possible involvement of stem-like populations with elevated ALDH1 in sarcomas for chemotherapeutic drug resistance. Oncol Rep. 2010;24:501–505. doi: 10.3892/or_00000885. [DOI] [PubMed] [Google Scholar]
- 153.Martins-Neves SR, Lopes ÁO, do Carmo A, et al. Therapeutic implications of an enriched cancer stem-like cell population in a human osteosarcoma cell line. BMC Cancer. 2012;12:139. doi: 10.1186/1471-2407-12-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chou AJ, Merola PR, Wexler LH, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2005;104:2214–2221. doi: 10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- 155.Alberts DS, Muggia FM, Carmichael J, et al. Efficacy and safety of liposomal anthracyclines in phase I/II clinical trials. Semin Oncol. 2004;31(Suppl 13):S53–S90. doi: 10.1053/j.seminoncol.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 156.Maes H, Rubio N, Garg AD, Agostinis P. Autophagy: shaping the tumor microenvironment and therapeutic response. Trends Mol Med. 2013;19:428–446. doi: 10.1016/j.molmed.2013.04.005. [DOI] [PubMed] [Google Scholar]