Abstract
Purpose
Recent evidence suggests that angiogenesis and inflammation contribute to the development and progression of osteoarthritis (OA). The purpose of this study was to investigate vascular endothelial growth factor (VEGF) levels in plasma and synovial fluid of patients with knee OA and to determine the relationship of VEGF levels with disease severity in knee OA.
Methods
A total of 100 subjects were enrolled in this study (80 knee OA patients and 20 healthy controls). Plasma and synovial fluid VEGF levels were analysed using enzyme-linked immunosorbent assay. VEGF expressions in synovial membrane and articular cartilage samples were assessed using immunohistochemistry.
Results
VEGF level in synovial fluid of knee OA patients was tenfold higher than that in paired plasma (P < 0.001). Both plasma and synovial fluid VEGF exhibited a positive correlation with radiographic severity (r = 0.454 and r = 0.727, P < 0.001, respectively). VEGF expression was highly detectable in synovial lining cells and articular chondrocytes of knee OA patients.
Conclusions
VEGF levels in both plasma and synovial fluid were positively correlated with the severity of knee OA. Therefore, VEGF may be useful for monitoring OA severity and could play a substantial role in the development and progression of knee OA.
Keywords: Osteoarthritis, Plasma, Severity, Synovial fluid, Vascular endothelial growth factor
Introduction
Osteoarthritis (OA) is a common age-related degenerative joint disease characterised by articular cartilage degradation, subchondral bone sclerosis, osteophyte formation and synovial membrane inflammation. The clinical symptoms and signs of OA include pain, stiffness, joint swelling, limited range of motion and joint disability [1]. The diagnosis of OA generally relies on clinical and radiographic alterations. A radiograph is a global measurement for determining the affected joint by grading joint destruction [2]. The precise aetiology and pathogenesis of OA remain largely unclear. Nevertheless, various environmental, biomechanical and biochemical factors including cytokines and growth factors have been recognised as playing a substantial role in OA development [3, 4].
Angiogenesis is the growth of new capillary blood vessels from pre-existing vasculature. Angiogenesis and chronic inflammation are closely integrated processes in OA and may affect disease progression. Inflammation could be both a primary event in OA and secondary to other aspects of the disease. Previous studies showed that synovial inflammation could be detected in the early and advanced stage of OA, indicating that synovitis is an early feature in OA and not restricted to patients with end-stage OA [5–7].
Vascular endothelial growth factor (VEGF) is one of the most potent pro-angiogenic growth factors secreted by endothelial cells. The VEGF family encompasses seven members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F and placental growth factor. Among them, VEGF-A commonly called VEGF exists in seven homodimeric isoforms resulting from alternative splicing of eight exons of the VEGF gene [8]. VEGF exerts its effects by binding to the signalling tyrosine kinase receptors VEGFR1 and VEGFR2 leading to angiogenesis, mitogenesis and cell survival [8, 9]. In addition, VEGF acts specifically on endothelial cells to activate proliferation, migration and tube formation. VEGF has been known to play an important role in endochondral ossification by coupling angiogenesis with hypertrophic cartilage remodelling and bone formation [9]. In growing cartilage, both VEGF and its receptors have recently been reported to be expressed in chondrocytes within the superficial layer of the articular cartilage [9]. They are not found in mature articular cartilage. However, VEGF and its receptors have been found to be expressed in human osteoarthritic articular cartilage accompanying the progression of OA [10–12]. The demonstration of VEGF and its receptors in osteoarthritic articular cartilage suggests their potential involvement in articular cartilage destruction and OA development.
In recent years, several cytokines have been shown to be associated with clinical parameters of disease severity in knee OA and may play possible roles in the pathogenesis of OA [13–16]. To the best of our knowledge, there are no published data with regard to the relationship between VEGF expression and radiographic severity in primary knee OA. We have postulated that VEGF in plasma and synovial fluid could be correlated with disease severity in knee OA patients. To prove this hypothesis, we investigated plasma, synovial fluid and tissue expression of VEGF in primary knee OA patients.
