Abstract
Purpose
Aseptic loosening of the tibial component remains a limitation to the highly successful procedure of total knee arthroplasty (TKA). Pulsed lavage improves bone cement penetration and interface strength in tibial tray cementation. This study tested whether pressurized cement application with a cement gun can compensate the use of jet lavage for bone surface preparation.
Methods
Tibial components were implanted in six pairs of cadaveric tibiae. On one side, pulsed lavage of the tibial bone was combined with finger packing of bone cement; on the other side, syringe lavage and gun cementing was used. Cement penetration into the bone was determined from computed tomography scans, and Interface strength was determined by pull-out testing.
Results
Cement penetration was greater (p = 0.004) and interface strength was higher (p = 0.028) in the pulsed lavage group.
Conclusion
Pressurization of cement by gun application could not compensate for the omission of pulsed lavage. Thus, pulsed lavage should be considered a crucial factor in TKA to improve implant fixation, which cannot be compensated for by cement application technique.
Keywords: Total knee arthroplasty, Cementing technique, Interface strength, Cement gun, Bone cement penetration, Pulsed lavage
Introduction
Total knee arthroplasty (TKA) is a highly successful procedure for treating osteoarthritis of the knee, with large registries reporting an overall revision rate of 10 % over 15 years [10]. Cemented fixation is the gold standard in TKA, and cementless designs show inferior success [7]. As shown in total hip arthroplasty (THA), cementing technique is an important factor in improving initial stability and interface strength at the time of surgery and could improve long-term implant survival. However, there is a lack of general recommendations regarding technical details, and controversy surrounds the methods of cement administration to the bone surface, particularly on the tibial side [3]. Various authors advocate application of a cementing gun to improve bone-cement penetration into the tibial bone [2, 13]. This technique was proposed to improve cement penetration and consistency [2, 13]. However, concerns remain that the technique could lead to overpenetration of cement, with subsequent thermal damage to the cancellous bone [9]. Pulsed lavage (PL) significantly improved interface strength and cement penetration compared wtih syringe lavage (SL) in a cadaveric investigation [19]. A survey of cementing techniques in TKA revealed that only 68 % of surgeons questioned routinely used PL [12]. There is concern that pressurisation techniques could be applied to substitute or compensate for the omission of pulsatile lavage (PL) for surface preparation. As financial pressure on hospitals increases, there may be the danger of avoiding PL systems. To date, there is no information in the literature addressing the question of whether pressurization without respective surface preparation can achieve equivalent tibial tray fixation. The purpose of this study was therefore to compare finger packing of bone cement in combination with PL to gun application combined with syringe lavage. Comparison was made in terms of fixation strength and cement penetration in a biomechanical setting using cadaveric human tibiae.
Materials and methods
In this study 12 paired human cadaveric tibiae were tested. The specimens were fresh frozen and stored at −27 °C. They were thawed overnight before experiments were carried out. Mean donor age was 71 years [standard deviation (SD) = 2.9 years)] Prior to experimental preparation, computed tomography (CT) scans were made. Osseous abnormalities were ruled out, and bone mineral density (BMD) was assessed in the proximal region near the tibial surface using a normal calibration (Mx8000, Philips, Best, The Netherlands). Analysis is described in detail elsewhere [19]. In all cases, PFC Sigma® MBT Keeled tibial components were implanted (PFC Sigma® Knee System, DePuy Orthopaedics, Warsaw, IN, USA). This implant is a cobalt chromium mobile-bearing design. To facilitate pullout testing on a servohydraulic machine, the tip of the stem was removed and replaced by an identically shaped screw, allowing fixation of a custom-made pull-out adapter (Fig. 1).
Fig. 1.
Tibial tray modification for testing
All surgical procedures were carried out following the manufacturer’s guidelines using a 0° slope cutting block and related punches (DePuy). Correctly sized implants were used, ranging from sizes 2.5 to 5. Following randomization, one side of each tibia pair underwent PL using 1,800 ml normal saline. The corresponding side was treated with syringe lavage (SL) using the identical fluid volume. The surfaces were then thoroughly dried. A surface cementing technique leaving the stem cementless was used in all cases to simplify cement-penetration analysis. Bone cement (SmartMix Cemvac 40 g, DePuy) was vacuum mixed for 30 seconds, with a subsequent waiting phase of 120 seconds to reach the sticky phase prior to application to the bone. For SL specimens, the tube of the mixing system was loaded into a cement gun. Application to the bone was performed under constant pressurization, with a short nozzle being placed directly onto the tibial bone surface at defined spots around the stem channel while ensuring that the entire surface was covered (Fig. 2). For PL specimens, an appropriate volume of cement was hand pressurized on the bone surface covering a similar area as in the gun cemented group. All trays were then impacted by ten mallet blows. Immediately afterwards, a steel mass was attached to allow cement curling under a constant load of 50 N.
