Abstract
Purpose
The purpose of this review is to provide a better understanding of biomechanical changes induced by reverse shoulder arthroplasty (RSA), discuss the different techniques of radiographic assessment of upper limb lengthening after RSA and determine the ideal soft tissue tension that provides the best functional outcome without increasing the risk of complications.
Methods
Inclusion criteria were articles in which the primary interest was the technique of measuring upper-extremity lengthening after complications related to lengthening and its role in postoperative function; those written in English, French or German; and those that provided evidence levels I–IV relevant to search terms.
Results
Seven articles met our inclusion criteria. Postoperatively, changes in humeral length varied from minus five to five millimetres, and changes in upper-extremity length varied from 15 mm to 27 mm. The acromiohumeral distance averaged 23 mm. Humeral and arm shortening increased the risk of dislocation and led to poor anterior active elevation. The type of surgical approach did not play a role in postoperative function. Subclinical neurological lesions were frequent.
Conclusions
Studies in this systematic review indicate that deltoid tensioning by restoring humeral length and increasing the acromiohumeral distance is critical for adequate postoperative function and to prevent dislocation. Excessive arm lengthening should be avoided, with zero to two centimetres of lengthening being a reasonable goal to avoid postoperative neurological impairment.
Keywords: Reverse Shoulder Arthroplasty, Grammont prosthesis, Lengthening, Arm, Humerus and acromiohumeral length, Review, Complication, Instability, Acromial fracture, Function
Introduction
During evolution, the development of the permanently upright posture has freed the human shoulder girdle of its quadruped functions. The anterior limbs became the upper limbs with the characteristics of a non-weight-bearing joint [1]. Major bony and muscular adaptations occurred. The rotator cuff is the most common structure that becomes compromised. When detached from the bone, the musculotendinous unit retracts medially [2], and the muscle may atrophy [3, 4] or develop fatty infiltration [3, 5–8]. In the absence of concavity compression and humeral head depression exerted by the rotator cuff, the unopposed contraction of the deltoid creates a force vector that displaces the humeral head superiorly rather than creating abduction. With large rotator cuff lesions, the patient may present with pseudoparalysis [9, 10]. To compensate for the loss of rotator cuff function, several options have been proposed. The preferred option, whenever possible, is to repair the rotator cuff. Good results are obtained in the vast majority of rotator cuff repairs [11–15], with healing of the cuff to the tuberosities [16] and successful reversal of the associated pseudoparalysis [17]. In some circumstances, rotator cuff repair is contraindicated, technically impossible or fails. In severe rotator cuff deficiency, the only remaining muscle able to elevate the arm is the deltoid. In order to allow anterior forward elevation above 90°, the abduction role of the deltoid has to be increased. Reverse shoulder arthroplasty (RSA) was developed to medialise and lower the glenohumeral centre of rotation, thereby increasing the lever arm of the deltoid muscle [18]. Deltoid tension, increased by the lower centre of rotation, increases muscle-fibre recruitment of the anterior and posterior deltoid, compensating for a deficient rotator cuff [19]. Due to the semiconstrained design of the prosthesis, adequate deltoid tension is critical to avoid dislocation. The lever arm of the deltoid muscle is almost doubled following RSA, and therefore, abduction efficiency of the deltoid increases. Under such tension, the reverse glenoid component provides the stable fulcrum essential for shoulder anterior elevation and prosthesis stability [19]. The increase in compressive force between the humeral and glenoid components also has a stabilising effect [20]. Failure to adequately tension the deltoid may result in prosthetic instability, one of the most common clinically significant complications. Moreover, other complications following RSA, such as neurological lesions, fractures of the acromion or fixed abduction of the arm [19–24], have also been described and could be related to excessive deltoid tension.
Few studies have been published about biomechanical implications and consequences of upper-extremity and humeral lengthening following RSA. This article provides a comprehensive review of current concepts pertaining to upper-extremity lengthening in RSA, including a review of pertinent biomechanical changes induced by the implant, risks related to lengthening and techniques to measure arm and humeral lengthening. Lastly, this article determines recommended deltoid tension to provide the best functional outcome without increasing the risk of complications.
Materials and methods
We identified all studies addressing techniques of measuring upper-extremity lengthening and its effect in RSA by conducting a search on PubMed from January 1970 to April 2013 using the combined terms “reverse shoulder arthroplasty”, “prosthesis”, “biomechanics”, “lengthening”, “complications” and “function”. We did not seek to perform a review of all studies documenting biomechanics but instead included only articles in which the primary interest was the technique of measuring lengthening after RSA, complications related to upper-extremity lengthening and the role of lengthening in postoperative function. Studies were included in this systematic review if they were published in English, French or German and provided levels I–IV evidence relevant to the search terms.
Results
The literature search identified seven articles that met the inclusion criteria (Table 1). Four articles described both upper-extremity and humeral lengthening following RSA, with its consequences on function and complication rate [25–28]. One article described the relationship between acromiohumeral distance and deltoid lengthening and postoperative function [29]. Another study limited data to a correlation between acromiohumeral distance and postoperative function [30]. One study described a technique of measuring arm length [31]. We also identified one article that reported the relationship of surgical approach on upper-extremity lengthening [25], and one study focused on the relationship between lengthening and postoperative neurological lesions [26].
Table 1.
Description of studies on upper-extremity lengthening after reverse shoulder arthroplasty (RSA)
| Study | Year | No. of RSAs | Design | Level of evidence |
|---|---|---|---|---|
| Renaud et al. [30] | 2001 | 21 | Retrospective cohort | IV |
| Boileau et al. [19] | 2005 | 45 | Retrospective cohort | IV |
| Lädermann et al. [28] | 2009 | 58 | Retrospective cohort | IV |
| Lädermann et al. [26] | 2011 | 42 | Prospective non randomised study | II |
| Lädermann et al. [25] | 2011 | 144 | Retrospective cohort | IV |
| Lädermann et al. [27] | 2012 | 183 | Retrospective cohort | IV |
| Jobin et al. [29] | 2012 | 49 | Prospective cohort design, treatment study | II |
Factors contributing to upper-extremity lengthening
Adequate deltoid tension is accepted as being critical to prosthetic function and stability [19, 27, 28]. This tension is determined by arm length. Arm length is dependant upon:
Position of the glenosphere in the frontal plane (Fig. 1)
Status of the acromion
Size of the glenosphere
Use of an eccentric or inferiorly tilted glenosphere
Use of an augment or spacer
Thickness of the polyethylene
Type of stem
Height of the humeral cut and consequent level of stem implantation (Fig. 2) [27, 28].
