Abstract
Purpose
Our aim was to explore the effect of varying in vitro culture conditions on general chondrogenesis of minced cartilage (MC) fragments.
Methods
Minced, fibrin-associated, bovine articular cartilage fragments were cultured in vitro within polyurethane scaffold rings. Constructs were maintained either free swelling for two or four weeks (control), underwent direct mechanical knee-joint-specific bioreactor-induced dynamic compression and shear, or they were maintained free swelling for two weeks followed by two weeks of bioreactor stimulation. Samples were collected for glycosaminoglycan (GAG)/DNA quantification; collagen type I, collagen type II, aggrecan, cartilage oligomeric matrix protein (COMP), proteoglycan-4 (PRG-4) messenger RNA (mRNA) analysis; histology and immunohistochemistry.
Results
Cellular outgrowth and neomatrix formation was successfully accomplished among all groups. GAG/DNA and collagen type I mRNA were not different between groups; chondrogenic genes collagen type II, aggrecan and COMP revealed a significant downregulation among free-swelling constructs over time (week two through week four). Mechanical loading was able to maintain chondrogenic expression with significantly stronger expression at long-term time points (four weeks) in comparison with four-week control. Histology and immunohistochemistry revealed that bioreactor culture induced stronger cellular outgrowth than free-swelling constructs. However, weaker collagen type II and aggrecan expression with an increased collagen type I expression was noted among this outgrowth neotissue.
Conclusions
The method of MC culture is feasible under in vitro free-swelling and dynamic loading conditions, simulating in vivo posttransplantation. Mechanical stimulation significantly provokes cellular outgrowth and long-term chondrogenic maturation at the mRNA level, whereas histology depicts immature neotissue where typical cartilage matrix is expected.
Keywords: Minced cartilage, Bioreactor, Scaffold, Fibrin, Cartilage repair
Introduction
In order to maintain typical benefits of autologous chondrocyte implantation (ACI), mainly being tissue quality and the possibility of covering large defective areas, novel off-the-shelf treatment modalities are being offered to the treating physician aiming to accomplish one-stage chondrocyte transplantation. Advantages of single-stage ACI are the avoidance of two surgeries, in vitro culturing and shipping processes, all implicating high cost, risk of infection and potential chondrocyte dedifferentiation and senescence. As early as 1983, Albrecht and colleagues reported on the usage of cartilage fragments to heal defective cartilage [1, 2]. As an alternative to the traditional two-stage surgery, the idea to transplant minced cartilage (MC) fragments was further investigated [3] and more recently documented as a safety trial [4]. The benefits are that particulate cartilage can be applied either off the shelf by using allogenic tissue [5] or prepared intra-operatively using a mincing device [4]. Cartilage fragments have been shown to be effective in healing animal-model cartilage defects [5–7] and in vitro when using co-culture of human adult and juvenile cartilage fragments [8]. However, despite generally favourable outcomes, there is still limited knowledge about the influence of external cues, such as mechanical environment on neocartilage formation.
Mechanical stimulation is one of the major regulators known to maintain in situ cartilage integrity and function, and various experiments have shown that physiological mechanical stimulation enhances chondrogenesis in vitro [9, 10]. In particular, specialised bioreactors are capable of reproducing a joint-like surrounding by providing compression, shear and fluid flow [11]. Consequently, tissue-engineered products for cartilage repair incorporate bioreactor systems for graft preconditioning prior to reimplantation [12]. Still, optimal mechanical stimulation protocols, with regards to achieving optimal cartilage maturation based on the particulate fragments, have not been established. Therefore, this in vitro study aimed to evaluate the effect of dynamic loading at different time points on cell outgrowth from cartilage fragments, cellular gene expression and neomatrix synthesis. Improving our understanding of cellular behaviour under mechanical load may optimise treatment regimes for MC therapy, particularly where maturation time points and postoperative rehabilitation are concerned.
