Abstract
Background
Women are twice as likely as men to suffer from stress-related affective disorders. Corticotropin-releasing factor (CRF) is an important link between stress and mood, in part through its signaling in the serotonergic dorsal raphe (DR). Development of CRF receptor-1 (CRFr1) antagonists has been a focus of numerous clinical trials, but has not yet been proven efficacious. We hypothesized that sex differences in CRFr1 modulation of DR circuits may be key determinants in predicting therapeutic responses and affective disorder vulnerability.
Methods
Male and female mice received DR infusions of the CRFr1 antagonist, NBI 35695, or CRF, and were evaluated for stress responsivity. Sex differences in indices of neural activation (cFos) and co-localization of CRFr1 throughout the DR were examined. Whole-cell patch-clamp electrophysiology assessed sex differences in serotonin neuron membrane characteristics and responsivity to CRF.
Results
Males showed robust behavioral and HPA axis responses to DR infusion of NBI 35695 and CRF, whereas females were minimally responsive. Sex differences were also found for both CRF induced DR cFos and CRFr1 co-localization throughout the DR. Electrophysiologically, female serotonergic neurons showed blunted membrane excitability, and divergent IPSC responses to CRF application.
Conclusions
These studies demonstrate convincing sex differences in CRFr1 activity in the DR, where blunted female responses to NBI 35695 and CRF suggest unique stress modulation of the DR. These sex differences may underlie affective disorder vulnerability and differential sensitivity to pharmacologic treatments developed to target the CRF system, thereby contributing to a current lack of CRFr1 antagonist efficacy in clinical trials.
Keywords: sex, corticotropin releasing factor, dorsal raphe nucleus, serotonin, stress, CRF receptor-1, GABA, parvalbumin
Introduction
Stress-mediated affective disorders such as depression and anxiety show a marked sex disparity, affecting women at nearly twice the rate of men (1, 2). Corticotropin-releasing factor (CRF) represents an important link between stress and mood regulation (3–6). Studies have suggested that stress-induced elevations in CRF contribute to neuropsychiatric disease development through excessive activation of its type 1 receptor, CRFr1 (7–15). Consequently, CRFr1 has received considerable attention as a novel pharmaceutical target for the treatment of stress-related affective disorders; GlaxoSmithKline, Pfizer, Neurocrine Biosciences, DuPont/Bristol-Myers Squibb, and others have developed CRFr1 small molecule antagonists toward this end (recently reviewed in (16)). However, despite compelling results for antidepressant-like and anxiolytic-like effects of these drugs in pre-clinical studies in rodents and nonhuman primates (17–25), none of the CRFr1 antagonists brought to clinical trial over the past decade have successfully completed a Phase III trial (reviewed in (26)).
Considerable evidence supports the involvement of CRFr1 in stress modulation of the serotonergic (5-HTergic) dorsal raphe nucleus (DR) in regulation of mood and affect (27–30). Robust sex differences exist across the stress-serotonin system, where females exhibit greater corticosterone and behavioral (anxiogenic) responses to acute selective 5-HT reuptake inhibitor (SSRI) treatment (31–34). A disruption in the ability of CRF to regulate 5-HT circuits during chronic stress is implicated in affective disorder pathophysiology (35–38). Thus, we hypothesized that sex differences in CRFr1 activation within the DR may contribute, in part, to an increased female predisposition to stress-induced affective disorders, and may underlie disparities between predicted outcomes from preclinical studies and those in clinical trials for CRFr1 antagonists.
Materials and Methods
Subjects
A total of 268 adult male and female littermate mice were used for all experiments. Mice were maintained under a 12-hour light/dark cycle with ad libitum access to food and water. For behavioral experiments and electrophysiological studies, C57Bl/6:129S/J F1 hybrid were obtained from the Jackson Laboratory or bred in house. For CRFr1 colocalization studies, mice with fluorescent-labeled CRFr1 containing neurons were generated as previously described (39). Mice were implanted between ages 7 and 8 weeks, allowd to recovery for at least one week, and behaviorally tested in age-matched cohorts at age 8 to 20 weeks. Mice were singly housed following cannulation to prevent disturbance of the cannulae. For electrophysiological experiments, slices were obtained from mice at 9 to 13 weeks of age. To mimic the housing conditions of behavioral studies, mice were individually housed for 7 to 12 days prior to recording. All studies were conducted in accordance with experimental protocols approved by the University of Pennsylvania Institutional Animal Use and Care Committee, and where applicable, by the Institutional Animal Care and Use Committee of the Weizmann Institute of Science.
Stereotaxic surgery and placement verification
Mice were anesthetized using isofluorane and implanted with a 26-gauge guide cannula (Plastics One, Roanoke, VA) using a stereotaxic instrument (Kopf, Tujunga, CA) positioned 1 mm from the DR using the following coordinates (from brain surface): AP −4.36 mm, ML +1.5 mm, DV −2.0 mm, angled 26 degrees (40). At the end of each study, mice were transcardially perfused and cannula placement was verified based on the termination point of the injector as estimated from the location of scar tissue in 50 μm sections through the DR. Mice with incorrect cannulae placement were dropped from the statistical analysis. Group sizes reported represent the final group size after subjects with incorrect placements were omitted.
Drugs and microinfusion
All drugs were reconstituted in distilled water, aliquotted, and frozen until the day of use. Fresh aliquots were dissolved in ACSF (artificial cerebrospinal fluid, Tocris) immediately prior to behavioral testing. NBI 35695 (Tocris), a highly selective CRFr1 antagonist, was used at 0.44 ng, 1000 times the Ki (41). Ovine CRF (Sigma) was used because of its higher affinity for CRFr1 (42). 1 ng and 50 ng doses were selected based on previous studies of DR infusion of this peptide to preferentially target CRFr1 (43, 44). Drug in 0.25 μL ACSF was infused over 1 min through a microinjector attached to polyethylene tubing connected to a 10 μL Hamilton syringe on an infusion pump (KD Scientific, Holliston, MA). 0.50 μL drug or ACSF was perfused through the microinjector to ensure patency between injections.
Hypothalamic-pituitary-adrenal axis assessment
Testing was performed during a 4-h period beginning 1-h after lights-on. 10 μL tail blood was collected immediately prior to DR infusion, and at 30, 45, 60, and 120 min post injection. Between the 30 and 45 min collections, mice in the NBI 35695 study were restrained in a 50 mL conical tube with a 5-mm air hole. Corticosterone was measured as described previously (45).
Behavioral testing
The tail suspension test (TST) and light-dark box (LD) were performed on separate cohorts of mice 30 min following drug or ACSF infusion. Methods were similar to those described previously (36, 46). Details in Supplement 1.
cFos Immunohistochemistry
To assess CRF-induced neuronal activation in the DR, double labeling immunohistochemistry for cFos and tryptophan hydroxylase (TPH) was performed on DR sections from mice sacrificed 90 min following CRF or ACSF infusion. Methods were similar to those described previously (47, 48). Details in Supplement 1.