Fig. 2.
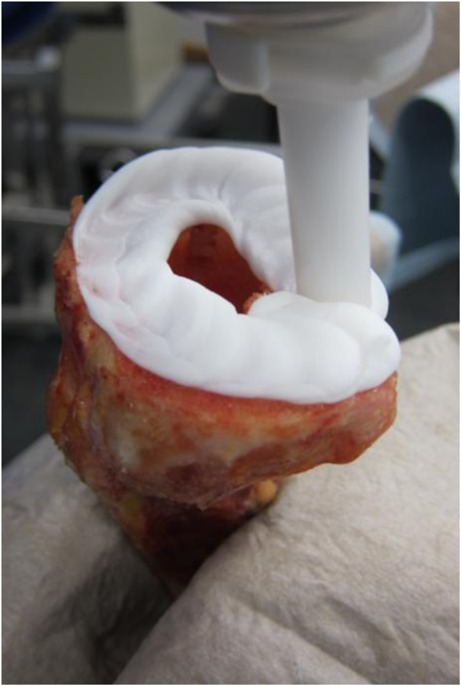
Gun cementation
As the focus of this study was the strength of the cement–bone interface, failure of that interface was to be prevented. To achieve this, a release agent was applied to the tray undersurface prior to implantation. After cement curing, the tray was removed manually, cleaned using acetone and then reattached to the cement mould using industrial polymethylmethacrylate (PMMA) glue (Pattex® Stabilit Express, Henkel AG & Co., Düsseldorf, Germany). Implant removal also enabled CT scanning of the specimens with the implant ex situ, thus providing scans without metal artefacts. These CT scans were processed using image analysis software to evaluate the cement mantle morphology (Avizo® 7, VSG, Burlington, MA, USA, and MATLAB®, The MathWorks Inc., Natick, MA, USA). Cement layer thickness (between implant undersurface and tibial bone surface) and cement penetration (from tibial surface into trabecular bone) were differentiated. The resolution was 0.6 mm in all spatial directions.
For mechanical testing, all samples were embedded distal to the tibial tuberosity using PMMA (Technovit® 4004, Heraeus Kulzer GmbH, Wehrheim Ts., Germany). A materials testing machine was used for pullout testing under displacement control at a rate of 0.5 mm/s (MTS 858, MTS Systems, Eden Prairie, MN, USA). Maximum forces to failure were recorded, with a sampling frequency of 50 Hz.
Statistical group comparisons regarding pullout strength, median cement layer thickness and median penetration depth was done using a t test for paired observations or Wilcoxon matched-pair signed-rank test, depending on data distribution (PASW 18, SPSS Inc./IBM Corporation, Armonk, NY, USA). BMD as a structural parameter was controlled for by the paired design but also compared between groups by one-way analysis of variance (ANOVA). A type I error probability of 5 % was used. Continuous data were described by mean and SD or, if indicated, by median and range.
Results
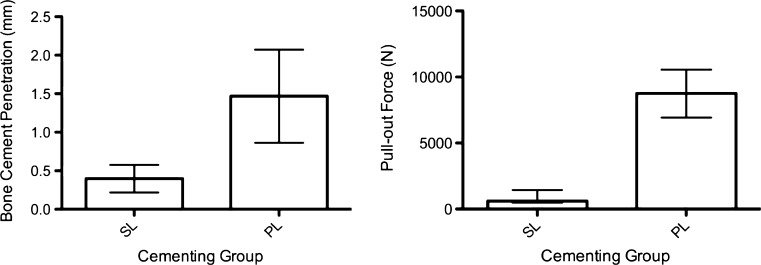
Mean BMD was similar between groups (PL 98 mg/cm3, SD = 27 mg/cm3; SL 104 mg/cm3, SD = 30 mg/cm3; p = 0.730, one-way ANOVA) (Table 1). Mean bone cement penetration was 1.47 mm (SD = 0.60 mm) in the PL group and 0.40 mm (SD = 0.18 mm) in the SL group (p = 0.004, paired t test) (Figs. 3, 4 and 5). Median bone cement layer thickness was 1.24 mm (range 0.92–2.57 mm) in the PL group compared with 2.60 mm (1.69–3.11 mm) in the SL group (p = 0.028, Wilcoxon).
Table 1.