Materials and methods
MC harvest and 3D culture system
Polyurethane (PU) scaffold rings (4 mm high; inner diameter 8 mm; outer diameter 14 mm) were prepared, as previously described [13]. Before MC seeding, PU rings were evacuated in the presence of Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % foetal bovine serum (FBS) under a vacuum for one hour to wet the hydrophobic polymer. Full-thickness articular cartilage was dissected aseptically from fetlock joints of three, three to four month-old calves directly after slaughter. Cartilage tissue was minced into small fragments (∼1 mm3) with a surgical scalpel in the presence of Tyrode’s balanced Salt Solution (TBSS). Isolated fragments of MC were suspended in fibrinogen solution (75 μl) and mixed with thrombin solution (100 μl) immediately prior to being transferred into the PU ring at about 80 mg cartilage fragments per ring. Final concentrations of the fibrin gel were 17 mg/ml fibrinogen and 0.5U/ml thrombin. The minced-cartilage-scaffold (MC-S) constructs were incubated for one hour to permit fibrin gel formation before adding growth medium consisting of DMEM containing 10 % FBS, 50 μg/ml ascorbic acid, 40 μg/ml L-proline, nonessential amino acids (Gibco) and 500 U/ml aprotinin (Sigma).
Bioreactor and loading protocol
Mechanical conditioning of MC-S composites was performed using a bioreactor system designed to approach the loading and motion characteristics of natural joints [11, 14]. The entire apparatus was placed in an incubator [37 °C, 5 % carbon dioxide (CO2) and 85 % humidity. A commercially available ceramic hip ball (32 mm diameter) was pressed onto the MC-S constructs. Surface motion was generated by oscillation of the ball around an axis perpendicular to the scaffold axis. Simultaneously, dynamic compression was applied along the cylindrical axis of the construct.
After one day of preculture, all MC-S constructs were transferred into bioreactor sample holders to provide identical experimental surroundings for all groups. For loading experiments, MC-S constructs were subjected to a standardised loading protocol within the bioreactor to simulate the articular milieu, as described previously [15]: preload 0.2 mm (5 % of scaffold height), dynamic sinusoidal oscillation 0.4 mm ± 0.2 mm (5–15 % of scaffold height) at 1Hz, surface shear at ±25° and 1Hz in phase difference for 1 h twice a day, with eight-hour rest between loadings, on every second day. During the nonloading periods, the constructs were kept free swelling, identical to the free-swelling groups, without any contact with the ball. Medium was changed every second day and collected for analysis of glycosaminoglycan (GAG) content. Upon termination of the experiment, every cell-matrix construct was cut vertically into two equal halves for further analysis.
Biochemical assays for GAG/DNA quantification
MC-fibrin constructs were digested with proteinase K (0.5 mg/ml) at 56 °C overnight for further DNA and GAG assay. DNA content was measured by spectrofluorometry using Hoechst 33258 dye solution against a calf-thymus DNA standard curve. The amount of sulphated GAG was measured with a dimethylmethylene blue (DMMB) colour reagent against bovine chondroitin sulfate standard. Conditioned culture medium was also analysed for GAG content to determine the amount of proteoglycan released from the construct into the medium. Given GAG/DNA values are in respect to values at day zero, prior to group division.
Gene-expression analysis
MC-fibrin constructs were homogenised using the Tissue-Lyser system (Qiagen, Retsch, Germany), and total RNA (tRNA) was extracted with TRI Reagent (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s specifications using the modified precipitation method with a high-salt precipitation solution (Molecular Research Center). Reverse transcription was performed with TaqMan reagents (Applied Biosystems, Foster City, CA, USA) using random hexamer primers and 1 μg of tRNA sample. Polymerase chain reaction (PCR) was performed on a 7,500 real-time PCR system (Applied Biosystems). Oligonucleotide primers and TaqMan probes (Table 1; all from Microsynth, Balgach, Switzerland) were designed with Primer Express Oligo Design software, version 1.5/2.0 (Applied Biosystems). The expression of 18S rRNA (Applied Biosystems) was measured as endogenous control. PCR was carried out with TaqMan gene expression master mix (Applied Biosystems), 900 nM primers (forward and reverse), 250nM TaqMan probe and 2 μl complementary DNA (cDNA) sample in a total reaction volume of 20 μl. Relative quantification of target mRNA was performed according to the comparative Ct method, with 18S rRNA as the endogenous control [16]. Data were normalised to expression levels at day 0 of construct culture.
Table 1.