Gene expression analysis
Brains were collected from experimentally naive adult male and female mice. Female brains were collected in diestrus. Gene expression of CRFr1, CRFr2, CRF binding protein, TPH2, GABA receptor subunits alpha-1, alpha-2, delta, and gamma-2 were determined by quantitative Taqman real-time PCR as previously described (49, 50). Details in Supplement 1.
Immunofluorescence and CRFr1 Localization
Dual immunofluorescence was performed to detect eGFP and TPH or parvalbumin in DR sections from paraformaldehyde-fixed male and female CRFr1-GFP mice (51). Details in Supplement 1.
Electrophysiology
A modified procedure based on the method of Challis et al. (52) was used. Details in Supplement 1.
Data analysis and statistics
Total corticosterone was analyzed by multivariate ANOVA (drug × time). Behavioral measures were analyzed by two-way ANOVA (sex × drug). Subsequent one-way analyses were performed on data within sex, with Dunnett’s test used to identify significant post-hoc comparisons. Student’s t-test was used to compare gene expression between males and females. To determine CRFr1 counts, a generalized linear mixed model was employed to analyze GFP count × sex × subregion using a Poisson distribution. Assuming a binomial distribution, further analyses were made to predict the likelihood that a given GFP-immunoreactive (-ir) cell co-expressed parvalbumin or TPH. Data are reported as estimated effect size ± 95% confidence intervals. Significance was determined as p < 0.05, with 95% confidence intervals not bounding 0. Statistics were performed in R software. For electrophysiological studies, results of 5-HTergic neuron response to CRF were compared between males and females via two-way rmANOVA and post hoc Tukey tests employing sex and drug (DNQX vs. DNQX + CRF) as the independent variables. Statistics were performed using JMP8 (SAS) software; data are reported as mean ± SEM.
Results
DR infusions of NBI 35695 or CRF preferentially alter male corticosterone production
5-HT output from the DR has modulatory activity on the HPA axis (34, 35). CRF regulation of DR neurons could therefore influence HPA responsiveness. Thus, we assessed the effect of CRFr1 antagonism within the DR on the corticosterone response to restraint stress (Fig. 1). NBI significantly blunted corticosterone levels in males (F(1,9) = 7.085, p = 0.026). The effect of NBI was manifested as a reduction in the rise time from 0 to 30 min prior to restraint (t(9) = 3.191, p = 0.011) and a reduction in total corticosterone produced throughout the course of the experiment (area under the curve, AUC) (t(9) = 2.794, p = 0.021). In females, NBI did not significantly impact corticosterone production (F(1,10) = 0.1180, p = 0.7383). We next tested the effect of CRF infusion on the HPA axis. In males, CRF significantly increased corticosterone (F(1,17) = 5.926, p = 0.026). In females, CRF did not significantly affect corticosterone (F(1,18) = 1.28, p = 0.27).
Figure 1.
Dorsal raphe infusion of the CRFr1 small molecule antagonist NBI 35965 (A–D) or 50 ng CRF (E–H) had male-specific effects on HPA responsiveness. (A, C) Time course of corticosterone response to a 15 min restraint (indicated by shaded region) in males (A) and females (C). (B, D) Area under the curve (AUC) analysis demonstrated a significant reduction of corticosterone output by NBI 35965 in males (B), but not in females (D). (E–H) Corticosterone response to infusion of 50 ng CRF infusion in males (E, F) and females (G, H). AUC analysis demonstrated that CRF enhancement of corticosterone release was specific to males (F). Data are presented as mean values ± SEM (n=8). *, P < 0.05 in comparison to ACSF.
Male-specific effects of DR infusion of NBI 35695 and CRF on stress coping and anxiety-like behaviors
To assess the role of DR CRFr1 in modulating stress coping behavior of male and female mice, the TST was performed 30 min following infusion of NBI 35695 or vehicle (ACSF) (Fig 2). We detected a significant interaction of sex and NBI on latency to become immobile (F(1,37) = 6.654, p = 0.014), where NBI increased latency in males (p = 0.015), but not in females (p = 0.37). There was a trend towards an interaction effect of NBI on immobility in the TST, where NBI reduced immobility in males and increased immobility in females, though this effect failed to reach statistical significance (F(1,40) = 2.651, p = 0.11).
Figure 2.
Males and females show divergent behavioral responses to CRFr1 antagonism (A–D) or CRF (E–H) infusion into the dorsal raphe. (A, B) Effects of CRFr1 antagonist NBI 35965 on total immobile time (A) and latency to first bout of immobility (B) in the tail suspension test (TST). Males showed a greater latency to immobility. (C, D) CRFr1 antagonism had no effect in the light-dark box (LD) on measures of total time spent in the light compartment (C) or latency to first exit from the light compartment (D). (E–H) Behavioral effects of 1 ng and 50 ng CRF infusion demonstrated no effect of the 1 ng dose, but male-specific effects of the 50 ng dose. 50 ng CRF infusion reduced immobile time (E) and increased latency to immobility (F) in males in the TST, and increased both total light time (G) and latency to exit the light (H) in males in the LD. Data are presented as mean values ± SEM (n=8). *, P < 0.05 in comparison to ACSF.
The observed sex difference in response to the CRFr1 antagonist suggested potential sex differences in the response to CRF. To address this possibility, we next assessed the effect of two doses (1 ng, 50 ng) of CRF infusion on behavior in the TST. The 1 ng dose of CRF was ineffective to change immobility or latency to become immobile on either test, suggesting ineffective local concentrations were achieved. The 50 ng dose of CRF decreased immobility (F(2,26) = 3.467, p = 0.046) and increased latency to become immobile (F(2,26) = 8.684, p = 0.001) in males, and was without effect in females.
To assess anxiety-like behavior, mice were tested in the LD. NBI had no effect in either sex on any parameter. However, as with behavioral outcomes in the TST, where 1 ng dose CRF was without effect, 50 ng dose CRF had sex-specific effects on behavior, increasing latency to exit the light compartment (F(2,26) = 5.313, p = 0.011), and total time spent in the light compartment (F(2,26) = 5.024, p = 0.014) in males but not females. In males, 50 ng CRF also reduced the number of transitions between compartments (F(2,26) = 4.915, p = 0.016), but did not affect distance traveled in the light (normalized to time spent in the light), indicating that the animals did not freeze while in the light compartment.