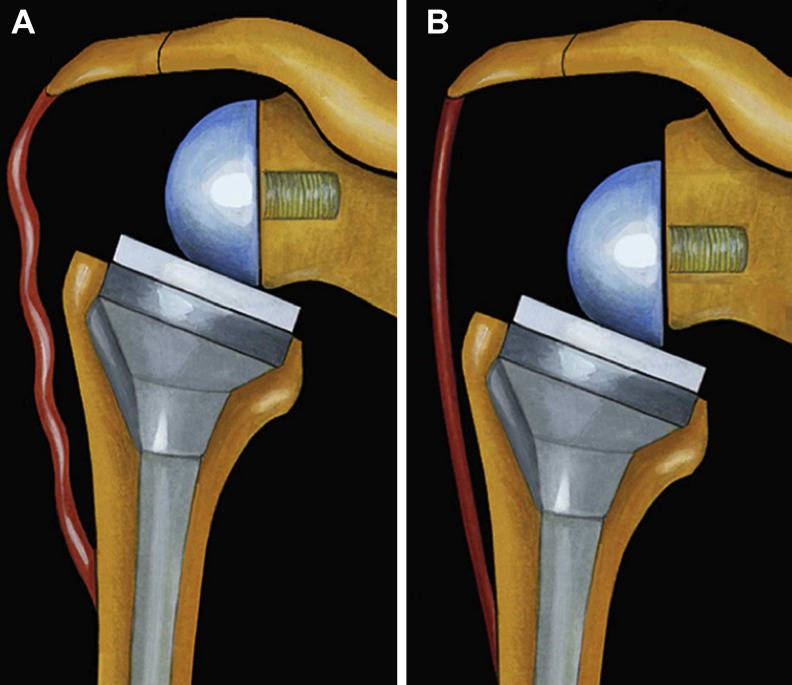
Fig. 1.
Influence of glenosphere position in the vertical plane. a A superior implantation of the baseplate or the use of a noneccentric glenosphere does not allow proper deltoid tensioning. b Use of an eccentric glenosphere or inferior positioning of the glenosphere in the vertical plane allows satisfactory deltoid tensioning. From [27], with permission
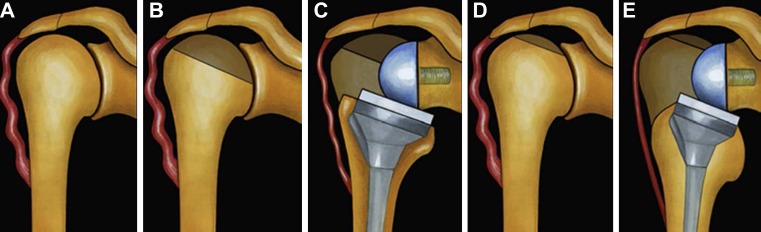
Fig. 2.
Influence of humeral cut on arm length. a Preoperative status with a lack of deltoid tension. b, c Aggressive humeral cut results in low implantation of the stem, with lack of deltoid tension. d, e Minimal humeral cut leads to high implantation of the prosthetic stem, with adequate deltoid tension. From [27], with permission
Glenosphere position is theoretically fixed, as it should be implanted on the lower part of the glenoid to avoid notching [32–36]. The type of glenosphere (size, eccentricity) allows adjustment of arm length by only several millimeters (about 1 % of arm length). Consequently, the key factor for arm length is humeral length determined by height and type of stem, polyethylene thickness and use of an augment or spacer. Collectively, these factors allow arm lengthening by up to several centimeters (about 10 % of arm length).
Measurement of arm, humerus or deltoid length in RSA
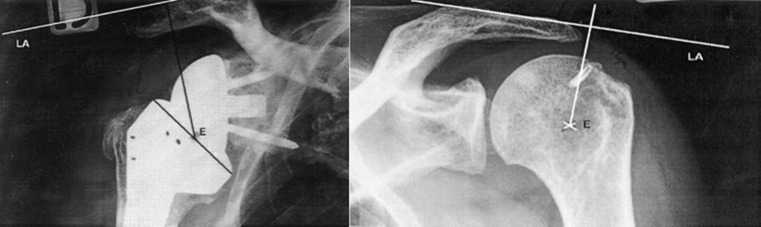
Few measurement techniques have yet been validated and can be either radiographic or clinical. Measurements can focus on upper-extremity (arm) length, humeral length or acromiohumeral distance. Renaud et al. were the first to propose the determination of a “radio-anatomical index” [30]. They described a measuring technique in which anteroposterior (AP) radiographs are compared (Fig. 3). This technique reported on acromiohumeral distance only and used radiographs that were not controlled for magnification. In cases of superior escape of the contralateral humeral head, the normal position of the humeral epiphysis was estimated using a horizontal line that passes perpendicular to the centre of the glenoid. The presence of superior glenoid erosion [37] renders this technique inaccurate.
Fig. 3.
