Abstract
Estrogen receptors [ERs (subtypes α and β)], classified as a nuclear receptor super family, are intracellular proteins with an important biological role as the transcription factors for estrogen target genes. For ER-induced transcription, an interaction must exist between ligand and coregulators. Coregulators may stimulate (coactivators) or inhibit (corepressors) transcription, following binding with a specific region of the gene, called the estrogen response element. Misbalanced activity of coregulators or higher ligand concentrations may cause increased cell proliferation, resulting in specific types of cancer. These are exhibited as overexpression of ER proteins. Breast cancer currently ranks first in the incidence and second in the mortality of cancer in females worldwide. In addition, 70% of breast tumors are ERα positive and the importance of these proteins for diagnostic use is indisputable. Early diagnosis of the tumor and its classification has a large influence on the selection of appropriate therapy, as ER-positive tumors demonstrate a positive response to hormonal therapy. Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI TOF MS) has been hypothesized to have great potential, as it offers reliable, robust and efficient analysis methods for biomarker monitoring and identification. The present review discusses ER protein analysis by MALDI TOF MS, including the crucial step of protein separation.
Keywords: matrix-assisted laser desorption/ionization time of flight, estrogen receptor, estrogen response element, cancer
1. Introduction
In 2011, breast cancer ranked first in the incidence and mortality of tumor diseases (1). Currently, it is the leading cause of cancer and continues to be the second most common cause of cancer mortality in females worldwide (2). In developed countries, the lower mortality rate is attributed to mammographic screening and advances in adjuvant therapy (3). The most commonly diagnosed breast cancer subtypes are luminal A and B tumors (4,5), together defined as the ER-positive (ER+) and progesterone receptor-positive (PgR+) tumors (6–8). In total, >70% of breast cancers are ER+ (9,10) and, thus, ERs remain the most informative biomarkers in specific subtypes of breast tumors (3,11). The ERs (subtypes α and β) are members of the nuclear receptor family of proteins modulating the expression of genes in response to ligand binding (12–14). ERα expression occurs in bones, the uterus, mammary gland, liver and adipose tissue, whereas ERβ is predominantly expressed in the ovary, mammary gland and intestinal tract. There is also expression of the two subtypes in the brain and cardiovascular system (15). ERs are located in the cell cytoplasm in a complex with the heat shock protein 90 chaperone, which dissociates following ligand binding (16). The ER-ligand complex is translocated into the nucleus, where it interacts with coregulators of transcription and binds to the estrogen response element (ERE) promoter region of a target gene and thereby activates mRNA transcription (17–19). Identification of biomarkers using matrix-assisted laser desorption/ionization (MALDI) is currently of increasing significance and has contributed to rapid advances in metabolomics (20). MALDI may also be a powerful tool for investigation of biomarkers in biological systems, through the direct analysis of thin tissue sections (21), for example ERs in breast tissue. The present review aimed to summarize the evidence for the use of MALDI time of flight mass spectrometry (TOF MS) for the identification of ER proteins in breast cancer tissues.
2. ER proteins and tumor diseases
ERs and cancer
Molecules acting as ER agonists generally exert a stimulatory effect on the proliferation of estrogen-sensitive breast carcinoma cells (12). In human breast cancer, ER+ tumors exhibit an overexpression of ERα as a result of transcription from a promoter inactive in normal breast epithelium. In addition, behavior of ERα depends on the structure of the bound ligand [e.g. estradiol, the most active estrogen (22)] modulating the transcriptional activity of the estrogen responsive genes (18,23). ERα, as a main target in breast cancer, is influenced by a number of types of coregulator following ligand binding, including coactivators and corepressors (24). The balance between coregulators is crucial for regulation of gene transcription by ERα (25). Overexpression of coactivators, for example coactivator-associated arginine methyltransferase 1, may also increase the expression of ERα target genes involved in breast tumor cell differentiation and proliferation (26), including breast cancer (BRCA) 1 and BRCA2 genes (27). In addition, reduction of ERα spliced variant 46 (46 kDa) and 36 (36 kDa) mRNA levels have been observed in colon tumors (28) and overexpression of ERα36 has been observed in gastric (29) and endometrial cancers (30).