DR infusion of CRF increases cFos in males but decreases cFos in females
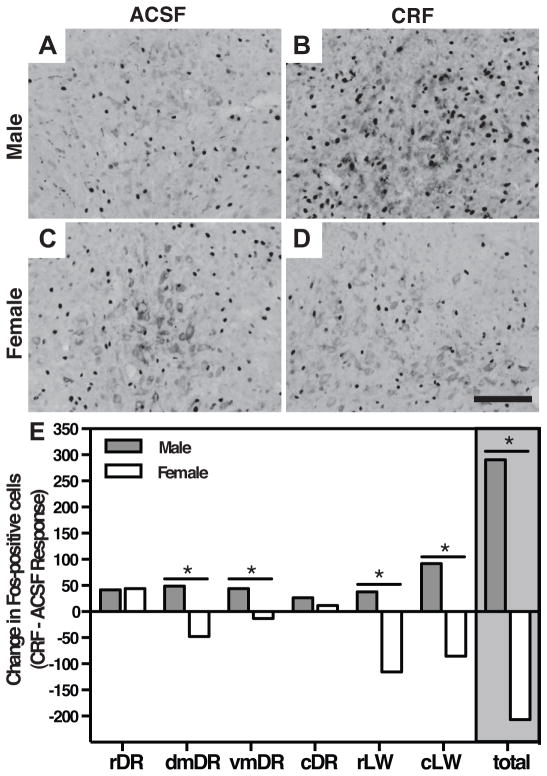
To determine if sex differences in behavioral responsiveness to CRF in the DR were associated with differential patterns of neuronal activation, we next assessed cFos immunoreactivity following infusion of CRF or ACSF (Fig 3, Table 1). In males, CRF increased the number of cFos-positive cells across the DR (F(1,65) = 14.79, p < 0.001), whereas in females, CRF reduced the number of cFos-positive cells (F(1,56) = 7.563, p = 0.008). Subregion analysis indicated that this interaction was present in dorsomedial (F(1,17) = 6.216, p = 0.023), ventromedial (F(1,19) = 5.590, p = 0.029), and lateral wing subregions of the DR (rostral, F(1,24) = 7.300, p = 0.013, caudal, F(1,21) = 7.359, p = 0.013). CRF had a similar effect of increasing cFos-positive cells in both male and female rostral DR (F(1,21) = 12.38, p = 0.002), and no significant main effects were found in the caudal DR.
Figure 3.
A 50 ng CRF infusion elicited differential patterns of neuronal activation in males and females across DR subregions. (A–D) Representative dorsomedial (dmDR) sections show cFos induction 90 min following CRF infusion is enhanced in males (A, B), but reduced in females (C, D). (E) Fos-positive cell counts from DR subregions demonstrate increased counts in males, but reduced counts in females, particularly in dmDR and rostral and caudal lateral wing (rLW, cLW) subregions. Values represent the difference in mean number of Fos-positive cells in sections from mice administered CRF or ASCF. Data represent the difference in averages from 7–9 mice per group. *, P < 0.05.
Table 1.
Clinical trials targeting CRFr1 for the treatment of major depression and anxiety disorders
| Compound | Trial Title | Indication | Sex | Total (M/F) | Phase | Status | Results | Identifier |
|---|---|---|---|---|---|---|---|---|
| 1 NBI-34041 | High-affinity CRF1 receptor antagonist NBI-34041: preclinical and clinical data suggest safety and efficacy in attenuating elevated stress response | Elevated stress response | Male | 24 (24/0) | Phase 2a | Completed | Success | Ising et al. (2007) |
| 2 BMS-562086 (Pexacerfont) | Multi-site, double-blind, placebo-controlled study in major depression | Depression | Female | 271 (0/271) | Phase 1/2 | Completed | Undisclosed | NCT00135421 |
| 2 BMS-562086 (Pexacerfont) | Multi-site, double-blind, placebo-controlled study in generalized anxiety disorder | Anxiety | Female | 260 (0/260) | Phase 2/3 | Completed | Fail | NCT00481325 |
| 3 GSK561679 (Verucerfont) | Multi-site, double-blind, placebo-controlled study in major depression | Depression | Female | 150 (0/150) | Phase 2 | Completed | Undisclosed | NCT00733980 |
| 3 GSK561679 (Verucerfont) | Effects of Corticotropin-Releasing Hormone Receptor 1 (CRH1) Antagonism on Stress-Induced Craving in Alcoholic Women With High Anxiety | Anxiety | Female | 37 (0/37) | Phase 2 | Suspended | Undisclosed | NCT01187511 |
| 4 NBI-30775 (R121919) | Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated | Depression | Both | 24 (13/11) | Phase 2a | Terminated | Elevated liver enzymes | Zobel et al. (2000) |
| 5 CP-316,311 | Multi-site, double-blind, placebo controlled study in major depression | Depression | Both | 28 (17/11) | Phase 2 | Terminated | Fail | NCT00143091 |
| 3 GW876008 (Emicerfont) | A 12 Week Flexible Dose Study of GW876008, Placebo and Active Control (Paroxetine) in the Treatment of Social Anxiety Disorder (SocAD) | Anxiety | Both | 280 (undisclosed) | Phase 2 | Completed | Undisclosed | NCT00397722 |
| 6 ONO-2333Ms | Placebo-Controlled Study of ONO-2333Ms in Patients With Recurrent Major Depressive Disorder | Depression | Both | 278 (undisclosed) | Phase 2 | Completed | Undisclosed | NCT00514865 |
| 7 SSR125543A | A Trial Evaluating the Efficacy and Tolerability of SSR125543 in Outpatients With Major Depressive Disorder | Depression | Both | 580 (undisclosed) | Phase 2 | Completed | Undisclosed | NCT01034995 |
Sponsors:
Neurocrine/GlaxoSmithKline,
Bristol-Myers-Squibb,
GlaxoSmithKline,
Neurocrine/Janssen,
Pfizer,
Ono Pharma USA Inc,
Sanofi-Aventis
CRFr1 is expressed differentially throughout subdivisions of the DR in males and females
We next assessed whether the observed sex differences in responsiveness the CRFr1 antagonist or CRF were due to differences in transcript levels of genes relevant to CRF and 5-HT signaling. We quantified the relative expression of CRFr1, CRFr2, and CRF-binding protein mRNA in DR micropunches from experimentally-naïve male and female mice (Fig 4a). We observed no difference in the mRNA levels of any of these relevant transcripts. As we predict that some of our observed behavioral and physiological differences may be GABA-mediated, we also quantified the relative expression of GABA receptor subunits alpha-1, alpha-2, delta, and gamma-2, which play important roles in receptor kinetics. We observed no sex difference in mRNA in any of these receptor subunits. As has been previously described, we detected differences in TPH2, with females expressing 1.62-fold higher levels relative to males (t(1,8) = 2.652, p = 0.029) (53–55).
Figure 4.