Technique proposed by Renaud et al. Two main lines are placed for measurement: an acromial line that represents the superior cortex of the acromion, and a tangent line to the centre of the prosthetic epiphysis or to the centre of rotation of the humeral head perpendicular to the first line. The two latter lines represent the acromioepiphyseal distance and are compared to provide a ratio of lengthening. From [30], with permission
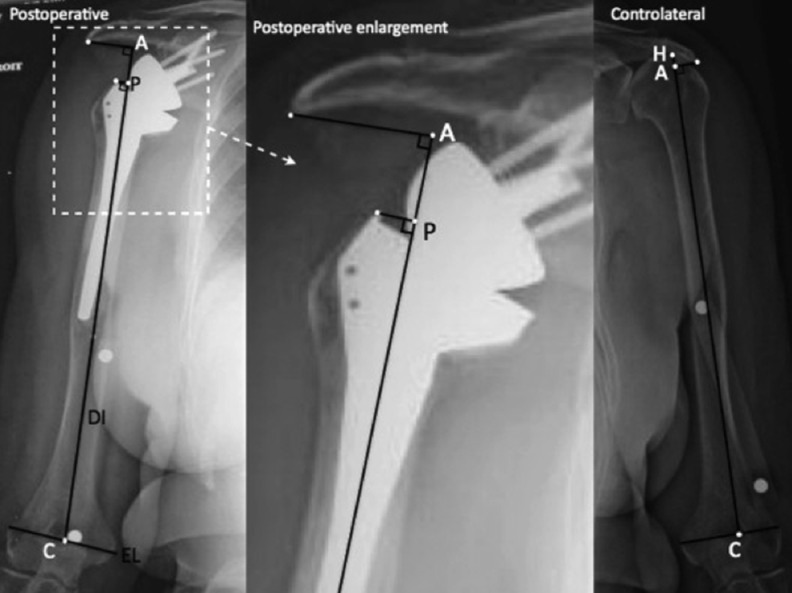
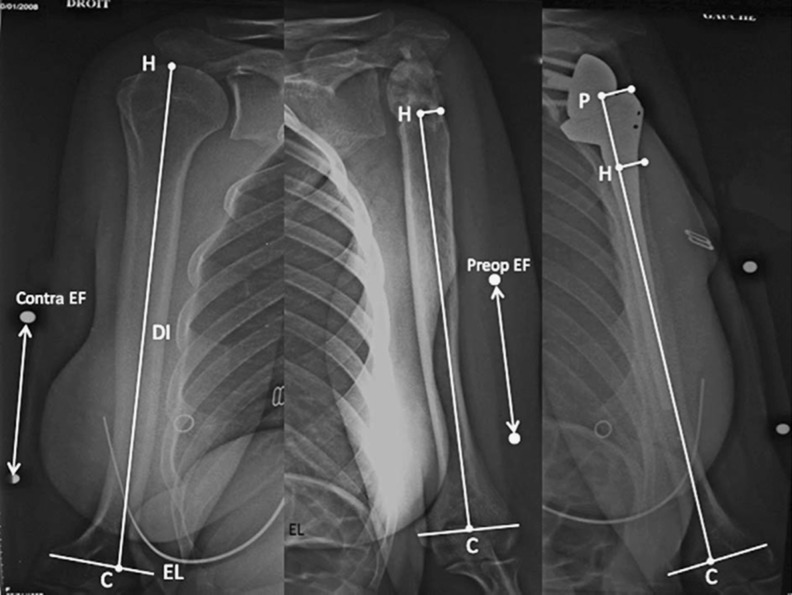
Lädermann et al. presented a technique to determine arm and humeral length using plain radiography [28]. Measurements were taken from bilateral preoperative and postoperative magnification and fluoroscopically controlled AP radiographs of the humerus (Fig. 4) and were made to determine relative arm length using points along the humerus and the acromion. A similar technique to assess the amount of lengthening of the humerus was subsequently reported by Greiner et al. [31]. Lädermann et al. compared the lengths of the affected and contralateral humeral shafts to determine whether the contralateral humerus may be used reliably as a reference for determining prosthetic height in complex cases with humeral bone loss, or when performing a postoperative assessment in revision cases in which preoperative scaled radiographs of the humerus are unavailable [28]. One disadvantage of this technique is the need to perform magnification-controlled radiographs of the entire humerus. As the X-ray beam is centred on the middle third of the humerus, radiographs do not provide an accurate depiction of the acromiohumeral interval. Consequently, this technique accurately reflects humeral length, but accuracy of acromiohumeral interval measurements is compromised. Moreover, this technique requires drawing an epicondylar reference line, which can be difficult if the humerus is not in neutral rotation.
Fig. 4.
Technique of Lädermann et al. [28]. Preoperative and postoperative true anteroposterior, bilateral, magnification-controlled radiographs of the humeri with neutral rotation and the patient standing. An epicondylar line (EL) defined as being between the most lateral part of the medial and lateral epicondyle. The diaphyseal axis (DI) is determined by a line drawn in the centre of the proximal humeral medullary canal. The intersection between the EL and DI represents point C. The intersection between the DI and the top of the humeral head is point H. Point Ais the intersection between the DI and a perpendicular line passing through the most lateral and inferior point of the acromion (A). A, C and H are represented by small white points; large white points correspond to the magnification control marker on the skin of the arm. C condyles, preop preoperative, contra contralateral, EF enlargement factor
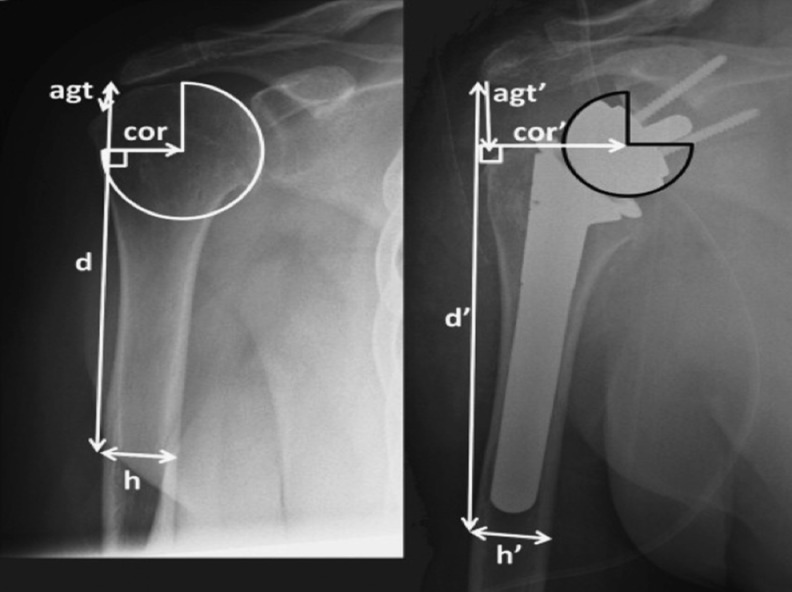
Jobin et al. recently proposed another technique to evaluate subacromial and deltoid length postoperatively [29]. In their study, complete preoperative and postoperative true AP radiographs of the glenohumeral joint in neutral rotation were collected. The subacromial length (acromion to greater tuberosity distance) was measured as the distance from the inferolateral acromial tip to the most prominent superolateral aspect of the greater tuberosity (Fig. 5). The middle deltoid length was defined as the distance between the inferolateral tip of the acromion to the midpoint of the deltoid tuberosity with the arm in neutral rotation and 0° abduction, as proposed initially by De Wilde [38]. Length was calibrated by the known diameter of the glenosphere and the fixed bony distances of the humeral shaft width, and the fixed bony distance from the greater tuberosity to the deltoid tuberosity. The technique of Jobin et al. calibrates each radiograph to the glenosphere diameter. Consequently, one inconvenience is the impossibility of determining humeral and subacromial length preoperatively. This technique is therefore not useful in preoperative planning of difficult cases. Furthermore, the greater tuberosity was selected for the proximal reference point. This anatomical landmark may be absent preoperatively, or if it is present, it may be difficult to visualise because of arm rotation. Moreover, humeral radiolucencies, stem subsidence, radiological signs of stress shielding and resorption of tuberosities are common complications after RSA [34] that can compromise anatomical landmarks used in this technique.