Specimen data summary and results. All donors were male
| Specimen | Age | Pulsatile lavage | BMD (mg/cm3) | Pullout force (N) | Penetration (mm) | Thickness (mm) |
|---|---|---|---|---|---|---|
| 1R | 73 | No | 110 | 1,088 | 0.55 | 2.52 |
| 1L | 73 | Yes | 104 | 10,337 | 2.36 | 0.92 |
| 2R | 68 | Yes | 112 | 9,371 | 1.54 | 1.19 |
| 2L | 68 | No | 118 | 495 | 0.31 | 2.67 |
| 3R | 69 | Yes | 142 | 8,150 | 1.41 | 1.28 |
| 3L | 69 | No | 154 | 517 | 0.20 | 2.14 |
| 4R | 76 | Yes | 78 | 6,934 | 0.46 | 2.57 |
| 4L | 76 | No | 79 | 608 | 0.27 | 3.11 |
| 5R | 71 | No | 85 | 613 | 0.67 | 1.69 |
| 5L | 71 | Yes | 83 | 7,320 | 1.55 | 1.19 |
| 6R | 69 | No | 77 | 1,442 | 0.38 | 2.69 |
| 6L | 69 | Yes | 69 | 10,550 | 1.49 | 1.40 |
Fig. 3.
Cement penetration depths (left) and pull-out forces (right). For penetration, mean and standard deviation (SD) is displayed; pull-out force is displayed as median and range due to nonparametric distribution. SL syringe lavage and gun cementing, PL pulsed lavage and finger packing
Fig. 4.
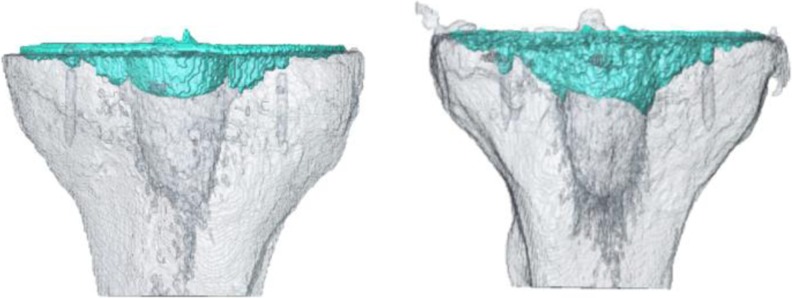
Three-dimensional reconstruction of bone-cement mantle for a typical specimen pair, left SL side, right PL side
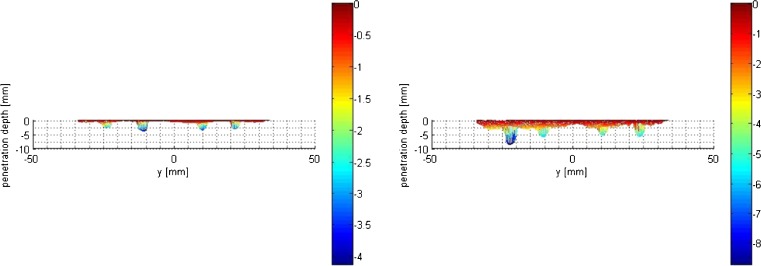
Fig. 5.
Anterior-posterior projection of cement penetration depth extracted from 3D reconstructions shown in Fig. 4. Stem and keel geometry were removed for penetration depth and thickness determination. Left SL side, right PL side
Mechanical testing revealed a median pull-out force of 8,760 N (range 6,934–10,550 N) for PL and 611 N (range 495–1,442 N) for SL (p = 0.028, Wilcoxon) (Fig. 3). Four of six specimens in the PL group failed at the cement–bone interface. The remaining two failed at the implant–cement interface. Five specimens in the SL group failed at the cement–bone interface and one at the implant–cement interface.
Discussion
The introduction of modern cementing techniques has contributed to reduced loosening rates in THA, but this has not been observed for TKA [17]. There is evidence that thorough surface preparation using PL improves bone cement penetration and thus, initial fixation strength, in TKA [5, 14, 19]. In a German survey investigating cementing techniques in THA, only 66.5 % of respondents used PL as a standard procedure [6]. Assuming these numbers transfer to TKA, there is almost no improvement when compared with an earlier Australian survey analysing cementing technique in TKA [12]. Considering that a third of surgeons omit PL in TKA or use other or no surface preparation techniques, it might be tempting to use cement pressurization techniques to improve cement penetration.