Therefore, the objective of this study was to evaluate both plasma and synovial fluid levels of VEGF in patients with primary knee OA and to examine the possible relationships with the radiographic grading of knee OA.
Materials and methods
Study population
This study was approved by the Institutional Review Board on Human Research of the Faculty of Medicine, Chulalongkorn University, and was conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from the patients and healthy volunteers prior to their participation in the study.
A total of 80 patients with primary knee OA (63 women and 17 men, mean age 69.8 ± 0.9 years) who were diagnosed with clinical and radiographic evidence of knee OA according to the criteria of the American College of Rheumatology were registered in this study. We also recruited 20 gender- and age-matched subjects (15 women and five men, mean age 68.2 ± 1.1 years) with normal knee radiographs as controls. None of the participants had underlying diseases such as diabetes, histories of corticosteroid medication, other forms of arthritis, cancer or other chronic inflammatory diseases.
Knee radiography was performed when each participant was standing on both legs with fully extended knee and the X-ray beam was centred at the level of the joint. Disease severity assessment was performed using the Kellgren and Lawrence (KL) grading system [17]. Depending on changes observed in conventional weight-bearing anteroposterior radiographs of the affected knee in extension, OA was divided into 5 grades (0–4): grade 0 (normal findings), no X-ray changes; grade 1 (questionable), doubtful narrowing of joint space and possible osteophytic lipping; grade 2 (mild), definite osteophytes and possible joint space narrowing; grade 3 (moderate), multiple moderate osteophytes, definite narrowing of joint space, bone sclerosis and possible deformity of bone contour; grade 4 (severe), large osteophytes, marked joint space narrowing, severe sclerosis and deformity of bone contour. OA patients were defined as having radiographic knee OA of KL grade ≥ 2 in at least one knee. Controls were defined as having neither radiographic hip OA nor knee OA, as indicated by KL grades of 0 for both hips and both knees. The grading of the worst affected knee in each patient was used for data analysis.
Sample collection
Synovial fluid was aspirated from the affected knee using sterile knee puncture just prior to surgery, when a total knee replacement was performed, centrifuged to remove cells and joint debris and stored immediately at −80 °C until the day of measurement. No synovial fluid was extracted from the controls due to ethical reasons. Venous blood samples collected from the same patients on the day of surgery were centrifuged and stored at −80 °C until analysed. Additionally, osteoarthritic cartilage and synovial tissues collected from the OA patients undergoing total knee replacement were cut approximately 0.3 × 0.3 cm2 in size and immersed in 10 % formalin for haematoxylin and eosin staining and immunohistochemical assay.
Determination of VEGF protein concentration
Double-blind quantitative determination of VEGF concentration in plasma and synovial fluid was performed using a commercially available enzyme-linked immunosorbent assay (ELISA) (Quantikine R&D systems, Minneapolis, MN, USA). According to the manufacturer’s protocol, 100 μl of recombinant human VEGF standards, plasma and synovial fluid samples were pipetted into 96-well microtitre plates precoated with mouse monoclonal antibody against human VEGF and incubated for two hours at room temperature. The wells were then washed three times with washing buffer and incubated for two hours at room temperature with a horseradish peroxidase-conjugated monoclonal antibody specific for VEGF. After three washes, substrate solution was added to each well, and the plate was incubated for 25 minutes at room temperature in the dark. Eventually, the reaction was stopped with the stop solution and the optical density was measured at 450 nm using an automated microplate reader. A standard optical density-concentration curve (range 31.2–2,000 pg/ml) was drawn for the determination of VEGF concentration. The manufacturer-reported precision was 4.5 %–6.7 % (intra-assay) and 6.2 %–8.8 % (inter-assay). The assay sensitivity of VEGF was 3.25 pg/ml.
Immunohistochemistry of the VEGF-stained specimens
The articular cartilage and synovial specimens from knee OA patients were immediately fixed in 10 % formaldehyde and embedded in paraffin. Serial sections of paraffin-embedded tissues were cut in 5-μm thickness and processed for VEGF staining.