CRFr1 is expressed on different cell populations in males and females. (A) Expression of genes relevant to CRF and serotonergic signaling in the dorsal raphe There were no sex differences in mRNA levels of CRFr1, CRFr2, CRF-binding protein (CRF-BP), GABA receptor (GABR) subunits alpha-1 (α1), α2, delta (δ), or gamma-2 (γ2); however TPH2 was significantly higher in females. Data are presented as fold change relative to the mean value in males ± SEM (n=5). *, P < 0.05 compared to males. (B) Regional, but no sex differences in number of GFP-ir cells throughout subregions of the DR. (C, D) Brain atlas diagrams depict the region viewed in the representative images of (C) the rostral lateral wings (rLW) and (D) the dorsomedial (dm) DR. Atlas diagrams from (49) reprinted with permission from Elsevier. (E–L) Representative, split channel confocal images from the rLW (E, G) and the dmDR (I, K) highlight these regional differences in GFP expression. Sections were dual-labeled with anti-parvalbumin (PV), (F, G) and anti-tryptophan hydroxylase (TPH) (J, K) to identify the phenotype of GFP+ cells in the DR. Arrows denote location of GFP-ir cells enlarged to illustrate co-localization (single arrow, GFP only; double arrow, co-localized). Examples of GFP-ir cell bodies with (left) or without (right) co-localization of PV (H) and TPH (L) show that GFP is transcribed by a subset of both types. (M, N) Probability that a given GFP-ir cell colocalizes with PV (M) or TPH (N). Females presented with an overall reduction in the probability that a given GFP-ir cell colocalizes with PV (N), and reduced likelihood that GFP-ir cells within the rostral DR (rDR) colocalize with TPH. Data are presented as an average probability ± SEM. *, P < 0.05 in comparison to male.
After finding no differences in CRFr1 gene expression, we hypothesized that sex differences in responsivity to CRFr1 antagonist or CRF may be due to differences in the neurotransmitter cell type expressing CRFr1. As current available antibodies are unable to distinguish between CRFr1 and CRFr2, we utilized a CRFr1-GFP transgenic mouse in which GFP is transcribed under the control of the CRFr1 promoter to identify CRFr1 positive neurons in the DR (Fig 4b–n) (39). Sections throughout the DR from these mice were dual labeled for either GFP and parvalbumin to identify putative GABAergic neurons expressing CRFr1, or GFP and TPH to identify serotonergic neurons expressing CRFr1. In accordance with our CRFr1 mRNA data, there were no sex differences in overall number of GFP-ir cells (−0.542 ± −1.59, +0.053; p=0.0796). However, we observed a sex difference in co-localization of GFP-ir cells throughout regions of the DR. In females, the probability that a given GFP-ir cell co-expressed parvalbumin was lower than in males (−2.535 ± −4.819, −0.259; p=0.0291). The probability that a given GFP-ir cell co-expressed TPH was less than 25% in all subdivisions, regardless of sex, except within the rDR, where males displayed significantly more co-localization with TPH than females (−2.16 ± −3.736, −0.583; p=0.007).
5-HT neurons in females demonstrate reduced excitability and a blunted response to CRF
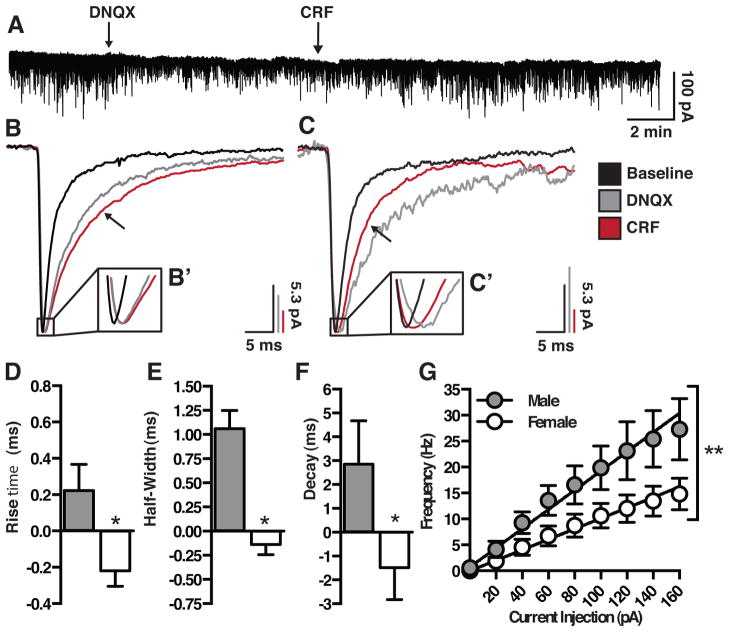
To investigate physiological differences in DR 5-HT neurons of males and females, whole-cell electrophysiological recordings were conducted using current-clamp and voltage-clamp techniques (Fig 5, Table 2). Data from a total of 21 neurons, using current-clamp recordings, were analyzed (9 from 6 male mice, 12 from 7 female mice). Cellular characteristics that were measured included resting membrane potential, resistance, time constant (tau), after hyperpolarization, and action potential amplitude, width, and threshold. Characterization of male and female 5-HT neurons revealed females to have a significantly depolarized resting membrane potential (t(13) = 2.498, p = 0.027) and a reduced tau (t(12) = 2.225, p = 0.046) relative to males. No other membrane characteristics differed between males and females. However, a non-linear regression on the frequency-current (F–I) plot of male and female 5-HT neurons revealed male neurons to have a greater excitability (slope) compared to females (F(1,158) = 8.929, p = 0.003), firing at 27.28 Hz vs. 14.83 Hz, to a maximal injected current of 160 pA.
Figure 5.
Males and females exhibit differences in baseline characteristics, as well as divergent responses to bath applied CRF. (A) Representative IPSC trace. (B–C) Scaled IPSC averaged from a single male (B) and female (C) illustrating the effect of DNQX and CRF on half-width. Insets (B′, C′) illustrate sex difference in effect of CRF on rise time. (D–F) CRF increases rise (D), half-width (E) and decay time (F) in males, and decreases these measures in females. (G) Males exhibit increased excitability compared to females to a series of current injections. Values represent the difference in response of CRF – DNQX. (n= 7–9). *, P < 0.05.
Table 2.