Sequences of oligonucleotide primers and probes used for real-time polymerase chain reaction (PCR)
| Gene (bovine) | Symbol | Forward primer (5′–3′) | Reverse primer (5′–3′) | Probe (5′FAM/3′TAMRA) |
|---|---|---|---|---|
| Collagen 1A2 | COL1A2 | TGC AGT AAC TTC GTG CCT AGC A | CGC GTG GTC CTC TAT CTC CA | CAT GCC AAT CCT TAC AAG AGGRECAN CAA CTG C |
| Collagen 2A1 | COL2A1 | AAG AAA CAC ATC TGG TTT GGA GAA A | TGG GAG CCA GGT TGT CAT C | CAA CGG TGG CTT CCA CTT CAG CTA TGG |
| Aggrecan | ACAN | CCA ACG AAA CCT ATG ACG TGT ACT | GCA CTC GTT GGC TGC CTC | ATG TTG CAT AGA AGA CCT CGC CCT CCA T |
| Cartilage oligomeric matrix protein | COMP | CCA GAA GAA CGA CGACCA GAA | TCT GAT CTG AGT TGG GCA CCT T | ACG GCG ACC GGA TCC GCA A |
| Proteoglycan-4 (SZP) | PRG-4 | GAG CAG ACC TGA ATC CGT GTA TT | GGT GGG TTC CTG TTT GTA AGT GTA | CTG AAC GCT GCC ACC TCT CTT GAA A |
Histology and immunohistochemistry (IHC)
MC-fibrin constructs were fixed in 70 % methanol and incubated in 5 % sucrose-PBS solution overnight at 4 °C before microtoming at 6 μm serial cryosections. Sections were stained with toluidine blue to visualise cell morphology and extracellular matrix (ECM) accumulation. Deposition of aggrecan and types I and II collagen was identified according to immunohistochemical (IHC) analysis. IHC staining was performed using the Vectastain Elite ABC Kit (Vector Laboratories). Horse serum was used for blocking nonspecific sites. Sections were probed with primary antibodies against collagen type I (Sigma-Aldrich, Saint Louis, MO, USA), collagen type II and aggrecan (Developmental Studies Hybridoma Bank, Iowa City, IA, USA) [17]. The primary antibody was applied for 30 min, followed by biotinylated secondary horse anti-mouse antibody and then incubation with avidin–biotin–peroxidase complex for 30 min at room temperature. Colour was developed using 3–3′-diaminobenzidine (DAB) monomer. For negative controls, the primary antibody was replaced by PBS.
Experimental design
The experiment was repeated three times under identical conditions; for each run, one calf fetlock joint was utilised. Following biopsy and after in vitro processing, MC pieces were embedded in fibrin and transferred to 3D scaffold rings. MC-fibrin constructs were either cultured under free-swelling conditions (F) or under loaded surroundings within a bioreactor (L). Each experiment was divided into five groups: group 1, unloaded control, free-swelling culture for two weeks (F2); group 2, direct bioreactor loading for two weeks (L2); group 3, unloaded control, free-swelling culture for four weeks (F4); group 4, direct bioreactor loading for four weeks (L4); group 5, free swelling for two weeks, followed by bioreactor loading for two weeks (F2L2).
Statistical analysis
Statistical analysis was performed using the software package SPSS (Version 18; SPSS Inc., Chicago, IL, USA). All data were tested for normal distribution using the Kolmogorov–Smirnov test. Data were compared using t test or Mann–Whitney U test. Group data were compared using one-way analysis of variance (ANOVA). Unless otherwise stated, descriptive results were demonstrated as mean ± standard deviation (SD). Significance level was defined at P < 0.05 or indicated a trend at 0.05 <P < 0.1 for all tests. Significance within the graphs is noted by an asterisk connecting the statistical comparison.