Role of ERs in diagnostics
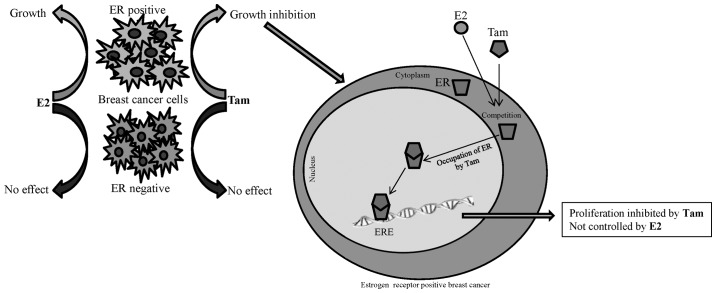
It is well known that ER levels and emplacement of breast tumor metastasis are the fundamental and critical determinants of clinical outcome, with high prognostic values having the greatest impact on patient survival chances (31,32). The importance of ERs as breast carcinoma biomarkers is also due to the ability of the hormone receptor protein to provide detailed information about breast tumor subtype. ER+ breast cancer types exhibit favorable responses to hormone therapy (33–35), for example tamoxifen (36), or to aromatase inhibitors (37), designed to block aberrant signaling within oncogenic pathways (Fig. 1). The use of neoadjuvant chemotherapy for the treatment of ER+ tumors is associated with a major obstacle; chemoresistance (38,39). Hence, the identification of cancer subtypes using protein analysis is likely to enable the treatment effects of chemotherapy to be maximized (40). At present, the most utilized method for ER protein analysis in practice is immunohistochemistry (41–45). Great potential has also been attributed to MALDI TOF MS offering reliable, robust and efficient analysis, renowned for its ease of operation and inexpensive matrixes required for sample preparation, as well as its derivative, surface-enhanced laser desorption/ionization spectrometry (20).
Figure 1.
Schematic representation of hormone treatment action in ER-positive breast cancer cells. E2, estradiol; Tam, tamoxifen; ER, estrogen receptor; ERE, estrogen response element.
3. MALDI TOF as a tool for analysis of ERs
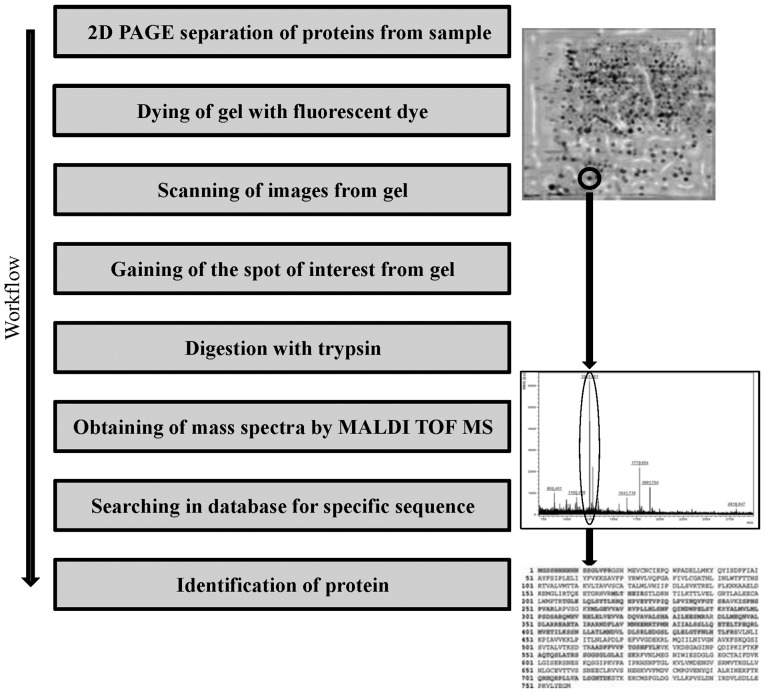
MALDI TOF has been hypothesized to represent one of the most comprehensive and versatile tools for investigation of new biomarkers and protein analysis (46). A key element of the proteomic application of MALDI TOF is the separation of proteins from a sample using two-dimensional gel electrophoresis, prior to subsequent analysis by MS (Fig. 2) (47,48). The MALDI TOF result, exhibited as protein peak spectra, may be quantitatively and statistically evaluated for determination of differential protein expression in response to a particular biological state (49). Nalvarte et al reported an approach for the isolation of ERα from MCF-7 cells based on the natural affinity of ER proteins towards the estrogen response element immobilized on a Sepharose column with subsequent two-dimensional electrophoresis, and identification using MALDI TOF mass spectrometry (50). This method provided a rapid method to identify ERα cofactor and transcription factor recruitment under various conditions. However, it has been hypothesized that the equivalent analysis of ER proteins in clinical samples is likely to be subject to extensive chemical noise that may invalidate results (51). The quantity and identity of biomarkers observed in tissue profiles are also influenced by a number of factors, including the volume of matrix solution used and the sites of laser shots application, used for ionization, which provides charge to molecules and thus enables proper mass detection, which facilitates rendering of the data into spatial distribution maps, or images for the many hundreds of ions measured in the mass spectra (52). A potential problem may be found also in the variability in sample preparation, leading to crystal heterogeneity, and thus to discrimination and suppression of certain signals. There are various approaches to minimize MALDI analysis, including the production of thin films by rapid drying of volatile solvents, or the use of electrospray with the ability to produce thin homogeneous films (51). The lack of further evidence associated with the diagnosis of breast cancer by MALDI TOF analysis of ERs highlights the issues associated with ER protein analysis in real biological samples. However, this method demonstrates excellent results for the visualization of protein expression (46,53), DNA methylation status (54), monitoring of ER interactions (55,56) and in searching for new biomarkers for breast cancer diagnosis (57,58).