Dorsal raphe nucleus cFos response
| ACSF | CRF (50 ng) | Delta (CRF-ACSF) | Sex Effect | CRF Effect | Interaction | |
|---|---|---|---|---|---|---|
| Rostral | 0.1418 | 0.002 | 0.6555 | |||
| Male (6,7) | 41.333 ± 7.923 | 83.857 ± 14.825 | 42.524 | |||
| Female (6,6) | 29.883 ± 6.161 | 62.667 ± 9.535 | 32.784 | |||
| Dorsomedial | 0.4301 | 0.713 | 0.0233 | |||
| Male (6,6) | 86.833 ± 22.237 | 151.167 ± 26.734 | 64.334 | |||
| Female (4,5) | 120.750 ± 8.958 | 73.200 ± 17.971 | −47.55 | |||
| Ventromedial | 0.5753 | 0.2026 | 0.0289 | |||
| Male (6,6) | 42.667 ± 10.899 | 90.167 ± 17.884 | 47.5 | |||
| Female (5,6) | 65.800 ± 10.874 | 52.333 ± 9.175 | −13.467 | |||
| Caudal | 0.0822 | 0.1358 | 0.5761 | |||
| Male (5,7) | 66.400 ± 12.335 | 91.286 ± 12.718 | 24.886 | |||
| Female (6,5) | 51.500 ± 9.258 | 63.000 ± 11.000 | 11.5 | |||
| rLW | 0.1836 | 0.1472 | 0.0125 | |||
| Male (7,7) | 212.429 ± 24.094 | 250.143 ± 36.476 | 37.714 | |||
| Female (6,8) | 254.167 ± 38.640 | 122.625 ± 26.071 | −131.542 | |||
| cLW | 0.7914 | 0.9231 | 0.013 | |||
| Male (7,7) | 124.714 ± 18.139 | 216.714 ± 36.973 | 92 | |||
| Female (4,7) | 204.750 ± 52.744 | 119.143 ± 25.842 | −85.607 |
Sample sizes vary because some sections were excluded from data analysis due to poor tissue quality
Voltage-clamp recordings of GABAergic IPSCs, isolated by the addition of DNQX, revealed several functional differences between male and female 5-HT neurons. In response to the addition of CRF (30 nM), males and females showed differential changes in the initial first decay time (t(10) = 2.673, p = 0.023), with males showing an increase (+2.148 ± 1.376) and females showing a decrease (−2.380 ± 1.045) compared to DNQX alone. Males and females also exhibited a divergent CRF-mediated change in rise time (t(11) = 2.802, p = 0.0187), and half width (t(11) = 5.994, p < 0.0001); in both measures males showed an increase, while females showed a decrease.
Discussion
Stress-mediated affective disorders display significant sex differences in incidence and treatment efficacy (1, 56). CRFr1 is a key mediator of neuroendocrine and behavioral stress responses, in part through signaling in the 5-HTergic DR (27–30). As there are known sex differences in the DR, dysregulation of 5-HTergic signaling may to contribute to increased disease risk in females (57, 58). Development of CRFr1 small molecule antagonists has been a major focus in clinical trials for more than a decade, but these compounds have been largely unsuccessful. While preclinical studies have predominantly been conducted in males, the majority of clinical trials have either focused on female patient populations, or have been underpowered to evaluate gender differences in study outcomes (Table 1). Therefore, we hypothesized that sex differences in CRFr1 regulation of DR circuits are a key determinant in affective disorder vulnerability, and may be important in predicting therapeutic outcomes.
In our studies, male and female mice received an infusion of the CRFr1 small molecule antagonist, NBI 35695, or one of two doses of CRF directly into the DR, and were evaluated for changes in physiological and behavioral stress responsiveness. NBI 35695 in the DR significantly blunted corticosterone levels in response to a restraint stress only in males. Similarly, CRF infused into the DR in the absence of restraint significantly elevated corticosterone production above the levels induced by intracranial injection only in males (34, 59). The 5-HT system is a known activator of the HPA axis, where selective SSRIs and 5-HT agonists increase corticosterone production during and independent of stress (60). While direct innervation of the PVN has been reported (61–63), 5-HTergic fibers from the DR also heavily innervate the GABAergic neurons of the PVN-surround (64). Therefore, CRF-mediated changes in 5-HT output could modulate this axis through a disinhibition of medial parvocellular neurons. We have previously demonstrated sex differences in this pathway, with females showing a greater corticosterone response to peripheral SSRI administration compared to males (34, 47). Thus, a male-specific response to administration of NBI 35695 and CRF directly into the DR suggests a unique sex-specific mechanism upstream of the PVN, including potential differences in CRFr1 co-localization or signaling within the DR.
In our assessment of behavioral stress coping strategies, including the TST, only males again showed a significant effect of NBI 35695 to increase the latency to immobility, and CRF infusion to reduce the immobile time. While NBI 35695 infusion produced no significant changes in male or female mice in the LD, CRF at the higher dose again increased time spent in the light and escape latency only in males. These findings are consistent with behavioral effects reported in previous studies for systemically administered CRFr1 antagonists and CRFr1 gene deletion, implicating the DR as a key brain region mediating these outcomes (9, 10). Interestingly, in our current studies, both CRF and NBI 35695 infusions produced similar effects in the TST. It may be that interactions with CRFr2, which is also found in the DR, and/or alternate DR projections may account for differences in this behavior (36, 65, 66). As the DR has a heterogeneous cell population involved in the complex regulation of 5-HT neurotransmission throughout the brain, the localization of CRF receptors on different cell types and their recruitment likely determines specificity in 5-HT release (27, 28). Similar paradoxical findings have been reported for other CNS receptor systems involved in complex behaviors (67, 68). In support of our hypothesis, these data demonstrate striking sex differences in stress responses to DR infusion of a CRFr1 antagonist or CRF.
To test whether sex differences in neuronal activation by CRF may underlie sex differences we detected in physiological and behavioral stress responses, we analyzed numbers of cFos positive cells following infusion of the behaviorally effective 50 ng dose of CRF into the DR. No significant sex differences in basal cFos staining following vehicle infusion were detected, suggesting similar basal cellular activation in the DR. However, CRF infusion into the DR resulted in a dramatic increase in the number of cFos positive cells in males, and a paradoxical reduction in females. This lack of an appreciable effect of CRF infusion in females supports the specificity of CRF administration to the DR with minimal diffusion into the cerebral aqueduct, as the latter would be expected to augment corticosterone irrespective of sex (34, 69). These sex differences were most apparent in the dorsomedial and lateral wings of the DR, regions previously implicated in uncontrollable stress and anxiety (28, 70, 71). Immunohistochemical evidence supports a dense CRF innervation of these DR subregions, where CRF fibers primarily contact GABA-containing dendrites (27, 72, 73). Thus, differences in the number of CRF-responsive GABAergic neurons in females could account for the observed reduction in activation detected in these mice.