Fig. 5.
Technique of Jobin et al. Radiographic measurement of deltoid length from the inferolateral acromion tip to the midpoint of the deltoid tuberosity preoperatively (left, d) and postoperatively (right, d’). From [29], with permission
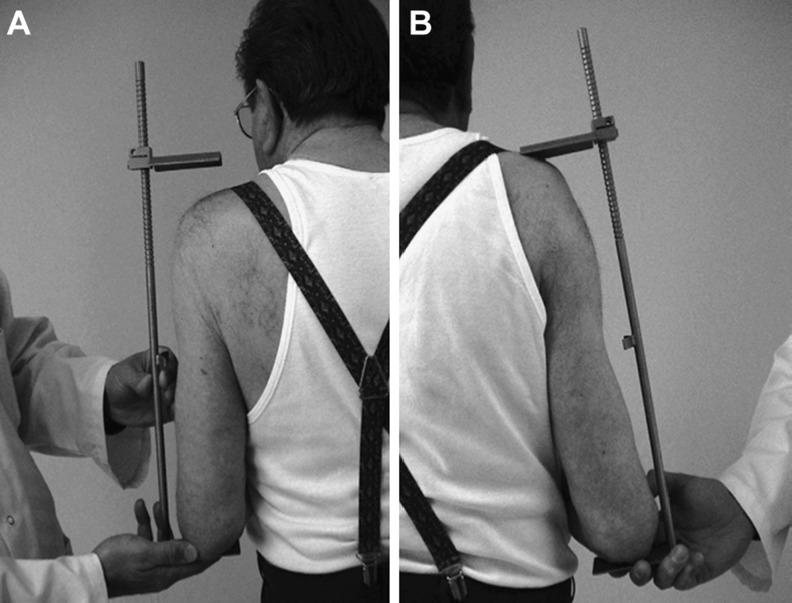
Lastly, Boileau et al. measured the postoperative length of the arm relative to the opposite side using a specially designed caliper (Fig. 6) [19]. This technique is noninvasive but neither gives information on humeral or subacromial length nor does it allow for preoperative planning.
Fig. 6.
Distance between acromion and olecranon with the elbow flexed is determined on a nonoperated and b operated sides. From [19], with permission
Results for arm, humerus and subacromial lengthening
Postoperative lengthening of the arm, humerus and subacromial space (acromiohumeral interval) is summarised in Table 2. Mean lengthening varied from 15 mm to 27 mm for the arm and from minus five to five millimetres for the humerus. The humeral cut was more aggressive when a transdeltoid surgical approach was performed; this was compensated for by an increase in thickness of the polyethylene liner. Mean subacromial lengthening reported in two studies was 23 mm [28, 29].
Table 2.
Mean lengthening of arm, humerus and subacromial space postoperatively in millimetres
| Study | Arm (deltoid lengthening) | Humerus | Subacromial space (acromiohumeral distance) |
|---|---|---|---|
| Renaud et al. [30] | NA | NA | NA |
| Boileau et al. [19] | 15 ± 11 (5–40)a | NA | NA |
| Lädermann et al. [28] | 23 ± 12 (4–47)b
20 ± 11 (−2 to 48)a |
2 ± 6 (−10 to16)a | 23 ± 9 (5–41)b |
| Greiner et al. [31] | 17 ± 13 (−10 to 45) | NA | NA |
| Lädermann et al. [26] | 27 ± 18 (0–59)a | NA | NA |
| Lädermann et al. [25] | DP 17 ± 17, TD 12 ± 1.4a | DP 5 ± 13, TD −5 ± 10a | NA |
| Lädermann et al. [27] | 16 ± 19 (−51 to 54)a | 2 ± 14 (−47 to 52)a | NA |
| Jobin et al. [29] | 21 ± 10b | NA | 23 ± 9b |
Values are mean ± standard deviation (range)
DP deltopectoral approach, NA not available, TD transdeltoid approach
aCompared with contralateral side
bCompared with ipsilateral side
Relationship between lengthening and postoperative function
Functional outcomes after RSA have shown variable results for range of motion (ROM) [23, 39–41]. Poor postoperative anterior elevation can be attributed to improper use, poor patient selection and preoperative and postoperative problems [41, 42]. Renaud et al. demonstrated a correlation between a subacromial space lengthening of 33–50 % and: (1) Constant score [43] ≥65.5 points (p = 0.024), (2) anterior elevation ≥120° (p = 0.001), and (3) gain in abduction ≥60° (p = 0.016) [30]. Lädermann et al. compared patients with arm lengthening and those with shortening and found that the postoperative active anterior elevation was significantly greater for arm lengthening (145° vs 122°), with a mean difference of 23° (p < 0.001) [27]. Jobin et al. also confirmed that deltoid lengthening correlated significantly (p = 0.002) with active anterior elevation [29]. In their study, deltoid lengthening that achieved an acromion-to-greater-tuberosity distance over 38 mm had a 90 % positive predictive value (PPV) of obtaining 135° of active anterior elevation. These clinical findings confirmed biomechanical studies that demonstrate the crucial role of the deltoid in postoperative function [18, 44]. However, arm lengthening showed no relationship to outcome scores, including Constant [43], Disabilities of the Arm, Shoulder and Hand (DASH) [45], American Shoulder and Elbow Surgeons (ASES) [46] or Simple Shoulder Test (SST) [29, 31] scores.
Relationship between lengthening and postoperative complications
Dislocation
Dislocation is one of the most common complications after RSA, with rates as high as 14 % and accounting for almost half of the complications in some series [21, 41, 47–53]. Most cases of dislocation occur during the first few months after implantation and are a result of a technical error [54]. The aetiology of dislocation is multifactorial. It can occur due to:
Lack of anterior restraints including subscapularis insufficiency, conjoint tendon weakness [55] and pectoralis major insufficiency
Component malpositioning
Impingement
Infection
Instability is more frequent in cases of revision arthroplasty [56]. Deltoid insufficiency can be caused by preoperative factors [41, 42] or result from a postoperative lack of deltoid tension, acromial or scapular spine fracture (Fig. 7), polyethylene wear, stem subsidence or postoperative neurological palsy. Interestingly, no previous studies have reported increased rates of postoperative dislocation after acromial or scapular spine fractures [57, 58]. Lädermann et al. noted a strong correlation (p < 0.0001) between preoperative humeral length and postoperative dislocation. Postoperative shortening of the humerus, compared with preoperative or contralateral humeral length, was observed in all cases of dislocation.