Figure 2.
Schematic representation of protein identification in cancer cells by MALDI TOF MS. MALDI TOF MS, matrix-assisted laser desorption/ionization time of flight mass spectrometry.
4. Immunohistochemistry versus MALDI TOF
At present, the most commonly used method for differentiation of breast cancer subtypes is immunohistochemical classification, based on the level of expression of ERs and progesterone receptors (41,42). This method provides relatively accurate results (false negativity, 15.1%), however, it can be time-consuming when analysis of a large number of samples is necessary, requiring sample staining, incubation, application of antibodies and visualization. By contrast, MALDI TOF MS may be useful for the analysis of large amounts of tissue samples. The greatest disadvantage of MALDI MS is the acquisition costs, however, this is balanced by reduced operating costs, reliability, robustness and efficiency. Additionally, ER isolation must be performed using two-dimensional gel electrophoresis (47) or a chromatographic system, in which several issues limit the isolation and proteomic analysis of ER complexes. The greatest of these is the low amount of endogenous ERs complexed with EREs, increasing the requirement for sensitivity of analytical methods used for isolation, and therefore it is necessary to find compromise between protein isolation efficiency and accuracy of the method utilized for its detection (50). However, MALDI may be useful for other applications, for example the monitoring of cancer gene expression (53,59).
5. Conclusions
ER proteins are important for diagnostics and classification of breast tumors subtypes. In particular, the need for identification of the cancer subtype is vital for selection of the appropriate treatment, and to predict the chemoresistance which is commonly noted in ER+ tumors. At present, immunohistochemistry provides good results, however, this technique is laborious. Large diagnostic potential has been attributed to MALDI TOF MS but, due to the relatively recent development and high cost, the use of this application in clinical practice remains uncommon.
Acknowledgements
This study was supported by grants from the Internal Grant Agency of the University of Veterinary and Pharmaceutical Sciences Brno (project 4/2013/FVHE) and MSMT (no. 6215712402).
Abbreviations
- ER
estrogen receptor
- ER+
estrogen receptor positive
- PgR+
progesteron receptor positive
- ERE
estrogen response element
- MALDI TOF
matrix-assisted laser desorption/ionization time of flight
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Patani N, Martin LA, Dowsett M. Biomarkers for the clinical management of breast cancer: international perspective. Int J Cancer. 2013;133:1–13. doi: 10.1002/ijc.27997. [DOI] [PubMed] [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 5.Yang XR, Chang-Claude J, Goode EL, et al. Associations of breast cancer risk factors with tumor subtypes: a pooled analysis from the Breast Cancer Association Consortium studies. J Natl Cancer Inst. 2011;103:250–263. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muss HB. Coming of age: breast cancer in seniors. Oncologist. 2011;16(Suppl 1):S79–S87. doi: 10.1634/theoncologist.2011-S1-79. [DOI] [PubMed] [Google Scholar]
- 7.Ma H, Wang Y, Sullivan-Halley J, et al. Use of four biomarkers to evaluate the risk of breast cancer subtypes in the women’s contraceptive and reproductive experiences study. Cancer Res. 2010;70:575–587. doi: 10.1158/0008-5472.CAN-09-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shubbar E, Helou K, Kovács A, et al. High levels of γ-glutamyl hydrolase (GGH) are associated with poor prognosis and unfavorable clinical outcomes in invasive breast cancer. BMC Cancer. 2013;13:47. doi: 10.1186/1471-2407-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian W, Chen J, He H, Deng Y. MicroRNAs and drug resistance of breast cancer: basic evidence and clinical applications. Clin Transl Oncol. 2013;15:335–342. doi: 10.1007/s12094-012-0929-5. [DOI] [PubMed] [Google Scholar]