Within the DR, there is a well-described topographical organization, where GABAergic interneurons exhibit tight control over the tonic and activity-mediated release of 5-HT (27, 28, 74). To test the hypothesis that divergent sex responses to CRF application within the DR may be due to differences in CRFr1 localization on functionally distinct neuronal populations, we used a CRFr1-driven GFP transgenic reporter mouse line to quantify sex differences in co-expression of CRFr1 (39). As the available antibodies for the CRF receptors are known to be of poor quality and lack sufficient specificity, this reporter mouse provided an excellent tool to identify CRFr1-positive neurons in the DR. We quantified sex differences in co-expression of GFP with TPH, a marker of 5-HTergic neurons, or with parvalbumin, a marker of a subset of GABAergic neurons. Parvalbumin was used to mark a GABAergic neurons as 87% of GAD67-ir neurons in the DR co-express parvalbumin (75). These neurons may display some functional differences compared to the broader population of GABAergic neurons, but were selected based on reliable somal immunoreactivity for co-expression analysis with CRFr1 (76). Overall numbers of CRFr1 neurons did not differ between males and females, as indicated by similar numbers of GFP-positive neurons in the DR. This was also confirmed by similar expression levels of CRFr1 mRNA in the DR of males and females. As expected, few GFP-positive neurons were co-expressed with TPH, consistent with previous reports that demonstrate CRF primarily acts on GABAergic neurons, and that CRFr1 shows little expression overlap with 5-HT neurons (77). Surprisingly, females had reduced GFP co-labeling with parvalbumin compared to males. This outcome supports a revised model; rather than females demonstrating greater CRFr1-mediated GABA tone, sex differences in CRF-induced neuronal activation may be due to differences in CRFr1 intracellular signaling, trafficking, or receptor kinetics (78). Such sex differences in CRF signaling have recently been demonstrated in the locus coeruleus (44, 79). Alternatively, CRF may be activating a population of parvalbumin-negative GABA neurons in the female DR.
Based on our observed sex differences in CRFr1 co-expression, we hypothesized that DR 5-HT neurons in males and females may receive differential GABA input in response to CRF. To examine this, we used whole-cell electrophysiological recordings to measure GABAergic IPSCs in 5-HT neurons before and after application of CRF. We observed a striking sex difference in CRF responsivity, where CRF increased IPSC decay time in males, but decreased decay time in females. IPSC decay time can correlate with the number of activated axonal inputs during the IPSC, suggesting sex differences in presynaptic GABAergic input onto 5-HT neurons (80). This is consistent with the differential co-expression of CRFr1 on parvalbumin neurons between males and females. Further, the reduced IPSC half-width in response to CRF exhibited by females also supports this sex difference in the number of GABAergic release sites onto 5-HT neurons. These differences in IPSC kinetics constitute a significant functional difference between males and females that could alter 5-HT neuron excitability and neurotransmission, and thereby influence stress physiological and behavioral measures.
In gathering basal characteristics prior to assessing sex differences in 5-HTergic neuron responses to CRF, we uncovered an unexpected sex difference in 5-HT neuronal excitability. Using whole-cell patch clamp recordings from 5-HT neurons from male and female dmDR slices, we found reduced neuronal excitability in females in response to a series of current injections. In addition to sex differences in CRFr1 localization and GABAergic responses to CRF, this suggests that 5-HT neurons in females require a greater depolarizing stimulus to generate neuronal firing and subsequent 5-HT release, even at baseline. Compared to males, this may translate to 5-HTergic hypofunction in females, an underlying risk factor for the development of affective disorders during stress exposure (81, 82).
Overall, these studies revealed intriguing sex differences in behavioral and physiological effects of CRFr1 antagonist and CRF in the DR, which were mechanistically associated with sex differences in receptor co-expression and divergent electrophysiological responses to CRF in 5-HTergic neurons. The blunted response of females points to a potential explanation for the lack of efficacy in CRFr1 antagonists in clinical trials, which have focused primarily on female participants due to their increased disease prevalence (Table 1). These findings support the importance of identifying sex differences in central stress pathways in order to understand the heightened predisposition of females toward these disorders, and in identifying sex-appropriate, and potentially sex-specific, pharmacological treatments.
Supplementary Material
Table 3a.
Passive cell characteristics
| Sex (n) | Vm rest (mV) | R (mW) | Decay (ms) | AP Thresh (mV) | AP Amp (mV) | AHP Amp (mV) |
|---|---|---|---|---|---|---|
| M (7–9) | −68.41 ± 3.60 | −663.01 ± 102.1 | 28.18 ± 3.93 | −33.57 ± 1.75 | 53.67 ± 5.59 | 26.80 ± 2.82 |
| F (6–10) | −54.78 ± 3.50 | −629.89 ± 78.89 | 31.33 ± 2.95 | −35.02 ± 1.81 | 60.97 ± 7.40 | 25.71 ± 2.56 |
Table 3b.
Active cell characteristics
| Amplitude (pA) | Frequency (Hz) | AUC (pA) | Rise (ms) | Half-Width (ms) | Decay (ms) | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| Male | 20.3.75 ± 2.49 | 4.30 ± 0.69 | 146.39 ± 18.25 | 1.80 ± 0.12 | 3.40 ± 0.31 | 2.69 ± 0.72 |
| Female | 23.60 ± 2.48 | 4.69 ± 1.47 | 131.89 ± 15.01 | 2.07 ± 0.22 | 2.92 ± 0.28 | 1.73 ± 0.19 |
| DNQX | ||||||
| Male | 24.58 ± 2.71 | 0.98 ± 0.21 | 254.44 ± 17.81 | 2.27 ± 0.16 | 7.02 ± 0.85 | 5.29 ± 1.06 |
| Female | 22.26 ± 2.49 | 0.86 ± 0.27 | 216.67 ± 20.70 | 2.65 ± 0.25 | 7.16 ± 0.55 | 6.38 ± 1.56 |
| CRF | ||||||
| Male | 28.75 ± 5.48 | 1.03 ± 0.20 | 340.03 ± 62.35 | 2.49 ± 0.22 | 8.08 ± 0.70 | 8.14 ± 2.81 |
| Female | 27.85 ± 3.58 | 1.48 ± 0.67 | 252.62 ± 26.35 | 2.49 ± 0.24 | 7.03 ± 0.61 | 4.90 ± 0.82 |
| Delta (CRF - DNQX) | ||||||
| Male | 4.17 ± 4.22 | 0.05 ± 0.11 | 85.59 ± 58.52 | 0.22 ± 0.14 | 1.06 ± 0.19 | 2.85 ± 1.83 |
| Female | 5.59 ± 3.48 | 0.62 ± 0.45 | 35.95 ± 9.38 | −0.16 ± 0.10 | −0.14 ± 0.11 | −1.48 ± 1.35 |
Bold indicates significant sex differences based on t-test
Acknowledgments
This study was supported by National Institutes of Health Grant MH073030. We gratefully acknowledge the assistance of Drs. B. A. Rood and C. L. Howerton for their consultation in electrophysiological experiments and data analysis, respectively, and C.M. Powell and C.R. Taylor for their assistance in data collection.