Sections were deparaffinised and rehydrated in Tris-buffered saline. Endogenous peroxidase activity was blocked with 0.3 % H2O2 for ten minutes. For antigen retrieval, tissue sections were microwave heated in 10 mmol/L citrate buffer for five minutes. Non-specific binding was blocked for ten minutes with 5 % goat serum (Dako, Glostrup, Denmark), followed by incubation with antibody to VEGF (1:500; Santa Cruz Biotechnology, Dallas, Texas, USA) in Tris-buffered saline containing 2 % rabbit serum and 1 % bovine serum albumin for two hours. Tissues were incubated with the same buffer without the antibody to serve as negative controls. Sections were subsequently stained with biotinylated goat anti-rabbit immunoglobulins (1:400; Dako) and streptavidin/horseradish peroxidase complex (1:400; Dako) and incubated at room temperature for 45 minutes. Reaction products were visualised using diaminobenzidine (Sigma, St. Louis, MO, USA) as the chromogen. The sections were subsequently counterstained with Mayer’s haematoxylin and mounted onto microscope slides using a permanent medium.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software, version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). Student’s unpaired t test was used to compare the means of two independent groups, and one-way analysis of variance (ANOVA) was used to compare the means of more than two independent groups. Correlations between plasma and synovial fluid VEGF with disease severity were calculated using Pearson’s correlation coefficient (r). Data were expressed as mean ± standard error of the mean (SEM). P values < 0.05 were considered to be statistically significant for differences and correlations.
Results
Correlation between plasma and synovial fluid VEGF and disease severity
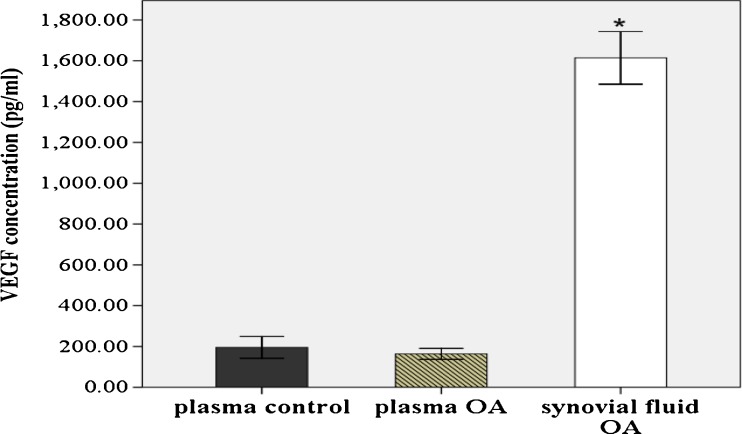
For measurement of VEGF concentrations, 80 plasma and synovial fluid samples from primary knee OA patients and 20 plasma samples from healthy controls were recruited. Characteristics of the study population are shown in Table 1. As illustrated in Fig. 1, plasma VEGF levels were lower in OA patients than in plasma controls; however, there was no statistically significant difference (163.5 ± 13.7 vs 195.4 ± 26.7 pg/ml, P > 0.05). VEGF levels in synovial fluid of knee OA patients were significantly tenfold higher than that in corresponding blood samples (1,614.3 ± 64.5 vs 163.5 ± 13.7 pg/ml, P < 0.001).
Table 1.
Plasma and SF VEGF in OA patients
| Total | KL grade 2 | KL grade 3 | KL grade 4 | P value | |
|---|---|---|---|---|---|
| n | 80 | 29 | 27 | 24 | |
| Age (years) | 69.9 ± 0.9 | 70.2 ± 1.3 | 68.4 ± 1.3 | 71.1 ± 2.1 | 0.5 |
| Gender (M/F) | 17/63 | 6/23 | 6/21 | 5/19 | 0.2 |
| BMI (kg/m2) | 26.7 ± 0.5 | 26.6 ± 0.7 | 26.2 ± 0.8 | 27.5 ± 0.9 | 0.4 |
| Plasma VEGF (pg/ml) | 163.5 ± 13.7 | 100.5 ± 10.1 | 166.2 ± 19.2 | 236.6 ± 32.9 | P < 0.01 |
| SF VEGF (pg/ml) | 1,614.3 ± 64.5 | 1,153.5 ± 76.1 | 1,602.5 ± 7.6 | 2,184.6 ± 80.1 | P < 0.01 |
Data represent mean and standard error of the mean. P values for differences among Kellgren and Lawrence (KL) subgroups
SF synovial fluid, VEGF vascular endothelial growth factor, BMI body mass index
Fig. 1.