Results
GAG, DNA synthesis
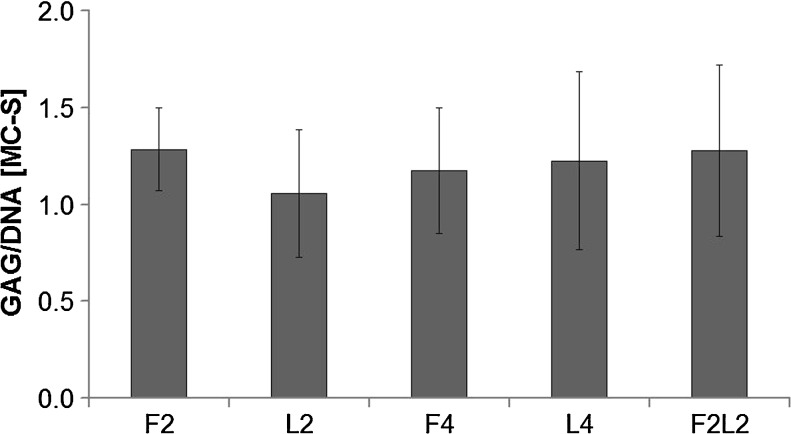
When comparing the GAG/DNA ratio relative to that of immediate harvesting at day 0, no increase was detected in any group (P > 0.05) (Fig. 1). Moreover, the proportion of GAG released from loaded MC fragment into medium remained at the same level between groups (data not shown).
Fig. 1.
Glycosaminoglycan (GAG)/DNA ratio relative to directly harvesting at day 0 (D0) in unloaded constructs (F2, F4) after loading for 2 or 4 weeks (L2, L4) or free swelling for 2 weeks followed by loading for 2 weeks (F2L2) (P > 0.05). Data from three experiments each run in triplicates are shown
Gene expression in conditioned MC-S constructs
Compared with respective unloaded controls, dynamic mechanical stimuli showed no major effect on mRNA expression levels of COL1A2. With the exception of PRG-4 expression, F2 scaffolds constantly had significantly stronger chondrogenic (COL2A1, ACAN, COMP) gene expression when comparing with F4; the F2 and L2 groups were similar for all genes analysed, and the same was true for L2, L4 and F2L2. Consequently, chondrogenic gene expression among L2, L4 and F2L2 was mostly stronger when compared with F4 products. The highest COMP and PRG-4 expression was detected in F2L2 MC-S, where the increase reached the level of significance (Fig. 2).
Fig. 2.
Relative messenger RNA (mRNA) expressions of cartilage-related genes in conditioned minced-cartilage (MC)-fibrin construct under different loading regimes. Data from three experiments each run in triplicate are shown. # P < 0.05, *P < 0.01
Histology and IHC of sections from MC-S constructs
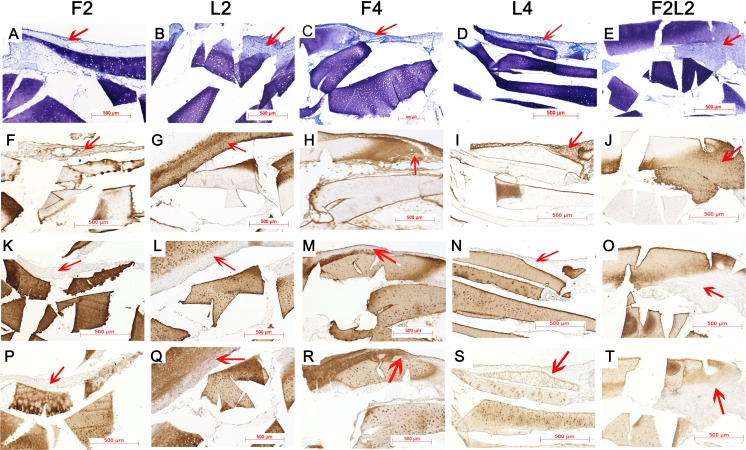
As shown in Fig. 3, higher chondrocyte quantity and stronger matrix accumulation were regenerated around MC fragments after direct loading for two weeks (b) compared with the unloaded group (a). Furthermore, after four weeks of loading (d), more chondrocytes appeared in the surrounding PU ring in addition to the regenerated neocartilage tissue compared with 2-week (L2) constructs. Moreover, regenerated tissue from the group subjected to free swelling for 2 weeks followed by loading for 2 weeks (F2L2) appeared to present the largest areas of newly formed matrix surrounding the native cartilage fragments (e). Specimens exhibited slightly denser immunostaining of collagen type I within the newly formed tissue in MC-S among L2, L4 and F2L2 constructs when compared with respective unloaded control (f–j). In contrast, collagen type II and aggrecan immunostaining was almost invisible in the neocartilage-like tissue surrounding the MC fragments in all the loaded constructs (k–t).
Fig. 3.