- 10.Ombra MN, Di Santi A, Abbondanza C, Migliaccio A, Avvedimento EV, Perillo B. Retinoic acid impairs estrogen signaling in breast cancer cells by interfering with activation of LSD1 via PKA. Biochim Biophys Acta. 2013;1829:480–486. doi: 10.1016/j.bbagrm.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Fasching PA, Heusinger K, Haeberle L, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer. 2011;11:486. doi: 10.1186/1471-2407-11-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharan S, Nikhil K, Roy P. Effects of low dose treatment of tributyltin on the regulation of estrogen receptor functions in MCF-7 cells. Toxicol Appl Pharmacol. 2013;269:176–186. doi: 10.1016/j.taap.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Yan Y, Liu H, Wen H, et al. The novel estrogen receptor GPER regulates the migration and invasion of ovarian cancer cells. Mol Cell Biochem. 2013;378:1–7. doi: 10.1007/s11010-013-1579-9. [DOI] [PubMed] [Google Scholar]
- 14.Oh Y, Chung KC. Zinc finger protein 131 inhibits estrogen signaling by suppressing estrogen receptor α homo-dimerization. Biochem Biophys Res Commun. 2013;430:400–405. doi: 10.1016/j.bbrc.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Komm BS, Mirkin S. Evolution of the tissue selective estrogen complex (TSEC) J Cell Physiol. 2013;228:1423–1427. doi: 10.1002/jcp.24324. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Q, Chang JT, Geradts J, et al. Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer. Breast Cancer Res. 2012;14:R62. doi: 10.1186/bcr3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coughlan N, Thillainadesan G, Andrews J, Isovic M, Torchia J. β-Estradiol-dependent activation of the JAK/STAT pathway requires p/CIP and CARM1. Biochim Biophys Acta-Mol Cell Res. 2013;1833:1463–1475. doi: 10.1016/j.bbamcr.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Sengupta S, Obiorah I, Maximov PY, Curpan R, Jordan VC. Molecular mechanism of action of bisphenol and bisphenol A mediated by oestrogen receptor alpha in growth and apoptosis of breast cancer cells. Br J Pharmacol. 2013;169:167–178. doi: 10.1111/bph.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirman DA, Efuet E, Ding XP, et al. Changes in cancer cell metabolism revealed by direct sample analysis with MALDI mass spectrometry. PLoS One. 2013;8:e61379. doi: 10.1371/journal.pone.0061379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornett DS, Reyzer ML, Chaurand P, Caprioli RM. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat Methods. 2007;4:828–833. doi: 10.1038/nmeth1094. [DOI] [PubMed] [Google Scholar]
- 22.Wang HS, Wu HM, Cheng BH, et al. Functional analyses of endometriosis-related polymorphisms in the estrogen synthesis and metabolism-related genes. PLoS One. 2012;7:e47374. doi: 10.1371/journal.pone.0047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasan S, Nwachukwu JC, Parent AA, et al. Ligand-binding dynamics rewire cellular signaling via estrogen receptor-α. Nat Chem Biol. 2013;9:326–332. doi: 10.1038/nchembio.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aust S, Horak P, Pils D, et al. The prognostic value of estrogen receptor beta and proline-, glutamic acid- and leucine-rich protein 1 (PELP1) expression in ovarian cancer. BMC Cancer. 2013;13:115. doi: 10.1186/1471-2407-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borjesson AE, Farman HH, Engdahl C, et al. The role of activation functions 1 and 2 of estrogen receptor-α for the effects of estradiol and selective estrogen receptor modulators in male mice. J Bone Miner Res. 2013;28:1117–1126. doi: 10.1002/jbmr.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng H, Wu JC, Bedford MT, et al. A TR-FRET-based functional assay for screening activators of CARM1. Chembiochem. 2013;14:827–835. doi: 10.1002/cbic.201300029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meric-Bernstam F, Gutierrez-Barrera AM, Litton J, et al. Genotype in BRCA-associated breast cancers. Breast J. 2013;19:87–91. doi: 10.1111/tbj.