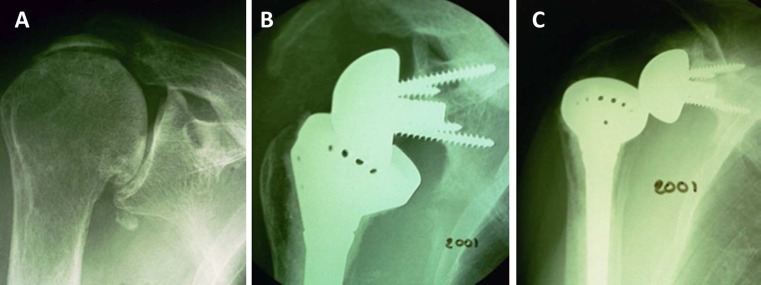
Fig. 7.
a Preoperative anteroposterior X-ray of a right shoulder with an acromial fatigue fracture. b At 2 years of follow-up, a postoperative tilt of the acromion and a grade 4 scapular notch are noted. c Prosthetic dislocation could be related to the lack of deltoid tension
Acromial or scapular spine fractures
The arm is lengthened by approximately 15–27 mm following RSA (Table 2). Biomechanically, tension on the deltoid and acromion is subsequently increased as a result of this lengthening. Preoperative and/or postoperative acromial pathology, which could compromise deltoid function and consequently affect the function of the prosthesis, is of legitimate concern. Postoperative fractures occur in at least in 3 % of cases [59], and their causes are theoretically numerous. Preoperatively, the acromion may be subject to a congenital or acquired abnormality, such as an os acromiale [58]. It may also already be eroded, fragmented or even fractured from the superiorly migrated humeral head in cases of cuff-tear arthropathy or osteoporosis-induced insufficiency. The superior base-plate fixation screw may function as a stress riser that results in acromial fractures (Fig. 8) [60, 61]. It seems that the most significant risk factor is preoperative osteoporosis [61].
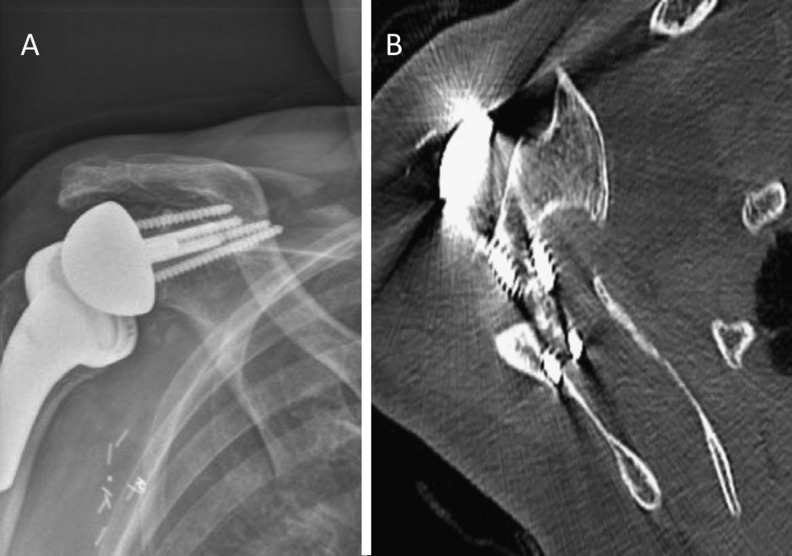
Fig. 8.
a Postoperative anteroposterior X-ray of a right shoulder with a scapular spine fracture. b Axial computed tomography scan reveals the superior metaglene fixation screw may function as a stress riser that results in fracture
Neurological lesions
Clinically relevant neurological complications involving the brachial plexus or the axillary nerve are considered rare [21, 62–65]. A prospective study determined the incidence of peripheral nerve lesions as determined by electromyographical analysis following RSA [26]. If one also takes into account subclinical deterioration of preoperative lesions, 63 % of patients in this study had postoperative neurological lesions. The prevalence of peripheral nerve lesions determined by electromyographical analysis following RSA is thus common, but patients usually recover. Arm lengthening during RSA, because of its nonanatomical design and/or manoeuvre of glenohumeral reduction, may be a major factor responsible for the increased prevalence of neurological injury.
Discussion
RSA is a commonly performed procedure, and its indications continue to expand. Despite the relatively high complication rate [22, 54, 66–68], RSA continues to be performed because of the significant postoperative improvement in shoulder function and the high rate of patient satisfaction. Ways to prevent complications associated with RSA require further investigation. A better understanding of the biomechanical implications of inserting an RSA may help avoid some of these complications. Obtaining an improved understanding of the relationship between these biomechanical effects and complications was the purpose of this review.
At present, there is no described standardised preoperative planning technique for determining appropriate implant position based on deltoid tension or length. Intraoperative criteria have been proposed by other authors to assess prosthetic stability. Recommendations are numerous and include:
Implanting the prosthesis in such a way that it is difficult to reduce
Absence of pistoning of the prosthesis when applying axial traction on the arm
Stability throughout a full ROM
Passive adduction of the arm to neutral with the elbow at the side
Palpation of tension in the conjoint tendon after reduction, with the arm at the side and the elbow extended [19]
No asymmetric subluxation or tilting of the proximal humeral component on the glenosphere during adduction [18]
Free glenohumeral motion without scapula–thoracic motion between 0° and 60° of abduction [69]
These intraoperative criteria, however,are qualitative, subjective and depend more on patient relaxation (i.e. depth of anaesthesia and quality of muscle relaxation) and preoperative scar tissue (i.e. post-traumatic arthritis or revision arthroplasty versus primary arthroplasty) than on objective measurements to assess the appropriate length of the deltoid or the arm. Some authors even recommend the use of a “Jedi skill that involves using the Force”, rather than the previously mentioned criteria [70]. A preoperative guide, useful in complex cases such as revision arthroplasty or post-traumatic arthritis where scar tissue and bone loss prevent making an accurate determination of humeral length, has thus been proposed (Fig. 9) [28]. Preoperative planning is probably not necessary in all primary cases; its use in revision cases, however, seems mandatory. To guarantee the best possible functional results, restoration of the appropriate humeral and arm length should be the goal [27, 29, 30]. Failure to restore sufficient deltoid tension may be responsible for poor anterior elevation and prosthetic instability [27, 28, 54]. Implantation of the humeral stem at the level of the humeral cut using the thickness of the polyethylene insert to obtain appropriate deltoid tension seems to be a reasonable option [25, 28].