Representative cryosections from the different conditioned minced-cartilage (MC)-fibrin constructs. Toluidine blue staining (a–e), collagen type I immunostaining (f–j), collagen type II immunostaining (k–o), aggrecan immunostaining (p–t). Red arrows indicate newly formed matrix. Bar 500 μm
Discussion
Results of this study show that chondrocyte outgrowth culture is feasible under in vitro free swelling as well as dynamic loading conditions. When comparing with unloaded control, increased cell and matrix neotissue was seen among loaded samples, in which chondrogenic gene expression was alwo maintained over time, an effect not seen among four-week, free-swelling products. Whereas there were no effects on GAG/DNA, PCR analysis demonstrated that selected gene expression (ACAN, COMP, PRC-4) was significantly downregulated in the absence of bioreactor-applied mechanical stimulus. Strongest expression was observed among constructs cultured for two weeks under free swelling followed by two weeks of bioreactor culture (F2L2). Prolonged free-swelling culture (F2–F4) resulted in significant downregulation of chondrogenic genes. Microscopic evaluation revealed weaker collagen type II and aggrecan with an elevated collagen type I matrix staining among all loaded MC-S in comparison with control. Tissue-based cartilage repair in this in vitro study generally exhibited a relatively inferior response to mechanical stimuli (< four weeks). The response of particulate cartilage to loading may differ from the performance of (isolated- and single-) cell-scaffold-based chondrogenesis, where it was recently demonstrated that free swelling followed by loading may result in the most efficient chondrogenesis [18].
MC provides native cartilage with multiple cell-matrix fragments. The principle of the MC technique is to accomplish hyaline-like cartilage regeneration via a cellular outgrowth mechanism using minced pieces of autologous cartilage as a cell source, often supplemented with a scaffold delivery system when aiming for clinical application [19–21]. Cole and coworkers presented satisfying two year clinical outcome data when using MC fragments in display of general functionality [4]. In our study, the MC experiment was performed similar to a previously reported cell-based setup in which a macroporous PU scaffold contained cell-seeded fibrin gel [18]. Application of a relatively short-term loading protocol in our study may be one reason no effective promotion of GAG biosynthesis in MC was noticeable. Hypothetically, cartilage fragments isolated from the native joint environment might take some time to adapt to new culture conditions. Furthermore, almost half of total GAG (45∼65 %) was retained in the minced fragments (data not shown). The possible reason might be that in MC-fibrin constructs, the major proportion of chondrocytes, was embedded in the original MC, whereas few chondrocytes grew out of the surrounding minced fragments, which could be visually observed in histological sections. The majority of GAG might be synthesised by MC-originated chondrocytes rather than by outgrown chondrocytes, so most GAG will be retained in the original ECM, and mechanical loading described here can hardly release them from the highly organised, dense collagen network into the medium. Expression levels of COL2A1, ACAN and COMP were downregulated in MC-fibrin constructs after free-swelling culture for four weeks, which in turn confirms the fact that dynamic stimulation is necessary to maintain the chondrogenic phenotype. The potential of particular chondrogenic gene regulation, such as ACAN or COMP, was enhanced in L4 and F2L2 groups, which might indicate that MC presented an elevated responsiveness to mechanical stimulation after adaption to culture conditions.
The histological evaluation enables us to confirm that chondrocyte migration or proliferation from tissue fragments was effectively elicited via mechanical loading. The reparative benefits of MC fragments are conceivable, as some neocartilaginous tissues were regenerated surrounding the minced fragments. Therefore, the promising outcome achieved in this study indicates that morselising the cartilage in approximately 1-mm3 pieces might provide ideal movement of chondrocytes around cartilage fragments and subsequent multiplication and matrix production in the loading regime. This is consistent with previous studies, which report an inverse relationship exists between cartilage fragment size and amount of cartilage-like tissue outgrowth (smaller size, more chondral growth) [3, 6]. Moreover, immunohistochemistry visualised that neocartilaginous tissue outgrown from the surrounding minced fragments was stained with stronger collagen type I and weaker collagen type II and aggrecan, which illustrated that the newly formed tissue within the setting described here was immature. Hypothetically, collagen and aggrecan synthesis on the protein level, however, requires longer time spans, so mRNA for, i.e. ACAN, was significantly upregulated among loaded constructs, whereas matrix immunostaining was weaker compared with free-swelling control. A longer time-span for bioreactor culture/dynamic in vivo settings may direct neomatrix formation into a cartilage-typical phenotypically correct direction.