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Teng R, Wang Q, et al. Transcriptional analysis of estrogen receptor alpha variant mRNAs in colorectal cancers and their matched normal colorectal tissues. J Steroid Biochem Mol Biol. 2008;112:20–24. doi: 10.1016/j.jsbmb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Li J, Fang R, Xie S, Wang L, Xu C. Expression of ERα36 in gastric cancer samples and their matched normal tissues. Oncol Lett. 2012;3:172–175. doi: 10.3892/ol.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tu BB, Lin SL, Yan LY, Wang ZY, Sun QY, Qiao J. ER-α36, a novel variant of estrogen receptor α, is involved in EGFR-related carcinogenesis in endometrial cancer. Am J Obstet Gynecol. 2011;205:227.e1–e6. doi: 10.1016/j.ajog.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Kammerer M, Gutzwiller S, Stauffer D, Delhon I, Seltenmeyer Y, Fournier B. Estrogen receptor α (ERα) and estrogen related receptor α (ERRα) are both transcriptional regulators of the Runx2-I isoform. Mol Cell Endocrinol. 2013;369:150–160. doi: 10.1016/j.mce.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Gamucci T, Vaccaro A, Ciancola F, et al. Recurrence risk in small, node-negative, early breast cancer: a multicenter retrospective analysis. J Cancer Res Clin Oncol. 2013;139:853–860. doi: 10.1007/s00432-013-1388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME. Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev. 2004;13:1558–1568. [PubMed] [Google Scholar]
- 34.Thrane S, Lykkesfeldt AE, Larsen MS, Sorensen BS, Yde CW. Estrogen receptor α is the major driving factor for growth in tamoxifen-resistant breast cancer and supported by HER/ERK signaling. Breast Cancer Res Treat. 2013;139:71–80. doi: 10.1007/s10549-013-2485-2. [DOI] [PubMed] [Google Scholar]
- 35.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez-Ardila DE, Helmijr JC, Look MP, et al. Hotspot mutations in PIK3CA associate with first-line treatment outcome for aromatase inhibitors but not for tamoxifen. Breast Cancer Res Treat. 2013;139:39–49. doi: 10.1007/s10549-013-2529-7. [DOI] [PubMed] [Google Scholar]
- 37.Hiscox S, Davies EL, Barrett-Lee P. Aromatase inhibitors in breast cancer. Maturitas. 2009;63:275–279. doi: 10.1016/j.maturitas.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Hodgkinson VC, Agarwal V, El Fadl D, et al. Pilot and feasibility study: comparative proteomic analysis by 2-DE MALDI TOF/TOF MS reveals 14-3-3 proteins as putative biomarkers of response to neoadjuvant chemotherapy in ER-positive breast cancer. J Proteomics. 2012;75:2745–2752. doi: 10.1016/j.jprot.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 39.Kim SI, Sohn J, Koo JS, Park SH, Park HS, Park BW. Molecular subtypes and tumor response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Oncology. 2010;79:324–330. doi: 10.1159/000322192. [DOI] [PubMed] [Google Scholar]
- 40.Haddock CL, Holtz B, Senzer N, Nemunaitis J. Applications of HPLC-MALDI-TOF MS/MS phosphoproteomic analysis in oncological clinical diagnostics. Curr Proteomics. 2011;8:153–167. [Google Scholar]
- 41.Tangjitgamol S, Tanvanich S, Srijaipracharoen S, Manusirivithaya S. Expression of estrogen receptor, progesterone receptor, and Her-2/neu in primary and extra-corporeal endometrial cancer. Histol Histopathol. 2013;28:787–794. doi: 10.14670/HH-28.787. [DOI] [PubMed] [Google Scholar]
- 42.Alkner S, Bendahl PO, Grabau D, et al. The role of AIB1 and PAX2 in primary breast cancer: validation of AIB1 as a negative prognostic factor. Ann Oncol. 2013;24:1244–1252. doi: 10.1093/annonc/mds613. [DOI] [PubMed] [Google Scholar]
- 43.Seferina SC, Nap M, van den Berkmortel F, Wals J, Voogd AC, Tjan-Heijnen VC. Reliability of receptor assessment on core needle biopsy in breast cancer patients. Tumour Biol. 2013;34:987–994. doi: 10.1007/s13277-012-0635-5. [DOI] [PubMed] [Google Scholar]
- 44.Wik E, Ræder MB, Krakstad C, et al. Lack of estrogen receptor-α is associated with epithelial-mesenchymal transition and PI3K alterations in endometrial carcinoma. Clin Cancer Res. 2013;19:1094–1105. doi: 10.1158/1078-0432.CCR-12-3039. [DOI] [PubMed] [Google Scholar]