Fig. 9.
Proposition for determining the height at which the prosthesis should be implanted by planning of the operation with a 10-cm marker: A = corrected length of contralateral humerus = CHcontra × 10: contra EF = 314 mm. B = corrected length of the preoperative humerus = CHipsi × 10: preop EF = 264 mm. A-B = corrected length of the missing bone. PHipsi = A-B = 50 mm. PHipsi is the exact distance in millimetres that we must measure at the time of implantation between the lateral cortex of the humerus (Hipsi) and the superolateral part of the metallic stem (P). A acromion, C condyles, Hcontra head, EP epicondylar line, DI diaphyseal axis, pre-op preoperative, contra contralateral, EF enlargement factor
Excessive lengthening of the arm may be responsible for neurological lesions, acromial or scapular spine fractures or fixed arm abduction [26, 28]. One study demonstrated a high prevalence of acute postoperative subclinical neurological lesions after RSA [26]. Lengthening of the arm during this procedure, because of its nonanatomical design and/or manoeuvre of glenohumeral reduction, might be a major factor responsible for the high prevalence of neurological injury. The risk of neurological lesions increases drastically with more than four centimetres of lengthening. An absolute lengthening threshold expressed in centimetres is, however, difficult to determine. Seemingly, a ratio that takes into consideration the total length of the upper limb of the patient, thus representing a percentage of lengthening, would be more accurate. However, this concept must be applied with caution, as lengthening beyond two centimetres compared with preoperative measurement may increase the frequency of postoperative neurological injury [26]. As a result, strategies have been developed to limit upper-extremity lengthening in RSA. In cases with a high risk of dislocation, such as revisions or proximal humeral bone loss, use of larger-diameter glenoid components, a superior approach and prosthetic or bony lateralisation of the glenosphere can be considered to avoid excessive tension [71, 72]. Nevertheless, if the preoperatively planned lengthening is over four centimetres, the authors recommend using intraoperative nerve monitoring [73].
Conclusion
Studies in this systematic review indicate that adequate deltoid tension obtained through restoration of humeral length and increase of the acromiohumeral interval is the key for adequate postoperative function preventing instability. Arm lengthening should be controlled, with zero to two centimetres being a reasonable goal to avoid postoperative neurological impairment. Current conventional radiographic preoperative planning techniques are inaccurate. Development of new preoperative and intraoperative aides for surgeons, using software, intraoperative guides and other imaging modalities such as computed tomography and magnetic resonance imaging, are required.
Acknowledgments
Disclaimer
A. Lädermann, his immediate family, and any research foundation with which he is affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article. Tom Bradley Edwards received royalties from, serves as a paid consultant to or is an employee of, or has received research or institutional support from Tornier. Gilles Walch received royalties from Tornier.
References
- 1.Baulot E, Sirveaux F, Boileau P. Grammont’s idea: the story of Paul Grammont’s functional surgery concept and the development of the reverse principle. Clin Orthop Relat Res. 2011;469(9):2425–2431. doi: 10.1007/s11999-010-1757-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer DC, Farshad M, Amacker NA, Gerber C, Wieser K. Quantitative analysis of muscle and tendon retraction in chronic rotator cuff tears. Am J Sports Med. 2012;40(3):606–610. doi: 10.1177/0363546511429778. [DOI] [PubMed] [Google Scholar]
- 3.Williams MD, Lädermann A, Melis B, Barthelemy R, Walch G. Fatty infiltration of the supraspinatus: a reliability study. J Shoulder Elbow Surg. 2009;18(4):581–587. doi: 10.1016/j.jse.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Zanetti M, Gerber C, Hodler J. Quantitative assessment of the muscles of the rotator cuff with magnetic resonance imaging. Investig Radiol. 1998;33(3):163–170. doi: 10.1097/00004424-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 6.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty infiltration of disrupted rotator cuff muscles. Rev Rhum. 1995;62(6):415–422. [PubMed] [Google Scholar]
- 7.Goutallier D, Postel JM, Lavau L, Bernageau J. Impact of fatty degeneration of the suparspinatus and infraspinatus msucles on the prognosis of surgical repair of the rotator cuff. Rev Chir Orthop Reparatrice Appar Mot. 1999;85(7):668–676. [PubMed] [Google Scholar]
- 8.Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2012;21(7):847–858. doi: 10.1016/j.jse.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boileau P, Baque F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747–757. doi: 10.2106/JBJS.E.01097. [DOI] [PubMed] [Google Scholar]
- 10.Lädermann A, Collin P, Walch G (2012) Correlation of the involved compartments of massive rotator cuff tear and loss of active shoulder range of motion. Knee Surg Sports Traumatol Arthrosc 20(Suppl 1):S1–S3. doi: 10.1007/s00167-012-1934-5
- 11.Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of a consecutive series of subscapularis tendon tears repaired arthroscopically. Arthroscopy. 2012 doi: 10.1016/j.arthro.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 12.Denard PJ, Jiwani AZ, Lädermann A, Burkhart SS. Long-term outcome of arthroscopic massive rotator cuff repair: the importance of double-row fixation. Arthroscopy. 2012;28(7):909–915. doi: 10.1016/j.arthro.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Denard PJ, Lädermann A, Burkhart SS. Arthroscopic management of subscapularis tears. Sports Med Arthrosc Rev. 2011;19(4):333–341. doi: 10.1097/JSA.0b013e31822d41c6. [DOI] [PubMed] [Google Scholar]
- 14.Lädermann A, Denard PJ, Burkhart SS. Revision arthroscopic rotator cuff repair: systematic review and authors’ preferred surgical technique. Arthroscopy. 2012;28(8):1160–1169. doi: 10.1016/j.arthro.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Lädermann A, Denard PJ, Burkhart SS. Midterm outcome of arthroscopic revision repair of massive and nonmassive rotator cuff tears. Arthroscopy. 2011;27(12):1620–1627. doi: 10.1016/j.arthro.2011.08.290. [DOI] [PubMed] [Google Scholar]