Cartilage regeneration potential of a one-stage procedure is likely dependent on multiple parameters, such as size and loading density of minced fragments, method of harvest, culture time/method and material properties of scaffolds [19]. This may be further modified by the use of growth factors such as transforming growth factor (TGF)- β1 and granulocyte colony-stimulating factor (G-CSF) [22]. Our short-term (four month) animal study demonstrated no difference in histology scores, tissue filling (35 %) or repair tissue stiffness between cell-based regeneration and MC technique. General tissue regeneration was limited, probably due to the early time point of investigation and the challenging mechanical environment [23]. Furthermore, MC fragments loaded onto an hyaluronic acid derivative felt/fibrin glue/platelet-rich plasma (HA felt/FG/PRP) scaffold could provide an efficient cell source with higher histological scoring at six months in a rabbit model [7]. In contrast to in vivo animal experiments that indicate long-term (maximum 12 months) repair potential of cartilage fragments delivered on scaffolds in large weight-bearing animals undergoing a controlled, strenuous-exercise protocol [6], we performed a relatively short-term in vitro study simulating the one-stage procedure of MC. Nevertheless, a partially promising outcome regarding MC-derived chondrogenesis was evident. However, GAG synthesis was no different between groups, and histology and immunohistochemistry visually revealed that neocartilaginous tissue was regenerated in each group; PCR data showed an increase in some chondrogenic gene expression, such as ACAN and COMP, within L4 and F2L2 constructs. In our previous study, we declared that preculture (F2L2) was most effective where in vitro chondrogenesis was concerned [18]; however, in the study reported here, only total amount of outgrowth-related neomatrix was seen in histological sections. Significantly different cartilage-typical gene expression was not provoked when MC was applied under identical in vitro settings. Based on these results, it was unjustifiable to consider that the stimulation mode of free swelling followed by loading could be superior in mechanically induced MC-derived chondrogenesis. Such results would only give baseline data on chondrocyte response to loading. As the major aim of MC products is to avoid ex vivo processing, future work might consider a comparison between free-swelling and bioreactor-conditioned MC constructs, which are retransplanted in vivo in order to better close the gap from bench to bedside.
Acknowledgments
Acknowledgments
This work was funded by AO foundation, Davos, Switzerland. The authors thank Robert Peter, AO Research Institute, for excellent technical assistance with biochemical analysis; to Andrea Oswald, AO Research Institute, for assistance in immunohistochemistry; and to Patrick Trüssel for support in histology.
Conflict of interest
None.
References
- 1.Albrecht FH. Closure of joint cartilage defects using cartilage fragments and fibrin glue. Fortschr Med. 1983;101(37):1650–1652. [PubMed] [Google Scholar]
- 2.Albrecht F, Roessner A, Zimmermann E. Closure of osteochondral lesions using chondral fragments and fibrin adhesive. Arch Orthop Trauma Surg. 1983;101(3):213–217. doi: 10.1007/BF00436773. [DOI] [PubMed] [Google Scholar]
- 3.Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, Binette F. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261–1270. doi: 10.1002/jor.20135. [DOI] [PubMed] [Google Scholar]
- 4.Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, De Deyne PG. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1170–1179. doi: 10.1177/0363546511399382. [DOI] [PubMed] [Google Scholar]
- 5.Farr J, Cole BJ, Sherman S, Karas V. Particulated articular cartilage: CAIS and DeNovo NT. J Knee Surg. 2012;25:23–29. doi: 10.1055/s-0031-1299652. [DOI] [PubMed] [Google Scholar]
- 6.Frisbie DD, Lu Y, Kawcak CE, DiCarlo EF, Binette F, McIlwraith CW. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med. 2009;37(Suppl 1):71S–80S. doi: 10.1177/0363546509348478. [DOI] [PubMed] [Google Scholar]