- 45.Kinsella MD, Birdsong GG, Siddiqui MT, Cohen C, Hanley KZ. Immunohistochemical detection of estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 in formalin-fixed breast carcinoma cell block preparations: correlation of results to corresponding tissue block (needle core and excision) samples. Diagn Cytopathol. 2013;41:192–198. doi: 10.1002/dc.21815. [DOI] [PubMed] [Google Scholar]
- 46.Sang QX, Man YG, Sung YM, et al. Non-receptor tyrosine kinase 2 reaches its lowest expression levels in human breast cancer during regional nodal metastasis. Clin Exp Metastasis. 2012;29:143–153. doi: 10.1007/s10585-011-9437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin Y, Zhang X, Lu D, et al. Histopathological and proteomic analysis of hepatic tissue from adult male zebrafish exposed to 17β-estradiol. Environ Toxicol Pharmacol. 2010;29:91–95. doi: 10.1016/j.etap.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Pietrowska M, Marczak L, Polanska J, et al. Mass spectrometry-based serum proteome pattern analysis in molecular diagnostics of early stage breast cancer. J Transl Med. 2009;7:60. doi: 10.1186/1479-5876-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouchal P, Dvorakova M, Scherl A, Garbis SD, Nenutil R, Vojtesek B. Intact protein profiling in breast cancer biomarker discovery: protein identification issue and the solutions based on 3D protein separation, bottom-up and top-down mass spectrometry. Proteomics. 2013;13:1053–1058. doi: 10.1002/pmic.201200121. [DOI] [PubMed] [Google Scholar]
- 50.Nalvarte I, Schwend T, Gustafsson JA. Proteomics analysis of the estrogen receptor alpha receptosome. Mol Cell Proteomics. 2010;9:1411–1422. doi: 10.1074/mcp.M900457-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atsriku C, Benz CC, Scott GK, Gibson BW, Baldwin MA. Quantification of cysteine oxidation in human estrogen receptor by mass spectrometry. Anal Chem. 2007;79:3083–3090. doi: 10.1021/ac062154o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cornett DS, Mobley JA, Dias EC, et al. A novel histology-directed strategy for MALDI-MS tissue profiling that improves throughput and cellular specificity in human breast cancer. Mol Cell Proteomics. 2006;5:1975–1983. doi: 10.1074/mcp.M600119-MCP200. [DOI] [PubMed] [Google Scholar]
- 53.Kabbage M, Trimeche M, Bergaoui S, et al. Calreticulin expression in infiltrating ductal breast carcinomas: relationships with disease progression and humoral immune responses. Tumour Biol. 2013;34:1177–1188. doi: 10.1007/s13277-013-0661-y. [DOI] [PubMed] [Google Scholar]
- 54.Castilla MÁ, Diaz-Martin J, Sarrió D, et al. MicroRNA-200 family modulation in distinct breast cancer phenotypes. PLoS One. 2012;7:e47709. doi: 10.1371/journal.pone.0047709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paramanik V, Thakur MK. Estrogen receptor β and its domains interact with casein kinase 2, phosphokinase C, and N-myristoylation sites of mitochondrial and nuclear proteins in mouse brain. J Biol Chem. 2012;287:22305–22316. doi: 10.1074/jbc.M112.351262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bovet C, Plet B, Ruff M, et al. Towards high-throughput identification of endocrine disrupting compounds with mass spectrometry. Toxicol In Vitro. 2009;23:704–709. doi: 10.1016/j.tiv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Collodoro M, Lemaire P, Eppe G, et al. Identification and quantification of concentration-dependent biomarkers in MCF-7/BOS cells exposed to 17β-estradiol by 2-D DIGE and label-free proteomics. J Proteomics. 2012;75:4555–4569. doi: 10.1016/j.jprot.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 58.Lai TC, Chou HC, Chen YW, et al. Secretomic and proteomic analysis of potential breast cancer markers by two-dimensional differential gel electrophoresis. J Proteome Res. 2010;9:1302–1322. doi: 10.1021/pr900825t. [DOI] [PubMed] [Google Scholar]
- 59.Obazee O, Justenhoven C, Winter S, et al. Confirmation of the reduction of hormone replacement therapy-related breast cancer risk for carriers of the HSD17B1_937_G variant. Breast Cancer Res Treat. 2013;138:543–548. doi: 10.1007/s10549-013-2448-7. [DOI] [PubMed] [Google Scholar]