VEGF levels in plasma and synovial fluid of OA patients (n = 80) and healthy controls (n = 20). *P < 0.001 when compared with plasma OA
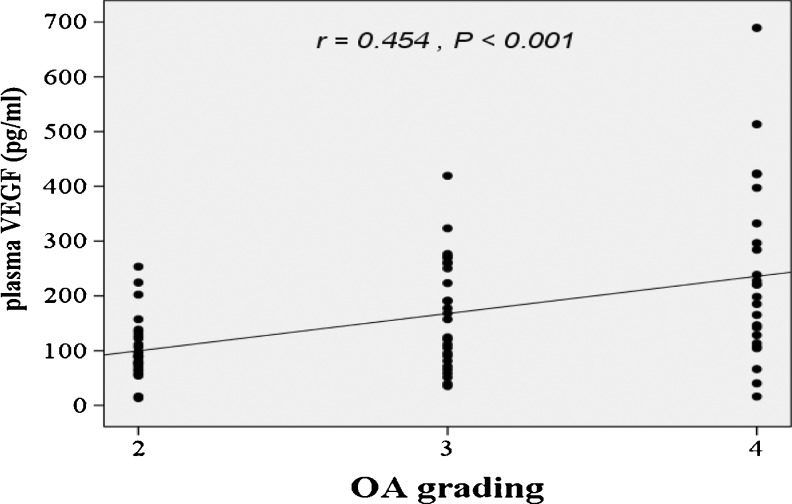
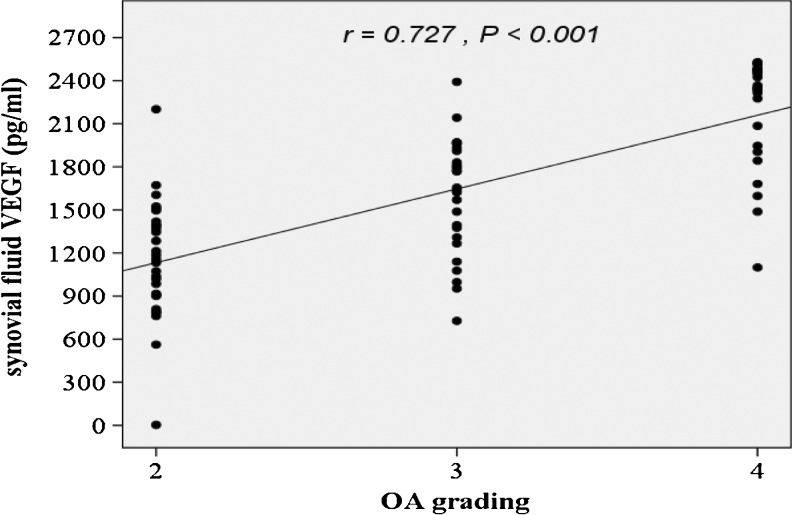
According to the radiographic KL grading criteria, OA patients were classified into three groups in relation to OA grading: 29 patients were categorised as grade 2, 27 as grade 3 and 24 as grade 4. As displayed in Table 1, knee OA patients with higher radiographic severity had significantly greater VEGF levels in both plasma and synovial fluid (P < 0.01). In addition, the associations between plasma and synovial fluid levels of VEGF and the radiographic severity were statistically investigated. The plasma VEGF levels exhibited a positive correlation with radiographic severity of knee OA (r = 0.454, P < 0.001) (Fig. 2). Furthermore, synovial fluid VEGF levels of knee OA patients were positively correlated with the radiographic severity (r = 0.727, P < 0.001) (Fig. 3).
Fig. 2.
Positive correlation between plasma VEGF levels in OA patients and severity classified according to KL grading scale (r = 0.454, P < 0.001)
Fig. 3.