- 16.Zumstein MA, Jost B, Hempel J, Hodler J, Gerber C. The clinical and structural long-term results of open repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2008;90(11):2423–2431. doi: 10.2106/JBJS.G.00677. [DOI] [PubMed] [Google Scholar]
- 17.Denard PJ, Lädermann A, Jiwani AZ, Burkhart SS. Functional outcome after arthroscopic repair of massive rotator cuff tears in individuals with pseudoparalysis. Arthroscopy. 2012;28(9):1214–1219. doi: 10.1016/j.arthro.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 18.Grammont PM, Trouilloud P, Latfay J, Deries X. Etude et réalisation d’une nouvelle prothèse d’épaule. Rhumatologie. 1987;39:407–418. [Google Scholar]
- 19.Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 Suppl S):147S–161S. doi: 10.1016/j.jse.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250–257. doi: 10.1097/00003086-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 21.Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527–540. doi: 10.1016/j.jse.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Scarlat MM. Complications with reverse total shoulder arthroplasty and recent evolutions. Int Orthop. 2013;37(5):843–851. doi: 10.1007/s00264-013-1832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirveaux F, Favard L, Oudet D, Huguet D, Lautman S. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive and non repairable cuff rupture. In: Walch G, Boileau P, Molé D, editors. 2000 shoulder prostheses: Two to ten year follow-up. Montpellier: Sauramps Medical; 2001. pp. 247–252. [Google Scholar]
- 24.Valenti PH, Boutens D, Nerot C. Delta 3 reversed prosthesis for osteoarthritis with massive rotator cuff tear: Long term results (> 5 years) In: Walch G, Boileau P, Molé D, editors. 2000 shoulder prostheses: Two to ten year follow-up. Montpellier: Sauramps Medical; 2001. pp. 253–259. [Google Scholar]
- 25.Lädermann A, Lubbeke A, Collin P, Edwards TB, Sirveaux F, Walch G. Influence of surgical approach on functional outcome in reverse shoulder arthroplasty. Orthop Traumatol Surg Res. 2011;97(6):579–582. doi: 10.1016/j.otsr.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Lädermann A, Lubbeke A, Mélis B, Stern R, Christofilopoulos P, Bacle G, Walch G. Prevalence of neurologic lesions after total shoulder arthroplasty. J Bone Joint Surg Am. 2011;93(14):1288–1293. doi: 10.2106/JBJS.J.00369. [DOI] [PubMed] [Google Scholar]
- 27.Lädermann A, Walch G, Lubbeke A, Drake GN, Mélis B, Bacle G, Collin P, Edwards TB, Sirveaux F. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(3):336–341. doi: 10.1016/j.jse.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Lädermann A, Williams MD, Mélis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(4):588–595. doi: 10.1016/j.jse.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Jobin CM, Brown GD, Bahu MJ, Gardner TR, Bigliani LU, Levine WN, Ahmad CS. Reverse total shoulder arthroplasty for cuff tear arthropathy: the clinical effect of deltoid lengthening and centre of rotation medialization. J Shoulder Elbow Surg. 2012;21(10):1269–1277. doi: 10.1016/j.jse.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 30.Renaud P, Wahab H, Bontoux L, Dauty M, Richard I, Bregeon C. Total inverted shoulder prosthesis and rotator cuff insufficiency: evaluation and determination of anatomical parameters predictive of good functional outcome in 21 shoulders. Ann Readapt Med Phys: Rev Sci Soc Fr Reeducation Fonctionnelle Readaptat Med Phys. 2001;44(5):273–280. doi: 10.1016/s0168-6054(01)00102-7. [DOI] [PubMed] [Google Scholar]
- 31.Greiner SH, Back DA, Herrmann S, Perka C, Asbach P. Degenerative changes of the deltoid muscle have impact on clinical outcome after reversed total shoulder arthroplasty. Arch Orthop Trauma Surg. 2010;130(2):177–183. doi: 10.1007/s00402-009-1001-y. [DOI] [PubMed] [Google Scholar]
- 32.De Biase CF, Ziveri G, Delcogliano M, de Caro F, Gumina S, Borroni M, Castagna A, Postacchini R. The use of an eccentric glenosphere compared with a concentric glenosphere in reverse total shoulder arthroplasty: two-year minimum follow-up results. Int Orthop. 2013;37(10):1949–1955. doi: 10.1007/s00264-013-1947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levigne C, Garret J, Boileau P, Alami G, Favard L, Walch G. Scapular notching in reverse shoulder arthroplasty: is it important to avoid it and how? Clin Orthop Relat Res. 2011;469(9):2512–2520. doi: 10.1007/s11999-010-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mélis B, DeFranco M, Lädermann A, Mole D, Favard L, Nerot C, Maynou C, Walch G. An evaluation of the radiological changes around the Grammont reverse geometry shoulder arthroplasty after eight to 12 years. J Bone Joint Surg Br. 2011;93(9):1240–1246. doi: 10.1302/0301-620X.93B9.25926. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297–1304. doi: 10.2106/JBJS.L.00820. [DOI] [PubMed] [Google Scholar]
- 36.Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg. 2005;14(5):524–528. doi: 10.1016/j.jse.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388–395. doi: 10.1302/0301-620x.86b3.14024. [DOI] [PubMed] [Google Scholar]
- 38.De Wilde LF, Audenaert EA, Berghs BM. Shoulder prostheses treating cuff tear arthropathy: a comparative biomechanical study. J Orthop Res. 2004;22(6):1222–1230. doi: 10.1016/j.orthres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Dedy NJ, Stangenberg M, Liem D, Hurschler C, Simmen B, Riner M, Marquardt B, Steinbeck J. Effect of posterior offset humeral components on range of motion in reverse shoulder arthroplasty. Int Orthop. 2011;35(4):549–554. doi: 10.1007/s00264-010-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortmaier R, Resch H, Hitzl W, Mayer M, Blocher M, Vasvary I, Mattiassich G, Stundner O, Tauber M. Reverse shoulder arthroplasty combined with latissimus dorsi transfer using the bone-chip technique. Int Orthop. 2013 doi: 10.1007/s00264-013-2139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 42.Lädermann A, Walch G, Denard PJ, Collin P, Sirveaux F, Favard L, Edwards TB, Kherad O, Boileau P. Reverse shoulder arthroplasty in patients with pre-operative impairment of the deltoid muscle. Bone Joint J. 2013;95-B(8):1106–1113. doi: 10.1302/0301-620X.95B8.31173. [DOI] [PubMed] [Google Scholar]