Footnotes
Conflict of Interest Statement
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 4.Sutton RE, Koob GF, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioural activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- 5.Groenink L, Dirks A, Verdouw PM, Schipholt M, Veening JG, van der Gugten J, et al. HPA axis dysregulation in mice overexpressing corticotropin releasing hormone. Biol Psychiatry. 2002;51:875–881. doi: 10.1016/s0006-3223(02)01334-3. [DOI] [PubMed] [Google Scholar]
- 6.Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britton KT, Lee G, Vale W, Rivier J, Koob GF. Corticotropin releasing factor (CRF) receptor antagonist blocks activating and ‘anxiogenic’ actions of CRF in the rat. Brain Res. 1986;369:303–306. doi: 10.1016/0006-8993(86)90539-1. [DOI] [PubMed] [Google Scholar]
- 8.Skutella T, Probst JC, Renner U, Holsboer F, Behl C. Corticotropin-releasing hormone receptor (type I) antisense targeting reduces anxiety. Neuroscience. 1998;85:795–805. doi: 10.1016/s0306-4522(97)00682-9. [DOI] [PubMed] [Google Scholar]
- 9.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 10.Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 11.Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, et al. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci U S A. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- 13.Keck ME, Welt T, Muller MB, Landgraf R, Holsboer F. The high-affinity non-peptide CRH1 receptor antagonist R121919 attenuates stress-induced alterations in plasma oxytocin, prolactin, and testosterone secretion in rats. Pharmacopsychiatry. 2003;36:27–31. doi: 10.1055/s-2003-38092. [DOI] [PubMed] [Google Scholar]
- 14.Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, et al. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol. 2005;509:145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Contarino A, Dellu F, Koob GF, Smith GW, Lee KF, Vale W, et al. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- 16.Paez-Pereda M, Hausch F, Holsboer F. Corticotropin releasing factor receptor antagonists for major depressive disorder. Expert Opin Investig Drugs. 2011;20:519–535. doi: 10.1517/13543784.2011.565330. [DOI] [PubMed] [Google Scholar]
- 17.Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, et al. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- 18.Spina MG, Basso AM, Zorrilla EP, Heyser CJ, Rivier J, Vale W, et al. Behavioral effects of central administration of the novel CRF antagonist astressin in rats. Neuropsychopharmacology. 2000;22:230–239. doi: 10.1016/S0893-133X(99)00108-6. [DOI] [PubMed] [Google Scholar]
- 19.Harro J, Tonissaar M, Eller M. The effects of CRA 1000, a non-peptide antagonist of corticotropin-releasing factor receptor type 1, on adaptive behaviour in the rat. Neuropeptides. 2001;35:100–109. doi: 10.1054/npep.2001.0851. [DOI] [PubMed] [Google Scholar]
- 20.Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, et al. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- 21.Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:625–631. doi: 10.1016/S0278-5846(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen DM, Carey GJ, Gold LH. Antidepressant-like activity of corticotropin-releasing factor type-1 receptor antagonists in mice. Eur J Pharmacol. 2004;499:135–146. doi: 10.1016/j.ejphar.2004.07.091. [DOI] [PubMed] [Google Scholar]
- 23.Lelas S, Wong H, Li YW, Heman KL, Ward KA, Zeller KL, et al. Anxiolytic-like effects of the corticotropin-releasing factor1 (CRF1) antagonist DMP904 [4-(3-pentylamino)-2,7-dimethyl-8-(2-methyl-4-methoxyphenyl)-pyrazolo-[1,5-a]-pyr imidine] administered acutely or chronically at doses occupying central CRF1 receptors in rats. J Pharmacol Exp Ther. 2004;309:293–302. doi: 10.1124/jpet.103.058784. [DOI] [PubMed] [Google Scholar]
- 24.Overstreet DH, Keeney A, Hogg S. Antidepressant effects of citalopram and CRF receptor antagonist CP-154,526 in a rat model of depression. Eur J Pharmacol. 2004;492:195–201. doi: 10.1016/j.ejphar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Jutkiewicz EM, Wood SK, Houshyar H, Hsin LW, Rice KC, Woods JH. The effects of CRF antagonists, antalarmin, CP154,526, LWH234, and R121919, in the forced swim test and on swim-induced increases in adrenocorticotropin in rats. Psychopharmacology (Berl) 2005;180:215–223. doi: 10.1007/s00213-005-2164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koob GF, Zorrilla EP. Update on corticotropin-releasing factor pharmacotherapy for psychiatric disorders: a revisionist view. Neuropsychopharmacology. 2012;37:308–309. doi: 10.1038/npp.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valentino RJ, Liouterman L, Van Bockstaele EJ. Evidence for regional heterogeneity in corticotropin-releasing factor interactions in the dorsal raphe nucleus. J Comp Neurol. 2001;435:450–463. doi: 10.1002/cne.1043. [DOI] [PubMed] [Google Scholar]
- 28.Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- 29.Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18:492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- 30.Oshima A, Flachskamm C, Reul JM, Holsboer F, Linthorst AC. Altered serotonergic neurotransmission but normal hypothalamic-pituitary-adrenocortical axis activity in mice chronically treated with the corticotropin-releasing hormone receptor type 1 antagonist NBI 30775. Neuropsychopharmacology. 2003;28:2148–2159. doi: 10.1038/sj.npp.1300267. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson M, Carlsson A. A regional study of sex differences in rat brain serotonin. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:53–61. doi: 10.1016/0278-5846(88)90061-9. [DOI] [PubMed] [Google Scholar]
- 32.Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A. 1997;94:5308–5313. doi: 10.1073/pnas.94.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEuen JG, Semsar KA, Lim MA, Bale TL. Influence of sex and corticotropin-releasing factor pathways as determinants in serotonin sensitivity. Endocrinology. 2009;150:3709–3716. doi: 10.1210/en.2008-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goel N, Bale TL. Sex differences in the serotonergic influence on the hypothalamic-pituitary-adrenal stress axis. Endocrinology. 2010;151:1784–1794. doi: 10.1210/en.2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- 36.McEuen JG, Beck SG, Bale TL. Failure to mount adaptive responses to stress results in dysregulation and cell death in the midbrain raphe. J Neurosci. 2008;28:8169–8177. doi: 10.1523/JNEUROSCI.0004-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood SK, Zhang XY, Reyes BA, Lee CS, Van Bockstaele EJ, Valentino RJ. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73:1087–1094. doi: 10.1016/j.biopsych.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008;511:479–496. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABA(B) receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J Neurosci. 2010;30:11771–11780. doi: 10.1523/JNEUROSCI.1814-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutton SW, Behan DP, Lahrichi SL, Kaiser R, Corrigan A, Lowry P, et al. Ligand requirements of the human corticotropin-releasing factor-binding protein. Endocrinology. 1995;136:1097–1102. doi: 10.1210/endo.136.3.7867564. [DOI] [PubMed] [Google Scholar]