Positive correlation between synovial fluid VEGF levels in OA patients and severity classified according to KL grading scale (r = 0.727, P < 0.001)
Localisation of VEGF in synovium and articular cartilage from OA patients
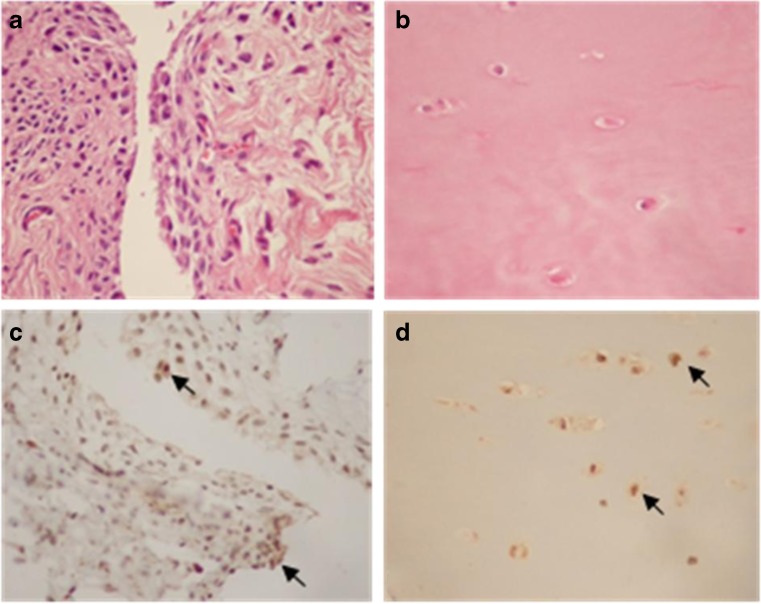
To further assess VEGF expression and tissue distribution, immunohistochemical analysis of synovial membrane and articular cartilage samples obtained from knee OA patients was performed. The results revealed that VEGF had strong expression on cells of the superficial aspects of synovial membrane samples. The VEGF staining was clearly observed in the cytoplasm of the synoviocytes in the lining and sublining from synovial membrane and in the chondrocytes of the articular cartilage from knee OA patients (Fig. 4).
Fig. 4.
Representative photomicrographs of synovium and articular cartilage samples from knee OA patients. a, b Haematoxylin and eosin staining in synovium and articular cartilage, respectively. c, d VEGF immunohistochemical staining in synovium and articular cartilage of knee OA patients demonstrated the presence of VEGF expression in synovial lining cells and chondrocytes (arrows), respectively (×400)
Discussion
VEGF, a heparin-binding dimeric glycoprotein, can act not only on endothelial cells but also on many cell types such as haematopoietic stem cells, monocytes, megakaryocytes, osteoblasts and chondrocytes [9, 11]. It has essential functions in embryonic vasculature, tissue remodelling, wound healing, cancer and endochondral ossification [10]. Previous studies reported that VEGF was upregulated in inflammatory arthritic conditions including rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis and OA [18, 19]. Furthermore, VEGF and its receptors have been shown to be expressed in articular cartilage of OA patients [10–12]. Corrado et al. reported that VEGF played an important role in bone formation via acting on osteoblasts [20]. Hypertrophic chondrocytes in osteophytes also expressed VEGF, indicating that VEGF plays a role in angiogenesis during osteophyte development in OA [21]. More recently, an increase of VEGF expression was evident on chondrocytes in osteoarthritic articular cartilage in experimental OA animals, suggesting that VEGF has been implicated in the development and progression of OA [22, 23].
Previous investigations have shown that VEGF was present in synovial fluid of the temporomandibular joint with internal derangement, rheumatoid arthritis and OA [24, 25]. However, both plasma and synovial fluid VEGF in patients with knee OA have not been investigated, and their correlations with disease severity have never been documented. This study is the first to demonstrate that VEGF was detected in both plasma and synovial fluid obtained from knee OA patients and that VEGF in both plasma and synovial fluid were positively correlated with the radiographic severity of knee OA.