Pressurization of cement into the bone surface using a cement gun has been described as a useful technique to achieve improved penetration [11, 13]. In contrast, no significant advantage regarding penetration could be detected in a porcine model using gun cementing. However, the technique has been proposed to result in a more reproducible cement mantle by eliminating local variations in penetration [2]. In a clinical study, PL and gun cementing resulted in mean bone cement penetration depths of 5 mm [13]. In an artificial bone model (Sawbone), similar mean penetration depths of 5 mm were reported when pressurizing cement [20]. Those penetration depths seem considerably higher when compared with the values in our study. The greatest median penetration depth observed in this study was 2.36 mm, which challenges results of earlier studies, in which a minimum penetration of 3–4 mm was observed and advocated [21]. As most recommendations are based on plain radiographs, care should be taken when interpreting the cement geometry. The transition of bone cement under the tibial tray when penetrating the cancellous bone is continuous and thus hinders the assessment of isolated penetration depths. Summing the median cement-layer thickness and penetration depth for each specimen in this study reveals a total cement thickness of 2.87 mm in the PL group and 2.89 mm in SL group, which matches results of other authors [1, 13, 20]. However, cement layer thickness under the tibial plateau has a minor impact on initial fixation strength in contrast to bone penetration.
Pull-out testing showed an increase in force to failure by a factor of >13 using PL. As bone cement penetration was 1.47 mm in the PL group, there is some doubt that former recommendations stating that penetration depths <2 mm could lead to reduced interface strength are justified [21]. Cement penetration in the SL group was 0.40 mm, which is unacceptable and emphasizes the role of surface preparation. The different bone-failure patterns for the PL and SL groups indicate different failure mechanisms, probably caused by penetration depth and extent of interdigitation with the trabeculae. The lower penetration depth in the SL group was accompanied by a cement layer thickness almost double that of the PL group. This indicates that the bone cement rather remains on the surface due to insufficient surface preparation. A median penetration of ~1.5 mm is deemed to be sufficient from a biomechanical view in this in vitro scenario.
The pull-out testing we used is a biomechanical simplification to estimate strength at the cement interfaces, as this is difficult to assess under axial or shear loading. An increase in tensile strength is proposed here to decrease micromotion under compression and shear, which should improve long-term implant performance [15, 21]. It has to be acknowledged that three implants failed at the implant–cement interface. The measured strength was compared with the respective side of the pair with cement–bone interface failure, although comparability of the retrieved data could be debated. However, if an implant fails at the implant interface, it can be assumed that failure strength at the cement–bone interface is equal or even higher. Considering this fact, the difference may have been even more distinct if all specimens failed at the bone interface—even though one implant interface failure occurred in the SL group. For this reason, it was decided to keep the retrieved values for strength assessment in the analysis. A surface cementing technique applying bone cement on the tibial surface, leaving the stem cementless, was chosen for this study. However, controversy exists regarding initial fixation strength and metaphyseal stress distribution for any of those techniques, although both are still in clinical use [4, 8, 18]. Finally, good clinical results indicate the suitability of baseplate cementation in this study’s experimental setting [8, 16].
Our study demonstrates that PL combined with finger packing improves bone cement penetration by a factor four and interface strength by a factor of almost 12 when compared with SL combined with pressurizing-gun cementing. This emphasises the role of PL as an important preparation step. Cement gun pressurization alone does not achieve sufficient tray fixation. Use of finger packing combined with PL is warranted based on results of this study. PL must be considered a crucial factor to improve tibial implant fixation in TKA, which cannot be compensated for by cement application technique.
Acknowledgments
Financial support for this study was received from DePuy Orthopaedics.