- 43.Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerber AR, Bale TL. Antiinflammatory treatment ameliorates HPA stress axis dysfunction in a mouse model of stress sensitivity. Endocrinology. 2012;153:4830–4837. doi: 10.1210/en.2012-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 47.Goel N, Plyler KS, Daniels D, Bale TL. Androgenic influence on serotonergic activation of the HPA stress axis. Endocrinology. 2011;152:2001–2010. doi: 10.1210/en.2010-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faulconbridge LF, Grill HJ, Kaplan JM, Daniels D. Caudal brainstem delivery of ghrelin induces fos expression in the nucleus of the solitary tract, but not in the arcuate or paraventricular nuclei of the hypothalamus. Brain Res. 2008;1218:151–157. doi: 10.1016/j.brainres.2008.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. Amsterdam; Boston: Elsevier Academic Press; 2004. Compact. [Google Scholar]
- 50.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci. 2013;33:9003–9012. doi: 10.1523/JNEUROSCI.0914-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sztainberg Y, Kuperman Y, Justice N, Chen A. An anxiolytic role for CRF receptor type 1 in the globus pallidus. J Neurosci. 2011;31:17416–17424. doi: 10.1523/JNEUROSCI.3087-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, et al. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci. 2013;33:13978–13988. doi: 10.1523/JNEUROSCI.2383-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlsson M, Svensson K, Eriksson E, Carlsson A. Rat brain serotonin: biochemical and functional evidence for a sex difference. J Neural Transm. 1985;63:297–313. doi: 10.1007/BF01252033. [DOI] [PubMed] [Google Scholar]
- 54.Rosecrans JA. Differences in brain area 5-hydroxytryptamine turnover and rearing behavior in rats and mice of both sexes. Eur J Pharmacol. 1970;9:379–382. doi: 10.1016/0014-2999(70)90239-6. [DOI] [PubMed] [Google Scholar]
- 55.Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Khan A, Brodhead AE, Schwartz KA, Kolts RL, Brown WA. Sex differences in antidepressant response in recent antidepressant clinical trials. J Clin Psychopharmacol. 2005;25:318–324. doi: 10.1097/01.jcp.0000168879.03169.ce. [DOI] [PubMed] [Google Scholar]
- 57.Dominguez R, Cruz-Morales SE, Carvalho MC, Xavier M, Brandao ML. Sex differences in serotonergic activity in dorsal and median raphe nucleus. Physiol Behav. 2003;80:203–210. doi: 10.1016/j.physbeh.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 58.Felton TM, Auerbach SB. Changes in gamma-aminobutyric acid tone and extracellular serotonin in the dorsal raphe nucleus over the rat estrous cycle. Neuroendocrinology. 2004;80:152–157. doi: 10.1159/000082356. [DOI] [PubMed] [Google Scholar]
- 59.Kim DH, Jung JS, Song DK, Suh HW, Huh SO, Kim YH. Intracerebroventricular injection-induced increase in plasma corticosterone levels in the mouse: a stress model. J Pharmacol Toxicol Methods. 1998;39:71–73. doi: 10.1016/s1056-8719(97)00105-6. [DOI] [PubMed] [Google Scholar]
- 60.Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, et al. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci. 2007;27:6956–6964. doi: 10.1523/JNEUROSCI.2584-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrov T, Krukoff TL, Jhamandas JH. Chemically defined collateral projections from the pons to the central nucleus of the amygdala and hypothalamic paraventricular nucleus in the rat. Cell Tissue Res. 1994;277:289–295. doi: 10.1007/BF00327776. [DOI] [PubMed] [Google Scholar]
- 62.Petrov T, Krukoff TL, Jhamandas JH. The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. J Comp Neurol. 1992;318:18–26. doi: 10.1002/cne.903180103. [DOI] [PubMed] [Google Scholar]
- 63.Williamson M, Viau V. Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J Comp Neurol. 2007;503:717–740. doi: 10.1002/cne.21411. [DOI] [PubMed] [Google Scholar]
- 64.Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983;277:355–360. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- 65.Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, et al. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neufeld-Cohen A, Kelly PA, Paul ED, Carter RN, Skinner E, Olverman HJ, et al. Chronic activation of corticotropin-releasing factor type 2 receptors reveals a key role for 5-HT1A receptor responsiveness in mediating behavioral and serotonergic responses to stressful challenge. Biol Psychiatry. 2012;72:437–447. doi: 10.1016/j.biopsych.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dragatsis I, Zioudrou C, Gerozissis K. Specific delta-opioid antagonists exert an agonist-independent inhibitory effect, similar to the agonist, on the release of GnRH in vitro. Cell Mol Neurobiol. 1995;15:389–400. doi: 10.1007/BF02071875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alleweireldt AT, Weber SM, Kirschner KF, Bullock BL, Neisewander JL. Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2002;159:284–293. doi: 10.1007/s002130100904. [DOI] [PubMed] [Google Scholar]
- 69.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 70.Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 71.Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- 72.Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waselus M, Valentino RJ, Van Bockstaele EJ. Ultrastructural evidence for a role of gamma-aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J Comp Neurol. 2005;482:155–165. doi: 10.1002/cne.20360. [DOI] [PubMed] [Google Scholar]
- 74.Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- 75.Shikanai H, Yoshida T, Konno K, Yamasaki M, Izumi T, Ohmura Y, et al. Distinct neurochemical and functional properties of GAD67-containing 5-HT neurons in the rat dorsal raphe nucleus. J Neurosci. 2012;32:14415–14426. doi: 10.1523/JNEUROSCI.5929-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McKenna JT, Yang C, Franciosi S, Winston S, Abarr KK, Rigby MS, et al. Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse. J Comp Neurol. 2013;521:1225–1250. doi: 10.1002/cne.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, et al. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bangasser DA. Sex differences in stress-related receptors: “micro” differences with “macro” implications for mood and anxiety disorders. Biol Sex Differ. 2013;4:2. doi: 10.1186/2042-6410-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valentino RJ, Bangasser D, Van Bockstaele EJ. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol Pharmacol. 2013;83:737–745. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balakrishnan V, Kuo SP, Roberts PD, Trussell LO. Slow glycinergic transmission mediated by transmitter pooling. Nat Neurosci. 2009;12:286–294. doi: 10.1038/nn.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kapitany T, Schindl M, Schindler SD, Hesselmann B, Fureder T, Barnas C, et al. The citalopram challenge test in patients with major depression and in healthy controls. Psychiatry Res. 1999;88:75–88. doi: 10.1016/s0165-1781(99)00082-7. [DOI] [PubMed] [Google Scholar]
- 82.Van de Kar LD. Neuroendocrine aspects of the serotonergic hypothesis of depression. Neurosci Biobehav Rev. 1989;13:237–246. doi: 10.1016/s0149-7634(89)80056-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.