Our study revealed a marked increase of VEGF levels in synovial fluid of knee OA patients compared to the paired plasma levels, suggesting that there is enhanced local VEGF production in knee OA. In agreement with this study, the elevated synovial fluid levels of VEGF as compared to paired plasma samples were also found in juvenile idiopathic arthritis [19]. The remarkable elevation of VEGF in synovial fluid could be possibly attributed to either the release of VEGF residing in extracellular matrix, the production of the cells in synovial membrane and/or articular cartilage or both processes. The source of VEGF in the synovial fluid presumably originated from synovial cells and chondrocytes in the local tissues (inflamed synovium and articular cartilage) and extra-articular tissues. The immunohistochemical study showed VEGF expression in synovial lining cells of synovial membrane and chondrocytes of articular cartilage in OA. Synovitis and degenerative articular cartilage are likely to be facilitating factors in the release of VEGF into the synovial fluid. Since synovial cells and articular chondrocytes express various cytokines, they are thought to be the primary source of cytokines secreted into joint cavities.
Limitations of our study revolved around the relatively small number of participants. Future studies conducted on a larger scale and multi-centre research are necessary to make a more definite conclusion. Additionally, this study was cross-sectional in its design and, therefore, no conclusions regarding cause and effect relationships can be drawn. Finally, synovial fluid samples from healthy controls were not taken due to ethical concerns. More investigations on synovial samples from controls will be essential for a better understanding of our findings.
In summary, our study illustrated that VEGF levels in synovial fluid were substantially higher with regard to paired plasma VEGF. Both plasma and synovial fluid VEGF levels were positively correlated with the radiographic severity in knee OA patients. Therefore, VEGF may serve as a promising biochemical marker for determining the disease severity in knee OA. Further prospective longitudinal research projects are in progress to elucidate the exact role of VEGF in the pathogenesis of the degenerative process of OA. Modulation of VEGF might be a potential target of OA therapy for the future.
Acknowledgments
This investigation has been facilitated by the Ratchadapiseksompotch Fund (RA55/22), Faculty of Medicine, Chulalongkorn University, and the 90th anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund). Natthaphon Saetan has been supported by grant under the program Strategic Scholarships for Frontier Research Network for the Ph.D. Program Thai Doctoral degree from the Office of the Higher Education Commission, Thailand.
References
- 1.Krasnokutsky S, Attur M, Palmer G, Samuels J, Abramson SB. Current concepts in the pathogenesis of osteoarthritis. Osteoarthritis Cartilage. 2008;16:S1–S3. doi: 10.1016/j.joca.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Golightly YM, Marshall SW, Kraus VB, Renner JB, Villaveces A, Casteel C, Jordan JM. Biomarkers of incident radiographic knee osteoarthritis: do they vary by chronic knee symptoms? Arthritis Rheum. 2011;63:2276–2283. doi: 10.1002/art.30412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura A, Hasegawa M, Kato K, Yamada T, Uchida A, Sudo A. Risk factors for the incidence and progression of radiographic osteoarthritis of the knee among Japanese. Int Orthop. 2011;35:839–843. doi: 10.1007/s00264-010-1073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krasnokutsky S, Samuels J, Abramson SB. Osteoarthritis in 2007. Bull NYU Hosp Jt Dis. 2007;65:222–228. [PubMed] [Google Scholar]
- 5.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005;44:7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- 7.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 9.Lingaraj K, Poh CK, Wang W. Vascular endothelial growth factor (VEGF) is expressed during articular cartilage growth and re-expressed in osteoarthritis. Ann Acad Med Singapore. 2010;39:399–403. [PubMed] [Google Scholar]