References
- 1.Askew MJ, Steege JW, Lewis JL, Ranieri JR, Wixson RL. Effect of cement pressure and bone strength on polymethylmethacrylate fixation. J Orthop Res. 1984;1:412–420. doi: 10.1002/jor.1100010410. [DOI] [PubMed] [Google Scholar]
- 2.Bauze AJ, Costi JJ, Stavrou P, Rankin WA, Hearn TC, Krishnan J, Slavotinek JP. Cement penetration and stiffness of the cement-bone composite in the proximal tibia in a porcine model. J Orthop Surg (Hong Kong) 2004;12:194–198. doi: 10.1177/230949900401200211. [DOI] [PubMed] [Google Scholar]
- 3.Cawley DT, Kelly N, McGarry JP, Shannon FJ. Cementing techniques for the tibial component in primary total knee replacement. Bone Joint J. 2013;95-B:295–300. doi: 10.1302/0301-620X.95B3.29586. [DOI] [PubMed] [Google Scholar]
- 4.Chong DY, Hansen UN, van der Venne R, Verdonschot N, Amis AA. The influence of tibial component fixation techniques on resorption of supporting bone stock after total knee replacement. J Biomech. 2011;44:948–954. doi: 10.1016/j.jbiomech.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Dorr LD, Lindberg JP, Claude-Faugere M, Malluche HH (1984) Factors influencing the intrusion of methylmethacrylate into human tibiae. Clin Orthop Relat Res 147–152 [PubMed]
- 6.Fischer CA, Kaszap B, Drexler C, Lehner B, Clarius M. Cemented total hip arthroplasty in Germany - update 2010. Z Orthop Unfall. 2012;150:309–317. doi: 10.1055/s-0031-1298261. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi R, Tsvetkov D, Davey JR, Mahomed NN. Survival and clinical function of cemented and uncemented prostheses in total knee replacement: a meta-analysis. J Bone Joint Surg (Br) 2009;91:889–895. doi: 10.1302/0301-620X.91B7.21702. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann AA, Goldberg TD, Tanner AM, Cook TM. Surface cementation of stemmed tibial components in primary total knee arthroplasty: minimum 5-year follow-up. J Arthroplasty. 2006;21:353–357. doi: 10.1016/j.arth.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Huiskes R, Sloof TJ. Thermal injury of cancellous bone following pressured penetration of acrylic cement. Trans Orthop Res Soc. 1981;6:134. [Google Scholar]
- 10.Knutson K, Lewold S, Robertsson O, Lidgren L. The Swedish knee arthroplasty register. A nation-wide study of 30,003 knees 1976-1992. Acta Orthop Scand. 1994;65:375–386. doi: 10.3109/17453679408995475. [DOI] [PubMed] [Google Scholar]
- 11.Labutti RS, Bayers-Thering M, Krackow KA. Enhancing femoral cement fixation in total knee arthroplasty. J Arthroplasty. 2003;18:979–983. doi: 10.1016/S0883-5403(03)00450-9. [DOI] [PubMed] [Google Scholar]
- 12.Lutz MJ, Halliday BR. Survey of current cementing techniques in total knee replacement. ANZ J Surg. 2002;72:437–439. doi: 10.1046/j.1445-2197.2002.02449.x. [DOI] [PubMed] [Google Scholar]
- 13.Lutz MJ, Pincus PF, Whitehouse SL, Halliday BR. The effect of cement gun and cement syringe use on the tibial cement mantle in total knee arthroplasty. J Arthroplasty. 2009;24:461–467. doi: 10.1016/j.arth.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Majkowski RS, Miles AW, Bannister GC, Perkins J, Taylor GJ. Bone surface preparation in cemented joint replacement. J Bone Joint Surg (Br) 1993;75:459–463. doi: 10.1302/0301-620X.75B3.8496223. [DOI] [PubMed] [Google Scholar]
- 15.Ritter MA, Herbst SA, Keating EM, Faris PM. Radiolucency at the bone-cement interface in total knee replacement. The effects of bone-surface preparation and cement technique. J Bone Joint Surg Am. 1994;76:60–65. doi: 10.2106/00004623-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Rossi R, Bruzzone M, Bonasia DE, Ferro A, Castoldi F. No early tibial tray loosening after surface cementing technique in mobile-bearing TKA. Knee Surg Sports Traumatol Arthrosc. 2010;18:1360–1365. doi: 10.1007/s00167-010-1177-2. [DOI] [PubMed] [Google Scholar]
- 17.Russotti GM, Coventry MB, Stauffer RN (1988) Cemented total hip arthroplasty with contemporary techniques. A five-year minimum follow-up study. Clin Orthop Relat Res 141–147 [PubMed]
- 18.Ryd L, Hansson U, Blunn G, Lindstrand A, Toksvig-Larsen S. Failure of partial cementation to achieve implant stability and bone ingrowth: a long-term roentgen stereophotogrammetric study of tibial components. J Orthop Res. 1999;17:311–320. doi: 10.1002/jor.1100170304. [DOI] [PubMed] [Google Scholar]
- 19.Schlegel UJ, Siewe J, Delank KS, Eysel P, Puschel K, Morlock MM, de Uhlenbrock AG. Pulsed lavage improves fixation strength of cemented tibial components. Int Orthop. 2011;35:1165–1169. doi: 10.1007/s00264-010-1137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanlommel J, Luyckx JP, Labey L, Innocenti B, De Corte R, Bellemans J. Cementing the tibial component in total knee arthroplasty which technique is the best? J Arthroplasty. 2010;26:492–496. doi: 10.1016/j.arth.2010.01.107. [DOI] [PubMed] [Google Scholar]
- 21.Walker PS, Soudry M, Ewald FC, McVickar H (1984) Control of cement penetration in total knee arthroplasty. Clin Orthop Relat Res 155–164 [PubMed]