- 43.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 44.Schwartz DG, Kang SH, Lynch TS, Edwards S, Nuber G, Zhang LQ, Saltzman M. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2012 doi: 10.1016/j.jse.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Germann G, Wind G, Harth A. The DASH(Disability of Arm-Shoulder-Hand) Questionnaire–a new instrument for evaluating upper extremity treatment outcome. Handchir Mikrochir Plast Chir: Organ Deutschsprachigen Arbeitsgemeinschaft Handchir: Organ Deutschsprachigen Arbeitsgemeinschaft Mikrochir Peripheren Nerven Gefasse. 1999;31(3):149–152. doi: 10.1055/s-1999-13902. [DOI] [PubMed] [Google Scholar]
- 46.Michener LA, McClure PW, Sennett BJ. American shoulder and elbow surgeons standardized shoulder assessment form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587–594. doi: 10.1067/mse.2002.127096. [DOI] [PubMed] [Google Scholar]
- 47.Cazeneuve JF, Cristofari DJ. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2006;92(6):543–548. doi: 10.1016/s0035-1040(06)75911-6. [DOI] [PubMed] [Google Scholar]
- 48.Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244–1251. doi: 10.2106/JBJS.G.00775. [DOI] [PubMed] [Google Scholar]
- 49.rCuff DJ, Virani NA, Levy J, Frankle MA, Derasari A, Hines B, Pupello DR, Cancio M, Mighell M. The treatment of deep shoulder infection and glenohumeral instability with debridement, reverse shoulder arthroplasty and postoperative antibiotics. J Bone Joint Surg Br. 2008;90(3):336–342. doi: 10.1302/0301-620X.90B3.19408. [DOI] [PubMed] [Google Scholar]
- 50.De Wilde L, Sys G, Julien Y, Van Ovost E, Poffyn B, Trouilloud P. The reversed Delta shoulder prosthesis in reconstruction of the proximal humerus after tumour resection. Acta Orthop Belg. 2003;69(6):495–500. [PubMed] [Google Scholar]
- 51.Gohlke F, Rolf O. Revision of failed fracture hemiarthroplasties to reverse total shoulder prosthesis through the transhumeral approach : method incorporating a pectoralis-major-pedicled bone window. Oper Orthop Traumatol. 2007;19(2):185–208. doi: 10.1007/s00064-007-1202-x. [DOI] [PubMed] [Google Scholar]
- 52.Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89(2):292–300. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 53.Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476–1486. doi: 10.2106/JBJS.D.02342. [DOI] [PubMed] [Google Scholar]
- 54.Farshad M, Gerber C. Reverse total shoulder arthroplasty-from the most to the least common complication. Int Orthop. 2010;34(8):1075–1082. doi: 10.1007/s00264-010-1125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892–896. doi: 10.1016/j.jse.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Zumstein MA, Pinedo M, Old J, Boileau P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2011;20(1):146–157. doi: 10.1016/j.jse.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Mottier F, Wall B, Nove-Josserand L, Galoisy Guibal L, Walch G. Reverse prosthesis and os acromiale or acromion stress fracture. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(2):133–141. doi: 10.1016/s0035-1040(07)90216-0. [DOI] [PubMed] [Google Scholar]
- 58.Walch G, Mottier F, Wall B, Boileau P, Mole D, Favard L. Acromial insufficiency in reverse shoulder arthroplasties. J Shoulder Elbow Surg. 2009;18(3):495–502. doi: 10.1016/j.jse.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 59.Molé D, Favard L. Excentreed scapulohumeral osteoarthritis. Rev Chir Orthop Reparatrice Appar Mot. 2007;93(6 Suppl):37–94. doi: 10.1016/s0035-1040(07)92708-7. [DOI] [PubMed] [Google Scholar]
- 60.Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res. 2011;469(9):2544–2549. doi: 10.1007/s11999-011-1881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Otto RJ, Virani NA, Levy JC, Nigro PT, Cuff DJ, Frankle MA. Scapular fractures after reverse shoulder arthroplasty: evaluation of risk factors and the reliability of a proposed classification. J Shoulder Elbow Surg. 2013 doi: 10.1016/j.jse.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Boardman ND, 3rd, Cofield RH. Neurologic complications of shoulder surgery. Clin Orthop Relat Res. 1999;368:44–53. [PubMed] [Google Scholar]
- 63.Bohsali KI, Wirth MA, Rockwood CA., Jr Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279–2292. doi: 10.2106/JBJS.F.00125. [DOI] [PubMed] [Google Scholar]
- 64.Lynch NM, Cofield RH, Silbert PL, Hermann RC. Neurologic complications after total shoulder arthroplasty. J Shoulder Elbow Surg. 1996;5(1):53–61. doi: 10.1016/s1058-2746(96)80031-0. [DOI] [PubMed] [Google Scholar]
- 65.Plausinis D, Kwon YW, Zuckerman JD. Complications of humeral head replacement for proximal humeral fractures. Instr Course Lect. 2005;54:371–380. [PubMed] [Google Scholar]
- 66.Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517–524. doi: 10.1007/s00264-009-0780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure–midterm results. Int Orthop. 2011;35(1):53–60. doi: 10.1007/s00264-010-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ortmaier R, Resch H, Matis N, Blocher M, Auffarth A, Mayer M, Hitzl W, Tauber M. Reverse shoulder arthroplasty in revision of failed shoulder arthroplasty-outcome and follow-up. Int Orthop. 2013;37(1):67–75. doi: 10.1007/s00264-012-1742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valenti P, Katz D (2005) Comment implanter une prothèse inversée? Maîtrise Orthopédique. http://www.maitrise-orthop.com/viewPage.do?id=796
- 70.Phipatanakul W, Norris T. Complications and treatment of reverse shoulder prosthesis. In: Dines D, Williams G, Laurencin C, editors. Arthritis and arthroplasty: The shoulder. vol arthritis and arthroplasty. Philadelphia: Saunders; 2009. [Google Scholar]
- 71.Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469(9):2558–2567. doi: 10.1007/s11999-011-1775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clin Orthop Relat Res. 2011;469(9):2440–2451. doi: 10.1007/s11999-011-1892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagda SH, Rogers KJ, Sestokas AK, Getz CL, Ramsey ML, Glaser DL, Williams GR., Jr Neer Award 2005: peripheral nerve function during shoulder arthroplasty using intraoperative nerve monitoring. J Shoulder Elbow Surg. 2007;16(3 Suppl):S2–S8. doi: 10.1016/j.jse.2006.01.016. [DOI] [PubMed] [Google Scholar]