- 7.Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Rossi R, Piras L, Maiello A, Realmuto C, Peretti GM. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg Sports Traumatol Arthrosc. 2012;20(12):2590–2601. doi: 10.1007/s00167-012-1920-y. [DOI] [PubMed] [Google Scholar]
- 8.Bonasia DE, Martin JA, Marmotti A, Amendola RL, Buckwalter JA, Rossi R, Blonna D, Adkisson HD, Amendola A. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments: in vitro matrix production. Am J Sports Med. 2011;39:2355–2361. doi: 10.1177/0363546511417172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grad S, Gogolewski S, Alini M, Wimmer MA. Effects of simple and complex motion patterns on gene expression of chondrocytes seeded in 3-D scaffolds. Tissue Eng. 2006;12:3171–3179. doi: 10.1089/ten.2006.12.3171. [DOI] [PubMed] [Google Scholar]
- 10.Grad S, Eglin D, Alini M, et al. Physical stimulation of chondrogenic cells in vitro: a review. Clin Orthop Relat Res. 2011;469(10):2764–2772. doi: 10.1007/s11999-011-1819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wimmer MA, Grad S, Kaup T, Hanni M, Schneider E, Gogolewski S, et al. Tribology approach to the engineering and study of articular cartilage. Tissue Eng. 2004;10:1436–1445. doi: 10.1089/ten.2004.10.1436. [DOI] [PubMed] [Google Scholar]
- 12.Crawford DC, Heveran CM, Cannon WD, Jr, Foo LF, Potter HG. An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years. Am J Sports Med. 2009;37:1334–1343. doi: 10.1177/0363546509333011. [DOI] [PubMed] [Google Scholar]
- 13.Lee CR, Grad S, Gorna K, Gogolewski S, Goessl A, Alini M. Fibrin-polyurethane composites for articular carti -lage tissue engineering: a preliminary analysis. Tissue Eng. 2005;11:1562–1573. doi: 10.1089/ten.2005.11.1562. [DOI] [PubMed] [Google Scholar]
- 14.Wimmer MA, Alini M, Grad S. The effect of sliding velocity on chondrocytes activity in 3-D scaffolds. J Biomech. 2009;42:424–429. doi: 10.1016/j.jbiomech.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Salzmann GM, Nuernberger B, Schmitz P, Anton M, Stoddart MJ, Grad S, et al. Physicobiochemical synergism through gene therapy and functional tissue engineering for in vitro chondrogenesis. Tissue Eng Part A. 2009;15:2513–2524. doi: 10.1089/ten.tea.2008.0479. [DOI] [PubMed] [Google Scholar]
- 16.Lee CR, Grad S, Maclean JJ, Iatridis JC, Alini M. Effect of mechanical loading on mRNA levels of common endogenous controls in articular chondrocytes and intervertebral disk. Anal Biochem. 2005;341:372–375. doi: 10.1016/j.ab.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Grad S, Loparic M, Peter R, Stolz M, Aebi U, Alini M. Sliding motion modulates stiffness and friction coefficient at the surface of tissue engineered cartilage. Osteoar Cartil. 2012;20(4):288–295. doi: 10.1016/j.joca.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Wang N, Grad S, Stoddart MJ, Niemeyer P, Südkamp NP, Pestka J, Alini M, Chen J, Salzmann GM. Bioreactor-induced chondrocyte maturation is dependent on cell passage and onset of loading. Cartilage. 2013;4(2):165–176. doi: 10.1177/1947603512471345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick F, Yanke A, Provencher MT, Cole BJ. Minced articualr cartilage-basic science, surgical technique, and clinical application. Sports Med Arthtosc Rev. 2008;16:217–220. doi: 10.1097/JSA.0b013e31818e0e4a. [DOI] [PubMed] [Google Scholar]
- 20.Farr J, Cole B, Dhawan A, Kercher J, Sherman S. Clinical cartilage restoration: evolution and overview. Clin Orthop Relat Res. 2011;469:2696–2705. doi: 10.1007/s11999-010-1764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 22.Marmotti A, Bonasia DE, Bruzzone M, Rossi R, Castoldi F, Collo G, et al. Human cartilage fragments in a composite scaffold for single-stage cartilage repair: an in vitro study of the chondrocyte migration and the influence of TGF-β1 and G-CSF. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1819–1833. doi: 10.1007/s00167-012-2244-7. [DOI] [PubMed] [Google Scholar]
- 23.Lind M, Larsen A. Equal cartilage repair response between autologous chondrocytes in a collagen scaffold and minced cartilage under a collagen scaffold: an in vivo study in goats. Connect Tissue Res. 2008;49:437–442. doi: 10.1080/03008200802325037. [DOI] [PubMed] [Google Scholar]