- 10.Pufe T, Petersen W, Tillmann B, Mentlein R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001;44:1082–1088. doi: 10.1002/1529-0131(200105)44:5<1082::AID-ANR188>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Pfander D, Körtje D, Zimmermann R, Weseloh G, Kirsch T, Gesslein M, Cramer T, Swoboda B. Vascular endothelial growth factor in articular cartilage of healthy and osteoarthritic human knee joints. Ann Rheum Dis. 2001;60:1070–1073. doi: 10.1136/ard.60.11.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enomoto H, Inoki I, Komiya K, Shiomi T, Ikeda E, Obata K, Matsumoto H, Toyama Y, Okada Y. Vascular endothelial growth factor isoforms and their receptors are expressed in human osteoarthritic cartilage. Am J Pathol. 2003;162:171–181. doi: 10.1016/S0002-9440(10)63808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honsawek S, Yuktanandana P, Tanavalee A, Saetan N, Anomasiri W, Parkpian V. Correlation between plasma and synovial fluid basic fibroblast growth factor with radiographic severity in primary knee osteoarthritis. Int Orthop. 2012;36:981–985. doi: 10.1007/s00264-011-1435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honsawek S, Chayanupatkul M, Tanavalee A, Sakdinakiattikoon M, Deepaisarnsakul B, Yuktanandana P, Ngarmukos S. Relationship of plasma and synovial fluid BMP-7 with disease severity in knee osteoarthritis patients: a pilot study. Int Orthop. 2009;33:1171–1175. doi: 10.1007/s00264-009-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honsawek S, Tanavalee A, Sakdinakiattikoon M, Chayanupatkul M, Yuktanandana P. Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin Biochem. 2009;42:808–812. doi: 10.1016/j.clinbiochem.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Liao W, Li Z, Wang H, Wang J, Fu Y, Bai X. Proteomic analysis of synovial fluid: insight into the pathogenesis of knee osteoarthritis. Int Orthop. 2013;37:1045–1053. doi: 10.1007/s00264-012-1768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballara S, Taylor PC, Reusch P, Marmé D, Feldmann M, Maini RN, Paleolog EM. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis Rheum. 2001;44:2055–2064. doi: 10.1002/1529-0131(200109)44:9<2055::AID-ART355>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Bosco MC, Delfino S, Ferlito F, Puppo M, Gregorio A, Gambini C, Gattorno M, Martini A, Varesio L. The hypoxic synovial environment regulates expression of vascular endothelial growth factor and osteopontin in juvenile idiopathic arthritis. J Rheumatol. 2009;36:1318–1329. doi: 10.3899/jrheum.080782. [DOI] [PubMed] [Google Scholar]
- 20.Corrado A, Neve A, Cantatore FP. Expression of vascular endothelial growth factor in normal, osteoarthritic and osteoporotic osteoblasts. Clin Exp Med. 2013;13:81–84. doi: 10.1007/s10238-011-0170-5. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto S, Creighton-Achermann L, Takahashi K, Amiel D, Coutts RD, Lotz M. Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage. 2002;10:180–187. doi: 10.1053/joca.2001.0505. [DOI] [PubMed] [Google Scholar]
- 22.Tibesku CO, Daniilidis K, Skwara A, Paletta J, Szuwart T, Fuchs-Winkelmann S. Expression of vascular endothelial growth factor on chondrocytes increases with osteoarthritis—an animal experimental investigation. Open Orthop J. 2011;5:177–180. doi: 10.2174/1874325001105010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansen H, Meffert RH, Birkenfeld F, Petersen W, Pufe T. Detection of vascular endothelial growth factor (VEGF) in moderate osteoarthritis in a rabbit model. Ann Anat. 2012;194:452–456. doi: 10.1016/j.aanat.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Lee SS, Joo YS, Kim WU, Min DJ, Min JK, Park SH, Cho CS, Kim HY. Vascular endothelial growth factor levels in the serum and synovial fluid of patients with rheumatoid arthritis. Clin Exp Rheumatol. 2001;19:321–324. [PubMed] [Google Scholar]
- 25.Kumagai K, Hamada Y, Holmlund AB, Gotoh A, Nakaoka K, Arai G, Yamane S, Suzuki R. The levels of vascular endothelial growth factor in the synovial fluid correlated with the severity of arthroscopically observed synovitis and clinical outcome after temporomandibular joint irrigation in patients with chronic closed lock. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:185–190. doi: 10.1016/j.tripleo.2009.09.009. [DOI] [PubMed] [Google Scholar]